Abstract
We discovered that renal injury releases 2′,3′-cAMP (positional isomer of 3′,5′-cAMP) into the interstitium. This finding motivated a novel hypothesis: renal injury leads to activation of an extracellular 2′,3′-cAMP-adenosine pathway (i.e. metabolism of extracellular 2′,3′-cAMP to 3′-AMP and 2′-AMP, which are metabolized to adenosine, a retaliatory metabolite). In isolated rat kidneys, arterial infusions of 2′,3′-cAMP (30 μmol/liter) increased the mean venous secretion of 3′-AMP (3,400-fold), 2′-AMP (26,000-fold), adenosine (53-fold), and inosine (adenosine metabolite, 30-fold). Renal injury with metabolic inhibitors increased the mean secretion of 2′,3′-cAMP (29-fold), 3′-AMP (16-fold), 2′-AMP (10-fold), adenosine (4.2-fold), and inosine (6.1-fold) while slightly increasing 5′-AMP (2.4-fold). Arterial infusions of 2′-AMP and 3′-AMP increased secretion of adenosine and inosine similar to that achieved by 5′-AMP. Renal artery infusions of 2′,3′-cAMP in vivo increased urinary excretion of 2′-AMP, 3′-AMP and adenosine, and infusions of 2′-AMP and 3′-AMP increased urinary excretion of adenosine as efficiently as 5′-AMP. The implications are that 1) in intact organs, 2′-AMP and 3′-AMP are converted to adenosine as efficiently as 5′-AMP (previously considered the most important adenosine precursor) and 2) because 2′,3′-cAMP opens mitochondrial permeability transition pores, a pro-apoptotic/pro-necrotic process, conversion of 2′,3′-cAMP to adenosine by the extracellular 2′,3′-cAMP-adenosine pathway would protect tissues by reducing a pro-death factor (2′,3′-cAMP) while increasing a retaliatory metabolite (adenosine).
introduction
Our past work supports the existence of a biochemical mechanism of adenosine biosynthesis that involves 3′,5′-cAMP and that contributes to the extracellular levels of adenosine, i.e. the extracellular 3′,5′-cAMP-adenosine pathway (Fig. 1, left side) (1–20). In this regard, we propose that the extracellular 3′,5′-cAMP-adenosine pathway involves four sequential steps that are spatially linked and occur on a quasi-solid surface (i.e. the membrane surface). The four sequential steps are: 1) receptor-mediated intracellular production of 3′,5′-cAMP; 2) active transport of 3′,5′-cAMP from the intracellular compartment to the cell surface; 3) extracellular metabolism of 3′,5′-cAMP to 5′-AMP; and 4) extracellular conversion of 5′-AMP to adenosine. Independent laboratories now confirm the existence of the extracellular 3′,5′-cAMP-adenosine pathway in pial microvessels (21) and more recently in skeletal muscle (22) and the gastrointestinal tract (23). Moreover, Müller et al. (24) report the existence of a 3′,5′-cAMP-adenosine pathway in which the sequential conversion of 3′,5′-cAMP to 5′-AMP and 5′-AMP to adenosine occurs on the outward facing membrane surface of lipid droplets within adipocytes.
FIGURE 1.
Schematic comparing the biochemical steps in the 3′,5′-cAMP-adenosine pathway (left side) versus the postulated 2′,3′-cAMP-adenosine pathway (right side). MRP4/5, multidrug resistance proteins 4 and 5; PDEs, phosphodiesterases; CNPase, 2′,3′-cAMP-3′-phosphodiesterase.
To study in more detail the role of 3′,5′-cAMP-adenosine pathway in the kidney, we recently developed a novel assay to measure 3′,5′-cAMP and other purines in the renal venous effluent from isolated, perfused kidneys (25). Unlike commercially available kits for 3′,5′-cAMP that rely upon the selectivity of antibody recognition, our assay was based on the platform technology of high performance liquid chromatography-tandem mass spectrometry. While investigating the production of 3′,5′-cAMP from isolated, perfused rat kidneys, we observed a dominant chromatographic peak that was due to an endogenous substance that was not 3′,5′-cAMP yet had the same parent ion as 3′,5′-cAMP and fragmented to the same daughter ion (adenine) as 3′,5′-cAMP (26). We identified the substance to be 2′,3′-cAMP (26).
Our discovery of 2′,3′-cAMP release into the extracellular compartment by an intact organ motivates our new hypothesis that there may exist, in addition to the extracellular 3′,5′-cAMP-adenosine pathway, an extracellular 2′,3′-cAMP-adenosine pathway (Fig. 1, right side). One rationale for this hypothesis is that stimulation of mRNA turnover involves the actions of multiple RNases that first cleave the phosphodiester bonds in the polyadenine (poly-A)2 tail of mRNA, followed by 5′-decapping and then degradation of the mRNA (27). Moreover, 2′,3′-cAMP may be the intermediate adenine nucleotide formed by this process (28). Because of the large mass of adenine nucleotides in mRNA (particularly in the poly-A tail), mRNA turnover would doubtless produce large quantities of 2′,3′-cAMP/molecule of mRNA degraded. Another rationale for this hypothesis is that transporters, such as multidrug resistance proteins 4 and 5, rapidly transport 3′,5′-cAMP out of the cell (29–31). However, these transporters carry many nucleosides and nucleotides, including cyclic nucleotides of diverse chemical structures. Thus it is nearly certain that they would also actively extrude 2′,3′-cAMP form the cell. The final rationale is that there exist various enzymes that could serve as ecto-2′,3′-cAMP-phosphodiesterases and ecto-2′/3′-nucleotidases to hydrolyze extracellular 2′,3′-cAMP to 2′-AMP and 3′-AMP and extracellular 2′-AMP and 3′-AMP to adenosine, respectively. For example 2′,3′-cAMP-3′-phosphodiesterase is a membrane-bound enzyme that specifically converts 2′,3′-cAMP to 2′-AMP (32), and some RNases, such as ptRNase 1 are secreted by cells into the extracellular compartment and can hydrolyze 2′,3′-cAMP to 3′-AMP (33, 34). Finally, there is a large family of ecto-nucleotidases, for example ecto- nucleoside-triphosphate-diphosphohydrolases, ecto-nucleotide pyrophosphatase/phosphodiesterases and alkaline phosphatases, that could possibly function not only as ecto-2′,3′-cAMP-phosphodiesterases to process 2′,3′-cAMP to 2′-AMP and 3′-AMP, but also could conceivably process 2′-AMP and 3′-AMP to adenosine (35–37). This putative extracellular 2′,3′-cAMP-adenosine pathway could be extremely important in producing extracellular adenosine whenever cells are exposed to stressful stimuli that enhance mRNA turnover, thus providing the protective, “retaliatory” metabolite, adenosine, to mitigate cellular damage. Here we establish purine metabolomics using liquid chromatography-tandem mass spectrometry and using this approach demonstrate that the intact kidney, both in isolation and in vivo, expresses an extracellular 2′,3′-cAMP-adenosine pathway.
EXPERIMENTAL PROCEDURES
Animals
The studies utilized adult (∼16 weeks-of-age), male Wistar-Koto rats that were obtained from Taconic Farms (Germantown, NY).
Isolated, Perfused Rat Kidney
The rats were anesthetized with Inactin (90 mg/kg intraperitoneally; Sigma-Aldrich), and the left kidney was isolated and perfused with Tyrode's solution (137 mm NaCl, 2.7 mm KCl, 1.8 mm CaCl2, 1.1 mm MgCl2, 12 mm NaHCO3, 0.42 mm NaH2PO4, 5.6 mm d(+)-glucose) using a Hugo Sachs Elektronik-Harvard Apparatus GmbH (March-Hugstetten, Germany) kidney perfusion system as previously described (38). Briefly, all branches of the left renal artery and vein were ligated. A polyethylene (PE)-50 cannula was placed into the left renal artery, and a PE-90 cannula was placed into the left renal vein. The left kidney was removed and attached to the perfusion system, the kidneys were perfused (single pass mode) at a constant flow (5 ml/min), and perfusion pressure was monitored with a pressure transducer.
In Vivo Rat Kidney
The rats were anesthetized with Inactin (90 mg/kg intraperitoneally) and placed on an isothermal pad, and body temperature was monitored with a rectal probe thermometer and kept at 37 °C with a heat lamp. The trachea was cannulated to facilitate respiration, and a PE-50 cannula was inserted into the left carotid artery and connected to a digital blood pressure analyzer (Micro-Med, Inc., Louisville, KY) for continuous measurement of mean arterial blood pressure and heart rate. A PE-50 cannula was placed in the jugular vein, and an infusion of 0.9% saline was begun at 50 μl/min for fluid replacement. A transit time flow probe (model 1 RB; Transonic Systems, Inc., Ithaca, NY) was positioned around the left renal artery and connected to a transit-time flowmeter (Transonic Systems, Inc.) to monitor renal blood flow. A 30-gauge needle connected to an infusion pump was inserted into the left renal artery, and an infusion of 0.9% saline was begun at 20 μl/min. Also, a PE-10 cannula was inserted into the left ureter for the collection of urine. After a 2-h rest period, the experiments were begun. cAMPs and AMPs were dissolved in 0.9% saline and infused into the kidney at 20 μl/min.
High Performance Liquid Chromatography Tandem Mass Spectrometry
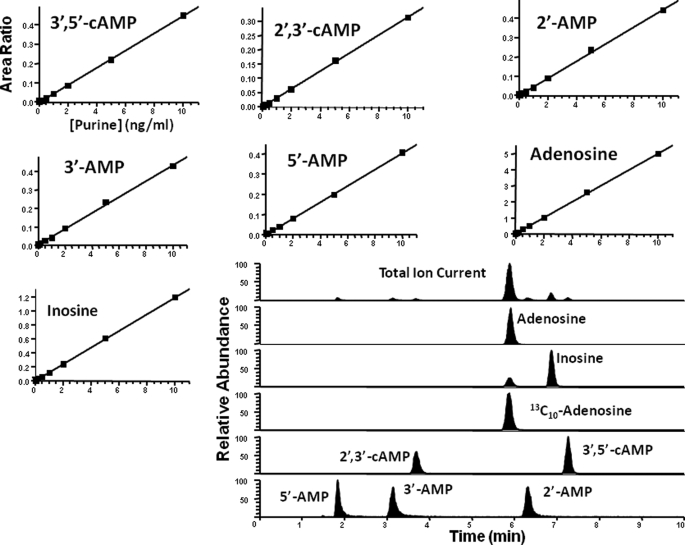
Purines (2′,3′-cAMP, 2′-AMP, 3′-AMP, 3′,5′-cAMP, 5′-AMP, adenosine, and inosine) were purchased from Sigma-Aldrich. The internal standard (13C10-adenosine) was from Medical Isotopes Inc. (Pelham, NH). Purines were resolved by reversed phase liquid chromatography (Agilent Zorbax eclipse XDB-C-18 column, 3.5 μm beads; 2.1 × 100 mm) and quantified using a triple quadrupole mass spectrometer (TSQ Quantum-Ultra; ThermoFisher Scientific, San Jose, CA) operating in the selective reaction monitoring mode with a heated electrospray ionization source. The mobile phases were delivered by an ultra pressure liquid chromatographic system (Accela; ThermoFisher Scientific, San Jose, CA) and consisted of linear gradient changes involving two buffers: Buffer A was 0.1% formic acid in water, and Buffer B was 0.1% formic acid in methanol. The mobile phase flow rate was 300 μl/min. The gradient (A/B) was: 0–2 min, 98.5%/1.5%; 2–4 min, to 98%/2%; 5–6 min, to 92%/8%; 7–8 min, to 85%/15%; and 9–11.5 min, to 98.5%/1.5%. The sample tray temperature was set at 4 °C, and the column temperature was kept at 20 °C. For maximum sensitivities, 5′-AMP and adenosine were used for optimization of parameters of the ion source. Two separate tune files were used in the process of determination: 0–4.5 min, tune file 1 for the monitoring of 5′-AMP, 3′-AMP and 2′,3′-cAMP; and 4.5–11.5 min, tune file 2 for monitoring 2′-AMP, 3′,5′-cAMP, adenosine, internal standard, and inosine. The following parameters were the same in the TSQ tune files 1 and 2: ion spray voltage, 3.8 kilovolts; ion transfer tube temperature, 270 °C; source vaporization temperature, 220 °C; Q2 CID gas, argon at 1.5 mTorr; sheath gas, nitrogen at 50 p.s.i.; auxillary gas, nitrogen at 40 p.s.i.; Q1/Q3 width, 0.7/0.7 units at full-width half-maximum; source CID, off; scan width, 0.5 units; and scan time, 0.05 s. The tube lens offset was 131 V for tune file 1 and 123 V for tune file 2. Five mass transitions were monitored: 330 → 136 for 2′,3′-cAMP and 3′,5′-cAMP with a collision energy of 28 volt; 348 → 136 for 5′-AMP, 3′-AMP, and 2′-AMP with a collision energy of 21 volts; 268 → 136 for adenosine with a collision energy of 19 volts; 278 → 141 for 13C10-adenosine as internal standard with a collision energy of 19 volts; and 269 → 137 for inosine with a collision energy of 20 volts. Calibration standard curves standards were constructed at concentrations of 0.02, 0.05, 0.1, 0.2, 0.5, 1, 2, 5, and 10 pg/μl in ultrapure water. Fig. 2 shows typical standard curves for the seven purines of interest along with a chromatogram illustrating the separation and detection of the seven purines.
FIGURE 2.
Typical standard curves for 3′,5′-cAMP, 2′,3′-cAMP, 2′-AMP, 3′-AMP, 5′-AMP, adenosine, and inosine and a representative chromatogram illustrating the separation and detection of these purines.
Statistical Analyses
Statistical comparisons were performed on a priori contrasts with the nonparametric Wilcoxon Signed-Rank test. The criterion of significance was p < 0.05.
RESULTS
Metabolism of Exogenous 3′,5′-cAMP, 2′,3′-cAMP, 5′-AMP, 3′-AMP, and 2′-AMP in the Isolated, Perfused Rat Kidney
The isolated rat kidney is a stable model for studying the vascular metabolism of purines because sampling from the renal vein is easily accomplished without perturbing the experimental system. In contrast, blood sampling from the rat renal vein in vivo would lead to exsanguination and hemodynamic instability caused by the large sample size required for analysis. Moreover, our previous studies show that purines are metabolized extensively by the vasculature of the isolated rat kidney. For example more than 65% of etheno-5′-AMP is converted to etheno-adenosine during a single pass through the vascular system of the isolated, perfused rat kidney (17). It is important to note that measurement of a given renal venous purine metabolite during an infusion of the parent purine into the renal artery does not capture the extent of renal metabolism because of downstream metabolism and uptake of the purine metabolite itself. Therefore, to compare the conversion of 3′,5′-cAMP to 5′-AMP versus 2′,3′-cAMP to 2′-AMP and 3′-AMP, we compared the effect of each cAMP on renal venous secretion of its corresponding AMP to the renal venous secretion induced by the same concentration of its corresponding AMP. Similarly we assessed the metabolism of 3′-AMP and 2′-AMP to adenosine and inosine by comparing the effects of 5′-AMP versus 3′-AMP versus 2′-AMP on renal venous secretion of adenosine and inosine because 5′-AMP is considered the most important adenosine precursor and is extensively metabolized to adenosine in the isolated, perfused rat kidney (17). Said differently, we benchmarked the metabolism of 2′-AMP and 3′-AMP to that of 5′-AMP.
Rat kidneys were isolated and perfused with Tyrode's solution. After a 1-h stabilization period, renal venous perfusate was collected for 1 min. Then either 3′,5′-cAMP, 2′,3′-cAMP, 5′-AMP, 3′-AMP, or 2′-AMP (10 μmol/liter) was administered into the renal artery, and 4 min into the treatment, another 1-min venous perfusate sample was collected. After a rest period of 20 min with no treatments, this protocol was performed with another purine. This procedure was repeated every 20 min until all five purines had been administered. The purines were administered in random order. The base-line renal perfusion pressure was 47 ± 3 mm Hg, which is normal for the isolated, perfused rat kidney at physiological flow rates because the isolated kidney is maximally vasodilated in the absence of endogenous vasoconstrictors and renal sympathetic tone (39). None of the treatments affected renal perfusion pressure. Renal artery administration of 3′,5′-cAMP increased renal venous 5′-AMP secretion (p = 0.0051; Fig. 3A), but not 3′-AMP secretion (Fig. 3B). The renal artery administration of 5′-AMP and 3′-AMP also increased renal venous secretion of 5′-AMP (p = 0.0195; Fig. 3A) and 3′-AMP (0.0118; Fig. 3B), respectively. The administration of 3′,5′-cAMP increased 5′-AMP secretion about half as effectively as an infusion of 5′-AMP (p = 0.0322), suggesting that approximately half of the 3′,5′-cAMP was converted to 5′-AMP during a single pass through the intact renal vasculature. Renal artery administration of 2′,3′-cAMP increased renal venous 3′-AMP secretion (p = 0.0117; Fig. 3C) and 2′-AMP secretion (p = 0.0222; Fig. 3D). The renal artery administration of 3′-AMP and 2′- AMP also increased renal venous secretion of 3′-AMP (p = 0.0118; Fig. 3C) and 2′-AMP (p = 0.0222; Fig. 3D), respectively. The administration of 10 μmol/liter of 2′,3′-cAMP increased 3′-AMP secretion numerically less, but not statistically less, than an infusion of 3′-AMP and increased 2′-AMP secretion approximately one-third as effectively as an infusion of 2′-AMP (p = 0.0269), suggesting that 2′,3′-cAMP was converted quantitatively to 3′-AMP plus 2′-AMP during a single pass through the intact renal vasculature, with 3′-AMP being the dominant pathway at 10 μmol/liter.
FIGURE 3.
Bar graphs comparing the effects of renal artery administration (10 μmol/liter) of 5′-AMP versus 3′,5′-cAMP on renal venous secretion of 5′-AMP (A), 3′-AMP versus 3′,5′-cAMP on renal venous secretion of 3′-AMP (B), 3′-AMP versus 2′,3′-cAMP on renal venous secretion of 3′-AMP (C), and 2′-AMP versus 2′,3′-cAMP on renal venous secretion of 2′-AMP (D) in the isolated, perfused rat kidney. The values represent the means ± S.E. for the indicated number of experiments (n). a, p < 0.05, compared with respective basal value; b, p < 0.05, compared with the corresponding noncyclic monophosphate.
Because both 2′,3′-cAMP and 3′,5′-cAMP were converted efficiently into their respective AMPs, we examined whether 3′-AMP and 2′-AMP were as efficiently converted to adenosine and inosine as was 5′-AMP (the most important precursor of adenosine and previously considered the main source of adenosine). Importantly, renal artery administration of all three AMPs increased renal venous secretion of adenosine (p = 0.0015, p = 0.002, and p = 0.0022 for 5′-AMP, 3′-AMP, and 2′-AMP, respectively; Fig. 4A) and inosine (p = 0.0022, p = 0.0022, and p = 0.0068 for 5′-AMP, 3′-AMP, and 2′-AMP, respectively; Fig. 4B), and the ability of 3′-AMP and 2′-AMP to increase renal venous secretion of adenosine was not significantly different compared with 5′-AMP or each other (Fig. 4A). Also, both 3′-AMP and 2′-AMP increased renal venous secretion of inosine, and the ability of 3′-AMP to increase inosine secretion was similar to that achieved by 5′-AMP, although 2′-AMP was less efficient in this regard (p = 0.0068 and p = 0.0005 versus 5′-AMP and 3′-AMP, respectively; Fig. 4B). None of the AMPs altered renal perfusion pressure. These results suggested that 2′-AMP and 3′-AMP were converted to adenosine and inosine in the intact renal vasculature as efficiently as was 5′-AMP.
FIGURE 4.
Bar graphs comparing the effects of renal artery administration (10 μmol/liter) of 5′-AMP, 3′-AMP, and 2′-AMP on renal venous secretion of adenosine (A) and inosine (B) and of 3′,5′-cAMP versus 2′,3′-cAMP on renal venous secretion of adenosine (C) and inosine (D) in the isolated, perfused rat kidney. The values represent the means ± S.E. for the indicated number of experiments (n). a, p < 0.05, compared with respective basal value; b, p < 0.05, compared with 5′-AMP; c, p < 0.05, compared with 3′-AMP.
Inasmuch as the cAMPs were converted efficiently into AMPs and the AMPs were converted efficiently to adenosine plus inosine, we predicted that the cAMPs would increase adenosine and inosine levels comparably. In support of this prediction, renal artery infusions of both 3′,5′-cAMP and 2′,3′-cAMP increased both adenosine (p = 0.0022 and p = 0.0015 for 3′,5′-cAMP and 2′,3-cAMP, respectively; Fig. 4C) and inosine (p = 0.0022 and p = 0.0024 for 3′,5′-cAMP and 2′,3-cAMP, respectively; Fig. 4D) secretion, without affecting renal perfusion pressure. The increases in adenosine and inosine induced by 3′,5′-cAMP and 2′,3′-cAMP were not significantly different (p = 0.4697 and p = 0.6772 for adenosine and inosine, respectively).
The aforementioned experiments were conducted with cAMPs at a concentration of 10 μmol/liter. To determine whether the profile of metabolism of 3′,5′-cAMP versus 2′,3′-cAMP to AMPs, adenosine and inosine would differ at higher concentrations, in a separate group of isolated, perfused kidneys we examined the effects of 30 μmol/liter of 3′,5′-cAMP and 2′,3′-cAMP on downstream metabolites. The base-line renal perfusion pressure was 48 ± 1 mm Hg, and the cAMPs at 30 μmol/liter did not alter renal perfusion pressure. 3′,5′-cAMP profoundly increased renal venous secretion of 5′-AMP (p = 0.0033; Fig. 5A), but not 3′-AMP (Fig. 5B) nor 2′-AMP (Fig. 5C), indicating that even at very high concentrations, 3′,5′-cAMP was not metabolized to 3′-AMP but only to 5′-AMP. In contrast, 30 μmol/liter of 2′,3′-cAMP had no effect on 5′-AMP secretion (Fig. 5A) but greatly increased renal venous secretion of 3′-AMP (p = 0.0033; Fig. 5B) and 2′-AMP (p = 0.0033; Fig. 5C). With regard to 2′,3′-cAMP conversion to AMPs, with 10 μmol/liter of 2′,3′-cAMP the dominant recovered product was 3′-AMP (p = 0.0010 versus 2′-AMP; Fig. 4, C and D), whereas at 30 μmol/liter the dominant recovered product was 2′-AMP (p < 0.0001 versus 3′-AMP; Fig. 5, B and C). This indicated that whether 3′-AMP or 2′-AMP was the dominant recovered product depended in part on the concentration of 2′,3′-cAMP. At this 3-fold higher concentration, both cAMPs markedly increased the renal venous secretion of adenosine (p = 0.0033 for both cAMPs; Fig. 5D) and inosine (p = 0.0033 for both cAMPs; Fig. 5E) with 2′,3′-cAMP being more effective with regard to increasing inosine secretion (p = 0.0003; Fig. 5E). Importantly, the increases in AMPs, adenosine, and inosine induced by the cAMPs were not linearly related to input concentrations (compare Figs. 3 and 4 with Fig. 5), suggesting that at higher levels of input the downstream metabolites were less likely to be captured by alternative metabolic pathways.
FIGURE 5.
Bar graphs comparing the effects of renal artery administration (30 μmol/liter) of 3′,5′-cAMP versus 2′,3′-cAMP on renal venous secretion of 5′-AMP (A), 3′-AMP (B), 2′-AMP (C), adenosine (D), and inosine (E) in the isolated, perfused rat kidney. The values represent the means ± S.E. for the indicated number of experiments (n). a, p < 0.05, compared with respective basal value; b, p < 0.05, compared with 3′,5′-cAMP.
Effects of Metabolic Poisoning on the Release of Endogenous 3′,5′-cAMP, 2′,3′-cAMP, 5′-AMP, 3′-AMP, 2′-AMP, Adenosine, and Inosine in the Isolated, Perfused Rat Kidney
Experiments in intact tissues suggested that energy depletion in tissues stimulated the degradation of mRNA (40–42), and studies with enzyme preparations indicated that 2′,3′-cAMP was a product of mRNA degradation (28). Therefore, we examined the effects of energy depletion on the renal venous secretion of 2′,3′-cAMP and its downstream metabolites. We treated isolated, perfused rat kidneys with two metabolic poisons, i.e. iodoacetate plus 2,4-dinitrophenol (50 μmol/liter each) for 30 min. The base-line renal perfusion pressure was 52 ± 4 mm Hg, and the metabolic poisons did not affect renal perfusion pressure. Renal injury using metabolic inhibitors increased the mean venous secretion of 2′,3′-cAMP by 29-fold (p = 0.0277; Fig. 6A), 3′-AMP by 16-fold (p = 0.0277; Fig. 6B), 2′-AMP by 10-fold (p = 0.0277; Fig. 6C), adenosine by 4.2-fold (p = 0.0277; Fig. 6D), and inosine by 6.1-fold (p = 0.0277; Fig. 6E), while decreasing 3′,5′-cAMP by ∼64% (p = 0.0312; Fig. 6F) and increasing 5′-AMP by only 2.4-fold (0.0277; Fig. 6G). These findings were consistent with the concept that when organs/tissues were injured, endogenous 2′,3′-cAMP was formed from mRNA and was metabolized to 3′-AMP and 2′-AMP, which were metabolized to adenosine and inosine.
FIGURE 6.
Bar graphs summarizing the effects of inhibiting energy production with the metabolic poisons iodoacetate plus 2,4-dinitrophenol (each at 50 μmol/liter) on the renal venous secretion of 2′,3′-cAMP (A), 3′-AMP (B), 2′-AMP (C), adenosine (D), inosine (E), 3′,5′-cAMP (F), and 5′-AMP (G) in the isolated, perfused rat kidney. The values represent the means ± S.E. for the indicated number of experiments (n). a, p < 0.05, compared with respective basal value.
Metabolism of Exogenous 3′,5′-cAMP, 2′,3′-cAMP, 5′-AMP, 3′-AMP, and 2′-AMP in the In Vivo Rat Kidney
In the experiments using isolated, perfused kidneys, we did not collect urine samples because of the well known instability and unreliability of tubular function in this model system. It was important, therefore, to compare the metabolism of 2′,3′-cAMP versus 3′,5′-cAMP in the tubular compartment by examining the effects of the cAMPs and AMPs on urinary excretion of purines in the intact animal. Accordingly, we anesthetized rats, and while maintaining body temperature and monitoring arterial blood pressure, heart rate, and renal blood flow to assure hemodynamic stability, we infused into the renal artery 3′,5′-cAMP or 2′,3′-cAMP. We used a cross-over experimental design with 30-min basal, treatment, and recovery periods for each cAMP (90 nmol/kg/min) while collecting urine via a polyethylene tubing inserted into the left ureter. The order of treatment was random. The basal arterial blood pressure, heart rate, renal blood flow, and urine flow rate were physiologically normal (126 ± 8 mm Hg, 369 ± 5 beats/min, 6.4 ± 1.0 ml/min, and 0.18 ± 0.05 ml/30 min, respectively) and were not altered by the cAMPs. Administration of 2′,3′-cAMP into the renal artery did not increase the urinary excretion rate of 3′,5′-cAMP but did increase the urinary excretion rate of 2′,3′-cAMP (p = 0.0010; Fig. 7). In contrast, renal artery infusions of 3′,5′-cAMP increased urinary excretion of 3′,5′-cAMP (p = 0.0022) but not 2′,3′-cAMP (Fig. 7). Importantly, 3′,5′-cAMP increased the urinary excretion of 3′,5′-cAMP more so than 2′,3′-cAMP increased the urinary excretion of 2′,3′-cAMP (p = 0.0049). These data suggested that in vivo the tubular compartment metabolized 2′,3′-cAMP more efficiently than 3′,5′-cAMP.
FIGURE 7.
Bar graph summarizing the effects of renal artery administration of 2′,3′-cAMP and 3′,5′-cAMP (90 nmol/kg/min) on the urinary excretion rate of 3′,5′-cAMP (left set of bars) and 2′,3′-cAMP (right set of bars) in the in vivo rat kidney. The values represent the means ± S.E. for the indicated number of experiments (n). a, p < 0.05, cAMP infusion period or recovery period versus respective basal period; b, p < 0.05, recovery period versus respective cAMP infusion period; c, p < 0.05, levels of 2′,3′-cAMP induced by infusions of 2′,3′-cAMP versus levels of 3′,5′-cAMP induced by infusions of 3′,5′-cAMP with respect to their corresponding periods.
To examine the metabolism of the cAMPs to AMPs by the in vivo kidney, we measured the urinary excretion rate of 5′-AMP, 3′-AMP, and 2′-AMP in response to the renal artery administration of the cAMPs. 2′,3′-cAMP did not increase the urinary excretion of 5′-AMP but profoundly increased the urinary excretion of 3′-AMP (p = 0.0022) and 2′-AMP (p = 0.0010; Fig. 8), with 2′-AMP greater than 3′-AMP (p = 0.0010). 3′,5′-cAMP did not increase the urinary excretion of either 3′-AMP or 2′-AMP but slightly increased the urinary excretion of 5′-AMP (p = 0.0269; Fig. 8). The 2′,3′-cAMP-induced increases in 3′-AMP and 2′-AMP were greater than the 3′,5′-cAMP-induced increases in 5′-AMP (p = 0.0015 and p = 0.0010, respectively). 2′,3′-cAMP markedly and significantly increased the urinary excretion rate of adenosine (p = 0.0022), whereas 3′,5′-cAMP did not (Fig. 9). The 2′,3′-cAMP-induced increase in adenosine was significantly greater that the 3′,5′-cAMP-induced increase in adenosine (p = 0.0005). These data suggested that in vivo in the tubular compartment, 2′,3′-cAMP was metabolized to AMPs and hence to adenosine more efficiently than was 3′,5′-cAMP.
FIGURE 8.
Bar graph summarizing the effects of renal artery administration of 2′,3′-cAMP and 3′,5′-cAMP (90 nmol/kg/min) on the urinary excretion rate of 5′-AMP (left set of bars), 3′-AMP (middle set of bars), and 2′-AMP (right set of bars) in the in vivo rat kidney. The values represent the means ± S.E. for the indicated number of experiments (n). a, p < 0.05, cAMP infusion period or recovery period versus respective basal period; b, p < 0.05, recovery period versus respective cAMP infusion period; c, p < 0.05, levels of 3′-AMP or 2′-AMP induced by infusions of 2′,3′-cAMP versus levels of 5′-AMP induced by infusions of 3′,5′-cAMP with respect to their corresponding periods; d, p < 0.05, levels of 2′-AMP versus 3′-AMP induced by infusions of 2′,3′-cAMP with respect to their corresponding periods.
FIGURE 9.
Bar graph showing the effects of renal artery administration of 2′,3′-cAMP and 3′,5′-cAMP (90 nmol/kg/min) on the urinary excretion rate of adenosine (left set of bars) and inosine (right set of bars) in the in vivo rat kidney. The values represent the means ± S.E. for the indicated number of experiments (n). a, p < 0.05, cAMP infusion period or recovery period versus respective basal period; b, p < 0.05, recovery period versus respective cAMP infusion period; c, p < 0.05, levels of adenosine induced by infusions of 3′,5′-cAMP versus levels of adenosine induced by infusions of 2′,3′-cAMP with respect to their corresponding periods.
In another protocol, we infused into the renal artery, in a cross-over experimental design, either vehicle, 5′-AMP, 3′-AMP, or 2′-AMP. In this experiment, we had to decrease the infusion rate of AMPs to 9 nmol/kg/min (one-tenth the dose of cAMPs) to avoid hemodynamic effects (hypotension and bradycardia), which occurred with all three AMPs when infused at 90 nmol/kg/min. The basal arterial blood pressure, heart rate, renal blood flow, and urine flow rate were physiologically normal (128 ± 9 mm Hg, 392 ± 9 beats/min, 7.5 ± 0.9 ml/min, and 0.17 ± 0.03 ml/30 min, respectively) and were not altered by the AMPs at 9 nmol/kg/min. Importantly, all three AMPs similarly increased the urinary excretion rate of adenosine (p = 0.0391, p = 0.0273, and p = 0.0078 for 2′-AMP, 3′-AMP, and 5′-AMP, respectively; Fig. 10). These findings indicated that in vivo 2′-AMP and 3′-AMP were as efficacious as 5′-AMP with regard to being converted to adenosine.
FIGURE 10.
Bar graph illustrating the effects of renal artery administration of 2′-AMP, 3′-AMP, and 5′AMP (9 nmol/kg/min) on the urinary excretion rate of adenosine (left set of bars) and inosine (right set of bars) in the in vivo rat kidney. The values represent the means ± S.E. for the indicated number of experiments (n). a, p < 0.05, AMP infusion periods versus basal period; b, p < 0.05, 5′-AMP versus 2′-AMP; c, p < 0.05, 5′-AMP versus 3′-AMP.
DISCUSSION
Tissue Injury Activates mRNA Breakdown by RNases
Tissue injury increases mRNA breakdown. For example, Almeida et al. (42) report that incubation of liver tissue at 37 °C results in extensive RNA degradation, with mRNA levels falling to one-tenth those before incubation. Akahane et al. (40, 41) report that normothermic ischemia/reperfusion injury increases mRNA breakdown within 1–3 h in bone and skeletal muscle. Studies by Chevyreva et al. (43) show that in human brain tissue, low pH markedly increases mRNA breakdown, and Catts et al. (44) report similar findings in mouse brain. mRNA breakdown is most likely a universal response to cellular, tissue, and organ injury.
2′,3′-cAMP May Be Formed by RNA Breakdown
mRNA is degraded by the action of RNases that catalyze the hydrolysis of the P-O5′ bond of mRNA. This hydrolysis reaction may involve transphosphorylation of mRNA to form 2′,3′-cyclic nucleotides (such as 2′,3′-cAMP). Thompson et al. (28) used 31P NMR spectroscopy to monitor the accumulation of the 2′,3′-cyclic nucleotides during the transphosphorylation and hydrolysis reactions catalyzed by various RNases. 2′,3′-Cyclic nucleotides were found to accumulate during catalysis by RNAses of widely disparate phylogenetic origin. Thus at least with isolated RNases acting on isolated mRNA, it appears that 2′,3′-cyclic nucleotides are not enzyme-bound intermediates but are true reaction products that are released by RNases.
mRNA Breakdown Would Likely Produce Large Quantities of 2′,3′-cAMP
Most mRNAs contain a poly-A tail of ∼150 adenine repeats (45), and mRNA turnover is initiated by hydrolysis of the poly-A tail by RNases (27). A typical mammalian cell contains 363,000 molecules of mRNA (45). Therefore, a typical mammalian cell would contain 5.445 × 107 potential molecules of 2′,3′-cAMP stored in mRNA poly-A tails, which when divided by Avogadro's number (6.022 × 1023) is 0.9042 × 10−16 mol/cell. The average mammalian cell has a water volume of 2.800 × 10−12 liter (46). Therefore, the potential concentration of 2′,3′-cAMP achieved in a mammalian cell by releasing the 2′,3′-cAMP stored in the poly-A tails of mRNA would be 32.29 μmol/liter. In addition, the average mRNA is 1500 bases in length (45), 25% of which is adenine. Therefore, there are 1500 × 0.25 × 363,000 = 1.361 × 108 potential molecules of 2′,3′-cAMP in the non-poly-A tail part of mRNAs. If these were mobilized, then the concentration of 2′,3′-cAMP would increase by another 80.71 μmol/liter. Therefore, each cell has the capacity to generate a total concentration of about 113.0 μmol/liter of 2′,3′-cAMP by degrading mRNA. Importantly, the poly-A tail would provide a rapidly releasable pool of 2′,3′-cAMP, whereas the body of the mRNA would provide a slowly releasable form.
Serendipitous Discovery That Intact Tissues/Organs Release 2′,3′-cAMP
While investigating 3′,5′-cAMP secretion by the isolated, perfused rat kidney, we noticed a chromatographic peak while measuring 3′,5′-cAMP by selective reaction monitoring using high performance liquid chromatograpy-tandem mass spectrometry that was at the incorrect retention time for 3′,5′-cAMP (26). We investigated this unknown substance and confirmed that it was 2′,3′-cAMP and that two different stimuli that are known to activate RNA breakdown, i.e. energy depletion and rapamycin, increased the release of 2′,3′-cAMP into the extracellular compartment (26).
The Extracellular 2′,3′-cAMP-Adenosine Pathway
Our long term interest in the renal extracellular 3′,5′-cAMP-adenosine pathway (Fig. 1, left side) caused us to conceive by analogy the possibility of a renal 2′,3′-cAMP-adenosine pathway (Fig. 1, right side), and the goal of the present study was to test this hypothesis. In this regard, the results of the present study show that the intact kidney metabolizes exogenous 2′,3′-cAMP to 3′-AMP, 2′-AMP, adenosine, and inosine and converts exogenous 3′-AMP and 2′-AMP to adenosine and inosine. Importantly, these findings apply to both kidneys perfused in vitro as well as kidneys in vivo. Moreover, at least in the intact kidney, 3′-AMP and 2′-AMP generate adenosine as readily as 5′-AMP, hitherto considered the most important adenosine precursor. However, the relative efficacy of 3′-AMP, 2′-AMP, and 5′-AMP as adenosine precursors may change under different conditions and in different cells/tissues/organs.
Our results suggest that whether exogenous 2′,3′-cAMP is metabolized predominantly down the 2′-AMP limb or 3′-AMP limb of the extracellular 2′,3′-cAMP-adenosine pathway is concentration-dependent. Low concentrations of 2′,3′-cAMP may favor the 3′-AMP limb, whereas higher concentrations may tilt the flux of 2′,3′-cAMP metabolism toward the 2′-AMP limb. An alternative, but not mutually exclusive, possibility is that with higher concentrations of 2′,3′-cAMP, 2′-AMP accumulates more than 3′-AMP because downstream metabolism of 2′-AMP becomes saturated. Thus concentration-dependent recovery of 2′-AMP versus 3′-AMP likely stems from the relative kinetic parameters and expression levels of the enzymes involved in metabolizing 2′,3′-cAMP, 2′-AMP, and 3′-AMP.
In the isolated perfused kidney, renal artery infusions of 2′,3′-cAMP and 3′,5′-cAMP similarly increase renal venous levels of adenosine. In contrast, infusions of 2′,3′-cAMP into the renal artery of kidneys in vivo increase urinary excretion of adenosine much more than do comparable infusions of 3′,5′-cAMP. Because comparable infusions of 2′-AMP, 3′-AMP, and 5′-AMP similarly increase urinary adenosine excretion in vivo, the relative inability of 3′,5′-cAMP to elevate urinary adenosine excretion is likely due to inefficient conversion of 3′,5′-cAMP to 5′-AMP rather than inefficient conversion of 5′-AMP to adenosine. This conclusion is confirmed by the greater urinary recovery of 3′,5′-cAMP and the lower urinary excretion of 5′-AMP following renal artery infusions of 3′,5′-cAMP compared with the lesser urinary recovery of 2′,3′-cAMP and the greater urinary excretion of 2′-AMP and 3′-AMP during renal artery infusions of 2′,3′-cAMP. The reason for the similar ability of 2′,3′-cAMP versus 3′,5′-cAMP to produce adenosine in isolated kidneys but the greater ability of 2′,3′-cAMP to do so in the in vivo kidney may relate to the fact that in the isolated, perfused kidney we measure metabolites in the renal vein, whereas in the kidney in vivo we examine metabolites in urine. Renal vein levels of metabolites likely reflect vascular metabolism, whereas urine levels of metabolites likely reflect renal epithelial metabolism. At any rate, the extracellular 2′,3′-cAMP pathway appears to be quantitatively important regardless of the site of sampling.
The Physiological Role of 2′,3′-cAMP-Adenosine Pathway
Studies in isolated mitochondria demonstrate that 2′,3′-cAMP opens mitochondrial permeability transition pores (47), and the opening of these mitochondrial pores is considered to be a significant contributor to ischemia/reperfusion injury leading to cell death via both apoptosis and necrosis (48). The extracellular 2′,3′-cAMP-adenosine pathway would protect cells from apoptosis and necrosis by two mechanisms: 1) removal of 2′,3′-cAMP from the cell (efflux to the extracellular compartment) and 2) conversion of extracellular 2′,3′-cAMP to 2′-AMP and 3′-AMP and hence to adenosine. Because adenosine is well known to act on cell surface receptors to induce cellular protection by engaging a host of signal transduction systems (49), the production of adenosine by the extracellular 2′,3′-cAMP-adenosine pathway would provide this retaliatory metabolite to protect both the injured cell (autocrine action) and neighboring cells (paracrine action). This concept is supported by the findings of the present study that metabolic poisons increase the release from the intact kidney of 2′,3′-cAMP, 3′-AMP, 2′-AMP, and adenosine. However, it is conceivable that there are three different types of 2′,3′-cAMP-adenosine pathways that serve to protect cells (Fig. 11), and the present study addresses the existence of only one, i.e. the extracellular 2′,3′-cAMP-adenosine pathway. In this regard, it is likely that some 2′,3′-cAMP is metabolized intracellularly, before efflux can occur, to produce intracellular 2′-AMP and 3′-AMP. Intracellularly produced 2′-AMP and 3′-AMP could be transported to the extracellular compartment and metabolized to adenosine (transcellular 2′,3′-cAMP-adenosine pathway) or metabolized intracellularly to adenosine followed by efflux of adenosine to the extracellular compartment (intracellular 2′,3′-cAMP- adenosine pathway). Also, although the present study focuses on the renal 2′,3′-cAMP-adenosine pathway, it is likely that this pathway is applicable to many, if not all, organ systems.
FIGURE 11.
Schematic summarizing the possible biochemical steps in the postulated 2′,3′-cAMP-adenosine pathways. The figure illustrates the concept that 2′,3′-cAMP may be exported from the cell and metabolized to adenosine extracellularly (extracellular 2′,3′-cAMP-adenosine pathway), may be metabolized to adenosine intracellularly (intracellular 2′,3′-cAMP-adenosine pathway), or may be metabolized to 2′-AMP and 3′-AMP inside the cell followed by export of 2′-AMP and 3′-AMP to the interstitial compartment with subsequent metabolism of these AMPs to adenosine (transcellular 2′,3′-cAMP-adenosine pathway). These processes would reduce the intracellular levels of 2′,3′-cAMP (a breakdown product of mRNA that is known to open mitochondrial permeability transition pores (mPTP), thus causing apoptosis and necrosis) while increasing the extracellular levels of adenosine (a retaliatory metabolite that protects cells from injury in an autocrine and paracrine fashion). (a), breakdown of mRNA (e.g. by RNases); (b) and (f), active transport (e.g. by multidrug resistance proteins); (c) and (e), hydrolysis of cyclic phosphodiester (e.g. by CNPase or ptRNase 1), (d) and (g) dephosphorylation (e.g. by various ecto- and endo-2′/3′-nucleotidases); and (h), transport of adenosine (e.g. by equilibrative nucleoside transporters).
This work was supported, in whole or in part, by National Institutes of Health Grants DK068575, DK077777, and HL069846.
- poly-A
- polyadenine
- PE
- polyethylene.
REFERENCES
- 1.Dubey R. K., Mi Z., Gillespie D. G., Jackson E. K. (1996) Hypertension 28, 765–771 [DOI] [PubMed] [Google Scholar]
- 2.Dubey R. K., Gillespie D. G., Mi Z., Jackson E. K. (1997) Hypertension 30, 506 [Google Scholar]
- 3.Dubey R. K., Gillespie D. G., Jackson E. K. ( 1998) Hypertension 31, 296– 302 [DOI] [PubMed] [Google Scholar]
- 4.Dubey R. K., Gillespie D. G., Mi Z., Jackson E. K. (2000) Hypertension 36, 337–342 [DOI] [PubMed] [Google Scholar]
- 5.Dubey R. K., Gillespie D. G., Mi Z., Rosselli M., Keller P. J., Jackson E. K. (2000) Hypertension 35, 262–266 [DOI] [PubMed] [Google Scholar]
- 6.Dubey R. K., Gillespie D. G., Mi Z., Jackson E. K. (2001) Hypertension 37, 1095–1100 [DOI] [PubMed] [Google Scholar]
- 7.Jackson E. K. (1991) Annu. Rev. Pharmacol. Toxicol. 31, 1–35 [DOI] [PubMed] [Google Scholar]
- 8.Jackson E. K., Mi Z., Gillespie D. G., Dubey R. K. (1997) J. Pharmacol. Exp. Ther. 283, 177–182 [PubMed] [Google Scholar]
- 9.Jackson E. K., Mi Z. (2000) J. Pharmacol. Exp. Ther. 295, 23–28 [PubMed] [Google Scholar]
- 10.Jackson E. K., Dubey R. K. (2001) Am. J. Physiol. Renal Physiol. 281, F597–F612 [DOI] [PubMed] [Google Scholar]
- 11.Jackson E. K., Mi Z., Zhu C., Dubey R. K. (2003) J. Pharmacol. Exp. Ther. 307, 888–896 [DOI] [PubMed] [Google Scholar]
- 12.Jackson E. K., Raghvendra D. K. (2004) Annu. Rev. Physiol. 66, 571–599 [DOI] [PubMed] [Google Scholar]
- 13.Jackson E. K., Zacharia L. C., Zhang M., Gillespie D. G., Zhu C., Dubey R. K. (2006) J. Pharmacol. Exp. Ther. 317, 1219–1229 [DOI] [PubMed] [Google Scholar]
- 14.Jackson E. K., Mi Z., Zacharia L. C., Tofovic S. P., Dubey R. K. (2007) J. Pharmacol. Exp. Ther. 321, 799–809 [DOI] [PubMed] [Google Scholar]
- 15.Jackson E. K., Mi Z., Dubey R. K. (2007) J. Pharmacol. Exp. Ther. 320, 117–123 [DOI] [PubMed] [Google Scholar]
- 16.Jackson E. K., Ren J., Zacharia L. C., Mi Z. (2007) J. Pharmacol. Exp. Ther. 321, 810–815 [DOI] [PubMed] [Google Scholar]
- 17.Jackson E. K., Mi Z. (2008) J. Pharmacol. Exp. Ther. 325, 210–216 [DOI] [PubMed] [Google Scholar]
- 18.Mi Z., Herzer W. A., Zhang Y., Jackson E. K. (1994) Life Sci. 54, 277–282 [DOI] [PubMed] [Google Scholar]
- 19.Mi Z., Jackson E. K. (1995) J. Pharmacol. Exp. Ther. 273, 728–733 [PubMed] [Google Scholar]
- 20.Mi Z., Jackson E. K. (1998) J. Pharmacol. Exp. Ther. 287, 926–930 [PubMed] [Google Scholar]
- 21.Hong K. W., Shin H. K., Kim H. H., Choi J. M., Rhim B. Y., Lee W. S. (1999) Am. J. Physiol. 276, H376–H382 [DOI] [PubMed] [Google Scholar]
- 22.Chiavegatti T., Costa V. L., Jr., Araújo M. S., Godinho R. O. (2008) Br. J. Pharmacol. 153, 1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giron M. C., Bin A., Brun P., Etteri S., Bolego C., Florio C., Gaion R. M. (2008) Gastroenterology 134, 1116–1126 [DOI] [PubMed] [Google Scholar]
- 24.Müller G., Wied S., Over S., Frick W. (2008) Biochemistry 47, 1259–1273 [DOI] [PubMed] [Google Scholar]
- 25.Ren J., Mi Z., Jackson E. K. (2008) J. Pharmacol. Exp. Ther. 325, 920–926 [DOI] [PubMed] [Google Scholar]
- 26.Ren J., Mi Z., Stewart N. A., Jackson E. K. (2009) J. Pharmacol. Exp. Ther. 328, 855–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilusz C. J., Wormington M., Peltz S. W. (2001) Nat. Rev. Mol. Cell Biol. 2, 237–246 [DOI] [PubMed] [Google Scholar]
- 28.Thompson J. E., Venegas F. D., Raines R. T. (1994) Biochemistry 33, 7408–7414 [DOI] [PubMed] [Google Scholar]
- 29.Borst P., de Wolf C., van de Wetering K. (2007) Pflugers Arch. 453, 661–673 [DOI] [PubMed] [Google Scholar]
- 30.Deeley R. G., Westlake C., Cole S. P. (2006) Physiol. Rev. 86, 849–899 [DOI] [PubMed] [Google Scholar]
- 31.Kruh G. D., Zeng H., Rea P. A., Liu G., Chen Z. S., Lee K., Belinsky M. G. (2001) J. Bioenerg. Biomembr. 33, 493–501 [DOI] [PubMed] [Google Scholar]
- 32.Vogel U. S., Thompson R. J. (1988) J. Neurochem. 50, 1667–1677 [DOI] [PubMed] [Google Scholar]
- 33.Sorrentino S., Libonati M. (1997) FEBS Letters 404, 1–5 [DOI] [PubMed] [Google Scholar]
- 34.Sorrentino S. (1998) Cell Mol. Life Sci. 54, 785–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zimmermann H., Braun N. (1999) Prog. Brain Res. 120, 371–385 [PubMed] [Google Scholar]
- 36.Zimmermann H. (2006) Novartis Found. Symp. 276, 113–128 [PubMed] [Google Scholar]
- 37.Atkinson B., Dwyer K., Enjyoji K., Robson S. C. (2006) Blood Cells Mol. Dis. 36, 217–222 [DOI] [PubMed] [Google Scholar]
- 38.Gao L., Zhu C., Jackson E. K. (2003) J. Pharmacol. Exp. Ther. 305, 581–586 [DOI] [PubMed] [Google Scholar]
- 39.Jackson E. K., Mi Z. (2009) Hypertension 54, 270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akahane M., Ono H., Ohgushi H., Takakura Y. (2001) J. Reconstr. Microsurg. 17, 203–209 [DOI] [PubMed] [Google Scholar]
- 41.Akahane M., Ono H., Ohgushi H., Tamai S. (2001) J. Orthop. Res. 19, 559–564 [DOI] [PubMed] [Google Scholar]
- 42.Almeida A., Paul Thiery J., Magdelénat H., Radvanyi F. (2004) Anal. Biochem. 328, 101–108 [DOI] [PubMed] [Google Scholar]
- 43.Chevyreva I., Faull R. L., Green C. R., Nicholson L. F. (2008) Exp. Mol. Pathol. 84, 71–77 [DOI] [PubMed] [Google Scholar]
- 44.Catts V. S., Catts S. V., Fernandez H. R., Taylor J. M., Coulson E. J., Lutze-Mann L. H. (2005) Brain Res. Mol. Brain Res. 138, 164–177 [DOI] [PubMed] [Google Scholar]
- 45.Alberts B., Bray D., Lewis J., Raff M., Roberts K., Watson J. D. (1989) in Molecular Biology of the Cell, pp. 481– 550,Garland Publishing, Inc., New York [Google Scholar]
- 46.Alberts B., Bray D., Lewis J., Raff M., Roberts K., Watson J. D. (1989) in Molecular Biology of the Cell, pp. 87– 134,Garland Publishing, Inc., New York [Google Scholar]
- 47.Azarashvili T., Krestinina O., Galvita A., Grachev D., Baburina Y., Stricker R., Evtodienko Y., Reiser G. (2009) Am. J. Physiol. Cell Physiol 296, C1428–C1439 [DOI] [PubMed] [Google Scholar]
- 48.Kroemer G., Galluzzi L., Brenner C. (2007) Physiol. Rev. 87, 99–163 [DOI] [PubMed] [Google Scholar]
- 49.Linden J. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 775–787 [DOI] [PubMed] [Google Scholar]