Abstract
Astrocytes are in close contact to excitatory synapses and express transporters which mediate the sodium-dependent uptake of glutamate. In cultured astrocytes, selective activation of glutamate transport results in sodium elevations which stimulate Na+/K+-ATPase and glucose uptake, indicating that synaptic release of glutamate might couple excitatory neuronal activity to glial sodium homeostasis and metabolism. Here, we analysed intracellular sodium transients evoked by synaptic stimulation in acute mouse hippocampal slices using quantitative sodium imaging with the sodium-sensitive fluorescent indicator dye SBFI (sodium-binding benzofuran isophthalate). We found that short bursts of Schaffer collateral stimulation evoke sodium transients in the millimolar range in both CA1 pyramidal neurons and in SR101-positive astrocytes of the stratum radiatum. At low stimulation intensities, glial sodium transients were confined to one to two primary branches and adjacent fine processes and only weakly invaded the soma. Increasing the number of activated afferent fibres by increasing the stimulation intensity elicited global sodium transients detectable in the processes as well as the somata of astrocytes. Pharmacological analysis revealed that neuronal sodium signals were mainly attributable to sodium influx through ionotropic glutamate receptors. Activation of ionotropic receptors also contributed to glial sodium transients, while TBOA-sensitive glutamate transport was the major pathway responsible for sodium influx into astrocytes. Our results thus establish that glutamatergic synaptic transmission in the hippocampus results in sodium transients in astrocytes that are mainly mediated by activation of glutamate transport. They support the proposed link between excitatory synaptic activity, glutamate uptake and sodium signals in astrocytes of the hippocampus.
Introduction
At glutamatergic synapses in the brain, astrocytes mediate the clearance of glutamate from the extracellular space by the high-affinity transporters GLAST and GLT-1 (Danbolt, 2001). Glial glutamate uptake is central to synaptic function because it shapes the time course of synaptic conductance, moderates the activation of extrasynaptic receptors and limits spillover of glutamate to adjacent synapses (Barbour et al. 1994; Diamond & Jahr, 1997; Huang & Bergles, 2004; Tzingounis & Wadiche, 2007). It is energized by the inward transport of three sodium ions and one proton, while one potassium ion is transported out of the cell, generating an inward current (Brew & Attwell, 1987; Szatkowski et al. 1990; Barbour et al. 1991; Zerangue & Kavanaugh, 1996; Bergles & Jahr, 1997).
In astrocytes in culture, bath application of glutamate or d-aspartate activates glutamate uptake and inward transport of sodium, resulting in an increase in the intracellular sodium concentration (Rose & Ransom, 1996b; Chatton et al. 2000; Voutsinos-Porche et al. 2003). Such sodium elevations stimulate Na+/K+-ATPase and cause increased ATP consumption and glucose uptake by astrocytes (Pellerin & Magistretti, 1994; Chatton et al. 2000; Loaiza et al. 2003; Porras et al. 2008). Based on these observations, it was suggested that glutamate-induced sodium signals in astrocytes might serve as key signals in the coupling of excitatory synaptic activity with increased glial metabolism (Tsacopoulos & Magistretti, 1996; Magistretti & Chatton, 2005). On the other hand side, glutamate uptake activity is directly dependent on the inwardly directed electrochemical gradient for sodium, and decreasing this gradient by increasing intracellular sodium provides a negative feedback (Szatkowski et al. 1990; Zerangue & Kavanaugh, 1996; Kelly et al. 2009). Furthermore, sodium influx, associated with prolonged activation of glutamate uptake, was reported to trigger the formation of clusters of GLT-1 at the plasma membrane of astrocytes co-cultured with neurons (Nakagawa et al. 2008). The sodium-dependent clustering was accompanied by a decrease in cell surface expression and increased intracellular expression of GLT-1. The aforementioned studies identified sodium elevations in astrocytes as relevant signals influencing glutamate uptake capacity as well as glial metabolism and glucose uptake and its coupling to neuronal activity.
The properties and spatial profiles of sodium transients in glial cells that accompany synaptic transmission in the intact tissue, however, are still largely unexplored. Until now, synaptically induced sodium transients have been described only from Bergmann glial cells, a specialized type of astrocytes in the cerebellum (Kirischuk et al. 2007; Bennay et al. 2008). In the present study, we analysed sodium signals evoked by afferent synaptic stimulation in SR101-positive, passive astrocytes of the CA1 area by performing quantitative sodium imaging in acute tissue slices of the mouse hippocampus. We demonstrate that, dependent on the stimulation parameters, synaptic stimulation results in either local or global sodium transients in hippocampal astrocytes. We also provide a pharmacological analysis which shows that these glial sodium transients are partly due to activation of ionotropic glutamate receptors, while most of the sodium influx is mediated by sodium-dependent glutamate transport.
Methods
The study and all experiments were carried out in accordance with The Journal of Physiology's policy (Drummond, 2009) and with the institutional guidelines of the Heinrich-Heine-University Düsseldorf as well as the European Communities Council Directive (86/609/EEC).
Tissue preparation and identification of astrocytes
Experiments were performed on acute tissue slices (250 μm) of mouse hippocampus (Mus musculus, Balb/C; postnatal days 12–21). Animals were decapitated following anaesthesia with CO2 and their brains were rapidly removed. Transverse hippocampal slices were prepared as described previously (Edwards et al. 1989). Sectioning was performed in standard saline at 4–6°C. The standard saline was composed of (in mm): 125 NaCl, 2.5 or 4 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3 and 20 glucose, bubbled with 95% O2 and 5% CO2, resulting in a pH of 7.4. After sectioning, slices were kept at 34°C for 20 min in standard saline that contained 0.5–1 μm sulforhodamine 101 (SR101), followed by a 10 min incubation in saline without SR101 at 34°C. This procedure results in the selective staining of passive astrocytes as described earlier (Nimmerjahn et al. 2004; Kafitz et al. 2008). Afterwards, slices were kept in standard saline at room temperature (19–22°C) until they were used for experiments, which were also performed at room temperature.
Electrical stimulation of Schaffer collaterals was performed by square pulses (150 μs duration) delivered at 50 Hz via a thin borosilicate glass pipette (Hilgenberg, Waldkappel, Germany) filled with saline (pipette resistance: 2–4 MΩ). The stimulation pipette was placed at a distance of 50–200 μm from the somata of selected SR101-positive astrocytes in the stratum radiatum of the CA1 area, unless stated otherwise. d-Aspartate was applied by a pressure application device (PDES-02D, NPI Electronic GmbH, Tamm, Germany) coupled to standard micropipettes (Hilgenberg, Waldkappel, Germany) placed 20–100 μm from cell bodies of selected cells in the presence of 0.5 μm tetrodotoxin. All other substances were applied with the bath perfusion system. Chemicals were purchased from Sigma, except for tetrodotoxin (Alomone Labs, Jerusalem, Israel or Biotrend Chemicals, Cologne, Germany) and dl-threo-ß-benzyloxyaspartatic acid (TBOA; Tocris/BIOZOL Diagnostica Vertrieb GmbH, Eching, Germany).
Electrophysiology
Standard whole-cell recordings were obtained from the somata of SR101-positive astrocytes with an EPC10 amplifier (HEKA Elektronik, Lambrecht, Germany); PatchMaster software (HEKA Elektronik, Lambrecht, Germany) was used for data acquisition. The pipette solution contained (in mm): 120 KMeSO3, 32 KCl, 10 N-(2-hydroxyethyl)piperazine-N′-(2-ethanesulfonic acid (Hepes), 4 NaCl, 4 Mg-ATP, 0.4 Na3-GTP and 0.5–1 sodium-binding benzofuran isophthalate (SBFI); the pH was adjusted to pH 7.3. Astrocytes were generally held at a membrane potential of −85 mV; liquid junction potential was not corrected. To ensure sufficient diffusion of SBFI into distal branches, cells were filled for at least 20 min before starting the sodium imaging.
Na+ imaging and calibration of SBFI fluorescence
Conventional, wide-field fluorescence imaging was performed using a variable scan digital imaging system (TILL Photonics, Martinsried, Germany) attached to an upright microscope (Olympus BX51Wi, 40×/60× water immersion objective, NA 0.80/0.90, Olympus Europe, Hamburg, Germany) and a CCD camera as sensor (TILL Imago VGA, TILL Photonics, Martinsried, Germany). Cells were dye-loaded by bolus injection of the membrane-permeable form of SBFI (SBFI-AM (the acetoxymethyl ester); Molecular Probes/Invitrogen, Karlsruhe, Germany) into the CA1 region of the hippocampus as described earlier (Meier et al. 2006). When performing patch-clamp experiments, individual cells were loaded via the patch pipette that contained 0.5 or 1 mm of the membrane-impermeable form of SBFI.
In situ calibration of intracellular SBFI fluorescence in bolus-loaded cells was performed as described earlier (Meier et al. 2006). Following loading with SBFI-AM, slices were perfused with calibration solutions containing different sodium concentrations as well as ionophores (3 μm gramicidin, 10 μm monensin) to equilibrate extra- and intracellular sodium concentrations. Excitation spectra, recorded from astrocyte somata in bolus-loaded slices at sodium concentrations of 0 mm and 30 mm, indicated an isosbestic point around or slightly below 340 nm, while at longer wavelengths, fluorescence emission decreased with increasing sodium concentration (n= 9; Fig. 1B). This ‘in situ’ spectrum is very similar to those obtained by others (e.g. Borzak et al. 1992) and confirms earlier reports showing that the spectral properties of SBFI inside cells differ from those in vitro (Harootunian et al. 1989; Rose & Ransom, 1996a; Diarra et al. 2001; Meier et al. 2006). Proper determination of the isosbestic point in the ‘in situ’ condition was not feasible because the performance of our imaging equipment (optical parts, lenses, monochromator) quickly drops below 340 nm.
Figure 1. Identification of astrocytes and calibration of sodium signals.
A, upper left: transmitted light image of the hippocampal CA1 area. Cell bodies of CA1 pyramidal neurons can be recognized in the stratum pyramidale (SP). A pipette positioned for electrical stimulation of Schaffer collaterals is visible in the stratum radiatum (SR). Upper right: image of the SBFI fluorescence taken from the same area. Both neuronal and glial cell bodies are stained in green. Lower left: image of the SR101 fluorescence in which astrocytes are labelled in red. Lower right: merged picture in which SBFI-loaded, SR101-positive astrocytes appear in yellow. B, excitation spectrum of intracellular SBFI fluorescence measured for excitation wavelengths between 320 and 415 nm in calibration solutions containing ionophores and 0 mm or 30 mm sodium. C, calibration of the sodium sensitivity of SBFI in astrocytes. Cells were bolus-loaded and then subjected to calibration solution containing ionophores. Stepwise changes in the extracellular sodium concentration from 0 to 30 mm and back caused stepwise changes in the fluorescence ratio of SBFI (F345/F385). D, relationship between changes in fluorescence ratio (ΔF345/F385) and [Na+]i, normalized to the ratio in sodium-free calibration saline. Shown are mean values ±s.e.m. (n= 44). The fit reveals a virtually linear relationship between 10 and 30 mm sodium. Within this range, a 10% change in fluorescence emission corresponds to a change of about 7.1 mm sodium. Dotted lines point to baseline sodium concentrations determined for neurons and astrocytes.
To validate the contribution of autofluorescence, that is the fluorescence of unstained tissue, to the fluorescence generated in SBFI-loaded slices, we obtained excitation spectra in slices before and after loading with SBFI. These spectra revealed that at excitation wavelengths between 330 and 390 nm, autofluorescence contributed only about 10% to the fluorescence signal obtained from the stratum radiatum of SBFI-loaded slices.
Based on this, we choose 345 nm, at which SBFI emission is only weakly sodium sensitive, and 385 nm, at which SBFI is strongly sodium sensitive, as alternate excitation wavelengths for ratio calculation (see also Fig. 2B).
Figure 2. Synaptically induced sodium transients in somata of neurons and astrocytes.
A, images of the SR101 fluorescence (left) and of the SBFI fluorescence (right) taken from the hippocampal CA1 area, double-labelled with SBFI and SR101. Dashed circles in the right image indicate the regions of interest analysed in the experiment depicted in B and C (SP: stratum pyramidale; SR: stratum radiatum). B, changes in SBFI fluorescence emission after excitation at 385 nm and 345 nm in the background region (bg), and in neuronal cell bodies (n1–n4) and astrocytes (a1–a5) induced by short-burst electrical stimulation of afferent Schaffer collaterals as indicated by the arrowheads. Virtually no signal could be detected in areas free of clearly visible cellular processes (bg). In regions positioned over neuronal and astrocyte cell bodies, the stimulation induced a large drop in the emission at 385 nm and a significantly smaller decrease at 345 nm. C, ratio changes in SBFI fluorescence (F345/F385) indicate the occurrence of somatic sodium transients in different neurons (n1–n4) and astrocytes (a1–a5) upon synaptic stimulation as indicated by the arrowheads. Note the different scale for the amplitude of transients of regions n1 and a1 and all other regions.
For wide-field imaging with SBFI, fluorescence emission (>440 nm) was collected at 3–4 Hz from defined regions of interest (ROI) containing cellular structures following alternate excitation at 345 nm and at 385 nm. After background correction, the fluorescence ratio (F345/F385) was calculated for individual ROIs and analysed off-line by employing OriginPro Software (OriginLab Corporation, Northhampton, MA, USA).
Standard background correction was performed as described earlier (Garaschuk et al. 1998). In brief, for each excitation wavelength (345 and 385 nm), fluorescence emission was collected from a region within the field of view which was free of clearly visible SR101-positive cellular processes (cf. region marked as ‘bg’ in Fig. 2A and B) and the determined background values were then subtracted from the fluorescence intensity of the ROIs for each frame. Background fluorescence is generated by both cellular autofluorescence (see above) and extracellular dye. Thus, background fluorescence is significantly larger than autofluorescence. Only cells with fluorescence values that were at least 30% higher than the background fluorescence were included in the study.
When bolus-loaded slices were subjected to Na+-free calibration saline containing ionophores (see above), ratio levels in the ROIs dropped rapidly, reaching a new steady state within about 20 min (n= 6 neurons, n= 51 astrocytes; not shown), as reported before (Rose & Ransom, 1996a; Meier et al. 2006; Bennay et al. 2008). Subsequent stepwise changes in the sodium concentration of the calibration saline then caused stepwise changes in the SFBI ratio, indicating stepwise changes in the intracellular sodium concentration (n= 44 astrocytes; Fig. 1C). When normalized to the fluorescence ratio obtained in sodium-free saline, the relationship between changes in SBFI fluorescence and intracellular sodium concentration was virtually linear for sodium concentrations between 10 and 30 mm (n= 44; Fig. 1D). Within this range, a change in the fluorescence ratio by 10% corresponded to a change in the intracellular sodium concentration by 7.1 mm.
Baseline intracellular sodium was determined by a direct comparison between the fluorescence ratios of single ROIs in physiological saline with that following perfusion with calibration saline at different sodium concentrations (Fig. 1D). Activity-induced changes in SBFI ratio, ranging from 0.4% to 33%, were generally expressed as changes relative to baseline in per cent. Because of the linearity of the ratio signal between 10 and 30 mm sodium, ratio changes in per cent were then multiplied by a factor of 0.71 (see above) to reflect absolute changes in the sodium concentration. Calibration parameters obtained with the bolus-loading technique were also used for quantification of SBFI signals obtained during patch-clamp experiments, because earlier studies in our laboratory have shown that, for a given imaging set-up, calibration parameters of intracellular SBFI fluorescence are comparable for bolus-loading of SBFI-AM and loading of SBFI through a patch pipette (Meier et al. 2006; Bennay et al. 2008).
Unless otherwise specified, data are presented as means ±s.e.m. and were statistically analysed by Student's paired or unpaired t tests, as appropriate. If not stated otherwise, n represents the number of analysed cells. Each set of experiment was performed on at least three different slice preparations.
Results
For analysis of activity-induced intracellular sodium transients, tissue slices were first incubated with the red fluorescent dye SR101, which selectively stains passive astrocytes (Fig. 1A; Nimmerjahn et al. 2004; Kafitz et al. 2008). Passive astrocytes are characterized by linear I–V relationships and a prominent expression of electrogenic glutamate transport (Zhou & Kimelberg, 2001; Matthias et al. 2003). At about 2–3 weeks postnatally, they comprise more than 90% of cells with glial morphology in the stratum radiatum of the hippocampus, thus representing the most prominent astrocyte subtype in this area (Bordey & Sontheimer, 1997; D’Ambrosio et al. 1998; Zhou et al. 2006; Kafitz et al. 2008). After staining with SR101, additional loading with the sodium-sensitive indicator dye SBFI was performed by bolus-injection of its membrane-permeable form (SBFI-AM) into the stratum radiatum of the CA1 area. This technique results in a good-quality staining of astrocytes as well as CA1 pyramidal neurons and thus enables the study of both cell types at the same time (Figs 1A and 2A; Kafitz et al. 2008; Meier et al. 2008).
Synaptically induced sodium transients in neurons and astrocytes
In situ calibration experiments (see Methods; Fig. 1D) revealed that baseline intracellular sodium concentration was 12.0 ± 1.1 mm (n= 6) in somata of CA1 pyramidal neurons and 10.8 ± 1.2 mm in SR101-positive astrocytes (n= 8), which is in good agreement with values reported earlier (e.g. Rose & Ransom, 1996a; Chatton et al. 2001; Sheldon et al. 2004). To evoke synaptic activity, afferent Schaffer collaterals were stimulated by current pulses delivered at 50 Hz through a fine micropipette positioned in the stratum radiatum. Short-burst stimulation of Schaffer collaterals (50 Hz, 200 ms) induced a significant transient reduction in fluorescence emission at 385 nm in both neurons and astrocytes, while fluorescence emission at 345 nm only slightly decreased (Fig. 2A and B). Fluorescence in background regions was virtually unchanged (Fig. 2A and B). Thus, synaptic activity caused a transient increase in the SBFI fluorescence ratio (F345/F385), indicating an increase in the sodium concentration in the somata of pyramidal neurons as well as of SR101-positive astrocytes (Fig. 2C).
The amplitude of sodium transients detected from ROIs positioned over neuronal somata ranged from 0.9 to 23.6 mm (average 5.1 ± 0.3 mm; n= 223). Peak sodium concentrations were reached only 5–20 s after the stimulation (Fig. 2C). Sodium transients detected from ROIs positioned over the somata of SR101-positive astrocytes ranged from 0.6 to 7.8 mm (average 2.1 ± 0.1 mm; n= 122) and peak concentrations were reached within 0.6–10 s (Fig. 2C).
In cultured astrocytes, intercellular waves of sodium, propagating with a speed of about 15 μm s−1, can be evoked in response to mechanical or direct electrical stimulation of single cells (Bernardinelli et al. 2004).
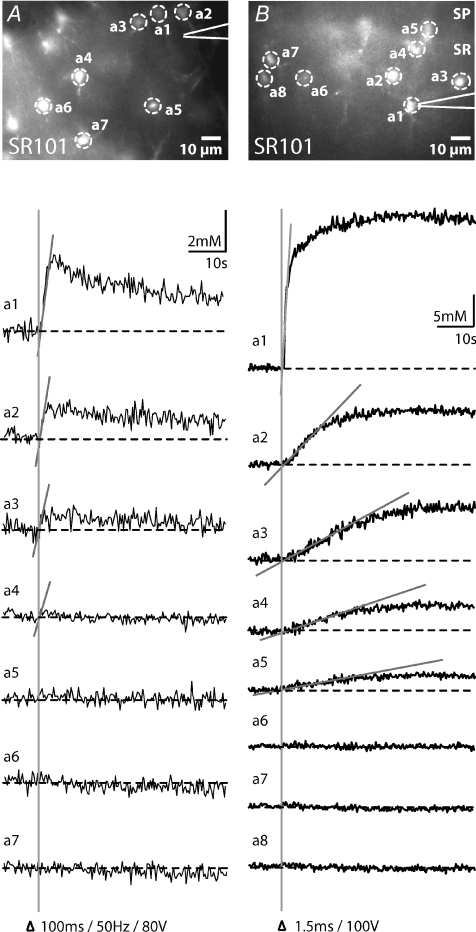
In the present study, stimulation pipettes were generally positioned 50–200 μm from the somata of SR101-positive, SBFI-loaded astrocytes. Under these conditions and with the given imaging frequency of 3–4 Hz, we did not observe sodium signals propagating in a wave-like manner from an individual astrocyte to its neighbours within the field of view (222 μm × 168 μm), indicating simultaneous activation of these astrocytes in response to synaptic stimulation. To reduce the number of activated cells in the field of view, we positioned the stimulation pipette close (∼20 μm) to SBFI-loaded astrocyte cell bodies. Under these conditions, detectable synaptically induced sodium signals were restricted to astrocytes located within a radius of about 50 μm around the stimulation pipette (n= 6 slices, Fig. 3A). While the amplitude of these sodium transients typically decreased with increasing distance from the stimulation pipette, no difference in their delay or rise time, indicative of a wave-like propagation from cell to cell, was visible (Fig. 3A). Thus, these data suggest that the observed sodium signals were again primarily caused by simultaneous synaptic activation of the involved astrocytes. To selectively induce a sodium elevation in a single astrocyte, we then placed the stimulation pipette in direct physical contact with their soma. Electrical stimulation resulted in an immediate, strong and long-lasting increase in the intracellular sodium concentration in this cell (n= 8; Fig. 3B). In addition, we observed sodium elevations in neighbouring astrocytes within a radius of about 40–50 μm. Both the peak amplitudes and the slopes of the rising phase of sodium elevations in surrounding cells typically decreased with increasing distance from the stimulated cell, suggesting propagation in a wave-like manner.
Figure 3. Astrocyte sodium transients evoked by focal synaptic and direct electrical stimulation.
A, top, image of the SR101 fluorescence from the stratum radiatum, double-labelled with SBFI and SR101. Dashed circles indicate the regions of interest (a1–a7) analysed in the experiment depicted below. The stimulation pipette, positioned close to an astrocyte cell body, is indicated schematically. Traces below depict intracellular sodium in the astrocytes a1–a7. Synaptic stimulation (100 ms, 50 Hz, 80 V) was performed as indicated by the arrowhead and the perpendicular line. Please note that synaptically induced sodium signals were restricted to astrocytes located within a radius of about 50 μm around the stimulation pipette. Grey lines indicate that the slope of the rising phase of sodium transients was similar in all cells. B, image of the SR101 fluorescence of the CA1 area, double-labelled with SBFI and SR101 (SP: stratum pyramidale; SR: stratum radiatum). Dashed circles indicate the regions of interest (a1–a8) analysed in the experiment depicted below. The stimulation pipette was positioned in direct physical contact with the cell a1. Traces below show intracellular sodium in the astrocytes a1–a8 in response to direct electrical stimulation of the cell a1 (100 V for 1.5 ms) as indicated by the arrowhead and the perpendicular line. Please note that the electrical stimulation induced an immediate and large increase in the intracellular sodium concentration in a1, whereas neighbouring astrocytes within a radius of about 40 μm (a2–a5) responded as well. Grey lines indicate that the slope of the rising phase of sodium transients decreased with increasing distance from the stimulated cell.
Specificity of cellular sodium signals
In a widefield fluorescence imaging system, fluorescence emission is generated (and collected) not only in the entire plane of focus, but also above and below. In addition, light scattering causes significant distortion of stained structures, and small astrocyte processes and neuronal dendrites cannot be resolved. As our bolus-loading technique stains both astrocytes and neurons at the same time, we carried out several sets of experiments to ensure that fluorescence signals obtained from ROIs positioned over cellular somata indeed primarily reflect intracellular signals from the chosen cell without significant distortions by fluorescence emission from out-of-focus structures.
To evaluate a possible crosstalk between neuronal and astrocyte SBFI fluorescence signals in bolus-loaded slices, we applied d-aspartate. d-Aspartate is a non-metabolized substrate of sodium-dependent glutamate uptake which is also transported (Danbolt, 2001). Its application thus results in sodium transients and inward currents in astrocytes if functional transporters are expressed (Rose & Ransom, 1996b; Chatton et al. 2001; Bennay et al. 2008; Kelly et al. 2009). Moreover, d-aspartate is an agonist of NMDA receptors, the activation of which causes sodium transients in CA1 pyramidal neurons (Rose & Konnerth, 2001). Pressure application of d-aspartate (1 mm) evoked transient increases in the SBFI fluorescence ratio in ROIs positioned over neuronal and astrocyte cell bodies, indicating intracellular sodium transients in both cell types (Fig. 4A). For a puff duration of 400 ms, these transients amounted to 13.4 ± 0.9 mm in neurons (n= 79) and 4.8 ± 0.3 mm in astrocytes (n= 95). d-Aspartate-induced sodium transients could be evoked repetitively in both cell types, while their amplitude decayed by 10–30% with five applications within 30 min (n= 15 neurons and 14 astrocytes; Fig. 4A). When APV (dl-2-amino-5-phosphonopentanoic acid, 50 μm), a selective blocker of NMDA receptors was applied, neuronal sodium transients were strongly reduced (reduction by 88.8 ± 3.2%; n= 10; Fig. 4B). At the same time, APV only slightly reduced the amplitude of glial sodium transients (reduction by 16.8 ± 3.6%; n= 13; Fig. 4B). While this could indicate some overlay of glial with dendritic signals under control conditions, this apparent effect of APV on astrocytes might also – at least partly – be explained by the observed slight rundown of the response under control conditions. Furthermore, it could arise from activation of NMDA receptors on astrocytes themselves, as recently described (Serrano et al. 2008).
Figure 4. Cell specificity of sodium signals.
A, left, sodium transients induced by repetitive local pressure application of d-aspartate (1 mm, 400 ms) detected in regions of interest positioned over a neuronal and an astrocyte cell body. Right, histogram depicting the mean amplitudes ±s.e.m. of sodium signals in neurons and astrocytes upon repetitive application of d-aspartate, normalized to the amplitude of the first response. B, left, influence of the NMDA receptor blocker APV (50 μm) on sodium transients induced by local pressure application of d-aspartate (1 mm, 400 ms). Whereas neuronal signals are strongly reduced, there is only a slight reduction of astrocyte sodium signals. Right, histogram depicting the effect of APV on the mean amplitudes of sodium transients induced by application of d-aspartate in neurons and astrocytes as per cent of control values. The number of investigated cells is indicated in the bars. *** indicates a significant difference as compared to control, P < 0.001.
To further test the cell type specificity of fluorescence signals, we performed a bolus injection of SBFI-AM into the stratum oriens. This resulted in the loading of somata of CA1 pyramidal neurons of the stratum pyramidale as well as their apical dendrites in the stratum radiatum, whereas astrocytes in the stratum radiatum did not visibly load with SBFI. Upon application of d-aspartate, SBFI-stained neuronal cell bodies showed transient changes in SBFI fluorescence emission, indicating an increase in sodium concentration. Fluorescence emission from the somata of SR101-stained astrocytes in the stratum radiatum, in contrast, was not different from background and did not change in response to stimulation (n= 11; not shown). This indicates that fluorescence emission from apical CA1 pyramidal cell dendrites located above or below the plane of focus of astrocyte somata had no detectable influence on glial fluorescence emission under these conditions.
We also carried out widefield imaging experiments on astrocytes individually loaded with SBFI. To this end, the membrane-impermeable form of SBFI was loaded into SR101-positive astrocytes through a patch pipette in the whole-cell patch-clamp mode. Twenty to thirty minutes after breakthrough, SBFI fluorescence was visible in the entire cell, including primary and secondary processes as well as fine processes arising from them, generating out-of-focus light of a cloud-like appearance (Figs 5A and 6A). As reported before, burst stimulation of Schaffer collaterals (50 Hz, 200 ms) induced long-lasting inward currents in astrocytes (n= 12; Fig. 5B), that largely reflect the activation of electrogenic glutamate transport (Brew & Attwell, 1987; Bergles & Jahr, 1997; Diamond et al. 1998; Luscher et al. 1998). Inward currents were accompanied by transient changes in SBFI emission in astrocyte somata, indicating sodium increases ranging from 0.4 to 4.3 mm (average: 1.4 ± 0.3; n= 12; see ROI 3 (r3) in Fig. 5B), which is similar to values obtained from bolus-loaded slices (see above). Fluorescence emission (at excitation with 345 and 385 nm) from surrounding SR101-positive astrocytes (Fig. 5C: ROIs r8, r9, r10) as well as from background regions (Fig. 5C: bg) did not change, indicating the absence of activity-dependent changes in autofluorescence. No significant differences in the time course and kinetics of sodium transients detected from astrocyte somata were observed when comparing signals from bolus-loaded and pipette-loaded astrocytes.
Figure 5. Synaptically induced sodium signals in different astrocytic regions.
A, image of the SR101 staining (top) and the SFBI fluorescence (centre) of a patch-clamped astrocyte. The patch pipette can be weakly seen on the left. Bottom, inversed picture of the SBFI fluorescence. Coloured dashed lines indicate the regions of interest (r1–r7) analysed for the experiment depicted in B. The stimulation pipette was positioned at the lower right hand side at a distance of about 40 μm from the cell body. B, somatic currents (upper traces) and sodium signals in the different cellular regions r1–r7 (bottom) in response to synaptic stimulation at 20 V and 50 V (200 ms, 50 Hz) as indicated by the arrowheads. Please note that low intensity stimulation causes a significant sodium signal in region r1 only. Higher intensity stimulation induces sodium transients in all cellular regions, which, however, differ in both rise times and amplitudes (see arrows). C, left, image of the SR101 staining (top) and the SFBI fluorescence (bottom) of the same area as in A, taken at smaller magnification. In the SR101 staining, three more astrocyte cell bodies (r8–r10) are now visible, which are absent in the SBFI fluorescence image. Right, the synaptic stimulation neither induces changes in the fluorescence ratio in these additional SR101-positive astrocytes, nor in a region free of clearly visible cellular processes, defined as background (bg), demonstrating the specificity of ratio signals obtained from the SFBI-loaded cell.
Figure 6. Sodium signals induced by d-aspartate in different astrocytic regions.
A, image of the SR101 staining (top) and the SFBI fluorescence (centre) of a patch-clamped astrocyte. The patch pipette can be weakly seen on the left. Bottom, inversed picture of the SBFI fluorescence. Coloured dashed lines indicate the regions of interest (r1–r7) analysed for the experiment depicted in B. The position of the stimulation pipette is indicated in the centre picture. B, somatic currents (upper traces) and sodium signals in the different cellular regions r1–r7 (bottom) in response to puff application of d-aspartate for 100 and 200 ms as indicated by the arrowheads. Please note that sodium signals increase with longer application and are largest in regions r1 and r2, which are located closest to the application pipette. Peak amplitudes are reached later in regions located further away from the application pipette (see arrows). C, left, image of the SR101 staining (top) and the SFBI fluorescence (bottom) of the same area as in A, taken at smaller magnification. In the SR101 staining, three more astrocyte cell bodies (r8–r10) are now visible, which are absent in the SBFI fluorescence image. Right, the D-aspartate application neither induces changes in the fluorescence ratio in these additional SR101-positive astrocytes, nor in a region free of clearly visible cellular processes, defined as background (bg), demonstrating the specificity of ratio signals obtained from the SFBI-loaded cell.
Taken together, these experiments demonstrate that synaptic activity generates sodium signals in astrocytes. Furthermore, they show that under our experimental conditions, fluorescence signals from ROIs positioned over CA1 pyramidal cell bodies in the stratum pyramidale and astrocyte cell bodies in the stratum radiatum primarily represent sodium signals from these cell types in bolus-loaded slices (cf. also later experiments and Figs 8B and 9Aa).
Figure 8. Influence of receptor blockers and action potential generation.
A, left, effects of bicuculline methiodide (20 μm) and tetrodotoxin (TTX, 0.5 μm) on synaptically induced somatic sodium transients in a pyramidal neuron (upper trace) and an astrocyte (lower trace). Stimulation was performed at 200 ms–50 Hz as indicated by the arrowheads. Right, bar chart depicting normalized mean values ±s.e.m. of the peak amplitudes of evoked sodium signals during perfusion with bicuculline and TTX as per cent of control values. B, left, influence of the AMPA receptor blocker CNQX (10 μm) on sodium transients in neurons and astrocytes induced by synaptic stimulation at 200 ms–50 Hz as indicated by arrowheads. Right, bar chart depicting the effect of CNQX on the amplitudes of activity-induced sodium transients in neurons and astrocytes as per cent of control values. The number of analysed cells is indicated in the bars; ***P < 0.001; **P < 0.01.
Figure 9. Role of electrogenic glutamate uptake.
Aa, experiments illustrating the influence of TBOA, a blocker of glutamate uptake, on baseline sodium concentration and on synaptically induced sodium transients in a neuron (upper row) and an astrocyte (lower row). Please note the difference in scales for amplitudes of sodium changes. Upper row, the inset shows the neuronal control signal at an enlarged scale for better comparison with the glial signal. The area enclosed by the dashed grey lines illustrates the effect of TBOA on baseline sodium at a condensed time scale. Right, histogram depicting the effect of TBOA on the mean amplitudes of sodium transients induced by Schaffer collateral stimulation in astrocytes as per cent of control values. Ab, left, influence of combined application of CNQX (10 μm) and APV (50 μm) as well as of an additional application of the glutamate uptake blocker TBOA (200 μm) on synaptically induced sodium signals in an astrocyte. Stimulation was performed as indicated by the arrowheads. Right, bar chart depicting the effect of combined application of the blockers on the amplitudes of activity-induced sodium transients in astrocytes as per cent of control values. Ba, left, sodium transients induced by local pressure application of d-aspartate (1 mm, 400 ms) and the influence of TBOA. Right, histogram depicting the effect of TBOA on the mean amplitude of sodium transients induced by application of d-aspartate in astrocytes as per cent of control values. Bb, left, sodium transients induced by local pressure application of d-aspartate (1 mm, 400 ms) in the control, during application of APV and CNQX, and during additional application of TBOA. Right, histogram depicting the effects of the indicated blockers on the amplitudes of d-aspartate-induced sodium transients in astrocytes as per cent of control values. The number of analysed cells is indicated in the bars; ***P < 0.001; **P < 0.01.
Sodium transients in cellular subdomains of astrocytes
Loading of astrocytes through a patch pipette enabled the visualization not only of astrocyte somata, but also of their primary and secondary processes and surrounding structures with a low background. Using this loading technique, we asked whether sodium transients differ in different cellular domains by analysing the amplitude and time course of fluorescence signals from the soma, and from several primary processes together with the diffuse fluorescence surrounding them, individually (Figs 5A and 6A).
We found large differences in the amplitude and kinetics of activity-induced sodium transients between the different cellular domains in single astrocytes (n= 12; Fig. 5B). The sodium signals were largest in one to two cellular domains that represented a primary branch with its fine processes (r1 and r2, Fig. 5). Maximal sodium transients were characterized by a fast rise time and a defined peak, which reached up to 6.0 mm. In other cellular domains (r4–r7, Fig. 5), the amplitudes of the sodium transients were smaller and their peaks only reached many seconds later (see arrows, Fig. 5B). In the soma (r3, Fig. 5), intermediate sodium signals in terms of amplitude and time to peak were recorded. We also found that, with low stimulation intensities, sodium signals were largely restricted to a primary branch and its associated fine processes and only weakly invaded the soma and other cellular domains (compare r1 and r2–7, Fig. 5).
Differences in amplitude, rise time and decay time of sodium transients between different cellular domains of astrocytes were also observed upon selective activation of electrogenic glutamate uptake. Local pressure application of d-aspartate (1 mm) evoked a somatic inward current in whole-cell patch-clamped, SBFI-loaded astrocytes (n= 30 applications, 8 cells; Fig. 6B). The average peak amplitude of this inward current was dependent on the duration of the d-aspartate application and amounted to 1.0 ± 0.4 nA when d-aspartate was applied for 200 ms (n= 13 applications, 8 cells). Amplitude and time course of evoked sodium transients depended not only on the duration of the application, but also on the spatial relationship between application pipette and analysed region of interest. We consistently found that sodium signals were largest and their peaks reached fastest in cellular domains located close to the application pipette (n= 8 cells, Fig. 6B). Again, no signals were detectable in surrounding SR101-positive, SBFI-unstained astrocytes nor in background regions (Fig. 6C).
Pharmacology of synaptically induced sodium transients
Our results obtained so far demonstrate that Schaffer collateral stimulation evokes sodium transients in the millimolar range in CA1 pyramidal neurons and SR101-positive astrocytes. We next characterized the basic pharmacological properties of these activity-induced sodium transients in bolus-loaded slices to elucidate the mechanisms of their generation. To this end, we first established that sodium signals can be evoked repetitively in neurons and astrocytes in the course of an experiment (n= 37 neurons and 32 astrocytes; Fig. 7A). Increasing the stimulation intensity increased the amplitude of synaptically induced sodium transients in both cell types (n= 53 neurons and 25 astrocytes; Fig. 7B; cf. Fig. 5).
Figure 7. Sodium signals in response to repetitive synaptic stimulation.
A, top, somatic sodium transients in a neuron (upper traces) and an astrocyte (lower traces) induced by three consecutive stimulations at 200 ms and 50 Hz as indicated by the arrowheads. Bottom, histogram depicting the mean amplitudes of sodium transients induced by consecutive stimulations in neurons (dark grey) and astrocytes (light grey) normalized to the amplitude of the first response. The number of investigated cells is indicated in the first bars. B, somatic sodium transients in a neuron (upper traces) and an astrocyte (lower traces) induced by short-burst electrical stimulation (200 ms, 50 Hz) of increasing stimulation intensity as indicated by the arrowheads.
Both neuronal and glial sodium transients induced by synaptic stimulation were blocked during perfusion with 0.5–1 μm tetrodotoxin, confirming their dependence on action potential generation (n= 42 neurons, 14 astrocytes; Fig. 8A). During perfusion with bicuculline methiodide (20 μm), a specific antagonist of GABAA receptors, the amplitudes of synaptically evoked neuronal and glial signals were increased to about 2- and 1.3-fold in neurons (n= 42) and astrocytes (n= 14), respectively (Fig. 8A). Astrocytes express GABAA receptors and their activation depolarizes the cells (e.g. Meier et al. 2008), thereby reducing the driving force for sodium-dependent glutamate uptake. Blocking this depolarization by bicuculline might thus result in increased activation of glutamate uptake and sodium influx. In addition, bicuculline methiodide might increase neuronal excitability due to its effect on calcium-activated potassium currents (Johansson et al. 2001).
Earlier work has established that suprathreshold short-burst Schaffer collateral stimulation induces sodium transients in dendrites and spines of CA1 pyramidal neurons that are dependent on AMPA receptor activation (Rose & Konnerth, 2001). While hippocampal astrocytes are characterized by a strong expression of sodium-dependent glutamate transport (Matthias et al. 2003), there is also some evidence for expression of AMPA receptors (Zhou & Kimelberg, 2001). We hence tested the influence of AMPA receptor activation on synaptically induced sodium signals in neurons and astrocytes. In CA1 pyramidal neurons, inhibition of AMPA receptors by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 10 μm) diminished sodium transients by 76.4 ± 3.7% (n= 31; Fig. 8B). At the same time, the amplitude of sodium transients in astrocytes was reduced by only 17.5 ± 5.9% (n= 16; Fig. 8B).
To study the involvement of sodium-dependent glutamate transport, we applied the non-transported, competitive glutamate-uptake blocker TBOA (200 μm). We found that, within minutes after application of TBOA, neuronal sodium concentrations began to rise to values of about 30 mm (n= 19; Fig. 9Aa). Synaptic stimulation resulted in further persistent sodium increases in only part of the neurons (n= 10; Fig. 9Aa). TBOA-induced neuronal sodium loads did not recover within 60 min after removal of TBOA from the saline (not shown). In contrast, TBOA did not alter the steady-state intracellular sodium concentration of astrocytes (n= 25; Fig. 9Aa). Schaffer collateral stimulation only triggered a minor increase in the sodium concentration in astrocytes in the presence of TBOA (n= 20; Fig. 9Aa). Compared to control, the amplitude of synaptically induced glial sodium transients was reduced by 93.2 ± 3.2% (n= 20; Fig. 9Aa). It recovered to about 65% of control values upon removal of TBOA (n= 15; Fig. 9Aa).
The persistent neuronal sodium elevation in the presence of TBOA was probably the consequence of an activation of ionotropic glutamate receptors following failure of glutamate uptake and accumulation of extracellular glutamate, as shown earlier (Rothstein et al. 1996; Jabaudon et al. 1999). To prevent sodium influx through ionotropic glutamate receptors, the experiment was repeated in the presence of the receptor blockers CNQX (10 μm) and APV (50 μm). Under these conditions, addition of TBOA did not significantly influence baseline intracellular sodium of neurons (n= 38 neurons in 6 slices).
As reported before (Rose & Konnerth, 2001); see also Fig. 8B), postsynaptic neuronal sodium transients were nearly completely diminished during combined application of both receptor blockers (reduction by 88.3 ± 1.7%; n= 42; not shown). In the presence of CNQX and APV, the amplitude of glial sodium signals was reduced by 35.8 ± 6.9% (n= 24; Fig. 9Ab). After addition of TBOA, stimulation-induced sodium transients in astrocytes were reduced by 86.8 ± 2.8% as compared to control without blockers (n= 24, Fig. 9Ab). In contrast to the recovery seen in experiments in which just TBOA was used, activity-induced sodium transients did not recover within a period of 60 min following removal of the three blockers.
To further establish the influence of an activation of electrogenic glutamate transport on sodium concentration in astrocytes in situ, we repeated experiments with puff application of d-aspartate. In the sole presence of TBOA, the amplitude of d-aspartate-induced sodium transients in astrocytes was reduced by 78.0 ± 4.3% (n= 12, Fig. 9Ba), confirming that they were largely mediated by activation of glutamate transport (cf. Fig. 4B). Following removal of TBOA, transients recovered to about 90% of control values (n= 12; Fig. 9Ba). In the presence of APV and CNQX, the amplitude of d-aspartate-induced sodium transients was reduced by 10.4 ± 3.2% (n= 37; Fig. 9Bb), which is not different from the reduction seen during APV alone reported above (see Fig. 4B). After addition of TBOA, signals were reduced by 82.0 ± 3.2% of control values without blockers (n= 24, Fig. 9Bb). As observed with synaptic stimulation, we did not obtain a recovery of the signals within 60 min after washout of the three blockers.
Discussion
Using quantitative intracellular sodium imaging we demonstrate that short-burst stimulation of Schaffer collaterals evokes sodium transients in the millimolar range in the somata of SR101-positive, passive astrocytes as well as in CA1 pyramidal neurons of the mouse hippocampus. Following selective activation of glutamate uptake by d-aspartate as well as following synaptic stimulation, we observed that with low stimulation intensities sodium signals were confined to primary branches and adjacent fine processes and only weakly invaded the soma. AMPA receptor activation was a prerequisite for the generation of synaptically induced neuronal sodium signals and also partly contributed to those in astrocytes. Sodium signals in astrocytes were diminished in the presence of TBOA, indicating that electrogenic glutamate transport is a major mechanism responsible for their generation.
Mechanisms of activity-induced neuronal sodium transients
Our data confirm earlier work which demonstrated that suprathreshold short-burst Schaffer collateral stimulation induces detectable sodium transients in CA1 pyramidal neurons (Rose & Konnerth, 2001). Their study found that while these synaptically induced neuronal sodium transients are mainly caused by influx of sodium through NMDA receptor channels, they are strongly sensitive to CNQX, a blocker of ionotropic AMPA receptor channels. This is because AMPA receptor activation by synaptically released glutamate is necessary to provide sufficient depolarisation to relieve the magnesium block of NMDA receptors (Rose & Konnerth, 2001). In the present study, we observed a reduction of neuronal sodium transients by about 80% in the presence of CNQX, again confirming the earlier data.
In apical CA1 pyramidal cell dendrites, sodium transients determined from dendritic regions of interest close to the stimulation pipette reached an amplitude of about 10 mm and peaked within 1–2 s following short-burst stimulation of Schaffer collaterals (5 pulses at 50 Hz; Rose & Konnerth, 2001). The peak amplitude of these dendritic sodium transients decayed monoexponentially with increasing distance from the region of maximal response and their rise time was prolonged. In the present study, the amplitude of sodium transients detected in neuronal somata averaged 5 mm with 10 stimulation pulses and peak concentrations were reached only 5–20 s after the stimulation. Based on the earlier observations mentioned above (Rose & Konnerth, 2001), this indicates that somatic sodium signals might be largely mediated by diffusion of sodium from the apical dendritic tree and active spines following activation of postsynaptic cells.
The present study shows that TBOA, a widely used competitive transport blocker of sodium-dependent glutamate transport, caused a strong and persistent rise in the intracellular sodium concentration of CA1 pyramidal neurons, emphasizing the vital role of glutamate uptake for glutamate clearance and neuronal function (Maragakis & Rothstein, 2001; Marcaggi & Attwell, 2004; Schousboe et al. 2004; Seifert et al. 2006). In line with this, earlier work has established that addition of TBOA causes glutamate accumulation and activation of ionotropic glutamate receptors in the intact tissue (Rothstein et al. 1996; Jabaudon et al. 1999). This is supported by the fact that blocking ionotropic receptors prevented the strong rise in intracellular sodium evoked by TBOA in the present study.
Activity-induced sodium transients in astrocytes
Our experiments demonstrate that glutamatergic synaptic activity induces sodium transients in astrocytes in a hippocampal slice preparation. Only recently, two reports established the occurrence of activity-induced sodium signals in cell bodies (Kirischuk et al. 2007) and in processes (Bennay et al. 2008) of Bergmann glial cells of the cerebellum. Synaptically induced sodium transients in hippocampal astrocytes reached amplitudes of up to 8 mm, a value which is similar to that determined in Bergmann glial processes upon short-burst stimulation (Bennay et al. 2008).
The time course of intracellular sodium transients as compared to calcium transients is surprisingly slow, a phenomenon observed and described by several other authors (e.g. Kirischuk et al. 1997; Regehr, 1997). The present study shows that sodium signals in astrocytes are strongly determined by the spatial relationship between the region of influx and the area where measurements are taken (see Figs 5 and 6). A similar dependence was described for synaptically induced sodium transients in CA1 pyramidal cell dendrites and dendritic spines (Rose & Konnerth, 2001). In addition, time constants of activity-induced intracellular sodium signals are dependent on the temperature (Rose et al. 1999; Bennay et al. 2008). In Bergmann glial cells, a Q10 of about 1.7 was determined for rise and decay times, while the peak amplitudes of sodium signals were similar at room temperature and at 32°C (Bennay et al. 2008). This indicates the dominating role of membrane transport in their generation and recovery and suggests that, at physiological temperature, sodium signals in astrocytes will be accelerated by a factor of about 2.
Earlier work had shown that bath application of glutamate evokes an elevation of sodium in cultured astrocytes (Kimelberg et al. 1989; Rose & Ransom, 1996b; Chatton et al. 2000). In such cultures, intracellular sodium concentrations are equalized between astrocytes by diffusion of sodium through gap junctions (Rose & Ransom, 1997) and intercellular waves of sodium, propagating with a speed of 15 μm s−1, can be evoked in response to mechanical or direct electrical stimulation of single cells (Bernardinelli et al. 2004). It has been proposed that these sodium waves induce metabolic waves by increasing glucose uptake within the astrocytic network (Bernardinelli et al. 2004). In the present study, we could not detect differences in the rise time of astrocytic sodium signals upon Schaffer collateral stimulation, indicating simultaneous activation of these astrocytes in response to synaptic stimulation. In contrast, when a single astrocyte was directly stimulated by current injection through a pipette positioned in physical contact to its soma, we not only observed a rapid increase in the intracellular sodium concentration in the stimulated cell, but also sodium elevations in neighbouring astrocytes. In this case, the slope of the rising phase in surrounding cells decreased with increasing distance from the stimulated cell, a behaviour similar to that described from intercellular sodium waves in culture (Bernardinelli et al. 2004). This suggests that, in the intact tissue, sodium can indeed travel in a wave-like manner within the astrocytic syncytium from regions of high to regions of low activity.
When single astrocytes were dye-filled through a patch-pipette, we could detect differences in both amplitude and time course of sodium signals induced by Schaffer collateral stimulation as well as following focal application of d-aspartate between different cellular regions of astrocytes represented by the somata and the main primary branches and their adjacent fine processes. For synaptic stimulation, these differences were most evident with low stimulation intensities. Here, sodium signals could be largely restricted to single domains, with only weak invasion of the cell body, indicating that these regions were close to synaptic release sites for glutamate, and the main regions of sodium influx. Similar observations were reported from branches of Bergmann glial cells upon local stimulation of parallel fibres (Bennay et al. 2008). These results suggest that in cellular domains close to activated synapses, sodium-dependent processes could be regulated locally and independent from other cellular regions.
In cultured astrocytes, sodium transients upon application of glutamate are primarily generated by activation of glutamate uptake (Rose & Ransom, 1996b; Chatton et al. 2000, 2001). Pharmacological inhibition with TBOA results in a reduction of these signals by 70–80% (Chatton et al. 2001). In addition, glutamate-induced sodium signals in cultured astrocytes are partly sensitive to CNQX, suggesting that AMPA receptor activation also plays a role (Rose & Ransom, 1996b; Chatton et al. 2000, 2001). Our data show a similar pharmacological profile of sodium transients evoked by synaptic stimulation in hippocampal astrocytes in acute tissue slices. Synaptically induced sodium signals were partly reduced in the presence of CNQX, indicating that activation of ionotropic AMPA receptors following synaptic release of glutamate is also a pathway for sodium influx into astrocytes in situ. Application of TBOA to the slice preparation nearly completely blocked synaptically induced sodium signals in astrocytes, while it did not influence baseline sodium concentrations. This suggests that sodium-dependent glutamate uptake mediates the majority of sodium influx into astrocytes in situ in response to Schaffer collateral stimulation.
These conclusions are supported by the experiments in which TBOA was added in the presence of the ionotropic glutamate receptor blockers CNQX and APV. Again, TBOA largely blocked glial sodium transients. While we could not obtain a recovery under these conditions, earlier experiments had demonstrated that synaptically induced sodium signals can be evoked repetitively if no blockers are present (Fig. 7), and can – at least partly – recover after application and washout of TBOA alone (Fig. 8Ba). A similar failure of recovery was observed upon application of d-aspartate under these conditions: signals induced by d-aspartate could be evoked repetitively in the absence of blockers (Fig. 4A), and showed a nearly complete recovery after washout of either APV (Fig. 4B) or TBOA (Fig. 9Ba) alone, while no effect of washout was obtained after combined application of the blockers. Thus, the failure of recovery was most probably caused by a failure of sufficient washout of the blockers from the slice tissue.
Taken together, our results suggest that sodium-dependent glutamate uptake mediates the majority of sodium influx into astrocytes in situ in response to Schaffer collateral stimulation. Furthermore, they indicate that activation of AMPA receptors is an additional relevant factor contributing to sodium signals in astrocytes in the intact tissue.
Possible consequences of intracellular sodium transients in astrocytes
The inwardly directed sodium gradient energizes many transport processes across the plasma membrane, and increases in the intracellular sodium concentration reduce the driving force for these transporters. Thus, sodium transients influence the recovery of calcium transients mediated by Na+/Ca2+ exchange (Kirischuk et al. 1997; Golovina et al. 2003). Furthermore, intracellular sodium transients will influence sodium-dependent reuptake of transmitters. Even minor elevations in the sodium concentration will reduce the driving force for sodium-dependent GABA uptake, and might promote the release of GABA by transport reversal (Wu et al. 2007). As glutamate transport is coupled to the inward transport of three sodium ions, the electrochemical sodium gradient provides its major driving force (Nicholls & Attwell, 1990; Levy et al. 1998; Danbolt, 2001; Maragakis & Rothstein, 2001). Together with a membrane depolarization induced by synaptic stimulation, sodium transients will significantly reduce the activity of glutamate uptake (Zerangue & Kavanaugh, 1996). In addition, a recent study has provided evidence that intracellular sodium elevations following prolonged activation of the glutamate transporter GLT-1 trigger a clustering and a decrease of the surface expression of this transporter (Nakagawa et al. 2008).
Another well-documented consequence of glial sodium elevations is increased ATP hydrolysis by the Na+/K+-ATPase. In cultured astrocytes, this has been suggested to promote aerobic glycolysis, glucose uptake and glycogen breakdown (Pellerin & Magistretti, 1994; Chatton et al. 2000; Voutsinos-Porche et al. 2003; Magistretti & Chatton, 2005). Interestingly, activation of GABA uptake, in contrast to glutamate, is not associated with significant sodium loads, nor with a measurable metabolic response (Chatton et al. 2003). Recent studies have demonstrated that the stimulation of GLUT-1-mediated glucose uptake by glutamate requires the concurrence of both sodium and calcium signals in astrocytes (Loaiza et al. 2003; Porras et al. 2008). Our finding that long-lasting sodium signals are present in astrocytes following neuronal release of glutamate supports the proposed tight link between excitatory neuronal activity, glutamate transport, sodium signals and glucose utilization by astrocytes (Pellerin & Magistretti, 1994; Tsacopoulos & Magistretti, 1996).
Acknowledgments
We thank Dr Silke D. Honsek for help with the establishment of imaging experiments and Drs Honsek and Karl W. Kafitz for critical discussion of the manuscript. This study was supported by grants from the Deutsche Forschungsgemeinschaft (Ro 2327/4-2, 4-3).
Glossary
Abbreviations
- CA1
cornu ammonis area 1
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- F
, fluorescence intensity
- GLAST
l-glutamate/l-aspartate transporter
- GLT-1
glutamate transporter 1
- GLUT1
glucose transporter 1
- KMeSO3
potassium methanesulfonate
- R
regression coefficient
- SBFI
sodium-binding benzofuran isophthalate
- SBFI-AM
sodium-binding benzofuran isophthalate acetoxymethyl ester
- SP
stratum pyramidale
- SR
stratum radiatum
- SR101
sulforhodamine 101
- TBOA
threo-ß-benzyloxyaspartate
Author contributions
All authors contributed to the design, analysis and interpretation of data, drafting and critical revision of manuscript for intellectual content and final approval of version to be published. All experimental work was carried out in the Institute for Neurobiology at the Heinrich-Heine-University Duesseldorf.
References
- Barbour B, Brew H, Attwell D. Electrogenic uptake of glutamate and aspartate into glial cells isolated from the salamander (Ambystoma) retina. J Physiol. 1991;436:169–193. doi: 10.1113/jphysiol.1991.sp018545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Bennay M, Langer J, Meier SD, Kafitz KW, Rose CR. Sodium signals in cerebellar Purkinje neurons and Bergmann glial cells evoked by glutamatergic synaptic transmission. Glia. 2008;56:1138–1149. doi: 10.1002/glia.20685. [DOI] [PubMed] [Google Scholar]
- Bergles D, Jahr C. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Bernardinelli Y, Magistretti PJ, Chatton JY. Astrocytes generate Na+-mediated metabolic waves. Proc Natl Acad Sci U S A. 2004;101:14937–14942. doi: 10.1073/pnas.0405315101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Postnatal development of ionic currents in rat hippocampal astrocytes in situ. J Neurophysiol. 1997;78:461–477. doi: 10.1152/jn.1997.78.1.461. [DOI] [PubMed] [Google Scholar]
- Borzak S, Reers M, Arruda J, Sharma VK, Sheu SS, Smith TW, Marsh JD. Na+ efflux mechanisms in ventricular myocytes: measurement of [Na+]i with Na+-binding benzofuran isophthalate. Am J Physiol Heart Circ Physiol. 1992;263:H866–874. doi: 10.1152/ajpheart.1992.263.3.H866. [DOI] [PubMed] [Google Scholar]
- Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature. 1987;327:707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Marquet P, Magistretti PJ. A quantitative analysis of L-glutamate-regulated Na+ dynamics in mouse cortical astrocytes: implications for cellular bioenergetics. Eur J Neurosci. 2000;12:3843–3853. doi: 10.1046/j.1460-9568.2000.00269.x. [DOI] [PubMed] [Google Scholar]
- Chatton JY, Pellerin L, Magistretti PJ. GABA uptake into astrocytes is not associated with significant metabolic cost: implications for brain imaging of inhibitory transmission. Proc Natl Acad Sci U S A. 2003;100:12456–12461. doi: 10.1073/pnas.2132096100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatton JY, Shimamoto K, Magistretti PJ. Effects of glial glutamate transporter inhibitors on intracellular Na+ in mouse astrocytes. Brain Res. 2001;893:46–52. doi: 10.1016/s0006-8993(00)03286-8. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio R, Wenzel J, Schwartzkroin PA, McKhann GM, 2nd, Janigro D. Functional specialization and topographic segregation of hippocampal astrocytes. J Neurosci. 1998;18:4425–4438. doi: 10.1523/JNEUROSCI.18-12-04425.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Bergles DE, Jahr CE. Glutamate release monitored with astrocyte transporter currents during LTP. Neuron. 1998;21:425–433. doi: 10.1016/s0896-6273(00)80551-6. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically released glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra A, Sheldon C, Church J. In situ calibration and [H+] sensitivity of the fluorescent Na+ indicator SBFI. Am J Physiol Cell Physiol. 2001;280:C1623–C1633. doi: 10.1152/ajpcell.2001.280.6.C1623. [DOI] [PubMed] [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurones of the mammalian central nervous system. Pflugers Arch. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol. 1998;507:219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina V, Song H, James P, Lingrel J, Blaustein M. Regulation of Ca2+ signalling by Na+ pumpa-2 subunit expression. Ann N Y Acad Sci. 2003;986:509–513. doi: 10.1111/j.1749-6632.2003.tb07236.x. [DOI] [PubMed] [Google Scholar]
- Harootunian AT, Kao JP, Eckert BK, Tsien RY. Fluorescence ratio imaging of cytosolic free Na+ in individual fibroblasts and lymphocytes. J Biol Chem. 1989;264:19458–19467. [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gahwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci U S A. 1999;96:8733–8738. doi: 10.1073/pnas.96.15.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S, Druzin M, Haage D, Wang MD. The functional role of a bicuculline-sensitive Ca2+-activated K+ current in rat medial preoptic neurons. J Physiol. 2001;532:625–635. doi: 10.1111/j.1469-7793.2001.0625e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafitz KW, Meier SD, Stephan J, Rose CR. Developmental profile and properties of sulforhodamine 101–labelled glial cells in acute brain slices of rat hippocampus. J Neurosci Methods. 2008;169:84–92. doi: 10.1016/j.jneumeth.2007.11.022. [DOI] [PubMed] [Google Scholar]
- Kelly T, Kafitz KW, Roderigo C, Rose CR. Ammonium-evoked alterations in intracellular sodium and pH reduce glial glutamate transport activity. Glia. 2009;57:921–934. doi: 10.1002/glia.20817. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Pang S, Treble DH. Excitatory amino acid-stimulated uptake of 22Na+ in primary astrocyte cultures. J Neurosci. 1989;9:1141–1149. doi: 10.1523/JNEUROSCI.09-04-01141.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A. Na+/Ca2+ exchanger modulates kainate-triggered Ca2+ signalling in Bergmann glial cells in situ. Faseb J. 1997;11:566–572. doi: 10.1096/fasebj.11.7.9212080. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kettenmann H, Verkhratsky A. Membrane currents and cytoplasmic sodium transients generated by glutamate transport in Bergmann glial cells. Pflugers Arch. 2007;454:245–252. doi: 10.1007/s00424-007-0207-5. [DOI] [PubMed] [Google Scholar]
- Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J Neurosci. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loaiza A, Porras OH, Barros LF. Glutamate triggers rapid glucose transport stimulation in astrocytes as evidenced by real-time confocal microscopy. J Neurosci. 2003;23:7337–7342. doi: 10.1523/JNEUROSCI.23-19-07337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Malenka RC, Nicoll RA. Monitoring glutamate release during LTP with glial transporter currents. Neuron. 1998;21:435–441. doi: 10.1016/s0896-6273(00)80552-8. [DOI] [PubMed] [Google Scholar]
- Magistretti PJ, Chatton JY. Relationship between L-glutamate-regulated intracellular Na+ dynamics and ATP hydrolysis in astrocytes. J Neural Transm. 2005;112:77–85. doi: 10.1007/s00702-004-0171-6. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rothstein JD. Glutamate transporters in neurologic disease. Arch Neurol. 2001;58:365–370. doi: 10.1001/archneur.58.3.365. [DOI] [PubMed] [Google Scholar]
- Marcaggi P, Attwell D. Role of glial amino acid transporters in synaptic transmission and brain energetics. Glia. 2004;47:217–225. doi: 10.1002/glia.20027. [DOI] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, Huttmann K, Matyash M, Kettenmann H, Steinhauser C. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier SD, Kafitz KW, Rose CR. Developmental profile and mechanisms of GABA-induced calcium signalling in hippocampal astrocytes. Glia. 2008;56:1127–1137. doi: 10.1002/glia.20684. [DOI] [PubMed] [Google Scholar]
- Meier SD, Kovalchuk Y, Rose CR. Properties of the new fluorescent Na+ indicator CoroNa Green: comparison with SBFI and confocal Na+ imaging. J Neurosci Methods. 2006;155:251–259. doi: 10.1016/j.jneumeth.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Otsubo Y, Yatani Y, Shirakawa H, Kaneko S. Mechanisms of substrate transport-induced clustering of a glial glutamate transporter GLT-1 in astroglial-neuronal cultures. Eur J Neurosci. 2008;28:1719–1730. doi: 10.1111/j.1460-9568.2008.06494.x. [DOI] [PubMed] [Google Scholar]
- Nicholls D, Attwell D. The release and uptake of excitatory amino acids. Trends Pharmacol Sci. 1990;11:462–468. doi: 10.1016/0165-6147(90)90129-v. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods. 2004;1:31–37. doi: 10.1038/nmeth706. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras OH, Ruminot I, Loaiza A, Barros LF. Na+-Ca2+ cosignalling in the stimulation of the glucose transporter GLUT1 in cultured astrocytes. Glia. 2008;56:59–68. doi: 10.1002/glia.20589. [DOI] [PubMed] [Google Scholar]
- Regehr WG. Interplay between sodium and calcium dynamics in granule cell presynaptic terminals. Biophys J. 1997;73:2476–2488. doi: 10.1016/S0006-3495(97)78276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Konnerth A. NMDA receptor-mediated Na+ signals in spines and dendrites. J Neurosci. 2001;21:4207–4214. doi: 10.1523/JNEUROSCI.21-12-04207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Kovalchuk Y, Eilers J, Konnerth A. Two-photon Na+ imaging in spines and fine dendrites of central neurons. Pflugers Arch. 1999;439:201–207. doi: 10.1007/s004249900123. [DOI] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Intracellular Na+ homeostasis in cultured rat hippocampal astrocytes. J Physiol. 1996a;491:291–305. doi: 10.1113/jphysiol.1996.sp021216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Mechanisms of H+ and Na+ changes induced by glutamate, kainate, and D-aspartate in rat hippocampal astrocytes. J Neurosci. 1996b;16:5393–5404. doi: 10.1523/JNEUROSCI.16-17-05393.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose CR, Ransom BR. Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia. 1997;20:299–307. doi: 10.1002/(sici)1098-1136(199708)20:4<299::aid-glia3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Bak LK, Waagepetersen HS, Larsson OM. Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem Int. 2004;45:521–527. doi: 10.1016/j.neuint.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7:194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- Serrano A, Robitaille R, Lacaille JC. Differential NMDA-dependent activation of glial cells in mouse hippocampus. Glia. 2008;56:1648–1663. doi: 10.1002/glia.20717. [DOI] [PubMed] [Google Scholar]
- Sheldon C, Cheng YM, Church J. Concurrent measurements of the free cytosolic concentrations of H+ and Na+ ions with fluorescent indicators. Pflugers Arch. 2004;449:307–318. doi: 10.1007/s00424-004-1344-8. [DOI] [PubMed] [Google Scholar]
- Szatkowski M, Barbour B, Attwell D. Non-vesicular release of glutamate from glial cells by reversed electrogenic glutamate uptake. Nature. 1990;348:443–446. doi: 10.1038/348443a0. [DOI] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. J Neurosci. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Wadiche JI. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat Rev Neurosci. 2007;8:935–947. doi: 10.1038/nrn2274. [DOI] [PubMed] [Google Scholar]
- Voutsinos-Porche B, Bonvento G, Tanaka K, Steiner P, Welker E, Chatton JY, Magistretti PJ, Pellerin L. Glial glutamate transporters mediate a functional metabolic crosstalk between neurons and astrocytes in the mouse developing cortex. Neuron. 2003;37:275–286. doi: 10.1016/s0896-6273(02)01170-4. [DOI] [PubMed] [Google Scholar]
- Wu Y, Wang W, Diez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–865. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- Zhou M, Kimelberg HK. Freshly isolated hippocampal CA1 astrocytes comprise two populations differing in glutamate transporter and AMPA receptor expression. J Neurosci. 2001;21:7901–7908. doi: 10.1523/JNEUROSCI.21-20-07901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Schools GP, Kimelberg HK. Development of GLAST+ astrocytes and NG2+ glia in rat hippocampus CA1: mature astrocytes are electrophysiologically passive. J Neurophysiol. 2006;95:134–143. doi: 10.1152/jn.00570.2005. [DOI] [PubMed] [Google Scholar]