Abstract
Uterine leiomyomas are benign uterine tumors characterized by extracellular matrix remodeling, increased collagen deposition, and increased smooth muscle cell (SMC) proliferation. The reactive oxygen species (ROS) producing NADPH oxidase complex has been shown to be involved in the signaling pathways of several growth factors, cytokines, and vasoactive agents that stimulate proliferation of a variety of cell types. Our objective was to test the hypothesis that ROS derived from NADPH oxidase is a necessary component of the MAP kinase mitogenic pathway activated by platelet derived growth factor (PDGF) and epidermal growth factor (EGF) in leiomyoma SMCs (LSMCs). Primary cell cultures of LSMCs were used as our experimental model. Our results showed that stimulation of these cells with PDGF or EGF caused a marked increase in intracellular ROS production and that the NADPH oxidase inhibitor, DPI, blocks ROS production. In addition, inhibition of ROS production by NADPH oxidase inhibitors blocked, in a dose-dependent manner, the EGF- and PDGF-induced increase in [3H]thymidine incorporation by LSMCs. Furthermore, an exogenous source of ROS, hydrogen peroxide, was sufficient to stimulate [3H]thymidine incorporation in LSMCs but did not affect COL1A2 and COL3A1 mRNA levels. Inhibition of the NADPH oxidase complex decreased PDGF-induced MAPK1/MAPK3 activation, whereas exogenous hydrogen peroxide induced MAPK1/MAPK3 activation. This article is the first report suggesting the presence of the NADPH oxidase system and its importance in mitogenic signaling pathways in LSMCs. The necessity of NADPH oxidase-derived ROS for EGF and PDGF signaling pathways leading to cell proliferation points to another potential therapeutic target for treatment and/or prevention of uterine leiomyomas.
Keywords: growth factors, hydrogen peroxide, leiomyoma smooth muscle cells, proliferation, reactive oxygen species
Reactive oxygen species mediate the proliferative effects of growth factors in leiomyoma smooth muscle cells through activation of mitogen-activated protein kinases.
INTRODUCTION
Uterine leiomyomas, or fibroids, are characterized by an increase in SMC proliferation and excessive deposition of extracellular matrix proteins, primarily collagens type I and III [1–3]. Recent survey studies have reported that over 600 000 hysterectomies are performed in the United States and that uterine leiomyomas represent 29.4% and 41.4% of the hysterectomies in women ages 18–44 and 45–64, respectively [4]. Epidemiological studies have shown that 7 out of every 10 Caucasian women and 8 out of every 10 African American women will eventually develop uterine leiomyomas [5].
Ovarian steroid hormones are known to play a central role in the regulation of uterine leiomyoma growth because leiomyomas develop only in postpubertal and premenopausal women. A significant reduction in tumor size is observed after the onset of menopause and in response to treatment with gonadotropin-releasing hormone or progesterone antagonists [6, 7]. Steroid hormones induce mitogenic effects directly through their receptors or by regulating expression of growth factors such as EGF and PDGF and their respective receptors [7, 8]. Both EGF and PDGF receptors have previously been identified in LSMCs [9, 10]. Furthermore, EGF and PDGF have been shown by numerous investigators to stimulate the proliferation of LSMCs [9, 11]. Vascular SMCs show similar mitogenic responses to these same growth factors [12, 13].
The pathophysiology of uterine leiomyomas is similar to that of other fibrotic conditions such as atherosclerosis, vascular restenosis, and liver, pancreatic, and renal interstitial fibrosis, in which an injury triggers normally quiescent cells to dedifferentiate into a myofibroblast-like, more proliferative phenotype [14–17]. It is likely that the development of leiomyomas may parallel these pathological conditions, occurring in response to some type of injury to the myometrium such as hypoxia.
Reactive oxygen species (ROS), which were once regarded as purely cytotoxic, are now recognized as effective second-messenger molecules regulating protein modifications, gene expression, cell proliferation, migration, and differentiation as well as tissue remodeling in a variety of cell types [18–27]. ROS activation is biphasic [28], and the signaling effects can be both immediate and long lasting. NADPH oxidase has been shown to serve as the primary source of intracellular ROS, specifically hydrogen peroxide, in a variety of cell types [29, 30]. Sundaresan and colleagues [31] were the first to uncover the importance of increasing intracellular ROS levels in response to specific growth factor ligands. This group showed that on treatment with PDGF, ROS levels in vascular SMCs rose rapidly and returned to baseline within approximately 30 min. Furthermore, after blocking the PDGF-stimulated generation of intracellular ROS, they noted a marked inhibition of MAPK activation and proliferation. More recent studies have shown that growth factors (including EGF and PDGF), chemokines, cytokines, and vasoactive agents such as angiotensin can induce NADPH oxidase-dependent ROS production, which in turn has been shown to activate various MAPKs that regulate downstream cell proliferation or matrix production [19, 22, 25].
The focus of our research is to gain a better understanding of the signaling pathways involved in regulation of uterine leiomyoma growth. Our goals in this study were to determine 1) whether ROS are necessary components of the signaling pathways for PDGF and EGF in LSMCs and 2) the potential involvement of MAPK1/MAPK3 as intermediate molecules mediating the effects of ROS on leiomyoma SMC proliferation. Our results show that NADPH oxidase-derived ROS are indeed an important component of thesesignaling pathways.
MATERIALS AND METHODS
Chemicals and Reagents
PDGF (120-HD) and EGF (236-EG) were purchased from R&D Systems. Diphenyleneiodonium chloride (DPI; D2926) and DMSO (D2650) were purchased from Sigma. Tritiated thymidine ([3H]thymidine) (NET-027) was purchased from PerkinElmer. Thirty percent hydrogen peroxide (H325‐100) and bovine serum albumin (BP1600‐100) were purchased from Fisher Scientific. Restore Western Blot Stripping Buffer (21059), BCA protein assay kit, and SuperSignal West Pico Chemiluminescent Substrate (34080) were purchased from Thermo Scientific. Anti-MAPK1/MAPK3 and antiphosphorylated MAPK1/MAPK3 antibodies and anti-mouse and anti-rabbit HRP-linked antibodies were purchased from Cell Signaling. Dulbecco modified Eagle medium (DMEM; 12–614F), penicillin-streptomycin (17‐602E), phenol-red-free DMEM (PRF-DMEM, 12‐917F), and l-glutamine (17‐605E) were purchased from Biowhittaker. Dubelco PBS/Modified (SH30264.01), fetal bovine serum (FBS; SH30071.03) and bovine calf serum (BCS; SH30072.03) were purchased from Hyclone. Carboxy-H2DCFDA (5-[and-6]-carboxy-2′,7′-dichlorodihydrofluorescein diacetate) (C400), dihydroethidium stabilized in DMSO (D23107), and Trizol Reagent (15‐596‐018) were purchased from Invitrogen.
Cell Culture
Leiomyomas were obtained from patients undergoing hysterectomy who had given written consent in accordance with the University of Illinois and Northwestern University institutional review board committees. Tissues were minced, digested in collagenase overnight, and then placed into flasks in DMEM + 5% FBS + 5% BCS, which will be referred to as DMEM/10% serum. Cells were characterized for expression of the smooth muscle cell specific markers α-actin and desmin, and for lack of expression of the fibroblast-specific marker vimentin. These primary cells were then cultured in DMEM/10% serum, supplemented with BCS, penicillin-streptomycin (10 000 U pen/ml, 10 000 μg strep/ml), and l-glutamine (200 mM) in 75 cm2 cell culture flasks (Midwest Scientific, 90075). Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were used in experiments between passages 4 and 8.
Measurement of Intracellular ROS Production: Dihydroethidium
LSMCs were cultured on two-well collagen-coated glass slides (Becton Dickinson, 354627) in 2 ml per well of DMEM/10% serum at a density of 50 000 cells per well. Cells were tested once they attained approximately 50% confluence to prevent fluorescent dye from becoming trapped in the extracellular matrix. The generation of intracellular ROS was measured using dihydroethidium bromide (DHE) as a fluorescent probe.
To determine the effects of PDGF or EGF on intracellular ROS production, cells received the following treatments for increasing time increments of 0, 5, 10, or 15 min: DMEM + 0.1% BSA, 10 ng/ml PDGF (maximal dose), or 100 ng/ml EGF (maximal dose). All treatments were applied using PRF-DMEM containing 0.1% BSA, pen-strep, and l-glutamine. Cells were washed once using PRF-DMEM/0.1% BSA. Cells were then loaded with the fluorescent DHE dye (10 μM) in PRF-DMEM/0.1% BSA for 20 min. This dye can passively enter cells and produces a red fluorescent signal on its oxidation by intracellular ROS. The oxidized ethidium bromide then becomes intercalated within the cell's DNA, producing a long-lasting fluorescence. After removal of the dye, cells were washed twice with PRF-DMEM. The medium was then removed and cells were visualized using a Zeiss Axiovert 25 fluorescence microscope. Pictures were taken using a Zeiss Axiocam camera to record fluorescent images. An increase in intracellular fluorescence indicated an increase in intracellular ROS production.
Measurement of Intracellular ROS Production: Carboxy-H2DCFDA
LSMCs were cultured in 35-mm μ-dishes (Ibidi) in 800 μl of DMEM/10% serum at a density of 2000 cells per dish. Once cells attained 50% confluence the experiment was performed. Cells were washed three times in warm DPBS, and carboxy-H2DCFDA dye was added to the cells in 800 μl of DPBS at a 10 μM concentration. After 15 min of incubation, dye was removed, cells were washed two times, and treatment was added. Cells were exposed to either DPBS alone (negative control), H2O2 (10 μM) (positive control), EGF (50 ng/ml) or PDGF (10 ng/ml) in DPBS for 15 min. Growth factors were added in absence or presence of 25 μM DPI. After treatment, cells were washed two times, and images were immediately captured using a fluorescence microscope (Zeiss Axiovert 200M).
Densitometric Quantification of Fluorescence Images
Individual cells were randomly selected from several different microscopic fields, and fluorescence as well as DIC-Nomarski images were captured and saved as TIFF files. Images were loaded and analyzed on Axiovision software (Zeiss). The intracellular compartment of cells was determined by using DIC images. To be consistent with regards to the areas of fluorescence, measurements circles were drawn around the nucleus of every cell. The average of the fluorescence measurements from each cell was taken and used for statistical analysis.
[3H]Thymidine Incorporation Assays: Effect of Exogenous ROS
LSMCs were cultured in 96-well plates in 200 μl of DMEM/10% serum at a density of 4000 cells per well. Once cells attained 80% confluence, they were growth arrested in DMEM/0.5% FBS for 48 h to synchronize the cells.
To examine stimulation of cell proliferation by exogenous ROS, cells were placed in fresh DMEM/0.5% FBS and were pulse-treated once each hour for 5 h with hydrogen peroxide (10, 50, 100, or 200 μM). [3H]thymidine (0.4 μCi) was added to the cells at the beginning of the 5-h treatment. Cells were then harvested and counted in a beta-scintillation counter to quantitate [3H]thymidine incorporation. DNA synthesis was recorded as counts per minute (cpm).
[3H]Thymidine Incorporation Assays: Effect of ROS Inhibitor
LSMCs were cultured as described in the previous section. To determine whether proliferation observed in response to EGF or PDGF could be inhibited by treatment with a ROS inhibitor, cells were maintained in DMEM/0.5% FBS and pretreated with 200 μL of diphenyleneiodonium chloride (DPI) at increasing concentrations of 0, 5, 25, or 50 μM for 60 min. DPI was then removed and cells were washed twice with DMEM/0.5% FBS and then treated with either 50 ng/ml EGF or 10 ng/ml PDGF for 24 h. Control cells that were not pretreated with the inhibitor were treated with either EGF (50 ng/ml), PDGF (10 ng/ml), or no growth factor. Cells were labeled during the final 6 h of treatment with [3H]thymidine (0.4 μCi). Cells were then harvested and counted using a beta-scintillation counter to measure the rate of [3H]thymidine incorporation. DNA synthesis was recorded as cpm.
Assessment of Proliferation Using Cell Counts
LSMCs were plated in six-well plates in 2 ml of DMEM/10% serum at a density of 50 000 cells per well. On attaining 80% confluence, cells were growth arrested in DMEM/0.5% FBS for 24 h, and experiments were performed. To determine the effects of H2O2 on cell numbers, cells were given new medium every hour for 8 h per day over a 2-day period. Treatments consisted of DMEM/0.5% FBS or H2O2 (20 μM and 100 μM). To determine the effects of DPI on cell numbers of EGF- and PDGF-stimulated cells, cells were exposed to either DMEM/0.5% FBS, EGF (50 ng/ml), or PDGF (10 ng/ml) for 48 h. Growth factors were added in the absence or presence of DPI (25 μM). At the end of the treatment period, cells were detached with 1 ml of trypsin for 8 min, mixed with 1 ml of DMEM/10% serum, and counted using a hemocytometer.
COL1A2 and COL3A1 Gene Expression
LSMCs were grown in 60-mm culture dishes and allowed to attain 80% confluence. Cells were then washed and switched to DMEM/0.5% FBS. Cells then were given hourly pulses of hydrogen peroxide (20 μM) for 5 h or were treated with one 200-μM hydrogen peroxide pulse for 24 h. Total RNA was isolated from cells using Trizol Reagent. Complementary DNA synthesis was performed and followed by qRT-PCR to determine relative fold differences in collagen type 1 alpha 2 (COL1A2) and collagen type 3 alpha 1 (COL3A1) mRNA expression in response to ROS (Applied Biosystems TaqMan Gene Expression Assay for COL3A1: Hs00164103_m1; Applied Biosystems TaqMan Gene Expression Assay for COL1A2: Hs00164099_m1).
Immunoblotting
LSMCs were serum starved for 1 h in 60-mm dishes. Two hundred microliters of concentrated hydrogen peroxide (10 μM final concentration), EGF (50 ng/ml final concentration), or PDGF (10 ng/ml final concentration) were added directly to the cells, and treatments continued for the specified time periods. When pretreatment with NADPH oxidase inhibitor was necessary, cells were exposed to DPI for 60 min, and concentrated growth factors were then added to the cells in the presence of the inhibitor. Cell lysates were harvested by removing medium and adding warm (95°C) 1X-Laemmli sample buffer (LSB) (2% SDS, 10% glycerol, 62.5 mM Tris, pH 6.8). Cells were scraped off the dishes, added to a 1.5-ml tube, sonicated (10 pulses), and stored at −20°C. BCA assays were performed on the cell lysates to determine protein concentrations. Either 5 μg (MAPK1/MAPK3 blots) or 45 μg (EGF-R or PDGF-R blots) of total protein were loaded on SDS-PAGE gels (12%) and run initially at 80 V for 30 min, then at 150 V for 90 min. Protein transfer was carried out at 60 mA for 16 h at room temperature. Primary antibody (antiphosphorylated MAPK1/MAPK3 1:2000 in 5% milk, anti-MAPK1/MAPK3 1:1000 in 3% BSA, antiphoshporylated EGF-R and anti-PDGF-R 1:500 in 5% milk, anti-EGF-R and anti-PDGF-R 1:500 in 3% BSA) incubation was performed for 90 min at room temperature. HRP-conjugated secondary antibody was incubated with membranes in 5% milk for 60 min at room temperature (1:10 000). The SuperSignal West Pico Chemiluminescent Substrate kit was used as substrate for HRP. Membranes were first probed with antiphosphorylated MAPK1/MAPK3 antibody and then reprobed with anti-total MAPK1/MAPK3 as a loading control. Membranes were stripped with Restore Western Blot Stripping buffer from Thermo Scientific before being reprobed.
Densitometric Analysis of Western Blots
Films developed after exposure to chemiluminescence on the membranes were scanned and images saved at high resolution in a TIFF format. Images were analyzed using the ImageJ software from the National Institutes of Health (available at http://rsbweb.nih.gov/ij/download.html). Software was calibrated for optical density measurements using a Kodak no. 3 calibrated step tablet available at http://rsb.info.nih.gov/ij/docs/examples/calibration. Bands of interest were measured by drawing the smallest rectangle capable of measuring the optical density of all the bands. The same rectangle was used to measure all the bands in one film. Measurements of bands referring to the activated form of the proteins (phosphorylated form) were normalized for loading differences using measurements obtained from the bands referring to total protein (loading control). Graphs represent LSmeans and SEM.
Statistical Analysis
Quantitative RT-PCR experiments were performed using the relative standard curve method. To show changes in gene expression relative to control level (control set as 1), each biological replicate was normalized to the average of the control group within each experiment. Relative fold difference values from qRT-PCR experiments and cpm values from [3H]thymidine incorporation experiments in each experiment were checked for normality and common variances, respecting the assumptions for performing analysis of variance. All distributions were normal. Statistical analysis for all experiments followed the same approach. A completely randomized design corresponding to the following linear model was used: Xik = μ + τi + εik, where Xik = an observation, μ = population mean, τi = effect of ith treatment, and εik = error term. The linear model for densitometry analysis of the Western blots used values for total MAPK1/MAPK3, EGF-R, or PDGF-R as a covariate. A priori comparisons determined by contrasts were used to minimize testing the null hypothesis multiple times (α = 0.05). The contrasts were only performed when analysis of variance indicated significant effect due to treatment. The data analyses for this article were generated using SAS software (SAS Institute Inc.).
RESULTS
EGF and PDGF Stimulate Intracellular ROS Generation
To test our hypothesis that ROS are necessary mediators of EGF and PDGF signaling pathways, we first examined whether EGF or PDGF would induce an increase in intracellular ROS production in LSMCs. Figure 1A indicates an increase in fluorescence after 15 min of stimulation with PDGF, and the bright-field image shows that fluorescence was specifically observed intracellularly. Cells were loaded with DHE fluorescent dye following growth factor treatment, and the presence of fluorescence indicated oxidation of DHE and, therefore, the presence of ROS. Intracellular fluorescence was observed in cells following treatment with either 10 ng/ml PDGF or 100 ng/ml EGF, and fluorescence increased over time, with maximum fluorescence occurring after 15 min of treatment (Fig. 1B). However, the effects of EGF or PDGF treatment on ROS production were evident as early as 5 min.
FIG. 1.
Intracellular ROS production by LSMCs increases in response to treatment with EGF or PDGF. A) Left panel: Intracellular ROS production is indicated by the presence of red fluorescence in LSMCs in response to PDGF (10 ng/ml) treatment for 15 min; right panel: Bright-field image indicating that the fluorescence is located specifically within the LSMCs. B) LSMCs were exposed to DMEM/0.1% BSA (negative control), EGF (100 ng/ml), or PDGF (10 ng/ml) for increasing time periods up to 15 min. An increase in fluorescence indicated an increase in intracellular ROS production (n = 3; representative image of LSMC response).
NADPH Oxidase Inhibitor Blocks EGF- and PDGF-Stimulated ROS Production
To capture higher power images and get better intracellular resolution with our intracellular ROS detection experiments, we used another oxidation sensitive dye, carboxy-H2DCFDA. We first tested whether this dye was sensitive to oxidation by H2O2. Indeed, on addition of H2O2 (10 μM) to the culture medium, the compound entered the cells readily and induced a significant increase in intracellular fluorescence (Fig. 2A). In the same experiment, we also confirmed the increase in ROS production after LSMC treatment with EGF and PDGF. Similar results were obtained when using DHE dye (Figs. 2A and 1A, respectively). After validating the carboxy-H2DCFDA dye, our next goal was to validate the compound diphenyleneiodonium (DPI) as an inhibitor of the enzymatic complex, which we hypothesize is responsible for ROS production (NADPH oxidase). When cells were treated with EGF or PDGF in the presence of DPI (25 μM) for 15 min, there was a significant drop in the amount of fluorescence observed intracellularly. DIC-Nomarzki images indicate the localization of the cells and, when merged with the fluorescence channel, support the intracellular ROS localization. These results show that carboxy-H2DCFDA is a highly sensitive probe that can detect changes in intracellular ROS concentrations, that DPI can block growth factor-induced ROS production by LSMCs, and that the NADPH oxidase complex is active and potentially involved with EGF and PDGF signaling pathways. Figure 2B shows the densitometric analysis of the fluorescence images as a means of quantitatively comparing the different treatments.
FIG. 2.
The NADPH oxidase inhibitor DPI blocks PDGF- and EGF-stimulated intracellular ROS production. An increase in fluorescent signal indicates ROS production. A) Rows indicate different combinations of treatments; the left column shows bright-field DIC-Nomarski, the middle column shows the fluorescent signal (Carboxy-H2DCFDA), and the right column is the merged image indicating intracellular ROS production. B) Graphic representation of the LSmeans ± SEM of fluorescent signal from cells exposed to different treatments. Different capital letters indicate statistical difference (P < 0.05) (n = 3; representative images of LSMCs response were used for A).
NADPH Oxidase Inhibitor Blocks EGF- and PDGF-Stimulated Proliferation of LSMCs
Based on our results showing that ROS are produced in response to EGF and PDGF, the next logical step was to test the hypothesis that ROS are a necessary component of the EGF and PDGF mitogenic pathways and that NADPH oxidase is the enzymatic complex involved in this receptor-mediated ROS production. After validating DPI, we used this NADPH oxidase inhibitor to block ROS generation in the presence of either EGF or PDGF. We then assessed the effect of DPI on EGF- and PDGF-induced LSMC proliferation by measuring changes in DNA synthesis using [3H]thymidine incorporation assays. Pretreatment of cells with increasing concentrations of DPI before adding EGF resulted in significant inhibition of proliferation when compared to those cells treated with EGF alone (Fig. 3A). In fact, the levels of DNA synthesis at the two higher concentrations were reduced to levels below those observed in the controls (0.5% FBS only). Similarly, when cells were exposed to DPI prior to treatment with PDGF, a reduction in growth factor-stimulated proliferation was again observed (Fig. 3B).
FIG. 3.
The NADPH oxidse inhibitor DPI blocks PDGF- and EGF-stimulated proliferation of LSMCs. A) Effects of DPI on LSMC DNA synthesis in the presence of 50 ng/ml EGF. B) Effects of DPI on LSMC DNA synthesis in the presence of 10 ng/ml PDGF. C) Effects of DPI on LSMC numbers in response to EGF and PDGF. Cell numbers were determined by manual counting on a hemocytometer (n = 3; bars represent LSmeans ± SEM; different capital letters indicate statistical difference, P < 0.05).
The effects of DPI on EGF- or PDGF-stimulated cell proliferation were significant. This inhibitory effect was dose dependent, as the highest concentration of DPI blocked DNA synthesis almost completely.
To confirm whether the results obtained with the [3H]thymidine incorporation assays were paralleled by similar changes in cell number, we performed cell count experiments using a hemocytometer. Cells exposed to either EGF or PDGF in the presence of DPI had a dramatic decrease in cell number in comparison to growth factors alone (Fig. 3C).
Exogenous H2O2 Stimulates LSMC Proliferation
After demonstrating that inhibition of the ROS-producing NADPH oxidase complex inhibited growth factor-induced cell proliferation, we tested whether an exogenous source of ROS was sufficient to induce LSMC proliferation. ROS are extremely labile and, therefore, difficult to use as a treatment for cells in vitro. In light of this characteristic of hydrogen peroxide, we used increasing concentrations of exogenous hydrogen peroxide (10–200 μM) as pulse treatments once per hour for 5 h and then assessed change in DNA synthesis. Interestingly, hydrogen peroxide at 10 μM increased DNA synthesis, whereas higher concentrations of the compound (50 and 100 μM) had no effect compared to control, and 200 μM was inhibitory (Fig. 4A). These data indicate that within the range of concentrations used, it was possible to separate the specific mitogenic effects of hydrogen peroxide from its inhibitory effects, which may be related to oxidative stress.
FIG. 4.
Exogenous H2O2 affects LSMC proliferation. A) Effects of H2O2 pulses (10, 50, 100, and 200 μM) on DNA synthesis in LSMCs. B) Effects of H2O2 pulses (20 and 100 μM) in LSMC number. Cell numbers were determined by manual counting using a hemocytometer (n = 3; bars represent LSmeans ± SEM; different capital letters indicate statistical difference, P < 0.05).
Cell count experiments confirmed that at relatively low concentrations (20 μM) H2O2 causes in increase in cell number compared to untreated control, whereas at higher concentrations (100 μM) it inhibited cell proliferation (Fig. 4B).
NADPH Oxidase Inhibitor Blocks PDGF-Stimulated MAPK1/MAPK3 Activation
To elucidate the inhibitory pathway further, we investigated the involvement of the well-known mitogen-activated protein kinase pathway on ROS-dependent proliferative responses of LSMCs, specifically MAPK1/MAPK3. Both EGF and PDGF induced phosphorylation of MAPK1/MAPK3 within 5 min of treatment with maximum phosphorylation occurring between 10 and 15 min (Fig. 5). We hypothesized that activation of MAPK1/MAPK3 occurs downstream of the increase in ROS production and that inhibition of ROS production would reduce the level of MAPK1/MAPK3 phosphorylation in response to EGF or PDGF treatment. No effects of DPI treatment on EGF-induced MAPK1/MAPK3 activation were observed (Fig. 6, A and B). However, pretreatment of LSMCs with DPI reduced PDGF-induced MAPK1/MAPK3 activation. This result is indicated by the decrease in intensity of the phosphorylated MAPK1/MAPK3 band from the cells treated with 50 μM of DPI in comparison to that observed for growth factor treatment alone (Fig. 6, C and D). Our results showing that inhibition of PDGF-induced MAPK1/MAPK3 activation by DPI and lack of inhibition of EGF-induced MAPK1/MAPK3 activation suggest a specific regulation of PDGF signaling pathway by ROS.
FIG. 5.
EGF and PDGF induce phosphorylation of MAPK1/MAPK3 in LSMCs. A) Immunoblot for time course of MAPK1/MAPK3 activation in response to EGF. B) Time course of MAPK1/MAPK3 activation in response to EGF. C) Immunoblot for time course of MAPK1/MAPK3 activation in response to PDGF; anti-total MAPK1/MAPK3 (tMAPK1/MAPK3) were used as loading control. Graphs represent the densitometric analysis of the immunoblots. D) Time course of MAPK1/MAPK3 activation in response to PDGF (n = 3; bars represent LSmeans ± SEM; line above two bars indicates that both time points were merged and compared to the other time points; asterisk above line indicates statistical difference in comparison to other time points, P < 0.05).
FIG. 6.
The NADPH oxidase inhibitor DPI blocks PDGF-stimulated MAPK1-/MAPK3phosphorylation in LSMCs. A) Immunoblot for MAPK1/MAPK3 activation in response to EGF in the presence or absence of DPI. B) Effects of DPI on EGF-induced MAPK1/MAPK3 activation. C) Immunoblot for MAPK1/MAPK3 activation in response to PDGF in the presence or absence of DPI; anti-total MAPK1/MAPK3 (tMAPK1/MAPK3) were used as loading control. Graphs represent the densitometric analysis of the blots. D) Effects of DPI on PDGF-induced MAPK1/MAPK3 activation (n = 3; bars represent LSmeans ± SEM; asterisk above line connecting two treatment groups indicates statistical difference between the connected groups, P < 0.05).
To test whether DPI was potentially having toxic effects, we decided to focus on the activation of molecules that are not regulated by ROS and would not be affected by DPI unless the inhibitor is having unspecific effects. We measured the ability of EGF-R or PDGF-R to undergo autophosphorylation on exposure to EGF or PDGF in the presence of DPI in LSMCs. Figure 7 provides evidence that LSMCs were able to respond to both growth factors even in the presence of the highest DPI concentration. This result is supported by the lack of a statistically significant reduction of EGF-R and PDGF-R autophosphorylation (Fig. 7, B and D).
FIG. 7.
The NADPH oxidase inhibitor DPI does not affect EGF or PDGF receptor activation. A) Immunoblot for EGF-R activation in response to EGF in the presence or absence of DPI. B) Effects of DPI on EGF-R activation. C) Immunoblot for PDGF-R activation in response to PDGF in the presence or absence of DPI; anti-total EGF-R (tEGF-R) and anti-total PDGF-R (tPDGF-R) were used as loading control. Graphs represent the densitometric analysis of the blots. D) Effects of DPI on PDGF-R activation (n = 3; bars represent LSmeans ± SEM; asterisk above line connecting two treatment groups indicates statistical difference between the connected groups, P < 0.05).
Exogenous H2O2 Stimulates MAPK1/MAPK3 in LSMCs
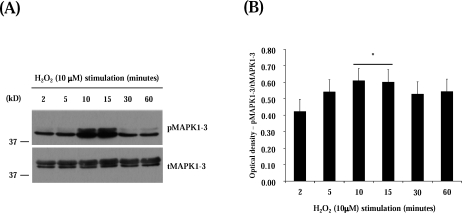
We next tested whether treatment with exogenous hydrogen peroxide was sufficient to induce MAPK1/MAPK3 phosphorylation. Similarly to the response observed in our [3H]thymidine incorporation experiments, exogenous hydrogen peroxide alone induced activation of the MAPK1/MAPK3 pathway (Fig. 8). MAPK1/MAPK3 reached maximum activation within 10 and 15 min after hydrogen peroxide treatment returning to basal levels after 30 min.
FIG. 8.
Exogenous H2O2 stimulates Erk1/2 phosphorylation in LSMCs. A) Immunoblot for time course of MAPK1/MAPK3 activation in response to H2O2; anti-total MAPK1/MAPK3 (tMAPK1/MAPK3) were used as loading control. Graphs represent the densitometric analysis of the blots showing the time-course of MAPK1/MAPK3 activation in response to exogenous H2O2 (n = 3; bars represent LSmeans ± SEM; line above two bars indicate that both time points were merged and compared to the other time points; asterisk above line indicates statistical difference in comparison to other time points, P < 0.05).
Effects of Exogenous H2O2 on COL1A2 and COL3A1 Gene Expression in LSMCs
Because our results showed that H2O2 had a positive effect on LSMC proliferation and MAPK1/MAPK3 activation, we tested whether H2O2 might also be involved in regulating collagen expression. Cells were either untreated, given five hourly pulses of 10 μM H2O2, or a higher concentration of H2O2 (100 μM) for 24 h. Total RNA was harvested and analyzed for changes in mRNA levels of COL1A2 and COL3A1 using qRT-PCR. Exogenous H2O2 did not alter COL1A2 and COL3A1 mRNA expression in LSMCs (data not shown). Treatment with either EGF or PDGF also did not affect levels of collagen mRNAs (data not shown).
DISCUSSION
The goal of our study was to determine whether ROS are necessary components of the PDGF and EGF signaling pathways for LSMC proliferation. Our results are the first to demonstrate that ROS generated by the NADPH oxidase system in LSMCs are involved as intermediates in the signaling pathway of these growth factors. The main findings of this study are that 1) LSMCs produce ROS in response to EGF and PDGF, 2) ROS are necessary and sufficient to induce LSMC proliferation, and 3) ROS are necessary and sufficient to induce a fraction of MAPK1/MAPK3 activation in LSMCs.
To determine the role of reactive oxygen species in the mitogenic signaling pathways of EGF and PDGF in LSMCs, we first needed to show that these growth factors induced ROS generation in such cells. EGF and PDGF have been shown previously to stimulate intracellular ROS production in other cells types [22, 24, 31, 32]. Our study is the first to show that both PDGF and EGF induce intracellular ROS generation in LSMCs. This receptor-mediated ROS production was initially discovered in cells of the immune system and shown to be derived from the plasma membrane flavohemoprotein complex NADPH oxidase [33]. One of the first reports of Matsubara and Ziff [34] focusing on nonimmune cells from showed that endothelial cells released superoxide in response to specific cytokines. Since this first evidence linking ROS generation to regulation of inflammatory responses, many other groups have become interested in the role of the NADPH oxidase complex in hyperproliferative disorders [35]. More recently, NADPH oxidase-derived ROS have been implicated as a necessary component of numerous signaling pathways and associated with specialized cell functions [32, 34–37].
If ROS are a necessary component of the PDGF and EGF signaling pathways, then the addition of exogenous ROS should mimic the effects of these growth factors. In fact, when treated with exogenous hydrogen peroxide, LSMCs exhibited a notable increase in DNA synthesis and cell number. The pathway by which exogenous ROS produced this effect in LSMCs is still unknown and may not be the same as that of EGF or PDGF. One of the first evidences of the participation of ROS, specifically hydrogen peroxide, in signaling pathways was provided by experiments showing hydrogen peroxide-dependent glucose oxidation in response to insulin [38]. ROS were later shown to be required for cell growth and transformation [21, 35, 36]. Hydrogen peroxide has been recognized as a signaling molecule since investigators determined that its concentration can rapidly and transiently increase in response to receptor-mediated signaling events. Furthermore, hydrogen peroxide undergoes quick enzymatic degradation, is diffusible, and can act in a specific and reversible fashion [39, 40]. Rao and Berk [41] demonstrated that hydrogen peroxide caused an increase in [3H]thymidine incorporation in vascular smooth muscle cells, in agreement with what our results have shown. In addition, other studies have demonstrated that a shift in intracellular ROS levels toward a more oxidizing environment stimulates the G1-to-S-phase transition in the cell cycle [42, 43].
Experiments focusing on the role of ROS in normal cell function are complicated by the fact that ROS are extremely short-lived molecules. In our study a low hydrogen peroxide concentration of 10 μM was added as a pulse, every hour for 5 h, in contrast to 200 μM every 3 h for 24 h by Rao and Berk [41]. Our replenishment system allowed us to overcome the problem with hydrogen peroxide being labile and degrading rapidly over time as well as to the use of concentrations that may more closely resemble physiological levels. Although it is difficult to determine what the physiological levels are and how specific hydrogen peroxide action is, we used concentrations that are 20 times lower than several other studies. Furthermore, COL1A2 and COL3A1gene expression was not affected by the same hydrogen peroxide administration regimen, suggesting that the higher proliferation rate was not due to increased collagen deposition or to a general nonspecific effect of hydrogen peroxide. We also observed that the use of concentrations above 50 μM abolished even basal levels of proliferation, confirming the negative effect of high levels of ROS on cells.
Pretreatment with the NADPH oxidase inhibitor DPI significantly inhibited EGF- and PDGF-induced proliferation of LSMCs. These data support our hypothesis that ROS are critical intermediates in the mitogenic signaling pathways of EGF and PDGF and also suggests for the first time the presence of the NADPH oxidase complex in the uterus. DPI has been used successfully by numerous other laboratories to prevent hormone/growth factor-induced, ROS-dependent cell proliferation in vascular smooth muscle cells [44–46]. DPI pretreatment did not appear to have caused cytotoxic effects at the concentrations used, as cells were able to respond to growth factor treatment with receptor autophosphorylation. This finding suggests a tight regulation of ROS production in the cell. When ROS levels are too high, it is believed that ROS function as potent oxidizing agents, potentially causing oxidative damage and cytotoxic effects. However, if intracellular ROS concentrations fall below a critical level, crucial cellular signaling events might be disrupted.
Inhibition of PDGF-induced MAPK1/MAPK3 activation by DPI and induction of MAPK1/MAPK3 activation by exogenous hydrogen peroxide suggest that ROS are necessary and sufficient to trigger the MAPK1/MAPK3 signaling pathway associated with PDGF. The fact that the responsiveness of LSMCs to EGF with regard to MAPK1/MAPK3 activation seemed unaffected by the presence of DPI may suggest another level of specificity to the role of ROS in regulating the PDGF signaling pathway. Although our study provides new information regarding the role of ROS in leiomyoma SMCs, MAPK1/MAPK3 activation in response to hydrogen peroxide has been observed previously in vascular smooth muscle cells, in agreement with our results [47]. Several studies have reported activation of a variety of specific downstream targets including ROS-dependent p38MAPK activation but not MAPK1/MAPK3 [19], activation of both MAPK1/MAPK3 and MAPK14 [48], as well as activation of only MAPK8 [25]. Some of the studies utilized NADPH oxidase inhibitors or ROS scavengers to confirm participation of ROS in these signaling pathways, whereas others tested the effects of exogenous hydrogen peroxide at concentrations ranging from 5- to 100-fold higher than those used in our study. The use of different hydrogen peroxide concentrations may explain the discrepancies between our results and those of others. ROS are involved in the activation of several signaling events including regulation of EGF and PDGF receptor activation or transactivation through induction of CSK activity [23, 49], VEGF-dependent HIF1 activation [50], IL1-dependent regulation of MP3K14 [51], TGFB1-dependent activation of EGR1 gene expression [52], and ultimately, the regulation of protein tyrosine phosphatase activity. Hydrogen peroxide cannot directly phosphorylate proteins, but it can specifically oxidize certain cysteine residues within protein tyrosine phosphatases, which then become unavailable to interact with phosphate substrates, rendering the enzyme inactive [39, 40]. Redox regulation of protein tyrosine phosphatases such as PTEN, PTPN1, ACP1, and CDC25C, has been reported [53–56]. Furthermore, the importance of PTPs in regulating the level of activation of growth factor receptors and the role of reductases in counteracting the effects of oxidants on PTPs during cell signaling have also been confirmed [57–59]. Whether NADPH oxidase-derived ROS affect proliferation and activation of MAPK1/MAPK3 pathways in LSMCs by regulating protein tyrosine phosphatase activity remains to be tested.
The involvement of ROS generated through the NADPH oxidase complex in the development of chronic diseases including fibrotic conditions such as pulmonary, hepatic, and pancreatic fibrosis has been proposed recently by other investigators [60]. Many of these disorders are associated with overproduction of ROS and are linked to higher expression of the NADPH oxidase subunits [60]. The increased production of ROS may occur as part of an inflammatory response and may contribute to the abnormal cell proliferation that is observed in conditions such as atherosclerosis or diabetic vascular disease. Drugs that can target the NADPH oxidase complex such as specific inhibitors of the NOX/DUOX family members represent potential therapeutic approaches for treatment of these chronic diseases [61]. Uterine leiomyomas are also an example of a chronic fibrotic disease, and thus the characterization and understanding of the NADPH oxidase complex in LSMCs may be important for future directions in treatment of these tumors.
In summary, the findings of our research suggest that reactive oxygen species are an important component of the EGF and PDGF signaling pathways involved in LSMC proliferation. Specifically regarding the PDGF signaling pathway, our data suggest that the activation of MAPK1/MAPK3 is an important step following PDGF-induced ROS production, which may be necessary for ROS-induced LSMC proliferation. This mechanism is likely occurring through the stimulation of NADPH oxidase and the subsequent generation of intracellular ROS. Our findings provide the first evidence of 1) ROS as a second messenger in LSMCs proliferation and 2) a nonphagocytic NADPH oxidase complex in leiomyoma smooth muscle cells. This research presents a novel target for the development of preventive strategies or therapeutic approaches to treat uterine leiomyomas through the targeting of intracellular ROS and, specifically, NADPH oxidase. Although no differences were observed between normal myometrial cells and leiomyoma cells in regard to the influence of NADPH oxidase-derived ROS on the signaling pathways examined, the presence of the complex in myometrial and leiomyoma cells provides the basis to investigate other pathways.
Acknowledgments
We would like to thank Professor Eddie Greene from the Mayo Clinic, Rochester, Minnesota, for his valuable advice in establishing the methodologies to measure changes in intracellular ROS production in these studies. We also want to thank Professor Janice Bahr from the Department of Animal Sciences at the University of Illinois, Urbana, for her thoughtful comments/suggestions and major help in editing this revised manuscript.
Footnotes
1Supported by NIH/NICHD RO1 HD046227 to R.A.N.
REFERENCES
- Stewart EA, Friedman AJ, Peck K, Nowak RA.Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab 1994; 79: 900–906. [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA.Uterine fibroids: the elephant in the room. Science 2005; 308: 1589–1592. [DOI] [PubMed] [Google Scholar]
- Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH.Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril 2004; 82(suppl 3):1182–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill RM.Hysterectomy Surveillance in the United States, 1997 Through 2005. Med Sci Monit 2008; 14: CR24–CR31. [PubMed] [Google Scholar]
- Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM.High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003; 188: 100–107. [DOI] [PubMed] [Google Scholar]
- Chegini N, Ma C, Tang XM, Williams RS.Effects of GnRH analogues, “add-back” steroid therapy, antiestrogen and antiprogestins on leiomyoma and myometrial smooth muscle cell growth and transforming growth factor-beta expression. Mol Hum Reprod 2002; 8: 1071–1078. [DOI] [PubMed] [Google Scholar]
- Barbarisi A, Petillo O, Di Lieto A, Melone MA, Margarucci S, Cannas M, Peluso G.17-Beta estradiol elicits an autocrine leiomyoma cell proliferation: evidence for a stimulation of protein kinase-dependent pathway. J Cell Physiol 2001; 186: 414–424. [DOI] [PubMed] [Google Scholar]
- Maruo T, Matsuo H, Samoto T, Shimomura Y, Kurachi O, Gao Z, Wang Y, Spitz IM, Johansson E.Effects of progesterone on uterine leiomyoma growth and apoptosis. Steroids 2000; 65: 585–592. [DOI] [PubMed] [Google Scholar]
- Rossi MJ, Chegini N, Masterson BJ.Presence of epidermal growth factor, platelet-derived growth factor, and their receptors in human myometrial tissue and smooth muscle cells: their action in smooth muscle cells in vitro. Endocrinology 1992; 130: 1716–1727. [DOI] [PubMed] [Google Scholar]
- Mangrulkar RS, Ono M, Ishikawa M, Takashima S, Klagsbrun M, Nowak RA.Isolation and characterization of heparin-binding growth factors in human leiomyomas and normal myometrium. Biol Reprod 1995; 53: 636–646. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Morita I, Kubota T, Murota S, Aso T.Human uterine myometrial smooth muscle cell proliferation and vascular endothelial growth-factor production in response to platelet-derived growth factor. J Endocrinol 2001; 169: 79–86. [DOI] [PubMed] [Google Scholar]
- Mitsumata M, Gamou S, Shimizu N, Yoshida Y.Response of atherosclerotic intimal smooth muscle cells to epidermal growth factor in vitro. Arterioscler Thromb 1994; 14: 1364–1371. [DOI] [PubMed] [Google Scholar]
- Ross R, Raines EW, Bowen-Pope DF.The biology of platelet-derived growth factor. Cell 1986; 46: 155–169. [DOI] [PubMed] [Google Scholar]
- Hirst SJ, Twort CH, Lee TH.Differential effects of extracellular matrix proteins on human airway smooth muscle cell proliferation and phenotype. Am J Respir Cell Mol Biol 2000; 23: 335–344. [DOI] [PubMed] [Google Scholar]
- Friedman SL.Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 2000; 275: 2247–2250. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Bonner G, Maeshima Y, Colorado P, Muller GA, Strutz F, Kalluri R.Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol 2001; 159: 1313–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, DeRuiter MC, Bergwerff M, Poelmann RE.Smooth muscle cell origin and its relation to heterogeneity in development and disease. Arterioscler Thromb Vasc Biol 1999; 19: 1589–1594. [DOI] [PubMed] [Google Scholar]
- Weber DS, Taniyama Y, Rocic P, Seshiah PN, Dechert MA, Gerthoffer WT, Griendling KK.Phosphoinositide-dependent kinase 1 and p21-activated protein kinase mediate reactive oxygen species-dependent regulation of platelet-derived growth factor-induced smooth muscle cell migration. Circ Res 2004; 94: 1219–1226. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Alexander RW, Akers M, Griendling KK.p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle cell hypertrophy. J Biol Chem 1998; 273: 15022–15029. [DOI] [PubMed] [Google Scholar]
- Greene EL, Houghton O, Collinsworth G, Garnovskaya MN, Nagai T, Sajjad T, Bheemanathini V, Grewal JS, Paul RV, Raymond JR.5-HT(2A) receptors stimulate mitogen-activated protein kinase via H(2)O(2) generation in rat renal mesangial cells. Am J Physiol Renal Physiol 2000; 278: F650–F658. [DOI] [PubMed] [Google Scholar]
- Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, Parthasarathy S, Petros JA, Lambeth JD.Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A 2001; 98: 5550–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svegliati S, Cancello R, Sambo P, Luchetti M, Paroncini P, Orlandini G, Discepoli G, Paterno R, Santillo M, Cuozzo C, Cassano S, Avvedimento EV, et al. Platelet-derived growth factor and reactive oxygen species (ROS) regulate Ras protein levels in primary human fibroblasts via ERK1/2. Amplification of ROS and Ras in systemic sclerosis fibroblasts. J Biol Chem 2005; 280: 36474–36482. [DOI] [PubMed] [Google Scholar]
- Catarzi S, Biagioni C, Giannoni E, Favilli F, Marcucci T, Iantomasi T, Vincenzini MT.Redox regulation of platelet-derived-growth-factor-receptor: role of NADPH-oxidase and c-Src tyrosine kinase. Biochim Biophys Acta 2005; 1745: 166–175. [DOI] [PubMed] [Google Scholar]
- Bae YS, Kang SW, Seo MS, Baines IC, Tekle E, Chock PB, Rhee SG.Epidermal growth factor (EGF)-induced generation of hydrogen peroxide: role in EGF receptor-mediated tyrosine phosphorylation. J Biol Chem 1997; 272: 217–221. [PubMed] [Google Scholar]
- Lo YY, Wong JM, Cruz TF.Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J Biol Chem 1996; 271: 15703–15707. [DOI] [PubMed] [Google Scholar]
- Fan J, Frey RS, Rahman A, Malik AB.Role of neutrophil NADPH oxidase in the mechanism of tumor necrosis factor-alpha-induced NF-kappa B activation and intercellular adhesion molecule-1 expression in endothelial cells. J Biol Chem 2002; 277: 3404–3411. [DOI] [PubMed] [Google Scholar]
- Colavitti R, Pani G, Bedogni B, Anzevino R, Borrello S, Waltenberger J, Galeotti T.Reactive oxygen species as downstream mediators of angiogenic signaling by vascular endothelial growth factor receptor-2/KDR. J Biol Chem 2002; 277: 3101–3108. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW.Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994; 74: 1141–1148. [DOI] [PubMed] [Google Scholar]
- Adachi T, Togashi H, Suzuki A, Kasai S, Ito J, Sugahara K, Kawata S.NAD(P)H oxidase plays a crucial role in PDGF-induced proliferation of hepatic stellate cells. Hepatology 2005; 41: 1272–1281. [DOI] [PubMed] [Google Scholar]
- Masamune A, Watanabe T, Kikuta K, Satoh K, Shimosegawa T.NADPH oxidase plays a crucial role in the activation of pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol 2008; 294: G99–G108. [DOI] [PubMed] [Google Scholar]
- Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T.Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 1995; 270: 296–299. [DOI] [PubMed] [Google Scholar]
- Baumer AT, Ten Freyhaus H, Sauer H, Wartenberg M, Kappert K, Schnabel P, Konkol C, Hescheler J, Vantler M, Rosenkranz S.Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem 2008; 283: 7864–7876. [DOI] [PubMed] [Google Scholar]
- Chanock SJ, el Benna J, Smith RM, Babior BM.The respiratory burst oxidase. J Biol Chem 1994; 269: 24519–24522. [PubMed] [Google Scholar]
- Matsubara T, Ziff M.Increased superoxide anion release from human endothelial cells in response to cytokines. J Immunol 1986; 137: 3295–3298. [PubMed] [Google Scholar]
- Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD.Cell transformation by the superoxide-generating oxidase Mox1. Nature 1999; 401: 79–82. [DOI] [PubMed] [Google Scholar]
- Mitsushita J, Lambeth JD, Kamata T.The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Res 2004; 64: 3580–3585. [DOI] [PubMed] [Google Scholar]
- Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, Goldschmidt-Clermont PJ.Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science 1997; 275: 1649–1652. [DOI] [PubMed] [Google Scholar]
- Mukherjee SP, Lane RH, Lynn WS.Endogenous hydrogen peroxide and peroxidative metabolism in adipocytes in response to insulin and sulfhydryl reagents. Biochem Pharmacol 1978; 27: 2589–2594. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS, Lee SR, Kwon J.Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE 2000; 2000: pe1 [DOI] [PubMed] [Google Scholar]
- Tonks NK.Redox redux: revisiting PTPs and the control of cell signaling. Cell 2005; 121: 667–670. [DOI] [PubMed] [Google Scholar]
- Rao GN, Berk BC.Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res 1992; 70: 593–599. [DOI] [PubMed] [Google Scholar]
- Deng X, Gao F, May WS., JrBcl2 retards G1/S cell cycle transition by regulating intracellular ROS. Blood 2003; 102: 3179–3185. [DOI] [PubMed] [Google Scholar]
- Menon SG, Sarsour EH, Spitz DR, Higashikubo R, Sturm M, Zhang H, Goswami PC.Redox regulation of the G1 to S phase transition in the mouse embryo fibroblast cell cycle. Cancer Res 2003; 63: 2109–2117. [PubMed] [Google Scholar]
- Bhunia AK, Han H, Snowden A, Chatterjee S.Redox-regulated signaling by lactosylceramide in the proliferation of human aortic smooth muscle cells. J Biol Chem 1997; 272: 15642–15649. [DOI] [PubMed] [Google Scholar]
- Bleeke T, Zhang H, Madamanchi N, Patterson C, Faber JE.Catecholamine-induced vascular wall growth is dependent on generation of reactive oxygen species. Circ Res 2004; 94: 37–45. [DOI] [PubMed] [Google Scholar]
- O'Donnell BV, Tew DG, Jones OT, England PJ.Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem J 1993; 290(pt 1):41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SL, Wang WW, Finlay GA, Fanburg BL.Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol 1999; 277: L282–L291. [DOI] [PubMed] [Google Scholar]
- Zhang J, Jin N, Liu Y, Rhoades RA.Hydrogen peroxide stimulates extracellular signal-regulated protein kinases in pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol 1998; 19: 324–332. [DOI] [PubMed] [Google Scholar]
- Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, Alexander RW.Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem 2001; 276: 48269–48275. [DOI] [PubMed] [Google Scholar]
- Biswas S, Gupta MK, Chattopadhyay D, Mukhopadhyay CK.Insulin-induced activation of hypoxia-inducible factor-1 requires generation of reactive oxygen species by NADPH oxidase. Am J Physiol Heart Circ Physiol 2007; 292: H758–H766. [DOI] [PubMed] [Google Scholar]
- Li Q, Engelhardt JF.Interleukin-1beta induction of NFkappaB is partially regulated by H2O2-mediated activation of NFkappaB-inducing kinase. J Biol Chem 2006; 281: 1495–1505. [DOI] [PubMed] [Google Scholar]
- Ohba M, Shibanuma M, Kuroki T, Nose K.Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. J Cell Biol 1994; 126: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitsky PA, Finkel T.Redox regulation of Cdc25C. J Biol Chem 2002; 277: 20535–20540. [DOI] [PubMed] [Google Scholar]
- Lee SR, Kwon KS, Kim SR, Rhee SG.Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J Biol Chem 1998; 273: 15366–15372. [DOI] [PubMed] [Google Scholar]
- Kappert K, Sparwel J, Sandin A, Seiler A, Siebolts U, Leppanen O, Rosenkranz S, Ostman A.Antioxidants relieve phosphatase inhibition and reduce PDGF signaling in cultured VSMCs and in restenosis. Arterioscler Thromb Vasc Biol 2006; 26: 2644–2651. [DOI] [PubMed] [Google Scholar]
- Seo JH, Ahn Y, Lee SR, Yeol Yeo C, Chung Hur K.The major target of the endogenously generated reactive oxygen species in response to insulin stimulation is phosphatase and tensin homolog and not phosphoinositide-3 kinase (PI-3 kinase) in the PI-3 kinase/Akt pathway. Mol Biol Cell 2005; 16: 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda M, Ihara Y, Murata H, Urata Y, Kono T, Yodoi J, Seto S, Yano K, Kondo T.Glutaredoxin modulates platelet-derived growth factor-dependent cell signaling by regulating the redox status of low molecular weight protein-tyrosine phosphatase. J Biol Chem 2006; 281: 28518–28528. [DOI] [PubMed] [Google Scholar]
- Chiarugi P, Fiaschi T, Taddei ML, Talini D, Giannoni E, Raugei G, Ramponi G.Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J Biol Chem 2001; 276: 33478–33487. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Morales FC, Kreimann EL, Georgescu MM.PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J 2006; 25: 910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD.Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 2007; 43: 332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth JD, Krause KH, Clark RA.NOX enzymes as novel targets for drug development. Semin Immunopathol 2008; 30: 339–363. [DOI] [PubMed] [Google Scholar]