Abstract
Vascular cognitive impairment is a term used to describe a heterogeneous group of diseases, including large vessel disease with strategic single and multiple strokes and small vessel disease with progressive damage to the deep white matter. Identification of patients with the progressive form of vascular cognitive impairment, referred to by some investigators as Binswanger disease, is important for treatment trials. Pathologically, Binswanger disease is associated with small vessel disease, extensive regions of demyelination, inflammatory cells around damaged blood vessels, and lacunar infarcts. Clinically, patients with Binswanger disease have impairments of gait and balance, focal neurological findings, and executive dysfunction on neuropsychological tests. White matter changes on MRI are thought to be due to hypoxic episodes related to hypoperfusion of the vulnerable deep white matter secondary to hypertension, diabetes, and other vessel diseases. Disruption of the blood– brain barrier suggests an inflammatory response. Matrix metalloproteinases are present in the brain of patients with vascular cognitive impairment and can be measured in the cerebrospinal fluid of some patients. Preliminary studies with quantification of the blood–brain barrier, using the multiple time graphical method (Patlak plots), supports disruption of the blood–brain barrier. Because no single clinical feature or diagnostic test is sufficient to identify patients with the small vessel form of vascular cognitive impairment, we propose that a multimodal approach will be needed to select patients for treatment trials.
Keywords: Binswanger’s, cognitive, disease, impairment, matrix, metalloproteinases, MRI, vascular
Vascular cognitive impairment (VCI) is an important cause of dementia that can occur in combination with Alzheimer disease, particularly in an aging population, creating a large public health problem.1 VCI is clinically heterogeneous, complicating diagnosis and treatment trials in the early stages, when treatment may be beneficial.2 For purposes of classification, the term VCI has recently replaced vascular dementia and includes all patients with a vascular component to the intellectual decline.3 VCI encompasses patients with large vessel strokes and small vessel disease. Research interest is currently focused on the patients with the progressive small vessel damage, which is referred to by some investigators as Binswanger disease, because the progressive nature of the illness makes it more amenable to clinical trials than the large vessel form, which is poorly predictable.4
Diagnosis of patients with Binswanger disease requires the use of clinical history, neurological examination, neuroimaging, and selective biomarkers. Because none of these are accurate alone, a multimodal approach can be used to optimize diagnosis of the progressive form of VCI. Useful information has come from clinical findings, neuropsychological test results, MRI with proton magnetic resonance spectroscopy, and cerebrospinal fluid (CSF) measurement of protein and matrix metalloproteinases (MMPs).5,6 MRI identifies patients with white matter hyperintensities, which, in studies of large populations, are associated with small strokes and cognitive decline.7 However, 30% of normal individuals over 65 years of age have moderate white matter hyperintensities on MRI and 7% have severe white matter hyperintensities, limiting the usefulness of MRI in diagnosis of an individual patient.8 These changes are strongly correlated with age and duration of hypertension and are improved with treatment of hypertension.9 Proton magnetic resonance spectroscopy identifies regions of white matter hyperintensities that are ischemic.6,10 Pathological studies in patients with VCI demonstrate an inflammatory response in the regions of myelin loss around blood vessels.11 MMPs are found in the inflammatory cells and in reactive astrocytes and microglia.12 Increased levels of MMPs are found in the CSF of patients with VCI.5 In patients with diabetes mellitus, changes in cognition are associated with abnormalities in the blood–brain barrier (BBB) that can be seen with contrast-enhanced scans, but such changes have not been seen in nondiabetic patients.13
Small Vessel Disease in the Clinical Spectrum of Vascular Cognitive Impairment
Hypertension and diabetes mellitus are the major factors related to small vessel disease in the brain. Rarer causes include vasculitis of the central nervous system, the antiphospholipid antibody syndrome and congenital diseases such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, and Sneddon syndrome. Clinical symptoms usually begin with gait problems, mild cognitive decline, and small strokes.14 Lacunar strokes and damage to the white matter lead to focal findings with imbalance and weakness. Neuropsychological testing shows abnormalities in multiple areas, including organizational or executive skills. Memory function may be intact or minimally affected in the early stages and language is generally preserved, which is an important finding that separates VCI small vessel patients from those with Alzheimer disease, particularly those with mild cognitive impairment. As the illness progresses, patients may have strokes and a few develop enlarged ventricles, leading to the diagnosis of normal pressure hydrocephalus. Neuroimaging shows attenuated white matter on CT and hyperintensities on fluid-attenuated inversion recovery or T2-weighted MRI.
Pathological Studies in Small Vessel Disease
Pathology studies provide the most reliable basis for diagnosis.15 In Binswanger disease, there is gliosis of the white matter with inflammatory cells in the white matter around blood vessels and in the vicinity of demyelination. There is white matter injury that is consistent with demyelination, but other causes for the loss of myelin are possible. The demyelination is generally seen around vessels associated with pathological changes in the blood vessels, particularly in the medullary arteries of the deep white matter. White matter shows extensive astrogliosis associated with fibrohyaline changes in the blood vessels, which contain serum components that suggest disruption of the BBB. Markers of inflammation such as cyclooxygenase-2 are seen in microglia/macrophages around blood vessels along with extravasated proteins, suggesting disruption of the BBB.16 MMPs are increased in macrophages and reactive astrocytes in VCI.12 Immunostaining showed hypoxia-inducing factor-1• in white matter suggestive of hypoxic hypoperfusion.17 Reactive astrocytes, fibrinogen, and microglia activation are found in regions of demyelination.18
Cerebrospinal Fluid Matrix Metalloproteinase in Vascular Cognitive Impairment
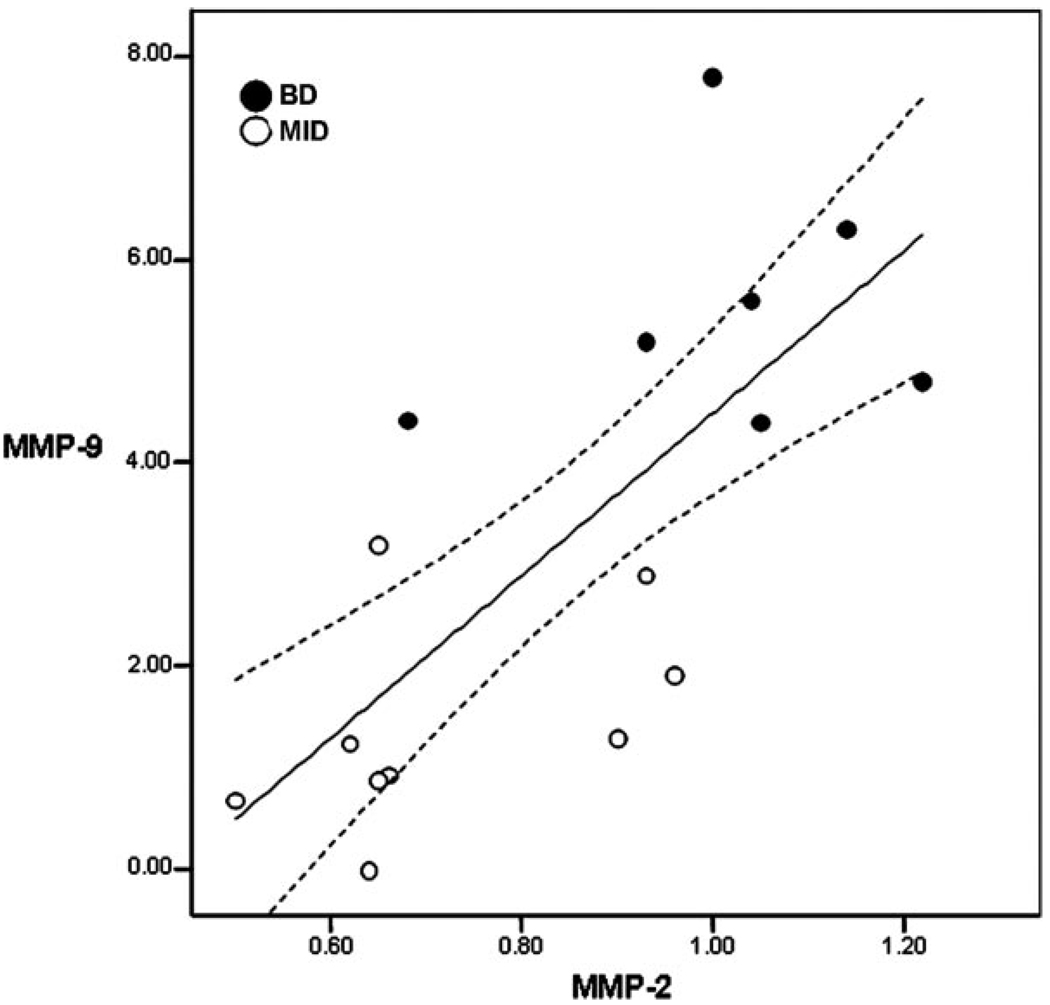
Matrix metalloproteinases are increased in the CSF in VCI.5 Gelatinase B (MMP-9) was found to be elevated compared with controls, but levels of gelatinase A (MMP-2) were not found to be significantly different than the controls.5 MMP-9 is a proinflammatory enzyme that is only produced under an injury stimulus, and MMP-2 is a constitutive enzyme that is present normally in a latent form and undergoes activation during injury and repair.19 These enzymes, which can be quantitatively measured with gelatin zymography, are elevated in CSF in a number of neuroinflammatory conditions, including acute stroke, infections, and multiple sclerosis.20 Although MMP-2 is present normally in CSF and is not elevated in groups of patients with VCI, in a small group of patients, plotting MMP-2 against MMP-9 showed that those with higher values of both were more likely to have the small vessel form of VCI than the large multistroke form (Figure 1). However, further studies will be needed to confirm these preliminary results.
Figure 1.
The relationship between the values for MMP-9 and MMP-2 in the patients with small vessel VCI or Binswanger disease (closed dots) and large vessel multi-infarct dementia (MID; open dots). Values have been normalized to control samples that were run on each gel so that samples collected at different times could be compared. Patients with VCI showed a significant correlation between the MMP-2 and the MMP-9 values. The patients with Binswanger disease tended to have higher values of both MMP-2 and MMP-9 placing them in the upper outer quadrant of the graph, whereas those with multiple infarcts tended to be in the lower inner quadrant. There was a significant positive correlation of MMP-9 and MMP-2 in the patients with VCI (r• 0.74; P• 0.001; N• 16). (Data extracted from reference 5.)
Blood–Brain Barrier Permeability
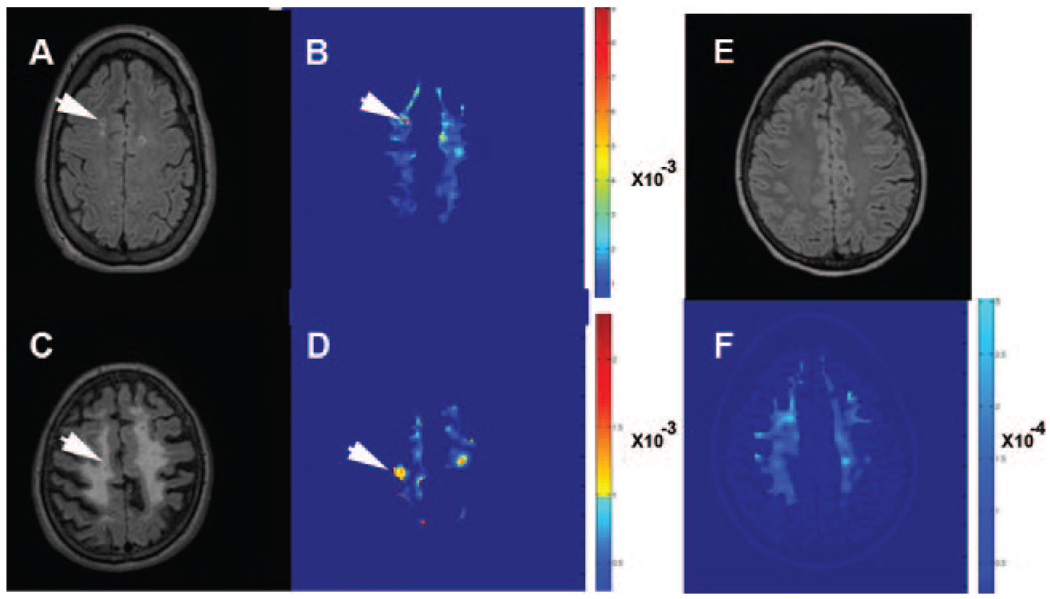
Opening of the BBB has been postulated from the finding of elevated albumin in the CSF and brain of patients with VCI, serum proteins in the brain at autopsy, and demonstration of leaky vessels with gadolinium-enhanced MRI in patients with diabetes.21 Quantification of BBB permeability has been done in animals with gadolinium DTPA using a fast T1-weighted imaging method based on multiple timed graphical plots (Patlak plots), which was originally developed for autoradiography with radiolabeled aminoisobutyric acid.22 We have adapted the Patlak plot method to humans for use in measurements of BBB permeability in patients with VCI; this is part of a long-term study to identify potential biomarkers in VCI that could be used for identification of inflammatory lesions. We have identified a group of patients with VCI who have increased permeability to gadolinium DTPA in the white matter compared with age-matched control subjects (Taheri et al, unpublished data). A contrast-enhanced MRI permeability map using the Patlak method reveals regions of increased permeability within the white matter hyperintensities (Figure 2). These preliminary data support the concept that a subset of patients with VCI could have an inflammatory process with disruption of the BBB. Further studies are underway to correlate clinical findings in the patients with and without enhanced permeability and to correlate that information with CSF findings.
Figure 2.
Multiple time graphical plots (Patlak plots) of BBB permeability with gadolinium DTPA-enhancing lesions in 2 patients with VCI. A, Fluid-attenuated inversion recovery (FLAIR) image from one patient with early changes in the white matter. B, White matter permeability maps with gray matter removed. The red regions represent increased BBB permeability in a region of white matter hyperintensity on the MRI (arrows). C, FLAIR image from a patient with advanced Binswanger disease and extensive changes in the white matter with relative sparing of the gray matter. D, Corresponding permeability map showing several smaller regions of enhanced permeability despite the extensive white matter changes (arrows). E, FLAIR image from a normal elderly control subject without white matter hyperintensities. F, Permeability map showing absence of increased permeability. Note that the color coding is not comparable in the patients and control subjects because the permeability scale is • 10• 3 in (mmol/kg• min) in the patients and • 10• 4 (mmol/kg• min) in control subjects, indicating the lack of enhancement in the control subjects. (Courtesy of Saeid Taheri, PhD.)
Matrix Metalloproteinases, Blood–Brain Barrier Damage, and White Matter Lesions
Several etiologies have been proposed to explain the progressive demyelination seen in VCI. Damage to the BBB with leakage of serum components in the white matter has been postulated, but the substrates in the blood that lead to the damage have not been identified. Hypoxic hypoperfusion is another possible mechanism. Because the long medullary arteries that supply the deep white matter may undergo fibrosis, cerebral blood flow to these end arteries in a watershed region could be compromised, leading to episodic induction of hypoxia-sensing substances such as hypoxia inducing factor-1•, which would induce inflammation. The presence of serum proteins, macrophages, and MMPs in the lesions supports an inflammatory etiology. Several potential triggers for the inflammation exist. Damage to the blood vessels from hypertension and diabetes could initiate repair of the vessels by the activation of astrocytes and microglia, leading to gliosis and inflammation. Reactive astrocytes and microglia secrete a wide variety of potentially damaging substances, including free radicals, cytokines, and proteases. Normally, inflammatory cells remove tissue debris from an injury site, but also participate in the repair process. Gliosis and fibrosis may take place as part of the remodeling of the extracellular matrix. As a consequence of this repair process, proteases may be released in the vicinity of the myelin. Several proteases, including the MMPs and serine protease, have been shown to cause demyelination.23,24
Challenges for Future Studies
Although there is no single measure that can be used to diagnose patients with the small vessel progressive form of VCI, combining information from the clinical examination, neuropsychological testing, white matter lesion pattern on MRI, proton magnetic resonance spectroscopy, BBB permeability, and CSF protein and MMPs will provide a comprehensive picture that improves accuracy of diagnosis early in the course. If inflammation can be proven to be an important component of the progressive white matter damage, treatment trials with novel approaches may be possible.
Acknowledgments
J. Adair, E. Edmonds, and J. Prestopnik helped with the clinical studies. J. Dencoff performed the CSF MMP measurements. S. Taheri performed the MRI permeability studies.
Sources of Funding
This study was supported by grants from the National Institutes of Health to G.A.R. (RO1 NS052305) and to the UNM General Clinical Research Center, and a grant from Bayer Pharmaceutical Industries.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
None.
References
- 1.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, DeCarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke–Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–2241. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 2.Bowler JV. Modern concept of vascular cognitive impairment. Br Med Bull. 2007;83:291–305. doi: 10.1093/bmb/ldm021. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, Rockwood K, Burns A, Gauthier S, DeKosky ST. Vascular cognitive impairment. Lancet Neurol. 2003;2:89–98. doi: 10.1016/s1474-4422(03)00305-3. [DOI] [PubMed] [Google Scholar]
- 4.Inzitari D, Erkinjuntti T, Wallin A, Del Ser T, Romanelli M, Pantoni L. Subcortical vascular dementia as a specific target for clinical trials. Ann NY Acad Sci. 2000;903:510–521. doi: 10.1111/j.1749-6632.2000.tb06407.x. [DOI] [PubMed] [Google Scholar]
- 5.Adair JC, Charlie J, Dencoff JE, Kaye JA, Quinn JF, Camicioli RM, Stetler-Stevenson WG, Rosenberg GA. Measurement of gelatinase b (MMP-9) in the cerebrospinal fluid of patients with vascular dementia and Alzheimer disease. Stroke. 2004;35:e159–e162. doi: 10.1161/01.STR.0000127420.10990.76. [DOI] [PubMed] [Google Scholar]
- 6.Brooks WM, Wesley MH, Kodituwakku PW, Garry PJ, Rosenberg GA. 1H-MRS differentiates white matter hyperintensities in subcortical arteriosclerotic encephalopathy from those in normal elderly. Stroke. 1997;28:1940–1943. doi: 10.1161/01.str.28.10.1940. [DOI] [PubMed] [Google Scholar]
- 7.Vermeer SE, Prins ND, Den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 8.Hunt AL, Orrison WW, Yeo RA, Haaland KY, Rhyne RL, Garry PJ, Rosenberg GA. Clinical significance of MRI white matter lesions in the elderly. Neurology. 1989;39:1470–1474. doi: 10.1212/wnl.39.11.1470. [DOI] [PubMed] [Google Scholar]
- 9.de Leeuw FE, de Groot JC, Oudkerk M, Witteman JC, Hofman A, van Gijn J, Breteler MM. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125:765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 10.MacKay S, Meyerhoff DJ, Constans JM, Norman D, Fein G, Weiner MW. Regional gray and white matter metabolite differences in subjects with AD, with subcortical vascular dementia, and elderly controls with 1H magnetic resonance spectroscopic imaging. Arch Neurol. 1996;53:167–174. doi: 10.1001/archneur.1996.00550020079018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H. Alterations in glia and axons in the brains of Binswanger’s disease patients. Stroke. 1997;28:1423–1429. doi: 10.1161/01.str.28.7.1423. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg GA, Sullivan N, Esiri MM. White matter damage is associated with matrix metalloproteinases in vascular dementia. Stroke. 2001;32:1162–1168. doi: 10.1161/01.str.32.5.1162. [DOI] [PubMed] [Google Scholar]
- 13.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood–brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–76. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan LR. Binswanger’s disease—revisited. Neurology. 1995;45:626–633. doi: 10.1212/wnl.45.4.626. [DOI] [PubMed] [Google Scholar]
- 15.Esiri MM, Wilcock GK, Morris JH. Neuropathological assessment of the lesions of significance in vascular dementia. J Neurol Neurosurg Psychiatry. 1997;63:749–753. doi: 10.1136/jnnp.63.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tomimoto H, Akiguchi I, Wakita H, Lin JX, Budka H. Cyclooxygenase-2 is induced in microglia during chronic cerebral ischemia in humans. Acta Neuropathol (Berl) 2000;99:26–30. doi: 10.1007/pl00007402. [DOI] [PubMed] [Google Scholar]
- 17.Fernando MS, Simpson JE, Matthews F, Brayne C, Lewis CE, Barber R, Kalaria RN, Forster G, Esteves F, Wharton SB, Shaw PJ, O’Brien JT, Ince PG. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 18.Simpson JE, Fernando MS, Clark L, Ince PG, Matthews F, Forster G, O’Brien JT, Barber R, Kalaria RN, Brayne C, Shaw PJ, Lewis CE, Wharton SB. White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathol Appl Neurobiol. 2007;33:410–419. doi: 10.1111/j.1365-2990.2007.00828.x. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham LA, Wetzel M, Rosenberg GA. Multiple roles for MMPs and TIMPs in cerebral ischemia. Glia. 2005;50:329–339. doi: 10.1002/glia.20169. [DOI] [PubMed] [Google Scholar]
- 20.Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6:931–944. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- 21.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood–brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 22.Ewing JR, Knight RA, Nagaraja TN, Yee JS, Nagesh V, Whitton PA, Li L, Fenstermacher JD. Patlak plots of Gd-DTPA MRI data yield blood–brain transfer constants concordant with those of 14c-sucrose in areas of blood–brain opening. Magn Reson Med. 2003;50:283–292. doi: 10.1002/mrm.10524. [DOI] [PubMed] [Google Scholar]
- 23.Cammer W, Bloom BR, Norton WT, Gordon S. Degradation of basic protein in myelin by neutral proteases secreted by stimulated macrophages: a possible mechanism of inflammatory demyelination. Proc Natl Acad Sci U S A. 1978;75:1554–1558. doi: 10.1073/pnas.75.3.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chandler S, Coates R, Gearing A, Lury J, Wells G, Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201:223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]