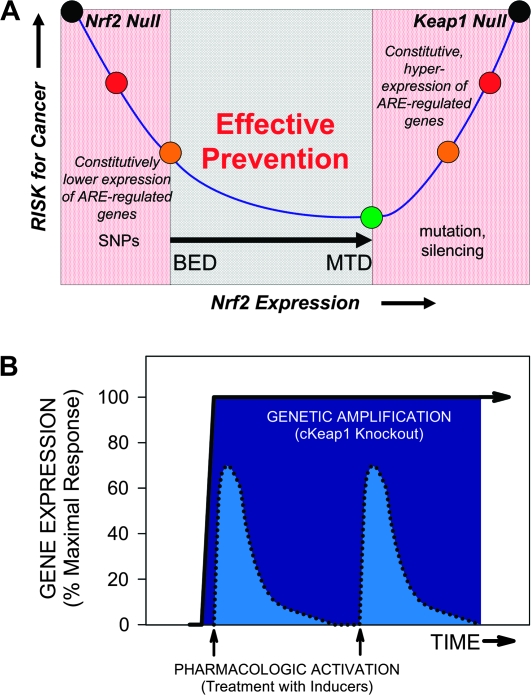
Fig. 3.
(A) U-Shaped modulation of cancer risk through the KEAP1–NRF2 pathway. Optimal activation of the pathway lies in a pharmacological range between the biologically effective dose (BED) that minimally activates the pathway and a maximal-tolerated dose (MTD) that not only activates the pathway but also may produce dose-limiting ‘off target’ toxicities as well. Single-nucleotide polymorphisms (SNPs) in the Nrf2 promoter may diminish constitutive or inducible capacity of the pathway, whereas mutations or epigenetic silencing of Keap1 leads to sustained hyperactivation. (B) Comparison of the kinetics of induction of NRF2-regulated genes by pharmacological intervention versus genetic disruption of KEAP1 function. Chemopreventive agents that activate NRF2 signaling are typically administered (in both preclinical and clinical settings) on daily to weekly schedules, leading to pronounced but transient induction of downstream genes (dotted lines). In contrast, genetic disruption of the pathway, such as by conditional, tissue-specific targeted disruption of Keap1 (cKeap1) in the mouse or through somatic mutations acquired by cancer cells in KEAP1 or NRF2, leads to markedly elevated and sustained activation of the pathway (solid line). Chemopreventive agents cannot replicate the magnitude of response seen with genetic perturbation of the pathway (95).