Abstract
Effective cell-mediated antitumor immunity requires the activation of tumor-reactive T cells and the trafficking of activated T cells to tumor sites. These processes involve the extravasation of lymphocytes from the blood and lymphatics, and their homing to lymph nodes and tumors. L-selectin (CD62L) is an important molecule in these processes. It directs naive lymphocytes to peripheral lymph nodes where they become activated and it traffics naive lymphocytes to inflammatory environments, such as tumors. Individuals with advanced cancer are immune suppressed due to myeloid-derived suppressor cells (MDSC), a population of immature myeloid cells that accumulate to high levels in response to tumor-secreted and proinflammatory factors. We now demonstrate that the reduction in T cell levels of L-selectin that is commonly seen in individuals with cancer inversely correlates with MDSC levels. Three lines of evidence demonstrate that MDSC directly down-regulate L-selectin on naive T cells: 1) naive T cells cocultured with tumor-induced MDSC have reduced L-selectin; 2) T cells in tumor-free aged mice with elevated levels of MDSC have reduced L-selectin, and 3) peritoneal exudate T cells of tumor-free mice treated with plasminogen activator urokinase to elevate MDSC have reduced levels of L-selectin. MDSC are likely to down-regulate L-selectin through their plasma membrane expression of ADAM17 (a disintegrin and metalloproteinase domain 17), an enzyme that cleaves the ectodomain of L-selectin. Therefore, MDSC down-regulate L-selectin levels on naive T cells, decreasing their ability to home to sites where they would be activated. This is another mechanism by which MDSC inhibit antitumor immunity.
Cell-mediated adaptive antitumor immunity and immune surveillance depend on the activation of naive lymphocytes in tumor-draining lymph nodes (TDLN)3 or within the tumor microenvironment (1–3). These activities involve the extravasation of lymphocytes from the blood and lymphatics and their homing to lymph nodes and tumor sites, processes that involve members of the selectin family of proteins (2–4). L-selectin, also known as CD62L, plays important roles in these processes. It directs naive lymphocytes to peripheral lymph nodes where they interact with Ag and become activated (5). If T cell expression of L-selectin is lost or decreased, naive T cells do not home to lymph nodes and T cell responses are diminished (6–8). L-selectin also directs naive L-selectinhigh lymphocytes to sites of acute and chronic inflammation (3, 9), such as the tumor microenvironment, where naive T cells can also become Ag activated and undergo firm adhesion and transmigration (10, 11). During inflammation, endothelial cells are activated by inflammatory cytokines to express adhesion molecules and synthesize chemokines, which results in the arrest of rolling leukocytes (10). The critical role of L-selectin in this process is demonstrated by the impaired recruitment of naive T cells to inflammatory sites in L-selectin-deficient mice (9, 11–15).
Many patients with advanced cancer are immune suppressed and are unable to activate tumor-reactive T cells (16). These immune defects are mediated in part by myeloid-derived suppressor cells (MDSC), profoundly immune suppressive cells which are ubiquitously present in cancer patients and experimental animals with malignant tumors (17–22). MDSC originate in the bone marrow of mice as Gr1+CD11b+CD31+ hematopoietic progenitor cells that under normal differentiation conditions mature to dendritic cells, macrophages, and/or granulocytes. In tumor-bearing individuals, tumor-derived factors, including proinflammatory mediators, block the differentiation of these immature cells, resulting in their accumulation and retention in the individual’s blood, lymphoid organs, and at tumor sites (23, 24). Immune suppression by MDSC has been attributed to multiple mechanisms including the production of arginase (25–29) and inducible NO synthase (17, 22, 30), nitration of the T cell Ag receptor (31), the skewing of macrophage activity (32), and the blocking of NK cytotoxicity (33). We now report that MDSC also facilitate immune suppression by down-regulating L-selectin on CD4+ and CD8+ T lymphocytes.
Materials and Methods
Mice
BALB/c mice (breeding stock obtained from The Jackson Laboratory), BALB/c clone 4 (αβ TCR specific for influenza hemagglutinin (HA) peptide 518–526 restricted to H-2Kd) (34), and BALB/c TS1 (αβ TCR specific for influenza HA peptide 110–119 restricted to I-Ed) (35) mice were bred and maintained in the University of Maryland Baltimore County Biology Department animal facility or at the Miller School of Medicine. Female mice <6 mo of age were used for all experiments except aged BALB/c mice which were 8 or 24 mo at the time of experiments. All animal procedures were approved by the Institutional Animal Care and Use Committees of the participating institutions.
Abs, cell lines, and reagents
Anti-mouse Abs CD3-PE, CD62L-FITC, CD44-FITC, CCR7-FITC, Gr-1-FITC, CD11b-PE, Vβ8.1, 8.2-PE (hereafter called Vβ8-PE), rat IgG2a-PE, and rat IgG2a-FITC were purchased from BD Pharmingen. CD4-tricolor (TC), rat IgG2a-TC, and CD8-TC were from Caltag. ADAM17 (a disintegrin and metalloproteinase domain 17) was purchased from Abcam and FITC-conjugated donkey anti-rabbit IgG from Valeant Pharmaceuticals. Saponin was purchased from Sigma-Aldrich. 4T1 mammary carcinoma cells were maintained in culture as previously described (36). The J774 macrophage cell line was obtained from the American Type Culture Collection and maintained as described elsewhere (28). BALB/c-derived DA-3 and D1-DMBA-3 mammary carcinomas were maintained as previously described (37). HA110–119 peptide was synthesized in the Biopolymer Core Facility at the University of Maryland Medical School (Baltimore, MD).
Tumor inoculations, tumor growth, surgery, organ preparations
BALB/c mice were inoculated in the abdominal mammary gland with the 4T1 tumor (7000 cells/50 μl/mouse) (36) or s.c. with the DA-3 or D1.DMBA tumors (1 × 106 cells/100 μl/mouse) (37) and followed for tumor progression as described. 4T1 primary mammary tumors were surgically removed as described elsewhere (38, 39), and wounds were closed with Nexaband liquid (H. Schein, Melville, NY). More than 95% of mice survived surgery. Mice in which primary tumors recurred at the site of the original tumor inoculation (<5% of operated mice) were omitted from the study. Mice were bled from the tail or submandibular region for MDSC when their primary tumors were 6–8 mm in diameter. Single-cell suspensions of lymph nodes (LNs) and spleens were prepared by dissociating cells through a 70-μm nylon cell strainer (BD Biosciences). RBCs were depleted from blood and tissues as previously described (39).
Gemcitabine treatment
Mice were inoculated i.p. with 1.5 mg of gemcitabine (provided as Gemzar from Eli Lilly) in 50 μl of saline twice per week for the first week and once per week thereafter. Treatment was started 1 day after surgery and continued until mice were sacrificed.
Flow cytometry
Cells were labeled for immunofluorescence and analyzed by flow cytometry for cell surface (40) and/or internal molecules (41) as described. Abs were diluted in PBS with 2% FCS (HyClone). Samples were analyzed on an Epics XL or Cyan ADP flow cytometer (Beckman Coulter) and analyzed using FCS Express V3 (De Novo Software) or Summit Software (Beckman Coulter). For L-selectin expression, cells were stained for CD3, CD8, or CD4 and L-selection, and the gated CD4+CD3+ and CD8+CD3+ cells were analyzed for L-selectin expression.
T cell proliferation
T cell proliferation was measured as previously described (28), with the following modifications: proliferation medium (HL1 medium; BioWhittaker) was supplemented with 1% penicillin, 1% streptomycin, and 1% GlutaMAX (Invitrogen). Splenocytes from transgenic mice were cocultured for 3 days with their cognate peptide at 1 × 105 cells/200 μl/well in 96-well plates. Tritiated thymidine (1μCi/50 μl/well) was added 18 h before harvesting. Data are the mean cpm ± SD of triplicate wells.
Urokinase plasminogen activator (uPA) recruitment of MDSC
Normal 12-wk-old BALB/c splenic T cells were purified using Thy1.2 microbeads (Miltenyi Biotec), and 4 × 106 T cells were coinjected i.p. in tumor-free BALB/c mice with either 1 μg of uPA or saline as control. Five hours later, peritoneal cells were collected as described elsewhere (42) and labeled for MDSC or T cell markers and L-selectin.
Statistical analysis
Student’s t test for unequal variance was done using Microsoft Excel 2000. The Pearson product-moment correlation coefficient test and the Kruskal-Wallis test were used to determine significance (see Figs. 2 and 5, respectively).
FIGURE 2.

Percentage of MDSC and T cell L-selectin levels are inversely correlated in tumor-bearing mice. A, BALB/c mice were inoculated on day 0 with 4T1 tumor cells and primary tumors were surgically resected on day 25 when metastatic disease was established and primary tumors were 6.5 ± 1.8 mm in diameter. Mice were bled on the indicated days and the percentage of Gr1+CD11b+ MDSC and the MCF for L-selectin on gated CD3+CD4+ and CD3+CD8+ T cells were assessed by flow cytometry. Each line represents the values for an individual mouse. Solid lines identify mice whose MDSC levels remained elevated after surgery. Dotted lines represent mice whose MDSC levels decreased after surgery. MDSC and L-selectin levels are inversely correlated (CD4+ T cells: r = −0.86; p < 0.0001; CD8+ T cells: r = −0.77, p < 0.0001). B, Experiment as in A, except BALB/c mice were treated with gemcitabine (1.5 mg/50 μl/mouse) at the indicated times starting 1 day after surgery. MDSC and L-selectin levels are inversely correlated (CD4+ T cells: r = −0.67; p < 0.0001; CD8+ T cells: r = −0.71; p < 0.0001). The data of A and B are pooled from three independent experiments.
FIGURE 5.
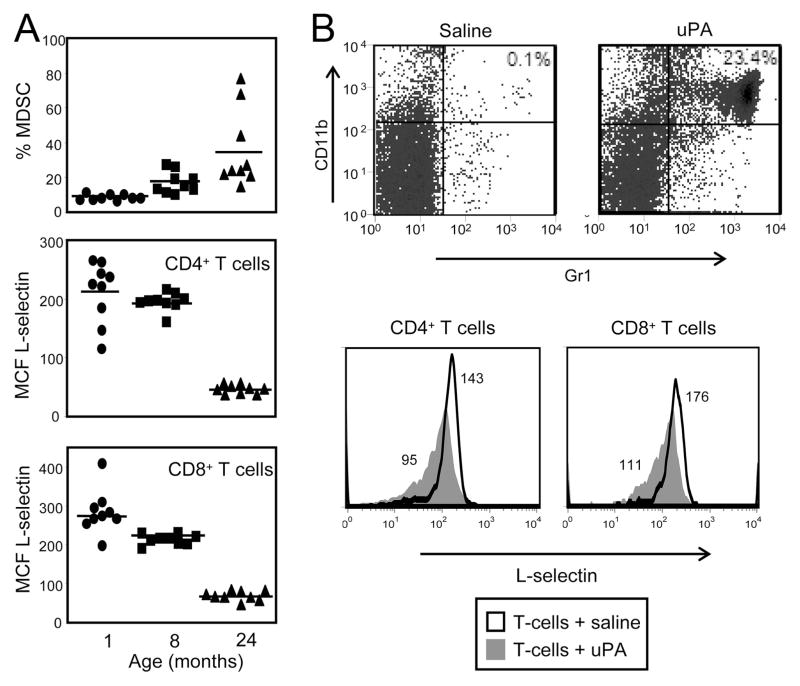
MDSC levels drive L-selectin expression on T cells in tumor-free mice. A, Blood was harvested from 1 (●)-, 8 (■)-, or 24 (▲)-mo-old tumor-free BALB/c mice and the cells were stained for CD4, CD8, CD3, and CD62L or Gr1 and CD11b. Gated CD3+CD4+ and CD3+CD8+ cells were analyzed for L-selectin expression. Percent MDSC (Kruskal-Wallis test, Hc9,9,9 = 19.82, p < 0.001), CD4 L-selectin levels (Kruskal-Wallis test, Hc9,9,9 = 18.10, p < 0.001), and CD8 L-selectin levels (Kruskal-Wallis test, Hc9,9,9 = 21.09, p < 0.001) are significantly different between ages for each group. Data are pooled from three independent experiments. B, Normal BALB/c splenic T cells were purified (97%) using Thy1.2 microbeads and 4 × 106 T cells were injected i.p. in normal BALB/c mice with either 1 μg of uPA or saline. Four hours later, peritoneal cells were collected and stained for CD4, CD8, and L-selectin or Gr1 and CD11b.
Results
CD8+ and CD4+ T cells of tumor-bearing mice with MDSC have an L-selectinlow phenotype
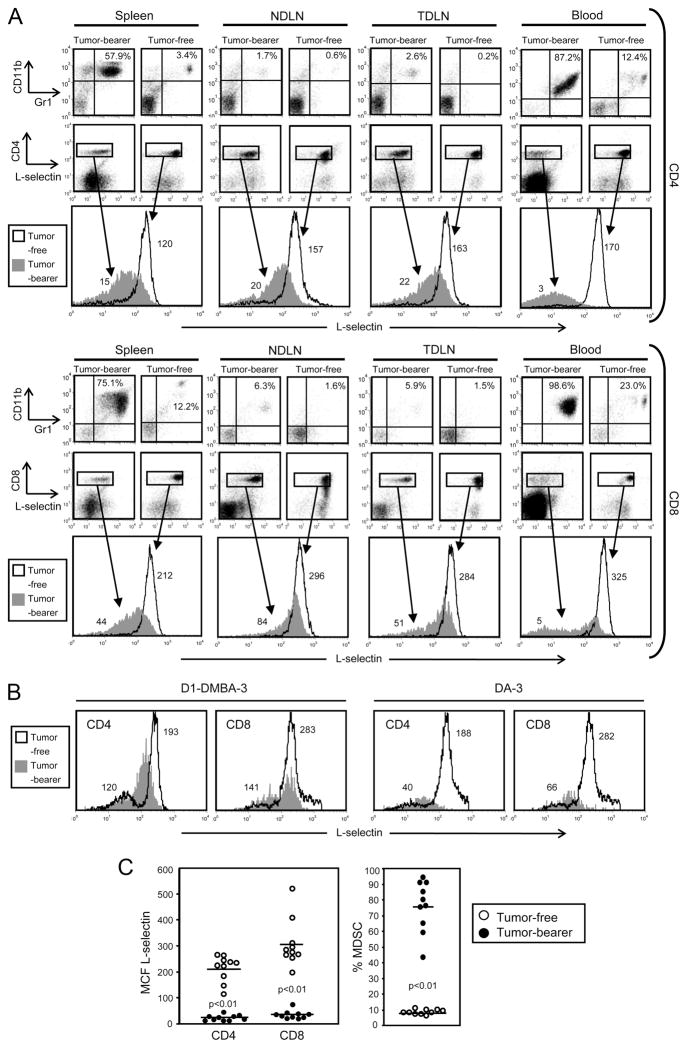
If MDSC alter L-selectin expression on T lymphocytes, then T lymphocytes from the spleen, LNs, and/or blood of tumor-bearing mice may show different L-selectin levels than lymphocytes from tumor-free mice. To test this hypothesis, BALB/c mice were inoculated in the abdominal mammary fat pad with 7000 4T1 mammary carcinoma cells and then splenocytes, TDLN (superficial inguinal), non-tumor draining lymph nodes (NDLN; brachial and axillary), and blood were harvested 28 days later when the mice had established primary tumors and metastatic disease (36). Single-cell suspensions were prepared from each tissue and following lysis of the RBC, the resulting white blood cells were stained for the markers of MDSC (Gr1 and CD11b), T cells (CD4 or CD8), and L-selectin (CD62L). Fig. 1A shows the results from an individual tumor-bearing mouse and an age- and sex-matched tumor-free mouse. MDSC are elevated in the organs of the tumor-bearing mouse compared with the tumor-free mouse. CD4+ and CD8+ T cells of the tumor-bearing mouse have an L-selectinlow phenotype (mean channel fluorescence (MCF) range of 3–22 and 5–84 for CD4+ and CD8+ T cells, respectively), while T cells from the tumor-free mouse have an L-selectinhigh phenotype (MCF range of 120–170 and 212–325 for CD4+ and CD8+ T cells, respectively). CD4+ and CD8+ T cells from BALB/c mice with D1-DMBA-3 or DA-3 mammary carcinomas (n = 5) showed a similar down-regulation of L-selectin (Fig. 1B), as did CD4+ and CD8+ T cells from all tested BALB/c mice with 4T1 tumors (n = 12).
FIGURE 1.
CD8+ and CD4+ T cells of tumor-bearing mice with MDSC have an L-selectinlow phenotype. A, Splenocytes, NDLN (brachial and axillary), TDLN (superficial inguinal), and blood were harvested from a 4T1-tumor bearing BALB/c mouse with a 28-day tumor (primary tumor diameter: 10.1 mm) or from a tumor-free BALB/c mouse. The resulting cells were stained for MDSC (Gr1+CD11b+) and for the T cell markers CD4 or CD8 and L-selectin. CD4+ and CD8+ populations were gated and analyzed for L-selectin expression. Numbers in the single parameter T cell histograms are the MCF of L-selectin for the gated populations. Data are representative of three independent experiments using 12 mice. B, Blood was harvested from BALB/c mice bearing the DA-3 or D1-DMBA-3 tumor and fluorescently labeled, gated, and analyzed for L-selectin expression as in A. Data are representative of two independent experiments using five mice. C, Blood was harvested from 10 tumor-free and 10 tumor-bearing mice (primary tumor diameter: 6.9 ± 2.4 mm) and labeled and analyzed for MDSC and L-selectin as in A (data in A and C are from separate cohorts of mice). Each symbol represents an individual mouse. Tumor-bearing mice have significantly higher levels of MDSC than tumor-free mice (p < 0.01) and CD3+CD4+ and CD3+CD8+ T cells of tumor-bearing mice have significantly lower levels of L-selectin than T cells of the tumor-free group (p < 0.01). Data are from one of four independent experiments.
Fig. 1C shows the MCF of L-selectin on CD4+ and CD8+ T cells (left panel) and the percent MDSC (right panel) in the blood of 4T1 tumor-bearing vs tumor-free BALB/c mice for all mice tested (n = 12). CD4+ and CD8+ T cells consistently have an L-selectinlow phenotype as compared with T cells from tumor-free mice. Therefore, CD8+ and CD4+ T cells of tumor-bearing mice with increased levels of MDSC have an L-selectinlow phenotype.
Percentage of MDSC and T cell L-selectin levels are inversely correlated in tumor-bearing mice
MDSC levels in 4T1 tumor-bearing BALB/c mice increase in proportion to primary and metastatic tumor burden, and surgical removal of primary tumor reduces MDSC levels in the subset of mice that have minimal metastatic disease (28). To determine whether L-selectin levels recover after surgery and reduction of MDSC, BALB/c mice were inoculated with 4T1 on day 0 and primary tumors were removed on day 25. Mice were bled 1 day before 4T1 injection (day −1), 1 day before surgery (day 24), and 10 days after surgery (day 35), and white blood cells were stained for Gr1 and CD11b, or CD3, CD4, CD8, and L-selectin. Stained cells were analyzed by flow cytometry for MDSC and the gated CD4+CD3+ and CD8+CD3+ populations were analyzed for L-selectin expression (Fig. 2A). As expected, the percent MDSC in the blood of tumor-free mice (day −1) was relatively low (<23%) and increased to >60% by 1 day before surgery (day 24) for six of seven tumor-bearing mice. Ten days after surgery (day 35), MDSC levels remained elevated in four of seven mice and decreased in three mice. CD4+ and CD8+ T cells of postsurgery mice with reduced MDSC were L-selectinhigh (dotted lines in Fig. 2A); while T cells of postsurgery mice with high levels of MDSC were L-selectinlow (solid lines in Fig. 2A). Therefore, T cell levels of L-selectin recover if MDSC are reduced, demonstrating that there is an inverse correlation between the quantity of MDSC and L-selectin levels on CD4+ and CD8+ T cells.
Since tumor burden drives MDSC levels, down-regulation of T cell L-selectin could be due to factors originating from tumor cells or from MDSC. To distinguish these alternative mechanisms, L-selectin levels were examined in tumor-bearing mice treated with the chemotherapeutic agent gemcitabine. Treatment with gemcitabine, a drug that inhibits DNA synthesis and induces apoptosis, reduces MDSC levels after surgery (32, 33), but tumor progression is not affected unless the mice are simultaneously treated with active immunotherapy (33) or also contain M1 macrophages (32). Therefore, gemcitabine treatment unlinks MDSC levels from tumor burden. To determine whether gemcitabine-mediated reduction of MDSC restores L-selectin levels, BALB/c mice were inoculated with 4T1 on day −1, primary tumors were surgically removed on day 25, and gemcitabine treatment was started on day 26 and continued through day 35. Mice were bled and percent Gr1+CD11b+ MDSC and L-selectin expression levels on CD3+CD4+ and CD3+CD8+ T cells were monitored by flow cytometry (Fig. 2B). As expected, gemcitabine treatment lowered MDSC levels in seven of eight postsurgery mice (dotted lines in Fig. 2B). Similar to the findings shown in Fig. 2A, T cells of gemcitabine-treated mice with reduced MDSC levels were L-selectinhigh (dotted lines in Fig. 2B), while T cells of gemcitabine-treated mice with high (unchanged) levels of MDSC remained L-selectinlow (solid lines in Fig. 2B). Since gemcitabine reduces MDSC levels but does not reduce tumor burden (32), these findings suggest that MDSC, rather than tumor burden, regulate L-selectin expression on T cells.
Down-regulation of L-selectin is not the result of Ag-driven T cell activation
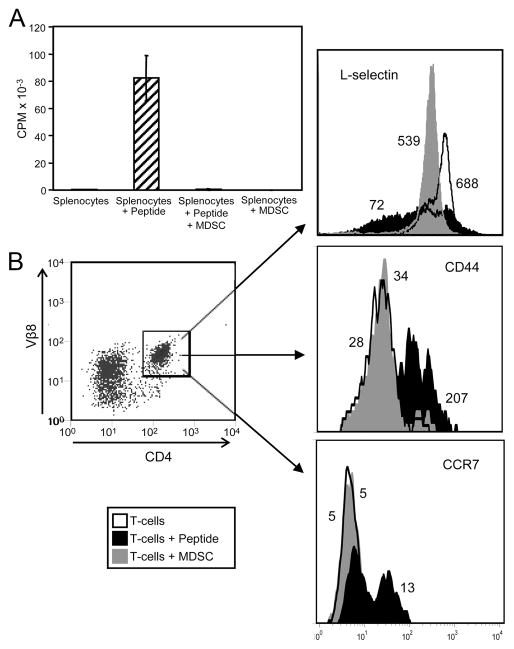
Down-regulation of L-selectin is a hallmark of newly activated T cells. Because MDSC suppress T cell activation, it is unlikely that the L-selectinlow phenotype induced by MDSC is the result of T cell activation. However, to test this possibility, splenocytes from TS1-transgenic mice were cultured with their cognate peptide (HA110–119) and/or with 4T1-induced MDSC, and T cell activation was measured by the incorporation of tritiated thymidine (Fig. 3A). Cells were also stained for L-selectin, CD4, Vβ8 (TCR of TS1-transgenic CD4+ T cells), and the CD4+Vβ8+ cells were gated and analyzed by flow cytometry for L-selectin and the activation markers CD44 and CCR7 (Fig. 3B). As expected, peptide-pulsed TS1 splenocytes proliferated in the absence of MDSC and their proliferation was suppressed in the presence of MDSC. CD4+ TS1 splenocytes cultured with peptide in the absence of MDSC had heterogeneous expression of L-selectin ranging from L-selectinlow to L-selectinhigh phenotypes, demonstrating that a subset of the splenocytes was activated. In contrast, TS1 splenocytes cocultured with MDSC and with or without peptide did not proliferate, demonstrating they were not activated. In agreement with Figs. 1 and 2, T cells cocultured with MDSC had a uniformly L-selectinlow phenotype. Peptide-activated CD4+ TS1 splenocytes had increased CD44 expression consistent with Ag-activated T cells. In contrast, TS1 splenocytes cocultured with MDSC did not have increased CD44. Similarly, peptide-activated CD4+ TS1 splenocytes had increased CCR7 expression, while TS1 splenocytes alone or cocultured with MDSC did not have increased CCR7. Thus, T cells cocultured with MDSC were not activated and did not show the same L-selectin, CD44, or CCR7 profile as peptide-activated T cells, indicating that down-regulation of L-selectin by MDSC was not due to Ag activation.
FIGURE 3.
Down-regulation of L-selectin is not the result of Ag-driven activation of T cells. Splenocytes from tumor-free TS1-transgenic mice were cultured alone, with HA110–119 peptide, or at a ratio of 1:1 splenocytes to Gr1+CD11b+ MDSC from the blood of 4T1-tumor bearing BALB/c mice. After 3 days of culture, cells were pulsed with [3H]thymidine to measure T cell proliferation (A). B, Cells from the cultures shown in A were stained for Vβ8, CD4, L-selectin, CD44, and CCR7, and the gated Vβ8+CD4+ T cells were analyzed for L-selectin, CD44, or CCR7 expression. Values are the MCF of L-selectin, CD44, or CCR7. Data for all panels are from one of three independent experiments.
T cells cocultured with MDSC have an L-selectinlow phenotype
The results of Figs. 1 and 2 suggested that MDSC may regulate T cell expression of L-selectin. To determine whether MDSC are sufficient to affect L-selectin levels, splenocytes from tumor-free mice were cultured in the presence or absence of MDSC from 4T1 tumor-bearing mice. After 48 h of culture, cells were harvested and stained with propidium iodide, CD4, CD8, L-selectin, Gr-1, and/or CD11b mAbs. Propidium iodide- negative CD4+ or CD8+ cells were gated and then analyzed for L-selectin expression by flow cytometry (Fig. 4). CD4+ or CD8+ splenocytes cocultured with MDSC displayed an L-selectinlow phenotype relative to splenocytes cultured alone. Coculture with J774 cells, a non-MDSC myeloid cell line, did not alter L-selectin expression. To determine whether the down-regulation by MDSC was limited to L-selectin, CD4 and CD8 levels were analyzed on the cocultured splenocytes. No significant changes were noted in the expression levels of CD4 or CD8, indicating that MDSC specifically decreased T cell expression of L-selectin.
FIGURE 4.
T cells cocultured with MDSC have an L-selectinlow phenotype. Splenocytes were harvested from tumor-free BALB/c mice and cocultured alone or with MDSC from the blood of 4T1-tumor bearing BALB/c mice (95% Gr1+CD11b+ cells) or with non-MDSC J774 myeloid cells at a ratio of 1:1 (T cells:myeloid cells). After 48 h of culture, cells were harvested and stained for L-selectin and CD4 or CD8, and the gated CD4+ and CD8+ cells were analyzed for L-selectin expression. Values are the MCF for L-selectin. Data are from one of three independent experiments.
MDSC levels drive L-selectin expression on T cells in tumor-free mice
If MDSC rather than tumors are driving L-selectin levels, then T cells in tumor-free mice with elevated MDSC levels should also have an L-selectinlow phenotype. To test this hypothesis, we used aged tumor-free mice, which also have increased numbers of MDSC (43, 44). One-, 8-, and 24-mo-old BALB/c mice were bled and the percentage of Gr1+CD11b+ cells and L-selectin levels on CD3+CD4+ and CD3+CD8+ T cells were determined by flow cytometry (Fig. 5A). As reported, MDSC levels increased with age. This increase was accompanied by a decrease in CD4+ and CD8+ T cell expression of L-selectin. Therefore, MDSC levels inversely correlated with L-selectin levels on both CD4+ and CD8+ T cells, consistent with the concept that MDSC, rather than tumor, directly reduce L-selectin levels.
To further determine whether MDSC, rather than tumor cells, are regulating L-selectin levels, mice were treated with uPA, a molecule that has recently been shown to increase MDSC levels in vivo (42). BALB/c mice were injected i.p. with a mixture of uPA and naive purified T cells, and peritoneal exudates were removed 4 h later. L-selectin levels on peritoneal exudate CD4+ and CD8+ T cells were determined by flow cytometry (Fig. 5B). As reported, MDSC levels increased in mice treated with uPA. The increase was accompanied by a decrease in L-selectin on CD4+ and CD8+ T cells. uPA did not directly affect L-selectin levels, as naive T cells cultured for 4 h with 200 ng/ml uPA did not have altered L-selectin levels (data not shown). Hence, MDSC induced by aging or by uPA alter T cell expression of L-selectin, demonstrating that MDSC and not tumors down-regulate L-selectin levels on CD4+ and CD8+ T cells.
MDSC express ADAM17 on their plasma membrane
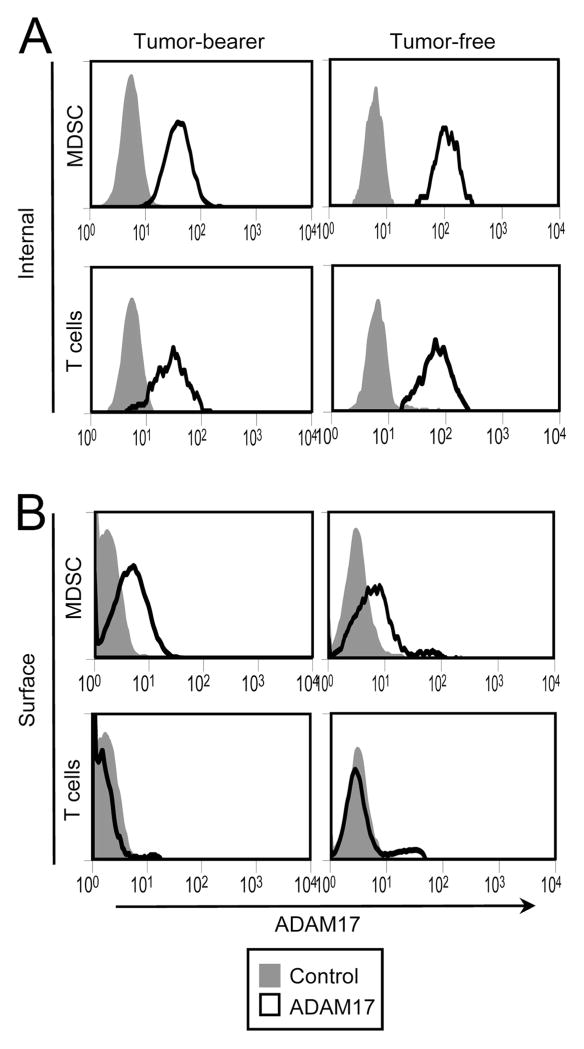
L-selectin down-regulation on T cells primarily occurs through proteolytic cleavage and subsequent shedding of the L-selectin ectodomain (45–49). A primary sheddase of L-selectin is the cell-membrane zinc-based protease ADAM17 (a disintegrin and metalloproteinase domain 17), also called TACE (TNF-α-converting enzyme). ADAM17 expression is common in the cytoplasm of many cells and its translocation to the plasma membrane coincides with L-selectin cleavage (48, 50). To determine whether MDSC down-regulate L-selectin through proteolytic cleavage and shedding, MDSC were tested by flow cytometry for the expression of ADAM17. Tumor-bearing and tumor-free mice were bled and white blood cells were stained for surface expression of CD3, CD11b, Gr1, and internal and surface expression of ADAM17. Gated CD3+ T cells and gated CD11b+Gr1+ MDSC were analyzed. Both T cells and MDSC showed internal expression of ADAM17 (Fig. 6A); however, only MDSC contained ADAM17 on the extracellular side of their plasma membrane (Fig. 6B). MDSC from all tumor-bearing (n = 8) and tumor-free (n = 6) mice tested displayed cell surface ADAM17 with a range of expression of 3- to 8-fold above background Therefore, MDSC express on their plasma membrane the enzyme that cleaves the ectodomain of L-selectin.
FIGURE 6.
MDSC express ADAM17 on their plasma membrane. Blood was harvested from 4T1 tumor-bearing and tumor-free BALB/c mice and the white blood cells were permeabilized and internally stained (A) or surface stained (B) for CD3, CD11b, Gr1, and ADAM17. Gated CD11b+Gr1+ MDSC and CD3+ T cells were analyzed for internal (A) or plasma membrane (B) expression of ADAM17 by flow cytometry. Data are representative of three experiments using seven tumor-free mice and eight tumor-bearing mice.
Discussion
L-selectin is a critical molecule for the leukocyte-endothelial cell interactions that result in the migration of naive T cells into peripheral LN and inflammatory locales such as the tumor microenvironment. Cancer patients (51, 52) and experimental animals with tumors (53, 54) are known to have T cells with an L-selectinlow phenotype; however, the mediators that down-regulate L-selectin were not previously identified. Using three mouse mammary carcinoma tumors that induce high levels of immune-suppressive myeloid cells, the in vivo and in vitro studies reported here demonstrate that T cell expression of L-selectin is down-regulated by MDSC.
MDSC-driven down-regulation of L-selectin on T cells has the potential to negatively impact antitumor immunity in several ways. An L-selectinhigh phenotype is required for naive T cells to home to peripheral LNs where they are activated by Ag (8, 55). However, T cells with reduced L-selectin levels may not efficiently traffic to TDLN where they will encounter tumor peptides and become activated. The critical role for L-selectin in activating tumor-specific T cells has been confirmed in two mouse tumor models. Using Moloney murine sarcoma virus-induced tumors, Rosato et al. (56) showed that blocking L-selectin prevented naive CTL from migrating through high endothelial venules and accumulating in peripheral LNs draining the tumor area. As a result, the generation of tumor-specific CTL was reduced (56). In a second report, homing and sticking of naive lymphocytes was shown to be impaired in the TDLN of C57BL/6 mice with B16 melanomas (54).
L-selectin down-regulation by MDSC is also likely to impair antitumor immunity by blocking the migration of naive T cells to the tumor site. Naive T cells with an L-selectinhigh phenotype are attracted to inflammatory locales (9, 13, 14). Since the tumor microenvironment is an inflammatory milieu (57, 58), T cells should accumulate within tumors. However, MDSC-mediated reduction of L-selectin potentially limits the efficiency of naive T cell migration to tumors. This scenario is supported by studies showing that L-selectin-deficient mice have decreased numbers of T cells infiltrating B16 primary tumors (15). L-selectin-deficient mice also demonstrate decreased leukocyte recruitment into an inflamed peritoneum at early and late time points (9, 59). Therefore, the down-regulation of L-selectin by MDSC may not only reduce naive T cell entry into lymph nodes and therefore reduce the quantity of activated tumor-specific T cells, but may also minimize naive T cell access to tumor microenvironments which are usually proinflammatory.
L-selectin down-regulation has been reported in response to various physiologically relevant soluble stimuli including proinflammatory mediators (60, 61), osmotic stress (62), and bacterial superantigens or toxins (63–65). To characterize the mechanism of down-regulation, investigators have decreased L-selectin expression by cross-linking with L-selectin-specific ligands (66–68). These studies demonstrated that down-regulation is through proteolytic cleavage and subsequent shedding of the L-selectin ectodomain (69). Depending on the experimental system and cross-linking ligand, shedding is triggered by different intracellular signaling pathways. In one system, the disintegrin and metalloproteinase ADAM17 was responsible for cleavage (70), while in another system TNF-α proteases were implicated (71, 72). Our results demonstrating that MDSC express ADAM17 on their cell surface are consistent with the concept that MDSC down-regulate L-selectin on T cells via the expression of ADAM17. However, MDSC are a large family of myeloid cells with suppressive activity and different MDSC subpopulations may down-regulate via different mechanisms. Regardless of how MDSC mediate their effect, since inflammation induces MDSC (24, 26, 73–75) and MDSC down-regulate L-selectin, it is possible that previous studies attributing L-selectin down-regulation to inflammation were in fact looking at effects mediated by inflammation-induced MDSC.
MDSC suppression of antitumor immunity has been attributed to various mechanisms. These include inhibiting T cell activation by the production of arginase (25, 30) and inducible NO synthase (17, 22, 30), nitration of the TCR (31), blocking NK cell cytotoxicity (33), as well as polarizing immunity toward a tumor-promoting type 2 response (32). The results described here demonstrate that MDSC may also suppress antitumor immunity by preventing naive T cells from entering LNs where they become activated and by reducing the trafficking of naive T cells to tumors. Therefore, MDSC reduce antitumor immunity through a diverse array of mechanisms that impact the activation as well as effector functions of both innate and adaptive immunity.
Acknowledgments
We thank Ephraim Fuchs for providing clone 4 and TS1 mice, Don Koonce, Jr. for technical assistance, Jeff Leips for statistical consultation, Sandy Mason and Terry King for excellent care of our mice, and Samuel Haile for critical reviews of this manuscript.
Footnotes
These studies were supported by National Institutes of Health Grants R01CA115880 and R01CA84232 and Susan G. Komen For the Cure Grant BCTR0503885. E.M.H. was supported by National Research Service Award 5F32CA119768-03.
Abbreviations used in this paper: TDLN, tumor-draining lymph node; ADAM17, a disintegrin and metalloproteinase 17 (also known as TACE); HA, hemagglutinin; LN, lymph node; MCF, mean channel fluorescence; MDSC, myeloid-derived suppressor cell; NDLN, nondraining lymph node; TC, tri-color; uPA, urokinase plasminogen activator.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Watanabe S, Deguchi K, Zheng R, Tamai H, Wang LX, Cohen PA, Shu S. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J Immunol. 2008;181:3291–3300. doi: 10.4049/jimmunol.181.5.3291. [DOI] [PubMed] [Google Scholar]
- 2.Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- 3.Khan AI, Landis RC, Malhotra R. L-selectin ligands in lymphoid tissues and models of inflammation. Inflammation. 2003;27:265–280. doi: 10.1023/a:1026056525755. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, I, Grewal S, Geba GP, Flavell RA. Impaired primary T cell responses in L-selectin-deficient mice. J Exp Med. 1996;183:589–598. doi: 10.1084/jem.183.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallatin WM, I, Weissman L, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983;304:30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- 6.Bradley LM, Watson SR, Swain SL. Entry of naive CD4 T cells into peripheral lymph nodes requires L-selectin. J Exp Med. 1994;180:2401–2406. doi: 10.1084/jem.180.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepault F, Gagnerault MC, Faveeuw C, Boitard C. Recirculation, phenotype and functions of lymphocytes in mice treated with monoclonal antibody MEL-14. Eur J Immunol. 1994;24:3106–3112. doi: 10.1002/eji.1830241229. [DOI] [PubMed] [Google Scholar]
- 8.Stremmel C, Sienel W, Eggeling S, Passlick B, Slavin A. Inhibition of T cell homing by down-regulation of CD62L and the induction of a Th-2 response as a method to prevent acute allograft rejection in mice. Eur J Cardiothorac Surg. 2006;30:362–369. doi: 10.1016/j.ejcts.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Tedder TF, Steeber DA, Pizcueta P. L-selectin-deficient mice have impaired leukocyte recruitment into inflammatory sites. J Exp Med. 1995;181:2259–2264. doi: 10.1084/jem.181.6.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 11.Hafezi-Moghadam A, Thomas KL, Prorock AJ, Huo Y, Ley K. L-selectin shedding regulates leukocyte recruitment. J Exp Med. 2001;193:863–872. doi: 10.1084/jem.193.7.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang ML, Hale LP, Steeber DA, Tedder TF. L-selectin is involved in lymphocyte migration to sites of inflammation in the skin: delayed rejection of allografts in L-selectin-deficient mice. J Immunol. 1997;158:5191–5199. [PubMed] [Google Scholar]
- 13.Arbones ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247–260. doi: 10.1016/1074-7613(94)90076-0. [DOI] [PubMed] [Google Scholar]
- 14.Steeber DA, Tang ML, Green NE, Zhang XQ, Sloane JE, Tedder TF. Leukocyte entry into sites of inflammation requires overlapping interactions between the L-selectin and ICAM-1 pathways. J Immunol. 1999;163:2176–2186. [PubMed] [Google Scholar]
- 15.Yamada M, Yanaba K, Hasegawa M, Matsushita Y, Horikawa M, Komura K, Matsushita T, Kawasuji A, Fujita T, Takehara K, et al. Regulation of local and metastatic host-mediated anti-tumour mechanisms by L-selectin and intercellular adhesion molecule-1. Clin Exp Immunol. 2006;143:216–227. doi: 10.1111/j.1365-2249.2005.02989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 17.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–785. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 18.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 19.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 20.Bronte V, Serafini P, Apolloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother. 2001;24:431–446. doi: 10.1097/00002371-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serafini P, De Santo C, Marigo I, Cingarlini S, Dolcetti L, Gallina G, Zanovello P, Bronte V. Derangement of immune responses by myeloid suppressor cells. Cancer Immunol Immunother. 2004;53:64–72. doi: 10.1007/s00262-003-0443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory s100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–999. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 26.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinha P, V, Clements K, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–645. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 29.Sinha P, V, Clements K, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–11751. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 30.Bronte V, Serafini P, Mazzoni A, Segal DM, Zanovello P. L-Arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 2003;24:302–306. doi: 10.1016/s1471-4906(03)00132-7. [DOI] [PubMed] [Google Scholar]
- 31.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sinha P, V, Clements K, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 34.Morgan DJ, Liblau R, Scott B, Fleck S, McDevitt HO, Sarvetnick N, Lo D, Sherman LA. CD8+ T cell-mediated spontaneous diabetes in neonatal mice. J Immunol. 1996;157:978–983. [PubMed] [Google Scholar]
- 35.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8+ single positive cells with a class II major histocompatibility complex-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58:1486–1493. [PubMed] [Google Scholar]
- 37.Ilkovitch D, Handel-Fernandez ME, Herbert LM, Lopez DM. Antitumor effects of mucin 1/sec involves the modulation of urokinase-type plasminogen activator and signal transducer and activator of transcription 1 expression in tumor cells. Cancer Res. 2008;68:2427–2435. doi: 10.1158/0008-5472.CAN-07-5651. [DOI] [PubMed] [Google Scholar]
- 38.Pulaski BA, Terman DS, Khan S, Muller E, Ostrand-Rosenberg S. Cooperativity of Staphylococcal aureus enterotoxin B superantigen, major histocompatibility complex class II, and CD80 for immunotherapy of advanced spontaneous metastases in a clinically relevant postoperative mouse breast cancer model. Cancer Res. 2000;60:2710–2715. [PubMed] [Google Scholar]
- 39.Pulaski BA, Ostrand-Rosenberg S, Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. Mouse 4T1 breast Tumor Model. Current Protocols in Immunology. 2000;4:20.2.1. doi: 10.1002/0471142735.im2002s39. [DOI] [PubMed] [Google Scholar]
- 40.Pulaski BA, V, Clements K, Pipeling MR, Ostrand-Rosenberg S. Immunotherapy with vaccines combining MHC class II/CD80+ tumor cells with interleukin-12 reduces established metastatic disease and stimulates immune effectors and monokine induced by interferon γ. Cancer Immunol Immunother. 2000;49:34–45. doi: 10.1007/s002620050024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Armstrong TD, V, Clements K, Martin BK, Ting JP, Ostrand-Rosenberg S. Major histocompatibility complex class II-transfected tumor cells present endogenous antigen and are potent inducers of tumor-specific immunity. Proc Natl Acad Sci USA. 1997;94:6886–6891. doi: 10.1073/pnas.94.13.6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilkovitch D, Lopez DM. Urokinase-mediated recruitment of myeloid-derived suppressor cells and their suppressive mechanisms are blocked by MUC1/sec. Blood. 2009;113:4729–4739. doi: 10.1182/blood-2008-08-176438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grizzle WE, Xu X, Zhang S, Stockard CR, Liu C, Yu S, Wang J, Mountz JD, Zhang HG. Age-related increase of tumor susceptibility is associated with myeloid-derived suppressor cell mediated suppression of T cell cytotoxicity in recombinant inbred BXD12 mice. Mech Ageing Dev. 2007;128:672–680. doi: 10.1016/j.mad.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Heithoff DM, Enioutina EY, Bareyan D, Daynes RA, Mahan MJ. Conditions that diminish myeloid derived suppressor cell activities stimulate cross-protective immunity. Infect Immun. 2008;76:5191–5199. doi: 10.1128/IAI.00759-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Herrera AH, Li Y, Belani KK, Walcheck B. Regulation of mature ADAM17 by redox agents for L-selectin shedding. J Immunol. 2009;182:2449–2457. doi: 10.4049/jimmunol.0802770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X, Perez L, Pan Z, Fan H. The transmembrane domain of TACE regulates protein ectodomain shedding. Cell Res. 2007;17:985–998. doi: 10.1038/cr.2007.98. [DOI] [PubMed] [Google Scholar]
- 47.Lee D, Schultz JB, Knauf PA, King MR. Mechanical shedding of L-selectin from the neutrophil surface during rolling on sialyl Lewis X under flow. J Biol Chem. 2007;282:4812–4820. doi: 10.1074/jbc.M609994200. [DOI] [PubMed] [Google Scholar]
- 48.Li Y, Brazzell J, Herrera A, Walcheck B. ADAM17 deficiency by mature neutrophils has differential effects on L-selectin shedding. Blood. 2006;108:2275–2279. doi: 10.1182/blood-2006-02-005827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smalley DM, Ley K. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9:255–266. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charbonneau M, Harper K, Grondin F, Pelmus M, McDonald PP, Dubois CM. Hypoxia-inducible factor mediates hypoxic and tumor necrosis factor α-induced increases in tumor necrosis factor-α converting enzyme/ADAM17 expression by synovial cells. J Biol Chem. 2007;282:33714–33724. doi: 10.1074/jbc.M704041200. [DOI] [PubMed] [Google Scholar]
- 51.Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–3494. [PubMed] [Google Scholar]
- 52.Saiki Y, Ohtani H, Naito Y, Miyazawa M, Nagura H. Immunophenotypic characterization of Epstein-Barr virus-associated gastric carcinoma: massive infiltration by proliferating CD8+ T-lymphocytes. Lab Invest. 1996;75:67–76. [PubMed] [Google Scholar]
- 53.Peng L, Kjaergaard J, Plautz GE, Weng DE, Shu S, Cohen PA. Helper-independent, L-selectinlowCD8+ T cells with broad anti-tumor efficacy are naturally sensitized during tumor progression. J Immunol. 2000;165:5738–5749. doi: 10.4049/jimmunol.165.10.5738. [DOI] [PubMed] [Google Scholar]
- 54.Carriere V, Colisson R, Jiguet-Jiglaire C, Bellard E, Bouche G, Al Saati T, Amalric F, Girard JP, M’Rini C. Cancer cells regulate lymphocyte recruitment and leukocyte-endothelium interactions in the tumor-draining lymph node. Cancer Res. 2005;65:11639–11648. doi: 10.1158/0008-5472.CAN-05-1190. [DOI] [PubMed] [Google Scholar]
- 55.Jung TM, Gallatin WM, Weissman IL, Dailey MO. Down-regulation of homing receptors after T cell activation. J Immunol. 1988;141:4110–4117. [PubMed] [Google Scholar]
- 56.Rosato A, Zambon A, Macino B, Mandruzzato S, Bronte V, Milan G, Zanovello P, Collavo D. Anti-L-selectin monoclonal antibody treatment in mice enhances tumor growth by preventing CTL sensitization in peripheral lymph nodes draining the tumor area. Int J Cancer. 1996;65:847–851. doi: 10.1002/(SICI)1097-0215(19960315)65:6<847::AID-IJC23>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 57.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J Exp Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science. 1989;245:1238–1241. doi: 10.1126/science.2551036. [DOI] [PubMed] [Google Scholar]
- 61.Jung TM, Dailey MO. Rapid modulation of homing receptors (gp90MEL-14) induced by activators of protein kinase C: receptor shedding due to accelerated proteolytic cleavage at the cell surface. J Immunol. 1990;144:3130–3136. [PubMed] [Google Scholar]
- 62.Rizoli SB, Rotstein OD, Kapus A. Cell volume-dependent regulation of L-selectin shedding in neutrophils: a role for p38 mitogen-activated protein kinase. J Biol Chem. 1999;274:22072–22080. doi: 10.1074/jbc.274.31.22072. [DOI] [PubMed] [Google Scholar]
- 63.Stibenz D, Buhrer C, Laufer D, Obladen M. CD45 engagement induces L-selectin down-regulation. Scand J Immunol. 1996;44:37–44. doi: 10.1046/j.1365-3083.1996.d01-282.x. [DOI] [PubMed] [Google Scholar]
- 64.Miethke T, Wahl C, Holzmann B, Heeg K, Wagner H. Bacterial superantigens induce rapid and T cell receptor Vβ-selective down-regulation of L-selectin (gp90Mel-14) in vivo. J Immunol. 1993;151:6777–6782. [PubMed] [Google Scholar]
- 65.Walev I, Tappe D, Gulbins E, Bhakdi S. Streptolysin O-permeabilized granulocytes shed L-selectin concomitantly with ceramide generation via neutral sphingomyelinase. J Leukocyte Biol. 2000;68:865–872. [PubMed] [Google Scholar]
- 66.Phong MC, Gutwein P, Kadel S, Hexel K, Altevogt P, Linderkamp O, Brenner B. Molecular mechanisms of L-selectin-induced co-localization in rafts and shedding [corrected] Biochem Biophys Res Commun. 2003;300:563–569. doi: 10.1016/s0006-291x(02)02886-3. [DOI] [PubMed] [Google Scholar]
- 67.Stoddart JH, Jr, Jasuja RR, Sikorski MA, von Andrian UH, Mier JW. Protease-resistant L-selectin mutants: down-modulation by cross-linking but not cellular activation. J Immunol. 1996;157:5653–5659. [PubMed] [Google Scholar]
- 68.Palecanda A, Walcheck B, Bishop DK, Jutila MA. Rapid activation-independent shedding of leukocyte L-selectin induced by cross-linking of the surface antigen. Eur J Immunol. 1992;22:1279–1286. doi: 10.1002/eji.1830220524. [DOI] [PubMed] [Google Scholar]
- 69.Galkina E, Tanousis K, Preece G, Tolaini M, Kioussis D, Florey O, Haskard DO, Tedder TF, Ager A. L-selectin shedding does not regulate constitutive T cell trafficking but controls the migration pathways of antigen-activated T lymphocytes. J Exp Med. 2003;198:1323–1335. doi: 10.1084/jem.20030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 71.Arribas J, Coodly L, Vollmer P, Kishimoto TK, Rose-John S, Massague J. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem. 1996;271:11376–11382. doi: 10.1074/jbc.271.19.11376. [DOI] [PubMed] [Google Scholar]
- 72.Borland G, Murphy G, Ager A. Tissue inhibitor of metalloproteinases-3 inhibits shedding of L-selectin from leukocytes. J Biol Chem. 1999;274:2810–2815. doi: 10.1074/jbc.274.5.2810. [DOI] [PubMed] [Google Scholar]
- 73.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, Voronov E, Apte RN. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1β-secreting cells. J Immunol. 2005;175:8200–8208. doi: 10.4049/jimmunol.175.12.8200. [DOI] [PubMed] [Google Scholar]
- 75.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]