Abstract
To successfully grow, neurons need to overcome the effects of hostile environments, such as the inhibitory action of myelin. We have evaluated the potential of exercise to overcome the intrinsic limitation of the central nervous system for axonal growth. In line with the demonstrated ability of exercise to increase the regenerative potential of neurons, here we show that exercise reduces the inhibitory capacity of myelin. Cortical neurons grown on myelin from exercised rats showed a more pronounced neurite extension compared with neurons grown on poly-D-lysine, or on myelin extracted from sedentary animals. The activity of cyclin-dependent kinase 5, a kinase involved in neurite outgrowth, was found to be increased in cortical neurons grown on exercise-myelin and in the lumbar spinal cord enlargement of exercised animals. Exercise significantly decreased the levels of myelin-associated glycoprotein (MAG), a potent axonal growth inhibitor, suggesting that downregulation of MAG is part of the mechanism through which exercise reduces growth inhibition. It is known that exercise elevates brain-derived neurotrophic factor (BDNF) spinal cord levels and that BDNF acts to overcome the inhibitory effects of myelin. Accordingly, we blocked the action of BDNF during exercise, which suppressed the exercise-related MAG decrease. Protein kinase A (PKA) has been related to the ability of BDNF to overcome growth inhibition; in agreement, we found that exercise increased PKA levels and this effect was reverted by blocking BDNF. Overall, these results show that exercise promotes a permissive cellular environment for axonal growth in the adult spinal cord requiring BDNF action.
Keywords: spinal cord injury, BDNF, MAG, neurite outgrowth, cdk5
INTRODUCTION
Injured axons in the adult central nervous system (CNS) have a very limited capacity for regeneration. To a large extent, the failure of CNS axons to regenerate is the result of a nonpermissive environment that includes multiple factors such as formation of glial scar, deficiency in trophic factors and growth-inhibitors produced by oligodendrocytes, reactive astrocytes and fibroblasts (Domeniconi and Filbin, 2005; Fawcett and Asher, 1999; Filbin, 2003; Horner and Gage, 2000, 2002; McGee and Strittmatter, 2003; Sandvig et al., 2004; Silver and Miller, 2004). Recently, myelin-derived growth-inhibitory signals have been shown to play a major role in hampering axonal growth across the site of injury (Liebscher et al., 2005; Merkler et al., 2001). Myelin-derived proteins (Chen et al., 2000; GrandPre et al., 2000; McKerracher et al., 1994; Mukhopadhyay et al., 1994; Prinjha et al., 2000; Schwab, 2004; Wang et al., 2002) have been identified as major sources of inhibition in the injured spinal cord. Neurons that were primed in vitro with brain-derived neurotrophic factor (BDNF) gain the ability to counteract the inhibitory capacity of myelin (Cai et al., 1999; Gao et al., 2003). Activation of the signal regulated kinase Erk by BDNF is required to overcome myelin-associated glycoprotein (MAG) inhibition. Its action is mediated by cyclic AMP (cAMP) and protein kinase A (PKA) (Gao et al., 2003). PKA is rapidly activated by cAMP in a transcription-independent fashion. In addition to its role on suppressing growth inhibition and supporting neuronal survival, BDNF can also contribute to neurite outgrowth by acting on growth associated pathways. For example, BDNF has been shown to elicit axonal growth and neurofilaments assembly (Segal and Greenberg, 1996). The dual action of BDNF in promoting growth and decreasing inhibition is critical for regulating CNS plasticity and repair.
Recent evidence supports the possibility that neural activity also has the potential to reverse the inhibitory action of MAG. For instance, electrophysiological activity modifies path finding cues on growth cones, turning repulsion into attraction in a cAMP-dependent manner (Ming et al., 2001). Evidence for the trophic effects of electrical activity on axonal growth has been forthcoming from studies showing that exogenous electrical activity increases neurite outgrowth from retinal ganglion cells (Goldberg et al., 2002) and peripheral motor axons (Al-Majed et al., 2000). The beneficial effects of physical activity on neural function have been increasingly recognized. For example, physical activity promotes adult neurogenesis (van Praag et al., 1999) and neural healing after CNS injury (Molteni et al., 2004). We have shown that physical activity elevates levels of BDNF in spinal cord areas activated by motor and sensory inputs derived from locomotion (Gomez-Pinilla et al., 2002). In the present studies, we investigated the influence of voluntary exercise on molecular systems responsible for growth inhibition such as MAG. Our data support the notion that managed physical activity may provide a realistic opportunity to influence the regenerative potential of the adult CNS under pathophysiological conditions.
MATERIALS AND METHODS
Animals
We used adult male Sprague-Dawley rats (Charles-River, Wilmington, MA) of approximately 2 months of age and 200–220 g weight. Sedentary animals were housed in standard rodent cages. For exercise conditioning, animals were housed in individual cages with voluntary access to a running wheel (Gomez-Pinilla et al., 2001). Animals were exposed to exercise for 3, 7, or 28 days, using a running wheel (diameter = 31.8 cm, width = 10 cm), according to published protocols (Vaynman et al., 2004). The rats were sacrificed by decapitation the morning after the last running period, and the lumbar enlargement of the spinal cord was quickly removed, frozen on dry ice, and stored at −70°C until used for Western blot and Real-time Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR). Three to seven rats per group were used for each time point. These studies were performed in accordance with the NIH guide for the Care and Use of Laboratory Animals, and approved by UCLA Animal Research Committee.
BDNF Blocking
BDNF was selectively sequestered during exercise using a specific immunoadhesin chimera (TrkB-IgG, R&D System, Minneapolis, MN), which comprises the extracellular domains of human TrkB and the Fc domain of immunoglobulin G (IgG), and has been shown to specifically antagonize BDNF action (Shelton et al., 1995). Infusion of TrkB-IgGs was achieved by coupling them to microbeads (Lumaflour Corp., Naples, FL), which we have previously used as a reservoir for the successful delivery of viable inhibitors into the hippocampus (Vaynman et al., 2003). Microbeads were coated via passive absorbency by incubating overnight at 4°C with a 1:5 mix of microbeads to TrkB-IgG 5 μg/μL in PBS with BSA, (Croll et al., 1998) or Cytochrome C (CytC) 100 ng/μL in sterile water (Lom and Cohen-Cory, 1999; Riddle et al., 1997). The solution was then centrifuged at 14,000g for 30 min and the microbeads were resuspended in sterile water at a 10% concentration. TrkBIgG or CytC fluorescent latex microbeads were injected bilaterally into the lumbar segment (L3; 0.6 mm lateral to the midline, and 1.5 mm vertical for the first injection, then 0.8 mm vertical for the second injection) in a volume of 2 μL over 15 min. The location of microbead injection was verified by histological examination using fluorescence microscopy. All animals were anaesthetized by isoflurane (2–2.5%) utilizing Mobile Laboratory Animal Anesthesia System, and positioned in a stereo-tactic apparatus. Exercised and sedentary rats received TrkB-IgG or the control CytC injection once prior to a 7-day running period. Injections were administered early in the morning such that an ample recovery time permitted all rats to begin running the same evening. The rats were divided into four groups: CytC/sed, sedentary animals injected with CytC; CytC/ex, exercised animals injected with CytC; TrkB-IgG/sed, sedentary animals that received TrkB-IgG injection; TrkB-IgG/ex, exercised animals that received TrkB-IgG injection.
Western Blot
Rat lumbar spinal cord was homogenized in lysis buffer containing 137 mM NaCl, 20 mM Tris-HCl pH 8.0, 1% NP40, 10% glycerol, 1 mM PMSF, 10 μg/lL aprotinin, 0.1 mM benzethonium chloride, and 0.5 mM sodium vanadate and western blotting analysis was performed as previously reported (Vaynman et al., 2004). The following primary antibodies were used: anti-MAG antibody (1:1,000; Chemicon, Temecula, CA), anti-MBP antibody (1:500; Chemicon), or anti-PKA antibody (1:1,000; Upstate Biotechnology, Lake Placid, NY). Protein bands were detected by chemiluminescence using horseradish peroxidase-conjugated secondary antibodies followed by the Amersham ECL kit (Amersham Pharmacia Biotech., Piscataway, NJ). Relative intensities of the protein bands were quantified by scanning densitometry using the NIH Image software (Image J, http://rsb.info. nih.gov/ij/). Actin levels were used as internal standards for comparison of different tissue samples.
Real-Time Reverse Transcriptase-Polymerase Chain Reaction
Total RNA was extracted from spinal cord tissue by using TRIzol (Invitrogen Life Science Technologies, Carlsbad, CA), following the manufacturer's protocol. Samples were further purified by DNAseI treatment (DNA-free™ kit, Ambion, Austin, TX) followed by a second extraction with phenol-chloroform. Total RNA was reverse-transcribed and TaqMan Real-time RT-PCR was performed using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Atlanta, GA). The sequences of forward, reverse primers and probes, designed by Integrated DNA Technologies (Coralville, IA) were: MAG: probe 5′-AGCCACCGCGTTGAAGCTG TCTGT-3′, forward: 5′-TGTGTAGCTGAGAAGGAGTAT GG-3′, reverse: 5′-ACAGTGCGATTCCAGAAGGATTAT-3′. An oligonucleotide probe (5′-CCGACTCTTGCCCTTC GAAC-3′) specific for the rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was used as an endogenous control to standardize the amount of sample RNA (Medhurst et al., 2000). For quantification of Real-time RT-PCR results, fluorescent signal intensities were plotted against the number of PCR cycles on a semilogaritmic scale. The amplification cycle at which the first significant increase of fluorescence occurred was designed as threshold cycle (Ct). The Ct value of each sample was then compared with those of the internal standard. The process was automatically finished by ABI sequence detector software version 1.6.3. Quantification of mRNA was normalized with corresponding GAPDH values.
Preparation of Myelin
Myelin was isolated from the lumbar part of the spinal cord of sedentary animals or animals that had exercised for 7 days, by sucrose density gradient centrifugation as previously reported (Carson et al., 1993; Norton and Poduslo, 1973; Shen et al., 1998). Briefly, after sacrifice, the rat spinal cord was rapidly removed and homogenized in 0.32 M sucrose using a Teflon/Glass homogenizer, then, layered over an equal volume of 0.85 M sucrose. After centrifugation at 40,000 rpm, myelin was collected from the interface, homogenized in water and recentrifuged. After the final hypotonic shock, the myelin membranes were centrifuged and resuspended in 10 mM Hepes pH 7.15, and protein concentrations were determined ((Bradford, 1976); BioRad, Hercules, CA). The purified myelin membranes were used as substrate in the neurite outgrowth assay.
Primary Rat Neuronal Cortical Culture
Cultures were prepared from E16 Sprague-Dawley rats (Charles-River) as previously described (Espinosa-Jeffrey et al., 2002; Ghiani et al., 2006). Primary cortical neurons were grown in a neuronal specific medium (Espinosa-Jeffrey et al., 2002; Ghiani et al., 2006), TII, supplemented with bFGF (10 ng/mL; Invitrogen Life Science Technologies), B27 (1:50; Invitrogen Life Science Technologies), and creatine (2 mg/mL; Sigma, Saint Louis, MO). Purity of the cultures was assessed by double immunostaining with neuronal and glial markers. The cultures were 98% immunopositive for Microtubuli-associated protein 2 (MAP2; mouse monoclonal, 1:500; Sigma) and the remaining immunostained for astrocytic or oligodendrocyte markers. No microglial cells were detected.
Immunocytochemistry and Neurite Outgrowth Analysis
Purified rat myelin membranes (0.5–2 μg) from exercise or sedentary animals were dried on glass coverslips previously coated for 1 h at RT with Poly-D-Lysine (PDL: 16.6 μg/mL; Sigma). Control cultures were plated onto glass coverslips coated only with PDL (Cai et al., 1999). Primary cortical neurons were fixed in 4% paraformaldehyde in PBS for 15 min and permeabilized in 5% acetic acid/95% ethanol for 10 min at −20°C. After blocking in 10% normal goat serum, primary antibodies: anti-GAP43 (rabbit polyclonal, 1:500; Chemicon) and anti-β Tubulin III (mouse monoclonal, 1:500; Covance, Berkeley, CA) were incubated overnight at 4°C, followed by secondary Affinipure fluorochrome-conjugated goat anti-mouse and goat anti-rabbit antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). The coverslips were mounted in Vectashield (Vector Laboratories, Burlin-game, CA) and pictures were taken using a 40X objective on a Zeiss Axioskop 2 microscope with an ORCA digital camera (Hamamatsu).
Analysis of neurite outgrowth was performed using the Carl Zeiss AxioVision Viewer. The total number and the length of the neurites were measured from all the cells in 10–15 fields/coverslip in double-blind. Experiments were performed in duplicate and repeated at least four times.
Kinase Assay
Cyclin-Dependent Kinase (cdk) 5-associated activity was measured as previously reported (Ghiani and Gallo, 2001; Tang et al., 1998; Tsai et al., 1993). Cells or tissues were lysed in 50 mM HEPES, pH 7.4, 250 mM NaCl, 5 mM EDTA, 0.5% NP-40, 10% Na4P2O4,1 mM Na3VO4, 4 mM NaF, 10 mg/mL leupeptin, 10 mg/mL aprotinin, 10 mg/mL pepstatin, and 1 mM AEBSF. Lysates (50 μg) were immunoprecipitated with a saturating concentration of a rabbit polyclonal antibody against cdk5 (C8; Santa Cruz Biotechnology, Santa Cruz, CA) plus 20 μL of protein A-agarose (Santa Cruz Biotechnology) for 2 h at 4°C. Kinase reaction mixtures (20 μL) contained 2 μg of histone H1 (Upstate Biotechmology) as substrates, 20 μM ATP, and 2 μCi [32P]-ATP (New England Nuclear, Boston, MA). Phosphorylated histone H1 were resolved on 4–20% mini-SDS polyacrylamide gels, which were dried and bands were visualized and quantitated by PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Statistical Analysis
All the results were converted to percent of control for presentation in bar graphs, and represent the mean ± SEM. Statistical analysis was performed using GraphPad Prism 4.01 software (GraphPad Software, San Diego, CA) by Friedman Test followed by Dunn's Multiple Comparison Test (Fig. 5), by Anova followed by Bonferroni's Multiple Comparison Test (Fig. 6) or ANOVA followed by Fisher's post-hoc test (Figs. 1, 2, and 3). An unpaired student's t test (Fig. 4) was used when only two conditions were compared.
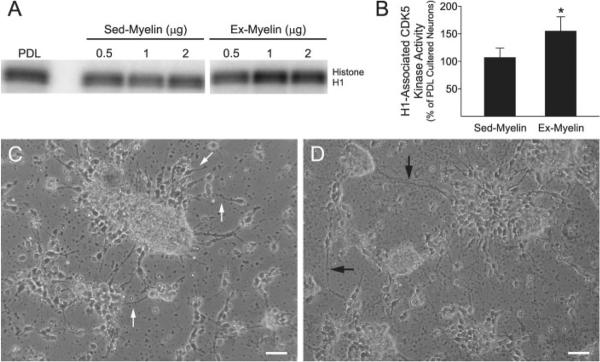
Fig. 5.
Cdk5 kinase activity is increased in primary cortical neurons grown on myelin extracted from the spinal cord of exercised animals. (A) Primary cortical neurons were grown for 24 h on PDL or on increasing concentration (0.5–2 μg) of myelin extracted from either sedentary (sed-myelin) or exercised animals (ex-myelin). Cdk5-associated kinase activity was determined after immunoprecipitation with anti-cdk5 antibodies and by using histone H1 as a substrate. Panel A shows a representative experiment. (B) Quantification of the effect of 1 μg myelin on Histone H1-associated cdk5 kinase activity in cortical neurons. Data were obtained by PhosphorImager analysis of the auto-radiographs and are expressed as percentage of cdk5 activity found in neuronal cells grown on PDL. Average of four independent experiment ± SEM is shown, *P < 0.05 vs. PDL cultured neurons (Friedman Test followed by Dunn's Multiple Comparison Test). (C, D) Cortical neurons plated for 24 h on exercise-myelin display longer processes compared with cells plated on sedentary-myelin. Pictures of live neuronal cells were taken on a Zeiss inverted microscope (Scale bar = 50 μm)
Fig. 6.
Exercise-myelin enhances neurite outgrowth in primary cortical neurons. Cortical neurons were grown for 24 h on sedentary-myelin (Sed), exercise-myelin (Ex) or Poly-D-Lysine (PDL). Cells were stained for GAP43, a marker for process elongation, and neurite length was determined. (A) Cortical neurons grown on exercise-myelin display longer processes than neurons on sedentary-myelin or PDL. Graph represents the average ± SEM of four independent experiments performed in duplicate, *P < 0.05 versus sedentary-myelin cultured neurons (Anova followed by Bonferroni's Multiple Comparison Test). (B) Neurons grown on exercise-myelin show more processes than cell plated on sedentary-myelin. Graph represents the average ± SEM of four independent experiments performed in duplicate, **P < 0.001 versus sedentary-myelin cultured neurons (Anova followed by Bonferroni's Multiple Comparison Test). (C) GAP43 immunoreactivity is increased in cortical neurons plated on exercise-myelin. Corresponding phase pictures are shown for each field. Representative pictures from four independent experiments are shown (Scale bar = 25 μm).
Fig. 1.
The decrease in MAG mRNA and protein levels induced by exercise in the lumbar region of rat spinal cord is “dose-dependent”. The animals were let free to run for 3, 7, or 28 days. At the end of the running period, the lumbar spinal cord was dissected out and used for Real-time RT-PCR or western blot analysis. (A) Levels of MAG mRNA and (B) protein are significantly decreased after 7 and 28 days of exercise in rat spinal cord. Data are mean ± SEM (*P < 0.05 vs. Sedentary animals (Sed); n = 6 animals/group). Insert in B shows a representative experiment. (C) The decrease in MAG mRNA levels is proportional to the amount of volunteer wheel running. The graph shows the correlation between individual variability in running and the decrease in MAG mRNA levels (P = 0.0001, r 5 0.833). MAG mRNA levels are plotted as function of the amount of running distance for each animal that ran for either 3 (open squares) or 7 (open triangles) days. (Filled triangles: Sedentary animals). (D) Exercise does not affect MBP protein levels in the lumbar spinal cord region. Data are mean ± SEM (n = 6 animals/group). Insert shows a representative experiment. Sed, sedentary animals.
Fig. 2.

The decrease in MAG expression induced by exercise is abolished by BDNF blockade. The BDNF inhibitor TrkB-IgG was injected into the spinal cord of animals prior to voluntary exercise for 7 days. Data are mean ± SEM (*P < 0.05; n = 6 animals/group). Insert shows a representative experiment. CytC/sed, sedentary animals that received CytC injection; CytC/ex, exercised animals that received CytC injection; TrkB-IgG/sed, sedentary animals that received TrkB-IgG injection; TrkB-IgG/ex, exercised animals that received TrkB-IgG injection. CytC, cytocrome C; ex, exercised animals; sed, sedentary animals.
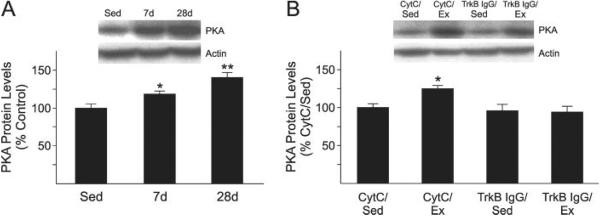
Fig. 3.
PKA levels are increased in the lumbar spinal cord of exercised rats. (A) PKA protein levels are increased after 7 and 28 days of exercise. Data are mean ± SEM (**P < 0.01, *P < 0.05 vs. sedentary animals (sed); n = 6 animals/group). (B) PKA increase is abolished by intraspinal injection of the BDNF inhibitor TrkB-IgG. The BDNF inhibitor TrkB-IgG was injected into the spinal cord of animals prior to voluntary exercise for 7 days. Data are mean ± SEM (*P < 0.05 vs. CytC/sed; n = 6 animals/group). Inserts in A and B show representative experiments.
Fig. 4.
Cdk5-associated kinase activity is greatly elevated by exercise in the lumbar enlargement of rat spinal cord. (A) Exercise is a positive modulator of cdk5 kinase activity in the rat lumbar spinal cord region. The animals were exposed to exercise for 7 days. Cdk5 activity was determined after immunoprecipitation with anti-cdk5 antibodies and using histone H1 as a substrate. Data were obtained by PhosphorImager analysis of the autoradiographs. Data are mean ± SEM and are expressed as percentage of cdk5 activity measured in sedentary animals (*P < 0.05 (Student's t-test); n = 3 animals/group). Insert shows representative gel bands. (B) The increase in cdk5-associated kinase activity is proportional to the amount of volunteer running. The graph shows the correlation between individual variability in running and the increase in cdk5 activity (p = 0.0011; r = 0.973). Changes in kinase activity were plotted as function of the amount of running distance for each animal that ran for 7 days (open triangles) or remained sedentary (filled triangles).
RESULTS
In Vivo Studies
Exercise decreases MAG mRNA and protein in the spinal cord
Given the powerful inhibitory action of MAG on axonal growth, we evaluated the capacity of exercise to modify MAG levels in the spinal cord. Levels of MAG mRNA and protein were measured in the lumbar spinal cord region in animals exposed to exercise or in sedentary conditions. Real-time RT-PCR revealed a significant decrease in MAG mRNA in the spinal cord, at both 7 and 28 days (72 and 51%, respectively) of exercise (Fig. 1A) when compared with controls (sedentary animals). The effects of exercise were also dramatic at the protein level. MAG protein levels were significantly decreased after 7 days (52%) and 28 days (62%) of exercise, when compared with protein levels in control rats (Fig. 1B). No changes in MAG mRNA or protein levels were found in the spinal cord of animals that had exercised for 3 days. Furthermore, we found that the effects of exercise on reducing the levels of MAG were proportional to the duration of running (Fig. 1C). MAG immunoreactivity showed a qualitative reduction in the spinal cord white matter of exercised animals (data not shown).
Exercise selectively affects myelin components
To determine whether exercise effects on myelin were specific for MAG, we analyzed the levels of myelin basic protein (MBP) in the lumbar segment of the spinal cord. Since MBP is a major and unique component of myelin, changes in its level would represent a change in myelin content. We did not detect any change in MBP protein levels after 3, 7, or 28 days of exercise (Fig. 1D). We further analyzed the composition of the myelin extracted from the spinal cord of exercise and sedentary rats by SDS page followed by coomassie-blue or silver staining. No differences were found in the bands for the main myelin components such as the four MBP isoforms, PLP/DM20, or CNPase (data not shown).
BDNF blockage counteracts the decrease in MAG after exercise in the spinal cord
BDNF has been shown to prime neurons in vitro to overcome the growth inhibitory action of myelin (Cai et al., 1999; Gao et al., 2003), and we have shown that exercise can increase the expression of BDNF in the intact and injured spinal cord (Gomez-Pinilla et al., 2001, 2002; Ying et al., 2005). We therefore determined the potential role of BDNF in mediating the effects of exercise on MAG expression by selectively blocking BDNF action using an immunoadhesin chimera (TrkB-IgG). TrkB-IgG microbeads were injected bilaterally into the lumbar spinal cord (L3 level) of rats just before exercising for 7 days. We chose to let the animals run only for 7 days, as MAG protein levels were already significantly reduced at this time point (Fig. 1B). MAG was significantly decreased in the control solution-injected exercised rats (CytC/ex) when compared with sedentary rats (CytC/sed; Fig. 2). TrkB-IgG injection completely abolished the reduction in MAG induced by exercise (TrkB-IgG-ex), and had no effect on MAG levels in rats maintained under sedentary conditions (TrkB-IgG/sed; Fig. 2). Most importantly, TrkB-IgG injection had no effect on MBP levels in the spinal cord of all experimental groups (data not shown), confirming that the effects of exercise are specific to MAG.
Exercise elevates protein levels of PKA
It has previously been reported that BDNF reduces the inhibitory ability of myelin using a mechanism mediated by cAMP and PKA (Gao et al., 2003). Therefore, we measured PKA levels in the lumbar region of the spinal cord of animals exposed to exercise or sedentary conditions. As shown in Fig. 3A, 7 and 28 days of exercise elicited a significant increase in PKA levels (18 and 40%, respectively). Furthermore, the BDNF inhibitor TrkB-IgG abolished the effects of exercise on PKA levels, reducing its levels to basal conditions (Fig. 3B).
Exercise increases cdk5 activity in the spinal cord
Considering the effects of exercise on increasing the regenerative potential of the spinal cord (Molteni et al., 2004), we further investigated possible mechanisms for these effects. Hence, we examined the ability of cdk5-complexes immunoprecipitated from lumbar spinal cord tissue extracts to phosphorylate the substrate Histone H1 as an indication of the effects that exercise might have on neurite outgrowth. Active cdk5-complexes localize to growth cones of postmitotic neurons and play an important role in the regulation of process elongation during neuronal differentiation (Dhavan and Tsai, 2001; Nikolic et al., 1996). Cdk5 activity was found significantly increased (371%; Fig. 4A) in the spinal cord of animals that had exercised for 7 days compared with that of sedentary controls. In addition, as shown above for the decrease in MAG mRNA levels, the changes in cdk5 kinase activity for each animal were proportional to the amount of volunteer wheel running (Fig. 4B).
In Vitro Studies
To determine how exercise modulates myelin influence on neuronal plasticity, we measured cdk5 activity in embryonic cortical neurons cultured for 24 h on myelin extracted from the lumbar spinal cord of sedentary (sedentary-myelin) or exercised (exercise-myelin) animals. First, we tested the effect of increasing concentrations of sedentary- and exercise-myelin on cdk5 activity in cortical neurons in comparison with cells cultured on PDL. The lowest concentration (0.5 μg) of exercise-myelin induced a modest increase in cdk5 activity (23%), whereas 1 and 2 μg greatly increased cdk5 activity (61 and 41%, respectively) after 24 h in culture when compared with sedentary-myelin (Fig. 5A). Therefore, in subsequent experiments only 1 μg of myelin was used. While exercise-myelin enhanced cdk5 activity (55%) compared with sedentary-myelin or PDL-plated neurons (Fig. 5B), no difference in cdk5 activity was found between neurons grown on sedentary myelin versus PDL.
Cortical neurons plated for 24 h on exercise-myelin displayed longer processes (Figs. 5D and 6A) compared with cells plated on myelin extracted from sedentary animals (Figs. 5C and 6A). Furthermore, neurons grown on exercise-myelin exhibited a higher number of neurites per microscopic field compared with cells on sedentary-myelin (Fig. 6B). Neurons plated on sedentary-myelin had shorter processes weakly immunostained for GAP43 (Fig. 6C) a protein associated with axonal growth and growth cone activity (Skene et al., 1986). In contrast, the processes of neurons grown on exercise-myelin displayed a strong GAP43 immunoreactivity (Fig. 6C).
DISCUSSION
It is widely acknowledged that CNS myelin is a major obstacle for axonal growth after injury. In line with the demonstrated influence of exercise on neural plasticity, we show that voluntary exercise modifies the properties of myelin. Here, we report that exercise acts as a positive modulator that makes spinal cord myelin permissive for neurite outgrowth. Our results show that exercise decreased the levels of MAG, a myelin component in part responsible for the inhibitory action of myelin, in a dose-like fashion. Application of a specific BDNF blocker to the spinal cord abolished the effects of exercise on MAG, suggesting that BDNF can mediate the effects of exercise on MAG. Furthermore, exercise increased PKA levels and these effects were prevented by application of BDNF inhibitors. These results are consistent with previous studies showing that the action of BDNF on reversing the inhibitory effects of myelin on neurite outgrowth is mediated by PKA and cAMP (Gao et al., 2003). Overall, these findings indicate a positive potential for exercise to neutralize growth inhibitory properties of the adult CNS via a BDNF-mediated mechanism. Neurons grown on exercise-myelin displayed greater process elongation than cells grown on sedentary-myelin. These differences in morphology were correlated with an increase in cdk5 activity. Hence, myelin extracted from the spinal cord of exercised animals appears to be a positive modulator of neurite outgrowth and enhances process elongations in cortical neurons in vitro. Our results suggest that exercise, in addition to affecting MAG levels, elicits critical functional changes in myelin. It is known that during development myelin has a stimulatory action on neurite outgrowth, a property that seems to be reinstated and enhanced by exercise.
Effects of Exercise on MAG
Exercise decreased MAG levels, such that changes in MAG protein followed closely changes in MAG mRNA at both 7 and 28 days of exercise. We also observed that MAG levels remained unchanged at exercise day 3, implying that longer periods of exercise are likely necessary to develop the “positive” effects of myelin on neurite outgrowth. As discussed below, the effects of exercise on MAG expression and function were associated with changes in various molecular systems previously identified as modulators of MAG function on growth inhibition. The discovery that only MAG levels were decreased in the lumbar spinal cord region of exercised rats identifies MAG as one of the main targets of exercise-positive modulatory effects. Indeed, we observed that exercise did not affect MBP levels, the major myelin-component, suggesting that exercise selectively downregulates MAG levels without affecting the total level of myelin in the CNS. Furthermore, preliminary results from our lab showed that both MAG and Nogo levels are significantly decreased by exercise after traumatic brain injury (Chytrova et al., 2006). Collectively, our results describe a selective effect of exercise on MAG and open the possibility that physical activity may have modulatory effects on other myelin inhibitors such as Nogo and Oligodendrocyte-Myelin glycoprotein (OMgp).
BDNF Can Mediate the Effects of Exercise on MAG
Blocking the action of BDNF counteracted the effects of exercise on MAG mRNA and protein levels, a strong evidence for an involvement of BDNF in the modulation of MAG by exercise. Exercise induces BDNF in the spinal cord (Gomez-Pinilla et al., 2001, 2002; Ying et al., 2005), and BDNF has been implicated in the mechanisms by which neurons become resistant to the inhibitory action of MAG. Specifically, neurons primed with BDNF can overcome myelin inhibition apparently associated with an increase in cAMP (Cai et al., 1999). Recently, Gao et al. (2003) reported that BDNF elevates cAMP to overcome the inhibition of MAG by involving activation of PKA. Our results showed that exercise elevated levels of PKA in the spinal cord and that BDNF blockade abolished the effects of exercise on PKA. Collectively these results suggest that exercise-induced BDNF can play a role in overcoming myelin inhibition by using PKA.
A recent study indicates that electrical activity can reverse the inhibitory effects of MAG and myelin on growth cones from repulsion to attraction in a cAMP dependent manner (Ming et al., 2001). Therefore, it is possible that neural activity associated with exercise may modify the molecular environment to increase the regenerative capacity of axons. The question arises as to how BDNF can signal to myelin-producing oligodendrocytes. It is possible that exercise elicits a general increase in BDNF content that may act directly on oligodendrocytes to regulate their functions such as myelin production and maintenance. We have previously reported that exercise increases the expression of BDNF in motorneurons, and glial-like cells in the white matter surrounding motoneuron axons (Gomez-Pinilla et al., 2002). In addition, it has been shown that long-term locomotor training upregulates BDNF and TrkB receptor expression in oligodendrocytes in the spinal cord (Skup et al., 2002). The fact that BDNF can be produced by oligodendrocytes opens the possibility that this neurotrophin may act in an autocrine fashion, or that neuronal BDNF as well as other signaling molecules could affect oligodendrocytes in a paracrine fashion (Condorelli et al., 1995; Dai et al., 2001; Du et al., 2006; Skup et al., 2002). It is known that neurotrophins affect oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord (McTigue et al., 1998).
Exercise Promotes a Suitable Neuronal Environment for Axonal Growth
Myelin obtained from exercised rats enhanced neurite outgrowth in cortical neuronal cultures and had a positive modulatory effect on cdk5-associated kinase activity. In addition, an increased cdk5 kinase activity was found in the lumbar region of the spinal cord of rats exposed to exercise when compared with that of sedentary controls. This observation suggests that exercise positively modulates cdk5 kinase activity and that this modulation might play a role in the action of exercise on neurite outgrowth.
Cdk5 is a serine-threonine kinase involved in process elongation and proper CNS development, whose dysfunction has been implicated in neurodegenerative diseases (Cruz and Tsai, 2004; Dhavan and Tsai, 2001). The facts that cdk5 is located at the growth cones (Nikolic et al., 1996) and that BDNF stimulates cdk5 activity in cortical neurons (Tokuoka et al., 2000) suggest the possibility that this kinase could be one of the effectors of the positive influence of exercise on neurite outgrowth in vivo. These results indicate that in addition to triggering changes in myelin composition, exercise may modify the extracellular environment and foster neuronal growth.
Exercise Can Increase the Regenerative Capacity of the CNS with a Positive Outcome for Neural Repair
Our findings support the notion that exercise may overcome some of the intrinsic conditions of the adult CNS responsible for limiting axonal growth and regeneration. Interestingly, they suggest that exercise may have a dual effect on regeneration, stimulating growth, and neutralizing factors (i.e. MAG) that inhibit axonal growth. Remarkably, BDNF seems to have the ability to support these two benefits of exercise. The potential action of exercise to increase growth-promoting signals concomitantly with reducing growth inhibitory signals emphasizes the therapeutic value of exercise after CNS injury. These findings have a therapeutic potential in terms of defining a molecular mechanism by which exercise may enhance the regenerative capacity of neurons and affect the ability of the spinal cord to compensate for the injury.
ACKNOWLEDGMENTS
The authors would like to thank Dr. James Waschek for critically reading the manuscript and insightful suggestions. We are grateful to Ruth Cole for her help with cortical neuronal cultures, to Dali Yin, Bieshia Chang, and Jemily S. Malvar for technical help and to Donna Crandall and Carol Gray of the UCLA MRRC media core for their help with the figures. The authors also thank Mark Mobilia and Tim Masloski from Carl Zeiss, Inc. for their help with the imaging software and quantification.
Grant sponsor: NIH; Grant numbers: NS45804, NS050465, HD-06576, HD-04612.
REFERENCES
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG, myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulinlike growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–740. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Chytrova G, Ying Z, Gomez-Pinilla F. Program No 22814 2006, Neuroscience Meeting Planner. Society for Neuroscience; Atlanta, GA: 2006. Exercise reduces levels of growth inhibitory molecules increased in the hippocampus after brain trauma. Online. [Google Scholar]
- Condorelli DF, Salin T, Dell' Albani P, Mudo G, Corsaro M, Timmusk T, Metsis M, Belluardo N. Neurotrophins and their trk receptors in cultured cells of the glial lineage and in white matter of the central nervous system. J Mol Neurosci. 1995;6:237–248. doi: 10.1007/BF02736783. [DOI] [PubMed] [Google Scholar]
- Croll SD, Chesnutt CR, Rudge JS, Acheson A, Ryan TE, Siuciak JA, DiStefano PS, Wiegand SJ, Lindsay RM. Co-infusion with a TrkB-Fc receptor body carrier enhances BDNF distribution in the adult rat brain. Exp Neurol. 1998;152:20–33. doi: 10.1006/exnr.1998.6836. [DOI] [PubMed] [Google Scholar]
- Cruz JC, Tsai LH. A Jekyll and Hyde kinase: Roles for Cdk5 in brain development and disease. Curr Opin Neurobiol. 2004;14:390–394. doi: 10.1016/j.conb.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Dai X, Qu P, Dreyfus CF. Neuronal signals regulate neurotrophin expression in oligodendrocytes of the basal forebrain. Glia. 2001;34:234–239. doi: 10.1002/glia.1057. [DOI] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Filbin MT. Overcoming inhibitors in myelin to promote axonal regeneration. J Neurol Sci. 2005;233:43–47. doi: 10.1016/j.jns.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Du Y, Lercher LD, Zhou R, Dreyfus CF. Mitogen-activated protein kinase pathway mediates effects of brain-derived neurotrophic factor on differentiation of basal forebrain oligodendrocytes. J Neurosci Res. 2006;84:1692, 1702. doi: 10.1002/jnr.21080. [DOI] [PubMed] [Google Scholar]
- Espinosa-Jeffrey A, Becker-Catania SG, Zhao PM, Cole R, Edmond J, de Vellis J. Selective specification of CNS stem cells into oligodendroglial or neuronal cell lineage: Cell culture and transplant studies. J Neurosci Res. 2002;69:810–825. doi: 10.1002/jnr.10344. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49:377–391. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Gao Y, Nikulina E, Mellado W, Filbin MT. Neurotrophins elevate cAMP to reach a threshold required to overcome inhibition by MAG through extracellular signal-regulated kinase-dependent inhibition of phosphodiesterase. J Neurosci. 2003;23:11770–11777. doi: 10.1523/JNEUROSCI.23-37-11770.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Gallo V. Inhibition of cyclin E-cyclin-dependent kinase 2 complex formation and activity is associated with cell cycle arrest and withdrawal in oligodendrocyte progenitor cells. J Neurosci. 2001;21:1274–1282. doi: 10.1523/JNEUROSCI.21-04-01274.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Lelievre V, Beltran-Parrazal L, Sforza DM, Malvar J, Smith DJ, Charles AC, Ferchmin PA, de Vellis J. Gene expression is differentially regulated by neurotransmitters in embryonic neuronal cortical culture. J Neurochem. 2006;97(Suppl 1):35–43. doi: 10.1111/j.1471-4159.2006.03713.x. [DOI] [PubMed] [Google Scholar]
- Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA. Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron. 2002;33:689–702. doi: 10.1016/s0896-6273(02)00602-5. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Opazo P, Roy RR, Edgerton VR. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur J Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407:963–970. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- Horner PJ, Gage FH. Regeneration in the adult and aging brain. Arch Neurol. 2002;59:1717–1720. doi: 10.1001/archneur.59.11.1717. [DOI] [PubMed] [Google Scholar]
- Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, Hamers FP, Schwab ME. Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol. 2005;58:706–719. doi: 10.1002/ana.20627. [DOI] [PubMed] [Google Scholar]
- Lom B, Cohen-Cory S. Brain-derived neurotrophic factor differentially regulates retinal ganglion cell dendritic and axonal arborization in vivo. J Neurosci. 1999;19:9928–9938. doi: 10.1523/JNEUROSCI.19-22-09928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee AW, Strittmatter SM. The Nogo-66 receptor: Focusing myelin inhibition of axon regeneration. Trends Neurosci. 2003;26:193–198. doi: 10.1016/S0166-2236(03)00062-6. [DOI] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medhurst AD, Harrison DC, Read SJ, Campbell CA, Robbins MJ, Pangalos MN. The use of TaqMan RT-PCR assays for semiquantitative analysis of gene expression in CNS tissues and disease models. J Neurosci Methods. 2000;98:9–20. doi: 10.1016/s0165-0270(00)00178-3. [DOI] [PubMed] [Google Scholar]
- Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G, Henley J, Tessier-Lavigne M, Song H, Poo M. Electrical activity modulates growth cone guidance by diffusible factors. Neuron. 2001;29:441–452. doi: 10.1016/s0896-6273(01)00217-3. [DOI] [PubMed] [Google Scholar]
- Molteni R, Zheng JQ, Ying Z, Gomez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci USA. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Nikolic M, Dudek H, Kwon YT, Ramos YF, Tsai LH. The cdk5/ p35 kinase is essential for neurite outgrowth during neuronal differentiation. Genes Dev. 1996;10:816–825. doi: 10.1101/gad.10.7.816. [DOI] [PubMed] [Google Scholar]
- Norton WT, Poduslo SE. Myelination in rat brain: Method of myelin isolation. J Neurochem. 1973;21:749–757. doi: 10.1111/j.1471-4159.1973.tb07519.x. [DOI] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, Michalovich D, Simmons DL, Walsh FS. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Riddle DR, Katz LC, Lo DC. Focal delivery of neurotrophins into the central nervous system using fluorescent latex microspheres. Biotechniques. 1997;23:928–934. 936–937. doi: 10.2144/97235rr02. [DOI] [PubMed] [Google Scholar]
- Sandvig A, Berry M, Barrett LB, Butt A, Logan A. Myelin-, reactive glia-, and scar-derived CNS axon growth inhibitors: Expression, receptor signaling, and correlation with axon regeneration. Glia. 2004;46:225–251. doi: 10.1002/glia.10315. [DOI] [PubMed] [Google Scholar]
- Schwab ME. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. J Neurosci. 1995;15(1 Part 2):477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YJ, DeBellard ME, Salzer JL, Roder J, Filbin MT. Myelin-associated glycoprotein in myelin and expressed by Schwann cells inhibits axonal regeneration and branching. Mol Cell Neurosci. 1998;12:79–91. doi: 10.1006/mcne.1998.0700. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Skene JH, Jacobson RD, Snipes GJ, McGuire CB, Norden JJ, Freeman JA. A protein induced during nerve growth (GAP-43) is a major component of growth-cone membranes. Science. 1986;233:783–786. doi: 10.1126/science.3738509. [DOI] [PubMed] [Google Scholar]
- Skup M, Dwornik A, Macias M, Sulejczak D, Wiater M, Czarkowska-Bauch J. Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp Neurol. 2002;176:289–307. doi: 10.1006/exnr.2002.7943. [DOI] [PubMed] [Google Scholar]
- Tang XM, Strocchi P, Cambi F. Changes in the activity of cdk2 and cdk5 accompany differentiation of rat primary oligodendrocytes. J Cell Biochem. 1998;68:128–137. doi: 10.1002/(sici)1097-4644(19980101)68:1<128::aid-jcb13>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Tokuoka H, Saito T, Yorifuji H, Wei F, Kishimoto T, Hisanaga S. Brain-derived neurotrophic factor-induced phosphorylation of neurofi-lament-H subunit in primary cultures of embryo rat cortical neurons. J Cell Sci. 2000;113(Part 6):1059–1068. doi: 10.1242/jcs.113.6.1059. [DOI] [PubMed] [Google Scholar]
- Tsai LH, Takahashi T, Caviness VS, Jr, Harlow E. Activity and expression pattern of cyclin-dependent kinase 5 in the embryonic mouse nervous system. Development. 1993;119:1029–1040. doi: 10.1242/dev.119.4.1029. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]