Abstract
Stress can trigger, intensify, and prolong drug consumption, as well as reinstate previously extinguished drug-taking behavior by directly impacting a neural circuit often referred to as a reward pathways. Animal models of drug abuse have been used to understand these neural circuits mediating stress-induced drug intake and relapse through examination of cellular and subcellular molecular mechanisms. Several types of intermittent stressors have been shown to induce cross-sensitization to psychomotor stimulants, enhance conditioned place preference under most conditions, increase self-administration of cocaine and amphetamine and induce reinstatement of heroin and cocaine seeking via activation of the mesocorticolimbic dopamine system.
Introduction
Considerable evidence points to a positive correlation between experience with stress and increased drug abuse in humans [1–6]. Substantiation of this relationship extends beyond anecdotal evidence and is further supported by experiments conducted in abstinent cocaine and alcohol abusers [7–12]. Acute administration of cortisol produces significant increases in reports of craving in cocaine dependent individuals, suggesting that cortisol can induce a state that is associated with drug seeking [13]. Rats will orally self-administer corticosterone [14], and adrenalectomy and corticosterone synthesis inhibitors reduce self-administration of psychostimulants [15;16]. Studies using rodent models of drug self-administration allow for a more systematic and intensive examination of the neurobiological mechanisms that link stress and various aspects of drug taking. Here, we will discuss the significance of stress in the addictive process, reviewing rodent studies that use the most common stressors administered in adulthood and examine the effects of these stressors on drug reinforcement, drug reinstatement, and subjective drug effects. Several types of brief intermittent stress experiences can consistently induce cross-sensitization to psychomotor stimulants, enhance conditioned place preference under most conditions, increase self-administration of cocaine and amphetamine, and induce reinstatement of heroin and cocaine seeking by acting on the ventral tegmental area (VTA)-nucleus accumbens (NAC)-medial prefrontal cortex (mPFC)-amygdala circuit., also known as the mesocorticolimbic dopamine system.
Dopamine Function and Addiction
The mesocorticolimbic dopamine system, originating in the VTA of the midbrain and connecting to the limbic system via the NAC and the amygdala, as well as to the mPFC, is activated by drugs of abuse as well as natural rewards such as palatable food, liquids and sex, with extracellular dopamine levels in the NAC increasing following acute administration [17–19]. The critical involvement of dopamine transmission in drug addiction and reward has been recognized for many years [18;20]. While the precise role of dopamine has been debated, dopamine is thought to be a key neurotransmitter involved in addiction. However, mere activation of this pathway is not sufficient for drug-seeking behavior. Therefore, dopamine release is thought merely to gate drug-seeking behavior but not to drive it [21;22], as accumbens dopamine modulates several functions related to motivated behavior, which include behavioral activation, exertion of effort, response allocation and effort-related decision making, maintenance of motivated behavior over time, responsiveness to conditioned stimuli, Pavlovian-instrumental interactions, coding of reward-prediction error, learning and cognition [22–25]. Indeed, the role of dopamine release in the NAC is more subtle and complex than merely being released as a result of intake of rewarding drugs, and different dopamine subsystems may mediate separate functions related to the behavior leading to drug delivery and the actual drug intake.
Stressors Used in Addiction Models
A paradox in need of resolution is how ostensibly aversive stress experiences promote powerfully rewarding drug consumption, activating the same or overlapping neural circuitry associated with processing reward information and seeking of natural and drug rewards [26]. Most studies in laboratory animals use intermittent electric shock pulses, applied to either the tail or feet, as well as restraint and forced swim stress, to produce stress effects that either increase drug intake or induce reinstatement of formerly extinguished drug seeking. The most attractive feature of these types of stressors is the ability to directly and precisely control all relevant stress parameters (i.e., intensity, duration, frequency, controllability), thereby greatly reducing the variability of each stress experience between subjects [27]. However, the need for stressors that translate more validly from the laboratory to real-world conditions led to the study of stressors such as maternal separation, food deprivation, or social defeat stress, either presented intermittently or continuously over an extended period of time [26]. Involuntary repeated maternal separation is an early-life stress that can trigger a cascade of neurobiological events that persist into adulthood [28;29]. Acute food deprivation stress can mimic conditions in the wild in which there is a moderate period of food shortage, an ecologically common experience for rodents [27;30]. For both rodents and humans, social status is important, and repeated social threats and intimidation can induce profound short-term and long-term changes in behavioral, cardiovascular, thermoregulatory, and chronobiological adjustments in rats, mice and tree shrews [31–34]. These intermittent stressors induce behavioral and neurobiological sequelae thought to directly affect the addiction process.
In vivo experimental models for the study of drugs with addiction liability
Behavioral Sensitization
As reviewed in detail elsewhere [35–37], behavioral sensitization is a long-term consequence of repeated exposure to a drug of abuse that is defined by an augmented locomotor response to a drug challenge. Briefly, the neuroadaptive changes that mediate behavioral sensitization to the effects of abused drugs have been postulated as a possible mechanism contributing to increased drug use [35;36]. Repeated intermittent administration of cocaine, amphetamine, morphine, phencyclidine (PCP), nicotine, and also ethanol can result in a progressive augmentation of the behavioral effects of the drug, particularly of locomotor activity [38–50]; [Fig. 1a]. Facilitated drug taking behavior can follow previous exposure to psychostimulants [51–55].
Figure 1.
The effect of repeated (a) amphetamine (1.0 mg/kg, i.p.) and (b) social defeat stress on horizontal locomotor behavior following amphetamine challenge (1.0 or 1.5 mg/kg, i.p., respectively) on day 20, 10 days after the last defeat experience. Locomotor activity 30 min after injection is shown. *P<0.05 compared with no stress or saline controls. From Yap et al. (2005).
In humans, sensitized behaviors following extended repetitive stimulant drug administration closely resemble symptoms of schizophrenia and may include psychotic symptoms of paranoia, ideas of reference (i.e., perception that irrelevant or innocuous phenomena in the world refer to them directly or have special personal significance), and auditory and visual hallucinations in otherwise healthy individuals [56;57]. However, sensitization is not always prominently exhibited by humans, and the applicability of behavioral sensitization to the human condition is confounded by retrospective reports from individuals who experienced psychosis following drug binges. Additionally, imaging studies using PET and fMRI techniques have generally shown a profile in limbic regions of reduced rather than augmented responses in cocaine addicted patients compared to controls to intravenous methylphenidate, a drug that, like cocaine, causes an increase in synaptic dopamine [58]. Still, it is a phenomenon that appears useful in studying the neural changes that occur with repeated drug or stress administration and that may contribute to the escalation of drug taking.
Exposure to stress can induce cross-sensitization to psychostimulants [Table 1], as indicated by an augmented locomotor response to a later psychostimulant challenge [59]. For example, repeated tail-pinch stress in rats results in behavioral sensitization to a later injection of amphetamine [60]. Sensitized behavioral responses to psychostimulants have also been observed after repeated foot shock [61;62], restraint [63], food restriction [30], social defeat stress [64;50]; [Fig. 1b] and maternal separation [29]. Many stress manipulations, including handling stress, swim stress, foot-shock and social defeat stress, increase the metabolic responses of dopamine neurons and change dopamine activity in the mesocorticolimbic system [61; 65–67], particularly in the NAC and prefrontal cortex (PFC) [68–75], in a way similar to drugs of abuse (reviewed in detail in [26]). The mesocorticolimbic dopamine system connects stress to subsequent escalated drug self-administration through changes in neural plasticity in this circuit [26;76].
Table 1.
Summary of emerging data using stress and in vivo models of drug addiction
| Behavioral Cross- sensitization |
Conditioned Place Preference |
Intravenous Self- administration |
Reinstatement of Drug Seeking |
|
|---|---|---|---|---|
| Electric Shock | ↑ [61;62] | ↑ morphine CPP [82;83] | ↑ morphine self- administration [126] | ↑ heroin and cocaine seeking [170;171] |
| No effect on cocaine, amphetamine or ethanol CPP [82;84] | ↑ heroin self- administration on progressive ratio schedule [127] | |||
| ↑ acquisition of cocaine self-administration [129] but see [128] | ||||
|
| ||||
| Food Deprivation | ↑ [30] | ↑ morphine CPP [86] | ↑ acquisition of heroin [131;132] | ↑ heroin and cocaine seeking [172;187] |
| ↑ cocaine CPP for 5 mg/kg but not for lower or higher doses [87] | ↑ heroin intake during daily limited access period [130] | |||
| ↑ amphetamine CPP for 0.85 mg/kg but | ↑ acquisition of amphetamine self- administration [133] | |||
| ↑ amphetamine CPP for higher doses [88] | ↑ acquisition and maintenance of cocaine self-administration [134–143] | |||
|
| ||||
| Social defeat | ↑ [50;64] | No morphine CPP in defeated or control rats [89] | ↑ cocaine self- administration under progressive ratio schedule and 24-hour binge [97;148;151] | ↑ reinstatement of morphine CPP [191] |
| ↑ cocaine CPP in mice [90] | ↑ reinstatement of alcohol seeking by cue associated with defeat [192] | |||
Conditioned place preference
As discussed in detail elsewhere [77–79], place preference or aversion is based on a Pavlovian conditioning procedure that is most often used with rodents to study the positive (rewarding) or negative (aversive) motivational effects of a particular stimulus. One of the earliest demonstrations of place preference was the observation by Olds and Milner [80] in which rats stimulated through an intracranial electrode would return to the location in which they received the stimulation. In brief, the standard procedure pairs a distinctive environmental cue (positive conditioned stimulus, CS+) with a significant event (unconditioned stimulus, US). In a simple version of the place preference paradigm, animals experience two distinct neutral environments that are subsequently paired spatially and temporally with distinct drug states (the US). The animal is later given an opportunity to choose, in the absence of the drug state, to enter and explore either environment, and the time spent in either environment is considered an index of the rewarding effect of the drug (the US). The animal’s choice to spend more time in the drug-paired environment is assumed to be an expression of the positively rewarding experience within that environment. The main advantages of conditioned place preference (CPP) are: (1) it tests animals in a drug-free state, relying on the memory of previous associations, (2) it is sensitive to both reward and aversion, (3) it is methodologically simple, (4) protocols are generally brief (ca. 2 weeks), and (5) locomotor activity can be concurrently measured, although changes in locomotor activity may confound preference measurements [78;79].
From a pharmacological perspective, one of the most serious disadvantages of this methodology is that it is difficult to produce graded dose-effect curves, with most drugs showing little effect across a range of low doses before suddenly showing a maximal effect across a range of higher doses, leaving many pharmacological questions unanswered [78;79]. Another disadvantage is dealing with a bias to one side of the apparatus, where the animal will prefer one of the two distinct contexts before conditioning occurs [77]. Despite these limitations, CPP provides good preliminary information about the rewarding effect of the environmental context associated with a drug stimulus, and the influence of stress experiences on this effect can be examined. The CPP technique is relatively inexpensive, non-invasive, and simple to use. Although the CPP method does not directly measure drug reinforcement, the concordance between CPP and self-administration studies is fairly good [81].
Results using various stressors and CPP have been intriguing, with an emerging trend towards enhancement of CPP [Table 1]. A single session of inescapable tail shock stress enhances morphine CPP [82;83], up to 200% at high doses, but not cocaine, amphetamine or ethanol CPP [82;84]. This effect of electric tail shock stress on morphine CPP is seen only when it is given 6–7 days prior to CPP training and not when given earlier (14–15 days). Repeated forced-swim stress preceding cocaine conditioning nearly doubles the subsequent place preference response in male C57Bl/6 mice [85]. Results from studies using food deprivation stress are mixed, depending on the drug used. One study showed that 3 days of food restriction greatly increases the rewarding motivational effects of morphine, 2–3 fold above the sated condition [86]. In another study, chronic food restriction to 80% of free-feeding body weight modestly increases cocaine CPP for a medium dose (5 mg/kg), but not for a lower (2.5 mg/kg) or higher (10 mg/kg) doses, highlighting the difficulty in using the CPP methodology for studying dose-dependent shifts in drug response [87]. Mild chronic food restriction of 15 g/day (ca. 90% of free-feeding body weight) enhances amphetamine CPP for a moderate dose (0.85 mg/kg), but decreases preference for two higher doses (1.7 and 3.4 mg/kg) [88]. Contrary to what is found using tail shock stress and food deprivation, social defeat stress has not been studied in a systematic manner in terms of its effect on CPP. In one study housed rats in pairs for at least 16 weeks -- enough time for the social hierarchy to be determined – only dominant, and not subordinate, rats demonstrated CPP for morphine [89], suggesting the important influence of social status on morphine-induced place conditioning. In a later study, social defeat stress-exposed C57Bl/6 mice conditioned with cocaine (15 mg/kg, s.c.) showed significant potentiation of place-preference for the drug-paired chamber over the responses of unstressed mice [90]. While CPP is enhanced by stress in most studies, it is only seen under specific stress and drug conditions.
Intravenous self-administration
The gold standard for modeling essential features of drug abuse in the laboratory is the intravenous self-administration methodology. Laboratory animals can be surgically prepared with intravenous catheters that permit automated drug injections [91]. Nearly all drugs that are addictive in humans are self-administered by laboratory animals, and drugs that are not self-administered by laboratory animals are generally not addictive in humans. Notable exceptions are LSD-like hallucinogens, although hallucinogens have not been found to cause physical or psychological dependence [92;93]. I.V. self-administration is used to study the neurobiological basis of drug taking and seeking, and to screen new psychoactive medications for possible abuse liability. This last application helps the pharmaceutical industry minimize the risk that new medications will be abused by humans.
As an individual rodent learns to self-administer drugs, there are time- and experience-dependent changes in the frequency and intensity of behavior. Thus, acquisition is a process with the essential feature of an increase in drug use over time. Indeed, acquisition is just one example of self-administration behavior in transition. Others include the escalation of drug use during extended access or “binge” conditions as physical dependence develops [94] or as brain reward systems change [64;95–100], the change in self-administration behavior when the pharmacological effects of the drug are blocked or removed (i.e., extinction, withdrawal, or compensatory increases in drug intake due to blockade of drug’s actions), and the reacquisition of behavior after a period of abstinence or extinction of previous drug taking. Both exposure to foot shock stress and social stress (e.g., [101] and [102] can facilitate acquisition of drug self-administration. Other pharmacological and environmental factors that influence acquisition are dose (e.g., [103]and [104]), availability of alternative reinforcement (e.g., [105]), sex (e.g., [106]and [107]), rat strain (e.g., [108]), developmental stage (e.g., [109]) and the presence of drug-paired sensory stimuli (e.g., [110]). Self-administration behavior during transition periods may provide unique information about individual differences in drug use that are not evident during stable drug-taking, and numerous factors, particularly specific stresses, can enhance acquisition of drug taking.
Studies using self-administration sessions of continuous access to the drug for an extended period of time or a progressive ratio schedule highlight different aspects of drug taking behavior. “Binge” self-administration in rodents is thought to mimic the compulsive drug taking behavior of human addicts. Unlike limited access conditions, prolonged access or “binges” in pre-clinical studies reveal a pattern of self-administration behavior similar to that by the compulsive drug user [111–113]. Specifically, rodent models of prolonged access to cocaine indicate an apparent shift from controlled, regulated drug intake to out-of- control or dysregulated intake [96;114]. Clinical literature suggests that the transition from recreational drug intake to compulsive drug seeking behavior is a hallmark of addiction [115]. Self-administration models of continuous access “binges” allow the animal to control the rate of consumption [113]. However, unlimited access to cocaine can, after several days or weeks, degenerate into severe toxicity resulting eventually in death [91;116;117]. While the behavior of animals given unlimited access models some important clinical features of cocaine use by human addicts, some limitations must be placed on the amount of drug the animal can administer to avoid toxicity. Some have studied the effect of binge intake by limiting access to a single session of 72 h [96] and, most often, less. Access has been examined over longer periods by limiting daily sessions to, for example, 6 [95;118] or 10 h [119;120]. Another procedure is the use of continuous discrete trials over the course of several days, as described and reviewed in detail by Olsen and Roberts in this special issue [121]. Due to the higher cumulative dose and different kinetics of binge self-administration protocols, they can produce some changes in gene expression not seen with noncontingent cocaine administration or limited access self-administration [122].
Progressive ratio schedules of reinforcement are thought to measure an animal’s motivation to self-administer a particular drug [123]. However, an equal interpretation for the progressively higher behavioral output expended in progressive ratio tests could be that of resistance to extinction (i.e. a resistance to cease responding in the absence of reward). In the simplest case, the first behavioral response is reinforced, and each subsequent reinforcement is delivered after meeting a doubling in behavioral demand. An example of this type of PR schedule would be 1, 2, 4, 8, 16, etc. The requirement increases until the behavioral demand eventually becomes so large that the subject fails to meet the response criterion for the next reinforcement within a criterion time of 30 or 60 min. The last completed ratio requirement after the subject stops being reinforced is defined as the “breaking point” or “break point.” Dynamic patterns of behavior are maintained by PR schedules of drug delivery. These response patterns reflect not only the influence of ratio requirement, but also the effects of increasing blood and brain concentrations of the self-administered drug, which can impact operant behavior by producing motor-behavior disruption or stimulation, temporary satiation, or other effects. The influence of accumulating blood levels of drug may be particularly substantial at the beginning of a session and soon thereafter, when several small-sized ratios can be completed in rapid succession [124]. The break point may be an indicator of the motivation to continue a self-administration bout, which is different from the motivation to initiate drug self-administration [121;125].
Several reports assessed the effect of intermittent shock on opiate (morphine and heroin) and cocaine i.v. self-administration during acquisition and daily limited access (i.e., maintenance) phases. Female rats trained to self-administer morphine for 3 weeks increased their response rate until they self-administered lethal doses of the drug when each lever press resulted in a brief mild shock to the leg. A possible reason for this result is the association of morphine delivery with pain relief [126]. In another study, rats received ten minutes of mild intermittent foot shock exposure before each of four daily heroin self-administration sessions [127]. Following acquisition of heroin-reinforced behavior (100 micrograms/kg per infusion), during which no group differences emerged, animals were placed on a progressive ratio schedule of reinforcement. Animals exposed to foot shock before each drug session had higher rates of lever pressing for heroin and achieved higher final ratios on the progressive ratio schedule than animals in the control group at the higher doses of heroin. Thus, under the conditions of this experiment, exposure to mild intermittent stress appeared to enhance the reinforcing effects of heroin, where animals responded at higher rates for the drug after foot shock. Under certain conditions, intermittent shock can increase cocaine self-administration behavior. Rats observing another rat receiving foot shock just prior to five daily sessions initiated cocaine self-administration reinforced by a very low dose that was insufficient for control rats or rats exposed to the shock to initiate cocaine self-administration (0.031 mg/kg/infusion) [128]. In another study, [129], rats given foot shock during sessions of food self-administration, just prior to the cocaine self-administration sessions showed enhanced acquisition of cocaine self-administration. This finding was extended, showing foot shock enhancing acquisition of cocaine self-administration, regardless of whether or not shocks were given contingently or non-contingently with lever pressing behavior [130]. Under most conditions, brief intermittent foot shocks facilitate the acquisition of cocaine self-administration and can increase responding for heroin under a progressive ratio schedule of reinforcement.
Several studies showed that acute food deprivation or restriction (24-hr deprivation of food or restriction of feeding amount to 5–8 g per day in rats) or chronic food restriction (i.e., multiple days or weeks of limited access to food) significantly increased the initiation and maintenance of i.v. self-administration of opiates and psychomotor stimulants. Chronic restricted feeding to 80% of free-feeding weight accelerated the acquisition of heroin and methadone self-administration [131;132], increased heroin intake during the maintenance phase [131], and increased the initiation of amphetamine self-administration over a range of unit doses (0.05–0.8 mg/kg) [133]. These findings have been extended to the initiation and maintenance of cocaine and amphetamine self-administration [134–138]. Using food restriction of 8–12 g of food either every third day or every day, rats showed increased cocaine self-administration behavior during the maintenance phase ([139]; [140–142], but see [134]). Also, 20 g of food per day accelerated acquisition of cocaine self-administration [143]. Daily food restriction, under most conditions, facilitates heroin, amphetamine and cocaine taking behavior during acquisition and daily limited access periods, and this may be attributed to neural changes that occur in the mesocorticolimbic dopamine system in response to stress [144].
Brief social stress can produce enduring neural sensitization as expressed through immediate early gene activation in the mesocorticolimbic circuit, and this genomic change due to stress may play a role in the transition to compulsive drug taking. Using a protocol of 4 intermittent defeat episodes over the course of 10 days in rats, repeated social defeat stress resulted in a decline in basal zif268 immediate early gene expression in the prefrontal cortex and an increase in zif268 expression in the central and medial amygdala for as long as 60 days after the last defeat episode [145]. Defeated rats also showed sensitized Fos labeling in the VTA and amygdala 60 days after the last stress experience [146]. In rats that previously experienced social defeat, exposure to olfactory, visual and auditory cues increased accumbens dopamine release; previously defeated rats also acquired intravenous cocaine self-administration in half the time non-stressed rats did [102]. Defeat stress-induced sensitization increased cocaine self-administration and intake [64]. Rats that experienced four social defeat episodes increased drug intake when compared to non-stressed control animals during a 24-hour continuous access session [64;145]; [Fig. 2]. Defeat stress-sensitized rats, but not mice, also achieved higher “break points” to a low dose of cocaine when placed on a progressive ratio schedule of reinforcement [64; 147; 148]. Recent studies point to the role of NMDA receptor activation in the VTA in the induction of behavioral sensitization and in prompting heightened cocaine intake during the binge [50;97].
Figure 2.
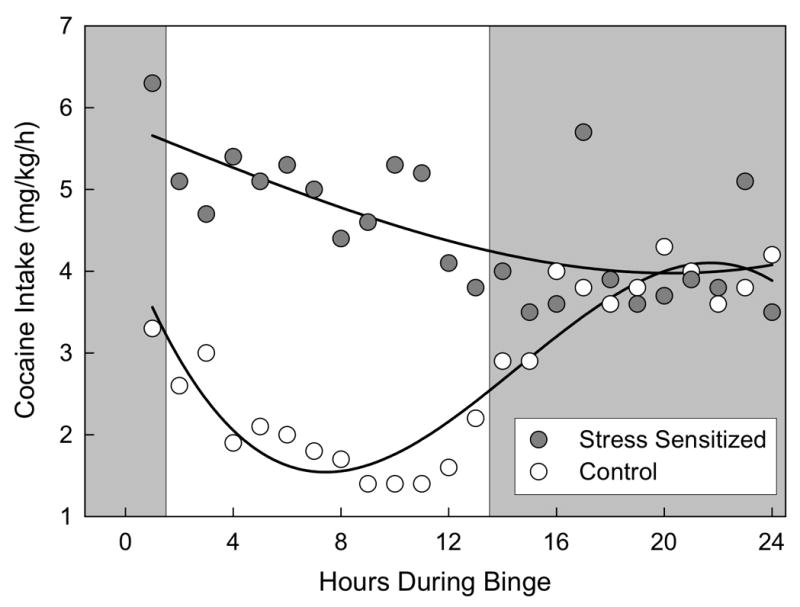
The effect of 4 brief, intermittent social defeat episodes on hourly cocaine self-administration during a 24-hour cocaine binge (0.3 mg/kg/infusion). Circadian-like cocaine self-administration behavior was maintained in control rats (open circles), whereas stressed rats (filled circles) self-administered cocaine intensely for 24 hours, effectively abolishing the circadian pattern of intake (p < 0.01). From Covington et al. (2005).
This escalation in cocaine self-administration during a 24-hour binge due to previous defeat stress experience can be attributed to the activity of the extracellular signal-regulated kinase (ERK) pathway, which when activated by repeated cocaine administration, can promote the downstream activation of the transcription factor cAMP response element binding protein (CREB) and enhance immediate early gene expression of c-fos and zif268 [149;150], genes also activated in response to repeated social defeat [145;146]. Using the MEK (mitogen-activated protein (MAP) kinase kinase; the immediate upstream activator kinase of ERK) inhibitor U0126 to prevent the phosphorylation of ERK in the VTA before each social defeat protected against the development of social defeat stress-induced sensitization and stress-induced increases in cocaine intake during the binge [151], highlighting the critical role of the VTA in the potentiation of cocaine self-administration due to social defeat. Therefore, the cascade of molecular and intracellular events that occur in the VTA in response to brief social defeat episodes can modulate subsequent intravenous cocaine taking behavior, particularly during a 24-hour continuous access binge.
Reinstatement
Clinical and epidemiological evidence documents the relapsing nature of drug addiction, and insights into the factors that promote and trigger relapse require adequate experimental models. There are two main versions of the reinstatement model that are employed with the aim to approximate the clinical phenomenon of relapse to drug abuse; one based on the operant self-administration procedure, and the other on the classical conditioned place preference procedure. In the last decade, the use of the latter version has become more widespread due to its ease of implementation, and the results obtained mostly complement those obtained in self-administration studies. It has been observed that the conditioned place preference induced by opioids, psychostimulants, nicotine, ethanol and other drugs of abuse can be extinguished and reinstated by drug priming or exposure to stressful events.
Over 25 years ago, de Wit and Stewart [152;153] used a reinstatement procedure in which the effect of acute non-contingent exposure to drug or non-drug stimuli on reinstatement of drug seeking was determined following training for drug self-administration and subsequent extinction of the drug-reinforced behavior. Non-contingent priming injections of the self-administered drug or related drugs reinstate lever-pressing behavior in rats with a history of cocaine or heroin self-administration. Based on these data, and those from earlier studies [154–156], de Wit and Stewart suggested that the reinstatement procedure may approximate an animal model of drug relapse. The reinstatement procedure has also been suggested as a model of drug-induced craving [157], although this subjective description can only be inferred. The appeal of the reinstatement model for basic scientists and clinicians derives from the observations that factors reported to reinstate drug seeking in laboratory animals also provoke relapse and craving in humans. These factors include re-exposure to drug or drug-associated cues ([158–161]; reviewed in detail by Fuchs et al. in this issue [162]) and exposure to certain stressors [3;163]. Both drugs and some types of stressors can reinstate drug seeking in laboratory animals even following prolonged withdrawal periods [164]. Furthermore, intracranial drug injections of morphine or amphetamine into the ventral tegmental area or the nucleus accumbens reinstate heroin or cocaine seeking, respectively [165;166], and conditioned cues previously paired with drug infusions reinstate morphine or cocaine seeking in rhesus monkeys or rats when extinction of lever pressing was conducted in the absence of these cues [167;168;156]. The most significant criticism of the early reinstatement model is that human addicts do not experience extinction of the drug habit, a condition in which the individual must actively learn that a response is no longer reinforced and inhibit the behavior leading to the drug delivery. Recent methods limit extinction learning, with extinction sessions and reinstatement testing conducted in the same day [157;164;169], as opposed to prolonged periods of extinction training over multiple daily sessions [154;156].
Most stress-induced reinstatement studies implement intermittent low-intensity foot shock as a form of stress because of its ease of use and its reliability in the reinstatement of drug seeking. This reinstating effect of mild foot shock [170;171] generalized to acute food deprivation but did not extend to restraint stress [172]. Transient inhibition of the central amygdala (CeA), NAC shell, NAC core, VTA, and the dorsal PFC blocked the effect of foot shock stress to reinstate lever pressing previously associated with cocaine delivery [173]. However, inhibition of the basolateral amygdala, mediodorsal nucleus of the thalamus, or the ventral prefrontal cortex had no effect on drug-seeking behavior. These data suggest that foot shock stress activates limbic circuitry of the CeA that, via the VTA, activates motor output circuitry responsible for producing lever press responding. Consistent with this proposal, the D1/D2 dopamine receptor antagonists fluphenazine and SCH 23390 blocked foot shock-induced reinstatement when infused into the PFC [173] or NAC [174], respectively. Further, inhibition of the NAC shell blocked the foot shock-induced increase in dopamine within the PFC and concomitantly blocked reinstatement responding [173]. Inactivation of the PFC was also shown to block stress-induced glutamate release within the NAC core while concurrently inhibiting reinstatement responding [173]. Taken together, these data suggest that foot shock activates limbic circuitry in the CeA, which in turn activates a VTA dopamine projection to the PFC. The rise in dopamine within the PFC initiates reinstatement via a glutamatergic projection to the NAC core.
Additional evidence points to the role of corticotropin-releasing factor (CRF) receptor activation in mesocorticolimbic areas, particularly the VTA and bed nucleus of the stria terminalis (BNST) [175–177]. Intracerebroventricular (i.c.v.) administration of the nonselective CRF antagonists α-helical CRF and d-Phe-CRF and systemic injections of the specific CRF1 receptor antagonist CP-154,526 attenuate foot shock-induced reinstatement of heroin, cocaine, and alcohol seeking [178–181]. I.c.v. and intraperitoneal (i.p.) blockade of the CRF receptors and specifically the CRF1 receptor similarly attenuates foot shock-induced reinstatement of morphine- and cocaine-conditioned place preference [182;183]. Foot shock reinstates cocaine seeking in cocaine-experienced rats by inducing corticotropin-releasing factor (CRF) and glutamate release in the VTA and thus activating VTA dopaminergic neurons [175]. Foot shock-induced VTA glutamate release, dopamine activation and reinstatements are blocked by VTA administration of alpha-helical CRF, a nonselective CRF receptor antagonist [175] as well as by infusion of the selective CRF2 receptor antagonist antisauvagine-30 into the VTA, while infusion of selective CRF1 receptor antagonists NBI-27914 or R121919 had no effect, suggesting that reinstatement of cocaine seeking and increases in dopamine and glutamate release in the VTA due to mild foot shock stress are primarily dependent on CRF2 receptor activation in the VTA [184]. The suggestion that CRF2 receptors are critical is not easily reconciled with: (1) the foot shock-induced reinstatement of morphine and cocaine conditioned place preference studies using i.c.v and systemic administration of CRF1 receptor antagonists [182;183] and (2) the findings that subcutaneous injections of a selective CRF1 receptor antagonist block reinstatement of cocaine, heroin, and alcohol seeking induced by footshock stress [179;181]. Moreover, CRF has a substantial weaker affinity for CRF2 receptors than for CRF1 receptors [185]. One of the possibilities may be that the CRF1 receptor-mediated effects are expressed in other brain regions that are also immunoreactive for CRF and CRF receptors [184]. Indeed, it has been shown that direct injections of CRF antagonists, including a selective CRF1 receptor antagonist, into the BNST are sufficient to block foot shock-induced reinstatements of either cocaine or morphine seeking [176] [186], where CRF1 and CRF2 receptors have both been identified.
Reliable reinstatement of heroin and cocaine seeking after exposure to acute, 24-hr food deprivation [172;187] was previously shown to be controlled by a leptin-dependent mechanism. Food deprivation-induced reinstatement of heroin seeking was completely blocked by i.c.v. administration of leptin [188]. However, leptin administration had no effect on foot shock- or heroin priming-induced reinstatement of heroin seeking [188], suggesting that the neural mechanisms that underlie food deprivation-induced reinstatement may be dissociable from those involved in reinstatement induced by foot shock stress or drug priming [189]. However, a later study showed that both foot shock-induced reinstatement of drug seeking [178] and reinstatement induced by acute food deprivation [190] are mediated by common stress-related neural pathways, with i.c.v. administration of CRF receptor antagonists dose-dependently attenuating both foot shock- and food deprivation-induced reinstatement of heroin seeking and adrenalectomy, which removes circulating corticosterone, affecting neither. Acute 1-day food deprivation stress also reinstates previously extinguished cocaine seeking, and in this case, this was attenuated by adrenalectomy [187], extending the finding that adrenalectomy attenuates foot shock-induced reinstatement of cocaine seeking [180]. While there is some overlap, there are differences in the way food deprivation and foot shock reinstate heroin- and cocaine-seeking behavior, particularly regarding the involvement of circulating corticosterone.
The only study to date using social defeat stress as the trigger for reinstatement has been with morphine CPP [191]. Adult male OF1 mice were conditioned with 10, 20, or 40 mg/kg of morphine or saline. Only morphine-conditioned animals acquired CPP, and all mice underwent extinction sessions. A single encounter with an aggressive resident male mouse was effective in reinstating morphine CPP. However, while an odor cue associated with defeat stress has been shown to reinstate alcohol drinking [192], reinstated cocaine or heroin seeking in rats that previously self-administered these drugs intravenously has yet to be established.
Conclusions
The neural circuit of VTA-NAC-PFC-amygdala represents overlapping elements mediating the rewarding effects of drugs and ostensibly stressful experiences. Brief intermittent stress experiences can facilitate intravenous drug self-administration and reinstate extinguished drug-seeking behavior under specific conditions. These intermittent stressors trigger a series of behavioral and cellular events which directly affect the animal’s: (1) CPP to opiates and psychomotor stimulants, (2) intravenous self-administration of morphine, heroin, cocaine and amphetamine, and (3) reinstatement of cocaine- and heroin-seeking behavior. While none of these models perfectly translate to the human condition, each model provides useful information about the conditions under which stress can affect particular aspects of drug taking, highlighting the complexity of the interplay between stress and addiction and the varying roles of mesocorticolimbic brain regions. Future studies need to identify the specific characteristics of stressors that promote drug self-administration, and delineate the exact cellular mechanisms for the interaction between aversive stress and intensely rewarding drug experiences.
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse (DA02623 and DA018478).
Footnotes
Conflict of Interest
Jasmine J. Yap and Klaus A. Miczek have no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kosten TR, Gawin FH, Rounsaville BJ, Kleber HD. Cocaine abuse among opioid addicts: demographic and diagnostic factors in treatment. Am J Drug Alcohol Abuse. 1986;12:1–16. doi: 10.3109/00952998609083739. [DOI] [PubMed] [Google Scholar]
- 2.Dembo R, Williams L, Schmeidler J, Berry E, Wothke W, Getreu A, Wish ED, Christensen C. Physical abuse, sexual victimization, and illicit drug use: replication of a structural analysis among a new sample of high-risk youths. Violence Vict. 1989;4:121–138. [PubMed] [Google Scholar]
- 3.Brown SA, Vik PW, Patterson TL, Grant I, Schuckit MA. Stress, vulnerability and adult alcohol relapse. Journal of Studies on Alcohol. 1995;56:538–545. doi: 10.15288/jsa.1995.56.538. [DOI] [PubMed] [Google Scholar]
- 4.Harrison PA, Fulkerson JA, Beebe TJ. Multiple substance use among adolescent physical and sexual abuse victims. Child Abuse & Neglect. 1997;21:529–539. doi: 10.1016/s0145-2134(97)00013-6. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: A review of the literature. American Journal of Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- 6.Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology. 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- 7.Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: Adrenocortical and pituitary glucocorticoid responsiveness. Alcoholism-Clinical and Experimental Research. 2005;29:517–527. doi: 10.1097/01.ALC.0000158940.05529.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 2: Response to ovine corticotropin-releasing factor and naloxone. Alcoholism-Clinical and Experimental Research. 2005;29:528–537. doi: 10.1097/01.ALC.0000158939.25531.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O’Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: A risk for relapse. Alcoholism-Clinical and Experimental Research. 2005;29:185–195. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady KT, Back SE, Waldrop AE, Mcrae AL, Anton RF, Upadhyaya HP, Saladin ME, Randall PK. Cold pressor task reactivity: Predictors of alcohol use among alcohol-dependent individuals with and without comorbid posttraumatic stress disorder. Alcoholism-Clinical and Experimental Research. 2006;30:938–946. doi: 10.1111/j.1530-0277.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 11.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Archives of General Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 12.Fox HC, Hong KIA, Siedlarz K, Sinha R. Enhanced sensitivity to stress and drug/alcohol craving in abstinent cocaine-dependent individuals compared to social drinkers. Neuropsychopharmacology. 2008;33:796–805. doi: 10.1038/sj.npp.1301470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elman I, Lukas SE, Karlsgodt KH, Gasic GP, Breiter HC. Acute cortisol administration triggers craving in individuals with cocaine dependence. Psychopharmacol Bull. 2003;37:84–89. [PubMed] [Google Scholar]
- 14.Deroche V, Piazza PV, Deminiere JM, Le Moal M, Simon H. Rats orally self-administer corticosterone. Brain Res. 1993;622:315–320. doi: 10.1016/0006-8993(93)90837-d. [DOI] [PubMed] [Google Scholar]
- 15.Piazza PV, Marinelli M, Jodogne C, Deroche V, Rouge-Pont F, Maccari S, Lemoal M, Simon H. Inhibition of corticosterone synthesis by Metyrapone decreases cocaine- induced locomotion and relapse of cocaine self-administration. Brain Research. 1994;658:259–264. doi: 10.1016/s0006-8993(09)90034-8. [DOI] [PubMed] [Google Scholar]
- 16.Goeders NE, Guerin GF. Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Res. 1996;722:145–152. doi: 10.1016/0006-8993(96)00206-5. [DOI] [PubMed] [Google Scholar]
- 17.DiChiara G, Imperato A. Drugs Abused by Humans Preferentially Increase Synaptic Dopamine Concentrations in the Mesolimbic System of Freely Moving Rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wise RA, Rompre PP. Brain dopamine and reward. Annual Review of Psychology. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- 19.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koob GF. Dopamine, addiction and reward. Seminars in the Neurosciences. 1992;4:139–148. [Google Scholar]
- 21.Wise RA. Dopamine, Learning and Motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 22.Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Current Opinion in Pharmacology. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology. 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- 24.Schultz W. Behavioral dopamine signals. Trends in Neurosciences. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 25.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends in Neurosciences. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Miczek KA, Yap JJ, Covington HE., III Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gambarana C. Experimental Protocols for the Study of Stress in Animals and Humans. In: Yehuda S, Mostofsky DI, editors. Nutrients. Stress and Medical Disorders Humana Press Inc; Totowa, NJ: 2007. pp. 21–35. [Google Scholar]
- 28.Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Seckl JR, Plotsky PM. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- 29.Meaney MJ, Brake W, Gratton A. Environmental regulation of the development of mesolimbic dopamine systems: a neurobiological mechanism for vulnerability to drug abuse? Psychoneuroendocrinology. 2002;27:127–138. doi: 10.1016/s0306-4530(01)00040-3. [DOI] [PubMed] [Google Scholar]
- 30.Cabib S, Orsini C, Le Moal M, Piazza PV. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289:463–465. doi: 10.1126/science.289.5478.463. [DOI] [PubMed] [Google Scholar]
- 31.Von Holst D. In: Coping behaviour and stress physiology in male tree shrews (Tupaia belangeri) Hölldobler B, Lindberg I, editors. Experimental Behavioral Ecology and Sociobiology Sinauer Associates; Sunderland, MA: 1985. pp. 461–470. [Google Scholar]
- 32.Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiology and Behavior. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- 33.Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on hypothalamic-pituitary-adrenal axis function and hippocampal serotonin release in mice. Journal of Neuroendocrinology. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- 34.Meerlo P, de Boer SF, Koolhaas JM, Daan S, van den Hoofdakker RH. Changes in daily rhythms of body temperature and activity after a single social defeat in rats. Physiology and Behavior. 1996;59:735–739. doi: 10.1016/0031-9384(95)02182-5. [DOI] [PubMed] [Google Scholar]
- 35.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 36.Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive- sensitization view. Addiction. 2000;95:S91–S117. doi: 10.1080/09652140050111681. [DOI] [PubMed] [Google Scholar]
- 37.Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 38.Shuster L, Webster GW, Yu G. Increased running response to morphine in morphine- pretreated mice. Journal of Pharmacology and Experimental Therapeutics. 1975;192:64–67. [PubMed] [Google Scholar]
- 39.Kilbey MM, Ellinwood EH. Reverse Tolerance to Stimulant-Induced Abnormal-Behavior. Life Sciences. 1977;20:1063–1075. doi: 10.1016/0024-3205(77)90294-6. [DOI] [PubMed] [Google Scholar]
- 40.Shuster L, Yu G, Bates A. Sensitization to cocaine stimulation in mice. Psychopharmacology (Berl) 1977;52:185–190. doi: 10.1007/BF00439108. [DOI] [PubMed] [Google Scholar]
- 41.Nabeshima T, Yamaguchi K, Yamada K, Hiramatsu M, Furukawa H, Kameyama T. Phencyclidine-Induced Stereotyped Behaviors in Rats Following Specific Neurotoxin Lesions of the Striatum. European Journal of Pharmacology. 1983;93:229–234. doi: 10.1016/0014-2999(83)90142-5. [DOI] [PubMed] [Google Scholar]
- 42.Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of “reverse tolerance” to cocaine and amphetamine by MK-801. Life Sciences. 1989;45:599–606. doi: 10.1016/0024-3205(89)90045-3. [DOI] [PubMed] [Google Scholar]
- 43.Phillips TJ, Dickinson S, Burkhart-Kasch S. Behavioral sensitization to drug stimulant effects in C57BL/6 and DBA/2J inbred mice. Behavioral Neuroscience. 1994;108:789–803. doi: 10.1037//0735-7044.108.4.789. [DOI] [PubMed] [Google Scholar]
- 44.Xu X, Domino EF. Phencyclidine-induced behavioral sensitization. Pharmacol Biochem Behav. 1994;47:603–608. doi: 10.1016/0091-3057(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 45.Itzhak Y, Martin JL. Effects of cocaine, nicotine, dizocipline and alcohol on mice locomotor activity: Cocaine-alcohol cross-sensitization involves upregulation of striatal dopamine transporter binding sites. Brain Research. 1999;818:204–211. doi: 10.1016/s0006-8993(98)01260-8. [DOI] [PubMed] [Google Scholar]
- 46.Olausson P, Engel JA, Soderpalm B. Behavioral sensitization to nicotine is associated with behavioral disinhibition; counteraction by citalopram. Psychopharmacology. 1999;142:111–119. doi: 10.1007/s002130050869. [DOI] [PubMed] [Google Scholar]
- 47.Domino EF. Nicotine induced behavioral locomotor sensitization. Progress in Neuro- Psychopharmacology & Biological Psychiatry. 2001;25:59–71. doi: 10.1016/s0278-5846(00)00148-2. [DOI] [PubMed] [Google Scholar]
- 48.Hoshaw BA, Lewis M. Behavioral sensitization to ethanol in rats: evidence from the Sprague- Dawley strain. Pharmacology Biochemistry and Behavior. 2001;68(4):685–690. doi: 10.1016/s0091-3057(01)00489-0. [DOI] [PubMed] [Google Scholar]
- 49.Fish EW, DeBold JF, Miczek KA. Repeated alcohol: behavioral sensitization and alcohol- heightened aggression in mice. Psychopharmacology (Berl) 2002;160:39–48. doi: 10.1007/s00213-001-0934-9. [DOI] [PubMed] [Google Scholar]
- 50.Yap JJ, Covington HE, III, Gale MC, Datta R, Miczek KA. Behavioral sensitization due to social defeat stress in mice: antagonism at mGluR5 and NMDA receptors. Psychopharmacology (Berl) 2005;179:230–239. doi: 10.1007/s00213-004-2023-3. [DOI] [PubMed] [Google Scholar]
- 51.Pierre PJ, Vezina P. Predisposition to self-administer amphetamine: the contribution of response to novelty and prior exposure to the drug. Psychopharmacology (Berl) 1997;129:277–284. doi: 10.1007/s002130050191. [DOI] [PubMed] [Google Scholar]
- 52.Mendrek A, Blaha CD, Phillips AG. Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology. 1998;135:416–422. doi: 10.1007/s002130050530. [DOI] [PubMed] [Google Scholar]
- 53.Suto N, Austin JD, Tanabe LM, Kramer MK, Wright DA, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in a D1 dopamine receptor dependent manner. Neuropsychopharmacology. 2002;27:970–979. doi: 10.1016/S0893-133X(02)00379-2. [DOI] [PubMed] [Google Scholar]
- 54.Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. Journal of Neuroscience. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suto N, Tanabe LM, Austin JD, Creekmore E, Vezina P. Previous exposure to VTA amphetamine enhances cocaine self-administration under a progressive ratio schedule in an NMDA, AMPA/kainate, and metabotropic glutamate receptor-dependent manner. Neuropsychopharmacology. 2003;28:629–639. doi: 10.1038/sj.npp.1300075. [DOI] [PubMed] [Google Scholar]
- 56.Ellinwood EH. Amphetamine Psychosis. I. Description of Individuals and Process. Journal of Nervous and Mental Disease. 1967;144:273. [Google Scholar]
- 57.Griffith JD, Cavanaugh J, Held J, Oates JA. Dextroamphetamine. Evaluation of psychomimetic properties in man. Arch Gen Psychiatry. 1972;26:97–100. doi: 10.1001/archpsyc.1972.01750200001001. [DOI] [PubMed] [Google Scholar]
- 58.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann AR, Chen AD, Dewey S, Pappas N. Decreased Striatal dopminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:831–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 59.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Research Reviews. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 60.Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- 61.Kalivas PW, Duffy P. Similar effects of daily cocaine and stress on mesocorticolimbic Dopamine Neurotransmission in the rat. Biological Psychiatry. 1989;25:913–928. doi: 10.1016/0006-3223(89)90271-0. [DOI] [PubMed] [Google Scholar]
- 62.Sorg BA, Kalivas PW. Effects of Cocaine and Footshock Stress on Extracellular Dopamine Levels in the Ventral Striatum. Brain Research. 1991;559:29–36. doi: 10.1016/0006-8993(91)90283-2. [DOI] [PubMed] [Google Scholar]
- 63.Hahn B, Zacharko RM, Anisman H. Alterations of amphetamine elicited perseveration and locomotor excitation following acute and repeated stressor application. Pharmacol Biochem Behav. 1986;25:29–33. doi: 10.1016/0091-3057(86)90225-x. [DOI] [PubMed] [Google Scholar]
- 64.Covington HE, III, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- 65.Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vunerability to amphetamine self- administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- 66.Thierry AM, Tassin JP, Blanc G, Glowinski J. Selective activation of the mesocortical DA system by stress. Nature. 1976;263:242–243. doi: 10.1038/263242a0. [DOI] [PubMed] [Google Scholar]
- 67.Claustre Y, Rivy JP, Dennis T, Scatton B. Pharmacological studies on stress-induced increase in frontal cortical dopamine metabolism in the rat. Journal of Pharmacology and Experimental Therapeutics. 1986;238:693–700. [PubMed] [Google Scholar]
- 68.Puglisi-Allegra S, Imperato A, Angelucci L, Cabib S. Acute Stress Induces Time-Dependent Responses in Dopamine Mesolimbic System. Brain Research. 1991;554:217–222. doi: 10.1016/0006-8993(91)90192-x. [DOI] [PubMed] [Google Scholar]
- 69.Bannon MJ, Roth RH. Pharmacology of mesocortical dopamine neurons. Pharmacological Reviews. 1983;35:53–68. [PubMed] [Google Scholar]
- 70.Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- 71.Imperato A, Puglisi-Allegra S, Zocchi A, Scrocco MG, Casolini P, Angelucci L. Stress activation of limbic and cortical dopamine release is prevented by ICS 205–930 but not by diazepam. Eur J Pharmacol. 1990;175:211–214. doi: 10.1016/0014-2999(90)90233-v. [DOI] [PubMed] [Google Scholar]
- 72.Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ. Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cereb Cortex. 1991;1:273–292. doi: 10.1093/cercor/1.4.273. [DOI] [PubMed] [Google Scholar]
- 73.Dazzi L, Motzo C, Imperato A, Serra M, Gessa GL, Biggio G. Modulation of basal and stress-induced release of acetylcholine and dopamine in rat brain by abecarnil and imidazenil, two anxioselective gamma-aminobutyric acidA receptor modulators. J Pharmacol Exp Ther. 1995;273:241–247. [PubMed] [Google Scholar]
- 74.Feenstra MGP, Botterblom MHA. Rapid sampling of extracellular dopamine in the rat prefrontal cortex during food consumption, handling and exposure to novelty. Brain Research. 1996;742:17–24. doi: 10.1016/s0006-8993(96)00945-6. [DOI] [PubMed] [Google Scholar]
- 75.Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: An in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- 76.Chao J, Nestler EJ. Molecular neurobiology of drug addiction. Annu Rev Med. 2004;55:113–132. doi: 10.1146/annurev.med.55.091902.103730. [DOI] [PubMed] [Google Scholar]
- 77.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: A comprehensive review of drug effects, recent progress and new issues. Progress in Neurobiology. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 78.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 79.Cunningham CL, Gremel CM, Groblewski PA. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;1:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- 80.OLDS J, MILNER P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- 81.Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neuroscience and Biobehavioral Reviews. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 82.Will MJ, Watkins LR, Maier SF. Uncontrollable stress potentiates morphine’s rewarding properties. Pharmacology Biochemistry and Behavior. 1998;60:655–664. doi: 10.1016/s0091-3057(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 83.Rozeske RR, Der-Avakian A, Bland ST, Beckley JT, Watkins LR, Maier SF. The Medial Prefrontal Cortex Regulates the Differential Expression of Morphine-Conditioned Place Preference Following a Single Exposure to Controllable or Uncontrollable Stress. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Der-Avakian A, Bland ST, Rozeske RR, Tamblyn JP, Hutchinson MR, Watkins LR, Maier SF. The effects of a single exposure to uncontrollable stress on the subsequent conditioned place preference responses to oxycodone, cocaine, and ethanol in rats. Psychopharmacology (Berl) 2007;191:909–917. doi: 10.1007/s00213-006-0678-7. [DOI] [PubMed] [Google Scholar]
- 85.McLaughlin JP, Marton-Popovici M, Chavkin C. kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. Journal of Neuroscience. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gaiardi M, Bartoletti M, Bacchi A, Gubellini C, Babbini M. Increased Sensitivity to the Stimulus Properties of Morphine in Food Deprived Rats. Pharmacology Biochemistry and Behavior. 1987;26:719–723. doi: 10.1016/0091-3057(87)90603-4. [DOI] [PubMed] [Google Scholar]
- 87.Bell SM, Stewart RB, Thompson SC, Meisch RA. Food-deprivation increases cocaine-induced conditioned place preference and locomotor activity in rats. Psychopharmacology (Berl) 1997;131:1–8. doi: 10.1007/s002130050258. [DOI] [PubMed] [Google Scholar]
- 88.Stuber GD, Evans SB, Higgins MS, Pu YP, Figlewicz DP. Food restriction modulates amphetamine-conditioned place preference and nucleus accumbens dopamine release in the rat. Synapse. 2002;46:83–90. doi: 10.1002/syn.10120. [DOI] [PubMed] [Google Scholar]
- 89.Coventry TL, D’Aquila PS, Brain P, Willner P. Social influences on morphine conditioned place preference. Behavioural Phamacology. 1997;8:575–584. doi: 10.1097/00008877-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 90.McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Deneau G, Yanagita T, Seevers MH. Self-administration of psychoactive substances by the monkey. Psychopharmacologia. 1969;16:30–48. doi: 10.1007/BF00405254. [DOI] [PubMed] [Google Scholar]
- 92.Harris RT, Claghorn JL, Schoolar JC. Self administration of monor tranquilizers as a functiion of conditioning. Psychopharmacologia. 1968;13:81–88. doi: 10.1007/BF00401621. [DOI] [PubMed] [Google Scholar]
- 93.Schuster CR, Thompson T. Self administration of and behavioral dependence on drugs. Annu Rev Pharmacol. 1969;9:483–502. doi: 10.1146/annurev.pa.09.040169.002411. [DOI] [PubMed] [Google Scholar]
- 94.Dai S, Corrigall WA, Coen KM, Kalant H. Heroin self-administration by rats: influence of dose and physical dependence. Pharmacol Biochem Behav. 1989;32:1009–1015. doi: 10.1016/0091-3057(89)90074-9. [DOI] [PubMed] [Google Scholar]
- 95.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: Change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 96.Tornatzky W, Miczek KA. Cocaine self-administration “binges”: transition from behavioral and autonomic regulation toward homeostatic dysregulation in rats. Psychopharmacology. 2000;148:289–298. doi: 10.1007/s002130050053. [DOI] [PubMed] [Google Scholar]
- 97.Covington HE, III, Tropea TF, Rajadhyaksha AM, Kosofsky BE, Miczek KA. NMDA receptors in the rat VTA: a critical site for social stress to intensify cocaine taking. Psychopharmacology (Berl) 2008;197:203–216. doi: 10.1007/s00213-007-1024-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci. 2002;5:625–626. doi: 10.1038/nn872. [DOI] [PubMed] [Google Scholar]
- 99.Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. NeuroReport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- 100.Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacology (Berl) 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- 101.Piazza PV, Deminiere JM, Le Moal M, Simon H. Stress- and pharmacologically- induced behavioral sensitization increases vulnerability to acquisition of amphetamine self-administration. Brain Research. 1990;514:22–26. doi: 10.1016/0006-8993(90)90431-a. [DOI] [PubMed] [Google Scholar]
- 102.Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology (Berl) 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- 103.Carroll ME, Lac ST. Acquisition of IV amphetamine and cocaine self-administration in rats as a function of dose. Psychopharmacology. 1997;129:206–214. doi: 10.1007/s002130050182. [DOI] [PubMed] [Google Scholar]
- 104.Campbell UC, Thompson SS, Carroll ME. Acquisition of oral phencyclidine (PCP) self-administration in rhesus monkeys: effects of dose and an alternative non-drug reinforcer. Psychopharmacology (Berl) 1998;137:132–138. doi: 10.1007/s002130050602. [DOI] [PubMed] [Google Scholar]
- 105.Carroll ME, Lac ST. Autoshaping i.v. cocaine self-administration in rats: effects of nondrug alternative reinforcers on acquisition. Psychopharmacology. 1993;110:5–12. doi: 10.1007/BF02246944. [DOI] [PubMed] [Google Scholar]
- 106.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 107.Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S, Mielke MM, Hoffman A, McCallum S. Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl) 2000;151:392–405. doi: 10.1007/s002130000497. [DOI] [PubMed] [Google Scholar]
- 108.Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- 109.Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self-administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- 110.Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- 111.Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreasing during cocaine withdrawal after unlimited-access self-administration. Brain Research. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- 112.O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 113.Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF. Animal-models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- 114.Kenny PJ. Brain reward systems and compulsive drug use. Trends Pharmacol Sci. 2007;28:135–141. doi: 10.1016/j.tips.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 115.Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 116.Johanson CE, Balster RL, Bonese K. Self-administration of psychomotor stimulant drugs: the effects of unlimited access. Pharmacology, Biochemistry and Behavior. 1976;4:45–51. doi: 10.1016/0091-3057(76)90174-x. [DOI] [PubMed] [Google Scholar]
- 117.Bozarth MA, Wise RA. Toxicity associated with long-term intravenous heroin and cocaine self- administration in the rat. Journal of the American Medical Association. 1985;254:81–83. [PubMed] [Google Scholar]
- 118.Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–312. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- 119.Mantsch JR, Schlussman SD, Ho A, Kreek MJ. Effects of cocaine self-administration on plasma corticosterone and prolactin in rats. Journal of Pharmacology and Experimental Therapeutics. 2000;294:239–247. [PubMed] [Google Scholar]
- 120.Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose-dependent. Psychopharmacology (Berl) 2001;157:31–39. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- 121.Oleson EB, Roberts DC. Drug Discovery Today: Disease Models. 2009 doi: 10.1016/j.ddmod.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Freeman WM, Brebner K, Patel KM, Lynch WJ, Roberts DCS, Vrana KE. Repeated cocaine self-administration causes multiple changes in rat frontal cortex gene expression. Neurochemical Research. 2002;27:1181–1192. doi: 10.1023/a:1020929526688. [DOI] [PubMed] [Google Scholar]
- 123.Hodos W. Progressive Ratio As A Measure of Reward Strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 124.Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self- administration: A review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- 125.Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. Journal of Neuroscience Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 126.Beck SG, O’Brien JH. Lethal self-administration of morphine by rats. Physiol Behav. 1980;25:559–564. doi: 10.1016/0031-9384(80)90122-5. [DOI] [PubMed] [Google Scholar]
- 127.Shaham Y, Stewart J. Exposure to mild stress enhances the reinforcing efficacy of intravenous heroin self-administration in rats. Psychopharmacology. 1994;114:523–527. doi: 10.1007/BF02249346. [DOI] [PubMed] [Google Scholar]
- 128.Ramsey NF, van Ree JM. Emotional but not physical stress enhances intravenous cocaine self- administration in drug-naive rats. Brain Research. 1993;608:216–222. doi: 10.1016/0006-8993(93)91461-z. [DOI] [PubMed] [Google Scholar]
- 129.Goeders NE, Guerin GF. Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology. 1994;114:63–70. doi: 10.1007/BF02245445. [DOI] [PubMed] [Google Scholar]
- 130.Goeders NE, Guerin GF. Role of corticosterone in intravenous cocaine self-administration in rats. Neuroendocrinology. 1996;64(5):337–348. doi: 10.1159/000127137. [DOI] [PubMed] [Google Scholar]
- 131.Madden C, Oei TP, Singer G. The effect of schedule removal on the maintenance of heroin self-injection. Pharmacol Biochem Behav. 1980;12:983–986. doi: 10.1016/0091-3057(80)90463-3. [DOI] [PubMed] [Google Scholar]
- 132.Oei TP, Singer G, Jefferys D. The interaction of a fixed time food delivery schedule and body weight on self-administration of narcotic analgesics. Psychopharmacology (Berl) 1980;67:171–176. doi: 10.1007/BF00431973. [DOI] [PubMed] [Google Scholar]
- 133.Takahashi RN, Singer G, Oei TP. Schedule induced self-injection of D-amphetamine by naive animals. Pharmacol Biochem Behav. 1978;9:857–861. doi: 10.1016/0091-3057(78)90369-6. [DOI] [PubMed] [Google Scholar]
- 134.De Vry J, Donselaar I, van Ree JM. Food deprivation and acquisition of intravenous cocaine self-administration in rats: effect of naltrexone and haloperidol. J Pharmacol Exp Ther. 1989;251:735–740. [PubMed] [Google Scholar]
- 135.Glick SD, Hinds PA, Carlson JN. Food deprivation and stimulant self-administration in rats: differences between cocaine and d-amphetamine. Psychopharmacology (Berl) 1987;91:372–374. doi: 10.1007/BF00518194. [DOI] [PubMed] [Google Scholar]
- 136.Oei TP. Effects of body weight reduction and food deprivation on cocaine self-administration. Pharmacol Biochem Behav. 1983;19:453–455. doi: 10.1016/0091-3057(83)90119-3. [DOI] [PubMed] [Google Scholar]
- 137.Papasava M, Oei TP, Singer G. Low dose cocaine self-administration by naive rats: effects of body weight and a fixed-time one minute food delivery schedule. Pharmacol Biochem Behav. 1981;15:485–488. doi: 10.1016/0091-3057(81)90281-1. [DOI] [PubMed] [Google Scholar]
- 138.Papasava M, Singer G. Self-administration of low-dose cocaine by rats at reduced and recovered body weight. Psychopharmacology (Berl) 1985;85:419–425. doi: 10.1007/BF00429657. [DOI] [PubMed] [Google Scholar]
- 139.Carroll ME. The role of food deprivation in the maintenance and reinstatement of cocaine- seeking behavior in rats. Drug Alcohol Depend. 1985;16:95–109. doi: 10.1016/0376-8716(85)90109-7. [DOI] [PubMed] [Google Scholar]
- 140.Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. Journal of Pharmacology and Experimental Therapeutics. 1981;217:241–247. [PubMed] [Google Scholar]
- 141.Carroll ME, Lac ST, Walker MJ, Kragh R, Newman T. Effects of naltrexone on intravenous cocaine self-administration in rats during food satiation and deprivation. Journal of Pharmacology and Experimental Therapeutics. 1986;238:1–7. [PubMed] [Google Scholar]
- 142.Comer SD, Lac ST, Wyvell CL, Curtis LK, Carroll ME. Food deprivation affects extinction and reinstatement of responding in rats. Psychopharmacology. 1995;121:150–157. doi: 10.1007/BF02245624. [DOI] [PubMed] [Google Scholar]
- 143.Carroll ME, Lac ST. Dietary additives and the acquisition of cocaine self-administration in rats. Psychopharmacology (Berl) 1998;137:81–89. doi: 10.1007/s002130050596. [DOI] [PubMed] [Google Scholar]
- 144.Carr KD. Augmentation of drug reward by chronic food restriction: behavioral evidence and underlying mechanisms. Physiol Behav. 2002;76:353–364. doi: 10.1016/s0031-9384(02)00759-x. [DOI] [PubMed] [Google Scholar]
- 145.Covington HE, III, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Jr, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- 146.Nikulina EM, Covington HE, III, Ganschow L, Hammer RP, Jr, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 147.Yap JJ, Miczek KA. Social defeat stress, sensitization, and intravenous cocaine self- administration in mice. Psychopharmacology (Berl) 2007;192:261–273. doi: 10.1007/s00213-007-0712-4. [DOI] [PubMed] [Google Scholar]
- 148.Quadros I, Miczek KA. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacology (Berl) 2009 doi: 10.1007/s00213-009-1584-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, Kreuter JD, Hope BT. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- 150.Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 151.Yap JJ, Chartoff EH, Hogenelst K, Carlezon WA, Jr, Miczek KA. Social defeat stress-induced sensitization and escalated cocaine self-administration: Role of ERK-CREB signaling in the mesocorticolimbic dopamine system. Abstr Soc Neurosci. 2008 [Google Scholar]
- 152.de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- 153.de Wit H, Stewart J. Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology (Berl) 1983;79:29–31. doi: 10.1007/BF00433012. [DOI] [PubMed] [Google Scholar]
- 154.Stretch R, Gerber GJ, Wood SM. Factors affecting behavior maintained by response-contingent intravenous infusions of amphetamine in squirrel monkeys. Can J Physiol Pharmacol. 1971;49:581–589. doi: 10.1139/y71-075. [DOI] [PubMed] [Google Scholar]
- 155.Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3:1055–1061. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- 156.Davis WM, Smith SG. Role of conditioned reinforcers in the initiation, maintenance and extinction of drug-seeking behavior. Pavlov J Biol Sci. 1976;11:222–236. doi: 10.1007/BF03000316. [DOI] [PubMed] [Google Scholar]
- 157.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ludwig AM, Wikler A, Stark LH. The first drink: psychobiological aspects of craving. Arch Gen Psychiatry. 1974;30:539–547. doi: 10.1001/archpsyc.1974.01760100093015. [DOI] [PubMed] [Google Scholar]
- 159.Jaffe JH, Cascella NG, Kumor KM, Sherer MA. Cocaine-induced cocaine craving. Psychopharmacology (Berl) 1989;97:59–64. doi: 10.1007/BF00443414. [DOI] [PubMed] [Google Scholar]
- 160.O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug- dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 161.Ehrman RN, Robbins SJ, Childress AR, O’Brien CP. Conditioned responses to cocaine- related stimuli in cocaine abuse patients. Psychopharmacology (Berl) 1992;107:523–529. doi: 10.1007/BF02245266. [DOI] [PubMed] [Google Scholar]
- 162.Fuchs RA. Drug Discovery Today: Disease Models. 2009 doi: 10.1016/j.ddmod.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sinha R, Fuse T, Aubin LR, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology (Berl) 2000;152:140–148. doi: 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- 164.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology (Berl) 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- 165.Stewart J. Reinstatement of heroin and cocaine self-administration behavior in the rat by intracerebral application of morphine in the ventral tegmental area. Pharmacol Biochem Behav. 1984;20:917–923. doi: 10.1016/0091-3057(84)90017-0. [DOI] [PubMed] [Google Scholar]
- 166.Stewart J, Vezina P. A comparison of the effects of intra-accumbens injections of amphetamine and morphine on reinstatement of heroin intravenous self-administration behavior. Brain Res. 1988;457:287–294. doi: 10.1016/0006-8993(88)90698-1. [DOI] [PubMed] [Google Scholar]
- 167.Schuster CR, Woods JH. Conditioned Reinforcing Effects of Stimuli Associated with Morphine Reinforcement. International Journal of the Addictions. 1968;3:223–230. [Google Scholar]
- 168.Goldberg SR. Conditioned behavioral and physiological changes associated with injections of a narcotic antagonist in morphine-dependent monkeys. Pavlov J Biol Sci. 1976;11:203–221. doi: 10.1007/BF03000315. [DOI] [PubMed] [Google Scholar]
- 169.Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL. Time-dependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacology. 1998;19:48–59. doi: 10.1016/S0893-133X(97)00205-4. [DOI] [PubMed] [Google Scholar]
- 170.Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and re-exposure to heroin in rats. Psychopharmacology. 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- 171.Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- 172.Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology (Berl) 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- 173.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and Motor Circuitry Underlying Footshock-Induced Reinstatement of Cocaine-Seeking Behavior. Journal of Neuroscience. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. Journal of Neuroscience. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- 178.Shaham Y, Funk D, Erb S, Brown TJ, Walker C, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. Journal of Neuroscience. 1997;17:0–4. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Shaham Y, Erb S, Leung S, Buczek Y, Stewart J. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998;137:184–190. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- 180.Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotropin-releasing factor instress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- 182.Lu L, Ceng XB, Huang MS. Corticotropin-releasing factor receptor type I mediates stress- induced relapse to opiate dependence in rats. NeuroReport. 2000;11:2373–2378. doi: 10.1097/00001756-200008030-00008. [DOI] [PubMed] [Google Scholar]
- 183.Lu L, Liu DH, Ceng XB. Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. European Journal of Pharmacology. 2001;415:203–208. doi: 10.1016/s0014-2999(01)00840-8. [DOI] [PubMed] [Google Scholar]
- 184.Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- 185.Eckart K, Jahn O, Radulovic J, Radulovic M, Blank T, Stiedl O, Brauns O, Tezval H, Zeyda T, Spiess J. Pharmacology and biology of corticotropin-releasing factor (CRF) receptors. Receptors Channels. 2002;8:163–177. [PubMed] [Google Scholar]
- 186.Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl) 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- 187.Shalev U, Marinelli M, Baumann MH, Piazza PV, Shaham Y. The role of corticosterone in food deprivation-induced reinstatement of cocaine seeking in the rat. Psychopharmacology (Berl) 2003;168:170–176. doi: 10.1007/s00213-002-1200-5. [DOI] [PubMed] [Google Scholar]
- 188.Shalev U, Yap J, Shaham Y. Leptin attenuates acute food deprivation-induced relapse to heroin seeking. J Neurosci. 2001;21:RC129. doi: 10.1523/JNEUROSCI.21-04-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 190.Shalev U, Finnie PS, Quinn T, Tobin S, Wahi P. A role for corticotropin-releasing factor, but not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2006;187:376–384. doi: 10.1007/s00213-006-0427-y. [DOI] [PubMed] [Google Scholar]
- 191.Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodríguez-Arias M, Armario A, Miñarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology. 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- 192.Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology. 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]



