Abstract
Interleukin-23 (IL-23) is integral to the pathogenesis of chronic inflammation. Resolution of acute inflammation is an active process mediated by specific signals and mediators, such as resolvin E1 (RvE1). Here, we provide the first evidence that RvE1, in nanogram quantities, promotes resolution of inflammatory airway responses in part by directly suppressing IL-23 and IL-6 production in the lung. Also contributing to the pro-resolving effects of RvE1 treatment were increased concentrations of interferon-γ in the lungs of RvE1-treated animals. These findings point to a pivotal role of IL-23 and IL-6—which promote survival and differentiation of TH-17 cells—in maintaining inflammation, and uncover an RvE1-initiated resolution program for allergic airway responses.
INTRODUCTION
Asthma is a chronic inflammatory disease of the airways characterized by infiltration of eosinophils and T lymphocytes that produce cytokines and pro-inflammatory lipid mediators, such as leukotrienes, which contribute to disease severity1, 2. The incidence of asthma is rising in Western countries1. Approximately 5% of individuals with asthma suffer from severe disease that is refractory to current medical treatment3. Importantly, no therapies are available to actively resolve the aberrant immune responses in asthmatic patients.
Epidemiological studies have indicated that diets rich in omega-3 fatty acids are associated with lower asthma prevalence4; however the mechanisms underlying this observation remain unclear. Resolvins are products of omega-3 fatty acids that were named based on their original identification in resolving exudates and their capacity to exert potent anti-inflammatory properties to accelerate the resolution phase of acute peritoneal inflammation5-8. Resolvin E1 (RvE1; 5S, 12R, 18R-tri-hydroxy-6Z, 8E, 10E, 14Z, 16E-eicosapaentanoic acid) is a potent inhibitor of polymorphonuclear leukocyte (PMN) transmigration across endothelial and epithelial barriers and promotes clearance of apoptotic PMNs and microbial products by macrophages9, 10. However, the impact of RvE1 on adaptive immunity is not known.
TH-17 cells, a recently identified subset of CD4+ T cells characterized by production of interleukin-17A (IL-17A), have been implicated in the pathogenesis of many inflammatory diseases including arthritis, experimental autoimmune encephalitis, inflammatory bowel disease and airway inflammation11. TH-17 cell population expansion and survival depends upon IL-23, which like IL-17 has been linked to the pathobiology of chronic inflammatory diseases12-15. IL-17 is present in asthmatic airways, can induce lung inflammation and stimulate mucus production16-23.
Here, we have investigated the impact of RvE1 on allergic airway inflammation in an experimental model of asthma. RvE1 both dampened the development and promoted the resolution of airway inflammation, in part by inhibiting IL-23 release from dendritic cells (DCs).
RESULTS
RvE1 impairs development of airway inflammation
Murine experimental models of asthma involve allergen sensitization and airway challenge24. Little information exists on the time course of natural resolution of allergic airway inflammation after discontinuation of allergen airway challenge. To determine the kinetics of spontaneous resolution of allergic lung inflammation, airway leukocyte numbers were monitored in bronchoalveolar lavage fluids (BALFs) 1, 4 and 8 days after the last of four daily allergen aerosol challenges. These time points corresponded to protocol days 18, 21 and 25 (Fig. 1a). Significant decrements in total BALF cell numbers were evident with a ~27% decrease between days 18 and 21 and a further ~60% decrease between days 21 and 25, (Fig. 1b). There was a distinct temporal regulation for each class of leukocyte. Similar to total leukocytes, eosinophils decreased by ~25% between days 18 and 21 and a further ~70% by day 25. In contrast, macrophages decreased by ~35% between day 18 and day 21, but no significant further decrease was evident by day 25. Of interest, lymphocytes increased significantly between day 18 and day 21 and then markedly decreased by ~85% by day 25. These findings indicate that the natural resolution of inflammatory cell trafficking in the airway is an active and highly regulated process.
Figure 1.
RvE1 dampens the development of allergic airway inflammation. a, Schematic diagram showing the protocol used to induce allergic airway inflammation. Mice were sensitized with OVA (i.p.) on day 0 and 7 and challenged with aerosolized OVA on days 14-17. b,c On protocol days 18 (white), 21 (gray) and 25 (black), mice were sacrificed and total BALF cells b and leukocyte subsets c were counted. d,e To some animals, RvE1 (200ng) (black) or vehicle (white) was given 30 min before aerosol challenge (days 14-17). On day 18, total BALF cells d and leukocyte subsets e were counted. Values represent the mean ± s.e.m. for n ≥ 3 mice, in ≥ 3 independent experiments *, P <0.05 by Student's t-test.
Because diets rich in eicosapentaenoic acid (EPA) are linked to lower asthma prevalence4 and the EPA-derived RvE1 regulates acute inflammatory responses10, we next determined the impact of RvE1 on inflammatory cell trafficking in allergic airway inflammation. Administration of RvE1 (200ng/day, i.v.) 30 minutes prior to OVA challenge on protocol days 14-17 (Fig. 1a) significantly decreased BALF leukocyte numbers at day 18 by ~80% compared to vehicle (0.1% ethanol) exposed animals (Fig. 1d). The numbers of BALF eosinophils, macrophages and lymphocytes were all markedly reduced by RvE1 treatment (Fig. 1e). These findings indicate that RvE1 can prevent the development of allergic airway inflammation. Of note, endogenous concentrations of RvE1 in normal and inflamed murine lung were below the limits of detection by liquid chromatography tandem mass spectrometry (LC-MS-MS).
RvE1 promotes resolution of airway inflammation
As these findings revealed potent immunomodulatory actions of RvE1, and in view of the current absence of pro-resolving therapies for asthma, we next determined the potential influence of RvE1 on the resolution of established airway inflammation. RvE1 (100ng/day, i.v.) or vehicle (0.1% ethanol) was administered for 3 consecutive days (protocol days 18-20) after the last allergen challenge and BALF leukocyte numbers were counted on days 21 and 25 (Fig. 2a). On day 21, RvE1-treated animals contained significantly decreased numbers of total BALF leukocytes, including eosinophils, macrophages and lymphocytes, compared to vehicle-treated animals (Fig. 2b,c). The resolution interval (Ri) is defined as the time required for cell numbers to reach 50% of the numbers observed at the peak of inflammation25. In vehicle-treated animals, between protocol days 18 and 25, the endogenous Ri for BALF leukocytes was ~5 days (Fig. 2d). Of interest, the Ri for RvE1 treated mice was markedly shortened to ~2.5 days. Together, these findings indicate that RvE1 exerts both anti-inflammatory and pro-resolving effects in this model of airway inflammation.
Figure 2.
RvE1 promotes resolution of airway inflammation. a, Schematic diagram showing the protocol used to determine the impact of RvE1 (100ng, i.v., days 18-20) or vehicle on the resolution of airway inflammation. b,c On day 21, total BALF cells b and leukocyte subsets c were counted. d, Using the mean number of BALF leukocytes on day 18, 21 and 25, the resolution interval (Ri) was calculated for RvE1 and vehicle cohorts. Values represent the mean ± s.e.m. for n ≥ 8 in ≥ 4 independent experiments, *, P <0.05 by Student's t-test.
At day 21 in the resolution protocol, histopathological analyses revealed marked decrements in leukocyte infiltration and adherence to vascular endothelial surfaces, and less reactive bronchial epithelial cells in the lungs of RvE1-treated animals (Fig. 3a,b and Supplementary Fig. 1, online). In addition, Periodic Acid Schiff (PAS) staining revealed that RvE1 also decreased airway epithelial mucus production (Fig. 3c,d). Thus, in the absence of continued allergen exposure RvE1 rapidly resolved airway leukocyte infiltration and lung morphological changes associated with allergic inflammation.
Figure 3.
RvE1 improves lung inflammation, airway mucus and airway hyper-reactivity. Representative (n ≥ 3) lung tissue sections from day 21 mice given RvE1 (100 ng) or vehicle were obtained from fixed, paraffin embedded lung tissue, prepared and stained with hematoxylin and eosin (a-b) or Periodic Acid Schiff (PAS) (c-d). Original magnification ×100 and ×200 as indicated. Br; bronchus, V; vein. Arrowheads denote mucus (magenta) containing Goblet cells. e, RvE1 (200ng) (filled circles) or vehicle (open circles) was administered on days 18-20 and AHR to methacholine was measured on day 21. Lung resistance was measured by flexiVent, and the change in mean lung resistance was plotted as a percentage of baseline (PBS alone). f, Log ED200 (the effective dose of MCh required to increase mean lung resistance 200% from baseline) for RvE1 (black) and vehicle (white) exposed cohorts. Values represent the mean ± s.e.m. for n ≥ 6 in ≥ 3 independent experiments *, P <0.05 by one-way ANOVA.
Airway hyper-responsiveness (AHR) is a functional hallmark of asthma3. At protocol day 21, AHR was measured in anesthetized mice that were mechanically ventilated in the presence of escalating doses of inhaled methacholine (MCh). Animals treated with RvE1 displayed significant protection from MCh-induced increases in mean lung resistance (Fig. 3e). The effective dose of MCh required to increase mean lung resistance 200% from baseline (ED200) was markedly higher for RvE1-treated mice than vehicle-treated counterparts (Fig. 3f). These results indicate that in addition to inflammation, RvE1 also accelerated resolution of allergic airway pathology and AHR.
RvE1 regulates cytokine and lipid mediators
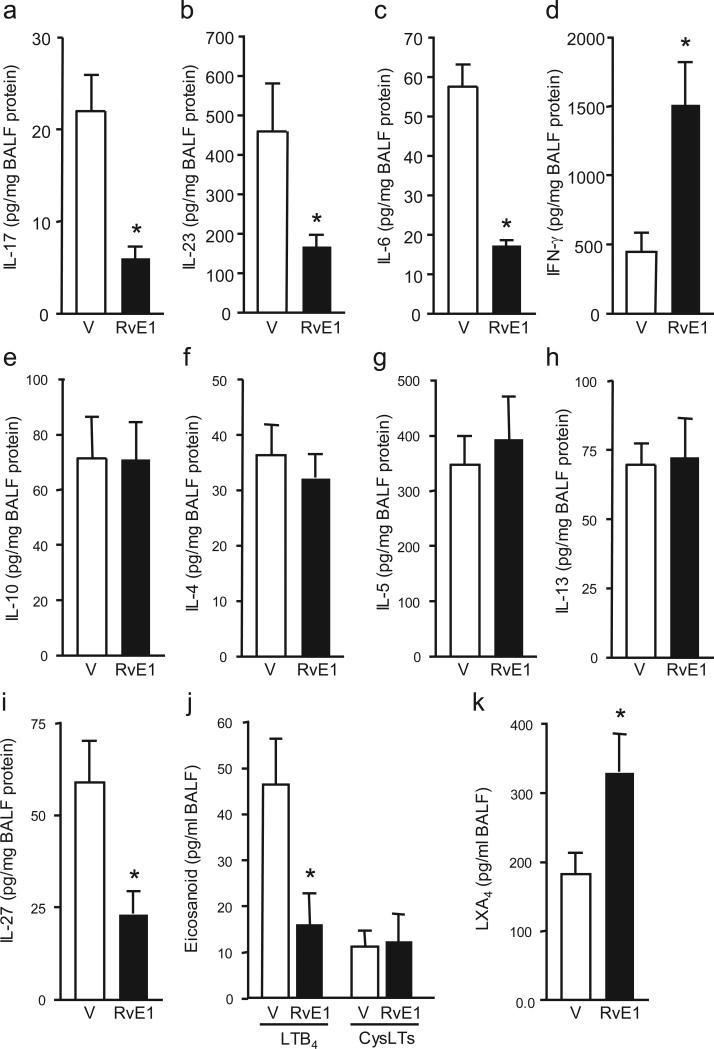
To uncover mechanisms through which RvE1 regulates airway responses, select cytokines and lipid mediators that modulate T cell population expansion and differentiation were measured by cytokine bead array in protocol day 21 BALF from vehicle or RvE1-treated mice. Notably, RvE1 significantly decreased IL-17A concentrations by ~70% (Fig. 4a). In control animals that were sensitized to OVA, but challenged with aerosol saline, IL-17A concentrations were undetectable at day 21. TH-17 T cell survival and differentiation is promoted by the cytokines IL-23 and IL-6, respectively26. Administration of RvE1 significantly decreased BALF IL-23 and IL-6 concentrations by ~65% and ~70%, respectively (Fig. 4b,c). Indicative of its agonist properties, RvE1 markedly increased interferon (IFN)-γ (http://www.signaling-gateway.org/molecule/query?afcsid=A001238) Fig. 4d). In sharp contrast to the marked RvE1-mediated regulation of IL-23, IL-6 and IFN-γ, RvE1 had no apparent effect at protocol day 21 on IL-10 or the TH2 cytokines IL-4, 5 and 13 (Fig. 4e-h). In addition, there was no significant difference in serum IgE concentrations (25.3 ± 2.7 ng/ml (vehicle) versus 26.5 ± 3.4 ng/ml (RvE1). Because IL-27 can block the development of both TH2 and TH-17 inflammatory responses27-29, we also measured BALF IL-27 concentrations. RvE1 decreased IL-27 by ~60% (Fig. 4i), indicating that RvE1 suppressed TH-17 responses in an IL-27–independent manner. Together, these results indicate that RvE1 is a potent and selective regulator of cytokines that are pivotal for airway inflammation.
Figure 4.
RvE1 selectively regulates cytokine and lipid mediators. a-i, Cytokines in day 21 BALF from vehicle (V) or RvE1 (100ng) treated mice were coded and measured by cytokine bead array. j,k BALF LTB4 and CysLTs j and LXA4 k concentrations were determined by ELISA. Results are expressed as mean ± s.e.m. for n ≥ 3 in ≥ 3 independent experiments, * P < 0.05 by Student's t-test.
Given their important roles in inflammation and asthma, the pro-inflammatory lipid mediators leukotriene B4 (LTB4) and cysteinyl leukotrienes (CysLTs) were also measured in the BALF of vehicle- and RvE1-treated mice3. RvE1 treatment led to a significant decrease in LTB4 (Fig. 4j). Of interest, endogenous concentrations of the counter-regulatory eicosanoid mediator lipoxin A4 (LXA4) increased from peak inflammation (day 18; 64.1 ± 12.9 pg/ml) to resolution (day 21; 183.7 ± 31.3 pg/ml). RvE1 administration led to further significant increases in LXA4 concentrations on protocol day 21 (330.00 ± 55.5 pg/ml (Fig. 4k). No significant differences in concentrations of the lipid mediator prostaglandin D2 (954.1 ± 34.0 (vehicle) versus 943.5 ± 24.5 pg/ml (RvE1)) were noted. Together, these findings uncover a “fingerprint” of selective mediator regulation by RvE1, and suggest that TH-17 cells and LTB4 may promote maintenance of inflammation in a manner distinct from the pathogenic roles played by TH2 cells and CysLTs in the initiation of allergic inflammatory responses.
RvE1 regulates T cell IL-17 and IFN-γ production
To investigate if changes in T cell phenotype were responsible for changes in IL-17A and IFN-γ production, lung, lung-draining mediastinal lymph nodes (MLNs) and spleen were excised during protocol day 21 and CD4+ T cell production of IL-17A and IFN-γ was measured by flow cytometry (Fig. 5a). Administration of RvE1 (days 18-20) significantly increased the ratio of CD4+ T cells expressing IFN-γ to those expressing IL-17A in all tissues (Fig. 5b). To determine the effect of IL-17A on the resolution of allergic airway inflammation, animals were given IL-17A–neutralizing antibody (10μg/day), isotype control antibody, or recombinant murine IL-17 (1μg/day) by intranasal administration each day on protocol days 18-20. Treatment with IL-17A–neutralizing antibody led to significant decreases in the total numbers of BALF leukocytes, eosinophils and macrophages relative to both vehicle and isotype control-treated animals (Fig. 5c and Supplementary Fig. 2a, online). BALF leukocyte numbers at protocol day 21 after treatment with IL-17A–neutralizing antibody were similar to those seen after RvE1 treatment (Fig. 5c). Administration of recombinant murine IL-17A had no significant effect on total BALF leukocytes, but administration of IL-17A in combination with RvE1 blocked the ability of RvE1 to induce resolution of leukocyte trafficking, as measured by BALF leukocyte numbers (Fig. 5c and Supplementary Fig. 2a, online). Administration of recombinant murine IL-23 together with RvE1 also blocked the pro-resolution effects of RvE1 (Fig. 5c and Supplementary Fig. 2b, online). As IL-6 promotes TH-17 cell differentiation30 and RvE1 decreased BALF IL-6 concentrations (Fig. 4c), we examined the effects of exposure to recombinant murine IL-6 on RvE1-mediated resolution. Of interest, like IL-23 and IL-17, IL-6 blocked RvE1-mediated decrements in BALF leukocyte numbers (Fig. 5d). Together, these findings reveal pivotal roles for IL-23 and TH-17 cells in the persistence of airway inflammation.
Figure 5.
RvE1 regulates pathogenic T cell IL-17A and IFN-γ production. a,b Representative FACS plots showing IFN-γ and IL-17A in CD4+ T cells from spleen, MLN and lung of day 21 mice treated with RvE1 or vehicle. Percentages of IFN-γ+ and IL-17A+ a and the ratio of IFN-γ+ to IL-17A+ b CD4+ T cells in spleen, MLN and lung are indicated after treatment with vehicle (white) or RvE1 (black). c, BALF cells at day 21 were counted after anti-IL-17A, rmIL-17A or RvE1 plus rmIL-17A (left) and in separate experiments after RvE1, rmIL-23 or RvE1 plus rmIL-23 (right) (See Methods) d, Mice were given IL-6, anti-IFN-γ alone or in combination with RvE1 on days 18-20 and BALF cells were enumerated. e, Representative lung tissue sections stained with H&E from day 21 mice treated with an isotype control antibody, anti-IL-17A, RvE1 or RvE1 plus anti-IFN-γ (see Methods). Original magnification ×100. Br; bronchus, V; vein. *, P < 0.05 compared to vehicle or isotype control; †, P < 0.05 compared to anti- IL-17, #, P < 0.05 compared to RvE1 by one-way ANOVA for n ≥ 4 in ≥ 2 independent experiments.
To determine if the RvE1-mediated increase in IFN-γ (Fig. 4d) contributed to the pro-resolving actions of RvE1, mice were given an IFN-γ–neutralizing antibody. Compared to treatment with RvE1, anti–IFN-γ led to significant increases in BALF total leukocytes (Fig. 5d) that were primarily the result of increased numbers of eosinophils (Supplementary Fig. 2c, online). Mice receiving anti–IFN-γ together with RvE1 contained fewer BALF leukocytes compared to mice treated with anti–IFN-γ and vehicle, but more BALF leukocytes than mice treated with RvE1 alone (Fig. 5d). However, anti–IFN-γ did not influence BALF IL-23 concentrations, and BALF IL-23 concentrations were similar in animals treated with anti-IFN-γ plus RvE1 and those treated with RvE1 alone (Supplementary Fig. 3). These findings indicate that IFN-γ does not regulate IL-23 in this setting, but does contribute to RvE1-mediated inflammation resolution.
Examination of lung histopathology revealed similar trends as mice treated with anti-IL-17A exhibited decreased leukocyte infiltration and hypertrophic changes to the airway epithelium, similar to mice treated with RvE1, and treatment with anti–IFN-γ reversed the ameliorative effects of RvE1 (Fig. 5e). Together, these findings implicate IL-23, IL-17 and IL-6 in the maintenance of airway inflammation and IFN-γ in its resolution after discontinuation of allergen challenge.
Overlapping yet distinct effects of RvE1 and LX
Because LXA4 concentrations increased during spontaneous resolution (day 21) and RvE1 treatment further increased LXA4 concentrations (Fig. 4k), we next investigated the impact of an LXA4 analog, 15-epi-lipoxin analog (ATLa), alone or in combination with RvE1 on airway inflammation. This LX stable analog prevents the development of allergic airway responses24, but its influence on resolution in this model has not been determined. ATLa significantly decreased BALF leukocyte numbers, measured at protocol day 21, by a similar magnitude as RvE1 (100ng/day) (Fig. 6a). Of interest, we noted no synergistic effect between RvE1 (50ng) and ATLa (100ng) (Fig. 6a). Quantification of BALF IL-17, IL-23 and IFN-γ concentrations revealed distinct regulation of these cytokines by RvE1 and ATLa. Both RvE1 and ATLa significantly decreased IL-17 concentrations (Fig. 6b), but RvE1 and not ATLa decreased IL-23 (Fig. 6c). In sharp contrast to the RvE1-induced increase in IFN-γ production, ATLa alone and together with RvE1 significantly decreased IFN-γ concentrations (Fig. 6d).
Figure 6.
RvE1 and LXA4 utilize distinct mechanisms to promote resolution of airway inflammation. a, Total BALF leukocytes from mice given RvE1, ATLa or RvE1 plus ATLa. b-d, Concentrations of IL-17, IL-23 and IFN-γ in BALF were measured by cytokine bead array. e, The inhibitory effect of increasing concentrations of RvE1 on IL-23 release from LPS-stimulated BMDCs was measured as a percentage of maximum (89.5 pg/ml/million cells). The LXA4 receptor antagonist BOC-2 (10 μM) was added where indicated. Results are expressed as mean ± s.e.m. *, P < 0.05. f, Concentrations of IL-6 and TNF released from LPS-activated BMDCs treated with vehicle or 1ng/ml RvE1 were measured by cytokine bead array. Results are expressed as mean ± s.e.m for n ≥ 3 in ≥ 2 independent experiments. *, P < 0.05 by one-way ANOVA.
RvE1 inhibited IL-23 production in vivo and this cytokine can be released by activated DCs31, 32. In addition, lung DCs removed from mice at protocol day 21 and bone marrow-derived DCs (BMDCs) expressed RNA transcripts encoding receptors for RvE1 (ChemR23 and BLT-1) and ATLa (ALX) (data not shown). Therefore, we measured the impact of RvE1 on IL-23 release from lipopolysaccharide-stimulated BMDCs. RvE1 potently inhibited IL-23 release in a concentration-dependent manner (Fig. 6e) Addition of BOC-2 peptide, an LXA4 receptor (ALX) antagonist33, did not block inhibition of IL-23 release by RvE1. Of note, RvE1 (1ng/ml) also inhibited release of IL-6 and tumor necrosis factor from these activated BMDCs (Fig. 6f). Together, these findings show that LXA4 and RvE1 both inhibit IL-17 in vivo, yet RvE1 alone regulates IL-23 in vivo and in vitro in a LXA4-independent manner.
DISCUSSION
Here, we provided evidence that RvE1 acts as an anti-inflammatory and pro-resolving lipid mediator for allergic airway inflammation. After antigen sensitization and aerosol challenge, there was a natural and spontaneous resolution of inflammation in the airway that was accelerated by administration of nanogram quantities of RvE1. In addition to inflammation, RvE1 also dampened airway mucus and hyper-reactivity, responses typical of asthma. RvE1 decreased IL-23, IL-6 and IL-17 and increased IFN-γ concentrations; these cytokine alterations were reflected in shifting percentages of TH-17 and IFN-γ–producing CD4+ T cells in lung, lung-draining MLNs and spleen. Antibody neutralization experiments implicated IL-17A as an essential driver of airway inflammation in vivo. Moreover, exogenous addition of recombinant IL-23, IL-6 or a neutralizing antibody against IFN-γ prevented RvE1-mediated resolution. RvE1 treatment also increased LXA4, and a stable LXA4 analog shared the pro-resolving and IL-17 counter-regulating actions of RvE1. Together, these findings point to IL-23 and TH-17 cells as essential for the maintenance of airway inflammation, and provide the first evidence for counter-regulatory lipid mediators with the capacity to control these pro-inflammatory cytokines and T cells.
IL-23 is involved in the pathobiology of numerous chronic diseases, including colitis, encephalitis, psoriasis, rheumatoid arthritis and cancer12, 14, 15, 34, and is critical for the survival of TH-17 cells 12, 30. These T cells release IL-17A, a cytokine that increases production of several proinflammatory molecules, including IL-1 and TNF35. RvE1 markedly decreased BALF concentrations of IL-6, IL-23 and IL-17A, indicating that the pathways promoting TH-17 cell differentiation and survival are regulatory checkpoints countered by RvE1 during resolution of airway inflammation. RvE1 alone regulated IL-23 both in vivo and in vitro. Administration of IL-23 in combination with RvE1 blocked its ability to promote resolution, indicating that IL-23 was important for prolonging airway inflammation. Of interest, eosinophils express the IL-23 receptor and IL-23 induces the release of pro-inflammatory chemokines from eosinophils22. Highlighting the importance of IL-23 and TH-17 cells in general inflammation, IL-23 deficient mice are protected in models of colitis13. Similar to IL-23, RvE1 can also protect against the development of colitis in model systems36, 37. Thus, regulation of IL-23 and TH-17 cells by RvE1 may serve as a common mechanism for its pro-resolving actions in mucosal inflammation.
IL-17 has a pivotal role in regulating airway inflammation and responses to allergen20, 21, 38. Expression of an IL-17 transgene driven by the airway epithelial promoter CC10 leads to spontaneous airway inflammation characterized by infiltration of eosinophils and lymphocytes, hypertrophic changes to the epithelium and increased mucus production18. In addition, IL-17 receptor knockout mice do not develop allergen-induced airway inflammation20. Moreover, elevated concentrations of IL-17 in human BALF and induced sputum are associated with asthma16, 20, 39. Of interest, IL-17 may have dual regulatory roles in allergic airway inflammation, as it can also inhibit TH2-driven immune responses20. Concurrent administration of allergen and recombinant IL-17 to sensitized animals dampens airway inflammation by decreasing IL-5, IL-13, CCL11 and CCL17 production in an IL-4 receptor dependent manner20. In contrast, in the experiments performed here, IL-17 BALF concentrations remained elevated in established asthma three days after allergen exposure. In promoting resolution, RvE1 significantly decreased both IL-17 and allergic airway responses. In addition, IL-17–neutralizing antibody significantly decreased in BALF leukocyte numbers and recombinant murine IL-17 blocked the capacity of RvE1 to promote resolution. RvE1 also downregulated IL-6 in BALF in vivo, and in vitro from LPS-stimulated BMDCs. IL-6 has been found to be increased in the serum of asthmatic subjects compared to nonasthmatic patients40 and IL-6 trans-signalling via soluble IL-6 receptor supports TH2 and TH-17 cell differentiation30, 40. Together these results indicate that RvE1-mediated regulation of IL-17 is important in promoting resolution of airway inflammation and suggest a pathogenic relationship between IL-17 and persistent airway inflammation in this model.
In contrast, RvE1 did not significantly impact concentrations of IL-10 and the TH2 cytokines IL-4, IL-5 and IL-13 during resolution. Thus, no longer exposed to allergen, the TH-17 cells and cytokines that maintained the inflammatory response exerted effects distinct from the TH2 cells and cytokines involved in the initiation of allergic inflammatory responses. Of note, RvE1 facilitated resolution despite decreased concentrations of IL-27, which can regulate both TH-17 and TH2 T cells27-29.
RvE1 increased IFN-γ production, and TH-17 immune responses are inhibited by IFN-γ18. IFN-γ can contribute to resolution by upregulating Fas (CD95) expression on lymphocytes to increase their rate of apoptosis41. Mice lacking IFN-γ or Fas exhibit defective resolution of inflammation in experimental models of asthma42, 43. As an IFN-γ–neutralizing antibody partially blocked RvE1-mediated resolution, IFN-γ is important for resolution of airway inflammation in this setting. However, as anti-IFN-γ did not reduce BALF IL-23 concentrations, IFN-γ promoted resolution via a mechanism distinct from that of RvE1.
ChemR23 is a cognate receptor for RvE1 and is expressed on several immune regulatory cell types, including DCs 44. ChemR23-deficient mice, in a model of zymosan induced peritonitis, exhibit increased inflammation, indicating that this receptor is important for counter-regulatory signalling45. The new findings presented here bolster the emerging concept of resolution as an active process with specific counter-regulatory signalling pathways.
The ideal outcome of resolution of inflammation is complete tissue catabasis46. RvE1-mediated increases in both IFN-γ and LXA4 concentrations are indicative of its agonist properties, and provide further evidence that signalling for inflammation resolution is an active process8. RvE1 displays autacoid properties as it is rapidly formed, rapidly inactivated and carries potent bioactions at sub-nanomolar concentrations8. Difficult to detect in vivo here in inflamed lungs, endogenous RvE1 has been detected in several models of spontaneously resolving inflammation5, 6. In addition, quantities of RvE1 can be increased by dietary modification, administration of aspirin or transgenic expression of the fat-1 gene36, 37, 44. Moreover, disruption of endogenous formation of RvE1 or LXA4 by biosynthetic enzyme inhibition disrupts resolution of acute inflammation, resulting in prolonged inflammatory responses10, 47. In addition, severe and uncontrolled asthma are characterized by a decreased capacity to generate these protective mediators 48, 49. During resolution of allergic airway inflammation, exogenous RvE1 increased production of LXA4 and inhibited production of the leukocyte chemoattractant and secretagogue LTB4. Of interest, both RvE1 and LTB4 can interact with the LTB4 receptor BLT-1 with RvE1 serving as a receptor antagonist50. In addition to its potent pro-resolving signals for airway inflammation24, 47, LXA4 can also block LTB4-mediated responses in leukocytes51. A stable lipoxin analog (ATLa) significantly decreased BALF leukocyte numbers and IL-17 concentrations, but the mechanism by which ATLa promoted resolution was distinct from RvE1-mediated regulation of IL-23 production, suggesting that IL-17 production is downstream of IL-23. These results show that two classes of pro-resolution lipid mediators target IL-17 via distinct mechanisms to promote resolution.
In summary, RvE1 is a potent regulator of both the initiation and resolution of complex airway inflammation. To promote resolution, RvE1 decreased production of the pro-inflammatory cytokines IL-23, IL-6 and IL-17, and increased production of the counter-regulatory mediators IFN-γ and LXA4. Regulation of adaptive immune responses by RvE1 points to important pathological roles for IL-23 and TH-17 cells in the persistent and unrestrained immune responses of asthma and other chronic inflammatory diseases.
METHODS
Sensitization and challenge protocols
Five to seven week old male FVB mice (Charles River Laboratories) were housed in isolation cages under viral antibody-free conditions. Mice were fed a standard diet (Laboratory Rodent Diet 5001, PMI Nutrition International) that contained no less than 4.5% total fat with 0.26% omega-3 fatty acids and <0.01% C20:4. After Harvard Medical Area IRB approval (Protocol #03618), mice were sensitized with intra-peritoneal injections of ovalbumin (OVA) (Grade III; Sigma) (10μg) plus 1mg aluminum hydroxide (alum) (Sigma) as adjuvant in 0.2ml saline on days 0 and 7. On days 14, 15, 16, 17 mice received aerosol challenge with 6% OVA for 25 minutes/day. To investigate inflammation development, some animals received RvE1 methyl ester (100ng) or vehicle (0.1% ethanol) in saline by intravenous injection 30 minutes before OVA aerosol challenge on days 14, 15, 16 and 17. This dose of RvE1 was chosen based on its regulation of murine peritoneal inflammation10. To investigate inflammation resolution, some mice received RvE1 methyl ester (50 ng or 100 ng), a 15 epi-LXA4 analog (15-epi-16 para-fluro-phenoxy-LXA4 methyl ester, ATLa) (100ng) or vehicle by intravenous injection on only days 18, 19 and 20. Some animals were treated with recombinant murine IL-17A (1μg/day) (eBiosciences), a neutralizing IL-17A-specific antibody (10 μg/day) (eBioMM17F3) or an isotype control antibody (eBiosciences), recombinant murine IL-23 (1μg/day) (R&D Systems), anti-IFN-γ (10 μg/day) (XMG1.2, Biolegend), or recombinant murine IL-6 (1μg/day) (PeproTech) by intranasal administration. Bronchoalveolar lavage was performed with 2 × 1 ml aliquots of phosphate buffered saline with 0.6mM EDTA, or tissues were harvested for histological analysis.
Measurement of lung resistance
To measure airway hyper-responsiveness, anesthetized mice were mechanically ventilated using a flexiVent (SciReq) and aerosolized methacholine (0, 1, 3, 10, 30 and 100mg/ml) was delivered in-line via the inhalation port for 10 sec. Lung resistance was measured as a percentage of baseline with PBS nebulization using the mean of 10 readings taken for each concentration of methacholine. No BALF or histological analysis was performed on those animals undergoing measurement of airway hyper-responsiveness.
Allergen-initiated respiratory inflammation
Murine lungs were fixed in 10% buffered formalin and paraffin embedded for hematoxylin and eosin and Periodic Acid Schiff staining (Sigma). Samples of cell-free BALF (centrifuged at 2000g for 10 minutes) were coded and cytokines were measured in deidentified aliquots by bead array (Pierce Biotechnology, Inc.); lipid mediators were measured by ELISA (LTB4, CysLTs and PGD2 Cayman Chemical; LXA4 Neogen). Cells in BALF were resuspended in PBS, enumerated by a hemocytometer. Cytospins were performed by cytocentrifuge (STATspin) (265×g). Cells were stained with a Wright-Giemsa stain (Sigma) to quantify leukocyte subsets, counting ≥ 200 cells per slide. Total serum IgE was measured by ELISA (BD Biosciences).
Intracellular cytokine flow cytometry
A single-cell suspension of lung, MLNs and spleen was generated by passing tissue through a 70μm filter (BD Pharmingen). Lung lymphocytes were enriched using Ficoll (Sigma). Cells were incubated (4 h, 37°C) in vitro with phorbol-12-myristate (50ng/ml; Sigma) and ionomycin (1μg/ml; Sigma) plus Golgi-stop (BD-Pharmingen). Fc receptors were blocked with anti-CD16/32 (Caltag), followed by cell surface staining with CD4 PE-Cy5 (eBiosciences). Cells were then resuspended in Fixation-Permeabilization solution (BD Cytofix/Cytoperm kit; BD Pharmingen) and intracellular staining with anti–IL-17A–FITC (eBio17B7; eBiosciences) and anti–IFN-γ–PE (XMG1.2; Caltag/Invitrogen) was performed at 4°C. A FACSCalibur (Becton Dickson) was used for flow cytometry and data was analysed using FloJo software (Tree Star).
Generation and purification of BMDCs
The femurs and tibia of FVB mice were opened at both ends and BM cells were extruded with ice cold RPMI-1640 and passed through a 70μm filter. Cells were cultured at a density of 1×106/ml for 7 days with IL-4 (1ng/ml and GM-CSF (10ng/ml) (R&D Systems)) in RPMI-1640 complete media (10% FCS, 2% Pen-Strep), changing the medium every other day. On day 7, the CD11c+ BMDCs were further purified from the non-adherent cells using anti-CD11c—coated magnetic beads according to the manufacturer's instructions (Miltenyi Biotech). The purity of the cells was assessed by flow cytometry (>93%). BMDCs were plated at a density of 1×106/ml and incubated with vehicle or RvE1 free acid (1, 10 or 100ng) in 1ml final volume for 24 h before the addition of LPS (Sigma) (1μg/ml) for a further 24 h (37°C, 5% CO2). IL-23 (p19/p40) release was determined by ELISA (eBiosciences). IL-6 and TNF were measured by cytokine bead array.
Statistical analysis
Results are expressed as the mean ± s.e.m. Statistical significance of differences was assessed by Student's t-test and one-way ANOVA. P <0.05 was set as the level of significance.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank G.L. Zhu, B. Ith and K. Gotlinger for expert technical assistance. This research was supported in part by the US National Institutes of Health grants AI068084 (B.D.L) and P50-DE016191 (B.D.L and C.N.S).
Footnotes
COMPETING INTERESTS STATEMENT
B.D. Levy, C.N. Serhan together with Brigham and Women's Hospital, have a pending patent on the use of RvE1 in the treatment of airway diseases and asthma.
References
- 1.Holgate ST. The epidemic of allergy and asthma. Nature. 1999 Nov 25;402(6760 Suppl):B2–4. doi: 10.1038/35037000. [DOI] [PubMed] [Google Scholar]
- 2.Martinez Molina D, Wetterholm A, Kohl A, McCarthy AA, Niegowski D, Ohlson E, et al. Structural basis for synthesis of inflammatory mediators by human leukotriene C4 synthase. Nature. 2007 Aug 2;448(7153):613–6. doi: 10.1038/nature06009. [DOI] [PubMed] [Google Scholar]
- 3.Busse WW, Lemanske RF., Jr. Asthma. N Engl J Med. 2001 Feb 1;344(5):350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz J, Weiss ST. The relationship of dietary fish intake to level of pulmonary function in the first National Health and Nutrition Survey (NHANES I). Eur Respir J. 1994 Oct;7(10):1821–4. doi: 10.1183/09031936.94.07101821. [DOI] [PubMed] [Google Scholar]
- 5.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000 Oct 16;192(8):1197–204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002 Oct 21;196(8):1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004 May;3(5):401–16. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- 8.Serhan CN. Resolution phase of inflammation: novel endogenous antiinflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–37. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 9.Campbell EL, Louis NA, Tomassetti SE, Canny GO, Arita M, Serhan CN, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. Faseb J. 2007 Oct;21(12):3162–70. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 10.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007 Jun 14;447(7146):869–74. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007 Apr;8(4):345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 12.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005 Jan 17;201(2):233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006 May;116(5):1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, et al. IL-23 promotes tumour incidence and growth. Nature. 2006 Jul 27;442(7101):461–5. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 15.Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006 Nov 27;203(12):2577–87. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, et al. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol. 2001 Sep;108(3):430–8. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 17.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002 Sep;17(3):375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 18.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005 Nov;6(11):1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Biol Chem. 2003 May 9;278(19):17036–43. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 20.Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, et al. Interleukin-17 is a negative regulator of established allergic asthma. J Exp Med. 2006 Nov 27;203(12):2715–25. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008 Feb 18;205(2):385–93. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung PF, Wong CK, Lam CW. Molecular Mechanisms of Cytokine and Chemokine Release from Eosinophils Activated by IL-17A, IL-17F, and IL-23: Implication for Th17 Lymphocytes-Mediated Allergic Inflammation. J Immunol. 2008 Apr 15;180(8):5625–35. doi: 10.4049/jimmunol.180.8.5625. [DOI] [PubMed] [Google Scholar]
- 23.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008 Apr 14; doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levy BD, De Sanctis GT, Devchand PR, Kim E, Ackerman K, Schmidt BA, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4). Nat Med. 2002 Sep;8(9):1018–23. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 25.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005 Apr 1;174(7):4345–55. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 26.Zhou L, Ivanov, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007 Sep;8(9):967–74. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 27.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006 Sep;7(9):937–45. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 28.Yoshimoto T, Yoshimoto T, Yasuda K, Mizuguchi J, Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007 Oct 1;179(7):4415–23. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 29.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006 Sep;7(9):929–36. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 30.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006 May 11;441(7090):235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 31.Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J. 2004 Aug;18(11):1318–20. doi: 10.1096/fj.03-1367fje. [DOI] [PubMed] [Google Scholar]
- 32.Muhlbauer M, Chilton PM, Mitchell TC, Jobin C. Impaired Bcl3 up-regulation leads to enhanced LPS-induced IL-23p19 gene expression in IL-10-/- mice. J Biol Chem. 2008 Mar 28; doi: 10.1074/jbc.M709029200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonnans C, Gras D, Chavis C, Mainprice B, Vachier I, Godard P, et al. Synthesis and anti-inflammatory effect of lipoxins in human airway epithelial cells. Biomed Pharmacother. 2007 Jun;61(5):261–7. doi: 10.1016/j.biopha.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Yago T, Nanke Y, Kawamoto M, Furuya T, Kobashigawa T, Kamatani N, et al. IL-23 induces human osteoclastogenesis via IL-17 in vitro, and anti-IL-23 antibody attenuates collagen-induced arthritis in rats. Arthritis Res Ther. 2007 Sep 23;9(5):R96. doi: 10.1186/ar2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998 Apr 1;160(7):3513–21. [PubMed] [Google Scholar]
- 36.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005 May 24;102(21):7671–6. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hudert CA, Weylandt KH, Lu Y, Wang J, Hong S, Dignass A, et al. Transgenic mice rich in endogenous omega-3 fatty acids are protected from colitis. Proc Natl Acad Sci U S A. 2006 Jul 25;103(30):11276–81. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanov S, Bozinovski S, Bossios A, Valadi H, Vlahos R, Malmhall C, et al. Functional relevance of the IL-23-IL-17 axis in lungs in vivo. Am J Respir Cell Mol Biol. 2007 Apr;36(4):442–51. doi: 10.1165/rcmb.2006-0020OC. [DOI] [PubMed] [Google Scholar]
- 39.Barczyk A, Pierzchala W, Sozanska E. Interleukin-17 in sputum correlates with airway hyperresponsiveness to methacholine. Respir Med. 2003 Jun;97(6):726–33. doi: 10.1053/rmed.2003.1507. [DOI] [PubMed] [Google Scholar]
- 40.Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005 Feb;115(2):313–25. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Rose V, Cappello P, Sorbello V, Ceccarini B, Gani F, Bosticardo M, et al. IFN-gamma inhibits the proliferation of allergen-activated T lymphocytes from atopic, asthmatic patients by inducing Fas/FasL-mediated apoptosis. J Leukoc Biol. 2004 Aug;76(2):423–32. doi: 10.1189/jlb.0503247. [DOI] [PubMed] [Google Scholar]
- 42.Tong J, Bandulwala HS, Clay BS, Anders RA, Shilling RA, Balachandran DD, et al. Fas-positive T cells regulate the resolution of airway inflammation in a murine model of asthma. J Exp Med. 2006 May 15;203(5):1173–84. doi: 10.1084/jem.20051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida M, Leigh R, Matsumoto K, Wattie J, Ellis R, O'Byrne PM, et al. Effect of interferon-gamma on allergic airway responses in interferon-gamma-deficient mice. Am J Respir Crit Care Med. 2002 Aug 15;166(4):451–6. doi: 10.1164/rccm.200202-095OC. [DOI] [PubMed] [Google Scholar]
- 44.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005 Mar 7;201(5):713–22. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cash JL, Hart R, Russ A, Dixon JP, Colledge WH, Doran J, et al. Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med. 2008 Apr 14;205(4):767–75. doi: 10.1084/jem.20071601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O'Neill LA, et al. Resolution of inflammation: state of the art, definitions and terms. Faseb J. 2006 Dec 13; doi: 10.1096/fj.06-7227rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005 Apr 15;174(8):5033–9. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 48.Levy BD, Bonnans C, Silverman ES, Palmer LJ, Marigowda G, Israel E. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005 Oct 1;172(7):824–30. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, et al. Protectin D1 Is Generated in Asthma and Dampens Airway Inflammation and Hyperresponsiveness. J Immunol. 2007;178(1):496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007 Mar 15;178(6):3912–7. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- 51.Levy BD, Fokin VV, Clark JM, Wakelam MJ, Petasis NA, Serhan CN. Polyisoprenyl phosphate (PIPP) signaling regulates phospholipase D activity: a ‘stop’ signaling switch for aspirin-triggered lipoxin A4. Faseb J. 1999 May;13(8):903–11. doi: 10.1096/fasebj.13.8.903. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.