Abstract
We have investigated cellular Ca2+ regulation during A2058 human melanoma cell chemotaxis to type IV collagen (CIV). We have identified α2β1-integrin as the primary mediator of A2058 cell response to CIV in vitro. Integrin ligation initiated a characteristic intracellular Ca2+ concentration ([Ca2+]i) response consisting of an internal release and a receptor-mediated Ca2+ entry. Thapsigargin (TG) pretreatment drained overlapping and CIV-inducible internal Ca2+ stores while initiating a store-operated Ca2+ release (SOCR). CIV-mediated Ca2+ entry was additive to TG-SOCR, suggesting an independent signaling mechanism. Similarly, ionophore application in a basal medium containing Ca2+ initiated a sustained influx. Elevated [Ca2+]i from TG-SOCR or ionophore significantly attenuated cell migration to CIV by recruiting the Ca2+/calcineurin-mediated signaling pathway. Furthermore, low [Ca2+]i induced by EGTA application in the presence of ionophore fully restored cell motility to CIV. Together, these results suggest that [Ca2+]i signaling accompanying A2058 cell response to α2β1-integrin ligation is neither necessary nor sufficient and that elevated [Ca2+]i downregulates cell motility via a calcineurin-mediated mechanism in A2058 cell chemotaxis to CIV.
Keywords: melanoma, chemotaxis, secondary messenger, signal transduction, intracellular Ca2+ concentration
Cell motility is regulated by a multiplex of signal transduction mechanisms and is coordinated through intracellular second messengers. Phenomenological aspects of cell motility and migration in response to cytokines, chemokines, and extracellular matrix (ECM) proteins in a variety of cell systems have been well documented; however, biochemical signaling and regulation of these processes require further elucidation (2, 5, 9, 10, 19, 29). Cell chemotaxis is mediated by coupling an extracellular agonist to its transmembrane receptor that is capable of activating cascades of intracellular second messenger signaling pathways. Of those receptors, G protein-linked receptor activation and integrin-mediated cell signaling have been suggested to play significant roles in tumor cell adhesion and migration over ECM protein substrates (5, 18, 35, 39). Whereas the G protein-mediated signal transduction cascade has been well characterized (2, 5, 9), the signaling pathway that activates tumor cell response mediated by integrin ligation remains to be elucidated.
The integrin superfamily consists of a variety of cell surface receptors, and those belonging to the VLA (very late antigen) subgroup have been identified as de facto ECM receptors (1, 31, 32, 39). The VLA integrin complex consists of a heterodimer of α- and β-subunits. In cell interactions that involve ECM proteins, the β-subunit of the VLA integrin complex is conserved, while the α-subunit is variable. The cytoplasmic tails of α- and β-subunits have been shown to associate with intracellular actin cytoskeleton filaments through the formation of focal adhesion complex (32). Cell attachment and spreading via the engagement of these integrin receptors initiate signaling pathways that result in mitotic and chemotactic cellular responses (11, 21, 22, 26, 32, 33, 36).
Recent findings indicated that the chemotaxis of A2058 human melanoma cells toward a member of ECM protein, type IV collagen (CIV), induced an intracellular Ca2+ second messenger pathway (28). The initial release of intracellular Ca2+ concentration ([Ca2+]i), which is triggered by a receptor engagement through the cell attachment and spreading, is followed by a prolonged period of Ca2+ influx from the extracellular compartments [store-operated Ca2+ release (SOCR) and receptor-mediated Ca2+ entry] (3, 4, 7, 27). However, the mechanism of initial [Ca2+]i release mediated by integrins remains poorly characterized in tumor cell chemotaxis. Evidently, A2058 cells do not significantly turn over phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2; PIP2] during CIV stimulation, ruling out the classic PIP2/d-myo-inositol 1,4,5-trisphosphate (IP3) [Ca2+]i release mechanism (28). Therefore, characterizing the source of cell Ca2+ will facilitate the elucidation of signaling events mediating tumor cell metastasis.
Here, we address the influence of Ca2+ signaling on integrin-mediated A2058 human melanoma cell migration toward CIV. We have identified a key integrin receptor initiating this specific chemotactic response of A2058 cells to CIV as α2β1 (VLA-2). We have shown that the initial [Ca2+]i release occurred from thapsigargin (TG)-sensitive intracellular stores. However, the receptor-mediated Ca2+ entry following the CIV stimulation utilized a distinctly different set of influx channels from those employed by TG-SOCR. TG, which specifically targets and irreversibly inactivates Ca2+-ATPases [sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)] on the endoplasmic reticulum (17), is used to assess the importance of intracellular store refilling during a cell response to collagen. Effects of the depletion of extracellular Ca2+ source by EGTA, as well as an enhancement of Ca2+ flux caused by Ca2+ ionophores, are addressed in this study and correlated to cellular chemotactic response toward CIV stimulation.
MATERIALS AND METHODS
Cell culture and preparation
A2058 human melanoma cells were maintained in tissue culture, as described previously, detached when subconfluent by a brief trypsinization, and allowed to regenerate for 1 h in culture medium (5, 35). Cells were resuspended in serum-free DMEM (Biofluids, Rockville, MD) containing 0.1% wt/vol fraction V bovine serum albumin with 0.02 M HEPES (Sigma Chemical, St. Louis, MO) at 1.3 × 106 cells/ml concentration and allowed to regenerate for one additional hour before assays. Cells used were in passages 14–18 for all experiments.
Antibodies
Integrin-specific monoclonal blocking antibodies (MAb) FB12 (anti-α1; Chemicon International, Temecula, CA), Gi9 (anti-α2; Beckman Coulter, Fullerton, CA), and P1B5 and 6S6 (anti-α3 and anti-β1; Chemicon) were obtained commercially. All antibodies are murine and of IgG1 isotype. Isotype control experiments were also performed with non-specific murine IgG.
Migration assay
A detailed procedure for chemotaxis assays using 48-well microchemotaxis chambers was described elsewhere (5, 13). In brief, 10-µm pore size polycarbonate filters (Neuro Probe, Cabin John, MD) were soaked overnight in 0.1% wt/vol poly-d-lysine solution (Sigma) to enhance cell adhesion. CIV (Becton Dickinson Labware, Bedford, MD) was dispersed into the experiment medium at a concentration of 100 µg/ml as the chemotactic solution. The pH of the chemotactic solution was adjusted to 7.4 immediately before the experiment. The chemotactic solutions were placed into the bottom wells of the 48-well chamber, and the cell suspension was placed into the top wells, separated by a filter. The chamber assembly was placed into a 5% CO2 37°C environment for 4 h. Upon completion of the experiment, the filter was stained with DiffQuik staining kit (Dade International, Miami, FL), and cells on the underside of the filter were visually counted under ×10 bright field magnification.
In cases involving EGTA (Sigma), the chemotactic solution contained EGTA at 3.2 mM to chelate 1.6 mM Ca2+ in basal medium. In this case, the medium additionally contained 3.2 mM Mg2+ to balance the depletion of Ca2+. Cells were transferred to medium that contained 3.2mMEGTA 30 min before an assay and placed into top wells of the migration chamber at the specified cell concentration. EGTA (3.2 mM at pH 7.4) yielded extracellular free Ca2+ of ~65 nM
| (1) |
where [Ca] is the concentration of extracellular free Ca2+, [Rt] is the concentration of EGTA, [Cat] is the total Ca2+ concentration in the basal medium, and is the apparent Ca2+ affinity constant for EGTA (6).
TG (Biomol Research Laboratories, Plymouth Meeting, PA) pretreatment involved TG exposure 30 min before an assay. Specifically, cells were centrifuged 30 min before an assay and resuspended in experiment medium that contained 1.0 µM TG. The chemotactic solution and the cell suspension both contained TG at 1.0 µM during the assay.
Ca2+ ionophore (4′-bromo-A-23187; Molecular Probes, Eugene, OR) was prepared into a stock solution and was subsequently diluted with experiment medium to a final concentration of 1.0 µM. Cells were transferred to this solution 15 min before an assay. The chemotactic solution and the cell suspension contained ionophore at the specified concentration during the assay.
Cyclosporin A (CsA; Sigma) was diluted to a final concentration of 1.5 µg/ml and placed in cell culture medium 20 h before an assay. During the chemotaxis assay, both upper and lower chambers contained CsA at the indicated concentration.
Functional blocking assays of integrin receptors using MAb involved a 30-min pretreatment of cells in medium that contained 10–25 µg/ml of MAb before an assay. The cell suspension and the CIV chemotactic solution both contained MAb at specified concentrations during the migration experiment.
Control experiments in which CIV was removed from the chemotactic solution were performed for all cases assayed. This reflected the nonspecific background motility and was subtracted from the resulting total cell count for all experiments. The migration data for all cases assayed were obtained at least in triplicates of 12 wells.
Ratiometric measurements of intracellular free Ca2+ in adherent cells
A2058 cells were cultured onto 25-mm round glass coverslips (pretreated with 0.1% wt/vol poly-d-lysine solution overnight) and maintained under standard culture conditions. The fluorescence measurements were conducted in a temperature-, humidity-, and gas (37°C, 100%, and 5%, respectively)-controlled chamber mounted on top of the microscope stage. The procedure for a digital Ca2+ ratiometric assay is detailed elsewhere (12, 28, 38). A computer software package (Axon Imaging Workbench 2.1; Axon Instruments, Foster City, CA) was used to control the excitation light (340- and 380-nm band pass filters; Chroma Technology, Brattleboro, VT), sample, and record the emitted fluorescence (510 nm) images from the fura 2-AM (Molecular Probes)-loaded cells in the field of view once every 6 s (once every 2 min for longtime Ca2+ assays). The background fluorescence was subtracted from each image. Ratio images of the cells at rest were collected initially for the first minute to establish an [Ca2+]i baseline. After the establishment of a baseline [Ca2+]i, above mentioned experiment solutions were selectively perfused into the chamber, and raw images were collected for an additional 10–12 min or up to 4 h. A calibration curve was constructed by acquiring 340/380 values (background subtracted) of 50 µM fura 2 pentapotassium salt solution using the calcium calibration kit no. 1 (Molecular Probes) and using an off-line calibration of the ratio data.
RESULTS
α2β1-integrin mediates A2058 cell chemotaxis to CIV
To identify and characterize the receptors mediating cell response to CIV, MAbs were used to functionally block the cell surface integrin receptors during a chemotaxis assay. In the absence of integrin-specific MAbs, A2058 melanoma cells actively migrated in response to 100 µg/ml of CIV (Fig. 1). At saturating MAb concentrations of 10–25 µg/ml, antibodies to α1- and α3-integrins mildly attenuated the cell response to CIV, while blocking α2 and β1 effectively abrogated the cell chemotaxis to CIV. These data indicate that A2058 cell chemotaxis to CIV is primarily mediated by α2β1-integrin.
Fig. 1.
Functional blocking of A2058 cell migration to 100 µg/ml of type IV collagen (CIV) stimulation using monoclonal antibodies. Data are normalized to the control CIV response without the antibody treatment and presented with means ± SE; n = 3–6 experiments. Each experiment consisted of results from 24–36 duplicate chemotaxis wells. Antibodies were diluted to 10 µg/ml (FB12, Gi9, and P1B5) and 25 µg/ml (6S6) in DMEM with 0.1% BSA at pH 7.4 and added to the cell suspension 30 min before the chemotaxis assay using the 48-well Boyden chamber as described in MATERIALS AND METHODS. Cell suspension and chemotactic solution (100 µg/ml of CIV) contained antibodies at the specified concentrations during assay. Upon completion of a 4-h migration assay, migrated cells on the bottom side of the filter were stained and visually counted under ×10 bright field magnification.
Cell migration abrogated during high [Ca2+]i conditions
To elucidate the regulatory role of Ca2+ in A2058 cell chemotaxis to CIV, intra- and extracellular [Ca2+] levels were controlled. As shown above, A2058 cells actively migrated to CIV (100 µg/ml) in 1.6 mM Ca2+ basal medium, and this robust response was used as the reference to normalize the migration assay data (Table 1; 100%). Cellular [Ca2+]i response corresponding to this control condition resulted in a peak [time (t) < 200 s] response and a plateau (t > 300 s) [Ca2+]i that decayed with time to approach the baseline level (100– 150 nM), as previously reported (28). [Ca2+]i assays were taken to a duration of 4 h to reflect the time course of a typical chemotaxis assay (Fig. 2). The data indicate that a CIV stimulation in this control condition results in a singular [Ca2+]i peak, occurring at the beginning, which subsides to the baseline by ~1,000– 1,500 s.
Table 1.
A2058 cell migration is attenuated at elevated [Ca2+]i
| %Migration | Baseline [Ca2+]i, nM | ΔPeak [Ca2+]i, nM | ΔPlateau [Ca2+]i, nM | |
|---|---|---|---|---|
| Control | 100 | 100–150 | 148.3 ± 26.2 | 127.9 ± 28.8 |
| Thapsigargin | 15.2 ± 3.5 | 600 – 800 | 41.6 ± 5.2 | 115.1 ± 23.4 |
| Ionophore | 21.1 ± 1.4 | 600 – 800 | n/s | n/s |
Values are means ± SD; n = 5–10. A2058 cell migration data are shown with the corresponding intracellular Ca2+ concentration ([Ca2+]i) response amplitudes. [Ca2+]i responses are indicated in terms of the baseline concentration before addition of type IV collagen (CIV; 100 µg/ml) to the assay chamber and the response amplitude difference above and beyond the baseline, indicated by peak and plateau [Ca2+]i “Δ”. Peak response occurred within t < 200 s after the addition of CIV to the experiment chamber, whereas plateau response was that of the average amplitude between 200 < t < 300 s. Thapsigargin (TG) pretreatment involved 1.0 µM TG in 1.6 mM Ca2+ basal medium applied to cells 30 min before CIV stimulation. Ionophore pretreatment involved 1.0 mM application in 1.6 mM Ca2+ basal medium 15 min before CIV stimulation. n/s, the response is negligible or within the error limits.
Fig. 2.
A2058 cell ratiometric intracellular Ca2+ concentration ([Ca2+]i) profile in response to CIV stimulation. Digital ratiometric fluorescence measurements of [Ca2+]i were performed on A2058 cells responding to CIV (100 µg/ml) as described in MATERIALS AND METHODS. Inset: long-duration (4 h) Ca2+ measurements mirror the corresponding migration assay. The peak [Ca2+]i response occurs within 200 s as shown, while later plateau dissipates to the baseline level by ~1,000–1,500 s into an assay. The frequency of ratiometric data acquisition was once every 6 s for t < 10 min and once every 2 min for the 4-h assay. Nonspecific background fluorescence was subtracted from each ratiometric image set before off-line data calibration and analysis. Due to photobleach of fura 2, short- and long-time Ca2+ assays were carried out independently using separate cell monolayers grown on glass microscope coverslips. Short-time data show representative profile of a single cell in a field of view (10–15 individual cells were simultaneously monitored per coverslip per experiment), whereas long-time data (inset) show a composite of 6 profiles of individual cells from a given field of view. Coverslips (5–10) of cell monolayers were assayed.
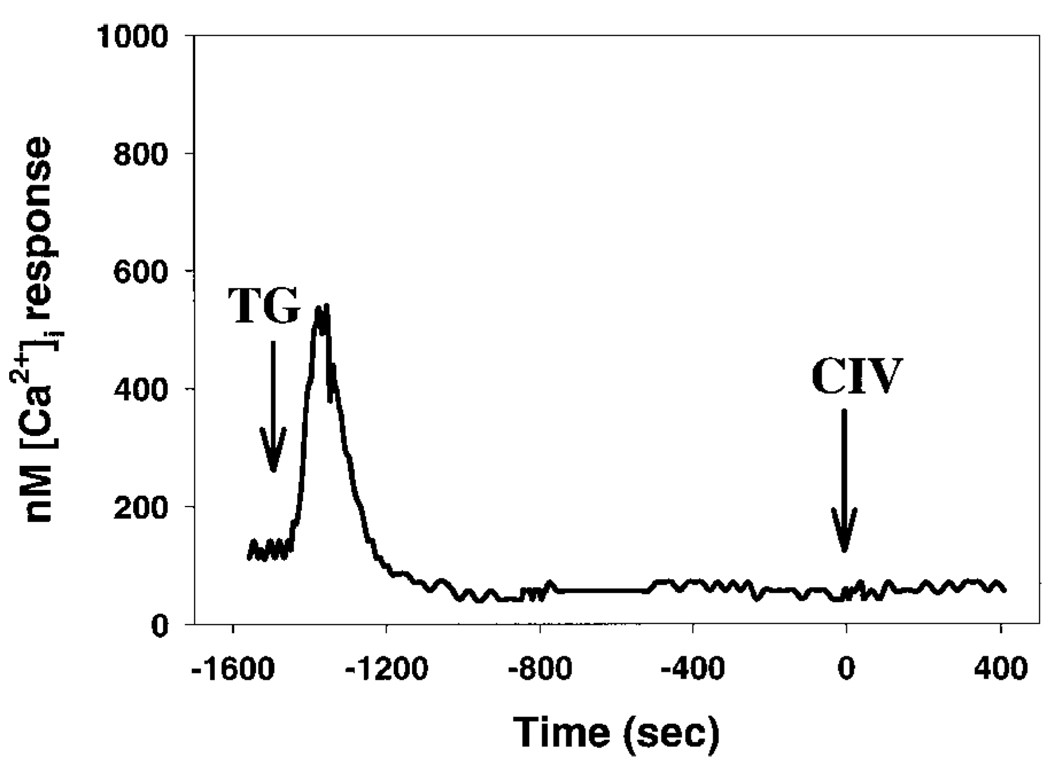
To determine the functional importance of the SERCA and to address the effects of SOCR occurring due to intracellular Ca2+ store drainage, we used TG to prevent refilling of the stores and to induce SOCR influx of extracellular Ca2+. TG pretreatment resulted in a substantial attenuation of cell migration to CIV (Table 1; 15.2 ± 3.5%). This result was accompanied by an elevated baseline [Ca2+]i from TG-SOCR before the CIV perfusion (Table 1; 600–800 nM). Upon stimulation with CIV, peak [Ca2+]i response was significantly attenuated, while the later plateau [Ca2+]i was additive to the elevated baseline due to TG-SOCR (Fig. 3). This indicated that integrin signaling recruited overlapping internal Ca2+ stores, while the signaling mechanism triggering the later influx utilized a distinctly different set of channels from TG-SOCR.
Fig. 3.
Thapsigargin (TG)-pretreated A2058 cell [Ca2+]i profile in response to CIV stimulation. Arrow indicates the time point of 100 µg/ml of CIV perfusion into the experiment chamber. TG (1.0 µM) was applied to the confluent monolayer of A2058 cells 30 min before the perfusion of CIV. The basal medium contained 1.6 mM Ca2+. Elevated baseline [Ca2+]i was due to TG-induced store-operated Ca2+ release (SOCR) influx. Perfusion of CIV induces a minimal peak response while later plateau [Ca2+]i is additive to TG-SOCR [Ca2+]i amplitude. Presented profile is of a representative single cell response from an experiment. Each experiment consisted of 10–15 individual cells assayed simultaneously in a given view field. Eight independent experiments were performed under this condition.
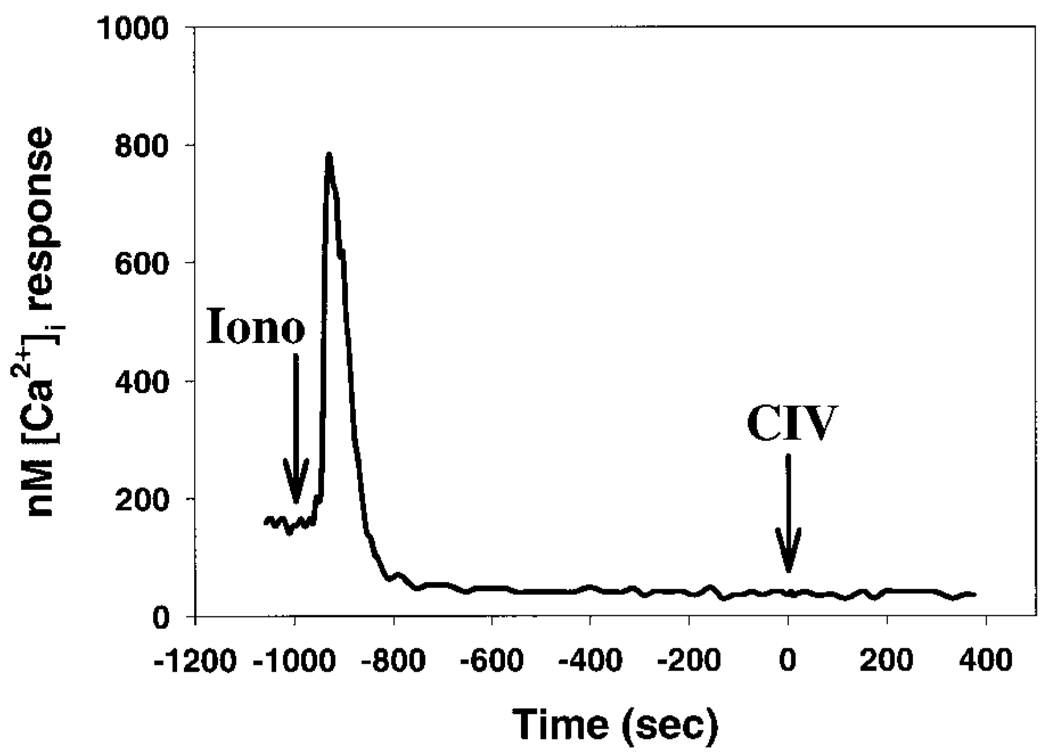
The mechanism by which TG induces SOCR is via inactivation of intracellular Ca2+-ATPases on endoplasmic reticulum (17). We wished to determine whether the elevated and sustained level of [Ca2+]i, and not the functional inactivation of Ca2+-ATPase, was inhibiting A2058 cell migration in response to α2β1-integrin ligation. Ca2+ ionophore A-23187 was used to enhance the transport of extracellular Ca2+ into cell cytosol, independent of SERCA activity. Cell migration was comparably attenuated when cells were pretreated with 1.0 µM A-23187 in 1.6 mM Ca2+ basal medium (Table 1; 21.1 ± 1.4%). The baseline [Ca2+]i was shown to be elevated to a high level, similar to TG-SOCR (Table 1; 600–800 nM), while the peak and plateau [Ca2+]i associated with CIV stimulation were eliminated. Together, these results indicated that the elevated level of [Ca2+]i is the primary inhibitor of A2058 cell motility in response to α2β1-integrin ligation.
Subbasal [Ca2+]i was sufficient for cell chemotaxis to CIV
We wished to determine the effects of low Ca2+ on A2058 cell migration to CIV. Chelating the Ca2+ in basal medium with 3.2 mM EGTA slightly reduced cell migration in response to CIV (Table 2; 69 ± 3.8%). Baseline [Ca2+]i and the peak [Ca2+]i were similar to the control case, while the plateau phase was eliminated due to lack of receptor-mediated Ca2+ entry (Table 2).
Table 2.
A2058 cell migration is restored in subbasal [Ca2+]i
| %Migration | Baseline [Ca2+]i, nM | ΔPeak [Ca2+]i, nM | ΔPlateau [Ca2+]i, nM | |
|---|---|---|---|---|
| Control | 100 | 100–150 | 148.3 ± 26.2 | 127.9 ± 28.8 |
| + EGTA | 69.0 ± 3.8 | 100–150 | 135.3 ± 22.6 | 13.3 ± 3.6 |
| + TG/EGTA | 66.4 ± 3.7 | 100–150 | n/s | −22.4 ± 3.1 |
| + Ionophore/EGTA | 94.5 ± 5.2 | 100–150 | n/s | −72.8 ± 17.2 |
Values are means ± SD; n = 5–10. Cell migration data are shown with the corresponding [Ca2+]i-response amplitudes. [Ca2+]i responses are indicated in terms of the baseline concentration before addition of CIV (100 µg/ml) to the assay chamber and the response amplitude difference above and beyond the baseline. Peak response occurred within t < 200 s after the addition of CIV to the experiment chamber, whereas plateau response was that of the average amplitude between 200 < t < 300 s. EGTA (3.2 mM) was applied to the medium (pH 7.4) to reduce the free extracellular Ca2+ to below 65 nM. Thapsigargin pretreatment involved 1.0 µM TG in EGTA-chelated medium applied to cells 30 min before CIV stimulation. Ionophore pretreatment involved 1.0 µM application in medium containing EGTA 15 min before CIV stimulation.
As shown previously, TG pretreatment significantly attenuated cell migration in 1.6 mM Ca2+ basal medium. Applying EGTA to chelate the extracellular Ca2+ after TG pretreatment resulted in a substantial recovery of cell migration (Table 2; 66.4 ± 3.7%). In this case, the baseline [Ca2+]i was restored to the control level in the absence of extracellular Ca2+. The addition of CIV did not elicit peak [Ca2+]i response, which further indicates that internal sources were overlapping with TG-inducible stores (Fig. 4). The plateau phase was also eliminated, which resulted in a subbaseline [Ca2+]i because of a lack of Ca2+ influx and a slow leakage of [Ca2+]i due to the absence of extracellular Ca2+ (Table 2).
Fig. 4.
TG-pretreated A2058 cells in EGTA do not release [Ca2+]i in response to CIV stimulation. Cells were placed in 3.2 mM EGTA-chelated medium, and TG (1.0 µM) was applied 30 min before CIV (100 µg/ml) perfusion. CIV application does not further induce internal release due to overlapping internal stores. The profile represents a typical response from a single cell in a field of view. Individual cells (10–15) were monitored simultaneously in every view field. Three independent experiments were conducted using separate coverslips of cell monolayers per experiment.
Previously, we attempted to chelate [Ca2+]i using 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA)-AM. This method of sequestering [Ca2+]i resulted in a low cell viability and loss of cell adhesion to substrate over the duration of a migration assay (unpublished observations). Kao (16) reported the use of Ca2+ ionophore A-23187 to facilitate the drainage of cellular Ca2+ while maintaining low extracellular Ca2+ using EGTA. We extended this technique to remove [Ca2+]i from A2058 cells without affecting the cell viability. Applying 3.2 mM EGTA to A-23187-pretreated cells resulted in a full recovery of cell chemo-taxis to CIV (Table 2; 94.5 ± 5.2%). The initial baseline [Ca2+]i before the perfusion of CIV was similar to the control baseline. CIV perfusion did not induce a peak response, and the plateau was eliminated to result in a subbasal [Ca2+]i (Table 2 and Fig. 5). These observations indicated that activation of A2058 cell chemotaxis to CIV, mediated by ligation of α2β1-integrin, did not require a cellular Ca2+ signaling mechanism.
Fig. 5.
Ionophore (Iono) A-23187-pretreated A2058 cells in EGTA do not elevate [Ca2+]i in response to CIV. Cells were placed in 3.2 mM EGTA-chelated medium, and A-23187 ionophore (1.0 µM) was applied 15 min before CIV (100 µg/ml) perfusion. CIV application does not induce additional [Ca2+]i response. The profile represents the response of a single cell in a field of view. Individual cells (10–15) were monitored in each view field per experiment. Five experiments were performed using separate coverslips of cell monolayers per experiment.
Elevated [Ca2+] i downregulates cell motility via calcineurin-mediated signaling
We have shown that a high [Ca2+]i is inhibiting A2058 cell migration in response to CIV. Calmodulin-dependent protein phosphatase IIB (calcineurin) has been implicated in an [Ca2+]i signaling pathway mediating neutrophil migration to ECM proteins fibronectin and vitronectin (14). Similarly, TG- and A-23187-induced prostatic carcinoma cell apoptosis was mediated reversibly by Ca2+/calmodulin-activated calcineurin (37). We therefore asked whether a signaling pathway involving calcineurin was recruited when TG or A-23187 increased [Ca2+]i in A2058 cells. Furthermore, we wished to determine whether activation of this particular pathway may downregulate cell chemotaxis in response to CIV. CsA specifically inhibits calcineurin activation by formation of a complex with cyclophilin A and then binds to calcineurin to inhibit the Ca2+/calmodulin-dependent activation of calcineurin (14, 20, 30, 37). Cells incubated with 1.5 µg/ml of CsA (20 h) were pretreated with 1.0 µM TG or 1.0 µM A-23187 as described and assayed for migration to 100 µg/ml of CIV in a 1.6 mM Ca2+ basal medium. CsA incubation of 1–2 h resulted in a negligible recovery of cell migration, while an overnight incubation (15–20 h) maximally restored cell migration under these conditions (Fig. 6). The recovery of cell migration in the presence of an elevated [Ca2+]i due to TG or A-23187 pretreatment indicated that Ca2+/calcineurin-mediated signaling events were recruited during an elevated [Ca2+]i to downregulate A2058 cell chemotaxis to CIV.
Fig. 6.
Effects of calcineurin inactivation on A2058 cell chemotaxis in elevated [Ca2+]i conditions. Cells were pretreated with cyclosporin A (CsA) for 20 h at 1.5 µg/ml before migration assays (+ or − indicates presence or absence of CsA treatment). CsA had no significant effect on control motility to 100 µg/ml of CIV. Cells were pretreated with TG (1.0 µM) or ionophore A-23187 (1.0 µM) in the presence of 1.6 mM Ca2+ basal medium as indicated in MATERIALS AND METHODS. Data are normalized by the control response and represent the average of 3–6 independent experiments containing 12–24 wells/condition as presented ± SD.
DISCUSSION
In this study, we examined the role of [Ca2+]i in chemotactic response of A2058 cells to CIV. It was previously shown that A2058 cells respond to CIV stimulation by a Ca2+ signal, and the source of internal release was suggested to be from non-IP3-sensitive stores (28). However, the causal relationship between the recruitment of an [Ca2+]i signaling mechanism and cell motility to CIV remained unclear. In this study, we attempted to elucidate whether such a link between cell motility and [Ca2+]i recruitment existed in A2058 cell chemotaxis to CIV. We further wished to identify putative chemotaxis receptors mediating this specific response to CIV. In our results, we have identified α2β1-integrin as the chemotaxis receptor of A2058 cells to soluble CIV. This CIV-mediated response was also confirmed in other tumor cell lines such as C8161 melanoma, 293T embryonic kidney carcinoma, and MDA-MB435 breast carcinoma (unpublished data).
Previously, Leavesley et al. (18) have shown that an endothelial cell spreading over immobilized CIV was mediated by α2β1-integrin and that this process was extracellular Ca2+ insensitive, while the same cell spreading over immobilized vitronectin recruited αvβ3 and was extracellular Ca2+ sensitive. Hendey and Maxfield (14) have shown that neutrophil migration over fibronectin and vitronectin resulted in [Ca2+]i transients, while inhibition caused the neutrophils to remain anchored to the substrate, resulting in a loss of motility. Whereas these studies focused on cell motility over immobilized ECM proteins to result in a haptotactic response, our system employed a polylysine-coated substrate with a soluble form of CIV as the chemoattractant following protocols of previous studies (5, 13). We have shown that chemotaxis of A2058 cells to CIV was mediated by the same set of integrin receptors that induced a haptotactic response in endothelial cells (18).
Unlike the neutrophils, A2058 cells did not exhibit repeated [Ca2+]i transients in our study. The cell response to CIV consisted of a singular [Ca2+]i peak followed by a decaying plateau [Ca2+]i that reached the baseline within 1,000–1,500 s. Hendey and Maxfield (14) have shown that cell spreading and pseudopodial extensions were not [Ca2+]i dependent in neutrophils. However, we have shown previously that A2058 cell pseudopodial activity to CIV was extracellular Ca2+ sensitive (15). Furthermore, neutrophil motility was mediated by calcineurin-calmodulin activation (14), whereas we have shown that tumor cells recruited a calcineurin signaling pathway only at an elevated [Ca2+]i to downregulate cell motility. Together, differential sensitivity of cell motility to [Ca2+]i in these cells was clearly identified and characterized in these cell types.
The most important finding we report is that A2058 cells migrated significantly when [Ca2+]i was maintained at a subbasal level throughout the duration of a chemotaxis assay. An application of ionophore in the absence of extracellular Ca2+ results in a complete efflux of [Ca2+]i, because this is the basis for obtaining the minimum ratio condition in an in situ ratiometric microscopy calibration (12, 16, 38). We have used this approach to deplete the [Ca2+]i instead of using more invasive [Ca2+]i chelators, such as BAPTA-AM or EGTA-AM. These chelating agents have been suggested to cause affects of reduced cell viability and cell adhesion (8, 40). Because of the concentration difference driving the [Ca2+]i drainage, the extent of extracellular free Ca2+ sequestration was maintained to result in a subresting baseline [Ca2+]i of ~65 nM. With these conditions, the cell viability and motility were maintained throughout the course of a migration assay. This demonstrated that the method was less hazardous to cells while sufficiently removing the cellular Ca2+. We have shown that abrogated [Ca2+]i signaling did not affect cell motility to CIV. This clearly indicates that A2058 cells do not require an [Ca2+]i signaling mechanism during the α2β1-mediated chemotaxis over polylysine substrate.
As previously reported, ligation of α2β1-integrin resulted in an [Ca2+]i response (28). The induction of this response was neither necessary nor sufficient to mediate cell migration to CIV in our results. The elevation of [Ca2+]i via the SOCR influx was shown to be inhibitory to cell motility via a calcineurin-mediated mechanism. Furthermore, the role of SERCA and the refilling of intracellular stores were insignificant during cell chemotaxis to CIV. These results indicated that cells possess many redundant signaling mechanisms, and [Ca2+]i induction in A2058 cells responding to CIV may be one such mechanism.
In an intact cell, regulatory mechanisms function to control the level and rate of Ca2+ flux. Evidently, Leavesley et al. (18) suggested that highly localized [Ca2+]i differences in a polarized cell undergoing chemotaxis may be responsible for the topical concentration-dependent differential role of Ca2+ in promoting adhesion and deadhesion at the sites of focal contacts. Furthermore, it appears that different cell lineages and different ECM chemotactic ligands present variable degrees of dependence on the levels of cellular Ca2+ (3, 4, 18, 28). Our results indicate that A2058 cell migration mediated by α2β1-integrin does not require the associated internal [Ca2+]i release nor later influx. Present work also demonstrates that the initial intracellular Ca2+ release in response to CIV originates from TG-sensitive stores, while the later receptor-mediated influx mechanism utilizes a distinctly separate set of signaling pathways. There are various theories that attempt to account for the nature of Ca2+ regulation encountered by nonexcitable cells. The likelihood of two distinctly separate signaling pathways recruited in Ca2+ influx regulation may be attributed to the signaling direction (23, 32). Depletion of intracellular stores is a signal that cells transmit via either the cytoskeletal network contraction or with yet another unidentified molecular signaling mechanism (24, 25). Conversely, integrin engagement and an ensuing focal contact formation with subsequent whole cell polarization originate from an extracellular stimulus. Hence, the formation of a focal adhesion complex initiated by an integrin receptor ligation may likely trigger different intracellular signaling intermediates than those driven by TG-induced store drainage. In our results, the influx Ca2+ amplitudes were additive to indicate that α2β1-integrin likely triggered its cognate receptor-mediated Ca2+-influx mechanism.
In summary, we have shown that CIV-induced chemotaxis in the A2058 human melanoma cell line was mediated by α2β1-integrin in vitro. The ligation of integrin receptors induced an [Ca2+]i response in these cells as previously reported (28). We report that an elevated [Ca2+]i, due to SOCR influx caused by an application of TG or A-23187 ionophore, down-regulated cell motility via the calcineurin-mediated mechanism in A2058 cells. While an elevated [Ca2+]i resulting from TG or ionophore treatment in a 1.6 mM Ca2+ basal medium caused a significant attenuation of cell motility, removing extracellular Ca2+ with EGTA restored cell motility to near control levels under these conditions. Most important, a sustained level of subbaseline [Ca2+]i did not affect cell motility to CIV via α2β1-integrin signaling. Together, these observations suggested that [Ca2+]i signaling recruited by α2β1-integrin ligation was neither necessary nor sufficient to mediate A2058 cell chemotaxis to CIV.
Acknowledgments
We acknowledge Andrew J. Henderson and Julie A. Cook at Penn State University, Veterinary Science Department, for reagents and Loretta L. Collins at University of Rochester, Department of Pathology, for reference work and helpful discussions.
This work was supported by National Cancer Institute Grant CA-76434 and National Science Foundation (NSF) Grant NSF-BES9502069. Cheng Dong is a recipient of the NSF Career Award.
REFERENCES
- 1.Akiyama SK, Yamada KM. Introduction: adhesion molecules in cancer. Semin Cancer Biol. 1993;4:215–218. [PubMed] [Google Scholar]
- 2.Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD. Molecular Biology of the Cell. New York: Garland; 1994. pp. 721–858.pp. 950–1006.pp. 1255–1269. [Google Scholar]
- 3.Alessandro R, Masiero L, Lapidos K, Spoonster J, Kohn EC. Endothelial cell spreading on type IV collagen and spreading-induced FAK phosphorylation is regulated by Ca2+ influx. Biochem Biophys Res Commun. 1998;248:635–640. doi: 10.1006/bbrc.1998.8705. [DOI] [PubMed] [Google Scholar]
- 4.Alessandro R, Masiero L, Liotta LA, Kohn EC. The role of calcium in the regulation of invasion and angiogenesis. In Vivo. 1996;10:153–160. [PubMed] [Google Scholar]
- 5.Aznavoorian S, Stracke ML, Krutzsch H, Schiffmann E, Liotta LA. Signal transduction for chemotaxis and hapto-taxis by matrix molecules in tumor cells. J Cell Biol. 1990;110:1427–1438. doi: 10.1083/jcb.110.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bers DM, Patton CW, Nuccitelli R. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 1994;40:3–29. doi: 10.1016/s0091-679x(08)61108-5. [DOI] [PubMed] [Google Scholar]
- 7.Cole K, Kohn EC. Calcium-mediated signal transduction: biology, biochemistry, and therapy. Cancer Metastasis Rev. 1994;13:31–44. doi: 10.1007/BF00690417. [DOI] [PubMed] [Google Scholar]
- 8.Dickens CJ, Gillespie JI, Greenwell JR, Hutchinson P. Relationship between intracellular pH (pHi) and calcium in avian heart fibroblasts. Exp Cell Res. 1990;187:39–46. doi: 10.1016/0014-4827(90)90113-o. [DOI] [PubMed] [Google Scholar]
- 9.Dong C, Aznavoorian S, Liotta LA. Two phases of pseudopod protrusion in tumor cells revealed by a micropipette. Microvasc Res. 1994;47:55–67. doi: 10.1006/mvre.1994.1005. [DOI] [PubMed] [Google Scholar]
- 10.Dong C, You J, Aznavoorian S, Savarese DMF, Liotta LA. In: Cell Mechanics and Cellular Engineering. Mow VC, Guilak F, Tran-Son-Tay R, Hochmuth RM, editors. New York: Springer-Verlag; 1994. pp. 515–533. [Google Scholar]
- 11.Fernandez R, Boxer LA, Suchard SJ. Beta2 integrins are not required for tyrosine phosphorylation of paxillin in human neutrophils. J Immunol. 1997;159:5568–5575. [PubMed] [Google Scholar]
- 12.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 13.Harvath L, Falk W, Leonard EJ. Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods. 1980;37:39–45. doi: 10.1016/0022-1759(80)90179-9. [DOI] [PubMed] [Google Scholar]
- 14.Hendey B, Maxfield FR. Regulation of neutrophil motility and adhesion by intracellular calcium transients. Blood Cells Mol Dis. 1993;19:143–164. [PubMed] [Google Scholar]
- 15.Hodgson L, Kohn EC, Dong C. Extracellular lipid-mediated signaling in tumor cell activation and pseudopod protrusion. Int J Cancer. 2000;88:593–600. doi: 10.1002/1097-0215(20001115)88:4<593::aid-ijc12>3.0.co;2-o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kao JPY. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- 17.Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem. 1992;267:17624–17630. [PubMed] [Google Scholar]
- 18.Leavesley DI, Schwartz MA, Rosenfeld M, Cheresh DA. Integrin β1- and β3-mediated endothelial cell migration is triggered through distinct signaling mechanisms. J Cell Biol. 1993;121:163–170. doi: 10.1083/jcb.121.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lester BR, McCarthy JB. Tumor cell adhesion to the extracellular matrix and signal transduction mechanism implicated in tumor cell motility, invasion and metastasis. Cancer Metastasis Rev. 1992;11:31–44. doi: 10.1007/BF00047601. [DOI] [PubMed] [Google Scholar]
- 20.Lotem J, Kama R, Sachs L. Proc Natl Acad Sci USA. Vol. 96. 1999. Suppression or induction of apoptosis by opposing pathways downstream from calcium-activated calcineurin; pp. 12016–12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. J Cell Biol. 1997;138:913–926. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masur SK, Idris A, Mechelson K, Antohi S, Zhu LX, Weissberg J. Integrin-dependent tyrosine phosphorylation in corneal fibroblasts. Invest Ophthalmol Vis Sci. 1995;36:1837–1846. [PubMed] [Google Scholar]
- 23.Putney JW., Jr Excitement about calcium signaling in inexcitable cells. Science. 1993;262:676–678. doi: 10.1126/science.8235587. [DOI] [PubMed] [Google Scholar]
- 24.Putney JW, Jr, Bird GJ. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr Rev. 1993;14:610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- 25.Randriamampita C, Tsien RY. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- 26.Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rink TJ. Receptor-mediated calcium entry. FEBS Lett. 1990;268:381–385. doi: 10.1016/0014-5793(90)81290-5. [DOI] [PubMed] [Google Scholar]
- 28.Savarese DMF, Russell JT, Fatatis A, Liotta LA. Type IV collagen stimulates an increase in intracellular calcium. J Biol Chem. 1992;267:21928–21935. [PubMed] [Google Scholar]
- 29.Schnaper HW, Kleinman HK. Regulation of cell function by extracellular matrix. Pediatr Nephrol. 1993;7:96–104. doi: 10.1007/BF00861587. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber SL, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz MA, Ingber DE. Integrating with integrins. Mol Biol Cell. 1994;5:389–393. doi: 10.1091/mbc.5.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz MA, Toksoz D, Khosravi-Far R. Transformation by Rho exchange factor oncogenes is mediated by activation of an integrin-dependent pathway. EMBO J. 1996;15:6525–6530. [PMC free article] [PubMed] [Google Scholar]
- 34.Sergeev IN, Rhoten WB. Regulation of intracellular calcium in human breast cancer cells. Endocrinology. 1998;9:321–327. doi: 10.1385/ENDO:9:3:321. [DOI] [PubMed] [Google Scholar]
- 35.Stracke ML, Murata J, Aznavoorian S, Liotta LA. The role of extracellular matrix in tumor cell metastasis. In Vivo. 1994;8:49–58. [PubMed] [Google Scholar]
- 36.Tawil N, Wilson P, Carbonetto S. Integrin in point contacts mediate cell spreading: factors that regulate integrin accumulation in point contacts vs. focal contacts. J Cell Biol. 1993;120:261–271. doi: 10.1083/jcb.120.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tombal B, Weeraratna AT, Denmeade SR, Isaacs JT. Thapsigargin induces a calmodulin/calcineurin-dependent apoptotic cascade responsible for the death of prostatic cancer cells. Prostate. 2000;43:303–317. doi: 10.1002/1097-0045(20000601)43:4<303::aid-pros10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 38.Tsien RY, Harootunian AT. Practical design criteria for a dynamic ratio imaging system. Cell Calcium. 1990;11:93–109. doi: 10.1016/0143-4160(90)90063-z. [DOI] [PubMed] [Google Scholar]
- 39.Vandenberg P, Kern A, Ries A, Luckenbill-Edds L, Mann K, Kuhn K. Characterization of a type IV collagen major binding site with affinity to alpha 1 beta 1 and the alpha 2 beta 1 integrins. J Cell Biol. 1991;113:1475–1483. doi: 10.1083/jcb.113.6.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xie L, Clunn GF, Lymn JS, Hughes AD. Role of intracellular calcium ([Ca2+]i) and tyrosine phosphorylation in adhesion of cultured vascular smooth muscle cells to fibrinogen. Cardiovasc Res. 1998;39:475–484. doi: 10.1016/s0008-6363(98)00079-0. [DOI] [PubMed] [Google Scholar]