Abstract
Nox family NADPH oxidases serve a variety of functions requiring reactive oxygen species (ROS) generation, including antimicrobial defense, biosynthetic processes, oxygen sensing, and redox-based cellular signaling. We explored targeting, assembly, and activation of several Nox family oxidases, since ROS production appears to be regulated both spatially and temporally. Nox1 and Nox3 are similar to the phagocytic (Nox2-based) oxidase, functioning as multicomponent superoxide-generating enzymes. Factors regulating their activities include cytosolic activator and organizer proteins and GTP-Rac. Their regulation varies, with the following rank order: Nox2 > Nox1 > Nox3. Determinants of subcellular targeting include: (a) formation of Nox-p22phox heterodimeric complexes allowing plasma membrane translocation, (b) phospholipids-binding specificities of PX domain-containing organizer proteins (p47phox or Nox organizer 1 (Noxo1 and p40phox), and (c) variably splicing of Noxo1 PX domains directing them to nuclear or plasma membranes. Dual oxidases (Duox1 and Duox2) are targeted by different mechanisms. Plasma membrane targeting results in H2O2 release, not superoxide, to support extracellular peroxidases. Human Duox1 and Duox2 have no demonstrable peroxidase activity, despite their extensive homology with heme peroxidases. The dual oxidases were reconstituted by Duox activator 2 (Duoxa2) or two Duoxa1 variants, which dictate maturation, subcellular localization, and the type of ROS generated by forming stable complexes with Duox. Antioxid Redox Signal. 11, 2607–2619.
Introduction
Reactive oxygen species (ROS) have long been considered in a pathological context as culprits that inflict tissue damage under conditions of chronic or acute inflammatory disease. Their production can be incidental to enzymatic reactions involving oxygen intermediates or, in the case of phagocytic cells, they are produced deliberately by NADPH oxidase activation to kill microbial pathogens during phagocytosis. Within the last decade, there has been a newfound appreciation of deliberate ROS production in a variety of essential biological processes. These include developmental and differentiation processes, extracellular matrix cross-linking, hormone biosynthesis, cellular senescence, apoptosis, responses to oxygenation (oxygen sensing), and cellular signaling responses to growth factors, hormones, and cytokines. The recognition of these new roles for ROS has occurred in parallel with the discovery of an entire family of ROS-generating NADPH oxidases related to the phagocytic system, now known as Nox enzymes. Among the seven known mammalian Nox family NADPH oxidases (Nox1‱Nox5, Duox1, and Duox2), several are known to serve essential roles based on the effects of spontaneous or disease-related mutations and targeted gene disruption. Defects in the phagocytic (Nox2-based) oxidase have been known for decades, resulting in chronic granulomatous disease (CGD), which is characterized by enhanced susceptibility to microbial infection and dysregulated inflammation. Recent Nox1 knockout and overexpression studies reveal a role of Nox1 in angiotensin II-based regulation of vascular tone (20, 25, 38, 68). A Nox3-based oxidase is required for development of otoconial structures in the inner ear; mutations in this system cause deficits in balance and gravity perception in the inner ear (60, 78, 80). The effects of Duox2 system mutations reveal its essential role in thyroid hormone biosynthesis in man and in the mouse (51, 74, 111). Recent surveys of the evolutionary origins of Nox family suggest that deliberate ROS production by these oxidases occurs in all multicellular organisms in both plant and animal kingdoms (9, 57).
In all of these Nox-related functions, it appears that ROS production is subject to tight regulation, both spatially and temporally, in order to avoid collateral damage to oxidant-sensitive host cell components. The respiratory burst oxidase mounted by phagocytic cells normally generates potent oxidants within the confines of the phagosome to kill ingested microbes (103). Recent studies on the composition, biosynthesis, subcellular targeting, and function of the novel Nox family oxidases suggest a variety of mechanisms by which oxidative tissue damage is minimized by controlling the amount, duration, and specific sites of ROS generation. Redox-based intracellular and extracellular signaling can be limited to particular membrane domains or compartments, designed for localized signaling at sites such as at the leading edge of migrating cells, in endosomes, or in receptor signaling complexes (105). Nox4 was proposed to function in some cells as an intracellular signaling oxidase, consistent with its detection in nuclear or perinuclear regions (3, 39, 61, 67). In cases involving Duox isozymes in polarized epithelium, high hydrogen peroxide (H2O2) production is limited to the apical plasma membrane for biosynthetic purposes or other reactions supported by extracellular peroxidases (e.g., thyroperoxidase (TPO), lactoperoxidase (LPO), ovoperoxidase) (26). Thus, a complete understanding of their function as deliberate ROS generators requires an appreciation of the factors that regulate their activity and target the active enzymes to particular subcellular compartments, as well as the types of ROS they generate at these sites. This review provides an update on the regulation and function of several Nox family NADPH oxidases, emphasizing our recent findings in reconstituted oxidase systems, where we have explored factors affecting subcellular localization of oxidase components and the type of ROS produced.
The ROS-Generating Catalytic Mechanism of Nox Family Oxidases
All Nox family NADPH oxidases are transmembrane electron carriers that use NADPH as an electron source and molecular oxygen as an acceptor (Fig. 1). They function as electrogenic enzymes that pass electrons from the cytoplasm through a membrane into some extracytoplasmic space, either the cell exterior or intracellular compartments such as phagosomes, endosomes, the endoplasmic reticulum, or perinuclear compartment. The core catalytic Nox components all contain C-terminal reductase domains, with conserved NADPH and FAD-binding sequences. These domains are anchored to a common heme-containing domain composed of six membrane-imbedded alpha-helical segments. In the case of the Nox enzymes, helix 3 and 5 each contain two invariant histidine residues located near the membrane interfaces that coordinate two heme molecules. The Dual oxidases (Duox1 and Duox2) share these features, but have an additional N-terminal extension with another transmembrane segment preceded by a large extracellular domain showing sequence similarities to several heme peroxidases.
FIG. 1.
Generation of reactive oxygen species (ROS) by Nox family NADPH oxidases. All are electrogenic enzymes that accept electrons from cytosolic NADPH, transport them through FAD and membrane-imbedded hemes, and donate single electrons to molecular oxygen, thereby producing superoxide anion. In the case of the dual oxidases (Duox1 and Duox2), a superoxide intermediate is not readily detected and the N-terminal peroxidase-like domain appears to affect its conversion into H2O2 by undefined mechanisms. The most structurally conservation features of these enzymes include regions of the C-terminal reductase domain that bind NADPH and FAD and the membrane-spanning helical segments thought to bind heme.
According to the proposed ROS-generating mechanism deduced from the best characterized Nox2-based or phox system of phagocytic cells, one NADPH molecule yields two superoxide ions in a stepwise transmembrane transfer of two single electrons to two molecules of oxygen (21). The effective range of superoxide released into phagosomes is limited, given its instability as a charged free radical and the high levels of superoxide dismutase in this compartment, although recent work suggests the ClC-3 anion channel transports superoxide through membranes (73, 76). Spontaneous or catalytic dismutation of superoxide into a more stable and membrane-permeable product, H2O2, permits its detection even outside of the cell. Most reconstituted cells expressing nonphagocytic Nox enzymes (i.e., Nox1, Nox3, and Nox5) release extracellular superoxide, suggesting these oxidases reach the plasma membrane and that the same Nox2-based ROS-generating mechanism also applies to these oxidases (7, 56, 100). In several studies, H2O2 is the predominant ROS detected in Nox4-reconstituted cells, consistent with its accumulation within intracellular sites (3, 67). Extracellular superoxide can be detected in Nox4-reconstituted cells by the sensitive Diogenes luminescence assay system (39) or by nitro-blue tetrazolium, a membrane-permeable reagent that reaches intracellular compartments (89). Other studies claim Nox4 is unique in its ability to produce H2O2 more directly, although the mechanisms involved are not clear (48, 108).
Dual oxidases exhibit an important feature distinct from the other Nox isozymes, in that they produce only H2O2, not superoxide. The Duox ancestor, the mysterious “thyroid H2O2 generator”, was known to be an NADPH oxidase producing only H2O2 in a calcium-dependent manner (24, 27, 77, 91, 107). Duox1 and Duox2 cloning from thyroid tissues confirmed the Nox-like features of these enzymes and revealed an additional transmembrane helix preceded by a long extracellular N-terminal region that is ∼20% homologous with hemoperoxidases (23, 28). This domain most likely confers the unique property of H2O2 production. Recent studies on Duox1 and Duox2 reconstituted in plasma membranes by co-expression of their respective maturation factors confirm these oxidases produce H2O2 from the cell surface, and no detectable superoxide (44, 72).
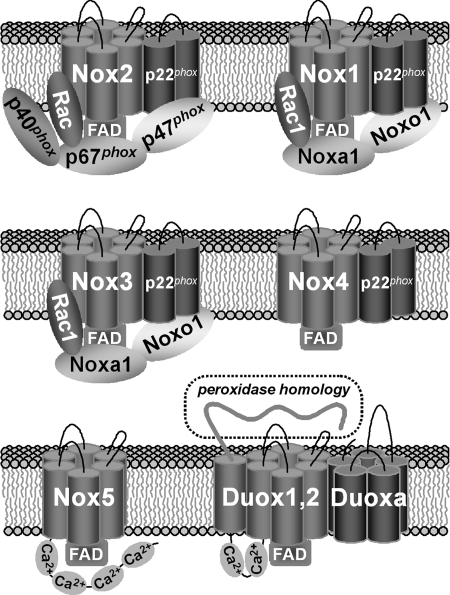
The Nox enzymes can be classified into three distinct functional groups (Fig. 2) related to the overall composition of the active enzymes: (a) Nox1, Nox2, and Nox3 function as multicomponent complexes involving cytosolic regulator proteins, (b) Nox4 exhibits high constitutive activity and is not dependent on any of the regulators that support Nox1, Nox2, or Nox3, and (c) Nox5, Duox1, and Duox2 are regulated by increased intracellular calcium levels due to the presence of calcium-binding EF-hands (8, 41, 63, 93). Direct interactions between the calcium-bound EF-hands and the C-terminal reductase domains were shown to activate Nox5 (7) and many presume the Duox isozymes are activated through a similar mechanism.
FIG. 2.
Molecular components of active Nox family NADPH oxidase complexes. Nox1, Nox2, and Nox3 function as regulated enzymes involving cytosolic adaptor proteins or “Nox organizers” (p47phox or Noxo1 and p40phox) and “Nox activators” (p67phox or Noxa1) that bind GTP-Rac and affect the flow of electrons. The p22phox component forms a stable heterodimeric complex with Nox core components (Nox1–4), required for post-translation processing or maturation into active oxidases. In Nox1–Nox3 systems, p22phox also promotes plasma membrane targeting of the oxidases and provides a docking site for Nox organizers. Nox5 and Duox are calcium-responsive oxidases that contain calcium-binding EF-hands. The Duox activators (Duoxa) are maturation factors functionally similar to p22phox recently shown to form stable complexes with Duox on the plasma membrane (72).
In most cases (excluding Nox5), the functional unit of these oxdases is considered a heterodimeric complex formed between the core oxidase flavocytochrome component and another membrane-imbedded chain, either p22phox or Duox activator (Duoxa) components, that serves as a “maturation factor” involved in their complete biosynthesis. The importance of Nox2 (gp91phox) and p22phox co-expression has been evident in CGD patients, where genetic defects affecting production of either component results in significantly reduced levels of the other chain, indicating that stabilization of the flavoenzyme involves formation of the heterodimeric complex (82, 87). Studies on the biosynthesis of Nox2 indicated that formation of this complex is a requisite for several post-translational modifications (heme incorporation and glycosylation), as well as for protein translocation beyond the endoplasmic reticulum (ER) (113). Subsequent studies suggested Nox1, Nox3, and Nox4 all form complexes with p22phox (3, 67, 98, 100). We showed that heterologous overexpression of either Nox1 or Nox3 results in cellular redistribution of p22phox from an ER-like pattern to accumulation along the plasma membrane, which is accompanied by enhanced p22phox detection by immunofluorescence and Western blotting (Fig. 3). The findings are consistent with release of superoxide by cells reconstituted with these oxidases (100) and plasma membrane co-localization of p22phox with Nox1, Nox3, or Nox4 in several tissues where the native oxidases are most abundant (58, 78). In addition to affecting oxidase targeting and stabilization, p22phox serves yet another function in the Nox1-, Nox2-, and Nox3-based systems in providing a docking site for their cytosolic regulators (see below). Mutagenesis experiments suggest this p22phox feature is not conserved in the Nox4-based enzyme, consistent with its ability to function independent of known oxidase cytosolic regulators (58, 108). Further studies are needed to clarify whether p22phox is essential for the Nox4-based enzyme or some other factor allows Nox4 to escape the ER.
FIG. 3.
Nox 1 and Nox 3 expression enables plasma membrane targeting and stabilization of p22phox. Immunofluorescence imaging of endogenous (A) and transfected (B) p22phox in HEK293 cells, showing reticular (ER) and nuclear membrane (arrows) staining patterns. (C) Transfection of Nox1 results in a redistribution of endogenous p22phox to the plasma membrane. (D) A similar redistribution of p22phox occurs in Nox3-transfected HEK293 cells. The untransfected cells (*) show primarily cytosolic staining patterns and overall weaker staining. Western blot analysis shows increased endogenous p22phox levels in Nox1- or Nox3- transfected cells (E), but not in mock or Noxo1β-transfected HEK293 cells (F). (Modified with permission from Fig. 6 of (100) and Fig. 6 of Ref. (102)).
The Duox isozymes also rely on maturation factors (a.k.a. Duox activators; Duoxa1, or Duoxa2) in order to reconstitute active Duox on the plasma membrane (44). Duoxa2 was initially proposed to act as an ER-resident protein required to export Duox beyond this compartment, however our recent work indicates the Duox maturation factors act in a similar fashion as the p22phox counterpart by forming stable complexes with the active oxidases on the plasma membrane (72).
Regulation of Multicomponent Oxidases: Nox1–Nox3
The activities of Nox1, Nox2, and Nox3 are all subject to the influence of cytosolic regulators. These regulatory mechanisms involve three types of factors: a) the small GTPase Rac bound to GTP, b) its GTP-dependent target proteins, referred to as Nox activators (p67phox or Noxa1), and c) the organizer or adaptor proteins (p47phox or Noxo1, and p40phox) responsible for bridging interactions of the Nox activators with the flavocytochrome components. The extent to which these oxidases are regulated varies, with the following rank order: Nox2 > Nox1 > Nox3, where Nox2 is completely inactive in the absence of cell stimulation, and Nox3 exhibits constitutive activity even in the absence these regulators. The importance of Rac2, p47phox, and p67phox as essential Nox2 system components is evident in autosomal recessive CGD patients whose genetic lesions affect these proteins (65, 79, 83). The maintenance of Nox2 cytosolic regulators in inactive conformations in large part accounts for the latency of this enzyme in unstimulated cells.
After the identification of p47phox and p67phox homologs (41), three groups explored the roles of these proteins (Noxo1 and Noxa1) in supporting Nox1 activity (7, 40, 96). The Nox organizers (p47phox and Noxo1) share common structures and function properties of binding to phospholipids through their PX (phox) domains, to proline-rich (PR) sequences of p22phox through the two SH3 domains, and to SH3 domains of the Nox activators through PR motifs near their carboxyl termini (Fig. 4). The PX domains of p47phox and Noxo1 exhibit different phospholipid-binding specificities: p47phox binds to phosphatidic acid (PA) and PI(3,4)P2 (54), whereas Noxo1 binds to PA and PI(4)P, PI(5)P, and PI(3,5)P2 (18, 95, 102). Noxo1 lacks sequence homologous to the C-terminal “auto-inhibitory (AIR)” domain of p47phox, encompassing a polybasic region with several serines that are phosphorylated during assembly and activation of the Nox2 complex (35). Both the absence of an AIR in Noxo1 and its phospholipid-binding specificity could explain its binding to the plasma membrane in the absence of cell stimulation (19). The Nox activators (p67phox and Noxa1) share homologous tetratricopeptide (TRP) scaffolding structures that present Rac-binding sequences, conserved activation domains (AD), and PB1 (Phox and Bem1) domains (Fig. 4). Noxa1 lacks a central SH3 domain and its PB1 domain can not bind p40phox (96), although the function of this SH3 domain of p67phox remains unknown. The p40phox component is a second adaptor protein that bridges contacts between membrane lipids (PI(3)P) through its PX domain and p67phox through PB1 domain heterodimerization (Fig. 4). Noxa1 shows a broader tissue-distribution pattern than Noxo1 (40, 96), whereas the expression of Noxo1 is upregulated by inflammatory agents, LPS and TNF-α (55, 62).
FIG. 4.
Schematic representation of multiple modular domains in Nox organizer and activator proteins in phagocytic (A) and non-phagocytic systems (B). The Nox organizers (p47phox and Noxo1) share common structures and functional properties of binding to phosphatidic acid (PA) and phosphoinositide lipids through PX domains, to p22phox through two SH3 domains, and to SH3 domains of Nox activators through the proline-rich (PR) motifs at their carboxyl termini. The Nox activators (p67phox and Noxa1) also share homologous tetratricopeptide repeat (TRP) scaffolds that present Rac binding sequences and activation domains (AD), and PB1 (Phox and Bem1) domain. The p40phox component is a secondary adaptor that bridges contacts between membrane (PI(3)P) and p67phox, using PX (phox) domain and PB1 domain heterodimerlization, respectively. Auto-inhibitory intramolecular interactions that maintain closed conformations in p47phox and p40phox are depicted with dashed lines. Striped box in p47phox between AIR and PR motif represents residues 341–360, which enhance the autoinhibitory interaction between the PX domain and the structure constructed by the tandem SH3s+ the AIR (101).
In unstimulated phagocytes, the phagocyte oxidase (Nox2) is dissociated and inactive: the membrane-penetrated flavocytochrome b558 (gp91phox-p22phox) is stored on intracellular granules (13), the other phox proteins (p47phox, p67phox, and p40phox) associate in a separate ternary cytosolic complex (64) in a dephosphorylated state (12, 85, 88), and Rac is maintained in a GDP-bound cytoplasmic complex dimerized with Rho-GDI (1). During phagocyte activation, intracellular granules containing flavocytochrome b558 fuse to newly forming phagosomes, and the ternary cytoplasmic phox complex and Rac bind to these membranes by independent mechanisms (49). p47phox is phosphorylated (2, 35, 103), thereby inducing conformational changes (unmasking) that promote the interaction of the ternary phox cytoplasmic complex with the flavocytochrome b558 through interactions between its SH3 domains and p22phox and between its PX domain and membrane phospholipids (45, 54, 66, 94). The “SuperSH3” model specifying AIR interactions responsible for masking of the tandem SH3 domains has been well recognized (45), however, details about precise intramolecular contacts responsible for masking the PX domain of p47phox remain unclear. We reported that residues 341–360 enhance autoinhibitory intramolecular interactions between the PX domain and a structure encompassing the tandem SH3s and the AIR (101) (Figs. 4 and 5). In addition, involvement of the linker region between the PX domain and N-terminal SH3 domain in masking was reported recently (90). Finally, Rac translocates to phagosomes (49, 99) and binds to its effector p67phox in a GTP-dependent manner in a critical regulatory point in Nox2 activation (112), resulting in generation of superoxide anion by the transfer of electrons from cytoplasmic NADPH to molecular oxygen.
FIG. 5.
Schematic representation of conformations changes in p47phox and p40phox allowing assembly of the ternary cytosolic complex into the active oxidase during the course of FcγR-mediated phagocytosis. The p47phox component acts early-phase adaptor protein following PKC-dependent phosphorylation-induced conformational change by binding to p22phox and membrane PA and PI(3,4)P2. In contrast, p40phox serves in the retention of the cytosolic complex on sealed phagosomes in later phases when PA and PI(3,4)P2 have disappeared, due to its PX domain-PI(3)P and PB1 domain-p67phox interactions (modified with permission from Fig. 11 of ref. 104). In the early phase, class I PI3-kinase (106) and PLD2 (15) serve in production of PI (3,4)P2 and PA, while in the later phase class III PI3-kinase (106) and early endosomes (43) serve in accumulation of PI (3)P.
Recent studies using p40phox knockout mice and FcγIIA receptor-reconstituted COSphox cells indicate that p40phox is also an essential positive regulator of the Nox2 system (33, 92). PI(3)P binding to the PX domain of p40phox plays critical roles in activation of Nox2 system (16, 32, 34, 52), particularly during FcγR-mediated phagocytosis (106). In contrast, a PX domain-independent, but PI(3)P-dependent, regulatory mechanism occurs in CD18-dependent activation of Nox2 system, and a PI(3)P-independent function of p40phox was reported recently (5, 10, 97). We demonstrated that a PX–PB1 domain intramolecular interaction within p40phox renders p40phox inaccessible to interact with PI(3)P in its resting state, and proposed that during Nox2 activation the PX–PB1 interaction can be disrupted, enabling p40phox to bind to PI(3)P-enriched phagosomes (104). A structural analysis of the PX-PB1 autoinhibitory contacts within p40phox was reported (50). Subsequently, we demonstrated that p40phox prolongs the retention of the p47phox-p67phox-p40phox complex on closed phagosomes and proposed a switching of phagosomal membrane adaptor functions from p47phox to p40phox: p47phox functions as the initial adaptor protein bringing the ternary complex to phagosomes, whereas p40phox functions as a late stage adaptor protein to retain the complex on phagosomes during the FcγR-mediated oxidative burst (101) (Fig. 5). However, there are still controversies and unanswered questions about this assembly process, such as the mechanisms promoting the p40phox conformational changes.
Nox1 dimerized with p22phox is activated by related cytosolic regulators, Noxo1, Noxoa1, and Rac1 by mechanisms that are less stringent but very similar to the Nox2 system. Interestingly, the murine Nox1 system exhibits high constitutive activity (6), whereas the human Nox1 system has a PMA-stimulated component showing 1.5- to 3.0-folds increases in activity (40, 100, 102). A negative regulatory mechanism by protein kinase A-mediated phosphorylation of Noxa1 Ser172 and Ser461 was described, which are not conserved in murine Noxa1 (59). Nox3 dimerized with p22phox show species specific differences: the reconstituted murine Nox3 system requires both Noxa1 and Noxo1 for its maximal activation (60), while the human Nox3 system shows maximal activity with Noxo1 alone (98, 100). In contrast to the well-recognized role of Rac in the Nox2 system, demonstration of Rac involvement in the Nox1 and Nox3 systems was less convincing using the same functional criteria. Constitutively active forms of Rac (RacQ61L or RacG12V) or dominant-negative Rac (RacT17N) did not affect these reconstituted cell systems, because of the lower demand for Rac by these oxidases. We demonstrated Rac1 involvement in Nox1 and Nox3 system based on three experiments designed in reconstituted cell models (100): (a) using a membrane-targeted form of Noxa1 (Noxa1pp) that supports Nox1 or Nox3 independent of Noxo1; this Noxa1 mutant was adapted with the C-terminal 10 amino acids of Rac1 (KKRKRKCLLL). This activity was dependent on an interaction with active Rac1; (b) using the Noxa1 mutant (Noxa1R103E) defective in Rac1 binding or the specific Rac1 mutant (Rac1G30S) defective in Noxa1 binding (mutations directed at effector contacts sites derived from crystallographic studies); and (c) using siRNA-mediated Rac1 knockdown accompanied by rescue-experiment by wild-type Rac1. Involvement of Rac1 in the Nox1 system was supported by similar findings in two subsequent reports (17, 69) and two previous studies showing effects of RacG12V and RacGEF on Nox1 (55, 81). Recognition of involvement of Rac1 in the human Nox3 system had been controversial because of the predominant role of Noxo1 (98), however, two subsequent reports confirmed that Nox3 is Rac-dependent to the same extent that it involves Noxa1 (53, 93). As in the Nox2 system where the binding of Rac to p67phox is a critical regulatory point in Nox2 activation (112), the Rac-Noxa1 interaction likely occurs at the membrane in a GTP-dependent manner.
Three groups described four structural variants of human Noxo1 (α, β, γ, and δ) that arise from alternative mRNA splicing on both boundaries of the third exon, resulting in protein sequence variations within their PX domains (18, 95, 102) (Fig. 6). Based on their distinct tissue expression patterns, subcellular localization, and Nox1- or Nox3-supporting abilities, it appears that Noxo1β and Noxo1γ are the most physiologically relevant isoforms among the four variants (102). The isolated PX domains or full-length Noxo1β and Noxo1γ proteins are targeted to the plasma membrane, whereas the Noxo1α and Noxo1δ isoforms showed a tendency to aggregate in the cytoplasm or localized on intracellular vesicles (Fig. 7). Interestingly, Noxo1γ also localized in nucleus in several transfected cell models. Plasma membrane targeting of these variants correlated closely with their relative efficiencies in supporting oxidase activity (102).
FIG. 6.
Predicted three-dimensional structures of alternatively spliced PX domain variants of Noxo1, based on alignments with sequences of p47phox and other PX domain-containing proteins. (A) Alignment of PX domain amino acid sequences, showing locations of splice site 1 (deleted from Noxo1α and δ) in the middle of conserved alpha-helix 1 and splice site 2 (present in Noxo1γ and δ) inserted within a variable loop region frequently containing the proline-rich motif in other PX domains. Conserved residues involved in p47phox binding to PA are marked with an asterisk (*) while those that bind phosphoinositide lipids are overlined (modified with permission from Fig. 2 of ref. 102). (B) Structural models of the Noxo1 isoforms based on the structure of p47phox (PDB ID: 1KQ6). The critical residues of the PA binding site are unchanged between the β and γ isoforms, despite the nearby insertion five amino acids in Noxo1γ. The electrostatic character of the PA binding site is significantly altered in the α isoform by the replacement of lysine by glutamic acid (red arrow heads). The PI binding site is basically unchanged between p47phox and the Noxo1 isoforms (blue arrows). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
FIG. 7.
Subcellular localization of PX domain and full-length Noxo1 alternative splice variants in HEK293 (A) and COS7 (B) cells. Noxo1α(PX)-GFP and Noxo1α-GFP show a tendency to aggregate or are localized on intracellular vesicles. Noxo1β(PX)-GFP and Noxo1β-GFP are localized predominately along the plasma membrane. Noxo1γ(PX)-GFP and Noxo1γ-GFP localize in the nucleus and along the plasma membrane. Noxo1δ(PX)-GFP and Noxo1δ-GFP show localization patterns similar to that of Noxo1α. Bar: 10 μm. (modified with permission from Fig. 2 of ref. 102).
PX domains are widely recognized as phosphoinositide lipid-binding modules in a variety of intracellular signaling proteins, although they also bind to some protein targets. As mentioned above, intramolecular protein contacts occur in both p47phox and p40phox involving their PX domains. The p47phox PX domain has another remarkable feature of having a second anionic phospholipid-binding pocket with specificity for PA (54). Interestingly, an alignment of the p47phox and Noxo1 PX domain protein sequences shows that residues involved in p47phox PA binding are also conserved in Noxo1 (Fig. 6A). Crystallographic studies showed that the p47phox residues lining the PA-binding pocket lie in α-helix1 and sequence preceding the PR motif: H51, K55, R70, P73, and H74 (Fig. 6B) (54); the corresponding aligned residues in Noxo1β and Noxo1γ are highly conserved: K49, K53, R68, P71, and K72 (Fig. 6A). Consistent with this feature, we showed that Noxo1β and Noxo1γ bind to PA, in addition to P1(4)P, P1(5)P, or PI(3,5)P2 binding previously demonstrated by others (18, 95).
Based on the close structural similarities between p47phox and Noxo1, we modeled the structures of Noxo1α, β, and γ-PX domains based on the solved structure of p47phox (PDB ID: 1KQ6) and the alignment in Figure 6A using DeepView and SwissModel (http://swissmodel.expasy.org) (46). Sulfate and phosphatidylinositol molecules were added to the models by structural alignment with the p47phox and p40phox PX domains (PDB ID: 1H6H; Fig. 6B). The deletion of K50 in the Noxo1α isoform shifts the register of all amino acids in the second half of helix-1, which would drastically alter the overall character of the PX domain surrounding this highly conserved helix. This changes the composition of the PA binding site by replacing the lysine side chain with that of glutamate. These changes provide a structural explanation of the diminished ability of this isoform to support Nox1 activity and its tendency to aggregate (99). The 5-residue insertion of the Noxo1γ isoform is near the PA binding site, but it does not change the placement of critical binding residues when compared to the β isoform. These features provide a structural explanation for the preserved lipid and plasma membrane-binding activity of the γ isoform, despite the insertion. Further studies are needed to understand the basis for nuclear targeting of Noxo1γ and its role in supporting oxidase activity at this site.
Targeting and Activation of Duox Isozymes
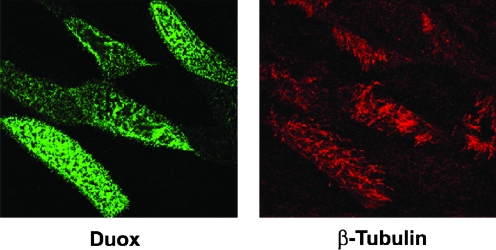
Duox enzymes are expressed at highest levels on epithelial surfaces of mucosal tissues, such as major airways, the digestive and urogenital tracts, and several endocrine and exocrine glands, such as the thyroid, salivary glands, the pancreas, and the prostate (23, 28, 29, 31, 37, 42, 86). In polarized epithelium, the Duox proteins accumulate along the apical plasma membrane, where they release H2O2 to support several dedicated extracellular hemoperoxidases (14, 22, 31, 37, 86). In the thyrocyte, Duox2-dependent H2O2 generation activates TPO, which catalyzes T3/T4 hormonogenesis (74). In the lung and salivary glands, Duox provides H2O2 to LPO, which converts thiocyanate anions into the microbicidal oxidant hypothiocyanite (37, 42, 75, 84). Primary human bronchial epithelial cells maintained long-term on membranes in air–liquid interface cultures redifferentiate into a mature airway phenotype, resulting in significant upregulation of Duox1 expression (84). Immunostaining of these cultures demonstrates that Duox is localized on the apical aspect of the mature ciliated cells (Fig. 8). We and other groups showed these differentiated airway models produce sufficient Duox1-derived H2O2 from the apical surface to effectively support LPO and thiocyanate-mediated microbicidal activity against several airway pathogens (37, 75, 84).
FIG. 8.
Laser scanning confocal immunofluorescence detection of Duox on the apical plasma membrane surface of ciliated primary human bronchial epithelial cells. Images show the apical X-Y plane of confluent cells grown on permeable transwells and re-differentiated for 25 days in an air–liquid interface culture system, as described (84). Ciliated cells, stained with anti β-tubulin antibody (red), are the same as those stained positive for Duox (green). 3-D reconstructed images depicting the rotation of the X-Y plane are available in the supplemental online video, showing Duox and β-tubulin accumulation within the apical aspect of this polarized cell layer. (See online supplemental video at www.liebertonline.com). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Initial attempts at heterologous expression of the recombinant Duox enzymes failed to reconstitute these H2O2 generating systems. Immunostaining and glycosylation studies showed the newly synthesized Duox was blocked inside the ER, whereas thyrocyte Duox accumulates at the cell surface and bears Golgi-modified carbohydrates (22). Co-expression of p67phox, p47phox, p22phox, or TPO did not rescue active Duox on the cell surface (22). Furthermore, inhibition of Rac by C. difficile toxin B had no effect on Duox activity (36). Using truncated Duox variants, a region encompassing the first transmembrane segment and the following 45 residues was shown to act as an ER retention signal (71). The missing Duox maturation factor was recently identified (44). Co-expression of the membrane protein Duox activator 2 (Duoxa2) enabled delivery of mature, glycosylated Duox2 to the cell surface, reconstituting oxidase activity (44). We cloned four alternatively spliced DUOXA1 variants and compared their abilities to support Duox maturation (72). Two of the four Duoxa1 variants (Duoxa1β and δ) that lacked the third coding exon (shortening the first extracellular loops by 45 aa) were incompletely glycosylated and did not support Duox activity. Interestingly, we found both Duox enzymes could be rescued by Duoxa1α, Duoxa1γ, or Duoxa2 and detected both Duox/Duoxa proteins at the cell-surface (Fig. 9). Immunostaining of Duoxa1 in human airway sections also confirmed that Duoxa1 accumulates on the apical surface of the airway epithelium (72). These localization results contrast with the initial study, where Duoxa2 was proposed to be an ER resident even when co-expressed with Duox2 (44). Interestingly, depending on the Duoxa variant expressed, co-localization of Duox and the Duoxa proteins in our models showed distinct subcellular patterns: Duoxa1α and Duoxa2 were efficient in targeting Duox1 and Duox2, respectively, to the plasma membrane and reconstituting high levels of H2O2 generation. In contrast, Duox1 or Duox2 accumulate with Duoxa1γ within some intracellular compartment distinct from the ER, as well as the plasma membrane. With the exception of Duox2/Duoxa1α or Duoxa1γ combinations, the reconstituted Duox produced solely H2O2, not superoxide, and could be immunoprecipitated in a heterodimer complex with Duoxa on the plasma membrane (72, 111). These findings are consistent with previous observations on native Duox, where no O2− could be detected from the thyroid (4), lung (42), and sea urchin enzymes (47, 110). Thus, these maturation factors not only function in enabling Duox exit from the ER, they appear to co-translocate with Duox, undergo carbohydrate modifications in the Golgi apparatus, and affect the type of ROS produced by Duox (72).
FIG. 9.
Role of Duox maturation factors in Duox isozyme biosynthesis and ROS-generating mechanism. DuoxA2 and DuoxA1α have similar sizes and predicted N-glycosylation sites. Duoxa1γ has an extended C-terminal sequence similar to that of Drosophila NIP (numb interacting protein). Duoxa1β and δ forms (bottom left) that lack exon 3-encoded sequence and two predicted gylcosylation sites remain in the ER and do not support biosynthesis of active Duox. Active Duox maturation factors (Duoxa1α, γ, Duoxa2) translocate to the plasma membrane, undergoing Golgi-based carbohydrate modifications and forming stable H2O2-generating Duox/Duoxa complexes (upper left). Less competent combinations of Duox2 overexpressed with Duoxa1α or Duoxa1γ do not form plasma membrane Duoxa complexes, show less carbohydrate processing, and produce superoxide (upper right).
The precise role of the Duox extracellular peroxidase homology domains has not yet been established. Contrary to its peroxidase-like designation, it does not appear to function as a peroxide-consuming core in Duox-reconstituted cells (72). Indeed, H2O2-dependent substrate oxidation by cell surface-exposed Duox required exogenous peroxidases (37, 44, 72, 84). In our hands, efficient Duox reconstitution correlated with cell surface exposure and always required an exogenous peroxidase to detect H2O2 production by oxidation of three different substrates (tyrosine, homovanillic acid, Amplex Red) (72). H2O2 formation most likely proceeds from formation of a superoxide intermediate by the Nox-like portion, with the peroxidase-like domains subsequently promoting intramolecular superoxide dismutation or other mechanisms leading to H2O2 release. Superoxide production could be measured from ER-blocked Duox2 in the absence of Duox maturation factors (4) or from surface-exposed Duox2/Duoxa1α or Duox2/Duoxa1γ combinations that do not form stable heterodimeric complexes (72, 111). In both cases, the Duox enzymes are incompletely processed in the absence of a stable Duox activator association. Thus, the Duox activators may function in processing of Duox isozymes into dedicated H2O2 generators or they could even serve as part of H2O2-forming Duox complexes (72).
Exactly where in the cell Duox becomes an active enzyme remains unclear, that is, within the Golgi apparatus or after reaching the plasma membrane. In reconstituted HEK293 cells, Duox reconstitution correlated closely with Golgi-based modification in both Duox and their maturation factors (72). As robust generators of toxic ROS, Duox enzymes may be designed for activation only on the cell surface, releasing high amounts of these products outside of the cell. Thyroid H2O2 is detected only on the apical pole, but not inside the cell (11, 30, 70). In other settings, lower levels of Duox activity may serve other functions within intracellular sites, such as cell signaling involved in migration or proliferation (i.e., would healing) (109), perhaps under the control of distinct targeting, maturation, or regulatory factors.
In conclusion, the diverse Nox family of NADPH oxidases serves a variety of essential roles requiring deliberate ROS generation in many tissues. Each of these enzymes is subject to unique mechanisms that control the site, duration, amount, and type of ROS generated to avoid collateral oxidative damage to host tissues. It has been known for years that the multicomponent Nox2-based oxidase is subject to tight regulation in order to direct potent microbicidal oxidants to the phagosomal compartment. Recent studies provide new insights on p40phox-based regulation of this system. Other closely related multicomponent oxidases, Nox1 and Nox3, are subject to similar mechanisms controlling the biosynthesis and sites of assembly and activation, involving participation of p22phox, Rac, and similar organizer and activator proteins. In contrast, the dual oxidases are specialized H2O2 generators that have developed distinct maturation, subcellular targeting, and activation mechanisms, enabling calcium-triggered ROS release from the cell surface to support several tissue-specific extracellular peroxidases.
Supplementary Material
Abbreviations Used
- AIR
auto-inhibitory region
- CGD
chronic granulomatous disease
- Duox
Dual oxidase
- Duoxa
Duox activator
- ER
endoplasmic reticulum
- Nox
NADPH oxidase
- PA
phosphatidic acid
- PB1
Phox and Bem1
- phox
phagocytic oxidase
- PX
phox
- ROS
reactive oxygen species
- SH3
Src homology 3
Acknowledgments
This work was supported by the National Institutes of Health Intramural Research Program, National Institute of Allergy and Infectious Diseases, and Grant-in-Aid for Scientific Research on (C), on Priority Areas, and on the Global-COE Program in Japan. We thank to Dr. Corinne Dupuy, Institut Gustave Roussy, Villejuif, France for kindly providing anti-Duox antibody.
References
- 1.Abo A. Pick E. Hall A. Totty N. Teahan CG. Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 2.Ago T. Nunoi H. Ito T. Sumimoto H. Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47phox. Triple replacement of serines 303, 304, and 328 with aspartates disrupts the SH3 domain-mediated intramolecular interaction in p47(phox), thereby activating the oxidase. J Biol Chem. 1999;274:33644–33653. doi: 10.1074/jbc.274.47.33644. [DOI] [PubMed] [Google Scholar]
- 3.Ambasta RK. Kumar P. Griendling KK. Schmidt HHHW. Busse R. Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004;279:45935–45941. doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 4.Ameziane–El-Hassani R. Morand S. Boucher JL. Frapart YM. Apostolou D. Agnandji D. Gnidehou S. Ohayon R. Noel–Hudson MS. Francon J. Lalaoui K. Virion A. Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 5.Anderson KE. Boyle K. Davidson K. Chessa TA. Kulkarni S. Jarvis GE. Sindrilaru A. Scharffetter–Kochanek K. Rausch O. Stephens LR. Hawkins PT. CD18-dependent activation of the neutrophil NADPH oxidase during phagocytosis of E. coli or S. aureus is regulated by Class III but not Class I or II PI3Ks. Blood. 2008;112:5205–5211. doi: 10.1182/blood-2008-04-149450. [DOI] [PubMed] [Google Scholar]
- 6.Banfi B. Clark RA. Steger K. Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 7.Banfi B. Tirone F. Durussel I. Knisz J. Moskwa P. Molnar GZ. Krause KH. Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 8.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 9.Bedard K. Lardy B. Krause KH. Nox family NADPH oxidase not just in mammals. Biochimie. 2007;89:1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Bissonnette SA. Glazier CM. Stewart MQ. Brown GE. Ellson CD. Yaffe MB. Phosphatidylinositol 3-phosphate-dependent and -independent functions of p40phox in activation of the neutrophil NADPH oxidase. J Biol Chem. 2008;283:2108–2119. doi: 10.1074/jbc.M706639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjorkman U. Ekholm R. Denef JF. Cytochemical localization of hydrogen peroxide in isolated thyroid follicles. J Ultrastruct Res. 1981;74:105–115. doi: 10.1016/s0022-5320(81)80113-x. [DOI] [PubMed] [Google Scholar]
- 12.Bolscher BG. van Zwieten R. Kramer IM. Weening RS. Verhoeven AJ. Roos D. A phosphoprotein of Mr 47,000, defective in autosomal chronic granulomatous disease, copurifies with one of two soluble components required for NADPH:O2 oxidoreductase activity in human neutrophils. J Clin Invest. 1989;83:757–763. doi: 10.1172/JCI113954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borregaard N. Heiple JM. Simons ER. Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caillou B. Dupuy C. Lacroix L. Nocera M. Talbot M. Ohayon R. Deme D. Bidart JM. Schlumberger M. Virion A. Expression of reduced nicotinamide adenine dinucleotide phosphate oxidase (ThoX, LNOX, Duox) genes and proteins in human thyroid tissues. J Clin Endocrinol Metab. 2001;86:3351–3358. doi: 10.1210/jcem.86.7.7646. [DOI] [PubMed] [Google Scholar]
- 15.Cheeseman KL. Ueyama T. Michaud TM. Kashiwagi K. Wang D. Flax LA. Shirai Y. Loegering DJ. Saito N. Lennartz MR. Targeting of protein kinase C-ε during Fcgamma receptor-dependent phagocytosis requires the epsilonC1B domain and phospholipase C-γ. Mol Biol Cell. 2006;17:799–813. doi: 10.1091/mbc.E04-12-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J. He R. Minshall RD. Dinauer MC. Ye RD. Characterization of a mutation in the Phox homology domain of the NADPH oxidase component p40phox identifies a mechanism for negative regulation of superoxide production. J Biol Chem. 2007;282:30273–30284. doi: 10.1074/jbc.M704416200. [DOI] [PubMed] [Google Scholar]
- 17.Cheng G. Diebold BA. Hughes Y. Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- 18.Cheng G. Lambeth JD. Alternative mRNA splice forms of NOXO1: differential tissue expression and regulation of Nox1 and Nox3. Gene. 2005;356:118–126. doi: 10.1016/j.gene.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Cheng G. Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem. 2004;279:4737–4742. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 20.Choi H. Leto TL. Hunyady L. Catt KJ. Bae YS. Rhee SG. Mechanism of angiotensin II-induced superoxide production in cells reconstituted with angiotensin type 1 receptor and the components of NADPH oxidase. J Biol Chem. 2008;283:255–267. doi: 10.1074/jbc.M708000200. [DOI] [PubMed] [Google Scholar]
- 21.Cross AR. Segal AW. The NADPH oxidase of professional phagocytes–prototype of the NOX electron transport chain systems. Biochim Biophys Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Deken X. Wang D. Dumont JE. Miot F. Characterization of ThOX proteins as components of the thyroid H2O2-generating system. Exp Cell Res. 2002;273:187–196. doi: 10.1006/excr.2001.5444. [DOI] [PubMed] [Google Scholar]
- 23.De Deken X. Wang D. Many MC. Costagliola S. Libert F. Vassart G. Dumont JE. Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 24.Deme D. Virion A. Hammou NA. Pommier J. NADPH-dependent generation of H2O2 in a thyroid particulate fraction requires Ca2+ FEBS Lett. 1985;186:107–110. doi: 10.1016/0014-5793(85)81349-1. [DOI] [PubMed] [Google Scholar]
- 25.Dikalova A. Clempus R. Lassegue B. Cheng G. McCoy J. Dikalov S. San Martin A. Lyle A. Weber DS. Weiss D. Taylor WR. Schmidt HH. Owens GK. Lambeth JD. Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 26.Donko A. Peterfi Z. Sum A. Leto T. Geiszt M. Dual oxidases. Philos Trans R Soc Lond B Biol Sci. 2005;360:2301–2308. doi: 10.1098/rstb.2005.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dupuy C. Kaniewski J. Deme D. Pommier J. Virion A. NADPH-dependent H2O2 generation catalyzed by thyroid plasma membranes. Studies with electron scavengers. Eur J Biochem. 1989;185:597–603. doi: 10.1111/j.1432-1033.1989.tb15155.x. [DOI] [PubMed] [Google Scholar]
- 28.Dupuy C. Ohayon R. Valent A. Noel-Hudson MS. Deme D. Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cDNAs. J Biol Chem. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 29.Dupuy C. Pomerance M. Ohayon R. Noel–Hudson MS. Deme D. Chaaraoui M. Francon J. Virion A. Thyroid oxidase (THOX2) gene expression in the rat thyroid cell line FRTL-5. Biochem Biophys Res Commun. 2000;277:287–292. doi: 10.1006/bbrc.2000.3671. [DOI] [PubMed] [Google Scholar]
- 30.Ekholm R. Iodination of thyroglobulin. An intracellular or extracellular process? Mol Cell Endocrinol. 1981;24:141–163. doi: 10.1016/0303-7207(81)90056-3. [DOI] [PubMed] [Google Scholar]
- 31.El Hassani RA. Benfares N. Caillou B. Talbot M. Sabourin JC. Belotte V. Morand S. Gnidehou S. Agnandji D. Ohayon R. Kaniewski J. Noel–Hudson MS. Bidart JM. Schlumberger M. Virion A. Dupuy C. Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol. 2005;288:G933–942. doi: 10.1152/ajpgi.00198.2004. [DOI] [PubMed] [Google Scholar]
- 32.Ellson C. Davidson K. Anderson K. Stephens LR. Hawkins PT. PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J. 2006;25:4468–4478. doi: 10.1038/sj.emboj.7601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellson CD. Davidson K. Ferguson GJ. O'Connor R. Stephens LR. Hawkins PT. Neutrophils from p40phox-/- mice exhibit severe defects in NADPH oxidase regulation and oxidant-dependent bacterial killing. J Exp Med. 2006;203:1927–1937. doi: 10.1084/jem.20052069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ellson CD. Gobert–Gosse S. Anderson KE. Davidson K. Erdjument-Bromage H. Tempst P. Thuring JW. Cooper MA. Lim ZY. Holmes AB. Gaffney PR. Coadwell J. Chilvers ER. Hawkins PT. Stephens LR. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40phox. Nat Cell Biol. 2001;3:679–682.. doi: 10.1038/35083076. [DOI] [PubMed] [Google Scholar]
- 35.Fontayne A. Dang PM. Gougerot–Pocidalo MA. El–Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 36.Fortemaison N. Miot F. Dumont JE. Dremier S. Regulation of H2O2 generation in thyroid cells does not involve Rac1 activation. Eur J Endocrinol. 2005;152:127–133. doi: 10.1530/eje.1.01815. [DOI] [PubMed] [Google Scholar]
- 37.Forteza R. Salathe M. Miot F. Conner GE. Regulated hydrogen peroxide production by Duox in human airway epithelial cells. Am J Respir Cell Mol Biol. 2005;32:462–469. doi: 10.1165/rcmb.2004-0302OC. [DOI] [PubMed] [Google Scholar]
- 38.Gavazzi G. Banfi B. Deffert C. Fiette L. Schappi M. Herrmann F. Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 39.Geiszt M. Kopp JB. Varnai P. Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geiszt M. Lekstrom K. Witta J. Leto TL. Proteins homologous to p47phox and p67phox support superoxide production by NAD(P)H oxidase 1 in colon epithelial cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 41.Geiszt M. Leto TL. The Nox family of NAD(P)H oxidases: Host defense and beyond. J Biol Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- 42.Geiszt M. Witta J. Baffi J. Lekstrom K. Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 43.Gillooly DJ. Morrow IC. Lindsay M. Gould R. Bryant NJ. Gaullier JM. Parton RG. Stenmark H. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 2000;19:4577–4588. doi: 10.1093/emboj/19.17.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grasberger H. Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 45.Groemping Y. Lapouge K. Smerdon SJ. Rittinger K. Molecular basis of phosphorylation-induced activation of the NADPH oxidase. Cell. 2003;113:343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- 46.Guex N. Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 47.Heinecke JW. Shapiro BM. Respiratory burst oxidase of fertilization. Proc Natl Acad Sci USA. 1989;86:1259–1263. doi: 10.1073/pnas.86.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Helmcke I. Heumuller S. Tikkanen R. Schröder K. Brandes RP. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxid Redox Signal. 2009;11:1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 49.Heyworth P. Bohl B. Bokoch G. Curnutte J. Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox. J Biol Chem. 1994;269:30749–30752. [PubMed] [Google Scholar]
- 50.Honbou K. Minakami R. Yuzawa S. Takeya R. Suzuki NN. Kamakura S. Sumimoto H. Inagaki F. Full-length p40phox structure suggests a basis for regulation mechanism of its membrane binding. EMBO J. 2007;26:1176–1186. doi: 10.1038/sj.emboj.7601561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson KR. Marden CC. Ward–Bailey P. Gagnon LH. Bronson RT. Donahue LR. Congenital hypothyroidism, dwarfism, and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol Endocrinol. 2007;21:1593–1602. doi: 10.1210/me.2007-0085. [DOI] [PubMed] [Google Scholar]
- 52.Kanai F. Liu H. Field SJ. Akbary H. Matsuo T. Brown GE. Cantley LC. Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- 53.Kao YY. Gianni D. Bohl B. Taylor RM. Bokoch GM. Identification of a conserved Rac-binding site on NADPH oxidases supports a direct GTPase regulatory mechanism. J Biol Chem. 2008;283:12736–12746. doi: 10.1074/jbc.M801010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karathanassis D. Stahelin RV. Bravo J. Perisic O. Pacold CM. Cho W. Williams RL. Binding of the PX domain of p47phox to phosphatidylinositol 3,4-bisphosphate and phosphatidic acid is masked by an intramolecular interaction. EMBO J. 2002;21:5057–5068. doi: 10.1093/emboj/cdf519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawahara T. Kohjima M. Kuwano Y. Mino H. Teshima–Kondo S. Takeya R. Tsunawaki S. Wada A. Sumimoto H. Rokutan K. Helicobacter pylori lipopolysaccharide activates Rac1 and transcription of NADPH oxidase Nox1 and its organizer NOXO1 in guinea pig gastric mucosal cells. Am J Physiol Cell Physiol. 2005;288:C450–457. doi: 10.1152/ajpcell.00319.2004. [DOI] [PubMed] [Google Scholar]
- 56.Kawahara T. Lambeth JD. Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol Biol Cell. 2008;19:4020–4031. doi: 10.1091/mbc.E07-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawahara T. Quinn MT. Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (Nox/Duox) family of enzymes. BMC Evol Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawahara T. Ritsick D. Cheng G. Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 59.Kim JS. Diebold BA. Babior BM. Knaus UG. Bokoch GM. Regulation of Nox1 activity via protein kinase A-mediated phosphorylation of NoxA1 and 14-3-3 binding. J Biol Chem. 2007;282:34787–34800. doi: 10.1074/jbc.M704754200. [DOI] [PubMed] [Google Scholar]
- 60.Kiss PJ. Knisz J. Zhang Y. Baltrusaitis J. Sigmund CD. Thalmann R. Smith RJ. Verpy E. Banfi B. Inactivation of NADPH oxidase organizer 1 results in severe imbalance. Curr Biol. 2006;16:208–213. doi: 10.1016/j.cub.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 61.Kuroda J. Nakagawa K. Yamasaki T. Nakamura K. Takeya R. Kuribayashi F. Imajoh–Ohmi S. Igarashi K. Shibata Y. Sueishi K. Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 62.Kuwano Y. Tominaga K. Kawahara T. Sasaki H. Takeo K. Nishida K. Masuda K. Kawai T. Teshima–Kondo S. Rokutan K. Tumor necrosis factor α activates transcription of the NADPH oxidase organizer 1 (NOXO1) gene and upregulates superoxide production in colon epithelial cells. Free Radic Biol Med. 2008;45:1642–1652. doi: 10.1016/j.freeradbiomed.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 63.Lambeth JD. Kawahara T. Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lapouge K. Smith SJ. Groemping Y. Rittinger K. Architecture of the p40-p47-p67phox complex in the resting state of the NADPH oxidase. J Biol Chem. 2002;277:10121–10128. doi: 10.1074/jbc.M112065200. [DOI] [PubMed] [Google Scholar]
- 65.Leto TL. In: Inflammation Basic Principles and Clinical Correlates. Gallin JI, editor; Snyderman R., editor. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 769–787. [Google Scholar]
- 66.Leto TL. Adams AG. de Mendez I. Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc Natl Acad Sci USA. 1994;91:10650–10654. doi: 10.1073/pnas.91.22.10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martyn KD. Frederick LM. von Loehneysen K. Dinauer MC. Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 68.Matsuno K. Yamada H. Iwata K. Jin D. Katsuyama M. Matsuki M. Takai S. Yamanishi K. Miyazaki M. Matsubara H. Yabe–Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 69.Miyano K. Ueno N. Takeya R. Sumimoto H. Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. J Biol Chem. 2006;281:21857–21868. doi: 10.1074/jbc.M513665200. [DOI] [PubMed] [Google Scholar]
- 70.Mizukami Y. Matsubara F. Matsukawa S. Cytochemical localization of peroxidase and hydrogen-peroxide-producing NAD(P)H-oxidase in thyroid follicular cells of propylthiouracil-treated rats. Histochemistry. 1985;82:263–268. doi: 10.1007/BF00501403. [DOI] [PubMed] [Google Scholar]
- 71.Morand S. Agnandji D. Noel–Hudson MS. Nicolas V. Buisson S. Macon–Lemaitre L. Gnidehou S. Kaniewski J. Ohayon R. Virion A. Dupuy C. Targeting of the dual oxidase 2 N-terminal region to the plasma membrane. J Biol Chem. 2004;279:30244–30251. doi: 10.1074/jbc.M405406200. [DOI] [PubMed] [Google Scholar]
- 72.Morand S. Ueyama T. Tsujibe S. Saito N. Korzeniowska A. Leto TL. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J. 2008;23:1205–1218. doi: 10.1096/fj.08-120006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moreland JG. Davis AP. Bailey G. Nauseef WM. Lamb FS. Anion channels, including ClC-3, are required for normal neutrophil oxidative function, phagocytosis, and transendothelial migration. J Biol Chem. 2006;281:12277–12288. doi: 10.1074/jbc.M511030200. [DOI] [PubMed] [Google Scholar]
- 74.Moreno JC. Bikker H. Kempers MJ. van Trotsenburg AS. Baas F. de Vijlder JJ. Vulsma T. Ris–Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N Engl J Med. 2002;347:95–102. doi: 10.1056/NEJMoa012752. [DOI] [PubMed] [Google Scholar]
- 75.Moskwa P. Lorentzen D. Excoffon KJ. Zabner J. McCray PB., Jr. Nauseef WM. Dupuy C. Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mumbengegwi DR. Li Q. Li C. Bear CE. Engelhardt JF. Evidence for a superoxide permeability pathway in endosomal membranes. Mol Cell Biol. 2008;28:3700–3712. doi: 10.1128/MCB.02038-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nakamura Y. Ogihara S. Ohtaki S. Activation by ATP of calcium-dependent NADPH-oxidase generating hydrogen peroxide in thyroid plasma membranes. J Biochem. 1987;102:1121–1132. doi: 10.1093/oxfordjournals.jbchem.a122150. [DOI] [PubMed] [Google Scholar]
- 78.Nakano Y. Longo–Guess CM. Bergstrom DE. Nauseef WM. Jones SM. Banfi B. Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J Clin Invest. 2008;118:1176–1185. doi: 10.1172/JCI33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nauseef WM. Assembly of the phagocyte NADPH oxidase. Histochem Cell Biol. 2004;122:277–291. doi: 10.1007/s00418-004-0679-8. [DOI] [PubMed] [Google Scholar]
- 80.Paffenholz R. Bergstrom RA. Pasutto F. Wabnitz P. Munroe RJ. Jagla W. Heinzmann U. Marquardt A. Bareiss A. Laufs J. Russ A. Stumm G. Schimenti JC. Bergstrom DE. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park HS. Lee SH. Park D. Lee JS. Ryu SH. Lee WJ. Rhee SG. Bae YS. Sequential activation of phosphatidylinositol 3-kinase, bPix, Rac1, and Nox1 in growth factor-induced production of H2O2. Mol Cell Biol. 2004;24:4384–4394. doi: 10.1128/MCB.24.10.4384-4394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parkos CA. Dinauer MC. Jesaitis AJ. Orkin SH. Curnutte JT. Absence of both the 91kD and 22kD subunits of human neutrophil cytochrome b in two genetic forms of chronic granulomatous disease. Blood. 1989;73:1416–1420. [PubMed] [Google Scholar]
- 83.Quinn MT. Ammons MC. Deleo FR. The expanding role of NADPH oxidases in health and disease: No longer just agents of death and destruction. Clin Sci (Lond) 2006;111:1–20. doi: 10.1042/CS20060059. [DOI] [PubMed] [Google Scholar]
- 84.Rada B. Lekstrom K. Damian S. Dupuy C. Leto TL. The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J Immunol. 2008;181:4883–4893. doi: 10.4049/jimmunol.181.7.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rotrosen D. Leto TL. Phosphorylation of neutrophil 47-kDa cytosolic oxidase factor. J Biol Chem. 1990;265:19910–19915. [PubMed] [Google Scholar]
- 86.Schwarzer C. Machen TE. Illek B. Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem. 2004;279:36454–36461. doi: 10.1074/jbc.M404983200. [DOI] [PubMed] [Google Scholar]
- 87.Segal A. Absence of both cytochrome b-245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature. 1987;326:88–91. doi: 10.1038/326088a0. [DOI] [PubMed] [Google Scholar]
- 88.Segal AW. Heyworth PG. Cockcroft S. Barrowman MM. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature. 1985;316:547–549. doi: 10.1038/316547a0. [DOI] [PubMed] [Google Scholar]
- 89.Serrander L. Cartier L. Bedard K. Banfi B. Lardy B. Plastre O. Sienkiewicz A. Forro L. Schlegel W. Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shen K. Sergeant S. Hantgan RR. McPhail LC. Horita DA. Mutations in the PX-SH3A linker of p47phox decouple PI(3,4)P2 binding from NADPH oxidase activation. Biochemistry. 2008;47:8855–8865. doi: 10.1021/bi8005847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sugawara M. Sugawara Y. Wen K. Giulivi C. Generation of oxygen free radicals in thyroid cells and inhibition of thyroid peroxidase. Exp Biol Med (Maywood) 2002;227:141–146. doi: 10.1177/153537020222700209. [DOI] [PubMed] [Google Scholar]
- 92.Suh C-I. Stull ND. Li XJ. Tian W. Price MO. Grinstein S. Yaffe MB. Atkinson S. Dinauer MC. The phosphoinositide-binding protein p40phox activates the NADPH oxidase during FcgIIA receptor-induced phagocytosis. J Exp Med. 2006;203:1915–1925. doi: 10.1084/jem.20052085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sumimoto H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008;275:3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- 94.Sumimoto H. Kage Y. Nunoi H. Sasaki H. Nose T. Fukumaki Y. Ohno M. Minakami S. Takeshige K. Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc Natl Acad Sci USA. 1994;91:5345–5349. doi: 10.1073/pnas.91.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Takeya R. Taura M. Yamasaki T. Naito S. Sumimoto H. Expression and function of Noxo1gamma, an alternative splicing form of the NADPH oxidase organizer 1. Febs J. 2006;273:3663–3677. doi: 10.1111/j.1742-4658.2006.05371.x. [DOI] [PubMed] [Google Scholar]
- 96.Takeya R. Ueno N. Kami K. Taura M. Kohjima M. Izaki T. Nunoi H. Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 97.Tian W. Li XJ. Stull ND. Ming W. Suh CI. Bissonnette SA. Yaffe MB. Grinstein S. Atkinson SJ. Dinauer MC. FcγR-stimulated activation of the NADPH oxidase: phosphoinositide-binding protein p40phox regulates NADPH oxidase activity after enzyme assembly on the phagosome. Blood. 2008;112:3867–3877. doi: 10.1182/blood-2007-11-126029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ueno N. Takeya R. Miyano K. Kikuchi H. Sumimoto H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: Its regulation by oxidase organizers and activators. J Biol Chem. 2005;280:23328–23339. doi: 10.1074/jbc.M414548200. [DOI] [PubMed] [Google Scholar]
- 99.Ueyama T. Eto M. Kami K. Tatsuno T. Kobayashi T. Shirai Y. Lennartz MR. Takeya R. Sumimoto H. Saito N. Isoform-specific membrane targeting mechanism of Rac during FcgR-mediated phagocytosis: Positive charge-dependent and independent targeting mechanism of Rac to the phagosome. J Immunol. 2005;175:2381–2390. doi: 10.4049/jimmunol.175.4.2381. [DOI] [PubMed] [Google Scholar]
- 100.Ueyama T. Geiszt M. Leto TL. Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ueyama T. Kusakabe T. Karasawa S. Kawasaki T. Shimizu A. Son J. Leto TL. Miyawaki A. Saito N. Sequential binding of cytosolic phox complex to phagosomes through regulated adaptor proteins: evaluation using the novel monomeric Kusabira–Green System and live imaging of phagocytosis. J Immunol. 2008;181:629–640. doi: 10.4049/jimmunol.181.1.629. [DOI] [PubMed] [Google Scholar]
- 102.Ueyama T. Lekstrom K. Tsujibe S. Saito N. Leto TL. Subcellular localization and function of alternatively spliced Noxo1 isoforms. Free Radic Biol Med. 2007;42:180–190. doi: 10.1016/j.freeradbiomed.2006.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ueyama T. Lennartz MR. Noda Y. Kobayashi T. Shirai Y. Rikitake K. Yamasaki T. Hayashi S. Sakai N. Seguchi H. Sawada M. Sumimoto H. Saito N. Superoxide production at phagosomal cup/phagosome through bI protein kinase C during FcγR-mediated phagocytosis in microglia. J Immunol. 2004;173:4582–4589. doi: 10.4049/jimmunol.173.7.4582. [DOI] [PubMed] [Google Scholar]
- 104.Ueyama T. Tatsuno T. Kawasaki T. Tsujibe S. Shirai Y. Sumimoto H. Leto TL. Saito N. A regulated adaptor function of p40phox: distinct p67phox membrane targeting by p40phox and by p47phox. Mol Biol Cell. 2007;18:441–454. doi: 10.1091/mbc.E06-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ushio–Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vieira OV. Botelho RJ. Rameh L. Brachmann SM. Matsuo T. Davidson HW. Schreiber A. Backer JM. Cantley LC. Grinstein S. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J Cell Biol. 2001;155:19–25. doi: 10.1083/jcb.200107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Virion A. Michot JL. Deme D. Kaniewski J. Pommier J. NADPH-dependent H2O2 generation and peroxidase activity in thyroid particular fraction. Mol Cell Endocrinol. 1984;36:95–105. doi: 10.1016/0303-7207(84)90088-1. [DOI] [PubMed] [Google Scholar]
- 108.von Lohneysen K. Noack D. Jesaitis AJ. Dinauer MC. Knaus UG. Mutational analysis reveals distinct features of the Nox4-p22phox complex. J Biol Chem. 2008;283:35273–35282. doi: 10.1074/jbc.M804200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wesley UV. Bove PF. Hristova M. McCarthy S. van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem. 2007;282:3213–3220. doi: 10.1074/jbc.M606533200. [DOI] [PubMed] [Google Scholar]
- 110.Wong JL. Creton R. Wessel GM. The oxidative burst at fertilization is dependent upon activation of the dual oxidase Udx1. Dev Cell. 2004;7:801–814. doi: 10.1016/j.devcel.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 111.Zamproni I. Grasberger H. Cortinovis F. Vigone MC. Chiumello G. Mora S. Onigata K. Fugazzola L. Refetoff S. Persani L. Weber G. Biallelic inactivation of the dual oxidase maturation factor 2 (DUOXA2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab. 2008;93:605–610. doi: 10.1210/jc.2007-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhao T. Benard V. Bohl BP. Bokoch GM. The molecular basis for adhesion-mediated suppression of reactive oxygen species generation by human neutrophils. J Clin Invest. 2003;112:1732–1740. doi: 10.1172/JCI19108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu Y. Marchal CC. Casbon AJ. Stull N. von Lohneysen K. Knaus UG. Jesaitis AJ. McCormick S. Nauseef WM. Dinauer MC. Deletion mutagenesis of p22phox subunit of flavocytochrome b558: Identification of regions critical for gp91phox maturation and NADPH oxidase activity. J Biol Chem. 2006;281:30336–30346. doi: 10.1074/jbc.M607191200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.