Abstract
Activation of STAT proteins by cytokines is initiated by their Src homology 2 domain-mediated association with phosphotyrosine residues from the cytoplasmic domain of a receptor. Here, we show that the C terminus of the interleukin-22 receptor (IL-22R) recruits in a tyrosine-independent manner the coiled-coil domain of STAT3. Mutation of all IL-22R cytoplasmic tyrosines did not abolish activation of STAT3, in contrast to that of STAT1 and STAT5. Coimmunoprecipitation and glutathione S-transferase pulldown experiments showed that the coiled-coil domain of STAT3 is constitutively associated with the C-terminal part of IL-22R, and a chimeric STAT3-STAT5 protein containing the coiled-coil domain of STAT3 could be activated by this tyrosine-independent mechanism. Deletion of the C-terminal part of IL-22R dramatically decreased its ability to activate STAT3 and to mediate IL-22 activity in cell lines, demonstrating that preassociation of STAT3 with this cytokine receptor, independent from the interaction between the Src homology 2 domain and phosphotyrosines, is required for its full activity.
Signal transducer and activator of transcription (STAT)3 proteins are a family of eukaryotic transcription factors composed of seven members that mediate the response to a large number of cytokines and growth factors. Upon activation by cell surface receptors, Janus tyrosine kinases (JAK) associated with the juxtamembrane domain of the receptors become activated and phosphorylate one or several tyrosine residues of the receptor, thus creating docking sites for proteins containing Src homology 2 (SH2) domains, such as STATs (1–3). STATs were originally considered latent monomeric proteins in the cytoplasm. However, accumulating data suggest that STAT factors could be preassociated in the cytosol as at least dimers (4) or even be included in high molecular weight complexes (5).
STAT factors share a highly conserved modular structure containing six functional domains: an N-terminal domain, a coiled-coil domain, a DNA-binding domain, a linker domain, an SH2 domain, and a transactivation domain (2). In STAT3, the N-terminal domain contains the N-terminal 130 amino acids and mediates dimer-dimer interactions that lead to cooperative DNA binding and nuclear translocation (6, 7). The coiled-coil domain consists of four α helices, which form a large predominantly hydrophilic surface that is available for specific interactions with other helical proteins and is required for nuclear translocation (8). The DNA-binding domain (amino acids 320–465) binds to specific enhancer sequences and seems to regulate nuclear export (9). The adjacent linker domain (amino acids 465–585) ensures the appropriate structure of the DNA-binding motif and may regulate nuclear export in resting cells. The SH2 domain (amino acids 585–688) is crucial for recruiting STATs to the activated cytokine receptors via receptor tyrosine motifs and for STAT dimerization subsequent to their phosphorylation. The C terminus of STAT factors contains an autonomously functioning transcriptional activation domain as well as at least one site of tyrosine phosphorylation.
Efficient phosphorylation of STATs by Janus kinases critically depends on the ability of the SH2 domain of the STAT factors to interact with phosphorylated tyrosines from a cytokine receptor. Which STAT is activated by a particular cytokine receptor depends on the amino acids surrounding the phosphotyrosines of the receptor. For instance, STAT3 is preferentially recruited by phospho pYXXQ and pYXPQ motifs, and mutation of the tyrosine or of the conserved glutamine residue in receptors such as gp130 or interleukin-9 receptor (IL-9R) abolishes STAT3 activation in response to IL-6 or IL-9, respectively (2, 10, 11). However, at high concentrations of G-CSF, a mutant of the G-CSFR in which all tyrosines were mutated, was shown to be able to recruit and activate STAT3 via an unknown mechanism involving the C-terminal part of G-CSFR (12).
IL-22R is a common cytokine receptor chain shared between IL-22 and IL-20 receptor complexes, which are potent STAT3 activators in hepatocytes and keratinocytes and potentially involved in psoriasis disease (13, 14). Here, we describe that STAT3 is constitutively associated via its coiled-coil domain with the C-terminal part of the IL-22R chain and that the tyrosines of the receptor are not necessary for the recruitment and activation of STAT3, illustrating a novel mechanism of STAT recruitment and activation by cytokine receptors.
EXPERIMENTAL PROCEDURES
Cell Culture and Cytokines
BW5147 lymphoma cells and HepG2 human hepatoma cells were grown as described previously (15). HEK293-EBNA cells and COS cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. HT-29 and colo205 human epithelial cell lines were grown in Iscove-Dulbecco medium supplemented with 10% fetal calf serum, 0.55 mm l-arginine, 0.24 mm l-asparagine, and 1.25 mm l-glutamine. Recombinant human IL-22 was produced in Escherichia coli as described previously (15). Recombinant hIL-9 and mouse IL-9 were produced in the baculovirus system and purified as described previously (16). To produce anti-hIL-22R polyclonal antibodies, P815 mastocytoma cells were transfected with the human IL-22R cDNA cloned into the pEF-BOS plasmid before injection into DBA/2 mice. After rejection of the tumors, the sera of these mice had high titers of neutralizing anti-IL-22R antibodies, and the spleens were used to generate hIL-22R-specific monoclonal antibodies.
Plasmid Constructions, Stable Transfections, and Analysis of Transfected Cells
The IL-22R cDNA was amplified by reverse transcription-PCR from the HepG2 hepatoma cell line before cloning into the pEF-BOSpuro expression vector. For the IL-22R 574ΔF receptor, the cDNA sequence corresponding to nucleotides 1–1050 of the IL-22R open reading frame was fused to nucleotides 1209–1725 using mutated primers to allow direct cloning into pEF-BOSpuro. IL-22R Tr490 and Tr490ΔF receptors were generated by using an antisense primer introducing a stop codon after amino acid 490. Mutagenesis was performed by changing tyrosine into phenylalanine residues using the GeneEditor in vitro site-directed mutagenesis system (Promega, Madison, WI). Clones obtained were sequenced using the DYEnamic ET dye terminator kit (Amersham Biosciences). BW5147 stable transfection was performed as described previously (11). Cell surface expression of the different IL-22R variants was checked by flow cytometry using an anti-IL-22R monoclonal antibody.
For the GST fusion protein, the intracytoplasmic part of IL-22R or fragment of IL-22R corresponding to amino acids located between 491 and 574 was cloned into pGMEX3-T vector (GE Healthcare). The pBS-STAT3 vector encoding the wild-type form of mouse STAT3 (provided by T. Hirano, Osaka University, Osaka, Japan) was subcloned into pCEP4 vector (Invitrogen). Truncated STAT3 isoforms were cloned into the same vector using antisense primers introducing a stop codon after amino acid 685, 585, 466, 326, or 130, according to the murine STAT3 amino acid numbers. The construct encoding the chimeric STAT1-STAT3 protein was generated by amplifying the cDNA sequences corresponding to amino acids 1–351 from STAT1 (N-terminal and coiled-coil domains) and to amino acids 356–770 of STAT3 (from DNA-binding to C-terminal domain) using mutated primers to allow direct cloning into pCEP4. A chimeric STAT3-STAT5 construct was generated similarly by amplifying the cDNA sequences corresponding to amino acids 1–325 from STAT3 and to amino acids 337–793 from STAT5. The chimeric IL-9RF116–22R was composed of a complete human IL-9R cDNA sequence with a Tyr-to-Phe mutation of residue 116 of the cytoplasmic domain and a mutation of the stop codon introducing a BstBI restriction site to insert the sequence coding for the C-terminal part of the IL-22R (amino acids 487–574, with Tyr491 mutated into Phe).
Western Blotting
BW5147 stable transfectants (106) were stimulated for 20 min with control medium or medium containing mouse IL-9 or human IL-9 (50 units/ml). Cells were lysed in 200 μl of Laemmli buffer (Bio-Rad), and anti-phospho-STAT3 Western blotting was performed as described previously (17).
Fluorescence-activated Cell Sorter Analysis
BW5147 cells (2 × 105) were left unstimulated or stimulated with IL-22 for 15 min at 37 °C. Cells were fixed in 2% paraformaldehyde for 10 min at 37 °C and then permeabilized in 90% methanol for 30 min on ice. Cells were washed twice with Hanks' medium and stained with anti-phosphorylated STAT3-Y705 (BD Biosciences). Cells were analyzed on a BD Biosciences FACSCaliburTM flow cytometer.
Immunoprecipitation
Twenty million cells were stimulated with hIL-22 (50 nm) or control medium for 5 min and washed, and lysates were obtained as described previously (17). Five micrograms of anti-IL-22R monoclonal antibody was added to the supernatant and incubated overnight at 4 °C. Lysates were then incubated with protein G-agarose for 2 h. Beads were washed, resuspended in Laemmli buffer (25 μl), and boiled. Proteins were separated in a 10% SDS-polyacrylamide gel (Novex, Carlsbad, CA) and transferred onto a nitrocellulose membrane. The membrane was blocked in 1% bovine serum albumin solution before overnight incubation with anti-STAT3 polyclonal antibody (Cell Signaling, Beverly, MA). Proteins were detected by chemiluminescence (SuperSignal West Pico, Pierce), and membranes were reprobed with anti-IL-22R antibody as a control.
BW5147 Bioassays
Cell proliferation assays and dexamethasone-induced apoptosis assay were performed as described previously (11). Then, cell proliferation was assessed either by a hexosaminidase assay or by thymidine proliferation as described previously (11).
Transient Cell Transfection and Luciferase Assay
The pGL3-Pap1 luciferase reporter plasmid was used as a specific promoter for STAT3 activation, whereas the pRL-TK plasmid (Promega), encoding Renilla luciferase, was cotransfected as an internal control of the transfection process. Transient COS cell transfections and luciferase assays were carried out as described previously (18). Briefly, 5 h after transfection, cells were stimulated with control medium or with human IL-22 (50 nm) for 16 h.
GST Purification Assay
To measure the interaction between GST fusion protein and STAT3 truncated mutants, the different proteins were produced independently in HEK293 cells by transient transfection using the Lipofectamine 2000 method (Invitrogen), according to the manufacturer's recommendations. Two days later, cells were lysed in 1 ml of lysis buffer (1% Triton X-100, 10% glycerol, 10 mm Tris (pH 7.6), 150 mm sodium chloride, 5 mm EDTA, 1 mm dithiothreitol, 1 mm sodium vanadate, 1 mm sodium fluoride, and inhibitor mixture (Roche Applied Science)). Lysates were homogenized as described above. Samples containing a GST fusion protein or a STAT3 protein were mixed, kept at 4 °C for 16 h, and purified on glutathione-Sepharose beads (Amersham Biosciences). After washing, specifically bound products were eluted from the beads with 20 mm reduced glutathione (Sigma). Eluted samples were subjected to SDS-PAGE, and proteins were transferred to nitrocellulose filters, probed with STAT3-specific antibodies, either specific for the C terminus (Cell Signaling Technology) or for the N terminus (Transduction Laboratories) depending on the truncated form of STAT3, and then reprobed with anti-GST monoclonal antibody.
RESULTS
Tyrosine Phosphorylation of IL-22R Is Not Required for STAT3 Activation
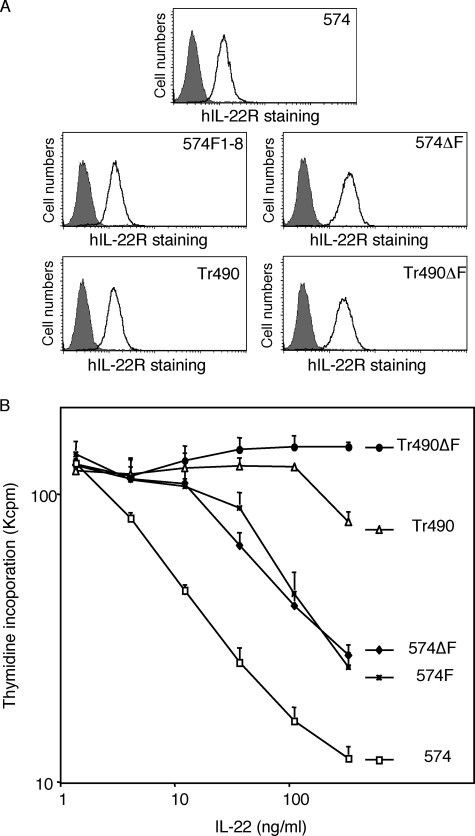
To determine whether STAT3 activation in response to IL-22 depends on the phosphorylation of IL-22R, two different types of IL-22R mutants were generated, either by mutating all of its cytoplasmic tyrosine residues into phenylalanine (574F) (Fig. 1) or by deleting a fragment of IL-22R containing five tyrosines from a repeated 13-amino acid motif located between Tyr351 and Tyr403 and mutating the three other tyrosines of the cytoplasmic domain (574ΔF). Unexpectedly, as shown in Fig. 2, A and C, both receptors lacking cytoplasmic tyrosines could still induce phosphorylation of STAT3 but not STAT5 or STAT1. Receptors lacking tyrosines (574F and 574ΔF) still activate STAT-dependent reporter gene transcription, and this activity requires the C terminus of IL-22R because deletion of the last 84 amino acids (Tr490ΔF) abolished the IL-22 response (Fig. 2B). As shown in Fig. 2C, a receptor lacking this C-terminal domain but keeping the cytoplasmic tyrosines (Tr490) still induced some STAT3 phosphorylation but to a lesser extent than receptors lacking tyrosines (574ΔF or 574F).
FIGURE 1.
Schematic representation of the wild-type and tyrosine-less mutated forms of IL-22R. Positions of tyrosine residues are indicated and numbered in IL-22R.
FIGURE 2.
STAT activation through two different mechanisms: independent and dependent of tyrosine residues. A, BW5147 stable transfectants (106) were stimulated with human IL-22 (50 nm) or with control medium for 30 min. Total lysates were analyzed by Western blotting with an antibody directed against tyrosine-phosphorylated STAT3 or STAT5 (P-STAT). The membranes were then reprobed with anti-STAT3 or anti-STAT5 antibodies (data not shown). B, COS cells (105) were seeded in 48-well plates. The day after, cells were transfected with the indicated receptor mutants, together with pGL3-pap1-luc and pRL-TK reporter plasmids. Cells were stimulated with IL-22 (50 nm) or with control medium for 16 h before a luciferase assay was performed. The experiments were repeated at least twice in triplicate, and data are presented as mean ± S.E. C, BW5147 stable transfectants (2 × 105) were stimulated in the presence of various concentrations of IL-22 for 15 min. Cells were fixed, permeabilized, and stained with Alexa Fluor 647 anti-phospho-STAT3. Cell fluorescence was assessed by flow cytometry, and STAT3 phosphorylation is shown as the difference of mean fluorescence intensity observed between unstimulated and IL-22-stimulated transfectants. Data correspond to the mean of triplicate cultures ± S.D. from one of three independent experiments.
The C-terminal Domain of IL-22R Is Sufficient for STAT3 Recruitment
To determine whether these 84 C-terminal residues are sufficient to recruit and activate STAT3 in the context of another receptor, we took advantage of a mutant form of human IL-9R that had lost the ability to activate STAT factors (h9RF116) (11). Different human IL-9 receptor constructs were transfected in BW5147 lymphoma cells, which endogenously respond to murine IL-9 but not to human IL-9. Transfection of wild-type (BWh9R) but not mutated BWh9RF116 IL-9R allowed hIL-9-dependent STAT3 phosphorylation. However, fusion of the C-terminal domain (residues 491–574) of IL-22R after the last amino acid of the mutated hIL-9R restored its ability to activate STAT3 at the same level as the wild-type hIL-9R (Fig. 3A). At the functional level, this correlated with a restored ability of this receptor to protect BW5147 transfectant cells against dexamethasone-induced apoptosis (Fig. 3B), an activity previously shown to be mediated by STAT3 (19). Taken together, these data indicate that the C-terminal domain of the IL-22R is sufficient to confer the ability to recruit STAT3 to an unrelated cytokine receptor, leading to functional STAT3 activation.
FIGURE 3.
The C-terminal domain of IL-22R is sufficient to restore STAT3 activation by IL-9RF116. A, 106 parental or stably transfected BW5147 (transfected with wild-type human IL-9R, the human IL-9RF116, or a chimeric IL-9RF116 receptor including the 84 C-terminal residues of IL-22R) were stimulated with human or murine (m) IL-9 (50 units/ml) or with control medium for 20 min. Total lysates were analyzed by Western blotting with an antibody directed against tyrosine-phosphorylated STAT3. The membranes were then reprobed with an anti-β-actin antibody (data not shown). B, parental or stably transfected BW5147 cells were seeded in 96-well plates. BW5147 cells were stimulated with 10 units/ml hIL-9 and mouse IL-9. After 72 h in the presence of dexamethasone (Dex), proliferation was measured by hexosaminidase assay. The data are representative of one of three independent experiments performed in triplicate and are presented as mean ± S.E.
Role of the C Terminus of IL-22R in IL-22 Bioactivity
To investigate whether the C-terminal domain of IL-22R is required for STAT3-dependent biological activities, we took advantage of BW5147 lymphoma cells in which prolonged STAT3 activation induces growth inhibition (19). In this model, IL-22 inhibited more than 90% of the proliferation of BW cells expressing the wild-type receptor (574), but deletion of the C-terminal domain of the receptor (Tr490) dramatically reduced this activity (Fig. 4B). Deletion or mutation of the tyrosines (574ΔF and 574F) decreased the activity of IL-22, but to a lesser extent, indicating that tyrosines were less critical than the C-terminal domain. Finally, a receptor mutant lacking both the C-terminal domain and the cytoplasmic tyrosines (Tr490ΔF) was completely devoid of activity as expected from the luciferase experiments described before. All transfectants had a similar cell surface expression of IL-22R, ruling out the possibility that these mutations affect the stability or trafficking of the protein (Fig. 4A).
FIGURE 4.
Both mechanisms of STAT3 activation are required for inhibition of cell proliferation. A, BW5147 transfectant cells (2 × 105) were stained with anti-IL-22R antibody (0.3 μg/ml) followed by fluorescein isothiocyanate goat anti-mouse antibody before fluorescence-activated cell sorter analysis. Shaded histograms correspond to staining with the secondary antibody alone, and open histograms correspond to staining with both antibodies. B, BW5147 cells stably transfected with IL-22R wild-type (574), IL-22R truncated at amino acid 490 (Tr490), tyrosine-less mutants (574ΔF or 574F) or a mutant lacking all tyrosines and the C terminus (Tr490ΔF) were stimulated in the presence of various concentrations of hIL-22 for 48 h followed by thymidine incorporation. The experiments were repeated at least twice in triplicate, and data are presented as mean ± S.E.
STAT3 Is Preassociated with IL-22R
To understand better this unconventional mechanism of STAT3 activation, we analyzed the interaction between IL-22R and STAT3 by immunoprecipitation. As shown in Fig. 5, STAT3 coprecipitated with IL-22R even in the absence of the ligand. Similar results were obtained in stable transfectants (Fig. 5A) and in cells endogenously expressing this receptor such as HepG2, HT-29, and colo205 (Fig. 5B). Deletion or mutation of all tyrosines in the IL-22R 574ΔF mutant did not affect the association with STAT3, but deletion of the C-terminal domain in the 490 and Tr490ΔF mutant abolished the preassociation with STAT3.
FIGURE 5.
STAT3 is preassociated with IL-22R. A, transfectant cells (2 × 107) were lysed, and immunoprecipitation (IP) was performed with an antibody directed against IL-22R or with an isotype control (CTL Ab). Western blot (WB) analysis was performed with an anti-STAT3 antibody or with an anti-IL-22R antibody. B, colo205, HT-29, or HepG2 cells (2 × 107) were lysed and immunoprecipitated with an antibody directed against IL-22R or a control IgG2a antibody. Western blot analysis was performed with an anti-STAT3 antibody.
To characterize further the interaction between the cytoplasmic domain of IL-22R and STAT3, we screened a series of GST fusion proteins that contained variants of the cytoplasmic domain of IL-22R for the binding of STAT3 (Fig. 6A). After purification on Sepharose-glutathione beads, STAT3 was found to copurify specifically with the GST fusion proteins that contained the C-terminal domain of IL-22R, irrespective of the presence of tyrosines. Thus, both immunoprecipitation and GST pulldown experiments indicate that STAT3 constitutively associates with the cytoplasmic domain of IL-22R.
FIGURE 6.
Role of the C terminus of IL-22R in STAT3 interaction. A, upper panel, schematic representation of GST fused to various forms of the cytoplasmic domain of IL-22R. Mutations of tyrosine residues into phenylalanine (F) are indicated. Lower panel, HEK293 cells (8 × 105) were seeded in 6-well plates. The next day, cells were transfected with a vector coding for one of the different GST-fused proteins or STAT3. Two days later, cells were lysed, and GST-fused proteins were mixed with STAT3 and glutathione-Sepharose beads. After 16 h at 4 °C, beads were washed. Proteins eluted with GST-fused proteins were analyzed by Western blotting with an antibody directed against STAT3. The membranes were then reprobed with an anti-GST antibody. B–D, same experiments as in A using different truncated forms of the C terminus of STAT3 (B), different truncated forms of the N terminus of STAT3 (C), a chimeric STAT factor with the N terminus (ND) and coiled-coil domain (CC) of STAT1 and the DNA-binding domain (DBD), linker domain (LD), SH2 domain (SH2), and C-terminal (CT) domain of STAT3 (D). A schematic representation of the domains from STAT3 is shown in B. Numbers 1, 2, 3, and 5 in B–D refer to the GST fusion constructs depicted in the upper panel of A. WB, Western blotting.
The Coiled-coil Domain of STAT3 Is Responsible for Its Association with IL-22
To identify which region of STAT3 is involved in this interaction, we generated STAT3 truncated mutants, successively deleting STAT3 domains from the C terminus, and analyzed their ability to interact with IL-22R-GST fusion proteins. As shown in Fig. 6B, a STAT3 variant lacking the C-terminal, SH2 and DNA-binding domain interacted like the wild type STAT3 with a tyrosine-less IL-22R-GST fusion protein (GST-574ΔF, lanes 2), but not with GST alone (lanes 1) or a fusion protein that lacked the C-terminal domain (GST-Tr491ΔF, lanes 3). STAT3 mutants that were truncated from the N terminus were used to dissect the role of coiled-coil and N-terminal domains (Fig. 6C). Although deletion of the N-terminal domain did not affect the interaction with IL-22R (STAT3770-N130), further deletion of the coiled-coil domain completely abolished the association of STAT3 with IL-22R (STAT3770-N326). To confirm these results in the context of a full-length STAT protein, we generated a chimeric STAT1-STAT3 protein in which the N-terminal and coiled-coil domains of STAT3 were replaced by those of STAT1. As shown in Fig. 6D, this chimeric protein failed to interact with GST fused to IL-22R, further demonstrating that the coiled-coil domain of STAT3 is specifically required for its interaction with C-terminal region of IL-22R.
The Coiled-coil Domain of STAT3 Is Sufficient to Confer Recruitment to IL-22R
To determine whether the coiled-coil domain of STAT3 might be sufficient to mediate association with IL-22R in vivo, we took advantage of HEK293 cells, which express very low levels of endogenous STAT5. As a result, IL-22 did not induce any detectable STAT5 phosphorylation in these cells, even when the wild-type IL-22R (574) is transfected (Fig. 7A). When STAT5 was cotransfected with different IL-22R mutants (Fig. 7B), STAT-5 phosphorylation was observed only with the wild-type receptor but not with the receptors lacking tyrosines (574ΔF, 574F, and Tr490ΔF), confirming that IL-22R tyrosines are required for STAT5 phosphorylation. However, when we replaced the N-terminal and coiled-coil domains of STAT5 with those of STAT3 (Fig. 7C), the resulting chimeric protein could be activated even by tyrosine-less receptors (such as 574ΔF and 574F) but not by the receptor lacking the C-terminal domain (Tr490ΔF). These data demonstrated that the coiled-coil domain of STAT3 conferred the ability to be recruited to the IL-22R C-terminal region independently of the SH2 domain of the STAT protein.
FIGURE 7.
The coiled-coil domain of STAT3 is sufficient to restore STAT5 phosphorylation in absence of tyrosine residues. HEK293 cells (2 ×105) were seeded in 6-well plates. Twenty-four hours later, cells were transfected with vectors coding for one of the different truncated forms of IL-22R and either the empty vector (A), STAT5B (B), or a chimeric protein containing the N-terminal and coiled-coil domains of STAT3 fused to the DNA-binding domain, linker domain, SH2 domain, and C-terminal domain of STAT5 (C). One day later, cells were stimulated with human IL-22 (50 nm) or with control medium for 20 min. Total lysates were analyzed by Western blotting (WB) with an antibody directed against tyrosine-phosphorylated STAT5 (PSTAT5). The membranes were then reprobed with an anti-STAT5 antibody followed by anti IL-22R monoclonal antibody.
DISCUSSION
The data reported here show that the C-terminal part of the IL-22R is constitutively associated with STAT3 and that the tyrosines of the receptor are not necessary for activation of STAT3 by IL-22. So far, the interaction between phosphotyrosines from the cytoplasmic domain of the receptor and the SH2 domain of STATs was considered to be critical for this process, and the activation of a particular STAT by a cytokine was shown to depend on amino acids surrounding phosphorylated tyrosines that are likely to be involved in the interaction with the SH2 domain. For instance, receptors that activate STAT3 are usually characterized by a YXXQ motif (3, 11). Mutation of the tyrosine or glutamine residue of this consensus motif from receptors such as gp130 and IL-9R abolished STAT3 recruitment and phosphorylation, supporting the idea that the recognition by the SH2 domain of STAT3 is mandatory for its activation (11, 20, 21). An SH2-independent interaction between a STAT protein and a cytokine receptor has been described previously for the type I interferon receptor, with STAT2 being constitutively associated with IFNAR2 (22). However, in this case, the interferon response remained dependent on the interaction between the SH2 domain of STAT2 and phosphorylated Tyr466 from IFNAR1 (23) or other tyrosines from both chains (24, 25). In addition, the loss of this constitutive interaction by mutations of the corresponding region of IFNAR2 does not decrease STAT2 activation by interferons (26).
Here, we show that mutation of all cytoplasmic tyrosine residues of the IL-22R only partially affects STAT3 activation and that STAT3 is constitutively associated with the C-terminal part of IL-22R via its coiled-coil domain. Our data show that the IL-22R uses two distinct and additive mechanisms to activate STAT3 because STAT3 phosphorylation is only abolished by both mutating all tyrosines and deleting the C-terminal domain of the receptor. This observation raises the hypothesis that other cytokine receptors may use a similar mode of STAT3 recruitment. Analysis of G-CSFR signaling showed some similarities with our model. Ward et al. (12) have shown that, at saturating G-CSF concentrations, STAT3 activation by the G-CSFR is efficiently mediated by the C-terminal domain in a manner independent of receptor tyrosines. However, we failed so far to confirm that STAT3 might associate constitutively with the C-terminal domain of G-CSFR by using immunoprecipitation of GST fusion approaches (data not shown). We also failed to detect any apparent amino acid sequence homology between the C-terminal domain of IL-22R and other cytokine receptors. Finally, activation of STAT3 by growth hormone or of STAT5 by IL-6 has been shown to be independent of tyrosines from the receptor but actually depends on a direct interaction with phosphorylated Janus kinase (27, 28).
A potential advantage for a cytokine receptor being preassociated with STAT3 might be to allow for a faster response or for efficient STAT3 activation in cells with lower endogenous STAT3 expression. Another potential advantage of the SH2-independent recruitment of STAT3 could be to escape negative feedback by proteins such as SOCS3, which can compete with STAT factors for phosphotyrosines (29). Although they are not required for STAT3 activation, four tyrosines from IL-22R are included in a YXXQ motif, which is a consensus recruitment motif of STAT3. These tyrosines might also recruit STAT3 via its SH2 domain, further increasing STAT3 activation. The use of these two complementary modes of STAT3 recruitment makes IL-22R a very potent activator of this transcription factor and suggests that STAT3 is critical for its biological activities. This observation might be particularly relevant for the protective effect of IL-22 during Citrobacter rodentium infection, which was shown to be mainly mediated by induction of Reg3β, a typical STAT3-dependent gene (30).
In addition, IL-22R mediates STAT3 activation in response to IL-22, IL-20, and IL-24 in various cell types, including keratinocytes, and might play an important role in psoriasis, a disease associated with a strong activation of STAT3 in such cells (31, 32). Moreover, IL-20, IL-22, and IL-22R are overexpressed in the skin of psoriatic patients, and transgenic mice for either IL-20 or IL-22 present a phenotype that mimics psoriasis lesions (33–37). Interestingly, IL-22-deficient mice are protected against dermal inflammation and acanthosis induced by IL-23 in an experimental model related to human psoriasis (14). Moreover, in a mouse model of psoriasis, blocking IL-22 prevents development of the disease (38). In this context, our observation that optimal STAT3 activation depends on the interaction between the C terminus of IL-22R and the coiled-coil domain of STAT3 opens some new perspectives of interfering specifically with this process without affecting the conventional mode of STAT3 activation used by other cytokine receptors.
Acknowledgments
We thank Dr. Hirano (Osaka University, Osaka, Japan) for providing the cDNA of STAT3 and Dr. S. Constantinescu for critical reading of the manuscript.
This work was supported in part by the Belgian Federal Service for Scientific Technical and Cultural Affairs and the Actions de Recherche Concertées, Communauté Française de Belgique, Direction de la Recherche Scientifique.
- STAT
- signal transducer and activator of transcription
- G-CSF
- granulocyte colony-stimulating factor
- G-CSFR
- G-CSF receptor
- GST
- glutathione S-transferase
- h
- human
- IL-9R
- interleukin-9 receptor
- SH2
- Src homology 2.
REFERENCES
- 1.Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J., Neel B. G., Birge R. B., Fajardo J. E., Chou M. M., Hanafusa H., Schaffhausen B., Cantley L. C. (1993) Cell 72, 767–778 [DOI] [PubMed] [Google Scholar]
- 2.Kisseleva T., Bhattacharya S., Braunstein J., Schindler C. W. (2002) Gene 285, 1–24 [DOI] [PubMed] [Google Scholar]
- 3.Stahl N., Farruggella T. J., Boulton T. G., Zhong Z., Darnell J. E., Jr., Yancopoulos G. D. (1995) Science 267, 1349–1353 [DOI] [PubMed] [Google Scholar]
- 4.Zhong M., Henriksen M. A., Takeuchi K., Schaefer O., Liu B., ten Hoeve J., Ren Z., Mao X., Chen X., Shuai K., Darnell J. E., Jr. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3966–3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ndubuisi M. I., Guo G. G., Fried V. A., Etlinger J. D., Sehgal P. B. (1999) J. Biol. Chem. 274, 25499–25509 [DOI] [PubMed] [Google Scholar]
- 6.Strehlow I., Schindler C. (1998) J. Biol. Chem. 273, 28049–28056 [DOI] [PubMed] [Google Scholar]
- 7.Becker S., Groner B., Müller C. W. (1998) Nature 394, 145–151 [DOI] [PubMed] [Google Scholar]
- 8.Ma J., Cao X. (2006) Cell. Signal. 18, 1117–1126 [DOI] [PubMed] [Google Scholar]
- 9.McBride K. M., McDonald C., Reich N. C. (2000) EMBO J. 19, 6196–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinrich P. C., Behrmann I., Haan S., Hermanns H. M., Müller-Newen G., Schaper F. (2003) Biochem. J. 374, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demoulin J. B., Uyttenhove C., Van Roost E., DeLestré B., Donckers D., Van Snick J., Renauld J. C. (1996) Mol. Cell. Biol. 16, 4710–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward A. C., Hermans M. H., Smith L., van Aesch Y. M., Schelen A. M., Antonissen C., Touw I. P. (1999) Blood 93, 113–124 [PubMed] [Google Scholar]
- 13.Renauld J. C. (2003) Nat. Rev. Immunol. 3, 667–676 [DOI] [PubMed] [Google Scholar]
- 14.Zheng Y., Danilenko D. M., Valdez P., Kasman I., Eastham-Anderson J., Wu J., Ouyang W. (2007) Nature 445, 648–651 [DOI] [PubMed] [Google Scholar]
- 15.Dumoutier L., Van Roost E., Colau D., Renauld J. C. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 10144–10149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Druez C., Coulie P., Uyttenhove C., Van Snick J. (1990) J. Immunol. 145, 2494–2499 [PubMed] [Google Scholar]
- 17.Lejeune D., Dumoutier L., Constantinescu S., Kruijer W., Schuringa J. J., Renauld J. C. (2002) J. Biol. Chem. 277, 33676–33682 [DOI] [PubMed] [Google Scholar]
- 18.Dumoutier L., Leemans C., Lejeune D., Kotenko S. V., Renauld J. C. (2001) J. Immunol. 167, 3545–3549 [DOI] [PubMed] [Google Scholar]
- 19.Demoulin J. B., Van Roost E., Stevens M., Groner B., Renauld J. C. (1999) J. Biol. Chem. 274, 25855–25861 [DOI] [PubMed] [Google Scholar]
- 20.Heinrich P. C., Behrmann I., Müller-Newen G., Schaper F., Graeve L. (1998) Biochem. J. 334, 297–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtani T., Ishihara K., Atsumi T., Nishida K., Kaneko Y., Miyata T., Itoh S., Narimatsu M., Maeda H., Fukada T., Itoh M., Okano H., Hibi M., Hirano T. (2000) Immunity 12, 95–105 [DOI] [PubMed] [Google Scholar]
- 22.Li X., Leung S., Kerr I. M., Stark G. R. (1997) Mol. Cell. Biol. 17, 2048–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan H., Krishnan K., Greenlund A. C., Gupta S., Lim J. T., Schreiber R. D., Schindler C. W., Krolewski J. J. (1996) EMBO J. 15, 1064–1074 [PMC free article] [PubMed] [Google Scholar]
- 24.Nadeau O. W., Domanski P., Usacheva A., Uddin S., Platanias L. C., Pitha P., Raz R., Levy D., Majchrzak B., Fish E., Colamonici O. R. (1999) J. Biol. Chem. 274, 4045–4052 [DOI] [PubMed] [Google Scholar]
- 25.Russell-Harde D., Wagner T. C., Rani M. R., Vogel D., Colamonici O., Ransohoff R. M., Majchrzak B., Fish E., Perez H. D., Croze E. (2000) J. Biol. Chem. 275, 23981–23985 [DOI] [PubMed] [Google Scholar]
- 26.Nguyen V. P., Saleh A. Z., Arch A. E., Yan H., Piazza F., Kim J., Krolewski J. J. (2002) J. Biol. Chem. 277, 9713–9721 [DOI] [PubMed] [Google Scholar]
- 27.Fujitani Y., Hibi M., Fukada T., Takahashi-Tezuka M., Yoshida H., Yamaguchi T., Sugiyama K., Yamanaka Y., Nakajima K., Hirano T. (1997) Oncogene 14, 751–761 [DOI] [PubMed] [Google Scholar]
- 28.Sotiropoulos A., Moutoussamy S., Renaudie F., Clauss M., Kayser C., Gouilleux F., Kelly P. A., Finidori J. (1996) Mol. Endocrinol. 10, 998–1009 [DOI] [PubMed] [Google Scholar]
- 29.Ilangumaran S., Ramanathan S., Rottapel R. (2004) Semin. Immunol. 16, 351–365 [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y., Valdez P. A., Danilenko D. M., Hu Y., Sa S. M., Gong Q., Abbas A. R., Modrusan Z., Ghilardi N., de Sauvage F. J., Ouyang W. (2008) Nat. Med. 14, 282–289 [DOI] [PubMed] [Google Scholar]
- 31.Sano S., Chan K. S., Carbajal S., Clifford J., Peavey M., Kiguchi K., Itami S., Nickoloff B. J., DiGiovanni J. (2005) Nat. Med. 11, 43–49 [DOI] [PubMed] [Google Scholar]
- 32.Sano S., Chan K. S., Kira M., Kataoka K., Takagi S., Tarutani M., Itami S., Kiguchi K., Yokoi M., Sugasawa K., Mori T., Hanaoka F., Takeda J., DiGiovanni J. (2005) Cancer Res. 65, 5720–5729 [DOI] [PubMed] [Google Scholar]
- 33.Wolk K., Witte E., Wallace E., Döcke W. D., Kunz S., Asadullah K., Volk H. D., Sterry W., Sabat R. (2006) Eur. J. Immunol. 36, 1309–1323 [DOI] [PubMed] [Google Scholar]
- 34.Boniface K., Lecron J. C., Bernard F. X., Dagregorio G., Guillet G., Nau F., Morel F. (2005) Eur. Cytokine Netw. 16, 309–319 [PubMed] [Google Scholar]
- 35.Boniface K., Bernard F. X., Garcia M., Gurney A. L., Lecron J. C., Morel F. (2005) J. Immunol. 174, 3695–3702 [DOI] [PubMed] [Google Scholar]
- 36.Blumberg H., Conklin D., Xu W. F., Grossmann A., Brender T., Carollo S., Eagan M., Foster D., Haldeman B. A., Hammond A., Haugen H., Jelinek L., Kelly J. D., Madden K., Maurer M. F., Parrish-Novak J., Prunkard D., Sexson S., Sprecher C., Waggie K., West J., Whitmore T. E., Yao L., Kuechle M. K., Dale B. A., Chandrasekher Y. A. (2001) Cell 104, 9–19 [DOI] [PubMed] [Google Scholar]
- 37.Wolk K., Haugen H. S., Xu W., Witte E., Waggie K., Anderson M., Vom Baur E., Witte K., Warszawska K., Philipp S., Johnson-Leger C., Volk H. D., Sterry W., Sabat R. (2009) J. Mol. Med. 87, 523–536 [DOI] [PubMed] [Google Scholar]
- 38.Ma H. L., Liang S., Li J., Napierata L., Brown T., Benoit S., Senices M., Gill D., Dunussi-Joannopoulos K., Collins M., Nickerson-Nutter C., Fouser L. A., Young D. A. (2008) J. Clin. Invest. 118, 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]