Abstract
Reductions in uterine perfusion pressure (RUPP) in pregnant rats is associated with increased tumor necrosis factor-α (TNF-α). This study was designed to determine the role of endogenous TNF-α in mediating changes in arterial pressure and endothelin-1 (ET-1) in RUPP rats. To achieve this goal we examined the effect of RUPP in the presence and absence of a TNF-α–soluble receptor, etanerecept (0.4 mg/kg). Mean arterial pressure increased from 102±1 mm Hg in normal pregnant (NP) rats to 134±3 mmHg (P<0.05) in RUPP rats. Serum TNF-α increased to 40±7.6 pg/mL in RUPP rats (n=24) versus 14.8±3.3 pg/mL (n=16; P<0.05) in NP rats. Administration of etanerecept decreased TNF-α in RUPP rats (n=20) to 17.2±3 pg/mL and mean arterial pressure to 118±2 mmHg (P<0.05). Tissue ET-1 decreased in etanerecept-treated RUPP rats compared with control RUPP rats. The direct effect of TNF-α blockade on endothelial activation in response to placental ischemia was examined in human umbilical vein endothelial cells. ET-1 secreted from human umbilical vein endothelial cells treated with RUPP serum was 59.2±16 pg/mg and decreased when etanerecept was added to the medium with RUPP serum (7.60±0.77 pg/mg), as well as in response to serum from etanerecept-treated RUPP rats (7.30±0.55 pg/mg; P<0.001). ET-1 secreted from human umbilical vein endothelial cells was 15.6±2 pg/mg when treated with NP serum. These data support the hypothesis that endogenous TNF-α is an important stimulus for ET-1 in response to placental ischemia and is important in mediating endothelial cell activation and hypertension during pregnancy.
Keywords: hypertension, pregnancy, inflammation, cytokines, endothelial activation
Preeclampsia has long been considered an immunologically based disease.1 During normal pregnancy, tumor necrosis factor (TNF)-α promotes expression of adhesion molecules in maternal endothelial cells and activates phagocytic cells that are important mediators of morphological changes in the uterine arteries. During preeclampsia, however, variable expression of adhesion molecules interferes with essential changes to the endothelial lining of the maternal vasculature.2,3 The compromised vascular remodeling characteristic of preeclampsia results in decreased placental perfusion and creates a hypoxic environment for placental and fetal tissues. Under hypoxic conditions, placental explants from preeclamptic women exhibit a 2-fold increase in TNF-α compared with explants from NP women.4–6 Previous studies have demonstrated that preeclamptic women have a 2-fold elevation in placental and plasma TNF-α protein levels compared with women with normal pregnancies.7,8 As a result, inflammatory cells are activated in the circulation and infiltrate into renal and placental tissues. These activated immune cells continue to release inflammatory cytokines, which mediate endothelial cell activation and dysfunction, thereby creating a milieu similar to that of chronic inflammatory diseases.9,10
Although elevated TNF-α is associated with preeclampsia, its importance in mediating the cardiovascular and endothelial dysfunction in response to placental ischemia during pregnancy has yet to be fully elucidated. We reported previously that chronic reductions in uterine perfusion pressure (RUPP) in pregnant rats increases arterial pressure and impairs endothelial function.11 Moreover, we reported recently that serum levels of TNF-α are elevated in RUPP rats, and chronic infusion of TNF-α into pregnant rats increases arterial pressure.12 One mechanism mediating TNF-induced hypertension during pregnancy is activation of the endothelin (ET) 1 system, ET-1 being the hallmark peptide of endothelial cell activation and dysfunction. The hypertension in response to elevated TNF-α in pregnant rats was associated with increased ET-1 production and was abolished by treatment with an ETA receptor antagonist.13 Furthermore, Alexander et al14 examined the role of ET-1 in mediating the hypertension in the RUPP rat. Alexander et al14 demonstrated that renal expression of preproendothelin was significantly elevated in both the medulla and the cortex of the RUPP pregnant rats compared with control pregnant rats and that hypertension associated with RUPP in pregnant rats was attenuated with administration of the ETA receptor antagonist. We previously demonstrated enhanced ET-1 secretion from endothelial cells in response to serum collected from RUPP rats compared with serum from NP rats,15 supporting the theory that circulating factors, such as TNF-α, released from the ischemic placenta potentially mediate endothelial cell activation and dysfunction that is associated with hypertension during pregnancy. Although serum levels of TNF-αare elevated in RUPP rats, the importance of endogenous TNF-α in mediating increases in ET-1 and arterial pressure in RUPP rats remains unclear. Therefore, the first objective of the present study was to determine the role of endogenous TNF-αin mediating the increases in local ET-1 production and arterial pressure in response to placental ischemia in pregnant rats. The experimental approach was to administer a soluble TNF-α receptor, etanerecept, to pregnant RUPP rats on day 18 of gestation and to analyze mean arterial pressure (MAP) and local ET-1 production to determine the role of endogenous TNF-α in mediating hypertension, via ET activation, in response to placental ischemia. The actions of etanerecept are to bind free TNF-α, thereby decreasing the availability of bioactive forms of the cytokine. A second objective was to determine a potential role for TNF-α as a mediator of endothelial activation and dysfunction in response to placental ischemia by analyzing ET-1 secretion from human umbilical vein endothelial cells (HUVECs) exposed to sera from normal pregnant (NP) and RUPP pregnant rats, with or without etanerecept treatment, or in response to NP and RUPP serum with etanerecept as an additive in the experimental medium.
Methods
All of the studies were performed in timed pregnant Sprague-Dawley rats purchased from Harlan Sprague Dawley Inc (Indianapolis, Ind). Animals were housed in a temperature-controlled room (23°C) with a 12:12 hour light/dark cycle. All of the experimental procedures executed in this study were in accordance with National Institutes of Health guidelines for use and care of animals and the institutional animal care and use committee at the University of Mississippi Medical Center, which approved all of the protocols.
Effect of TNF-α Blockade on Mean Arterial Pressure in Response to Chronic RUPP
Experiments were performed in the following groups of rats: pregnant control (n=16); NP treated with etanerecept (specifically Enbrel 0.4 mg/kg16,17), a soluble TNF-α receptor, on day 18 of gestation (n=15); RUPP pregnant rats (n=24); and RUPP pregnant rats treated with etanerecept (Enbrel 0.4 mg/kg) on day 18 (n=20) of gestation. This dose was shown previously to decrease circulating TNF-α levels and improve clinical uveitis scores in male rats,16 and a similar dose was shown to decrease serum bioactive TNF-α and increase modulation of vascular function by NO in ovariectomized Sprague-Dawley rats.17 All of the pregnant rats undergoing surgical procedures were anesthetized with 2% isoflurane (W.A. Butler Co) delivered by an anesthesia apparatus (Vaporizer for Forane Anesthetic, Ohio Medical Products). Pregnant rats entering the RUPP group underwent the clipping procedure at day 14 of gestation. After a midline incision, the lower abdominal aorta was isolated, and a surgical silver clip (0.203 mm ID) was placed around the aorta above the iliac bifurcation. Branches of both the right and left ovarian arteries were clipped using a silver clip (0.100 mm ID). Groups of rats treated with etanerecept received a single intraperitoneal injection on day 18 of gestation.
Measurement of Arterial Pressure in Chronically Instrumented Conscious Rats
Arterial pressure was determined in all of the groups of rats at day 19 of gestation. Pregnant rats were catheterized on day 18 of gestation under a short-acting anesthetic, with isoflurane delivered by an anesthesia apparatus. A catheter of V−3 tubing (SCI) was inserted into the carotid artery for blood pressure monitoring. The catheter was tunneled to the back of the neck and exteriorized after implantation. On day 19 of gestation, pregnant rats were placed in individual restraining cages for arterial pressure measurements. Arterial pressure was monitored with a pressure transducer (Cobe III Transducer CDX Sema) and was recorded continuously for a 2-hour period after 1 hour of stabilization. Rats were anesthetized using isoflurane delivered by an anesthesia apparatus for blood and tissue collection.
Determination of Serum TNF-α Levels
A rat TNF-α colorimetric sandwich ELISA (R&D Systems) was used for quantification of serum TNF-α levels between 12.5 and 800.0 pg/mL. This assay displayed a sensitivity level of 5 pg/mL and interassay variability of 10.0% and intra-assay of 5.1%. Both serial dilutions of etanerecept and concentrated TNF-α were used to test the efficiency of the ELISA, and we found positive binding of TNF-α, as expected, and no concentration of etanerecept interfered with the ELISA results.
Determination of Renal and Placental mRNA TNF-α Levels
The placenta, cortex, and medulla of the kidneys were snap frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted using the Totally RNA kit supplied by Ambion after the tissue was crushed in liquid nitrogen with a mortar and pestle. Isolation procedure was then performed as outlined in the instructions provided by the manufacturer. TNF-α message was analyzed using Quantikine mRNA colorimetric Quantitation Base kit and specific TNF-α oligonucleotide capture probes designed and produced by R&D Systems. Detection procedure was performed as outlined in the instructions provided by the manufacturer. Briefly, mRNA was hybridized with a gene-specific, biotin-labeled TNF-α oligonucleotide probe and a digoxigenin-labeled detection probe in a 96-well plate. The hybridization solution was transferred to a streptavidin-coated microplate where the RNA/probe was captured, unbound material washed away, and antidigoxigenin alkaline phosphatase conjugate added. Substrate and amplifier solution was added, and color developed in proportion to the amount of gene-specific mRNA in the sample as quantitated against a standard curve specific for TNF-α mRNA.
Determination of Placental TNF-α Protein Levels
Rat cytokine antibody microarray from Ray Biotech, Inc, was used to evaluate cytokine protein expression. Specifically, TNF-α protein levels were analyzed from 500-µg protein from placental tissue lysate isolated in buffer provided by the manufacturer. Placental lysate was hybridized to the anticytokine protein microarray overnight at 4°C. The membrane was washed and incubated with a cocktail of biotinylated anticytokine antibodies, incubated with horseradish peroxidase– conjugated streptavidin, and exposed to alkaline phosphotase. Cytokines were detected by chemiluminescence, normalized against an internal positive control, and analyzed by autoradiography.
Determination of Renal and Placental Preproendothelin mRNA Levels
The placenta, cortex, and medulla of the kidneys were snap frozen in liquid nitrogen and stored at −80°C. Total RNA was extracted using the Totally RNA kit supplied by Ambion after the tissue was crushed in liquid nitrogen with a mortar and pestle. Isolation procedure was then performed as outlined in the instructions provided by the manufacturer.
Genomic DNA was digested with DNase1 following instructions outlined by Ambion. RNA was quantified spectrophotometrically using an Eppendorf BioPhotometer. cDNA was synthesized from 1 µg of RNA with BioRad Iscript cDNA reverse transcriptase, and real-time PCR was performed using the BioRad Syber Green supermix and iCycler, as described previously.13 Levels of mRNA expression were calculated using the mathematical formula for Δ/Δ cycle threshold recommended by Applied Biosystems (Applied Biosystems User Bulletin, No. 2, 1997).
Effect of Sera From RUPP Rats or Etanerecept-Treated RUPP Rats on Endothelin Production: Cell Culture
HUVECs, passage 2, were cultured in 50:50 DMEM/M199 (Gibco BRL) with 10% FBS (Hyclone) and 1% antimycotic antibiotic (Gibco BRL) in a humidified atmosphere of 5% CO2–20% O2–75% N2 at 37°C. Seventy-percent confluent monolayers were incubated for 48 hours in serum-free medium before exposure to experimental conditions.
Experimental Protocol
Culture medium was removed, and experimental medium containing 1 mL of experimental rat serum and 1 mL of serum free medium was laid onto the cells for 24 hours. The experimental medium was removed, fresh serum free medium was added, and cells were cultured for an additional 6 hours. In addition, groups of cells were exposed to RUPP or NP serum with 10 µg/mL of etanerecept added to the medium to determine whether there was a direct effect of etanerecept to inhibit endothelial cell activation and dysfunction resulting from exposure to serum from rats with RUPP. Aliquots of sample were taken after 6 hours of cultivation to determine whether ET-1 secretion increased with time. Cells were trypsinized, and total protein was collected.
Determination of Endothelin Concentration
Endothelin concentration was determined using 100 µL of media collected and measured using the ET-1 Quantikine ELISA kit from R&D Systems. The assay displayed a sensitivity of 0.023 to 0.102 pg/mL, interassay variability of 8.9%, and intra-assay variability of 3.4%. Total protein was isolated and used to standardize immunoassay results. After trypsinization, cells were collected by centrifugation (5 minutes at 2 rpm), washed with of Dulbecco’s PBS, and centrifuged. Protein lysis buffer (200 µL) was added, and cells were disrupted by vortex. The lysate was placed on ice for 5 minutes, and cell debris was collected by centrifugation at full speed for 2 minutes. The protein lysate was extracted and placed in a clean tube. Total protein was quantitated using the BCA protein quantitation kit from Pierce.
Statistical Analysis
Differences between control and experimental groups were analyzed using ANOVA with the Tukey-Kramer multiple comparison test. Data were considered statistically different at P<0.05. Statistical analysis of real-time PCR results was performed using the mean normalized cycle threshold values and SDs analyzed by 1-way ANOVA and Tukey-Kramer multiple comparison test.
Results
Arterial Pressure Response to TNF-α Soluble Receptor, Etanerecept, in NP and RUPP Rats
Administration of the TNF-α soluble receptor, etanerecept (0.4 mg/kg), to NP rats on day 18 of gestation had no effect on MAP (Figure 1). Blood pressure in NP rats was unchanged in NP rats by administration of etanerecept. In sharp contrast, administration of etanerecept decreased MAP significantly, yet was not completely attenuated in RUPP rats.
Figure 1.
MAP and serum concentration of TNF-α in control pregnant rats (n=16), RUPP rats (n=24), control pregnant rats treated with etanerecept (n=15), and RUPP rats treated with etanerecept (n=20) at day 19 of gestation. *P<0.05 vs control pregnant rats; all of the data are expressed as means±SEMs.
TNF-α Levels in Control and Etanerecept-Treated NP and RUPP Rats
Circulating levels of TNF-α were significantly higher in RUPP rats (40±7.6 pg/mL) compared with NP rats (14.8±3.3 pg/mL; Figure 1) but was decreased to 17.2±3.0 pg/mL with administration of etanerecept. TNF-α messenger RNA was higher in the renal cortex and medulla of RUPP rats compared with NP rats (Figure 2). Placental TNF-α messenger RNA and protein were higher in RUPP rats compared with NP rats (Figure 3).
Figure 2.
Renal TNF-α messenger RNA level in RUPP rats (n=15) vs NP rats (n=14). *P<0.05 vs control pregnant rats. All of the data are expressed as means±SEMs.
Figure 3.
Placental levels of TNF-α mRNA are elevated in RUPP rats (n=15) vs NP rats (n=14). TNF-α protein is elevated in placental tissue lysates in RUPP (n=5) vs NP (n=3) rats. *P<0.05 vs control pregnant rats. All of the data are expressed as means±SEMs.
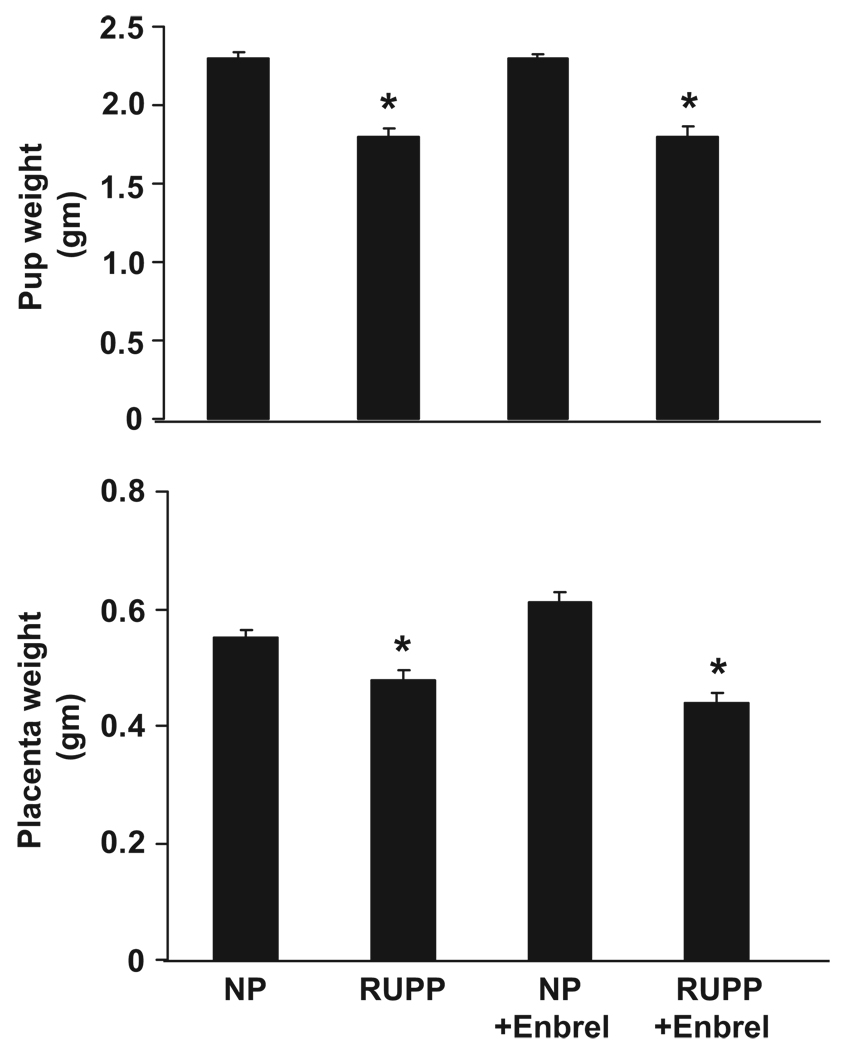
Pup and Placental Weights Decrease in Response to RUPP in Pregnant Rats and Were Unchanged by Administration of Etanerecept
Pup and placental weights are less in RUPP pregnant rats compared with control NP rats (Figure 4). Administration of etanerecept had no effect on pup or placental weights in either RUPP or control pregnant rats. Only viable pups were counted and weighed, and the average from each group is demonstrated in Figure 4. The average weight for pups from NP rats was 2.3±0.04 g and was 2.3±0.03 g in NP rats treated with etanerecept. The average weight of pups from RUPP rats was 1.88±0.04 g and was 1.88±0.07 g in RUPP rats treated with etanerecept. Placental weights in NP rats averaged 0.55±0.02 g and were 0.61±0.02 g in NP rats treated with etanerecept. Placental weights in RUPP rats averaged 0.48±0.02 g and were 0.44±0.02 g in RUPP rats treated with etanerecept (Figure 4).
Figure 4.
Pup weights and placental weights in control pregnant rats (n=16), RUPP rats (n=24), control pregnant rats treated with etanerecept (n=15), and RUPP rats treated with etanerecept (n=20) at day 19 of gestation.*P<0.05 vs control pregnant rats. All of the data are expressed as means±SEMs.
Effect of RUPP and Etanerecept on Local Production of Tissue Endothelin
Real-time PCR was used to measure preproendothelin in the renal cortex and medulla and the placenta of NP or RUPP rats. Preproendothelin was increased 3-fold in the cortex from RUPP rats compared with NP rats (P<0.02) and was not significantly decreased in RUPP rats treated with etanerecept (Figure 5). Expression of preproendothelin in RUPP medullas was significantly greater than NP rats and was not significantly different in the medulla of RUPP rats treated with etanerecept (Figure 5).
Figure 5.
Preproendothelin levels in the cortex, medulla, and placenta of control pregnant rats (n=5, n=9, n=7, respectively), NP rats treated with etanerecept (n=6, n=4, n=5, respectively), RUPP rats (n=6, P<0.02; n=7, P<0.004; n=6, P<0.011, respectively), and RUPP rats treated with etanerecept (n=9, n=5, n=10, respectively) at day 19 of gestation. *P<0.05 vs control pregnant rats. All of the data are expressed as means±SEMs.
Likewise, preproendothelin increased 4-fold in the placenta of RUPP rats compared with NP rats, and administration of etanerecept did not effect preproendothelin expression in NP rats. Although treatment of RUPP rats with etanerecept decreased placental levels of preproendothelin compared with control RUPP, the difference was not statistically significant (Figure 5). These data indicate that elevations in TNF-α in response to placental ischemia contribute to increased transcription of ET-1 in both the kidney and placentas of pregnant RUPP rats; however, additional factors influencing ET-1 expression exist in RUPP rats and play an equally important role as TNF-α.
Effect of Sera From RUPP Rats and/or Etanerecept-Treated RUPP Rats on Endothelin Production in Endothelial Cells
We demonstrated previously a 2-fold increase in ET-1 secretion, a marker for endothelial cell activation, from endothelial cells in response to 6 hours of exposure to serum from RUPP rats compared with serum from NP rats.15 After an 18-hour incubation, the ET-1 response increased 3-fold in response to RUPP-induced placental ischemia compared with NP rats. To examine the role of endogenous TNF-α in mediating endothelial activation and dysfunction in response to placental ischemia, we exposed HUVECs to serum collected from control RUPP rat serum from RUPP rats with exogenous etanerecept added to the experimental medium and RUPP rats treated with etanerecept. Samples were taken at 6 hours of cultivation after exposure to rat serum. In contrast to previous studies, there was increased mortality of HUVECs to rat serum, and 18-hour time points could not be collected. The ET-1 response of HUVECs to serum from rats with placental ischemia is significantly increased compared with ET-1 response of HUVECs to serum from NP rats after 6 hours of cultivation (59.2±16.5 pg/mg, n=10, versus 15.6±1.9, n=14; P<0.001). To determine the direct response to etanerecept on endothelial cells exposed to RUPP and NP serum, etanerecept was added to the experimental medium (10 µg/mL). Figure 6 illustrates that ET-1 secretion decreased in response to exogenous etanerecept added to experimental medium containing RUPP serum to the same degree as the HUVEC response to RUPP rats treated with etanerecept. Finally, HUVEC response to serum from NP rats was not different from the HUVEC response to exogenous etanerecept added to experimental medium containing NP or the HUVEC response to serum collected from NP rats treated with etanerecept (Figure 6) These results indicate that decreasing circulating TNF-α in pregnant rats with placental ischemia may provide protection, at the cellular level, against the actions of TNF-α to induce endothelial cell activation and dysfunction.
Figure 6.
ET-1 secretion from HUVECS at baseline and in response to etanerecept (E), to sera collected from control pregnant rats (n=14), control NP rats treated with etanerecept (n=8), sera from control pregnant rats with exogenous etanerecept added to the experimental media (n=12), RUPP rats (n=10; P<0.001), sera from RUPP rats treated with etanerecept (n=14), and sera from RUPP rats with exogenous etanerecept added to the experimental media (n=8). *P<0.001. All of the data are expressed as means±SEMs.
Discussion
It is well known that TNF-α plays an important role in mediating many chronic inflammatory diseases, such as rheumatoid arthritis, Crohn disease, ankylosing spondylitis, juvenile chronic arthritis, and psoriatic arthritis.18–20 Most recently, a role for TNF-α in mediating the endothelial dysfunction associated with the pathogenesis of a number of cardiovascular diseases, including hypertension, has become clear.21–23 An increase in the understanding of immunologic processes, such as receptor recognition and signaling, has lead to new chemotherapeutic agents targeting these cellular processes. TNF-α inhibitors varying from monoclonal antibodies to fusion proteins containing the soluble receptor such as etanerecept (Enbrel) have proven to be beneficial in the treatment of rheumatoid arthritis20 and to markedly improve the vasodilator capacity in patients with chronic heart failure.21 These inhibitors have also been used as experimental tools by investigators to quantify the relative importance of cytokines in the pathogenesis of cardiovascular disease. For example, Togashi et al24 reported recently that a TNF-converting enzyme inhibitor was effective in abolishing the hypertension in fructose-fed rats.
Although inflammatory cytokines such as TNF-α have been reported to be elevated in preeclamptic women,7,8 the importance of these cytokines in mediating the cardiovascular and endothelial dysfunction in response to placental ischemia during pregnancy has yet to be determined. We reported previously that chronic RUPP in the pregnant rat increases arterial pressure and impairs endothelial function.25 Here we demonstrated elevated TNF-α transcript in the renal cortex and medulla of RUPP rats compared with NP rats. We have reported previously that RUPP in pregnant rats results in a chronic inflammatory response, characterized by elevated previously and immune infiltrates in the placenta.12,26 We reported recently that chronic infusion of recently into pregnant rats increases arterial pressure and causes endothelial dysfunction.12,26 This current study was designed to examine the role of endogenous recently in mediating the increase in ET-1 and arterial pressure in response to placental ischemia. The soluble TNF-α receptor etanerecept was administered on day 18 of gestation to NP and to RUPP pregnant rats. Neither blood pressure nor circulating TNF-α in NP rats was altered by administration of etanerecept on day 18. In contrast, MAP was significantly lower in RUPP rats when circulating TNF-α levels were significantly decreased by the administration of etanerecept. Hypertension in response to RUPP is associated with increases in interleukin 6, agonistic autoantibodies to the angiotensin II type 1 receptor, and decreases in endothelial-dependent relaxation factors, and alterations in these mechanisms may lend an explanation as to why etanerecept did not completely abolish the blood pressure response to reductions in uterine perfusion. Nevertheless, the findings of the present study support an important role for TNF-α in the hypertensive response to RUPP in pregnant rats.
Cytokines such as TNF-α have been shown to directly increase transcription of the vasoconstrictor peptide endothelin, ET-1, from cultured endothelial cells.27 Because endothelial damage is a known stimulus for ET-1 synthesis, increases in the production of ET-1 and activation of ETA receptors have been proposed to participate in the pathophysiology of hypertension during preeclampsia.28 A role for ET-1 in mediating the pathophysiology induced by placental ischemia has been demonstrated by previous studies performed in our laboratory. In addition, hypertension in response to TNF-α during pregnancy is associated with significant increases in local production of ET-1 in the kidney, placenta, and vasculature.13 Furthermore, the increase in MAP in response to placental ischemia and TNF-α was completely abolished with an ETA receptor antagonist.13
Whether inhibition of TNF-α in response to placental ischemia in RUPP rats could lead to alterations in ET-1 levels had not been examined previously. However, Elmarakby et al29 reported recently that etanerecept lowered renal ET-1 production in deoxycorticosterone acetate+0.9% salt hypertensive rats. In the current study we found that ET-1 transcript, via real-time PCR, was indeed significantly elevated in cortices, medulla, and placenta of RUPP rats as compared with NP rats. In additional support of previous findings, we found that the ET-1 transcript was lower, albeit not significantly, in the cortex, medulla, and the placenta of RUPP rats treated with etanerecept, supporting a role for TNF-α stimulation of local ET-1 production. However, we recognize that hypertension in response to placental ischemia in RUPP rats is a multifactoral response with elevations in other inflammatory cytokines, production of angiotensin II type 1-antibody, and a decline in vasodilatory actions of the endothelium. Therefore, we suggest that the increase in local ET-1 transcript is but one mechanism whereby TNF-α could increase arterial pressure in response to placental ischemia and that factors in addition to TNF-α activate the ET-1 system in response to placental ischemia. Furthermore, we demonstrated that ET-1 protein secretion from cultured endothelial cells exposed to serum from RUPP rats was significantly decreased with administration of etanerecept to the experimental culture medium, as well as administration etanerecept to RUPP rats in vivo. These results support a potential role of TNF-α to orchestrate endothelial activation or dysfunction either directly or by some intermediary pathway. In conclusion, although these data provide additional support linking ET-1 production via TNF-α– stimulated pathways with hypertension in response to RUPP in pregnant rats, they also indicate the importance of additional factors in stimulating ET-1 as a mediator of hypertension during pregnancy.
Perspectives
Increases in inflammatory cytokines have been suggested to be an important link among placental ischemia, endothelial dysfunction, and hypertension in women with preeclampsia. Although previous studies have demonstrated that cytokines such as TNF-α are elevated in preeclamptic women and in pregnant rats with RUPP, the importance of these changes in circulating levels of TNF-α in mediating increases in arterial pressure and in causing endothelial cell activation has been unclear. Using a selective TNF-α inhibitor, we found that etanerecept was not only effective in lowering blood pressure but also in dampening the ET-1 transcript that is typically observed in response to RUPP in pregnant rats. Whether etanerecept and other cytokine inhibitors would have beneficial effects in preeclamptic women remains unknown, because elevated levels of cytokines and exaggerated inflammatory responses have been reported in some but not all studies. Interestingly, administration of etanerecept did not alter pup or placental weight in RUPP or NP rats. These findings are consistent with results from studies in pregnant women given etanerecept and other cytokine inhibitors in the treatment of rheumatological diseases.
Although soluble TNF-α antagonists have proven beneficial in the treatment of chronic inflammatory diseases and may be a potential treatment for improving endothelial function during cardiovascular disease, the efficacy of TNF-α inhibitors in treating preeclampsia will not be answered until well-controlled clinical studies are performed in pregnant women.
Acknowledgments
Sources of Funding
This work was supported in part by an individual National Research Service Award (HL78147 to B.L.) and the National Institutes of Health (HL051971 to J.P.G.).
Footnotes
This paper was sent to Friedrich C. Luft, associate editor, for review by expert referees, editorial decision, and final disposition.
Disclosures
None.
References
- 1.Minagawa M, Narita J, Tada T, Maruyama S, Shimizu T, Bannai M, Oya H, Hatakeyama K, Abo T. Mechanisms underlying immunologic states during pregnancy: possible association of the sympathetic nervous system. Cell Immunol. 1999;196:1–13. doi: 10.1006/cimm.1999.1541. [DOI] [PubMed] [Google Scholar]
- 2.Fisher SJ, Martin JM. Defects in placentation and placental perfusion. In: Lindheimer M, Roberts JM, Cunningham FG, editors. Chesley’s Hypertensive Disorders in Pregnancy. II Ed. Stamford, CT: Appleton and Lange; 1998. pp. 377–394. [Google Scholar]
- 3.Ashworth JR, Baker PN, Warrend AY. Plasma from preeclamptic women induces a functional change in myometrial resisitance arteries. J Soc Gynecol Invest. 1997;4:71. [Google Scholar]
- 4.August P, Lindheimer MD. Pathophysiology of preeclampsia. Hypertension. 1995;142:2407–2426. [Google Scholar]
- 5.Roberts JM, Lain KY. Recent Insights into the pathogenesis of preeclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- 6.Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States. Am J Obstet Gynecol. 1990;163:460–465. doi: 10.1016/0002-9378(90)91176-d. [DOI] [PubMed] [Google Scholar]
- 7.Benyo DF, Miles TM, Conrad KP. Hypoxia stimulates cytokine production by Villous explants from the human placenta. J Clin Endocrinol Metab. 1997;82:1582–1588. doi: 10.1210/jcem.82.5.3916. [DOI] [PubMed] [Google Scholar]
- 8.Conrad KP, Benyo DF. Placental cytokines and the pathogenesis of preeclampsia. Am J Reprod Immun. 1997;37:240–249. doi: 10.1111/j.1600-0897.1997.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 9.Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens. 1991;4:700–708. doi: 10.1093/ajh/4.8.700. [DOI] [PubMed] [Google Scholar]
- 10.Pober JS, Coltran RS. Cytokines and endothelial cell biology. Physiol Rev. 1990;70:427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- 11.Granger JP, LaMarca BBD, Cockrell K, Sedeek M, Balzi C, Chandler D, Bennett W. Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol Med. 2006;122:383–392. doi: 10.1385/1-59259-989-3:381. [DOI] [PubMed] [Google Scholar]
- 12.LaMarca BD, Bennett WA, Alexander BT, Cockrell K, Granger JP. Hypertension produced by reductions in uterine perfusion in the pregnant rat: role of tumor necrosis factor alpha. Hypertension. 2005;46:1022–1025. doi: 10.1161/01.HYP.0000175476.26719.36. [DOI] [PubMed] [Google Scholar]
- 13.LaMarca BB, Cockrell K, Sullivan E, Bennett W, Granger JP. Role of endothelin in mediating tumor necrosis factor-induced hypertension in pregnant rats. Hypertension. 2005;46:82–86. doi: 10.1161/01.HYP.0000169152.59854.36. [DOI] [PubMed] [Google Scholar]
- 14.Alexander BT, Rinewalt AN, Cockrell KL, Bennett WA, Granger JP. Endothelin-A receptor blockade attenuates the hypertension in response to chronic reductions in uterine perfusion pressure. Hypertension. 2001;37:485–489. doi: 10.1161/01.hyp.37.2.485. [DOI] [PubMed] [Google Scholar]
- 15.Roberts L, LaMarca B, Fournier L, Bain J, Cockrell K, Granger JP. Enhanced endothelin synthesis by endothelial cells exposed to sera from pregnant rats with decreased uterine perfusion. Hypertension. 2006;47:615–618. doi: 10.1161/01.HYP.0000197950.42301.dd. [DOI] [PubMed] [Google Scholar]
- 16.Mogolkoc R. etanerecept treatment in the endotoxin-induced uveitis of rats. Exp Eye Res. 2004;79:357–365. doi: 10.1016/j.exer.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Arenas AI, Armstrong SJ, Xu Y, Davidge ST. Chronic TNF–α inhibition enhances nitric oxide modulation of vascular function in estrogen deficient rats. Hypertension. 2005;46:76–81. doi: 10.1161/01.HYP.0000168925.98963.ef. [DOI] [PubMed] [Google Scholar]
- 18.Kalarz B, Targonska-Stepniak B, Darmochwal-Kolarz C, Majdan M. Autoimmune aspects of treatment with TNF-alpha inhibitors. Postepy Hig Med Dosw. 2007;28:478–484. [PubMed] [Google Scholar]
- 19.Agirbasli M, Inanc N, Byakan OA, Direskeneli H. The effects of TNF alpha inhibition on plasma fibrinolytic balance in patients with chronic inflammatory rheumatical disorders. Clin Exp Rheumatol. 2006;24:580–583. [PubMed] [Google Scholar]
- 20.Hamilton K, Clair EW. Tumor necrosis factor-α blockade: a new era for effective management of rheumatoid arthritis. Expert Opin Pharmacother. 2000;1:1041–1052. doi: 10.1517/14656566.1.5.1041. [DOI] [PubMed] [Google Scholar]
- 21.Fichtlscherer S, Rossig L, Breuere S, Vasa M, Dimmeler S, Zeiher AM. Tumor necrosis factor antagonism with etanerecept improves systemic endothelal vasoreactivity in patients with advanced heart failure. Circulation. 2001;25:3023–3025. doi: 10.1161/hc5001.101749. [DOI] [PubMed] [Google Scholar]
- 22.Granger JP. Inflammatory cytokines, vascular function, and hypertension. Am J Regul Intgr Comp Physiol. 2004;286:R989–R990. doi: 10.1152/ajpregu.00157.2004. [DOI] [PubMed] [Google Scholar]
- 23.Wang P, Ba ZF, Chaudry IH. Administration of TNF alpha in vivo depresses endothelium-dependent relaxation. Am J Physiol. 1994;266:H2535–H2541. doi: 10.1152/ajpheart.1994.266.6.H2535. [DOI] [PubMed] [Google Scholar]
- 24.Togashi N, Ura N, Higashiura K, Murakami H, Shimamoto K. Effect of TNF-alpha-converting enzyme inhibitor on insulin resistance in fructose-fed rats. Hypertension. 2002;39:578–580. doi: 10.1161/hy0202.103290. [DOI] [PubMed] [Google Scholar]
- 25.Khalil RA, Granger JP. Vascular mechanisms of increased arterial pressure in preeclampsia: lessons from animal models. Am J Physiol Regul Integr Comp Physiol. 2002;283:R29–R45. doi: 10.1152/ajpregu.00762.2001. [DOI] [PubMed] [Google Scholar]
- 26.LaMarca BD, Ryan MJ, Granger JP. Pathophysiology of hypertension during preeclampsia: role of inflammatory cytokines. Curr Hypertens Rev. 2007;3:1. doi: 10.1007/s11906-007-0088-1. [DOI] [PubMed] [Google Scholar]
- 27.Marsden PA, Brenner BM. Transcriptional regulation of the endothelin-1 gene by TNF-α. Am J Physiol. 1992;262:C854–C861. doi: 10.1152/ajpcell.1992.262.4.C854. [DOI] [PubMed] [Google Scholar]
- 28.Taylor RN, Varma M, Teng NN, Roberts JM. Women with preeclampsia have higher plasma endothelin levels than women with normal pregnancies. J Clin Endocrinol Metab. 1990;71:1675–1677. doi: 10.1210/jcem-71-6-1675. [DOI] [PubMed] [Google Scholar]
- 29.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-alpha inhibition reduces renal inflammation in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R76–R83. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]