Abstract
Background
The significant variability across studies of mild cognitive impairment (MCI) in rates of progression to Alzheimer's disease (AD) and reversion to normal cognition may be due to differences in specific neuropsychological tests and thresholds used to define MCI.
Methods
We assessed 115 subjects with amnestic (AMN) or non-amnestic (NON) MCI on a standardized neuropsychological battery at baseline and after a mean follow-up of 16.4 months to determine the prevalence and persistence of deficits identified with specific tests.
Results
The prevalence of impaired performance varied widely across tests. Deficits were more persistent in the AMN group than in the NON group. Baseline deficits in Visual Reproduction II and the California Verbal Learning Test were the best predictors of persistent memory impairment. Subjects who at baseline were impaired on multiple memory tests or had poorer overall memory performance were more likely to exhibit persistent memory deficits.
Conclusions
The use of different neuropsychological tests and thresholds to diagnose MCI identified subsets of subjects with different rates of persistence of cognitive impairment. Standardization of the operational definition of cognitive impairment in MCI may result in more consistent predictions of progression to AD.
Key Words: Alzheimer disease, Memory, Cognition, Dementia, Psychometrics
Introduction
Mild cognitive impairment (MCI) often represents a transitional stage between normal aging and Alzheimer's disease (AD) [1]. Subjects meeting diagnostic criteria for MCI progress to AD at significantly higher rates than normal elderly controls (10% vs. 1 to 2% per year) [2, 3]. These findings have led to increased efforts to identify subjects with MCI, particularly since therapeutic interventions initiated in this population may potentially prevent or delay progression to clinical AD [1].
These initiatives have been complicated by the variability in reported rates of progression from MCI to AD, which range from 2 to 32% per year [4, 5]. Of equal concern is the variability in rates of reversion from MCI to normal cognition, which range from 4 to 53% [6, 7]. High reversion rates in MCI indicate that many subjects may not have incipient AD and its utility for predicting progression to dementia reduce.
A number of factors are likely to contribute to the variance in reported rates of progression and reversion. Population-based samples [4, 7] often have lower rates of progression to AD than memory clinic-based samples [5, 8], which may include a higher percentage of subjects with preclinical AD [9]. Rates of progression may also depend on the length of follow-up, which can range from 1 to 10 years [10, 11]. Calculations of annual rates of progression typically assume linear rates of progression over time [2], but the risk of progression may be greatest over the first 18 months of follow-up [12].
Other potentially significant sources of variability are differences in the operational definition of MCI between individual studies [9]. The Petersen criteria establish general guidelines for the diagnosis of MCI: (1) subjective cognitive complaint, (2) essentially intact activities of daily living, (3) objective cognitive impairment, and (4) not demented [1]. Unfortunately, the operationalization of these criteria, particularly for objective cognitive impairment, differs widely across research groups.
Individual investigators may rely on clinician judgment [13], global assessment tools [14, 15], and/or formal neuropsychological testing [7, 16] to diagnose MCI [1]. Petersen's original cohort of MCI subjects averaged 1.5 standard deviations (SD) below age-adjusted normative means on memory testing [17]. Most subsequent studies have specified performance ≥1.5 SD below the mean as a threshold for impairment [7, 16, 18], but others have used cutoffs of ≥1.0 SD [19, 20]. Individuals diagnosed with MCI may need to demonstrate neuropsychological deficits in single tests [7, 21, 22], multiple tests [16], or averaged across cognitive domains [18]. Subjects subsequently diagnosed with AD are most frequently characterized by deficits in episodic memory, executive function, and perceptual speed [23]. However, the specific tests that best predict progression to AD vary across studies [21, 22,24,25,26,27,28], and subjects demonstrate differential impairments on tests putatively assessing the same cognitive domains [29].
Since MCI subjects with incipient AD exhibit consistent cognitive impairments [30, 31], another approach to optimizing the operationalization of the criterion for objective cognitive impairment is to focus on deficits that remain persistent across longitudinal assessments. The exploratory analyses presented here sought to determine whether commonly used neuropsychological tests effectively identify persistent cognitive impairments, and whether increasing the stringency of the operational definition of MCI improves the persistence of identified impairments. We were specifically interested in whether subjects fulfilling different arbitrary criteria for impaired performance on cognitive testing at baseline continued to exhibit similar impairments at follow-up assessment.
Methods
Research Participants
Subjects were drawn from a larger group of participants enrolled in an ongoing longitudinal and cross-sectional study through the University of California Los Angeles (UCLA) Alzheimer's Disease Research Center (ADRC). Participants meeting criteria for MCI were initially recruited from patients assessed in the UCLA Memory Disorders Clinic. Normal controls included individuals initially seen in the Memory Disorders Clinic and individuals recruited from the community who consistently performed in the normal range on neuropsychological assessments, irrespective of subjective cognitive complaints. Written consent, approved by the UCLA Institutional Review Board, was obtained from each subject.
Inclusion criteria included a multi-disciplinary evaluation resulting in a diagnosis of MCI or normal cognition and a subsequent follow-up evaluation. Exclusion criteria included: (1) age <50, (2) diagnosis of dementia by the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) criteria [32] or diagnosis of AD by the National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA) criteria [33], (3) MRI or CT of the brain demonstrating any major focal lesions (mild to moderate microvascular ischemic changes or isolated lacunes noted on clinical neuroradiology reports were permitted), (4) abnormal vitamin B12 or thyroid function tests, (5) premorbid history of DSM-IV Axis I psychiatric disorders [32], and (6) clinically significant neurological disease, systemic illnesses, or unstable medical conditions that could contribute to impaired cognition.
Evaluations included formal neuropsychological testing, physician interview, and neurological examination. MCI was a consensus diagnosis based on the modified Petersen criteria: (1) subjective cognitive complaint, (2) essentially intact activities of daily living, (3) objective cognitive impairment, and (4) not demented [1]. Cognitive performance in the domains of memory, attention, language, visuospatial, and executive function was assessed using the tests listed in table 1. All tests were singly administered and scored by psychologists and psychometrists under the supervision of the director of the UCLA ADRC Neuropsychology Laboratory. Subjects were considered cognitively impaired if their performance on at least 1 test in any domain was ≥1.5 SD below published age- and education-adjusted normative means [34,35,36,37,38,39,40,41,42]. MCI was retrospectively sub-classified as amnestic or non-amnestic based on the presence or absence of memory impairment. Global cognitive functioning was assessed using the Mini-Mental Status Examination (MMSE) [43]. Physician and psychologist/psychometrist assessments included questions directed to subjects and/or their informants regarding performance of basic and instrumental activities of daily living. The presence of essentially intact activities of daily living was determined through consensus clinician judgment during a subsequent multi-disciplinary conference. Depression was assessed using the Geriatric Depression Scale (GDS) [44]. A subset of subjects was assessed with the Wechsler Adult Intelligence Scale, third edition (WAIS-III) Vocabulary subtest [38], which was used to estimate premorbid intelligence [45].
Table 1.
Neuropsychological tests used to assess specific cognitive domains
| Cognitive domain | Neuropsychological tests |
|---|---|
| Memory | |
| Attention | |
| Language | |
| Visuo-spatial | |
| Executive | |
WMS-R/III = Wechsler Memory Scale, revised or third edition; WAIS-III = Wechsler Adult Intelligence Scale, third edition; COWAT = Controlled Oral Word Association Test.
WMS-R was administered to 5 subjects at baseline (2 NC, 2 AMN, 1 NON) and 8 subjects at follow-up (2 NC, 3 AMN, 3 NON). All other WMS testing was conducted with WMS-III.
Using these criteria, we retrospectively identified 115 subjects meeting criteria for MCI at baseline. Sixty-three subjects were diagnosed with amnestic MCI (AMN group). Thirty-seven AMN subjects exhibited impaired performance in at least 1 additional cognitive domain (AMN-MD subgroup). Fifty-two subjects were diagnosed with non-amnestic MCI (NON group); 41 had deficits limited to a single non-memory domain and 11 had deficits in multiple non-memory domains. Forty-one subjects exhibited normal performance on initial and follow-up neuropsychological testing and served as normal controls (NC group). The average interval between the initial assessment and the next follow-up visit was 16.4 months across all subjects (range 5–54 months). Subjects were re-evaluated at consensus conference after follow-up assessment to determine whether they had reverted to normal cognition, remained MCI, or progressed to possible or probable AD [33] or other dementing conditions. Baseline characteristics of longitudinally evaluated subjects were compared to 51 MCI (35 amnestic, 16 non-amnestic) and 17 NC subjects in our database who had been cross-sectionally evaluated to determine whether study participants were representative of the larger population of subjects at the UCLA ADRC.
Data Analysis
Statistical analyses were performed using SPSS 15.0 for Windows (SPSS Inc., Chicago, Ill., USA). Demographic and neuropsychological data were analyzed using Kruskal-Wallis tests and post-hoc Mann-Whitney U tests for dichotomous variables and one-way analyses of variance (ANOVAs) with Bonferroni correction for continuous variables. The persistence of baseline cognitive deficits (i.e. continued performance ≥1.5 SD below adjusted normative means) was determined by repeat neuropsychological testing and calculated for: (1) any deficit, (2) domain-specific deficits, and (3) test-specific deficits. Primary analyses incorporated stepwise logistic regression (adjusted for gender, race, baseline MMSE scores, and length of follow-up interval), which was used to determine which baseline neuropsychological impairments (previously adjusted for age and education) predicted persistent deficits at follow-up. Longitudinal change in performance in specific cognitive domains was examined using repeated-measures ANOVAs analyses of z-scores calculated from published age- and education-adjusted normative data [34,35,36,37,38,39,40,41,42]. For several tests, there were small numbers of missing data at baseline or follow-up (ranging from 0 to 7); subjects who had missing data were excluded from these analyses.
One approach to reduce the risk of false positives associated with using multiple neuropsychological tests to diagnose MCI [46,47,48] is to increase the number and/or severity of impaired performances necessary to constitute a significant deficit [49]. For the AMN group, we applied 2 more stringent criteria: (1) performance ≤–1.5 SD on ≥2 memory tests [16] and (2) average z-score ≤–1.5 across all 4 memory tests [18]. For the NON group, we applied an additional criterion of performance ≤–1.5 SD on ≥2 non-memory tests [16]. We investigated the prevalence, persistence, and longitudinal course of deficits identified with these more stringent criteria.
Results
Baseline Demographic and Neuropsychological Variables
Baseline demographic data are shown in table 2. The AMN group was significantly older (p < 0.01) and had significantly lower MMSE scores (p ≤ 0.001) than the NON and NC groups. GDS scores were significantly higher in the AMN group than in the NC group (p = 0.031), but there was no correlation between baseline GDS and memory z-scores in subjects with memory impairment [r(60) = 0.067; p = 0.61]. There were no differences between groups in gender distribution, years of formal education, or follow-up interval. Our subjects were predominantly Caucasian (90.4%), but racial distribution differed across groups [χ2(2,156) = 6.13, p = 0.047]. Post-hoc analyses revealed a significantly lower percentage of Caucasian subjects in the NON group relative to the NC group [Z(93) = −2.29, p = 0.022]. There was no difference in racial composition between the AMN and NON groups [Z(115) = −0.152, p = 0.128]. Race did not affect the persistence of amnestic [χ2(1,63) = 0.13, p = 0.71] or non-amnestic [χ2(1,52) = 0.02, p = 0.89] deficits.
Table 2.
Baseline demographic variables
| NC | AMN | NON | χ2(2,156)/F(2,153)1 | |
|---|---|---|---|---|
| Number | 41 | 63 | 52 | |
| M/F | 24/17 | 37/26 | 34/18 | 0.53 |
| Age, years (SD) | 68.6 (8.3)a | 73.6 (7.0)b | 68.4 (8.8)a | 7.72∗ |
| Education, years (SD) | 17.0 (2.9) | 16.5 (2.9) | 16.8 (2.8) | 0.38 |
| MMSE (SD) | 29.4 (0.6)a | 27.5 (2.1)b | 28.7 (1.8)a | 16.21∗ |
| GDS (SD) | 4.1 (3.7)a | 6.7 (5.7)b | 4.9 (4.8)a, b | 3.78∗ |
| Caucasian, % | 97.6a | 92.1a, b | 82.7b | 6.13∗ |
| Follow-up, months (SD) | 16.2 (6.1) | 17.1 (9.2) | 15.6 (5.5) | 0.63 |
p < 0.05. Groups denoted by different superscript letters differ by p < 0.05 after Bonferroni correction.
Degrees of freedom for GDS = 150 due to missing data for 3 subjects (2 AMN, 1 NON).
A subset of 113 subjects (47 AMN, 34 NON, 32 NC) underwent baseline assessment with the WAIS-III Vocabulary subtest. There was a significant effect of group [F(2,110) = 12.21, p < 0.001]; mean scores in the NC group (14.16; SD = 1.44) were significantly higher than in the AMN (11.87; SD = 2.22; p < 0.001) and NON (12.76; SD = 2.18; p = 0.018) groups. Vocabulary scores were similar in the 2 MCI groups (p = 0.16), and scaled scores in all groups were above normative means [38].
There were few differences between longitudinally and cross-sectionally assessed subjects. These 2 cohorts included similar percentages of AMN, NON and NC subjects [χ2(2,225) = 3.18, p = 0.20]. Amnestic MCI subjects assessed multiple times had more years of formal education than those assessed only once [16.5 vs. 15.0; t(97) = 2.54, p = 0.013]. Longitudinal NC subjects performed significantly better than their cross-sectional counterparts on the Wechsler Memory Scale (WMS) revised or third edition (R/III) Logical Memory II [LM-II; z-score: 1.49 vs. 0.76; t(56) = 3.40, p = 0.001]. There were no other differences in demographics or neuropsychological testing between the longitudinal and cross-sectional cohorts (p > 0.05).
Persistence of Memory Impairments in Amnestic MCI
At follow-up, 76% of AMN subjects demonstrated objective cognitive impairment and 73% continued to perform poorly on ≥1 memory test. AMN subjects with persistent memory deficits had lower baseline MMSE scores than subjects whose memory performance reverted to normal [27.0 vs. 28.8; t(61) = 3.21, p = 0.002]. AMN-MD subjects (31/37, 84%) were more likely to exhibit persistent memory deficits than AMN subjects with isolated memory deficits [15/26, 58%; χ2(1,63) = 5.28, p = 0.022]. Baseline demographic variables, length of follow-up interval, GDS, and WAIS-III Vocabulary scores did not distinguish between subjects with persistent versus unstable memory deficits (p > 0.1).
More rigorous operational definitions of amnestic MCI dramatically reduced the prevalence of memory deficits: 28 subjects (44%) were impaired on ≥2 memory tests and 18 subjects (29%) had average memory z-scores ≤−1.5. However, a moderate increase in persistence of these deficits was observed (fig. 1a). At follow-up, subjects with baseline deficits in multiple memory tests were significantly more likely than subjects with baseline deficits limited to a single memory test to exhibit impaired performance on any memory test [96 vs. 54%; χ2(1, 63) = 14.02, p < 0.001]. Likewise, subjects with average memory z-scores ≤−1.5 were more likely than subjects with average memory z-scores >−1.5 to exhibit any memory deficits at follow-up [100 vs. 61%; χ2(1,62) = 9.58, p = 0.002]. These findings remained robust after including the presence of any baseline deficits in attention, language, visuospatial, or executive tasks as covariates (p ≤ 0.005).
Fig. 1.
Persistence of memory deficits (a), average memory z-scores across baseline and follow-up assessments (b), and rates of progression to AD (c) in amnestic MCI subjects identified with increasingly stringent performance criteria. AMN = Subjects with baseline deficit in ≥1 memory test(s); ≥2 Imp Tests = subjects with baseline deficits in ≥2 memory tests; Avg ≤ −1.5 = subjects with a baseline average memory z-score ≤ −1.5. Error bars represent standard error of the mean.
More stringent criteria also identified subjects who demonstrated stable or worsening performance across assessments (fig. 1b). Average memory z-scores in the NC and AMN groups improved across assessments [F(1,99) = 6.64, p = 0.011], but there was no group × assessment interaction [F(1,99) = 0.50, p = 0.482]. When the NC group was compared to AMN subjects with baseline deficits in ≥2 memory tests, a marginally significant group × assessment interaction emerged [F(1,66) = 2.79, p = 0.099], as this subset of AMN subjects exhibited largely stable overall memory scores. When the NC group was compared to AMN subjects with average baseline memory z-scores ≤−1.5, the group × assessment interaction term became significant [F(1,56) = 6.50, p = 0.014], suggesting a relative deterioration of memory performance among these AMN subjects. Eight AMN subjects (13%) progressed to clinical AD at follow-up: 6 AMN-MD subjects (16%) and 2 AMN subjects with isolated memory deficits (8%). More stringent criteria for memory impairment resulted in numerically higher rates of progression to AD (fig. 1c) that failed to reach significance (p > 0.1).
There were significant overall differences in the prevalence of impairments on individual memory tests at baseline [LM-II: 29%; Visual Reproduction II (VR-II): 41%; California Verbal Learning Test (CVLT): 60%; Rey Complex Figure Test-Recall (RCFT-R): 55%; F(3,183) = 5.35, p = 0.001]. AMN subjects were more frequently impaired on the CVLT (p = 0.001) and RCFT-R (p = 0.011) than on LM-II.
The persistence of cognitive deficits in the AMN group at the follow-up evaluation was related to baseline performance on individual memory tests (fig. 2). Impaired performance on VR-II [odds ratio (OR) = 22.32; 95% confidence interval (CI) = 2.34−213.40; p = 0.007] or the CVLT (OR = 7.94; CI = 1.45−43.31; p = 0.017) was independently associated with impaired memory performance at follow-up (R2 = 0.535).
Fig. 2.
Persistence of deficits in the AMN group relative to baseline memory deficits and specificity of impairment at follow-up. Error bars represent standard error of the mean.
Persistence of Neuropsychological Testing Deficits in Non-Amnestic MCI
At follow-up, only 60% of NON subjects demonstrated objective cognitive impairment and only 58% continued to perform poorly on ≥1 non-memory test. Baseline demographic variables, length of follow-up interval, MMSE, GDS, and WAIS-III Vocabulary scores were similar between subjects with persistent versus unstable non-memory deficits (all p > 0.1).
There were significant differences in the prevalence of domain-specific deficits in the NON group [fig. 3a; F(3,153) = 5.67, p = 0.001]. Executive function was more frequently impaired than attention (p = 0.009) or language (p = 0.004). Impairment in individual tests in specific non-memory domains was highly variable (table 3). Fourteen subjects (27%) were impaired on ≥2 non-memory tests. Four subjects (8%) were impaired on multiple tests within a single non-memory domain.
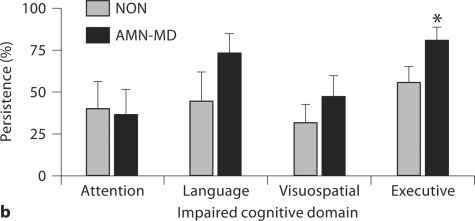
Fig. 3.
Prevalence (a) and persistence (b) of baseline impairment in non-memory cognitive domains in nonamnestic (NON) and multiple-domain amnestic (AMN-MD) MCI subjects. ∗ p < 0.05 vs. NON group; ∗∗ p < 0.05 vs. attention and language. Error bars represent standard error of the mean.
Table 3.
Prevalence and persistence of non-amnestic neuropsychological testing deficits in the NON group
| Prevalence, % | Persistence of impairment at follow-up, % |
|||
|---|---|---|---|---|
| any non-amnestic | specific domain | specific test | ||
| Attention (n = 10) | ||||
| Digit span | 0 | – | – | – |
| Digit symbol | 0 | – | – | – |
| TMT-A | 100 | 80 | 40 | 40 |
| Language (n = 9) | ||||
| BNT | 56 | 40 | 40 | 40 |
| Animals | 44 | 75 | 50 | 50 |
| Visuospatial (n = 19) | ||||
| Block design | 11 | 100 | 50 | 0 |
| RCFT-C | 95 | 44 | 33 | 33 |
| Executive (n = 27) | ||||
| TMT-B | 63 | 88 | 65 | 65 |
| Stroop C | 33 | 56 | 56 | 44 |
| COWAT | 19 | 80 | 60 | 40 |
The number of NON subjects that demonstrated impaired performance within a specific cognitive domain at baseline is indicated in parentheses. TMT-A = Trail Making Test, part A; BNT = Boston Naming Test; RCFT-C = Rey Complex Figure Test-Copy; TMT-B = Trail Making Test, part B; COWAT = Controlled Oral Word Association Test.
Baseline deficits in attention (OR = 8.11; CI = 1.16−56.54; p = 0.035) or executive function (OR = 7.81; CI = 1.86−32.74; p = 0.005) were independently associated with any non-amnestic impairment at follow-up (R2 = 0.335). The persistence of domain-specific (fig. 3b) and test-specific (table 3) non-amnestic deficits was quite low. Subjects with baseline deficits in ≥2 non-memory tests were more likely than subjects with baseline deficits in a single non-memory test to be impaired on any non-memory test at follow-up [86 vs. 47%; χ2(1,52) = 6.16, p = 0.013]. However, the persistence of impaired performance using this more stringent criterion remained poor (64%). Only 1 NON subject (2%) progressed to AD at time of follow-up.
Persistence of Non-Memory Deficits in Multiple-Domain Amnestic MCI
The poor persistence of deficits in the NON group prompted further investigation of whether non-amnestic deficits might exhibit greater persistence when seen in conjunction with memory dysfunction. At baseline, AMN-MD subjects were significantly more likely to have language deficits [χ2(1,89) = 5.92, p = 0.015] and marginally more likely to have executive deficits [χ2(1,89) = 3.02, p = 0.082] than NON subjects (fig. 3a). The AMN-MD subgroup was also more likely than the NON group to be impaired on multiple non-memory tests [57 vs. 27%; χ2(1,89) = 8.06, p = 0.005].
Relative to the NON group, the AMN-MD subgroup exhibited marginally greater persistence for any non-memory deficit [76 vs. 58%; χ2(1,89) = 3.08, p = 0.079]. However, when the more stringent criterion for non-amnestic impairment was applied, the persistence of non-memory deficits did not differ between these 2 groups [86 vs. 64%; χ2(1,35) = 2.19, p = 0.139]. When impairments in specific non-memory domains were considered, the AMN-MD subgroup exhibited significantly greater persistence for executive deficits than the NON group [fig. 3b; χ2(1,53) = 3.86, p = 0.049].
Discussion
The data presented here demonstrate that the use of different neuropsychological tests and thresholds for impairment in the diagnosis of MCI identifies subsets of subjects with different rates of persistent cognitive impairment. The prevalence of impaired performance varied widely across individual tests. Amnestic deficits were more persistent than non-amnestic deficits. Baseline deficits in VR-II and the CVLT were the best predictors of persistent memory impairment. Subjects who at baseline were impaired on multiple memory tests or had poorer overall memory performance were more likely to exhibit persistent memory deficits. Our work confirms and extends previous findings indicating that different methods of operationalizing ‘objective cognitive impairment for age and education’ for the Petersen criteria result in the identification of heterogeneous and incompletely overlapping subject populations [29].
The tests included in our neuropsychological battery exhibited significant variability in their ability to detect deficits, implying that the different assessments used to define MCI impact the composition of individual study populations. In particular, inclusion criteria for several previous clinical trials for MCI required impaired performance on delayed story recall tests similar to LM-II [50]. Although deficits in LM-II are likely to reflect persistent memory impairment, relatively few AMN subjects performed poorly on this test, which may reflect the more structured context in which information to be remembered is presented and/or the broad normative ranges reported for older subjects, particularly on the WMS-III, which was administered to the majority of our subjects [34, 35].
Cognitive deficits were more persistent in the AMN group than in the NON group. AMN subjects with deficits in ≥2 memory tests or average memory z-scores ≤−1.5 at baseline demonstrated higher frequencies of stable memory deficits. More stringent criteria for memory impairment may more specifically identify amnestic MCI subjects most likely to have incipient AD. However, increasing the number of impaired tests necessary to constitute a non-amnestic deficit did not appreciably improve the persistence of non-memory impairments. Our data are consistent with the lower rates of reversion to normal cognition [31, 51, 52] and higher rates of progression to AD [6, 15, 51, 53, 54] seen in amnestic MCI relative to non-amnestic MCI. Neuropathological studies indicate that neurofibrillary tangle deposition in medial temporal lobe structures suggestive of incipient AD is seen more frequently in amnestic MCI [55,56,57] than in non-amnestic MCI [58]. Non-memory testing in elderly subjects may be more susceptible to cognitive phenomena associated with normal aging, such as increased intra-individual variability in task performance [59].
There are several possible alternative explanations for the poorer persistence of non-amnestic deficits. Increasing the number of tests in a battery increases the likelihood that performance on at least 1 test will be in the impaired range [49]. The composition of our battery (4 memory tests vs. 10 non-memory tests) may have predisposed the NON group to include a greater proportion of false-positive MCI subjects than the AMN group, and the poorer persistence of deficits in the NON group may reflect regression to the mean. Nevertheless, with the notable exception of the RCFT-R, the persistence of individual test-specific deficits was consistently higher on memory tests than non-memory tests.
Subjects received essentially identical neuropsychological batteries at consecutive assessments; alternate versions were not used. Poorer persistence of deficits in the NON group might have been due to more substantial test-retest improvements in the non-memory tests. However, analyses of test-retest effects in the NC group using paired t tests revealed significant improvement in composite memory z-scores [baseline: mean = 1.18, SD = 0.60; follow-up: mean = 1.41, SD = 0.64; t(39) = 2.68; p = 0.011] but not on composite non-memory z-scores [baseline: mean = 0.87, SD = 0.37; follow-up: mean = 0.90, SD = 0.41; t(39) = 0.66; p = 0.51].
Although the importance of isolated non-amnestic deficits remains uncertain, the combined presence of both amnestic and non-amnestic deficits appears to be a marker of more advanced incipient AD. AMN-MD subjects were more likely to have persistent memory impairments than AMN subjects with isolated memory deficits, and more likely to have persistent non-memory deficits than NON subjects. These results are consistent with prior work demonstrating that progression rates to AD are highest in multiple-domain amnestic MCI [18, 27, 53, 54, 60, 61]. Alternatively, it remains possible that our baseline assessment of functional decline was insufficiently sensitive and some AMN-MD subjects were already mildly demented.
VR-II and the CVLT were the best predictors of persistent memory deficits. Our findings are consistent with previous work in MCI demonstrating that poorer performance on Visual Reproduction [21, 26] or delayed recall of word lists [22, 25] is associated with subsequent AD. Conversely, although some investigators have advocated the use of the RCFT-R for the identification of amnestic MCI [29, 62], and a significant proportion of AMN subjects exhibited baseline deficits in this task, the persistence of these deficits was poorer than that of the other memory measures, suggesting that the RCFT-R may be less useful for identifying incipient AD.
AMN subjects who performed poorly at baseline on the RCFT-R also performed poorly at baseline on the copy portion of the Rey Complex Figure Test [RCFT-C; χ2(1,62) = 5.06, p = 0.025], but exhibited similar improvements across assessments on both tasks. Poor performance on the RCFT-R may be due in part to deficits in visuospatial function, and poor persistence of RCFT-R deficits may mirror the poor persistence of visuospatial deficits in our MCI population. However, baseline visual memory deficits in both the RCFT-R and VR-II remained robust after correction for visuospatial performance (RCFT-C, Visual Reproduction I) using encoding scores [63].
Our administration of the RCFT-R incorporated a 3-min delay, which is shorter than the delays used in the LM-II, VR-II, and CVLT tasks. Although other studies of the RCFT-R in MCI populations have used longer delays [29, 62], performance on this task appears to be stable across delays ranging up to 60 min [45]. The RCFT-R also differs from the other memory tests in our battery, at least during subjects’ initial exposure, because it represents an incidental rather than intentional memory task. In subsequent administrations, the RCFT-R likely becomes an intentional memory task given subjects’ prior experience [64]. This may result in improved performance, particularly in older populations [65]. Impaired performance on the initial administration of this test may therefore be more indicative of poor attention and/or motivation than poor memory [64].
Since participants with MCI in this study were recruited from patients initially seen in the UCLA Memory Disorders Clinic, the base rate of underlying AD is likely to be higher in our sample than in the general elderly population [66]. Therefore, both the prevalence and persistence of deficits in our neuropsychological battery are also likely to be elevated in our MCI cohort relative to an unselected group of elderly individuals. Conversely, setting the threshold for impaired performance on individual tests at ≥1.5 SD below normative means results in approximately 7% of subjects with normal cognition appearing to exhibit deficits. The inclusion of such subjects in our MCI group may underestimate the true persistence of deficits in specific measures due to regression to the mean.
In evaluating for MCI, it is important to weigh the possible outcomes attached to false positive versus false negative errors. False positive diagnoses carry the potentially negative consequences associated with being ‘labeled’ with a cognitive disorder. Alternatively, if the criteria are excessively stringent, they may fail to identify subjects with incipient AD. Our data suggest that if a single memory test is used to diagnose amnestic MCI, VR-II, delayed recall of the CVLT, or similar visual or verbal memory paradigms achieve the optimal balance between these undesirable outcomes. The accuracy of clinical evaluations based on neuropsychological assessments may be further improved through the addition of biochemical and neuroimaging biomarkers [67]. The poor persistence of non-memory deficits in this study indicates that the optimal operationalization of non-amnestic MCI remains uncertain and further investigations are necessary to clarify the clinical significance of such impairments.
A number of factors may limit the interpretation of our results. Clinical diagnoses of MCI were not determined independently of subjects’ neuropsychological performance. Additionally, the cutoffs used to define impaired performance on each test were obtained from different published normative samples. Therefore, the relative prevalence of impairments on individual tests in our battery may not consistently reflect the utility of each test for diagnosing MCI. The interval between neuropsychological assessments was relatively short (16.4 months) and varied somewhat between individual subjects. However, the annualized progression rate from MCI to AD in the AMN group was 8.9%, comparable to that reported across other longitudinal studies [2], and reversion rates from MCI to normal cognition appeared to be relatively insensitive to length of follow-up both in the present study and in previous reports [68, 69]. Relatively few subjects were impaired on specific non-memory tests, raising the possibility that this study may be insufficiently powered to evaluate the persistence of deficits on these assessments. Nevertheless, persistence of non-memory deficits remained poor even when impairment in any test was considered. Practice effects and test-retest improvement may also have affected performance in our MCI subjects, potentially reducing the observed persistence of baseline cognitive deficits. However, previous work suggests that although normal aged control subjects demonstrate significant improvement when administered an identical verbal learning test over an interval of 2 to 3 years, subjects with MCI or AD do not [70]. Our study population was primarily Caucasian. There was a relatively higher proportion of non-Caucasian subjects in the NON group, consistent with previous work demonstrating poorer performance on non-memory neuropsychological testing in non-Caucasian samples [71, 72]. Thus, our results may not be generalizable to ethnically diverse populations, which may require assessment with specific community-based norms [73]. The NC group consisted of individuals who consistently demonstrated normal cognition to minimize the inclusion of subjects with incipient cognitive impairment. These criteria are likely to have selected for subjects with higher premorbid level of function and greater cognitive reserve, which may protect against cognitive decline and limit the generalizability of their results to a broader elderly population.
Our current findings add to the existing literature on the neuropsychological assessment of MCI by assessing the clinical utility of several common neuropsychological measures for the identification of persistent cognitive deficits. Ideally, these data will contribute to improving the operational definition of this heterogeneous condition. Additional work in larger populations of MCI subjects should focus on developing a consensus neuropsychological battery that yields predictable rates of progression to AD and low rates of reversion to normal cognition.
Acknowledgements
This research was supported by the National Institute on Aging (P50 AG 16570), the Alzheimer's Disease Research Centers of California, and the Sidell-Kagan Foundation. We would like to thank Charmaine Lowe, Myha Ngo, Alice Yau, and Michelle Zeller for their assistance with data management.
References
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Bruscoli M, Lovestone S. Is MCI really just early dementia? A systematic review of conversion studies. Int Psychogeriatr. 2004;16:129–140. doi: 10.1017/s1041610204000092. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 4.Solfrizzi V, Panza F, Colacicco AM, D'Introno A, Capurso C, Torres F, Grigoletto F, Maggi S, Del Parigi A, Reiman EM, Caselli RJ, Scafato E, Farchi G, Capurso A. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–1891. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- 5.Geslani DM, Tierney MC, Herrmann N, Szalai JP. Mild cognitive impairment: an operational definition and its conversion rate to Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;19:383–389. doi: 10.1159/000084709. [DOI] [PubMed] [Google Scholar]
- 6.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Pantieri G, Mariani E. Conversion of mild cognitive impairment to dementia: predictive role of mild cognitive impairment subtypes and vascular risk factors. Dement Geriatr Cogn Disord. 2006;21:51–58. doi: 10.1159/000089515. [DOI] [PubMed] [Google Scholar]
- 7.Kryscio RJ, Schmitt FA, Salazar JC, Mendiondo MS, Markesbery WR. Risk factors for transitions from normal to mild cognitive impairment and dementia. Neurology. 2006;66:828–832. doi: 10.1212/01.wnl.0000203264.71880.45. [DOI] [PubMed] [Google Scholar]
- 8.Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41:1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- 9.Luis CA, Loewenstein DA, Acevedo A, Barker WW, Duara R. Mild cognitive impairment: directions for future research. Neurology. 2003;61:438–444. doi: 10.1212/01.wnl.0000080366.90234.7f. [DOI] [PubMed] [Google Scholar]
- 10.Visser PJ, Scheltens P, Verhey FR, Schmand B, Launer LJ, Jolles J, Jonker C. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. J Neurol. 1999;246:477–485. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- 11.Visser PJ, Kester A, Jolles J, Verhey F. Ten-year risk of dementia in subjects with mild cognitive impairment. Neurology. 2006;67:1201–1207. doi: 10.1212/01.wnl.0000238517.59286.c5. [DOI] [PubMed] [Google Scholar]
- 12.Busse A, Angermeyer MC, Riedel-Heller SG. Progression of mild cognitive impairment to dementia: a challenge to current thinking. Br J Psychiatry. 2006;189:399–404. doi: 10.1192/bjp.bp.105.014779. [DOI] [PubMed] [Google Scholar]
- 13.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 14.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stageAlzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 15.DeCarli C, Mungas D, Harvey D, Reed B, Weiner M, Chui H, Jagust W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez OL, Jagust WJ, DeKosky ST, Becker JT, Fitzpatrick A, Dulberg C, Breitner J, Lyketsos C, Jones B, Kawas C, Carlson M, Kuller LH. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 17.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 18.Manly JJ, Tang MX, Schupf N, Stern Y, Vonsattel JP, Mayeux R. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol. 2008;63:494–506. doi: 10.1002/ana.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001;56:37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 20.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 21.Griffith HR, Netson KL, Harrell LE, Zamrini EY, Brockington JC, Marson DC. Amnestic mild cognitive impairment: diagnostic outcomes and clinical prediction over a two-year time period. J Int Neuropsychol Soc. 2006;12:166–175. doi: 10.1017/S1355617706060267. [DOI] [PubMed] [Google Scholar]
- 22.Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, Zamora D, Goodkind M, Bell K, Stern Y, Devanand DP. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 23.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer's disease: a meta-analysis. Neuropsychology. 2005;19:520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 24.Kluger A, Ferris SH, Golomb J, Mittelman MS, Reisberg B. Neuropsychological prediction of decline to dementia in nondemented elderly. J Geriatr Psychiatry Neurol. 1999;12:168–179. doi: 10.1177/089198879901200402. [DOI] [PubMed] [Google Scholar]
- 25.Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Cognitive tests that best discriminate between presymptomatic AD and those who remain nondemented. Neurology. 2000;55:1847–1853. doi: 10.1212/wnl.55.12.1847. [DOI] [PubMed] [Google Scholar]
- 26.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 27.Ahmed S, Mitchell J, Arnold R, Nestor PJ, Hodges JR. Predicting rapid clinical progression in amnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;25:170–177. doi: 10.1159/000113014. [DOI] [PubMed] [Google Scholar]
- 28.Sarazin M, Berr C, De Rotrou J, Fabrigoule C, Pasquier F, Legrain S, Michel B, Puel M, Volteau M, Touchon J, Verny M, Dubois B. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69:1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- 29.Alladi S, Arnold R, Mitchell J, Nestor PJ, Hodges JR. Mild cognitive impairment: applicability of research criteria in a memory clinic and characterization of cognitive profile. Psychol Med. 2006;36:507–515. doi: 10.1017/S0033291705006744. [DOI] [PubMed] [Google Scholar]
- 30.Backman L, Small BJ, Fratiglioni L. Stability of the preclinical episodic memory deficit in Alzheimer's disease. Brain. 2001;124:96–102. doi: 10.1093/brain/124.1.96. [DOI] [PubMed] [Google Scholar]
- 31.Hodges JR, Erzinclioglu S, Patterson K. Evolution of cognitive deficits and conversion to dementia in patients with mild cognitive impairment: a very-long-term follow-up study. Dement Geriatr Cogn Disord. 2006;21:380–391. doi: 10.1159/000092534. [DOI] [PubMed] [Google Scholar]
- 32.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, ed 4. Washington: American Psychiatric Association; 1994. [Google Scholar]
- 33.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 34.Wechsler D. Wechsler Memory Scale, Revised Edition: Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1981. [Google Scholar]
- 35.Wechsler D. Wechsler Memory Scale: Administration and Scoring Manual, ed 3. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 36.Delis DC, Kramer JH, Kaplan E, Ober B. The California Verbal Learning Test, ed 2. San Antonio: The Psychological Corporation; 2000. [Google Scholar]
- 37.Meyers J, Meyers K. Rey Complex Figure and Recognition Trial: Professional Manual. Odessa: Psychological Assessment Resources, Inc.; 1995. [Google Scholar]
- 38.Wechsler D. Wechsler Adult Intelligence Scale, ed 3: Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 39.Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 40.Tombaugh TN, Hubley AM. The 60-item Boston Naming Test: norms for cognitively intact adults aged 25 to 88 years. J Clin Exp Neuropsychol. 1997;19:922–932. doi: 10.1080/01688639708403773. [DOI] [PubMed] [Google Scholar]
- 41.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- 42.Demick J, Harkins D. American Association of Retired Persons Andrus Foundation Final Grant Report. Washington: American Association of Retired Persons; 1997. Role of cognitive style in the driving skills of young, middle-aged, and older adults. [Google Scholar]
- 43.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 44.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 45.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment, ed 4. New York: Oxford University Press; 2004. [Google Scholar]
- 46.de Rotrou J, Wenisch E, Chausson C, Dray F, Faucounau V, Rigaud AS. Accidental MCI in healthy subjects: a prospective longitudinal study. Eur J Neurol. 2005;12:879–885. doi: 10.1111/j.1468-1331.2005.01100.x. [DOI] [PubMed] [Google Scholar]
- 47.Loewenstein DA, Acevedo A, Ownby R, Agron J, Barker WW, Isaacson R, Strauman S, Duara R. Using different memory cutoffs to assess mild cognitive impairment. Am J Geriatr Psychiatry. 2006;14:911–919. doi: 10.1097/01.JGP.0000229651.62137.e2. [DOI] [PubMed] [Google Scholar]
- 48.Palmer BW, Boone KB, Lesser IM, Wohl MA. Base rates of ‘impaired’ neuropsychological test performance among healthy older adults. Arch Clin Neuropsychol. 1998;13:503–511. [PubMed] [Google Scholar]
- 49.Ingraham LJ, Aiken CB. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology. 1996;10:120–124. [Google Scholar]
- 50.Jelic V, Kivipelto M, Winblad B. Clinical trials in mild cognitive impairment: lessons for the future. J Neurol Neurosurg Psychiatry. 2006;77:429–438. doi: 10.1136/jnnp.2005.072926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fischer P, Jungwirth S, Zehetmayer S, Weissgram S, Hoenigschnabl S, Gelpi E, Krampla W, Tragl KH. Conversion from subtypes of mild cognitive impairment to Alzheimer dementia. Neurology. 2007;68:288–291. doi: 10.1212/01.wnl.0000252358.03285.9d. [DOI] [PubMed] [Google Scholar]
- 52.Loewenstein DA, Acevedo A, Agron J, Duara R. Stability of neurocognitive impairment in different subtypes of mild cognitive impairment. Dement Geriatr Cogn Disord. 2007;23:82–86. doi: 10.1159/000097304. [DOI] [PubMed] [Google Scholar]
- 53.Rasquin SM, Lodder J, Visser PJ, Lousberg R, Verhey FR. Predictive accuracy of MCI subtypes for Alzheimer's disease and vascular dementia in subjects with mild cognitive impairment: a 2-year follow-up study. Dement Geriatr Cogn Disord. 2005;19:113–119. doi: 10.1159/000082662. [DOI] [PubMed] [Google Scholar]
- 54.Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67:2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell TW, Mufson EJ, Schneider JA, Cochran EJ, Nissanov J, Han LY, Bienias JL, Lee VM, Trojanowski JQ, Bennett DA, Arnold SE. Parahippocampal tau pathology in healthy aging, mild cognitive impairment, and early Alzheimer's disease. Ann Neurol. 2002;51:182–189. doi: 10.1002/ana.10086. [DOI] [PubMed] [Google Scholar]
- 56.Guillozet AL, Weintraub S, Mash DC, Mesulam MM. Neurofibrillary tangles, amyloid, and memory in aging and mild cognitive impairment. Arch Neurol. 2003;60:729–736. doi: 10.1001/archneur.60.5.729. [DOI] [PubMed] [Google Scholar]
- 57.Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Jicha GA, Ivnik RJ, Smith GE, Tangalos EG, Braak H, Kokmen E. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 58.Riley KP, Snowdon DA, Markesbery WR. Alzheimer's neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002;51:567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 59.West R, Murphy KJ, Armilio ML, Craik FI, Stuss DT. Lapses of intention and performance variability reveal age-related increases in fluctuations of executive control. Brain Cogn. 2002;49:402–419. doi: 10.1006/brcg.2001.1507. [DOI] [PubMed] [Google Scholar]
- 60.Bozoki A, Giordani B, Heidebrink JL, Berent S, Foster NL. Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch Neurol. 2001;58:411–416. doi: 10.1001/archneur.58.3.411. [DOI] [PubMed] [Google Scholar]
- 61.Alexopoulos P, Grimmer T, Perneczky R, Domes G, Kurz A. Progression to dementia in clinical subtypes of mild cognitive impairment. Dement Geriatr Cogn Disord. 2006;22:27–34. doi: 10.1159/000093101. [DOI] [PubMed] [Google Scholar]
- 62.Kasai M, Meguro K, Hashimoto R, Ishizaki J, Yamadori A, Mori E. Non-verbal learning is impaired in very mild Alzheimer's disease (CDR 0.5): normative data from the learning version of the Rey-Osterrieth Complex Figure Test. Psychiatry Clin Neurosci. 2006;60:139–146. doi: 10.1111/j.1440-1819.2006.01478.x. [DOI] [PubMed] [Google Scholar]
- 63.Shorr JS, Delis DC, Massman PJ. Memory for the Rey-Osterrieth figure: perceptual clustering, encoding, and storage. Neuropsychology. 1992;6:43–50. [Google Scholar]
- 64.Hubley AM, Tremblay D. Comparability of total score performance on the Rey-Osterrieth Complex Figure and a modified Taylor Complex Figure. J Clin Exp Neuropsychol. 2002;24:370–382. doi: 10.1076/jcen.24.3.370.984. [DOI] [PubMed] [Google Scholar]
- 65.Gagnon M, Awad N, Mertens VB, Messier C. Comparing the Rey and Taylor Complex Figures: a test-retest study in young and older adults. J Clin Exp Neuropsychol. 2003;25:878–890. doi: 10.1076/jcen.25.6.878.16480. [DOI] [PubMed] [Google Scholar]
- 66.Smith GE, Cerhan JH, Ivnik RJ. Diagnostic validity. In: Tulsky DS, Saklofske DH, Chelune GJ, Heaton RK, Ivnik RJ, Bornstein R, Prifitera A, Ledbetter MF, editors. Clinical Interpretation of the WAIS-III and WMS-III. San Diego: Academic Press; 2003. pp. 273–301. [Google Scholar]
- 67.Brys M, Glodzik L, Mosconi L, Switalski R, De Santi S, Pirraglia E, Rich K, Kim BC, Mehta P, Zinkowski R, Pratico D, Wallin A, Zetterberg H, Tsui WH, Rusinek H, Blennow K, de Leon MJ. Magnetic resonance imaging improves cerebrospinal fluid biomarkers in the early detection of Alzheimer's disease. J Alzheimer's Dis. 2009;16:351–362. doi: 10.3233/JAD-2009-0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Visser PJ, Verhey FR, Ponds RW, Cruts M, Van Broeckhoven CL, Jolles J. Course of objective memory impairment in non-demented subjects attending a memory clinic and predictors of outcome. Int J Geriatr Psychiatry. 2000;15:363–372. doi: 10.1002/(sici)1099-1166(200004)15:4<363::aid-gps129>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 69.Larrieu S, Letenneur L, Orgogozo JM, Fabrigoule C, Amieva H, Le Carret N, Barberger-Gateau P, Dartigues JF. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology. 2002;59:1594–1599. doi: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 70.Schrijnemaekers AM, de Jager CA, Hogervorst E, Budge MM. Cases with mild cognitive impairment and Alzheimer's disease fail to benefit from repeated exposure to episodic memory tests as compared with controls. J Clin Exp Neuropsychol. 2006;28:438–455. doi: 10.1080/13803390590935462. [DOI] [PubMed] [Google Scholar]
- 71.Boone KB, Victor TL, Wen J, Razani J, Ponton M. The association between neuropsychological scores and ethnicity, language, and acculturation variables in a large patient population. Arch Clin Neuropsychol. 2007;22:355–365. doi: 10.1016/j.acn.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 72.Manly JJ, Jacobs DM, Touradji P, Small SA, Stern Y. Reading level attenuates differences in neuropsychological test performance between African American and White elders. J Int Neuropsychol Soc. 2002;8:341–348. doi: 10.1017/s1355617702813157. [DOI] [PubMed] [Google Scholar]
- 73.Manly JJ, Bell-McGinty S, Tang MX, Schupf N, Stern Y, Mayeux R. Implementing diagnostic criteria and estimating frequency of mild cognitive impairment in an urban community. Arch Neurol. 2005;62:1739–1746. doi: 10.1001/archneur.62.11.1739. [DOI] [PubMed] [Google Scholar]
- 74.Reitan RM, Wolfson D. Theory and Clinical Interpretation. Tucson, AZ: Neuropsychology Press; 1989. The Halstead-Reitan Neuropsychological Test Battery. [Google Scholar]
- 75.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 76.Benton AL, Hamsher KD. Multilingual Aphasia Examination. Iowa City: AJA Associates; 1989. [Google Scholar]
- 77.Comalli PE, Wapner S, Werner H. Interference effects of Stroop color-word test in childhood, adulthood, and aging. J Genet Psychol. 1962;100:47–53. doi: 10.1080/00221325.1962.10533572. [DOI] [PubMed] [Google Scholar]