Abstract
Regulation of cell differentiation and assembly remains a fundamental question in developmental biology. During development, tissues emerge from coordinated sequences of the renewal, differentiation, and assembly of stem cells. Likewise, regeneration of an adult tissue is driven by the migration and differentiation of repair cells. The fields of stem cells and regenerative medicine are starting to realize how important is the entire context of the cell environment, with the presence of other cells, three-dimensional matrices, and sequences of molecular and physical morphogens. The premise is that to unlock the full potential of stem cells, at least some aspects of the dynamic environments normally present in vivo need to be reconstructed in experimental systems used in vitro. We review here some recent work that utilized engineered environments for guiding the embryonic and adult human stem cells, and focus on vasculogenesis as a critical and universally important aspect of tissue development and regeneration.
Keywords: tissue engineering, human stem cells, scaffold, vasculogenesis
INTRODUCTION
With the aging population and increasing expectations for an improved quality of life, there is a growing need to develop new treatment modalities that are based on stem cells and can repair, replace, or regenerate a missing or defective tissue. Tissue engineering is responding to this need by developing methods for coaxing the stem cells toward predictive and controllable regeneration of functional tissue structures. At the same time, engineered tissues are finding new roles as models for fundamental research, studies of disease, testing of drugs, and many other applications (see Fig. 1).
Figure 1.
Microenvironments for adult and embryonic stem cells. Two distinct populations of stem cells—embryonic and adult—have emerged as promising for studying vascularization both in vitro and in vivo. Embryonic stem cells are expanded on both live feeder cell layers and defined substrates. Three-dimensional hydrogels have been used to direct and study differentiation of embryonic cells to vascular populations. Adult stem cells have been studied largely in the context of in vivo repair. The mechanisms driving their known ability to induce vascularization of tissue have been studied in various scaffolds in vitro. Hydrogels and biological matrices represent novel sources for controlling biological cues delivered to cells, while providing appropriate platforms for delivery of the cells to the in vivo setting. Various culture systems have been developed to culture stem cells in 3D environments, using biophysical stimulation. These culture systems must continue to be explored to condition cell-scaffold structures to develop functional tissue engineered constructs for in vivo delivery of stem cells to mediate repair.
In general, the designs of tissue engineered systems are inspired by biology, in an attempt to create “biomimetic” environments suitable to provide the cells with the environmental cues—molecular, structural, and physical—and thereby unlock their full potential for tissue development and regeneration. To direct cells to differentiate at the right time, in the right place, and into the right phenotype, one needs to recreate the right environment, with biology and engineering interacting at multiple levels. Tissue engineering thus opens several exciting possibilities: (1) to create functional grafts suitable for implantation and repair of failing tissues, (2) to study stem cell behavior and developmental processes in the context of controllable three dimensional (3D) models of engineered tissues, and (3) to utilize engineered tissues as models for studies of physiology and disease.
Culture environments that capture the molecular, structural, and physical factors regulating cellular processes are instrumental toward these goals. We review representative recent studies of human stem cells that utilized biomaterial scaffolds designed to mimic some aspects of the native developmental milieu.
3D ENVIRONMENTS
To fully elucidate the potential of stem cells for disease repair, it is necessary to provide experimental environments that mimic the physiological milieu in developing and adult tissues. Early methods for cultivating stem cells were restricted to simple two-dimensional settings, whereas in the physiological milieu differentiation occurs in a dynamically changing 3D domain. Most of the current in vitro methods for studying cell behavior and function do not recapitulate the in vivo environment in a way that allows researchers to understand and predict cell behavior in vivo. Researchers have sought to recapitulate the physiological architecture by directed differentiation in embryoid bodies and by using 3D matrices for the maintenance, proliferation, and differentiation of stem cells (Burdick and Vunjak-Novakovic, 2008).
In vivo, cells are surrounded by an extracellular matrix (ECM) which mediates cell–cell communication and provides appropriate biological cues to cells. The architecture of the ECM is responsible for long-term order in highly organized tissues. Various mechanical properties including stiffness and elasticity are determinants of cell function and differentiation. The ECM undergoes constant remodeling by cells, and disease processes are characterized by cell-mediated remodeling that, in the case of ischemia, leads to a turnover of the ECM to stiff and fibrous environments, which in turn negatively affect cell function.
To better predict and understand cell behavior, culturing cells in 3D scaffolds present an in vitro alternative to reconstructing the architecture and spatial domain that is seen in vivo. The growing field of biomaterials explores development of matrices that provide biological and mechanical signals to cells that are appropriate to the physiological environment they seek to mimic.
Scaffold Design Criteria
To capture the physiological milieu in a 3D culture environment, several criteria must be met. Importantly, the emerging field of tissue engineering seeks to both develop in vitro models and functional tissue replacements. Thus, it is important that 3D environments explored for the culture of stem cells in vitro may later be translated into a viable graft for cell delivery in vivo.
The first specification for 3D environments is the use of bio-compatible materials and chemical substances that do not prove detrimental or negatively alter cellular genotype. Importantly, materials tolerated by cells in controlled in vitro settings are not necessarily tolerated in the much more complicated in vivo environments. For in vivo use, biomaterials must not elicit an immune response and its reactivity and degradation properties in the body must be fully understood.
Ideally, tissue replacements are created by culturing cells on 3D scaffolds to develop functionality and then implanted in vivo. Depending on what tissue is to be replaced, the properties of scaffolds will vary along several parameters, including biological substances used, porosity, elasticity, stiffness, and specific anatomical shapes. Cultivation in vitro requires appropriate methods for delivering oxygen and nutrients to the cells (Radisic et al., 2005, 2006), including in particular perfusion of culture medium through constructs (Grayson et al., 2008; Radisic et al., 2008).
Scaffold Fabrication
Extracellular matrix components have been made into various matrices, including hydrogels and porous scaffolds, using a variety of methods. The field of biomaterials is enormous, and we may characterize the great variety of scaffolds based on fabrication method, structural properties, and material derivation.
Synthetic matrices made from polymers, including poly(ethylene glycol) (PEG) and poly(lactide-co-glycotide) (PLGA), have been explored as scaffolds for tissue engineering, because of their easily controlled and reproducible properties. Their use, however, is often accompanied by surface modifications to enhance cell adhesion. Additionally, unlike biologically derived matrices, these materials do not have the advantage of providing biological signaling.
Naturally derived materials, including silk, collagen, fibrin, and hyaluronic acid, can be used to make hydrogels. Gels made from liquid solutions polymerize via a chemical reaction (change in pH, redox reaction, or use of photoinitiators) or mechanically (sonication). Formation of hydrogels from a liquid suspension allows for cell encapsulation in the hydrogel before polymerization, as opposed to seeding cells onto 3D constructs. Naturally derived matrices may also be used to make electrospun scaffolds and porous scaffolds, as is done with silk. This allows for greater control over scaffold architecture and pore size.
Biologically derived matrices may enable a new approach for studying the microenvironment. Researchers in the Badylak laboratory have long investigated the role of ECM components in wound repair (Badylak, 2007), including in particular the ECM derived from decellularized tissues (Gilbert et al., 2006). They have used the ECM powder derived from decellularized porcine urinary bladder to develop an ECM-based hydrogel (Freytes et al., 2008). Extension of this technology to forming ECM-gels derived from various tissues could provide great insight into the differences in ECM composition among different organs in the body. For example, it may be useful to study cardiac differentiation of cells in an ECM derived from heart tissue.
More recently, whole organ decellularization and subsequent repopulation with rat myocytes were reported (Ott et al., 2008), demonstrating the ability to replace cells in a native environment. Small decellularized scaffolds, rather than entire decellularized organs, derived from native tissues, may provide a promising matrix for tissue engineering applications, by virtue of their appropriate biological cues, architecture, and mechanical properties.
Scaffold Modifications
Several studies have used scaffolds as a structure to provide signals to cells to improve cell adhesion and alter their behavior. RGD-peptides have been widely used as molecules tethered to scaffolds to increase cell adhesion (Burdick and Anseth, 2002). Various integrins have been investigated for promoting cell–matrix adhesion to otherwise nonadhesive matrices.
Ferreira et al. (2007a) developed “bioactive” hydrogels for the controlled release of growth factors (Ferreira et al., 2007a). It is possible to microencapsulate growth factors within a hydrogel, and depending on degradation rate of the hydrogel, growth factor release kinetics may be finely controlled. Thus, these scaffolds not only provide spatial control, but additionally have control over biochemical signaling, in the temporal domain. Such technology brings us a step closer to mimicking the physiological setting. This will be particularly interesting for studying stem cell differentiation, as the embryonic environment is characterized by highly controlled spatial and temporal signaling of growth factors. Control over biochemical signaling to cells is important for both in vitro studies of cell differentiation and in vivo therapies, where sustained release over a bolus injection of a drug is necessary to mediate repair.
TWO DIMENSIONAL (2D) SYSTEMS FOR DERIVATION AND PROPAGATION OF HUMAN EMBRYONIC STEM CELLS
Pluripotent human embryonic stem cells (hESCs) are characterized by their ability to proliferate in an unlimited manner while maintaining an undifferentiated state with normal karyotype. Derivation of hESCs typically occurs by extraction of totipotent cells from the blastocyst (Evans and Kaufman, 1981; Thomson et al., 1998).
Propagation of undifferentiated hESCs requires a specific micro-environment, which can be established in vitro by utilizing combinations of the following:
an appropriate substrate for the attachment of hESC colonies (such as those consisting of extracellular matrix proteins: laminin, fibronectin, vitronectin) (Xu et al., 2001; Ludwig et al., 2006; Braam et al., 2008);
regulatory molecules, produced by the feeder layer cells as paracrine factors and/or supplemented in the culture medium (Xu et al., 2001; Ludwig et al., 2006); and
autocrine factors and signals arising from the cell–cell interactions, provided by the maintenance of hESC in clumps of 10 or more cells.
Table 1 gives an overview of key methods currently used to derive and expand hESCs in 2D culture systems.
TABLE 1.
2D Approaches to Propagation of hESCs
| Feeder layer | Feeder specifics | Reference | Passage | hESC line | Common hESC evaluations |
|---|---|---|---|---|---|
| Animal feeder layer |
Mouse embryonic fibroblasts |
Thomson et al., 1998 | <5 | H1#, H7#, H9#, H13#, H14# | |
| Richards et al., 2002 | <5 | HES-3, HES-4 | |||
| Cell line feeder layer |
STO cell line | Park et al., 2003 | Line | Miz-hES1#, Miz-hES2#, Miz-hES3# |
|
| Autogeneic feeder layer |
hESC-derived feeder cells |
Wang et al., 2005 | 5, 15 | H1, SH1, SH2, SH7# | |
| Stojkovic et al., 2005a | 2–12 | hES-NCL1, H1 | |||
| Xu et al., 2004 | Line | H7, H9 | |||
| Human feeder layer |
Human embryonic fibroblasts |
Lee et al., 2004 | 3 | Miz-hES1 | Surface antigens: immunohistochemistry, flow cytometry |
| Human fetal skin fibroblasts |
Richards et al., 2002 | 4–9 | HES-3, HES-4 | SSEA-3, SSEA-4, SSEA-1, Tra-1-60, Tra-1-81 |
|
| Richards et al., 2003 | 4–8 | HES-3, HES-4 HES-3, HES-4 |
|||
| Human fetal muscle fibroblasts |
Richards et al., 2002 | 4–9 | HES-3, HES-4 | ||
| Richards et al., 2003 | 4–16 | HES-3, HES-4 | |||
| Human fallopian tube epithelial cells |
Richards et al., 2002 | 4–9 | HES-3, HES-4 | Transcription factors: immunohistochemistry, gene expression, Oct4, Nanog, Sox2, Rex1 |
|
| Richards et al., 2003 | 4–8 | HSF1, HSF6, H1, H9 | |||
| UCSF-1#, UCSF-2# Miz-hES1 |
|||||
| Miz-hES9# Miz-hES14#, | |||||
| Human placental cells | Genbacev et al., 2005 | 7 | Miz-hES15# H1 |
||
| Human uterine endometrial cells |
Lee et al., 2004 | 3 | Enzymes: immunohistochemistry, histochemistry, gene expression, alkaline phosphatase, telomerase |
||
| Lee et al., 2005 | 3–25 | ||||
| Human marrow stromal cells |
Cheng et al., 2003 | 2–5 | |||
| Human foreskin fibroblasts |
Amit et al., 2003 | ≥25 | I–3, I–6, H–9 | Karyotype: G-banding | |
| Hovatta et al., 2003 | HS181#, HS207# | ||||
| Inzunza et al., 2005 | HS293#, HS306# | ||||
| Human breast parenchymal cells |
Lee et al., 2004 | 3 | Miz-hES1 | Embryoid body differentiation: immunohistochemistry, gene expression |
|
| Human skin fibroblasts | Richards et al., 2003 | 4–16 | HES-3, HES-4 | Lineage-specific markers |
| Substrate | Substrate specifics | Reference | Media supplements | hESC line | Common hESC evaluations |
|---|---|---|---|---|---|
| Extracellular Matrix |
Human serum Matrigel | Stojkovic et al., 2005b | cond-AFL | hES-NCL1, H1 | Teratoma formation: histology |
| Xu et al., 2001 | cond-MEF | H1, H7, H9, H14 | Microbiological status: sterility testing, mycoplasma testing |
||
| Braam et al., 2008 | cond-MEF, mTESR1 | HUES1 | |||
| Stojkovic et al., 2005a | cond-AFL | hES-NCL1, H1 | |||
| Ludwig et al., 2006 | TeSR1 | H1, H7, H9, H14 | |||
| Laminin | Xu et al., 2001 | cond-MEF | H1, H7, H9, H14 | ||
| Braam et al., 2008 | cond-MEF | HUES1 | |||
| Fibronectin | Amit et al., 2004 | SR, TGFβ1, LIF, bFGF | I–3, I–6, H9 | ||
| Braam et al., 2008 | cond-MEF | HUES1 | |||
| Collagen IV | Braam et al., 2008 | cond-MEF | HUES1 | ||
| Laminin, fibronectin, collagen IV, vitronectin |
Ludwig et al., 2006 | TeSR1 | H1, H9, WA15#, WA16# | ||
| Vitronectin | Braam et al., 2008 | mTeSR1 | HUES1 |
An overview of representative recent studies that illustrates the range of experimental settings and components utilized for the in vitro propagation of hESC.
hESC line designations are listed as in the original references.
marks newly derived hESC lines; MEF, mouse embryonic fibroblasts; AFL, autogeneic feeder layer; cond, conditioned medium; SR, serum replacement, TGFβ1, transforming growth factor β1; LIF, leukemia inhibitory factor; bFGF, basic fibroblast growth factor; TeSR1/mTeSR1, defined medium developed for hESC culture; SSEA, stage-specific embryonic antigen; Tra, abbreviation for antigens present on hESC; Oct4, octamer-binding transcription factor 4; Sox2, SRY-related HMG-box gene 2; Rex-1, RNA exonuclease 1.
Conventional 2D systems for propagation of hESCs involve their culture on inactivated mouse embryonic fibroblasts (MEFs) as feeder layers, in culture medium containing serum or serum replacements, basic fibroblast growth factor (bFGF), and other supplements (Thomson et al., 1998, NSCB Technical Support 2008a, b, Lerou, 2008). Under these conditions, hESCs seem to have the ability to self-renew indefinitely, as well as give rise to any adult cell type (Thomson et al., 1998; Reubinoff et al., 2000; Hoffman and Carpenter, 2005).
A need arose to develop an animal cell and product-free culture system to prevent exposure of human cells to xenogenic pathogens, both for fundamental studies of hESCs and for the derivation of cells for clinical application. A recent study reports that hESCs cultured in animal serum replacements on MEFs incorporate non-human sialic acid (Martin et al., 2005).
In the context of hESCs as a possible source of differentiated cells for therapeutic uses, investigations have focused on establishing defined protocols based on the use of animal product-free reagents for the derivation and propagation of hESC lines. In one approach, hESC culture has been “humanized” by substituting the mouse feeder cells with primary human cells or immortalized human cell lines (Table 1).
A variety of cell types have been shown to support undifferentiated hESC growth, including primary human foreskin fibroblasts (Hovatta et al., 2003; Inzunza et al., 2005), fetal fibroblasts (Richards et al., 2002; Lee et al., 2004), breast parenchymal cells (Lee et al., 2004), fallopian tube epithelial cells (Richards et al., 2002), uterine endometrial cells (Lee et al., 2004), placental fibroblasts (Genbacev et al., 2005), bone marrow stromal cells (Cheng et al., 2003), and cells of the STO line (Park et al., 2003). Also, autogenic feeder cells, derived from differentiating hESCs themselves, have been successfully used (Stojkovic et al., 2005; Wang et al., 2005).
In these studies that minimized the use of animal-derived culture components, hESCs maintained properties similar to those of hESC cultured on MEF, as judged from the expression of surface markers (SSEA-4, SSEA-1, TRA-1-60, TRA-1-81), transcription factors (OCT4, SOX2, NANOG), enzymes (alkaline phosphatase, telomerase), and the retention of differentiation potential when cultured in embryoid bodies or implanted to form xenografted tumors.
Much less effort has been put into the investigation of the specific characteristics of various feeder cells and the microenvironments they produce (as for example, the profiles of cytokines they secrete) in coculture with hESCs. For example, vimentin expression (indicative of mesenchymal stem cells) has been detected in placental fibroblasts and autogeneic feeders (Genbacev et al., 2005; Wang et al., 2005). Some of the mesenchymal stem cell (MSC) markers were expressed by autogeneic feeders (Stojkovic et al., 2005a), and a typical MSC marker profile was detected on bone marrow stromal feeders (Cheng et al., 2003). In one study, production of collagen, laminin, and fibronectin was found comparable for inactivated MEF and STO feeder cells (Park et al., 2003).
In an attempt to develop alternatives to the rather undefined conditions of “humanized” culture, several studies focused on determining the exact substrate and medium components that support the propagation of undifferentiated hESCs. In one of the first studies, Xu et al. (2001) have shown that hESCs can be propagated on Matrigel (a basement membrane extract of Engelbreth-Holm-Swarm mouse tumor, consisting of laminin, collagen type IV, entactin, heparin sulfate proteoglycans, and growth factors) and that laminin alone could be used as the substrate if culture medium was preconditioned on MEF.
Alternatively, cells were successfully cultured on Matrigel in culture medium conditioned on autogeneic feeder cells (Stojkovic et al., 2005a). Several studies showed the essential role of bFGF supplementation for the maintenance of hESC pluripotency (Xu et al., 2005b; Levenstein et al., 2006). Notably, medium supplementation with high concentrations of bFGF could support the hESC propagation on Matrigel without medium preconditioning on feeder cells (Xu et al., 2005a).
Another alternative is to forego feeder layers and use a serum-free environment, attained by using serum replacement in combination with a variety of cytokines. hESCs have been successfully derived and cultured on Matrigel with defined media, and they expressed normal hESC markers and maintained a normal karyotype (Ludwig et al., 2006). Alternatively, a culture of hESCs in a defined matrix will allow strict control over what components are incorporated.
In 2006, Ludwig at al. published a protocol for defined culture of hESCs that utilized protein components either derived from recombinant sources or purified from human material. Using this protocol, the investigators derived two new lines of hESCs. Unfortunately, both lines were found karyotypically abnormal after 7 months in culture (Ludwig et al., 2006), warranting further investigation. More recently, recombinant vitronectin supported hESC propagation in defined medium (i.e., collagen IV, fibronectin, and laminin were all eliminated from the substrate) (Braam et al., 2008).
Our studies have shown that substrate topography also plays a major role in hESC proliferation and morphology (alignment and elongation) and that the cytoskeleton plays an active role in response to contact guidance (Gerecht et al., 2007a). Further studies are needed to elucidate how different culture conditions affect propagation and specific differentiation potentials of various cell lines, and whether the same defined culture protocols (Ludwig et al., 2006) could be used in maintenance of embryonic stem cell-like induced pluripotent stem cells (IPCs) (Takahashi et al., 2007; Yu et al., 2007).
3D HYDROGELS FOR CULTURE OF HESCS
Propagation of hESCs
During embryogenesis, hESCs are embedded in a 3D matrix, which regulates both their self-renewal and differentiation (Czyz and Wobus, 2001). To establish a single, controllable 3D culture system, in which hESCs can be maintained as undifferentiated and differentiate in response to specific cues, we explored encapsulation of hESC lines in hydrogel scaffolds composed of a biologically recognized molecule. Hyaluronic acid (HA) is a simple, nonsulfated glycosaminoglycan consisting of straight chains of alternating disaccharide units of β-1-glucuronic acid and N-acetylglucosamine polysaccharide; HA is ubiquitously distributed in the ECM and its content is greatest during early embryogenesis (Toole, 2001).
We encapsulated hESCs in hydrogels fabricated entirely of HA and constructs were placed within MEF-conditioned medium (MEF-CM) supplemented with bFGF (Gerecht et al., 2007). Human ESCs encapsulated in HA hydrogels and cultured in MEF-CM maintained their typical undifferentiated morphology (Fig. 2A), with high levels of expression of stem cell markers SSEA4 and alkaline phosphatase (AP) (Fig. 2B). To determine if the hESC-HA interactions, and not only the 3D morphology, are critical for controlled differentiation of hESCs encapsulated in hydrogel, hESCs were also encapsulated in networks formed from a different polysaccharide, dextran, using the same methodology of photopolymerization. In contrast for the maintenance of undifferentiated hESC colonies in the HA system, dextran hydrogels induced hESC differentiation and the formation of embryoid bodies (EB) (Fig. 2C).
Figure 2.
Vasculogenesis of hESC in HA hydrogels. A: Light microscopy revealed (i) uniform distribution of hESC colonies cultured in CM and (ii) proliforative cells (XTT assay). B: Encapsulation of hESCs was compared for HA and dextran hydrogels after 15 days of culture. Light microscope images of both cultures at low and high magnifications and histological sections (hematoxylin and eosin stain) demonstrate embryoid body formation in dextran hydrogels (left panel) vs. colony arrangements of undifferentiated hESCs in HA hydrogels (right panel). C: hESCs (line H9) were encapsulated in HA hydrogel and cultured in CM or endothelial growth medium (EGM) with VEGF for one week. (i) Cell sprouting was observed after 48 hours in gels in EGM containing VEGF (arrows) compared with (ii) gels cultured in CM. After one week of differentiation, sprouting elongating cells were mainly positive for (iii) vascular α-smooth muscle actin, while some were positive for (iv) early stage endothelial marker CD34 (in-situ 3D staining of gels). Scale bars: A–C(ii) 5 100 µm; A (lower panel) C(iii–iv) 5 25 µm (Gerecht et al., 2007)
Vascular Differentiation of hESCs
Vascular differentiation of hESCs can be induced using three different methods:
Spontaneous differentiation of hESCs grown in suspension in differentiation medium to form EBs that contain various cell types of all three germ layers, in an organizational manner that parallels embryonic development (Itskovitz-Eldor et al., 2000; Gerecht-Nir et al., 2005);
2D cultivation of hESCs on ECM proteins or specific feeder layers which can induce directed differentiation towards a specific lineage (Reubinoff et al., 2000; Gerecht-Nir et al., 2003). Vascular cells can then be isolated for further differentiation and maturation or for transplantation (Levenberg et al., 2002; Wang et al., 2004; Ferreira et al., 2007a,b);
Seeding or encapsulating hESCs in 3D porous scaffolds, followed by their release from the gel, and subculture for further differentiation (Levenberg et al., 2002; Gerecht-Nir et al., 2004; Ferreira et al., 2007a,b; Gerecht et al. 2007c).
HA has a crucial role in regulating the angiogenic process. Notably, the low-molecular weight degradation products of HA (3–10 disaccharide units) stimulate endothelial cell proliferation, migration, and sprouting (Rooney et al., 1995; Slevin et al., 1998, 2002), and induce angiogenesis in the infarcted myocardium and in the chick chorioallantoic membrane (West et al., 1985). Generation of this “angiogenic” HA from naturally occurring HA is mediated by the endoglycosidase, hyaluronidase (via polysaccharide degradation), by processes associated with tissue damage, inflammatory disease, and certain types of tumors (Stern, 2003).
After we demonstrated that hESCs express hyaluronidase, and in particular hyaluronidase 2 (Gerecht et al., 2007a,b), we sought to take advantage of the HA hydrogel culture system for controlled 3D vasculogenesis. Human ESCs encapsulated in HA hydrogels were first maintained as undifferentiated (in MEF-CM), and then cultured in angiogenic differentiation induction medium (endothelial growth medium supplemented with VEGF). After 48 h, cell sprouting and elongation from hESC colonies were observed (Fig. 2i–ii). After 1 week of differentiation, staining with specific vascular markers revealed that most sprouting cells were positive for smooth muscle actin (Fig. 2Ciii), while few were positive for CD34 (Gerecht et al., 2007a,b) (Fig. 2Civ). This offers a novel approach for controlling hESC fate by switching their environmental cues from maintaining pluripotency to inducing vasculogenesis.
VASCULARIZATION AND ADULT STEM CELLS
With ischemic injury, a loss of blood (and therefore oxygen and nutrient) supply is sustained by the affected organ. Hypoperfusion leads to rapid cell death or dysfunction of dependent cells. Cardiac ischemia leads to significant loss of cardiomyocytes and subsequent hypertrophy of surviving cells, and is followed by deterioration of heart function, in many cases leading into heart failure. The heart’s limited ability to recover from ischemic injury, through revascularization and replacement of cardiomyocytes, has prompted a surge in cell therapy clinical trials aimed at restoring the heart’s function. Mixed results from these trials suggest the need for the development of predictable methods for established vascular networks, which can sustain or even promote the regeneration of the injured heart.
In a more general sense, vascularization represents an obstacle common to all translational tissue engineering—both for cultivation of viable tissue grafts in vitro and for support of graft function in vivo. Establishing vascular networks in ischemic tissue may be sufficient to initiate repair in some cases. If, however, tissue replacement is necessary, a graft’s ability to integrate with host vasculature is key to preventing necrosis, particularly in metabolically active tissues such as the heart. Numerous studies have explored vascularization in the heart and hind limb models of ischemia (Tongers et al., 2008), as well as in tissue-engineered bone (Jabbarzadeh et al., 2008) and skeletal muscle (Levenberg et al., 2005) constructs, among others.
In this section, we first introduce adult stem cells as mediators of vascularization in the heart. Second, we address the role of the microenvironment in promoting vascularization by considering (a) 3D in vitro systems for studying vascular behavior of the in vivo microenvironment of ischemic tissue to which cells are currently delivered; and (b) the development of 3D in vitro systems for vascularization, as well as (c) cell delivery platforms as an alternative approach to cell therapy.
Adult Stem Cells in Heart Repair
Multipotent adult stem cell populations have been isolated from various sources, including, but not limited to, bone-marrow (hematopoietic and mesenchymal populations) (Berardi et al., 1995; Kocher et al., 2001; Pittenger and Martin, 2004), adipose tissue (Gimble et al., 2007), and umbilical cord blood (Harris and Rogers, 2007). Additionally, iPS cell technology allows reprogramming of terminally differentiated cells into “embryonic-like” populations (Nakagawa et al., 2008; Narazaki et al., 2008; Park et al., 2008).
Thus far, clinical trials of cardiovascular cell therapy have largely utilized bone-marrow derived stem cells in both autologous and allogeneic settings. Numerous studies report their in vitro differentiation into multiple lineages, including bone, fat, cartilage, and muscle (Ashton et al., 1980; Umezawa et al., 1992;Rickard et al. 1994; Ferrari et al., 1998). Although there have been reports of myogenic differentiation from bone-marrow derived elements, the process occurs with low efficiency and is not a major source of myogenesis in vivo. It remains unclear to what extent differentiation of MSCs occurs in vivo. However, the role of MSCs as sources of paracrine factors and recruitment of endogenous vascular cells may play a more important role in cell-mediated repair (Gnecchi et al., 2006).
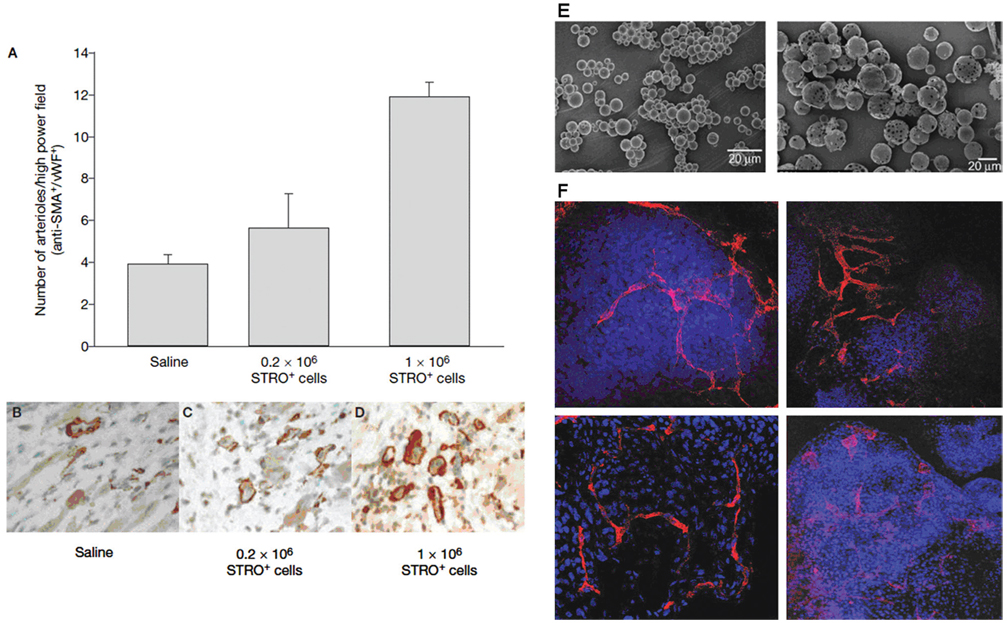
Promising results from animal studies have suggested a possible benefit of adult stem cells in repair through tissue vascularization. In vivo, several groups have reported vascular network formation following injection of MSCs (Amado et al., 2005; Martens et al., 2006) as evidenced by arteriole formation in the infarct (Fig. 3A–D). Importantly, these studies do not report differentiation of MSCs into vascular populations, but rather support their peripheral role in establishing vascular networks, either through mechanisms involving paracrine factors including SDF-1, which actively recruit endogenous cardiovascular populations (Hu et al., 2007; Xu et al., 2007; Zhang et al., 2007). To optimize the potential of MSCs to promote formation of vascular networks in vivo, it will be necessary to investigate methods of cultivation and delivery of cells.
Figure 3.
Vasculogenesis in adult human mesenchymal stem cells. (A–D) Stro-1 selected mesenchymal bone mononuclear cells were injected into the peri-infarct regions of ischemic athymic nude rats, two days after myocardial infarction. Animals exhibited dose dependent arteriogenesis in the heart (Martens et al., 2006). Anti-SMA, anti-smooth-muscle-actin; vWF, von Willebrand’s factor (B–D). (E) PLGA particles at Day 0 (i) and Day 10 (ii) during the release of VEGF165. (F) Endothelial markers on EBs (i, iii) and hESC aggregates encapsulated in dextran-based hydrogels (ii, iv) with 0.5 mM Acr-PEG-RGD containing 5 mg/mL of 7 mm micro-particles loaded with VEGF165. Confocal images of CD34+ (i, ii) and PECAM1+ (iii, iv) (Ferreira et al., 2007a)
In Vitro Microenvironments for Studying Vascularization
Encouraging results from in vivo studies suggest a strong role for adult stem cells in vascularization, most actively investigated with bone marrow-derived cells. To best understand the role of adult stem cells in vascularization, it will be necessary to return to the laboratory bench and study cell behavior in physiologically relevant settings.
In 2D settings, the “Matrigel assay” remains a standard for identification of a cell’s vascular potential. Endothelial cells cultured over the ECM-derived material readily assemble into “tube-like” structures. Culture of various stem cells alone or in cocultures with endothelial cells provides a means to assess the “stabilization” effects of stem cells on existing vascular networks (Ferreira et al., 2007b; Traktuev et al., 2008).
In a 3D setting, various scaffolds, discussed earlier in this review, can be used to recreate physiological milieus in which appropriate signals may be imparted to cells, via mechanical properties of the matrix (stiffness, porosity, fiber alignment, elasticity) or biological cues (Ingber et al., 2006), (extracellular matrix components in scaffold for adhesion and directed differentiation; encapsulated growth factors for sustained release (Fig. 3E,F, Ferreira et al., 2007a). Customization of scaffolds allows greater control over embedded cells in both a spatial and temporal context and may therefore enable the study of these cells in a more biomimetic fashion.
Previously, Ghajar et al. (2006) used 3D fibrin scaffolds to demonstrate a mechanism for MSC mediated angiogenesis in tissue, as well as the role of the microenvironment (stiffness) on the ability of cells to form networks. Similarly, other groups (Jabbarzadeh et al., 2008) demonstrated angiogenesis in vitro using VEGF-transfected adipose-derived stem cells in PLAGA scaffolds and studied the physical effects of the matrix on cell morphology, gene expression, and differentiation.
Cell–cell communication remains pivotal in directing behavior of stem cells by affecting differentiation pathways, cell migration, and secretion of factors. Using bio-chemical signaling and the spatial control provided by 3D scaffolds, hypotheses of SDF-1 dependent migration of MSCs were validated in an in vitro model (Schantz et al., 2007). 3D scaffolds provide the unique potential to study co-cultures of MSCs with various other cells types (endothelial cells) (Steingen et al., 2008). Additionally, micropatterning technologies (Du et al., 2008) may provide a novel way for controlling cell–cell communication to further our understanding of paracrine signaling under controlled conditions.
The Radisic group recently demonstrated the use of micofabricated templates for a rapid screening method for studying co-cultures of cells, demonstrating the importance of endothelial and fibroblast cells in maintaining functional cardiac organoids in vitro (Iyer et al., 2008). Rapid screening processes are increasingly accessible to researchers and with these home-made microbioreactors (Figallo et al., 2007), it is possible to study stem cell behavior under many experimental conditions.
3D systems provide unique ways for studying the role of stem cells as mediators of repair through vasculogenesis. What will be most interesting is the transition of these scaffolds from in vitro models for studying stem cells to in vivo platforms for delivering cells to repair ischemic tissues.
Engineering Microenvironments for In Vivo Studies
Current methods of cell delivery utilize liquid-phase cell suspensions. Cells are delivered directly or to the periphery of the infarct, which may account for the massive loss or death of delivered MSCs within hours after delivery (Siminiak et al., 2006). By introducing cells to the ischemic tissue, whose microenvironment is characterized by a stiff extracellular matrix to which cell adhesion is minimal (Hou et al., 2005), with high levels of “inflammation-related factors” (Laflamme et al., 2007) and hypoxia due to lack of intact vasculature, expectations of MSCs to function properly is, at best, unlikely.
Preconditioning with prosurvival cocktails (Laflamme et al., 2007) may provide a means of initially overcoming obstacles in the ischemic environment; however, long-term survival and function remain to be addressed. 3D microenvironments may be used to study the vascular properties of MSCs in vitro (Burdick and Vunjak-Novakovic, 2008), as discussed earlier; moreover, they present an alternative platform on which cells may be delivered to an ischemic environment to mediate repair.
To provide an appropriate microenvironment to condition cells for repair and protect them in ischemic tissue, the delivery of cells within a scaffolding material may prove the most viable solution. Such cell delivery platforms may be implanted onto regions of infarcted tissue or, in the case of hydrogels, an injectable scaffold that polymerizes in situ is possible (Suuronen et al., 2006). Cell delivery platforms also provide a potential advantage over current liquid-phase cell injections by increasing the number of viable cells that are retained in the heart on engraftment.
Several groups have explored creation of cardiac patches which may be used as implantable tissues for humans (Zimmermann et al., 2002, 2006; Radisic et al., 2004a,b, 2008). Development of a patch of clinically relevant size requires addressing vascularization of that tissue. Tissue engineers are actively investigating two guiding theories on vascularization of these patches. The first would require development of a patch that remains viable in ischemic conditions, while relying on endogenous mechanisms to stimulate vascular cell infiltration and network formation within the patch. The second requires establishing vascular conduits in vitro in the construct, such that an implanted patch may readily integrate with host vasculature upon implantation. This complicated, but more promising means of providing sufficient oxygen and nutrient supply is an area of active investigation in our laboratory.
Culturing vascularized tissues has been explored via in vitro culture of cells onto perfused channeled scaffolds (Radisic et al., 2008). Microfluidics technology may play a role in emerging scaffold design (Choi et al., 2007; Hwang et al. 2008) and utilizing mixed cell populations to include vascular progenitors may encourage angiogenesis in these constructs. It has become increasingly evident that various distinct cell types are needed to maintain functionally viable engineered tissues (Iyer et al., 2008).
A final requirement for developing functional tissues for implantation is the physiological conditioning of the implant. The biomaterial (scaffold) and various biochemical signals delivered via the culture medium provide various biophysical stimuli to the cells. However, especially for active tissues like the heart, electrical and dynamic mechanical stimuli may play an important role in developing functional implants. Previously, the role of electrical stimulation has been shown to enhance tissue assembly (Radisic et al., 2004a), and more recently, the parameters of electrical signals have proven an important role in conditioning cells. The Eschenhagen group (Zimmerman et al., (2002))first used mechanical stretch as a means of conditioning rat ven tricular myocytes to beat synchronously. Additionally, continuous perfusion of engineered tissues helps maintain viability through delivery of oxygen and nutrients (Figallo et al., 2007). Developing functional tissue replacements for the heart will require (1) established vasculature of the engineered construct through fabricated conduits seeded with vascular progenitors accustomed to shear, and in the case of delivering a functional cardiac tissue, and (2) maintenance of function through conditioning in vitro, before implantation, via biophysical signaling.
SUMMARY
In all, stem cells represent a multipotent, clinically approved cell population, which has been introduced into the clinical setting most notably for cardiac repair. The demonstrated ability of human stem cells to promote tissue vascularization has been severely under-utilized due to current methods of cell delivery. Three dimensional scaffold materials may provide appropriate microenvironments to promote cell viability and vascular network formation in vivo. Introduction of these microenvironments in vivo represents the goal of tissue engineering, as these cell delivery platforms may provide the most effective manner to address current challenges in cell therapy, while maximizing the cell’s potential to mediate the repair of the harsh environments they are introduced to through vascularization.
Acknowledgments
Grant sponsor: NIH; Grant numbers: HL076485; EB002520; CO21631; Grant sponsor: University of Ljubljana
Contributor Information
Amandine F. G. Godier, Department of Biomedical Engineering, Columbia University, New York, New York.
Darja Marolt, Department of Biomedical Engineering, Columbia University, New York, New York..
Sharon Gerecht, Department of Chemical & Biomolecular Engineering, Johns Hopkins University, Baltimore, Maryland.
Urska Tajnsek, Blood Transfusion Centre, University of Ljubljana, Ljubljana, Slovenia..
Timothy P. Martens, Department of Surgery, Columbia University Medical Center, New York.
Gordana Vunjak-Novakovic, Department of Biomedical Engineering, Columbia University, New York, New York..
REFERENCES
- Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intra-myocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amit M, Margulets V, Segev H, et al. Human feeder layers for human embryonic stem cells. Biol Reprod. 2003;68:2150–2156. doi: 10.1095/biolreprod.102.012583. [DOI] [PubMed] [Google Scholar]
- Amit M, Shariki C, Margulets V, et al. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- Ashton B, Allen T, Howlett C, et al. Formation of bone and cartilage by marrow stromal cells in diffusion chambers in vivo. Clin Orthop Relat Res. 1980;151:294–307. [PubMed] [Google Scholar]
- Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Berardi AC, Wang A, Levine JD, et al. Functional isolation and characterization of human hematopoietic stem cells. Science. 1995;267:104–108. doi: 10.1126/science.7528940. [DOI] [PubMed] [Google Scholar]
- Braam SR, Zeinstra L, Litjens S, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via αVβ5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
- Burdick JA, Anseth KS. Photoen-capsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials. 2002;23:4315–4323. doi: 10.1016/s0142-9612(02)00176-x. [DOI] [PubMed] [Google Scholar]
- Burdick J, Vunjak-Novakovic G. Review: Engineered microenvironments for controlled stem cell differentiation. Tissue Eng A. doi: 10.1089/ten.tea.2008.0131. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Hammond H, Ye Z, et al. Human adult marrow cells support prolonged expansion of human embryonic stem cells in culture. Stem Cells. 2003;21:131–142. doi: 10.1634/stemcells.21-2-131. [DOI] [PubMed] [Google Scholar]
- Choi NW, Cabodi M, Held B, et al. Microfluidic scaffolds for tissue engineering. Nat Mater. 2007;6:908–915. doi: 10.1038/nmat2022. [DOI] [PubMed] [Google Scholar]
- Czyz J, Wobus A. Embryonic stem cell differentiation: The role of extracellular factors. Differentiation. 2001;68(4–5):167–174. doi: 10.1046/j.1432-0436.2001.680404.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Lo E, Ali S, et al. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proc Natl Acad Sci. 2008;105:9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella G, Angelis D, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Ferreira LS, Gerecht S, Shieh HF, et al. Bioactive hydrogel scaffolds for controllable vascular differentiation of human embryonic stem cells. Biomaterials. 2007a;28:2706–2717. doi: 10.1016/j.biomaterials.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira LS, Gerecht S, Fuller J, et al. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circulat Res. 2007b;101:286–294. doi: 10.1161/CIRCRESAHA.107.150201. [DOI] [PubMed] [Google Scholar]
- Figallo E, Cannizzaro C, Gerecht S, et al. Micro-bioreactor array for controlling cellular microenvironments. Lab Chip. 2007;7:710–719. doi: 10.1039/b700063d. [DOI] [PubMed] [Google Scholar]
- Freytes DO, Martin J, Velankar SS, et al. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630–1637. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Genbacev O, Krtolica A, Zdravkovic T, et al. Serum-free derivation of human embryonic stem cell lines on human placental fibroblast feeders. Fertil Steril. 2005;83:1517–1529. doi: 10.1016/j.fertnstert.2005.01.086. [DOI] [PubMed] [Google Scholar]
- Gerecht-Nir S, Ziskind A, Cohen S, et al. Human embryonic stem cells as an in vitro model for human vascular development and the induction of vascular differentiation. Lab Invest. 2003;83:1811–1820. doi: 10.1097/01.lab.0000106502.41391.f0. [DOI] [PubMed] [Google Scholar]
- Gerecht-Nir S, Cohen S, Ziskind A, et al. Three-dimensional porous alginate scaffolds provide a conducive environment for generation of well-vascularized embryoid bodies from human embryonic stem cells. Biotechnol Bioeng. 2004;88:313–320. doi: 10.1002/bit.20248. [DOI] [PubMed] [Google Scholar]
- Gerecht-Nir S, Dazard J, Golan-Mashiach M, et al. Vascular gene expression and phenotypic correlation during differentiation of human embryonic stem cells. Dev Dynamics. 2005;232:487–497. doi: 10.1002/dvdy.20247. [DOI] [PubMed] [Google Scholar]
- Gerecht S, Bettinger CJ, Zhang Z, et al. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials. 2007a;28:4068–4077. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecht S, Burdick JA, Ferreira LS, et al. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci. 2007b;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerecht S, Townsend SA, Pressler H, et al. A porous photocurable elastomer for cell encapsulation and culture. Biomaterials. 2007c;28:4826–4835. doi: 10.1016/j.biomaterials.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Ghajar C, Blevins K, Hughes C, et al. Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early matrix metalloproteinase upregulation. Tissue Eng. 2006;12:2875–2888. doi: 10.1089/ten.2006.12.2875. [DOI] [PubMed] [Google Scholar]
- Gilbert TW, Sellaro TL, Badylak SF, et al. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Katz AJ, Bunnell BA, et al. Adipose-derived stem cells for regenerative medicine. Circulat Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- Grayson W, Bhumiratana S, Cannizzaro C, et al. Effects of initial seeding density and Fluid perfusion rate on formation of tissue-engineered bone. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2007.0255. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D, Rogers I. Umbilical cord blood: a unique source of pluripotent stem cells for regenerative medicine. Curr Stem Cell Res Ther. 2007;2:301–309. doi: 10.2174/157488807782793790. [DOI] [PubMed] [Google Scholar]
- Hoffman LM, Carpenter MK. Characterization and culture of human embryonic stem cells. Nat Biotechnol. 2005;23:699–708. doi: 10.1038/nbt1102. [DOI] [PubMed] [Google Scholar]
- Hou D, Youssef EA-S, Brinton TJ, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112(9 Suppl):I-150–I-156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- Hovatta O, Mikkola M, Gertow K, et al. A culture system using human foreskin fibroblasts as feeder cells allows production of human embryonic stem cells. Human Reprod. 2003;18:1404–1409. doi: 10.1093/humrep/deg290. [DOI] [PubMed] [Google Scholar]
- Hu X, Dai S, Tan W, et al. Stromal cell derived factor-1{alpha} confers protection against myocardial ischemia/reperfusion injury: Role of the cardiac stromal cell derived factor-1α CXCR4 axis. Circulation. 2007;116:654–663. doi: 10.1161/CIRCULATIONAHA.106.672451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang CM, Khademhosseini A, Park Y, et al. Microfluidic chip-based fabrication of PLGA microfiber scaffolds for tissue engineering. Langmuir. 2008;24:6845–6851. doi: 10.1021/la800253b. [DOI] [PubMed] [Google Scholar]
- Ingber D, Mow V, Butler D, et al. Tissue engineering and developmental biology: Going biomimetic. Tissue Eng. 2006;12:3265–3283. doi: 10.1089/ten.2006.12.3265. [DOI] [PubMed] [Google Scholar]
- Inzunza J, Gertow K, Stromberg MA, et al. Derivation of human embryonic stem cell lines in serum replacement medium using postnatal human fibroblasts as feeder cells. Stem Cells. 2005;23:544–549. doi: 10.1634/stemcells.2004-0201. [DOI] [PubMed] [Google Scholar]
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Molec Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Chiu L, Radisic M. Microfabricated poly(ethylene glycol) templates enable rapid screening of triculture conditions for cardiac tissue engineering. J Biomed Mater Res A. doi: 10.1002/jbm.a.32014. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Jabbarzadeh E, Starnes T, Khan YM, et al. Induction of angiogenesis in tissue-engineered scaffolds designed for bone repair: A combined gene therapyâ[euro]cell transplantation approach. Proc Natl Acad Sci. 2008;105:11099–11104. doi: 10.1073/pnas.0800069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cadiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Chen KY, Naumova AV, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotech. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- Lee JB, Song JM, Lee JE, et al. Available human feeder cells for the maintenance of human embryonic stem cells. Reproduction. 2004;128:727–735. doi: 10.1530/rep.1.00415. [DOI] [PubMed] [Google Scholar]
- Lee JB, Lee JE, Park JH, et al. Establishment and maintenance of human embryonic stem cell lines on human feeder cells derived from uterine endometrium under serum-free condition. Biol Reprod. 2005;72:42–49. doi: 10.1095/biolreprod.104.033480. [DOI] [PubMed] [Google Scholar]
- Lerou PH, Yabuuchi A, Huo H, et al. Derivation and maintenance of human embryonic stem cells from poor-quality in vitro fertilization embryos. Nat Protocols. 2008;3:923–933. doi: 10.1038/nprot.2008.60. [DOI] [PubMed] [Google Scholar]
- Levenberg S, Golub J, Amit M, et al. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci. 2002;99:4391–4396. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg S, Rouwkema J, Macdonald M, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- Levenstein ME, Ludwig TE, Xu R-h, et al. Basic fibroblast growth factor support of human embryonic stem cell self-renewal. Stem Cells. 2006;24:568–574. doi: 10.1634/stemcells.2005-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig TE, Levenstein ME, Jones JM, et al. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24:185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- Martens TP, See F, Schuster MD, et al. Mesenchymal lineage precursor cells induce vascular network formation in ischemic myocardium. Nat Clin Pract Cardiovasc Med. 2006;1(S1):S18–S22. doi: 10.1038/ncpcardio0404. [DOI] [PubMed] [Google Scholar]
- Martin MJ, Muotri A, Gage F, et al. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–232. doi: 10.1038/nm1181. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanebe K, et al. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008;26:101–106. doi: 10.1038/nbt1374. [DOI] [PubMed] [Google Scholar]
- Narazaki G, Uosaki H, Teranishi M, et al. Directed and systematic differentiation of cardiovascular cells from mouse induced pluripotent stem cells. Circulation. 2008;118:498–506. doi: 10.1161/CIRCULATIONAHA.108.769562. [DOI] [PubMed] [Google Scholar]
- NSCB Technical Support a: NSCB Protocols. Available at: http://www.wicell.org. 9/30/2008.
- NSCB Technical Support b: ESI hES Cell Culture Manual. Available at: http://www.wicell.org. 9/30/2008.
- Ott HC, Matthiesen TS, Goh S-K, et al. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- Park JH, Kim SJ, Oh EJ, et al. Establishment and maintenance of human embryonic stem cells on STO, a permanently growing cell line. Biol Reprod. 2003;69:2007–2014. doi: 10.1095/biolreprod.103.017467. [DOI] [PubMed] [Google Scholar]
- Park I-H, Arora N, Huo H, et al. Disease-specific induced pluripotent stem cells. Cell. 2008 doi: 10.1016/j.cell.2008.07.041. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circulat Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- Radisic M, Park H, Shing H, et al. From the cover: Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci. 2004a;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisic M, Yang L, Boublik J, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. AJP Heart Circulat Physiol. 2004b;286:H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- Radisic M, Deen W, Langer R, et al. Mathematical model of oxygen distribution in engineered cardiac tissue with parallel channel array perfused with culture medium containing oxygen carriers. AJP Heart Circulat Physiol. 2005;288:H1278–H1289. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- Radisic M, Malda J, Epping E, et al. Oxygen gradients correlate with cell density and cell viability in engineered cardiac tissue. Biotechnol Bioeng. 2006;93:332–343. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- Radisic M, Marsano A, Maidhof R, et al. Cardiac tissue engineering using perfusion bioreactor systems. Nat. Protocols. 2008;3:719–738. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong C-Y, et al. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Richards M, Fong C-Y, Chan W-K, et al. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–936. doi: 10.1038/nbt726. [DOI] [PubMed] [Google Scholar]
- Richards M, Tan S, Fong C-Y, et al. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–556. doi: 10.1634/stemcells.21-5-546. [DOI] [PubMed] [Google Scholar]
- Rickard DJ, Sullivan TA, Shenker BJ, et al. Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev Biol. 1994;161:218–228. doi: 10.1006/dbio.1994.1022. [DOI] [PubMed] [Google Scholar]
- Rooney P, Kumar S, Ponting J, et al. The role of hyaluronan in tumour neovascularization (review) Int J Cancer. 1995;60:632–636. doi: 10.1002/ijc.2910600511. [DOI] [PubMed] [Google Scholar]
- Schantz J, Chim H, Whiteman M, et al. Cell guidance in tissue engineering: SDF-1 mediates site-directed homing of mesenchymal stem cells within three-dimensional polycaprolactone scaffolds. Tissue Eng. 2007;13:2615–2624. doi: 10.1089/ten.2006.0438. [DOI] [PubMed] [Google Scholar]
- Siminiak T, Burchardt P, Kurpisz M, et al. Postinfarction heart failure: surgical and trans-coronary-venous transplantation of autologous myoblasts. Nat Clin Pract Cardiovasc Med. 2006;3 Suppl 1:S46–S51. doi: 10.1038/ncpcardio0403. [DOI] [PubMed] [Google Scholar]
- Slevin M, Krupinski J, Kumar S, et al. Angiogenic oligosaccharides of hyaluronan induce protein tyrosine kinase activity in endothelial cells and activate a cytoplasmic signal transduction pathway resulting in proliferation. Lab Invest. 1998;78:987–1003. [PubMed] [Google Scholar]
- Slevin M, Kumar S, Gaffney J, et al. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J Biol Chem. 2002;277:41046–41059. doi: 10.1074/jbc.M109443200. [DOI] [PubMed] [Google Scholar]
- Steingen C, Brenig F, Baumgartner L, et al. Characterization of key mechanisms in transmigration and invasion of mesenchymal stem cells. J Mol Cell Cardiol. 2008;44:1072–1084. doi: 10.1016/j.yjmcc.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Stern R. Devising a pathway for hyaluronan catabolism: Are we there yet? Glycobiology. 2003;13:105R–115R. doi: 10.1093/glycob/cwg112. [DOI] [PubMed] [Google Scholar]
- Stojkovic P, Lako M, Stewart R, et al. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005a;23:306–314. doi: 10.1634/stemcells.2004-0137. [DOI] [PubMed] [Google Scholar]
- Stojkovic P, Lako M, Przyborski S, et al. Human-serum matrix supports undifferentiated growth of human embryonic stem cells. Stem Cells. 2005b;23:306–314. doi: 10.1634/stemcells.2004-0326. [DOI] [PubMed] [Google Scholar]
- Suuronen EJ, Veinot JP, Wong S, et al. Tissue-engineered injectable collagen-based matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation. 2006;114(1 Suppl):I138–I144. doi: 10.1161/CIRCULATIONAHA.105.001081. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tongers J, Roncalli JG, Losordo DW, et al. Therapeutic angiogenesis for critical limb ischemia: Microvascular therapies coming of age. Circulation. 2008;118:9–16. doi: 10.1161/CIRCULATIONAHA.108.784371. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan in morphogenesis. Semin Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- Traktuev DO, Merfeld-Clauss S, Li J, et al. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circulat Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- Umezawa A, Maruyama T, Segawa K, et al. Multipotent marrow stromal cell line is able to induce hematopoiesis in vivo. J Cell Physiol. 1992;151:197–205. doi: 10.1002/jcp.1041510125. [DOI] [PubMed] [Google Scholar]
- Wang L, Li L, Shojaei F, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41. doi: 10.1016/j.immuni.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wang Q, Fang ZF, Jin F, et al. Derivation and growing human embryonic stem cells on feeders derived from themselves. Stem Cells. 2005;23:1221–1227. doi: 10.1634/stemcells.2004-0347. [DOI] [PubMed] [Google Scholar]
- West D, Hampson I, Arnold F, et al. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotech. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Xu C, Jiang J, Sottile V, et al. Immortalized fibroblast-like cells derived from human embryonic stem cell support undifferentiated cell growth. Stem Cells. 2004;22:972–980. doi: 10.1634/stemcells.22-6-972. [DOI] [PubMed] [Google Scholar]
- Xu C, Rosler E, Jiang J, et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005a;23:315–323. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- Xu R-H, Peck RM, Li D, et al. Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat Meth. 2005b;2:185–190. doi: 10.1038/nmeth744. [DOI] [PubMed] [Google Scholar]
- Xu M, Uemura R, Dai Y, et al. In vitro and in vivo effects of bone marrow stem cells on cardiac structure and function. J Molecul Cell Cardiol. 2007;42:441–448. doi: 10.1016/j.yjmcc.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mal N, Kierdrowski M, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- Zimmermann WH, Schneiderbanger K, Schubert P, et al. Tissue engineering of a differentiated cardiac muscle construct. Circulat Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- Zimmermann W-H, Melnychenko I, Wasmeier G, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]