Abstract
A dominant paradigm in neurological disease research is that the primary etiological factors for diseases such as Alzheimer’s (AD), Parkinson’s (PD), and amyotrophic lateral sclerosis (ALS) are genetic. Opposed to this perspective are the clear observations from epidemiology that purely genetic casual factors account for a relatively small fraction of all cases. Many who support a genetic etiology for neurological disease take the view that while the percentages may be relatively small, these numbers will rise in the future with the inevitable discoveries of additional genetic mutations. The follow up argument is that even if the last is not true, the events triggered by the aberrant genes identified so far will be shown to impact the same neuronal cell death pathways as those activated by environmental factors that trigger most sporadic disease cases. In this article we present a countervailing view that environmental neurotoxins may be the sole sufficient factor in at least three neurological disease clusters. For each, neurotoxins have been isolated and characterized that, at least in animal models, faithfully reproduce each disorder without the need for genetic co-factors. Based on these data, we will propose a set of principles that would enable any potential toxin to be evaluated as an etiological factor in a given neurodegenerative disease. Finally, we will attempt to put environmental toxins into the context of possible genetically-determined susceptibility.
Keywords: Neurological disease cluster, Atypical parkinsonism, Parkinsonism-dementia complex, ALS, Progressive subranuclear palsy, Guam, Guadeloupe, Cycad, Annonacin, Sterol glucosides , MPTP, Environmental toxins
Introduction
The notion that numerous human diseases arise primarily due to genetic abnormalities is widely accepted. The technical success of the human genome project has served to strengthen this view, albeit without providing much insight into post-sequencing mechanisms of action. In turn, this notion is widely touted in the media, leading to a general perception by the lay public that a widespread genetic basis for human disease causality is an established fact. The general perspective on neurological diseases tends to be similar. In part, this view is bolstered by neurological disorders such as Huntington’s disease that are clearly of genetic origin. The successful identification of mutations associated with familial forms of Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) similarly supports the notion of genetic etiology. Whatever individual scientists may say privately, it is the ‘‘gene’’ perspective that sends the vast bulk of extramural research funding—both public and private—into studies devoted to genetic rather than toxin etiologies.
In spite of this, epidemiology tells a somewhat different story in regard to sporadic cases of the age-dependent neurodegenerative disorders. Although estimates vary, in no case do the known gene mutations account for more than 10% for any of these diseases overall We cannot, however, rule out future discoveries that could serve to bring larger fractions of the sporadic forms of each disease into the genetic causality fold. Similarly, it is undeniable that the cell death cascades evoked by any form of genetic abnormality (i.e. gene mutations, deletions or duplications) could be identical to those triggered in other ways, for example by neurotoxins.
In the following review, we will summarize the evidence for a neurotoxin etiology in three well-established clusters of neurological disease and demonstrate that for each an animal model using the putative neurotoxin can faithfully reproduce the disease state. These outcomes argue in favor of such toxins being the sole necessary and sufficient condition for at least these disease clusters.
The clusters we will consider are the following: (1) The type of parkinsonism associated with the accidental use of MPTP by recreational drug users; (2) atypical parkinsonism of Guadeloupe in the French Antilles; (3) The separate and combined phenotypes of ALS and parkinsonism dementia of the Western Pacific, with a focus on the most studied of these among the Chamorro population of Guam. From these examples, we will try to derive basic principles to serve in the hunt for environmental toxins associated with other forms of sporadic neurodegenerative disease. Last, we will consider the combined effects of modifier genes and environmental toxins.
Environmental Determinants of Neurological Disease: Three Clusters
MPTP-Induced Parkinsonism
In 1983, Langston et al. reported the occurrence of an akinetic rigid syndrome responsive to Levodopa resembling the clinical features of PD in seven individuals after intravenous injection of an illicit synthetic heroin analog that contained high amounts of the by-product 1-methyl 4—phenyl 1,2,3, 6-tetradhydropyridine (MPTP) (Langston et al. 1983; Ballard et al. 1985). Subsequent studies demonstrated that systemic injection of MPTP into non-human primates (Langston et al. 1984a) and mice (Ricaurte et al. 1987) induced an irreversible, selective loss of dopaminergic neurons in the substantia nigra (SN), thus reproducing the main neuropathological hallmark of PD. MPTP is a highly lipophilic molecule (Riachi et al. 1989) and crosses the blood–brain-barrier in a matter of seconds after systemic administration (Markey et al. 1984). Within the brain, MPTP is rapidly converted to the hydrophilic metabolite 1-methyl-4-phenylpyridinium ion (MPP+) (Heikkila et al. 1984; Langston et al. 1984b) which is not lipophilic enough to cross biomembranes spontaneously (Riachi et al. 1989). The demonstration that an inhibition of the conversion of MPTP to MPP+ by monoamine oxidase B prevents MPTP-neurotoxicity, clearly identified MPP+ as the active toxic principle (Heikkila et al. 1984; Langston et al. 1984b). Further work demonstrated that the cellular specificity of MPTP for dopaminergic neurons resulted from the accumulation of MPP+ through uptake by the dopamine transporter (Javitch et al. 1985; Ricaurte et al. 1985; Bezard et al. 1999). Within the cell, MPP+ accumulates in the mitochondrial matrix (Ramsay and Singer 1986; Davey et al. 1992) where it impairs respiration by inhibiting complex I (NADH-ubiquinone oxidoreductase) of the electron transport chain (Nicklas et al. 1985; Singer et al. 1987). The downstream mechanisms of MPP+–induced complex I inhibition that lead ultimately to neuronal cell death and parkinsonism are reviewed elsewhere (Dauer and Przedborski 2003; Dawson and Dawson 2003).
The relevance of MPTP-induced parkinsonism as a model for sporadic PD comes from the demonstration of a similar impairment of mitochondrial complex I in PD (Schapira et al. 1989; Schapira et al. 1990; Mann et al. 1992; Abou-Sleiman et al. 2006; Keeney et al. 2006). The principal differences between PD and MPTP-induced parkinsonism in the routinely used acute and subacute intoxication protocols are the lack of chronic progression of neurodegeneration in experimental animals and the absence of formation of typical Lewy bodies, the protein-aceous inclusion bodies that are characteristic of PD and related disorders (Forno et al. 1993). Recent experimental approaches utilizing chronic MPTP administration protocols appear to demonstrate that MPTP-induced parkinsonism is even closer to ‘‘classical’’ PD in its absence of extra-nigral pathology (Fornai et al. 2005). At the same time, more detailed studies of the timecourse of PD pathology appears to suggest that a more widespread impact on the CNS occurs (Braak et al. 2004). MPTP may thus reflect an extremely selective form of a disease that may have multiple manifestations.
Guadeloupean Atypical Parkinsonism
The inhabitants of Guadeloupe, a French Caribbean island, experience an unusually high prevalence of atypical parkinsonism (Caparros-Lefebvre and Elbaz 1999; Caparros-Lefebvre et al. 2006; Lannuzel et al. 2007) which exceeds that observed in European or North American populations. Approximately one-third of the parkinsonian patients present with classical PD, one-third with a clinical manifestation resembling progressive supranuclear palsy (PSP), and one-third have an undetermined form of parkinsonism (Caparros-Lefebvre and Elbaz 1999; Caparros-Lefebvre et al. 2002; Lannuzel et al. 2007). In three of the patients with PSP-like clinical symptoms who have come to autopsy, neuropathological alterations consistent with PSP were found (Caparros-Lefebvre et al. 2002). The absence of Mendelian inheritance or of mutations in the MAPT or α-synuclein genes, as well as the ethnic heterogeneity of the affected population argues against a genetic causality (Caparros-Lefebvre and Elbaz 1999; Caparros-Lefebvre et al. 2002, 2006; Lannuzel et al. 2007). In contrast, the geographical clustering is consistent with an environmental etiology.
A case-control study (Caparros-Lefebvre and Elbaz 1999) linked atypical parkinsonism on Guadeloupe to the consumption of fruit and medicinal preparations of the leaves of plants of the Annonaceae family, in particular Annona muricata (soursop, corossol) the predominant Annonaceae on Guadeloupe. Associations between the consumption of Annonaceae and atypical parkinsonism have also been reported in New Caledonia (Angibaud et al. 2004) and in patients of Caribbean origin in living in London (Chaudhuri et al. 2000). These associations in widely separated populations with genetically distinct backgrounds further support the hypothesis that toxic compounds contained in Annonaceae may be responsible for the observed neurodegenerative syndrome.
Acetogenins and alkaloids are the two major groups of candidate toxins contained in plants from the Annonaceae family. In vitro, acetogenins are toxic to neurons at concentrations in the nanomolar range, whereas micromolar concentrations of the alkaloids were necessary to produce toxic outcomes (Lannuzel et al. 2002; Lannuzel et al. 2003). For this reason, acetogenins have received greater attention in recent years. Acetogenins penetrate into cells by passive diffusion because of their lipophilic character rather than servng as a substrate for the dopamine transporter (Lannuzel et al. 2003). These observations explain why these compounds are equally toxic to various dopaminergic as well as non-dopamine containing neurons (Lannuzel et al. 2003), acting in both as potent inhibitors of mitochondrial complex I (Degli Esposti 1998). Annonacin, the most abundant acetogenin in Annona muricata, inhibits complex I activity with an IC50 of about 30 nM and kills neurons by ATP-depletion. It is about 50 times more toxic to dopaminergic neurons and 2000 times more toxic to non-dopaminergic neurons than MPP+ (Lannuzel et al. 2003). When administered intravenously to rats for 28 days, annonacin enters the brain parenchyma, decreases brain ATP levels and induces pronounced and widespread neurodegeneration in basal ganglia and brainstem nuclei; hippocampus, cerebellum, and cerebral cortex are only moderately affected (Champy et al. 2004). These outcomes mimic the distribution of brain lesions seen in patients with atypical parkinsonism of Guadeloupe (Caparros-Lefebvre et al. 2002). Quantification of the acetogenin content in A. muricata fruits and products demonstrates that an adult who consumes one fruit or can of nectar per day will ingest in 1 year the amount of annonacin relative to body weight that induces brain lesion in rats (Champy et al. 2005). It is noteworthy that the chronic systemic administration to rats of rotenone, another plant-derived, lipophilic, and highly potent complex I inhibitor, produces the identical pattern of mitochondrial energy impairment and neurodegeneration (Höglinger et al. 2003). In addition, rotenone-treated rats also develop a cerebral tauopathy with ultrastructural and molecular features resembling those found in PSP-brains (Höglinger et al. 2005). Annonacin is also capable of inducing somatodendritic redistribution of abnormal tau protein in cultured neurons (Escobar-Khondiker et al. 2007). These observations strengthen the hypothesis that natural lipophilic complex I inhibitors, such as the acetogenins contained in annonaceous plants, are capable of inducing atypical parkinsonism of the PSP-type. The concept that mitochondrial dysfunction plays a role in the etiology of PSP-like syndromes is further supported by the observation that cybrid cells containing mitochondria from PSP patients have defective mitochondrial function (Swerdlow et al. 2000; Albers et al. 2001).
ALS-Parkinsonism Dementia Complex (ALS-PDC) of the Western Pacific
A high incidence of what at first appeared to be a classical form of ALS was identified by American Navy doctors among the Chamorro people of Guam shortly after World War II (Zimmerman 1945). Later clinical characterization (Kurland and Mulder 1954; Kurland et al. 1961) confirmed a near epidemic level of the disorder. A second disease with a similarly high incidence was soon added to the emerging picture. This was an atypical form of parkinsonism that presented with dementia and was named parkinsonism dementia complex (PDC) (Hirano et al. 1961). Marked similarities to PSP have since been noted (Steele et al. 2002), both in the abnormal expression of abnormal tau protein and in neuronal loss in regions not associated with classical PD. The spectrum of neurological disorders of both types was collectively referred to as ALS-PDC. Most cases were predominantly either ALS or PDC; however, there was notable overlap with about 7% of the patients suffering from both disorders (Kurland and Mulder 1954). A familial susceptibility to disease was noted, however affected members within one family could exhibit either of the separate disorders.
Disease incidence for ALS on Guam has declined dramatically and now is only marginally higher than the North American average. PDC appears to have declined in recent years, but remains higher than for PD in North America (Galasko et al. 2002). Controversy still exists concerning the extent of the decline (D. Galasko personal communication). Apparently pathologically similar forms of ALS-PDC have been described elsewhere in the Western Pacific, notably in Irian Jaya (western New Guinea) (Gajdusek and Salazar 1982) and the Kii Peninsula of Honshu Island in Japan (Kurland 1972). Histologically, both Guamanian ALS and PDC are characterized by the abundance of neurofibrillary tangles (NFT) of abnormal tau protein whose presence in Guamanian ALS was considered to mark a significant deviation from the classical form. However, the perception that ALS pathology does not involve abnormal tau expression may be changing with recent descriptions of tangles found in the temporal lobes of cognitively impaired ALS patients (Strong et al. 1999, 2006; Strong 2003). In a similar manner, the presence of NFT, together with the neuronal loss outside the nigro-striatal pathway observed in PDC, resembles both PSP and Guadeloupean atypical parkinsonism. The dementia associated with PDC was thought by Kurland and others (Kurland 1972) to resemble AD in a number of aspects, albeit with an underrepresentation of amyloid-β depositions (Winton et al. 2006), possibly making it more like frontotemporal dementia (FTD) than AD (Galasko et al. 2002).
The increased susceptibility to ALS-PDC development in some families suggested the influence of modifier genes, but no clear evidence of inheritance of a disease causing mutation has since emerged (Trojanowski et al. 2002; Hermosura et al. 2005). Instead, the strongest epidemiological data have historically supported an environmental factor(s) as causal to the disease. The strong epidemiological link was recently reconfirmed (Borenstein et al. 2007).
Various environmental hypotheses have been put forward over the years ranging from deficiencies in calcium and magnesium in ground water (Gajdusek and Salazar 1982) to the presence of toxic compounds contained in the gametophyte of the local variety of cycad tree (Cycas micronesica). Consumption of flour made from cycad seeds, termed fadang in Chamorro, has been a recognized feature of Chamorro culture for generations (Whiting 1963), but has declined considerably since the war.
Cycad gametophytes contain various known toxins, including the amino sugar cycasin whose active principle is the hepatotoxin methylazoxymethanol (MAM) as well as the free amino acids β-oxalyoamino alanine (BOAA) and β-methylamino alanine (BMAA). MAM ingestion in animals does not induce neural outcomes resembling ALS-PDC (Kurland et al. 1961) or indeed show any pronounced neural impact in adult animals. BOAA, an AMPA receptor agonist, has been linked to neurolathyrism (Spencer and Schaumburg 1983), but the features of this disease do not closely resemble those of ALS-PDC. BMAA appears to activate both NMDA and AMPA receptors (Weiss et al. 1989; Rao et al. 2006) in vitro, but does not generate ALS-PDC- like features in vivo (Cruz-Aguado et al. 2006). In this regard, Spencer et al. (1987) had originally suggested that BMAA in high doses could do so, but the behavioral deficits and apparent pathological outcomes observed by these investigators were neither consistent with ALS-PDC nor were they permanent once BMAA treatment ended. Later studies failed to replicate even these minimal outcomes (Perry et al. 1989; Duncan et al. 1990; Cruz-Aguado et al. 2006). An additional barrier to any of the above water-soluble toxins as etiologic agents of ALS-PDC is that all elute as the seeds are washed in the traditional methods of Chamorro cycad flour preparation (Duncan 1991; Wilson et al. 2002).
More recently, evidence for additional neurotoxins contained in cycad seeds from Guam has emerged. In vivo feeding of washed cycad flour (lacking any of the water-soluble toxins described above) to adult male mice gives rise to a progressive neurological disorder that recreates much of the spectrum of ALS-PDC. In particular, cycad-fed mice display motor deficits consistent with ALS and parkinsonism, including ALS-PDC olfactory and late stage cognitive deficits (Wilson et al. 2002). Magnetic imaging and histological examination reveal a decreased volume and neuron losses in spinal cord, SNpc, olfactory bulb, hippocampus, and some regions of the cortex (Wilson et al. 2002, 2004, 2005). The same regions show evidence of astrogliosis and/or microglial proliferation (Wilson et al. 2006).
Similar cycad samples fed to adult male rats generate an apparently pure parkinsonism phenotype. At the onset of behavioral symptoms, cycad-fed rats initially unilaterally rotate, with corresponding losses of tyrosine hydroxylase activity and neurons in SNpc and dopamine terminals in the striatum. Both lesions are on the contralateral side to the direction of rotation (Valentino et al. 2006). Once initiated, the disorder continues to progress in the absence of continued exposure to cycad neurotoxins with all of the rats showing a later behavioral stage characterized by freezing. This stage corresponds to the emergence of bilateral SNpc and striatal lesions.
The isolation of bioactive molecules from washed cycad flour suggests that at least three sterol glucoside variants are, individually or in combination, the active toxic principle (Khabazian et al. 2002). Sterol glucosides of various types are known to have neurotoxic properties (Ly et al. 2006) and due to their highly lipophilic nature may easily access the nervous system. The largest fraction of sterol glucosides in cycad is usually β-sitosterol β-D glucoside (BSSG), however the absolute amounts and ratios of the different sterol glucosides can vary considerably between batches of cycad seeds. These variations likely arise due to differences in the season harvested, locale, and, most crucially, seed age (Marler et al. 2005a): Cycad sterol glucoside concentrations, especially in young seeds, appear to be considerably higher than for most other plants studied to date (Marler et al. 2005b).
The identified cycad sterol glucosides are neurotoxic to primary neuronal and astroglial cultures (Khabazian et al. 2000; Khabazian et al. 2002) as well as to an motor neuron-derived cell line (NSC-34) (Ly et al. 2007; Ly and Shaw 2007). Studies using organotypic slices of spinal cord, SNpc/striatum, or hippocampus with BSSG in nanomolar concentrations or a cholesterol glucoside all show significant neuronal death over a period of days to weeks (S. Jafri, K. Andreassen, and C. Mathews, personal communications). Synthetic BSSG fed to mice at concentrations approximating those measured in cycad flour, gives rise to behavioral deficits consistent with an ALS-like phenotype with concomitant progressive motor neuron loss in spinal cord and later cell loss in the striatum (Wilson et al. 2006; Tabata et al. 2007). These outcomes occur even in the absence of further exposure to BSSG.
The data from the ALS-PDC models cited above strongly supports the interpretation that several water insoluble neurotoxic factors in cycad seeds from Guam are indeed causal to the human disease in its various forms. Whether or not the identified sterol glucosides are alone responsible for the all of the pathological outcomes observed in the animal experiments using cycad seed flour is still uncertain.
A Comparison of Guadeloupean Atypical Parkinsonsim and ALS-PDC
There is considerable overlap in observed features between the parkinsonism observed in Guadeloupean atypical parkinsonsim and ALS-PDC. For example, both can be defined as sporadic neurodegenerative tauopathies and both show some resemblance to PSP by involving CNS regions outside the nigro-striatal system. The differences in presentation may reflect the strong possibility that different neurotoxins, as cited in the previous sections, are involved. However, we cannot currently rule out the possibility that both C. micronesica and Annonaceae may contain significant concentrations of both sterol glucosides and acetongenins. We have also noted elsewhere the structural similarity of the sterol glucosides to at least one of the toxic alkaloids, reticuline, found in Annonaceae (Slow et al. 2003).
Criteria for the Identification of Environmental Factors as Causative Agents in Neurodegenerative Diseases
Based on the experimental approaches utilized to study ALS-PDC, Guadeloupean atypical parkinsonism, as well as MPTP-induced parkinsonism, we propose that the following criteria could be used to guide a search to identify and validate putative neurotoxins involved in the etiology of sporadic neurodegenerative disease. These criteria are:
Epidemiological validity: Clinical and pathological evidence consistent with disease segregation by exposure to the putative neurotoxin must exist in individuals or particular human populations;
Agent identification: Isolation, purification, and structural determination of the putative neurotoxin must be accomplished. The neurotoxin must be able to gain access to the CNS under realistic conditions, e.g., ingestion or inhalation, and must, in addition, exhibit plausible dosages and exposure periods;
Experimental modeling in vivo: Exposure of experimental animals to the neurotoxin must recreate the human disease, both behaviorally and histopathologically;
Extinction by prevention: The prevention of exposure to the neurotoxic agents should serve to eradicate the disease.
Environment-Gene Interactions and Neurodegenerative Disease
The last few years have seen an impressive increase in the knowledge of the genetic contribution to the etiology of some neurodegenerative disorders. For example, mutations in a single gene have been identified as being causative for Huntington’s disease. A transgenic animal model expressing this mutation has been developed that closely mimics the disease, thus allowing researchers to study potential neuroprotective interventions.
Most neurodegenerative disorders, however, are far more complex. In both AD and PD, for example, only a small percentage of cases are inherited in a simple Mendelian fashion, the vast majority remaining in the sporadic category. A similar situation exists in regard to ALS. Nonetheless, the identification and experimental study of the genes involved in the inherited forms of neurodegenerative diseases have allowed researchers to identify molecular pathways leading to neurodegeneration and the hope remains that these will turn out to be the same as those evoked in sporadic disease.
In AD for example, all gene defects identified so far appear to drive a common metabolic pathway that involves the production, processing, or clearance of amyloid protein (Mayeux 2006). Such observations can guide the search for neurotoxins that affect, directly, or indirectly the same pathway. In contrast, in PD the inherited forms of the disease do not map to a solitary pathway. Similarly, in ALS, gene-evoked neuropathological cascades seem more likely to involve multiple pathways. Thus for PD and ALS, the notion that identifying gene mutations will necessarily lead to any clearer understanding of the sporadic forms of the disease is not necessarily correct. Insofar as it is not correct, the best justification for genetic studies in these disorders is that the events triggered may converge on common downstream targets to give clinically and pathologically identical outcomes.
Putting Toxin-Gene Synergies into Perspective
The above discussion of the relative impact of environmental neurotoxins versus genetic factors in neurological disease leads to the following conclusions: Although various gene variants/mutations can induce a percentage of overall cases of ALS, PD, and AD, this fraction is not large. Nor is it absolutely clear even in such cases that the aberrant genes alone are solely causal to the disease since potential environmental factors acting in synergy cannot be discounted. This is well illustrated by the current failure of PD transgenetic models to generate the full pathological spectrum of the disease (Emborg 2004). The same lack of effect holds for the animal model of the ALS2 mutation to the protein alsin (Devon et al. 2006). Gene proponents often point to the success of the mSOD model of the various mutations involved in some familial forms of ALS in which homozygous mice and rats develop a rapidly fatal motor neuron loss and display many of the clinical and pathological features seen in human ALS. In this view, these data are strong evidence for a strict genetic etiology. However, it must be noted that high levels of expression of the mSOD mutation also impact a variety of other areas of CNS, including the SNpc, striatum, and hippocampus, suggesting that the intensity of the insult provided by the mutation is very great (Petrik et al. 2007). In contrast, low expressing mSOD mice show relatively minor or late expressing pathological outcomes (Julien and Kriz 2006).
If many of the demonstrated mutations are alone insufficient to re-create the human neurological disease in animal models, what are the chances that environmental factors alone do so? The models discussed above—as well as various others not considered in this review—clearly show that such potential exists. Cycad and Annona neurotoxins can be linked epidemiologically to ALS-PDC on Guam and elsewhere, and atypical parkinsonism in various locations, respectively. The problem here, however, is one of universality: Outside of these rare clusters of disease, can worldwide cases of ALS, PD/parkinsonism, or AD be linked to either group of potential toxins? The presence of various sterol glucosides from a variety of sources suggests that these may to some extent satisfy a criterion of universality (Ly et al. 2007). The same could be true of the Annonaceous toxins, but more likely reflect not a specific molecule but rather a number of molecules of different types that share common toxic mechanisms of action.
Thus, although such toxins—related by type or mechanism of action—could be causal to neurological diseases, a central question remains unanswered: Why are there not more clusters of neurological disease? One answer may be that although such toxins can kill neurons in the CNS at sufficient concentrations, such fatal concentrations may be rare, only occurring in places such as Guam or Guadeloupe. Hence, the causal toxins might be ubiquitously distributed, but the exposure for most people may occur at concentrations too low to induce disease. If so, then the obvious exception might be those who share some genetic susceptibility that influences toxin accessibility, transport, or degradation. Numerous examples of such genetic susceptibility factors exist, one of the most widely known that of the polymorphisms in the cholesterol transport gene apoE. In regard to the latter, cycad toxicity in mice seems to be in part regulated by apoE variants in a manner than appears to reflect the risk of ALS and AD in humans (Bédlâck et al. 2000; Wilson et al. 2005; Mayeux 2006).
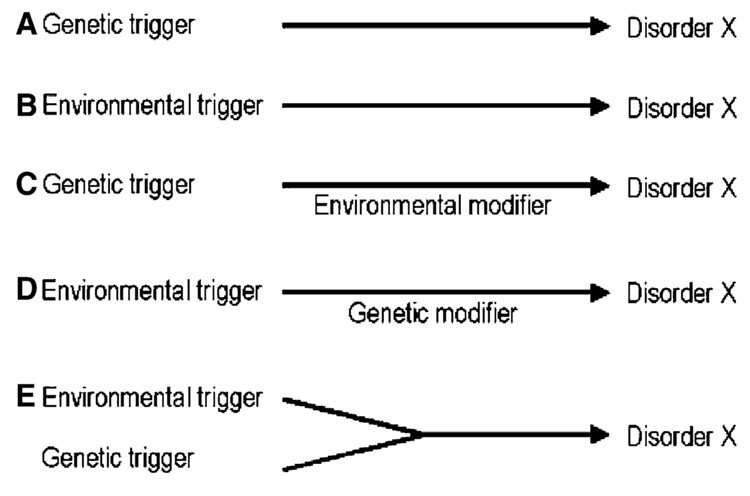
These speculations lead to a view that is rapidly gaining support, namely that environmental and genetic factors must interact to cause sporadic neurological diseases. This last consideration, in addition to toxin only or gene only etiologies is illustrated in the schematic of Fig. 1. It is also increasingly likely that toxins may directly alter gene function: Epigenetic modifications of chromatin leading to changes in DNA methylation may be a crucial factor in toxin-gene interactions (D’Alessio and Szyf 2006).
Fig. 1.
Schematic representation of different modes of putative gene-environment interactions in the etiology of a neurodegenerative disease ‘‘X’’, as defined by a unique clinical or neuropathological phenotype. (a) One of several genetic mutations may be the sole trigger the disease. (b) One of several environmental toxins may be the sole trigger of the disease. (c) One or several genetic mutations may be the prime trigger of the disease, the penetrance or severity of which may be modified by environmental factors. (d) One or several environmental toxins may be the prime trigger of the disease, the penetrance or severity of which may be modified by genetic factors. (e) Environmental and genetic factors, both being too weak individually to trigger the disease, may be required, interacting in a synergistic manner to trigger the disease
All of the above speculation is open to experimental scrutiny. It seems to us that an urgent future priority in the field will be to conduct a systematic exploration of the interactions identified neurotoxins and mutations. From such studies may come the ultimate answer and hope for prophylaxis to sporadic neurodegenerative disease.
Acknowledgments
This work was supported by the US Army Medical Research and Materiel Command (#DAMD17-02-1-0678), Scottish Rite Charitable Foundation of Canada, and the Natural Science and Engineering Research Council of Canada (NSERC), and NINDS to CAS and European Union Grant LSHM-CT-2003-503330 to GUH. The authors thank Michael Petrik, Dr. Reyniel Cruz-Aguado and Dr. Denis Kay for helpful suggestions and commentary.
Contributor Information
Christopher A. Shaw, Department of Ophthalmology, University of British Columbia, Vancouver, BC, Canada Department of Experimental Medicine and the Neuroscience Program, University of British Columbia, Vancouver, BC, Canada.
Günter U. Höglinger, Experimental Neurology, Philipps University, 35033 Marburg, Germany
References
- Abou-Sleiman PM, Muqit MM, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Natural Reviews Neuroscience. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- Albers DS, Swerdlow RH, Manfredi G, Gajewski C, Yang L, Parker WD, Jr., Beal MF. Further evidence for mitochondrial dysfunction in progressive supranuclear palsy. Experimental Neurology. 2001;168:196–198. doi: 10.1006/exnr.2000.7607. [DOI] [PubMed] [Google Scholar]
- Angibaud G, Gaultier C, Rascol O. Annonaceae consumption in New Caledonia. Movement Disorders. 2004;19:603–604. doi: 10.1002/mds.20104. [DOI] [PubMed] [Google Scholar]
- Ballard PA, Tetrud JW, Langston JW. Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): Seven cases. Neurology. 1985;35:949–956. doi: 10.1212/wnl.35.7.949. [DOI] [PubMed] [Google Scholar]
- Bédlâck RS, Strittniatter WJ, Morgenlandèr JC. ApoIipoprotem E and neuromuscular disease: A critical review of the literature. Archives of Neurology. 2000;57:1561–1565. doi: 10.1001/archneur.57.11.1561. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE, Fournier MC, Dovero S, Bloch B, Jaber M. Absence of MPTP-induced neuronal death in mice lacking the dopamine transporter. Experimental Neurology. 1999;155:268–273. doi: 10.1006/exnr.1998.6995. [DOI] [PubMed] [Google Scholar]
- Borenstein AR, Mortimer JA, Schofield MPH, Wu Y, Salmon DP, Gamst A, Olichney J, Thal LJ, Sibert L, Kaye J, Craig UL, Schellenberg GD, Galasko DR. Cycad exposure and risk of dementia, MCI, and PDC in the Chamorro population of Guam. Neurology. 2007;68:1764–1771. doi: 10.1212/01.wnl.0000262027.31623.b2. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell and Tissue Research. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Caparros-Lefebvre D, Elbaz A Caribbean Parkinsonism Study Group. Possible relation of atypical parkinsonism in the French West Indies with consumption of tropical plants: A case-control study. Lancet. 1999;354:281–286. doi: 10.1016/s0140-6736(98)10166-6. [DOI] [PubMed] [Google Scholar]
- Caparros-Lefebvre D, Sergeant N, Lees A, Camuzat A, Daniel S, Lannuzel A, Brice A, Tolosa E, Delacourte A, Duyckaerts C. Guadeloupean parkinsonism: A cluster of progressive supranuclear palsy-like tauopathy. Brain. 2002;125:801–811. doi: 10.1093/brain/awf086. [DOI] [PubMed] [Google Scholar]
- Caparros-Lefebvre D, Steele J, Kotake Y, Ohta S. Geographic isolates of atypical Parkinsonism and tauopathy in the tropics: Possible synergy of neurotoxins. Movement Disorders. 2006;21:1769–1771. doi: 10.1002/mds.21024. [DOI] [PubMed] [Google Scholar]
- Champy P, Höglinger GU, Feger J, Gleye C, Hocquemiller R, Laurens A, Guerineau V, Laprevote O, Medja F, Lombes A, Michel PP, Lannuzel A, Hirsch EC, Ruberg M. Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: Possible relevance for atypical parkinsonism in Guadeloupe. Journal of Neurochemistry. 2004;88:63–69. doi: 10.1046/j.1471-4159.2003.02138.x. [DOI] [PubMed] [Google Scholar]
- Champy P, Melot C, Guerineau Eng V, Gleye C, Fall D, Höglinger GU, Ruberg C, Lannuzel A, Laprevote O, Laurens A, Höcquemiller R. Quantification ofacetogenins in Annona muricata linked to atypical parkinsonismin Guadeloupe. Movement Disorders. 2005;20:1629–1633. doi: 10.1002/mds.20632. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Hu MT, Brooks DJ. Atypical parkinsonism in Afro-Caribbean and Indian origin immigrants to the UK. Movement Disorders. 2000;15:18–23. doi: 10.1002/1531-8257(200001)15:1<18::aid-mds1005>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Cruz-Aguado R, Winkler D, Shaw CA. Lack of behavioral and neuropathological effects of dietary beta-methylamino-L-alanine (BMAA) in mice. Pharmacology, Biochemistry, and Behavior. 2006;84:294–299. doi: 10.1016/j.pbb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- D’Alessio AC, Szyf M. Epigenetic tete-a-tete: The bilateral relationship between chromatin modifications and DNA methylation. Biochemistry and Cell Biology. 2006;84:463–476. doi: 10.1139/o06-090. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: Mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Davey GP, Tipton KF, Murphy MP. Uptake and accumulation of 1-methyl-4-phenylpyridinium by rat liver mitochondria measured using an ion-selective electrode. The Biochemistry Journal. 1992;288(Pt 2):439–443. doi: 10.1042/bj2880439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- Degli Esposti M. Inhibitors of NADH-ubiquinone reductase: An overview. Biochimica Biophysica Acta. 1998;1364:222–235. doi: 10.1016/s0005-2728(98)00029-2. [DOI] [PubMed] [Google Scholar]
- Devon RS, Orban PC, Gerrow K, Barbieri MA, Schwab C, Cao LP, Helm JR, Bissada N, Cruz-Aguado R, Davidson TL, Witmer J, Metzler M, Lam CK, Tetzlaff W, Simpson EM, McCaffery JM, El-Hussein AE, Leavitt BR, Hayden MR. Als2-deficient mice exhibit disturbances in endosome trafficking associated with motor behavioral abnormalities. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9595–9600. doi: 10.1073/pnas.0510197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MW. Role of the cycad neurotoxin BMAA in the amyotrophic lateral sclerosis-parkinsonism dementia complex of the western Pacific. Advances in Neurology. 1991;56:301–310. [PubMed] [Google Scholar]
- Duncan MW, Steele JC, Kopin IJ, Markey SP. 2-Amino-3-(methylamino)-propanoic acid (BMAA) in cycad flour: An unlikely cause of amyotrophic lateral sclerosis and parkinsonism-dementia of Guam. Neurology. 1990;40:767–772. doi: 10.1212/wnl.40.5.767. [DOI] [PubMed] [Google Scholar]
- Emborg ME. Evaluation of animal models of Parkinson’s disease for neuroprotective strategies. Journal of Neuroscience Methods. 2004;139:121–143. doi: 10.1016/j.jneumeth.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Escobar-Khondiker M, Hoöllerhage M, Michel PP, Muriel MP, Champy P, Respondek G, Yagi T, Lannuzel A, Hirsch EC, Oertel WH, Jacob R, Ruberg R, Hoöglinger GU. Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. Journal of Neuroscience. 2007;27:7827–7837. doi: 10.1523/JNEUROSCI.1644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, Pellegrini A, Nicoletti F, Ruggieri S, Paparelli A, Sudhof TC. Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forno LS, DeLanney LE, Irwin I, Langston CW. Similarities and differences between MPTP-induced parkinsonism and Parkinson’s disease Neuropathologic considerations. Advances in Neurology. 1993;60:600–608. [PubMed] [Google Scholar]
- Gajdusek DC, Salazar AM. Amyotrophic lateral sclerosis and parkinsonian syndromes in high incidence among the Auyu and Jakai people of West New Guinea. Neurology. 1982;32:107–126. doi: 10.1212/wnl.32.2.107. [DOI] [PubMed] [Google Scholar]
- Galasko D, Salmon DP, Craig UK, Thal LJ, Schellenberg G, Wiederholt W. Clinical features and changing patterns of neurodegenerative disorders on Guam, 1997–2000. Neurology. 2002;58:90–97. doi: 10.1212/wnl.58.1.90. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L, Cabbat FS, Duvoisin RC. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature. 1984;311:467–469. doi: 10.1038/311467a0. [DOI] [PubMed] [Google Scholar]
- Hermosura MC, Nayakanti H, Dorovkov MV, Calderon FR, Ryazanov AG, Haymer DS, Garruto RM. A TRPM7 variant shows altered sensitivity to magnesium that may contribute to the pathogenesis of two Guamanian neurodegenerative disorders. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11510–11515. doi: 10.1073/pnas.0505149102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano A, Kurland LT, Krooth RS, Lessell S. Parkinsonism-dementia complex, an endemic disease on the island of Guam. I. Clinical features. Brain. 1961;84:642–661. doi: 10.1093/brain/84.4.642. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Feger J, Prigent A, Michel PP, Parain K, Champy P, Ruberg M, Oertel WH, Hirsch EC. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. Journal of Neurochemistry. 2003;84:491–502. doi: 10.1046/j.1471-4159.2003.01533.x. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Lannuzel A, Khondiker ME, Michel PP, Duyckaerts C, Champy J, Prigent A, Medja F, Lombes A, Oertel WH, Ruberg M, Hirsch EC. The mitochondrial complex I inhibitor rotenone triggers a cerebral tauopathy. Journal of Neurochemistry. 2005;95:930–939. doi: 10.1111/j.1471-4159.2005.03493.x. [DOI] [PubMed] [Google Scholar]
- Javitch JA, D’Amato RJ, Strittmatter SM, Snyder SH. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: Uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proceedings of the National Academy of Sciences of the United States of America. 1985;82:2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien JP, Kriz J. Transgenic mouse models of amyotrophic lateral sclerosis. Biochimica Biophysica Acta. 2006;1762:1013–1024. doi: 10.1016/j.bbadis.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. Journal of Neuroscience. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khabazian I, Bains JS, Williams DE, Cheung J, Wilson JM, Pasqualotto BA, Pelech SL, Andersen RJ, Wang YT, Liu L, Nagai A, Kim SU, Craig UK, Shaw CA. Isolation of various forms of sterol beta-D-glucoside from the seed of Cycas circinalis: Neurotoxicity and implications for ALS-parkinsonism dementia complex. Journal of Neurochemistry. 2002;82:516–528. doi: 10.1046/j.1471-4159.2002.00976.x. [DOI] [PubMed] [Google Scholar]
- Khabazian I, Pelech SL, Williams DL, Andersen RJ, Craig UK, Krieger C, Shaw CA. Mechanisms of action of sitosterol glucoside in mammalian CNS. Society for Neuroscience Abstract. 2000 [Google Scholar]
- Kurland LT. An appraisal of the neurotoxicity of cycad and the etiology of amyotrophic lateral sclerosis on Guam. Federation Proceeding. 1972;31:1540–1542. [PubMed] [Google Scholar]
- Kurland LT, Hirano A, Malamud N, Lessell S. Parkinsonism-dementia complex, en endemic disease on the island of Guam. Clinical, pathological, genetic and epidemiological features. Transactions of the American Neurological Association. 1961;86:115–120. [PubMed] [Google Scholar]
- Kurland LT, Mulder DW. Epidemiologic investigations of amyotrophic lateral sclerosis. I. Preliminary report on geographic distribution, with special reference to the Mariana Islands, including clinical and pathologic observations. Neurology. 1954;4:355–378. doi: 10.1212/wnl.4.5.355. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Rebert CS, Irwin I. Selective nigral toxicity after systemic administration of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyrine (MPTP) in the squirrel monkey. Brain Research. 1984a;292:390–394. doi: 10.1016/0006-8993(84)90777-7. [DOI] [PubMed] [Google Scholar]
- Langston JW, Irwin I, Langston EB, Forno LS. Pargyline prevents MPTP-induced parkinsonism in primates. Science. 1984b;225:1480–1482. doi: 10.1126/science.6332378. [DOI] [PubMed] [Google Scholar]
- Lannuzel A, Höglinger GU, Verhaeghe S, Gire L, Belson S, Escobar-Khondiker M, Poullain P, Oertel WH, Hirsch EC, Dubois B, Ruberg M. Atypical Parkinsonism in Guadeloupe: A common risk factor for two closely related phenotypes? Brain. 2007;130:816–827. doi: 10.1093/brain/awl347. [DOI] [PubMed] [Google Scholar]
- Lannuzel A, Michel PP, Caparros-Lefebvre D, Abaul J, Hocquemiller R, Ruberg M. Toxicity of Annonaceae for dopaminergic neurons: Potential role in atypical parkinsonism in Guadeloupe. Movement Disorders. 2002;17:84–90. doi: 10.1002/mds.1246. [DOI] [PubMed] [Google Scholar]
- Lannuzel A, Michel PP, Höglinger GU, Champy P, Jousset A, Medja F, Lombes A, Darios F, Gleye C, Laurens A, Hocquemiller R, Hirsch EC, Ruberg M. The mitochondrial complex I inhibitor annonacin is toxic to mesencephalic dopaminergic neurons by impairment of energy metabolism. Neuroscience. 2003;121:287–296. doi: 10.1016/s0306-4522(03)00441-x. [DOI] [PubMed] [Google Scholar]
- Ly PT, Singh S, Shaw CA. Novel environmental toxins: Steryl glycosides as a potential etiological factor for age-related neurodegenerative diseases. Journal of Neuroscience Research. 2007;85:231–237. doi: 10.1002/jnr.21147. [DOI] [PubMed] [Google Scholar]
- Ly PT, Shaw CA. Steryl glycoside induced cytopathological changes in the motor neuron-derived NSC-34 cells. Society for Neuroscience Abstract. 2007 [Google Scholar]
- Ly PTT, Liang XB, Wang Q, Andreasson K, Shaw CA. The neurotoxic effects of -sitosterol glucosides in NSC 34 cells, a mouse motor neuron-derived cell line. Society for Neuroscience Abstract. 2006 [Google Scholar]
- Mann VM, Cooper JM, Krige D, Daniel SE, Schapira AH, Marsden CD. Brain, skeletal muscle and platelet homogenate mitochondrial function in Parkinson’s disease. Brain. 1992;115:333–342. doi: 10.1093/brain/115.2.333. [DOI] [PubMed] [Google Scholar]
- Markey SP, Johannessen JN, Chiueh CC, Burns RS, Herkenham MA. Intraneuronal generation of a pyridinium metabolite may cause drug-induced parkinsonism. Nature. 1984;311:464–467. doi: 10.1038/311464a0. [DOI] [PubMed] [Google Scholar]
- Marler TE, Lee V, Shaw CA. Spatial variation of steryl glucosides in Cycas micronesica plants-within and among plant sampling procedures. Hort Science. 2005a;40:1607–1611. [Google Scholar]
- Marler TE, Lee V, Shaw CA. Cycad toxins and neurological diseases in Guam: Defining theoretical and experimental standards for correlation human disease with environmental toxins. Hort Science. 2005b;33:1598–1606. [Google Scholar]
- Mayeux R. Genetic epidemiology of Alzheimer disease. Alzheimer Disease and Associated Disorders. 2006;20:S58–S62. doi: 10.1097/00002093-200607001-00008. [DOI] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sciences. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Perry TL, Bergeron C, Biro AJ, Hansen S. Beta-N-methylamino-L-alanine. Chronic oral administration is not neurotoxic to mice. Journal of the Neurological Sciences. 1989;94:173–180. doi: 10.1016/0022-510x(89)90227-x. [DOI] [PubMed] [Google Scholar]
- Petrik MS, Wilson JMB, Grant SC, Blackband SJ, Tabata RC, Shan X, Krieger C, Shaw CA. Magnetic resonance microscopy and immunohistochemistry of the CNS of the mutant SOD murine model of ALS reveal widespread neural deficits. Journal of Neuromolecular Medicine. 2007;9:216–229. doi: 10.1007/s12017-007-8002-1. [DOI] [PubMed] [Google Scholar]
- Ramsay RR, Singer TP. Energy-dependent uptake of N-methyl-4-phenylpyridinium,the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. The Journal of Biological Chemistry. 1986;261:7585–7587. [PubMed] [Google Scholar]
- Rao SD, Banack SA, Cox PA, Weiss JH. BMAA selectively injures motor neurons via AMPA/kainate receptor activation. Experimental Neurology. 2006;201:244–252. doi: 10.1016/j.expneurol.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Riachi NJ, LaManna JC, Harik SI. Entry of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine into the rat brain. The Journal of Pharmacology and Experimental Therapeutics. 1989;249:744–748. [PubMed] [Google Scholar]
- Ricaurte GA, DeLanney LE, Irwin I, Langston JW. Older dopaminergic neurons do not recover from the effects of MPTP. Neuropharmacology. 1987;26:97–99. doi: 10.1016/0028-3908(87)90051-7. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Langston JW, DeLanney LE, Irwin I, Brooks JD. Dopamine uptake blockers protect against the dopamine depleting effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse striatum. Neuroscience Letters. 1985;59:259–264. doi: 10.1016/0304-3940(85)90141-7. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269. doi: 10.1016/s0140-6736(89)92366-0. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD. Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. Journal of Neurochemistry. 1990;55:2142–2145. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- Singer TP, Castagnoli N, Jr., Ramsay RR, Trevor AJ. Biochemical events in the development of parkinsonism induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Journal of Neurochemistry. 1987;49:1–8. doi: 10.1111/j.1471-4159.1987.tb03384.x. [DOI] [PubMed] [Google Scholar]
- Slow EJ, van Raamsdonk J, Rogers D, Coleman SH, Graham RK, Deng Y, Oh R, Bissada N, Hossain SM, Yang YZ, Li XJ, Simposon EM, Gutekunst CA, Leavitt BR, Hayden MR. Selective striatal neuronal loss in a YACI28 mouse model of Huntington disease. Human Molecular Genetics. 2003;12:1555–1567. doi: 10.1093/hmg/ddg169. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH. Lathyrism: A neurotoxic disease. Neurobehavioral Toxicology and Teratology. 1983;5:625–629. [PubMed] [Google Scholar]
- Spencer PS, Nunn PB, Hugon J, Ludolph AC, Ross SM, Roy DN, Robertson RC. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- Steele JC, Caparros-Lefebvre D, Lees AJ, Sacks OW. Progressive supranuclear palsy and its relation to pacific foci of the parkinsonism-dementia complex and Guadeloupean parkinsonism. Parkinsonism & Related Disorders. 2002;9:39–54. doi: 10.1016/s1353-8020(02)00043-3. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Grace GM, Orange JB, Leeper HA, Menon RS, Aere C. A prospective study of cognitive impairment in ALS. Neurology. 1999;53:1665–1670. doi: 10.1212/wnl.53.8.1665. [DOI] [PubMed] [Google Scholar]
- Strong MJ. The basic aspects of therapeutics in amyotrophic lateral sclerosis. Pharmacology & Therapeutics. 2003;98:379–414. doi: 10.1016/s0163-7258(03)00040-8. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Yang W, Strong WL, Leystra-Lantz C, Jaffe H, Pant HC. Tau protein hyperphosphorylation in sporadic ALS with cognitive impairment. Neurology. 2006;66:1770–1771. doi: 10.1212/01.wnl.0000218161.15834.db. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Golbe LI, Parks JK, Cassarino DS, Binder DR, Grawey AE, Litvan I, Bennett JP, Jr., Wooten GF, Parker WD. Mitochondrial dysfunction in cybrid lines expressing mitochondrial genes from patients with progressive supranuclear palsy. Journal of Neurochemistry. 2000;75:1681–1684. doi: 10.1046/j.1471-4159.2000.0751681.x. [DOI] [PubMed] [Google Scholar]
- Tabata RC, Wilson JMB, Ly P, Zwiegers P, Kwok D, Van Kampen JM, Cashman N, Shaw CA. Chronic exposure to dietary sterol glucosides are neurotoxic to motor neurons and induce an ALS-PDC phenotype. Neurornolecular Medicine. 2007 doi: 10.1007/s12017-007-8020-z. (in submission) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojanowski JQ, Ishihara T, Higuchi M, Yoshiyama Y, Hong M, Zhang B, Forman MS, Zhukareva V, Lee VM. Amyotrophic lateral sclerosis/parkinsonism dementia complex: Transgenic mice provide insights into mechanisms underlying a common tauopathy in an ethnic minority on Guam. Experimental Neurology. 2002;176:1–11. doi: 10.1006/exnr.2002.7940. [DOI] [PubMed] [Google Scholar]
- Valentino KM, Dugger NV, Peterson E, Wilson JM, Shaw CA, Yarowsky PJ. Environmentally-induced parkinsonism in cycad-fed rats. Society for Neuroscience Abstract. 2006 [Google Scholar]
- Weiss JH, Koh JY, Choi DW. Neurotoxicity of beta-N-methylamino-L-alanine (BMAA) and beta-N-oxalylamino-L-alanine (BOAA) on cultured cortical neurons. Brain Research. 1989;497:64–71. doi: 10.1016/0006-8993(89)90970-0. [DOI] [PubMed] [Google Scholar]
- Whiting MG. Toxicity of cycads. Economic Botany. 1963;17:271–302. [Google Scholar]
- Wilson JM, Khabazian I, Wong MC, Seyedalikhani A, Bains JS, Pasqualotto BA, Williams DE, Andersen RJ, Simpson RJ, Smith R, Craig UK, Kurland LT, Shaw CA. Behavioral and neurological correlates of ALS-parkinsonism dementia complex in adult mice fed washed cycad flour. Journal of Neuromolecular Medicine. 2002;1:207–221. doi: 10.1385/NMM:1:3:207. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Petrik MS, Grant SC, Blackband SJ, Lai J, Shaw CA. Quantitative measurement of neurodegeneration in an ALS-PDC model using MR microscopy. Neuroimage. 2004;23:336–343. doi: 10.1016/j.neuroimage.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Petrik MS, Moghadasian MH, Shaw CA. Examining the interaction of apo E and neurotoxicity on a murine model of ALS-PDC. Canadian Journal of Physiology and Pharmacology. 2005;83:131–141. doi: 10.1139/y04-140. [DOI] [PubMed] [Google Scholar]
- Wilson JMB, Tabata RC, Shaw CA. In vivo sterol glucoside neurotoxicity: Implications for ALS-PDC and ALS. Society for Neuroscience Abstract. 2006 [Google Scholar]
- Winton MJ, Joyce S, Zhukareva V, Practico D, Perl DP, Galasko D, Craig U, Trojanowski JQ, Lee VM. Characterization of tau pathologies in gray and white matter of Guam parkinsonism-dementia complex. Acta Neuropathologica (Berl) 2006;111:401–412. doi: 10.1007/s00401-006-0053-0. [DOI] [PubMed] [Google Scholar]
- Zimmerman H. Reported to US Navy and Public Health Service: Washington; Progress report of work in the laboratory of pathology during May, 1945, Guam US Naval Medical Research Unit Number 2, June 1. 1945