Abstract
Late-onset Alzheimer’s disease (LOAD) is the most common cause of late-onset dementia in western societies. Despite remarkable achievements in human genetics throughout the years, in particular technological advances in gene mapping and in statistical methods that relate genetic variants to disease, to date only a small proportion of the genetic contribution to LOAD can be explained leaving several remaining genetic risk factors to be identified. A possible explanation for the difficulty in gene identification is that LOAD is a multifactorial complex disorder with both genetic and environmental components. Multiple genes with small effects each (“quantitative trait loci” [QTLs]) are likely to contribute to the quantitative traits associated with the disease, such as memory performance, amyloid/tau pathology, or hippocampal atrophy. The motivation for identifying the genetics of LOAD is clear. Not only could it shed light on disease pathogenesis, but it may also provide potential targets for effective treatment, screening, and prevention. Here, we review the usefulness of genetic variation as diagnostic tools and biomarkers in LOAD and discuss the potentials and difficulties researchers face in designing appropriate studies for gene discovery.
Keywords: Alzheimer’s, biomarkers, genetics
Introduction
Late-onset Alzheimer’s disease (LOAD) is among the most frequently encountered diseases in aging societies. It is estimated that approximately 5 million people in the United States and 17 million people worldwide suffer from the disease. By age 85 years and older 15–30% are affected, and the incidence rate increases from approximately 1% among people aged 65–70 years to approximately 68% for people aged 85 years and older.1,2 It is expected that these numbers will quadruple by the year 2040, by which 1 out of 45 Americans will be affected, leading to a considerable public health burden.3
To date, there are no definitive diagnostic tests or biological markers of the disease. The diagnosis of LOAD during life is based on clinical examination using the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association Work Group.4 Although these criteria have good reliability5–7 and validity,8,9 any measure that would increase diagnostic sensitivity and specificity would be highly valuable for improving early detection and intervention. Genetic variation can be particularly useful as it is stable across the lifespan and the disease process and is not influenced by confounding factors.
Twin studies suggest that 37% to as much as 78% of the variance in the age at onset of LOAD can be attributed to additive genetic effects.10 As a consequence, genes involved in LOAD could theoretically be highly valuable diagnostic tools. Untangling the genetics of LOAD would in addition help elucidate the disease pathogenesis and would provide potential targets for effective treatment and prevention.
Despite available improved analytic techniques, the continued pursuit of genetic variants associated with LOAD has, however, been limited. To date, only two genes have been implicated in the cause: the Apolipoprotein (APOE)-ε4 allele and the Sortilin-related receptor (SORL1) gene. Several additional genetic loci have been reported but remain to be confirmed. Together, these reported genes explain only a small proportion of the genetic contribution to LOAD leaving several genetic risk factors to be identified.
In this article, we review the genetic risk factors that have been implicated in LOAD, and review their usefulness as diagnostic tools and biomarkers in AD. In addition, we discuss the potentials and difficulties researchers face when performing studies for gene discovery in common complex diseases, such as LOAD.
Genetics of LOAD and Its Endophenotypes
Most of the studies assessing the role of genetic variants in dementia have used the diagnostic criteria of LOAD as the phenotype. The most frequently used endophenotypes are age at onset of dementia and cognitive test performance. Plasma amyloid β (Aβ) levels, a putative risk factor, have been studied rarely11–13 but have in particular been mapped to loci on chromosome 10q21–25. The rationale for use of endophenotypes in LOAD research is that quantitative traits provide more informative phenotypes than simply considering affection status and thus provide more statistical power to detect small polygenic effects.
Apolipoprotein E
APOE, a lipid-binding protein, is expressed in humans as three common isoforms coded for by three alleles, APOEε2, ε3, and ε4. The first reports linking APOE genotype with LOAD found a significant increase in the APOEε4 allele frequency in patients with the disease. The large body of epidemiological data that subsequently accumulated clarified this effect by demonstrating that APOEε4 decreases the age at onset of LOAD in a gene dosage-dependent manner,14–23 and that APOEε4 is associated with lower cognitive performance, in particular the memory domain. It is thought that APOE may account for as much as 20–50% of LOAD risk.24,25
In vitro studies have indicated that the APOE-ε4 isoform binds Aβ peptides with a higher avidity compared to APOE-ε3.26 Furthermore, there is a strong correlation between the presence of an APOE-ε4 allele and a higher Aβ burden in the brains of AD patients,27,28 suggesting that APOE interacts with Aβ in enhancing its deposition in plaques. This is supported by the observation that homozygous APOE knockout (APOE −/−) mice develop fewer and more diffuse, nonfibrillar Aβ deposits.29–31 Some, but not all, studies assessing the effect of different APOE isoforms on Aβ fibrillization, showed that the ε4 isoform leads to increased Aβ aggregation in vitro.32,33 Similarly, in vivo studies in APOE −/− mice indicated that Aβ fibrillization and plaques formation was increased in mice expressing human APOE-ε4 (APPV717F+/−, apo E−/−) compared to mice not expressing human APOE.34,35 Still, it is possible that APOE exerts its effects through different mechanisms, e.g., APOE is a major cholesterol transporter, and high cholesterol levels have been associated with an increased Aβ load in animal models36,37 and changes in amyloid precursor protein (APP) processing.38,39 Thus, APOE isoform-specific changes in cholesterol binding and transport in brain might also affect plaque formation in AD brains.
LOAD as the Endophenotype
A large amount of studies assessed the relation between APOE genotypes and LOAD in population-based settings. In a meta analysis40 that included data from 40 research teams on 5930 patients with LOAD and 8607 controls without dementia who were recruited from clinical, community, and brain bank sources, the risk of AD was significantly increased for Caucasians with genotypes ε2/ε4 (OR = 2.6, 95% CI = 1.6–4.0), ε3/ε4 (OR = 3.2, 95% CI = 2.8–3.8), and ε4/ε4 (OR = 14.9, 95% CI = 10.8–20.6), whereas the ORs were decreased for people with genotypes ε2/ε2 (OR = 0.6, 95% CI = 0.2–2.0) and ε2/ε3 (OR = 0.6, 95% CI = 0.5–0.8). The APOE-ε4-AD association was weaker among African-Americans and Hispanics, but there was significant heterogeneity in ORs among studies of African-Americans (P < 0.03). The APOE-ε4-AD association in Japanese subjects was stronger than in Caucasian subjects (ε3/ε4: OR = 5.6, 95% CI = 3.9–8.0; ε4/ε4: OR = 33.1, 95% CI = 13.6–80.5). The ε2/ε3 genotype appeared equally protective across ethnic groups. Figure 1 shows the pooled odds ratios (95% CI) of the 40 studies included in the paper. Taken together, it seems that one ε4 allele is associated with a two- to three-fold increased risk, while having two copies is associated with a five- to 10-fold increase.
Figure 1.
Pooled odds ratios (95% CI) of the 40 studies included in the meta-analysis by Farrer et al.40 relating APOE genotype with LOAD (ε4 allele versus ε3 allele). †No data provided; ‡Hardy-Weinberg-Equilibrium (HWE) deviation in controls (P ≤ 0.05). (In color in Annals online.)
Age at Onset as the Phenotype
In the vast majority of studies, both clinical and epidemiological, age at onset of LOAD was strongly related to the presence of the APOE-ε4 allele (Table 1).14–23 Taken together, these studies, which include both clinical and epidemiological studies, suggest that APOEε4 may decrease the age at onset by as much as 7–9 years per allele. They further suggest that this effect is present across the lifespan including children and adolescents19,20,41–46 and across various ethnic groups, although it may be stronger in Caucasians and Hispanics than African-Americans.23 Mak et al.47 studied the APOE allele frequencies in Hong Kong elderly Chinese (65 LOAD patients and 82 age-and sex-matched controls). Both the mean and the median age at onset tended to be lower in subjects with one or two copies of ε4 compared to persons without ε4 allele (mean age of onset [SD] no ε4 versus one ε4, one ε4 versus 2 ε4s: 73.3 [8.5] versus 72.0 [6.4] versus 71.2 [5.0]). There was in addition a tendency for the mean and median ages at onset to be higher in subjects with ε2/ε2 or ε2/ε3 than in subjects with ε3/ε3. Although these differences only approached statistical significance (P = 0.078, Z = 1.419), these findings suggest that APOE also exerts its effect in Chinese populations. This notion is supported by the fact that in the same study, the APOE-ε4 allele frequency was significantly higher in the AD group than in the control group (0.169 versus 0.067, P < 0.01), and the fact that in Chinese the ε4 frequency is low, which decreases the power to obtain statistical significant results.48
TABLE 1.
Summary of Studies Relating APOE Genotype with LOAD Endophenotypes
| Author | Subjects | Age in years, mean (range) | Endophenotype | Finding |
|---|---|---|---|---|
| AGE AT ONSET | ||||
| Lehtovirta et al.,117 1995 | 202 Finnish LOAD patients and 55 age- and sex-matched controls | Disease onset: ε4: −/− 76 ± 10, −/+: 77 ± 8, +/+: 71 ± 7 | Age at onset | Age at onset decreased from 76 to 69 as the number of ε4 alleles increased from 0 to 2 |
| Gomez-Isla et al.,16 1996 | 359 patients LOAD, age- and sex-matched 129 controls | LOAD group: mean age of 77.8 years; control group: mean age of 77.8 years | Age at onset | Age of onset declined significantly as number of ε4 alleles increased (P < 0.0001 for linear contrast ε3/ε3 to ε3ε4 to ε4/ε4 |
| Holmes et al.,17 1996 | 164 patients | 60 years and older | Age at onset | Trend for decreasing age at onset of 3–4 years in carriers of the APOEε4 –allele (mean age [SD]: no ε4− versus ε4: 78.7 [7.9] versus 75.5 [5.9], P = 0.004) |
| Murman et al.,20 1996 | 107 normal, elderly control subjects and 123 LOAD patients | 45 years and older | Age at onset | Increased APOEε4 frequencies associated with onset ages of 55 and 75 years, but not at the extremes of onset ages (i.e., onset between 45 and 54 years of age and after age 75) |
| Breitner et al.,14 1999 | 5677 elderly residents of Cache County, Utah | 65 years and older | Prevalence and Age at onset | Age-specific prevalence of LOAD reached in APOEε4 heterozygotes the maximum at age 87, in homozygotes at age 73 and in noncarriers at age 95 |
| Tang et al.,23 1996 | 305 LOAD patients, 485 nondemented controls | LOAD cases: 76.4 ± 9.1 years, controls: 72.9 ± 6.7 years | Relative risk of LOAD, Age at onset | Relative risk (RR) for LOAD associated with APOEε4 homozygosity increased in all ethnic groups (African-American RR = 3.0; 95% confidence interval [CI] = 1.5–5.9; Caucasian RR = 7.3, 95% CI = 2.5–21.6; and Hispanic RR = 2.5, 95% CI = 1.1–5.7), compared with those with APOE-ε3/ε3 genotypes. The risk was also increased for APOE-ε4 heterozygous Caucasians (RR = 2.9, 95% CI = 1.7–5.1) and Hispanics (RR = 1.6, 95% CI = 1.1–2.3), but not for African-Americans (RR = 0.6, 95% CI = 0.4–0.9). The age distribution of the proportion of Caucasians and Hispanics without LOAD was consistently lower for ε4 homozygous and heterozygous individuals than for those with other APOE genotypes |
| Kurz et al.,19 1996 | 91 patients, 69 healthy age-matched controls | 44–95 years | Age at onset | Inheritance of at least one ε4 allele associated with significant reduction of age at onset by 7.7 years among patients 83 years or older, and a weaker relationship among patients aged 44–63 years |
| Poirier et al.,21 1993 | 91 patients with LOAD and 74 controls | Mean age (SD): 75.1 (10.3) | Prevalence of LOAD, Age at onset | Significant association between ε4 and sporadic LOAD (ε4 frequency 0.380 in LOAD and 0.122 in controls, P < 0.01). Age at onset in ε4 carriers earlier than in ε2 or ε4 carriers |
| Mak et al.,47 1996 | 65 LOAD patients and 82 controls | Mean age of 76.5 years | Age at onset | Tendency toward lower age at onset in subjects with 1 or 2 copies of ε4 (mean age of onset [SD] −/− versus 4/− versus 4/4: 73.3 [8.5] versus 72.0 [6.4] versus 71.2 [5.0]), and higher in subjects with ε2/ε2 or ε2/ε3 than in subjects with ε3/ε3 but differences not statistically significant (P = 0.078, Z = 1.419) |
| do Couto et al.,49 1998 | 68 patients with LOAD | Mean age (SD): 68.8 (7.9) | Age at onset | Age at onset significantly higher in patients bearing the APOEε4 allele (ε3/ε4 and ε4/ε4, 65.7 [7.1], n = 40) compared with patients without ε4 allele (ε3/ε3, 61.6 [7.6], n = 28, P < 0.05) |
| Dal Forno et al.,501996 | 101 LOAD subjects | Mean age: 69.6 years | Age at onset | Age at onset highest for ε4 heterozygous subjects and least for ε4 negative subjects. Heterozygous subjects declined more rapidly on MMSE and the Category Fluency Test than subjects without ε4 or ε4 homozygosity |
| COGNITIVE PERFORMANCE | ||||
| Welsh-Bohmer et al.,51 2008 | 507 participants of the Cache County Study of Memory in Aging (CCMS) | 70–110 years | Cognitive performance | No association |
| Salo et al.,52 2001 | 46 nondemented persons | >85 years | Memory performance | No association |
| Murphy et al.,53 1997 | 86 subjects with LOAD | Mean age of onset (SD): based on caregiver report: 65.3 (7.4); based on age when MMSE < 23: 68.8 (7.0) | Rate of decline on MMSE | No association |
| Cosentino et al.,54 2008 | One incident (n = 199) and 2 prevalent samples (n = 215, n = 156) of LOAD patients | Age 65 years and older | Memory performance | Presence of an APOE ε4 allele associated with a more rapid decline in memory perfomance over a 7-year follow-up period |
| Wehling et al.,46 2007 | 70 LOAD patients | 50–75 years | Cognitive performance | APOEε4 carriers had slightly poorer performance than noncarriers on the MMSE (27.5 versus 28.4, P = 0.03) and learning trials of the California Verbal Learning Test (CVLT) (F [1,68] = 5.46, P = 0.022) |
| Hirono et al.,55 2003 | 64 LOAD patients | 60 years or older | Memory performance | Presence of the APOEε4 allele in dose–response fashion associated with accelerated memory decline on Word Recall subtest of ADAS-Cog (mean score −/− versus 4/− versus 4/4: −0.2 versus 0.4 versus 1.0, P = 0.008) |
| Mayeux et al.,56 2001 | 563 healthy elderly without LOAD or questionable dementia | 65 years and older | Memory performance over 7-year follow-up | APOEε4 allele associated with a more rapid decline in memory performance |
| Wilson et al.,57 2002 | 669 participants from the Religious Order Study | 65 years and older | Summary measures of episodic memory, semantic memory, working memory, perceptual speed, and visuospatial ability | Average annual increase of 0.016 units in the ε2 subgroup and annual decreases of 0.022 units in those with ε3/3 and of 0.073 units in the ε4 subgroup |
| Lehmann et al.,58 2006 | 2181 elderly of the Hordaland Health Study | 70–74 years | Episodic memory | APOEε4 effect on episodic memory: OR of cognitive impairment in women 1.8 (95% CI: 1.1–2.8) for heterozygotes and 1.1 (0.3–3.7) for homozygotes; OR in men 1.1 (95% CI 0.6–2.1) for heterozygotes and 10.7 (95% CI 4.7–24) for homozygotes |
| Liu et al.,44 2008 | 2208 related individuals | 50 years and older | Cognitive performance | APOEε4 significantly associated with reduced test scores for Adult Verbal Learning Test, particularly on the memory and learning subdomains |
| Bondi et al.,59 1995 | 52 elderly nondemented | 59–83 years | Performance on California Verbal Learning Test (CVLT) | APOEε4 associated with poorer performance on CVLT. Six of the 14 APOEε4 subjects developed either LOAD or questionable LOAD, whereas none of the 26 non-APOEε4 subjects demonstrated any cognitive decline |
| Dik et al.,60 2001 | 1168 subjects from the population-based Longitudinal Aging Study Amsterdam | 62–85 years | Performance on MMSE, immediate recall and delayed recall, and the Alphabet Coding Task-15 | APOEε4 carriers had a greater rate of cognitive decline shown by MMSE scores and slower information processing speeds after 6 years. The effects of both memory complaints and APOEε4 allele carriage were additive: subjects with both factors had a two times higher cognitive decline than did subjects without both factors |
| Hsiung et al.,61 2004 | 1469 cases with cognitive impairment, 582 controls | Control group: mean age 75.6, group with CIND: mean age 77.8, group with AD: mean age 82.7 | Progression from normal cognition to CIND and from CIND to AD or vascular dementia, age at onset of LOAD | Possession of an APOEε4 allele associated with increased risk of LOAD developing from CIND (OR 2.6, 95% CI 1.48–4.92), and associated with decrease in the age at onset of LOAD |
| Petersen et al.,62 1995 | 66 patients with MCI from Mayo Clinic | Mean age: 79.8 years | Conversion from MCI to dementia | APOEε4 strong predictor for conversion to dementia |
| Caselli et al.,42 1999 | 100 nondemented individuals | Mean age 56 years | Immediate and delayed recall | Tests sensitive to immediate and delayed recall showed significant negative correlation with age in the APOEε4 homozygote group relative to the noncarrier group |
| Flory et al.,43 2000 | 220 nondemented non-Hispanic Caucasian men and women | Aged 24–60 (average age = 46) | Verbal learning and memory (e.g., learning a list of words and recalling them 30 min later), visual memory (e.g., reproducing a previously copied figure from memory), and attention span memory | Performance on learning and memory tasks was significantly poorer in adults having any APOEε4 allele, relative to adults with APOEε2 or APOEε3 genotypes (P < 0.01) |
| Reynolds et al.,63 2006 | 478 nondemented twins from the Swedish Adoption/Twin Study of Aging (SATSA) | 50 years and older | Memory performance over 13 years | APOEε4 associated with working and recall memory ability levels and working memory rate of change, with ε4 homozygotes exhibiting the worst performance at all ages over 13-year follow-up |
| Schultz et al.,45 2008 | 626 male twins randomly drawn from the Vietnam Era Twin (VET) Registry | In their 50s | Memory performance | ε4-carriers: lower performance on immediate and delayed recall than noncarriers (mean [SD] comparing ε4+ versus ε4−: immediate recall 22.19 [5.37] versus 23.8 [6.2]; delayed recall: 19.5 [5.9] versus 20.12 [6.6]) |
In contrast to these studies, two studies found a higher age at onset for patients bearing the APOEε4 allele. In a study by do Couto et al.,49 among 68 patients with LOAD, the age at onset of disease was significantly higher in the patients with the ε4 allele (mean onset [SD] of ε3/ε4 and ε4/ε4, 65.7 [7.1], n = 40) compared with patients without the ε4 allele (mean onset [SD] ε3/ε3, 61.6 [7.6], n = 28, P < 0.05, two-tailed Student’s t-test). Among 101 LOAD patients,50 age at onset was highest for the ε4-heterozygous subjects and lowest for the ε4-negative subjects. The heterozygous subjects declined more rapidly on the Mini-Mental State Examination and the Category Fluency Test than the subjects without the ε4 allele or with ε4 homozygosity. The homozygous subjects declined only faster on the Physical Capacity subscale of the Psychogeriatric Dependency Rating Scale. It is important to note that these two studies included relatively younger patients. It remains possible that the presence of the ε4 allele represents a particularly high risk in the older patients. The bulk of data on age at onset is consistent with the large body of studies showing an association between the APOEε4 allele and risk of LOAD and suggests that the ε4 allele decreases age at onset of LOAD in a dose-dependent manner.
Cognitive Performance as the Phenotype
Few studies, including the Cache County Study of Memory in Aging (CCMS),51 a study among 46 nondemented persons aged 85 years or above from a randomly selected group of 128 subjects in Vantaa, Finland,52 and the study by Murphy et al.,53 observed no effect of the APOE locus on the rate of cognitive decline. It is important to note that these studies either had unspecific assessment of memory,53 small sample sizes,52,53 or consisted of samples prone to survival bias,51 which may limit their ability to detect harmful associations. However, most studies exploring the association of APOE with cognitive performance were consistent with the studies reporting an association of the APOE genotype with LOAD or age at onset of LOAD and showed a harmful effect of the APOEε4 variant with a dose–response relationship of the effect (Table 1). In general, these studies can be divided into studies including and excluding subjects with cognitive impairment or dementia. Studies that explore the effect of APOE on cognitive performance in nondemented subjects provide the ability to draw conclusions about the effect of genetic risk factors on cognition in cognitively normal persons or the preclinical stage of the disease.
Studies Including Subjects with Cognitive Impairment or Dementia
Cosentino et al.54 examined the impact of the APOEε4 variant on the rate of cognitive change in one incident (n = 199) and two prevalent samples (n = 215, n = 156) of LOAD patients 65 years and older. The presence of at least one ε4 allele was associated with faster cognitive decline in the incident LOAD group (P = 0.01). Similar results were observed for the two prevalent dementia samples when adjusting for disease severity or excluding the most impaired participants from the analyses, indicating that the APOEε4 may influence the rate of cognitive decline in both the early and late stages of LOAD. In a study by Wehling et al.,46 which comprised 70 consecutively referred patients aged 50–75 years, APOEε4 carriers showed a slightly poorer performance than noncarriers on the MMSE (27.5 versus 28.4, P = 0.03) and learning trials of the California Verbal Learning Test (CVLT; F (1,68) = 5.46, P = 0.022). Hirono et al.,55 who explored the effect of APOE on cognition in 64 LOAD patients using the Alzheimer Disease Assessment Scale-Cognitive subscale (ADAS-Cog), observed that the presence of the ε4 allele was in a dose–response fashion associated with accelerated memory decline (mean ADAS-Cog score −/− versus 4/− versus 4/4: −0.2 versus 0.4 versus 1.0, P = 0.008).
Studies Excluding Subjects with Cognitive Impairment or Dementia
Most studies exploring these associations among nondemented subjects yielded consistent results, indicating that APOE also exerts its effect in cognitively normal subjects or preclinical stages of the disease. In a study by Mayeux et al.,56 presence of an APOEε4 allele was in 563 nondemented elderly associated with a more rapid decline in a composite score of memory performance over a 7-year follow-up period. Among 669 participants of the Religious Order Study,57 possession of one or more ε4 alleles was over a 8-year follow-up associated with faster decline in episodic memory compared to the ε3/3 genotype, whereas possession of one or more APOEε2 alleles was associated with reduced decline. The rates of change in episodic memory were an average annual increase of 0.016 units in the ε2 subgroup and annual decreases of 0.022 units in those with ε3/3 and of 0.073 units in the ε4 subgroup. In 2181 elderly participants (aged 70–74 years) from the Hordaland Health Study, the APOEε4 allele was in a dose-dependent fashion also associated with lower episodic memory performance. The strongest effect was seen in homozygous men (OR 10.7; 95% CI 4.7–24.0).58 In a Dutch sample of 2208 related individuals, the ε4 variant was associated with reduced test scores for the Adult Verbal Learning Test and within this test was strongest for the memory and learning sub domains.44 Bondi et al.59 explored the effect of APOE on cognition in 52 nondemented elderly using the CVLT. Consistent with the studies described above, APOEε4 carriers demonstrated significantly poorer mean performances than non-carriers. Six of the 14 APOEε4 carriers who completed annual follow-up evaluations developed either LOAD or questionable LOAD, whereas none of the 26 noncarriers demonstrated any cognitive decline.
The longitudinal population-based Longitudinal Aging Study Amsterdam60 explored to what extent subjective memory complaints and APOEε4 allele carriage interact in their prediction of future cognitive decline. In this study of 1168 elderly subjects, APOEε4 carriers had after a 6-year follow-up a greater rate of cognitive decline measured by MMSE scores and slower information processing speeds. This effect appeared to be additive with the effect of memory complaints: subjects with both factors showed a two times higher cognitive decline than did subjects without memory complaints and ε4 allele.
In the Canadian Study of Health and Aging61 and a consecutive sample of 66 patients from the Mayo Clinic Alzheimer’s Disease Center/Alzheimer’s Disease Patient Registry who met criteria for a diagnosis of a mild cognitive impairment (MCI) and who had at least one clinical reevaluation,62 possession of an APOEε4allele increased the risk of conversion from cognitive impairment no dementia (CIND) or MCI to LOAD. In the Canadian Study of Health and Aging the presence of the APOEε4 allele was also associated with a decrease in the age at onset of LOAD.61
In two cross-sectional studies in younger subjects (average ages 46 and 56),42,43 the APOEε4 allele was relative to the noncarrier group associated with significantly poorer performance on learning and memory tasks and immediate and delayed recall, suggesting that age-related memory decline occurs earlier in cognitively healthy APOEε4 carriers than in noncarriers, and precedes clinically detectable LOAD.
Finally, these findings could also be replicated by twin studies. In a longitudinal study over 13 years63 among 478 twins from the Swedish Adoption/Twin Study of Aging (SATSA), the APOEε4 variant was in a dose-dependent fashion at all ages associated with worse working and recall memory and rate of change in working memory. In a second longitudinal twin study among 626 twins in their 50s,45 ε4-carriers showed significantly lower performance on immediate and delayed recall than noncarriers (mean (SD) comparing ε4+ versus ε4−: immediate recall 22.19 (5.37) versus 23.8 (6.2); delayed recall: 19.5 (5.9) versus 20.12 (6.6)), supporting the genetic contribution of APOE to LOAD.
Sensitivity and Specificity of APOE
Studies, assessing the usefulness of the APOE genotype in the diagnosis of AD among persons with dementia, reported specificities of the e4 allele between 81 and 100%64–67 when used in combination with clinical or autopsy criteria but lower specificities when used alone.65 Sensitivity estimates were lower and ranged between 19–75%.64,65,67,68
Sortilin-Related Receptor
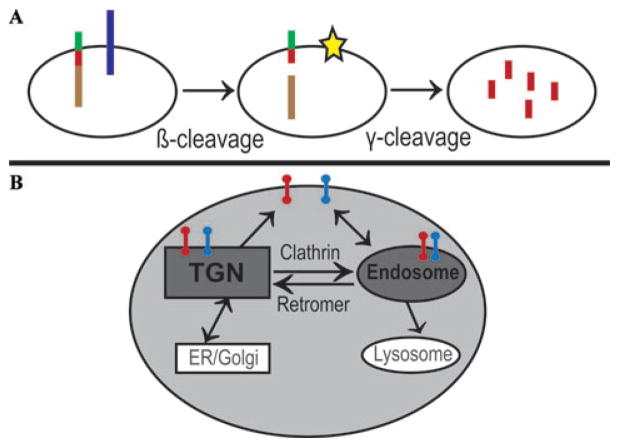
Identification of APP, presenilin 1 (PSEN1), and presenilin 2 (PSEN2) as susceptibility genes for early-onset AD (EOAD) has led to the initiation of the “amyloid cascade,” the basic biochemical formula for production of Aβ, the putative culprit of AD. The amyloid pathway involves two enzymatic steps (Fig. 2): In the first β-cleavage step, BACE cleaves APP near the N terminus of the Aβ peptide; in the second γ-cleavage step, the membrane-bound C-terminal APP fragment is cleaved by γ-secretase, a complex composed of transmembrane proteins PSEN1 and PSEN2, nicastrin, APH1, TMP21, and PEN2.69
Figure 2.
Protein sorting as a key mechanism in Alzheimer’s cell biology. (A) Aβ (red bar) is liberated from its parent protein, APP (multicolor bar), in two enzymatic steps. In the first β-cleavage step, BACE (blue bar) splits full-length APP into an sAPPβ fragment (brown bar) and a C-terminal fragment (CTFβ, red/green bar). Then, in the γ-cleavage step, the γ-secretase (star) splits CTFβ into Aβ (red bar) and amyloid intracellular domain (AICD, green bar). β-cleavage is the committed step in APP processing and may be upregulated in LOAD. (B) Both APP (red bar) and BACE (blue bar) are type-I transmembrane proteins that are sorted through multiple membranous compartments of the cell. The sorting triangle that interconnects the trans-Golgi network (TGN), cell surface, and the endosome is critically important for APP and BACE sorting. As indicated, clathrin is the coat complex that regulates transport from the cell surface and the TGN to the endosome, whereas the retromer is the coat complex that regulates transport from the endosome back to the TGN. APP and BACE interact within the membranes of the endosomal system, initiating the amyloidogenic pathway. (Illustration adapted from Small and Gandy.75) (In color in Annals online.)
It is notable that APP and the secretases are all integral transmembrane proteins. Further, they are dynamically sorted through the plasma membrane and the membranes of intracellular organelles, and the liberation of Aβ involves a transmembrane secretase enzyme acting on a transmembrane APP C-terminal fragment substrate. Thus, from a cell biology perspective, sorting mechanisms that cause APP and the secretases to colocalize in the same membranous compartment are expected to play important roles in the regulation of Aβ production. Over 30% of all proteins are transmembrane proteins,70 and most are typically sorted via the secretory and endocytic pathways.71,72 During the last two decades, the trans-Golgi network and the endosome were identified as the key organelles organizing the complex movement of the transmembrane proteins via secretory and endocytic pathways (Fig. 2B). Important coat complexes initiating the transport of APP and BACE through this sorting itinerary are the clathrin coat and the retromer.73–75 Clathrin coats are involved in the endocytic pathway connecting the cell surface to the endosome and the pathway connecting the trans-Golgi network to the endosome.76 The retromer is involved in the trafficking from the endosome to the trans-Golgi network.
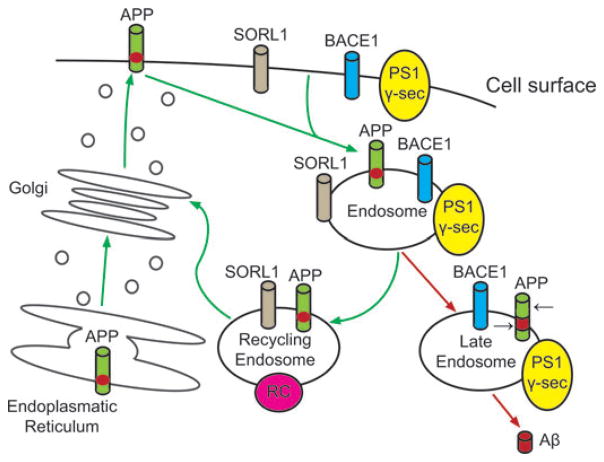
Recent studies showed that SORL1 is involved in trafficking of APP from the cell surface to the golgi-endoplasmic reticulum complex (Fig. 3). Under-expression of SORL1 leads to over-expression of Aβ and an increased risk of AD.77 Most studies exploring the effect of SORL1 on cognitive impairment or dementia used LOAD as a dichotomized trait in the analyses. Rogaeva and colleagues77 first reported the allelic and haplotypic associations among LOAD and variants in SORL1 (Table 2). Subsequently, several studies supported the initial finding by showing that genetic variants in SORL1 contribute toward LOAD.78–83 The original study included four different ethnic groups, ranging from North American and European Caucasians, Caribbean Hispanics, African-Americans, and Israeli-Arabs. With this investigation on over 6000 subjects, two different sets of haplotypes were identified: (1) SNPs in the 5′ end of the gene (SNP 8–10; 120873131 bp-120886175 bp) among Caribbean Hispanics (family study), Caucasians (case–control study), and Israeli-Arabs (case–control study); and (2) SNPs in the 3′ end of the gene (SNP 22–25; 120962172 bp-120988611 bp) among multiple Caucasian samples (family and case–control studies) and African-Americans (family study). Haplotype analysis strengthened the statistical support further. However, as observed in many common diseases, these candidate SNPs confer a modestly elevated risk of LOAD, ranging from an odds ratio of 1.4 to 2.2, and the allelic association was not uniform across data sets or ethnic groups. The authors strengthened their allelic association findings by functional cell biology findings, which showed that suppression of SORL1 led to elevation of Aβ levels.77 Two subsequent studies by the same group broadly supported 1 or both haplotypes or some variations of the 2: Haplotype C-G-C at SNPs 8–10 or haplotype T-T-C at SNPs 23–25 or both. Lee and colleagues80 showed that the same set of SNPs at SNPs 23–25 were associated with LOAD in Caucasians residing in northern Manhattan. They then confirmed the allelic and haplotypic associations in autopsy confirmed cases of Caucasian ethnicity for haplotype at SNPs 8–10 and haplotype at SNPs 23–25.79
Figure 3.
Role of SORL1 in transmembrane sorting of APP. The green arrows track re-entry of APP from the cell surface when SORL1 is present. The red arrows show that, when SORL1 is absent, more APP moves into domains, such as the late endosome/lysosome, where the black arrows show how it is subsequently cut by beta-secretase (BACE1) and gamma-secretase (PS1 γ-sec), generating the neurotoxic amyloid beta-peptide (Aβ). (Illustration adapted from Rogaeva et al.77) (In color in Annals online.)
TABLE 2.
Summary of Studies Relating SORL1 with LOAD
| Haplotype 1 |
Haplotype 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Author (Year) | Age | rs668387 SNP 8 1,2 | rs689021 SNP 9 | rs641120 SNP 10 | rs3824968 SNP 23 | rs2282649 SNP 24 | rs1010159 SNP 25 | Other Significant SNPs |
| Significant Association | ||||||||
| Rogaeva et al. (2007) | Mean AAO: 70 ± 9 – 77 ± 8 | |||||||
| Caucasians (family data set) | T | T | C | |||||
| Caribbean Hispanics | C | G | C | |||||
| Caucasians (case–control data sets) | C | G | C | T | T | C | ||
| Israeli Arabs | C | G | C | |||||
| African-Americans | ||||||||
| Lee et al. (2007) - Northern Manhattan | Mean AAO: 79.1 ± 5.1 – 84.4 ± 8.0 | |||||||
| Caucasians | C | A | T | T | T | C | rs3824966 (SNP 20) | |
| Hispanics | rs12285364 (SNP 12) | |||||||
| African-Americans | C | G | T | C | C | rs12285364, rs1784933 (SNP 26) | ||
| Meng et al. (2007)2 | Not released | |||||||
| Caucasians | + | + | + | |||||
| Lee et al. (2007) - Autopsy | Mean AAO: cases: 80.5, controls: 79.9 | |||||||
| Caucasians | C | G | C | A | T | C | ||
| Tan et al. (2007) | Mean AAO: 71.2 ± 8.9 | |||||||
| Han Chinese | A | T | ||||||
| Seshadri et al. (2007)3 | Mean age: 62 +9 | |||||||
| Caucasians | + | rs1131497 (SNP29) | ||||||
| Bettens et al. (2008) | Mean AAO: 79.0 ± 5.2 | |||||||
| Caucasians | C | G | C | rs560573 (SNP 6), rs1614735 (SNP 27) | ||||
| Lee et al. (2008), re-analyzing data by Shibata et al. (2008) | ||||||||
| Japanese | C | T | ||||||
| Weak Association | ||||||||
| Webster et al. (2008)2 | Age ≥ 65 years | |||||||
| Caucasians | + | + | ||||||
| Grupe et al. (2007)2 | Mean age: 77.4 ± 7.5 (WU), 76.4 ± 6.1 (UK1), 76.5 ± 5.6 (UK2) | |||||||
| Caucasians | T | rs2070045 (SNP 19) | ||||||
| No Association | ||||||||
| Li H (2008)4 | Mean AAO ≥60 | |||||||
| Caucasians | ||||||||
SNP numbers from Rogaeva et al. (2007) are presented. Alleles are presented only when significant alleles in bold were statistically significant in either allelic, genotypic, or haplotypic analysis.
Used the nearest SNPs (indicated with a “+” sign) when different SNPs were used.
Endophenotype was studied.
No specific marker information for SORL1 was available from the paper. AAO = age of onset.
Seven other groups examined the relation between SORL1 and LOAD or LOAD endophenotypes in different populations (Table 2).78,82–86 Three replication studies supported the initial findings, while the remaining three showed either negative or weak results. Three clearly positive studies included one by Tan et al.83 and the other by Seshadri et al.82 Bettens and colleagues78 directly replicated SNPs 8–10 and showed support for SNPs 25–27 in 550 Belgians with LOAD and 637 unaffected individuals. Tan et al.83 examined 223 cases and 263 controls from a Han Chinese population to show that haplotype G-C-A at SNP 19–22-23 were associated with LOAD (OR = 1.35; 1.04–1.74), but none of the haplotypes in SNP 8–10 were associated. Webster et al.86 and Li et al.85 reported weak associations.
Li et al.84 and Shibata et al.87 reported no associations between SORL1 and LOAD. However, in the latter study the negative results were based on genotypic association analyses only. When Lee et al.88 re-analyzed the data of this study using allelic association tests, SNPs 8 and 24 were significantly associated with LOAD, supporting the association in both the 3′ and 5′ regions of SORL1. Using the Framingham community-based family samples, Seshadri et al.82 extended the existing studies using cognitive performance as an endophenotype. The authors reported that SORL1 was significantly associated with abstract reasoning ability as measured by the Similarity test (P = 3.2 × 10−6). However, they did not observe an association with memory. A possible explanation for this discrepancy may be that this sample consisted of 705 related persons, which can lead to limited power to uncover associations as compared to larger samples that include unrelated subjects.
Overall, these genetic and functional genomic studies provide compelling evidence for a role of SORL1 in LOAD. Putative variants and their sensitivity and specificity for LOAD diagnosis, however, remain to be identified, as the reported variants do not affect coding sequence or splicing. In any case, the results of the above summarized studies imply that (a) there are several different LOAD–associated allelic variants in distinct regions of the SORL1 gene in different populations; (b) these variants are likely to be in intronic regulatory sequences that might govern cell type–specific or tissue-specific expression of SORL1; and (c) these variants affect this risk by altering the physiological role of SORL1 in the processing of APP holoprotein.
Other Genes
Several genes, in particular genes mapping to chromosome 10q21–25 have been reported to influence Aβ levels in LOAD. In a study by Ertekin-Taner et al.,12 Aβ42 levels were related to a missense C/T polymorphism in exon 6 in the urokinase-type plasminogen activator (PLAU) gene at chromosome 10q24. In a second study by the same group, genetic variants in a haplotype block spanning the insulin degrading enzyme (IDE) mapping to 10q23–25 were significantly associated with plasma Aβ42 levels.11 The latter finding is consistent with a study by Farris et al.,13 demonstrating that partial loss-of-function mutations in IDE, which induce diabetes, also impair degradation of Aβ protein. PLAU 12 and IDE11 were also associated with an increased risk of LOAD, supporting the usefulness of Aβ levels as a LOAD endophenotype. Additional genes and putative loci have been reported, but independent replication remains inconsistent. There is little concordance between case–control and family-based studies89–93 suggesting that both clinical and genetic heterogeneity influence the outcome of these analyses. LRP6, a coreceptor for Wnt signaling, has been associated with LOAD and confirmed in a case–control analysis.94 GAB2 may modify the risk of LOAD in APOEε4 carriers and has been associated with hyperphosporylation of tau protein.95 The P86L polymorphism in CALHM1, which encodes an essential component of a previously uncharacterized cerebral Ca2+ channel that may control Aβ levels, has been putatively associated with LOAD.96 Additional genes that have been reported but remain to be confirmed include CHRNB2, A2M, CTNNA3, GSTO1, GSTO2, and GAPD.97–100 There also continues to be support for linkage to LOAD at 6p, 9q, 10q and 12p and 19q,101–111 but specific causative loci could not yet be identified. In contrast, mutations in APP, PSEN1, and PSEN2 genes are associated with EOAD (onset ≤ 60 years) with an autosomal pattern of inheritance.
Discussion
Theoretically, genes involved in AD could be used as diagnostic tools or potential novel therapeutic targets for treatment. However, in contrast to EOAD, which is caused by mutations in APP, PSEN1, and PSEN2 that have almost complete penetrance (>85%), and a clear-cut autosomal dominant pattern of inheritance, LOAD is a complex genetic disorder, in which multiple genes with small effects each are likely to contribute. Although segregation analyses conducted in families of patients with LOAD support the presence of at least four to six major genes,112,113 only two genes have been firmly implicated as genetic risk factors: SORL1 and APOE. Additional genes and genetic loci that have been reported but remain to be confirmed include CHRNB2, A2M, CTNNA3, GSTO1, GSTO2, GAPD, and loci at 6p, 9q, 10q and 12p and 19q.
The difficulty in gene detection in LOAD may reflect the continued use of small cohorts of patients underpowered for genetic studies in this complex disease, in which multiple genes with small effects each quantitative trait loci (QTLs) are likely to contribute to the various quantitative traits associated with the disease, such as memory performance, amyloid/tau pathology, or hippocampal atrophy. Alternatively, it could reflect a failure to develop or make use of better quantitative endophenotypes. Endophenotypes are closer to the action of the gene than affection status, exhibit higher genetic signal-to-noise ratios,114 and thus provide greater power to localize and identify disease-related QTLs than does affection status alone.115 It is possible that the endophenotypes that are commonly used in dementia research are too heterogeneous to observe an effect. Finally, genetic research is often hampered by uncontrolled population stratification, pleiotropic effects (a single gene influences multiple phenotypic traits), or locus or allelic heterogeneity (a single disorder is caused by mutations in different genes or various mutations in a single gene, respectively), which frequently lead to null associations.
As described above, with a population attributable risk that is estimated at 20–50%,24,25 the ε4 allele increases LOAD risk in a dose-dependent fashion; one ε4 allele is associated with a two- to three-fold increased risk, having two copies is associated with a fiveto 10-fold increase. Also affected in a dose-dependent fashion is age of onset of AD. The two haplotypes in the 3′ and 5′ regions of SORL1 that repeatedly were found to be associated with LOAD among Caribbean Hispanic and Northern European families, in case–control studies of Europeans, Americans, African-Americans, and Israeli Arabs, had effect sizes ranging from odds ratios of 1.4 to 2.2. However, APOE has only moderate diagnostic sensitivity and specificity, and both APOE and SORL1 increase LOAD risk in a non-Mendelian fashion, are not fully penetrant, and environmental factors either exacerbate or modify their effects. Moreover, they are neither necessary nor sufficient by themselves to cause LOAD. The same is likely to be true for the remaining yet-to-be-identified genetic factors associated with LOAD. These facts suggest that genetic variants associated with LOAD are better suited as risk biomarkers than diagnostic tools.
While probably not suitable as diagnostic markers, both SORL1 and APOE may be targets for prevention and treatment. However, several issues must be resolved before development of a drug based on these genes can be considered. First, for both genes, it is necessary to clarify the exact mechanisms through which they increase LOAD risk. Second, it is necessary to further characterize the molecular pathways in which they are involved or with which they interact. Clarification of the biological functions, risk-factor activities and pathways of SORL1 and APOE will help to understand their role in LOAD and can provide targets for effective intervention. Third, for SORL1, the precise putative genetic variants have to be identified. The reported variants are nonfunctional and do not affect coding sequence or splicing. Fourth, the additional risk factor genes involved in LOAD need to be known. The accurate risks associated with each gene involved can only be estimated when all putative and protective genetic variants are known. Fifth, it has to be clarified whether the genes mediating each endophenotype involved in LOAD are distinct from each other. This is a key assumption of the endophenotype approach, yet empirical proof of this remains to be determined. A substantial degree of overlap appears likely for a number of the known genes associated with EOAD and LOAD, at least including the APP, PSEN1, PSEN2, APOE, and SORL1, given that these genes are involved in either the production or processing of the Aβ peptide. Nevertheless, each gene has a unique role in this cascade, and it thus seems likely these loci will differ in their magnitudes of influence across the brain systems affected in this disorder. Sixth, it has to be clarified how SORL1 and APOE (along with others that remain to be identified) coalesce in influencing liability to overt expression of LOAD. It remains unclear whether their effects are independent or additive or interactive. Finally, it has to be determined whether these genes are unique to cognitive impairment in LOAD or are shared by other diseases, such as Dementia with Lewy Bodies, Parkinson’s disease, or depression. Lewy body inclusions and Lewy neurites, the key pathological hallmarks of dementia with Lewy Bodies and Parkinson’s disease, are a frequent coexistent pathological change observed in autopsy-confirmed LOAD.
The issues posed above raise considerable challenges for investigators attempting to unravel the genetic complexity of LOAD. Only when these issues are better understood, development of preventive and treatment strategies based on genetic risk factors, including SORL1 and APOE, can be considered. Nevertheless, we have entered a new era in which conjoint advances in functional genomics and genetic epidemiological techniques are enabling rapid progress with multiple gene discoveries. The major advantage of genetic studies is the ability to overcome limitations of classic epidemiological techniques through “Mendelian randomization.”116 While in conventional observational studies establishing causal relationships between environmental exposures and common diseases is beset with unresolved confounding, reverse causation and selection bias that can result in spurious inferences, the laws of Mendelian genetics ensure that in a correctly designed genetic association study comparison of groups of individuals defined by genotype is equivalent to a randomized comparison. By definition, these groups will not differ systematically, except with respect to allelic associations (linkage disequilibrium) that extend through a short genomic region from the locus under study, as the inheritance of genetic variants is independent of the inheritance of other traits (i.e., “randomized”).
If correctly conducted and carefully interpreted, the merge of modern functional genomics with large-scale studies of genetically at-risk samples and sophisticated statistical algorithms can be a powerful tool for identification of genes, and therefore biomarkers, associated with common complex diseases, such as LOAD.
Acknowledgments
This work was supported by federal grants from the National Institute on Aging of the National Institutes of Health (P01AG07232, R37AG15473, P50 AG08702) and by grants from the Alzheimer Association, the Blanchette Hooker Rockefeller Fund, the Robertson Gift from the Banbury Fund, and the Merrill Lynch Foundation.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fratiglioni L, De Ronchi D, Aguero-Torres H. Worldwide prevalence and incidence of dementia. Drugs Aging. 1999;15:365–375. doi: 10.2165/00002512-199915050-00004. [DOI] [PubMed] [Google Scholar]
- 3.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 5.Lopez OL, Swihart AA, Becker JT, et al. Reliability of NINCDS-ADRDA clinical criteria for the diagnosis of Alzheimer’s disease. Neurology. 1990;40:1517–1522. doi: 10.1212/wnl.40.10.1517. [DOI] [PubMed] [Google Scholar]
- 6.Kukull WA, Larson EB, Reifler BV, et al. Interrater reliability of Alzheimer’s disease diagnosis. Neurology. 1990;40:257–260. doi: 10.1212/wnl.40.2.257. [DOI] [PubMed] [Google Scholar]
- 7.Schofield PW, Tang M, Marder K, et al. Consistency of clinical diagnosis in a community-based longitudinal study of dementia and Alzheimer’s disease. Neurology. 1995;45:2159–2164. doi: 10.1212/wnl.45.12.2159. [DOI] [PubMed] [Google Scholar]
- 8.Morris JC, McKeel DW, Jr, Fulling K, et al. Validation of clinical diagnostic criteria for Alzheimer’s disease. Ann Neurol. 1988;24:17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- 9.Burns A, Luthert P, Levy R, et al. Accuracy of clinical diagnosis of Alzheimer’s disease. BMJ (Clinical Research Ed) 1990;301:1026. doi: 10.1136/bmj.301.6759.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meyer JM, Breitner JC. Multiple threshold model for the onset of Alzheimer’s disease in the NAS-NRC twin panel. Am J Med Genet. 1998;81:92–97. doi: 10.1002/(sici)1096-8628(19980207)81:1<92::aid-ajmg16>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Ertekin-Taner N, Allen M, Fadale D, et al. Genetic variants in a haplotype block spanning IDE are significantly associated with plasma Abeta42 levels and risk for Alzheimer disease. Hum Mutat. 2004;23:334–342. doi: 10.1002/humu.20016. [DOI] [PubMed] [Google Scholar]
- 12.Ertekin-Taner N, Ronald J, Feuk L, et al. Elevated amyloid beta protein (Abeta42) and late onset Alzheimer’s disease are associated with single nucleotide polymorphisms in the urokinase-type plasminogen activator gene. Hum Mol Genet. 2005;14:447–460. doi: 10.1093/hmg/ddi041. [DOI] [PubMed] [Google Scholar]
- 13.Farris W, Mansourian S, Leissring MA, et al. Partial loss-of-function mutations in insulin-degrading enzyme that induce diabetes also impair degradation of amyloid beta-protein. Am J Pathol. 2004;164:1425–1434. doi: 10.1016/s0002-9440(10)63229-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- 15.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 16.Gomez-Isla T, West HL, Rebeck GW, et al. Clinical and pathological correlates of apolipoprotein E epsilon 4 in Alzheimer’s disease. Ann Neurol. 1996;39:62–70. doi: 10.1002/ana.410390110. [DOI] [PubMed] [Google Scholar]
- 17.Holmes C, Levy R, McLoughlin DM, et al. Apolipoprotein E: non-cognitive symptoms and cognitive decline in late onset Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1996;61:580–583. doi: 10.1136/jnnp.61.6.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyman BT, Gomez-Isla T, Rebeck GW, et al. Epidemiological, clinical, and neuropathological study of apolipoprotein E genotype in Alzheimer’s disease. Ann N Y Acad Sci. 1996;802:1–5. doi: 10.1111/j.1749-6632.1996.tb32592.x. [DOI] [PubMed] [Google Scholar]
- 19.Kurz A, Altland K, Lautenschlager N, et al. Apolipoprotein E type 4 allele and Alzheimer’s disease: effect on age at onset and relative risk in different age groups. J Neurol. 1996;243:452–456. doi: 10.1007/BF00900498. [DOI] [PubMed] [Google Scholar]
- 20.Murman DL, Foster NL, Kilgore SP, et al. Apolipoprotein E and Alzheimer’s disease: strength of association is related to age at onset. Dementia. 1996;7:251–255. doi: 10.1159/000106888. [DOI] [PubMed] [Google Scholar]
- 21.Poirier J, Davignon J, Bouthillier D, et al. Apolipoprotein E polymorphism and Alzheimer’s disease. Lancet. 1993;342:697–699. doi: 10.1016/0140-6736(93)91705-q. [DOI] [PubMed] [Google Scholar]
- 22.Roses AD. Alzheimer’s disease: the genetics of risk. Hosp Pract (Minneap) 1997;32:51–55. 58–63, 67–69. doi: 10.1080/21548331.1997.11443525. [DOI] [PubMed] [Google Scholar]
- 23.Tang MX, Maestre G, Tsai WY, et al. Relative risk of Alzheimer disease and age-at-onset distributions, based on APOE genotypes among elderly African Americans, Caucasians, and Hispanics in New York City. Am J Hum Genet. 1996;58:574–584. [PMC free article] [PubMed] [Google Scholar]
- 24.Slooter AJ, Cruts M, Kalmijn S, et al. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Arch Neurol. 1998;55:964–968. doi: 10.1001/archneur.55.7.964. [DOI] [PubMed] [Google Scholar]
- 25.Ashford JW, Mortimer JA. Non-familial Alzheimer’s disease is mainly due to genetic factors. J Alzheimers Dis. 2002;4:169–177. doi: 10.3233/jad-2002-4307. [DOI] [PubMed] [Google Scholar]
- 26.Strittmatter WJ, Weisgraber KH, Huang DY, et al. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:8098–8102. doi: 10.1073/pnas.90.17.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmechel DE, Saunders AM, Strittmatter WJ, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 29.Bales KR, Verina T, Cummins DJ, et al. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bales KR, Verina T, Dodel RC, et al. Lack of apolipoprotein E dramatically reduces amyloid beta-peptide deposition. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 31.Kindy MS, Rader DJ. Reduction in amyloid A amyloid formation in apolipoprotein-E-deficient mice. Am J Pathol. 1998;152:1387–1395. [PMC free article] [PubMed] [Google Scholar]
- 32.Ma J, Yee A, Brewer HB, Jr, et al. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994;372:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 33.Sanan DA, Weisgraber KH, Russell SJ, et al. Apolipoprotein E associates with beta amyloid peptide of Alzheimer’s disease to form novel monofibrils. Isoform apoE4 associates more efficiently than apoE3. J Clin Investig. 1994;94:860–869. doi: 10.1172/JCI117407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holtzman DM, Bales KR, Tenkova T, et al. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holtzman DM, Bales KR, Wu S, et al. Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer’s disease. J Clin Investig. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sparks DL, Scheff SW, Hunsaker JC, 3rd, et al. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 37.Refolo LM, Malester B, LaFrancois J, et al. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Disease. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- 38.Bodovitz S, Klein WL. Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J Biol Chem. 1996;271:4436–4440. doi: 10.1074/jbc.271.8.4436. [DOI] [PubMed] [Google Scholar]
- 39.Howland DS, Trusko SP, Savage MJ, et al. Modulation of secreted beta-amyloid precursor protein and amyloid beta-peptide in brain by cholesterol. J Biol Chem. 1998;273:16576–16582. doi: 10.1074/jbc.273.26.16576. [DOI] [PubMed] [Google Scholar]
- 40.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 41.Gozal D, Capdevila OS, Kheirandish-Gozal L, Crabtree VM. APOE epsilon 4 allele, cognitive dysfunction, and obstructive sleep apnea in children. Neurology. 2007;69:243–249. doi: 10.1212/01.wnl.0000265818.88703.83. [DOI] [PubMed] [Google Scholar]
- 42.Caselli RJ, Graff-Radford NR, Reiman EM, et al. Preclinical memory decline in cognitively normal apolipoprotein E-epsilon4 homozygotes. Neurology. 1999;53:201–207. doi: 10.1212/wnl.53.1.201. [DOI] [PubMed] [Google Scholar]
- 43.Flory JD, Manuck SB, Ferrell RE, et al. Memory performance and the apolipoprotein E polymorphism in a community sample of middle-aged adults. Am J Med Genet. 2000;96:707–711. doi: 10.1002/1096-8628(20001204)96:6<707::aid-ajmg1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 44.Liu F, Pardo LM, Schuur M, et al. The apolipoprotein E gene and its age-specific effects on cognitive function. Neurobiol Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.09.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Schultz MR, Lyons MJ, Franz CE, et al. Apolipoprotein E genotype and memory in the sixth decade of life. Neurology. 2008;70(19 Pt 2):1771–1777. doi: 10.1212/01.wnl.0000286941.74372.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wehling E, Lundervold AJ, Standnes B, et al. APOE status and its association to learning and memory performance in middle aged and older Norwegians seeking assessment for memory deficits. Behav Brain Funct. 2007;3:57. doi: 10.1186/1744-9081-3-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mak YT, Chiu H, Woo J, et al. Apolipoprotein E genotype and Alzheimer’s disease in Hong Kong elderly Chinese. Neurology. 1996;46:146–149. doi: 10.1212/wnl.46.1.146. [DOI] [PubMed] [Google Scholar]
- 48.Hallman DM, Boerwinkle E, Saha N, et al. The apolipoprotein E polymorphism: a comparison of allele frequencies and effects in nine populations. Am J Hum Genet. 1991;49:338–349. [PMC free article] [PubMed] [Google Scholar]
- 49.do Couto FS, de Mendonca A, Garcia C, et al. Age of onset in patients with Alzheimer’s disease with different apoE genotypes. J Neurol Neurosurg Psychiatry. 1998;64:817. doi: 10.1136/jnnp.64.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dal Forno G, Rasmusson DX, Brandt J, et al. Apolipoprotein E genotype and rate of decline in probable Alzheimer’s disease. Arch Neurol. 1996;53:345–350. doi: 10.1001/archneur.1996.00550040085017. [DOI] [PubMed] [Google Scholar]
- 51.Welsh-Bohmer KA, Ostbye T, Sanders L, et al. Neuropsychological performance in advanced age: influences of demographic factors and apolipoprotein E: findings from the Cache County Memory Study. Clin Neuropsychol. 2008:1–23. doi: 10.1080/13854040801894730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salo A, Ylikoski R, Verkkoniemi A, et al. Does apolipoprotein E influence learning and memory in the nondemented oldest old? Int Psychogeriatr. 2001;13:451–459. doi: 10.1017/s1041610201007864. [DOI] [PubMed] [Google Scholar]
- 53.Murphy GM, Jr, Taylor J, Kraemer HC, et al. No association between apolipoprotein E epsilon 4 allele and rate of decline in Alzheimer’s disease. Am J Psychiatry. 1997;154:603–608. doi: 10.1176/ajp.154.5.603. [DOI] [PubMed] [Google Scholar]
- 54.Cosentino S, Scarmeas N, Helzner E, et al. APOE epsilon 4 allele predicts faster cognitive decline in mild Alzheimer disease. Neurology. 2008;70(19 Pt 2):1842–1849. doi: 10.1212/01.wnl.0000304038.37421.cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirono N, Hashimoto M, Yasuda M, et al. Accelerated memory decline in Alzheimer’s disease with apolipoprotein epsilon4 allele. J Neuropsychiatry Clin Neurosci. 2003;15:354–358. doi: 10.1176/jnp.15.3.354. [DOI] [PubMed] [Google Scholar]
- 56.Mayeux R, Small SA, Tang M, et al. Memory performance in healthy elderly without Alzheimer’s disease: effects of time and apolipoprotein-E. Neurobiol Aging. 2001;22:683–689. doi: 10.1016/s0197-4580(01)00223-8. [DOI] [PubMed] [Google Scholar]
- 57.Wilson RS, Bienias JL, Berry-Kravis E, et al. The apolipoprotein E epsilon 2 allele and decline in episodic memory. J Neurol Neurosurg Psychiatry. 2002;73:672–677. doi: 10.1136/jnnp.73.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lehmann DJ, Refsum H, Nurk E, et al. Apolipoprotein E epsilon4 and impaired episodic memory in community-dwelling elderly people: a marked sex difference. The Hordaland Health Study. J Neurol Neurosurg Psychiatry. 2006;77:902–908. doi: 10.1136/jnnp.2005.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bondi MW, Salmon DP, Monsch AU, et al. Episodic memory changes are associated with the APOE-epsilon 4 allele in nondemented older adults. Neurology. 1995;45:2203–2206. doi: 10.1212/wnl.45.12.2203. [DOI] [PubMed] [Google Scholar]
- 60.Dik MG, Jonker C, Comijs HC, et al. Memory complaints and APOE-epsilon4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001;57:2217–2222. doi: 10.1212/wnl.57.12.2217. [DOI] [PubMed] [Google Scholar]
- 61.Hsiung GY, Sadovnick AD, Feldman H. Apolipoprotein E epsilon4 genotype as a risk factor for cognitive decline and dementia: data from the Canadian Study of Health and Aging. CMAJ. 2004;171:863–867. doi: 10.1503/cmaj.1031789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 63.Reynolds CA, Prince JA, Feuk L, et al. Longitudinal memory performance during normal aging: twin association models of APOE and other Alzheimer candidate genes. Behav Genet. 2006;36:185–194. doi: 10.1007/s10519-005-9027-6. [DOI] [PubMed] [Google Scholar]
- 64.Saunders AM, Hulette O, Welsh-Bohmer KA, et al. Specificity, sensitivity, and predictive value of apolipoprotein-E genotyping for sporadic Alzheimer’s disease. Lancet. 1996;348:90–93. doi: 10.1016/s0140-6736(96)01251-2. [DOI] [PubMed] [Google Scholar]
- 65.Mayeux R, Saunders AM, Shea S, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s disease centers consortium on apolipoprotein E and Alzheimer’s disease. N Engl J Med. 1998;338:506–511. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- 66.Roses AD. Apolipoprotein E genotyping in the differential diagnosis, not prediction, of Alzheimer’s disease. Ann Neurol. 1995;38:6–14. doi: 10.1002/ana.410380105. [DOI] [PubMed] [Google Scholar]
- 67.Slooter AJ, Breteler MB, Ott A, et al. APOE genotyping in differential diagnosis of Alzheimer’s disease. Lancet. 1996;348:334. doi: 10.1016/s0140-6736(05)64501-1. [DOI] [PubMed] [Google Scholar]
- 68.Kakulas BA, Wilton SD, Fabian VA, Jones TM. Apolipoprotein-E genotyping in diagnosis of Alzheimer’s disease. Lancet. 1996;348:483. doi: 10.1016/s0140-6736(05)64588-6. [DOI] [PubMed] [Google Scholar]
- 69.Edbauer D, Winkler E, Regula JT, et al. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- 70.Cobbold C, Monaco AP, Sivaprasadarao A, Ponnambalam S. Aberrant trafficking of transmembrane proteins in human disease. Trends Cell Biol. 2003;13:639–647. doi: 10.1016/j.tcb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 71.Harter C, Reinhard C. The secretory pathway from history to the state of the art. Sub-Cell Biochem. 2000;34:1–38. doi: 10.1007/0-306-46824-7_1. [DOI] [PubMed] [Google Scholar]
- 72.Le Borgne R, Hoflack B. Protein transport from the secretory to the endocytic pathway in mammalian cells. Biochim Biophys Acta. 1998;1404:195–209. doi: 10.1016/s0167-4889(98)00057-3. [DOI] [PubMed] [Google Scholar]
- 73.Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- 74.He X, Li F, Chang WP, Tang J. GGA proteins mediate the recycling pathway of memapsin 2 (BACE) J Biol Chem. 2005;280:11696–11703. doi: 10.1074/jbc.M411296200. [DOI] [PubMed] [Google Scholar]
- 75.Small SA, Gandy S. Sorting through the cell biology of Alzheimer’s disease: intracellular pathways to pathogenesis. Neuron. 2006;52:15–31. doi: 10.1016/j.neuron.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Traub LM. Common principles in clathrin-mediated sorting at the Golgi and the plasma membrane. Biochim Biophys Acta. 2005;1744:415–437. doi: 10.1016/j.bbamcr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 77.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bettens K, Brouwers N, Engelborghs S, et al. SORL1 is genetically associated with increased risk for late-onset Alzheimer disease in the Belgian population. Hum Mutat. 2008;29:769–770. doi: 10.1002/humu.20725. [DOI] [PubMed] [Google Scholar]
- 79.Lee JH, Cheng R, Honig LS, et al. Association between genetic variants in SORL1 and autopsy-confirmed Alzheimer disease. Neurology. 2008;70:887–889. doi: 10.1212/01.wnl.0000280581.39755.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee JH, Cheng R, Schupf N, et al. The association between genetic variants in SORL1 and Alzheimer disease in an urban, multiethnic, community-based cohort. Arch Neurol. 2007;64:501–506. doi: 10.1001/archneur.64.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Meng Y, Lee JH, Cheng R, et al. Association between SORL1 and Alzheimer’s disease in a genome-wide study. Neuroreport. 2007;18:1761–1764. doi: 10.1097/WNR.0b013e3282f13e7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seshadri S, DeStefano AL, Au R, et al. Genetic correlates of brain aging on MRI and cognitive test measures: a genome-wide association and linkage analysis in the Framingham Study. BMC Med Genet. 2007;8(Suppl 1):S15. doi: 10.1186/1471-2350-8-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tan EK, Lee J, Chen CP, et al. SORL1 haplotypes modulate risk of Alzheimer’s disease in Chinese. Neurobiol Aging. 2009;30:1048–1051. doi: 10.1016/j.neurobiolaging.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 84.Li H, Wetten S, Li L, et al. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Arch Neurol. 2008;65:45–53. doi: 10.1001/archneurol.2007.3. [DOI] [PubMed] [Google Scholar]
- 85.Li Y, Rowland C, Catanese J, et al. SORL1 variants and risk of late-onset Alzheimer’s disease. Neurobiol Dis. 2008;29:293–296. doi: 10.1016/j.nbd.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Webster JA, Myers AJ, Pearson JV, et al. Sorl1 as an Alzheimer’s disease predisposition gene? Neurodegener Dis. 2008;5:60–64. doi: 10.1159/000110789. [DOI] [PubMed] [Google Scholar]
- 87.Shibata N, Ohnuma T, Baba H, et al. Genetic association between SORL1 polymorphisms and Alzheimer’s disease in a Japanese population. Dement Geriatr Cogn Disord. 2008;26:161–164. doi: 10.1159/000149821. [DOI] [PubMed] [Google Scholar]
- 88.Lee JH, Shibata N, Cheng R, Mayeux R. Possible association between SORL1 and Alzheimer disease? Reanalyzing the data of Shibata et al. Dement Geriatr Cogn Disord. 2008;26:482. doi: 10.1159/000167792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bertram L, McQueen MB, Mullin K, et al. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 90.Grupe A, Abraham R, Li Y, et al. Evidence for novel susceptibility genes for late-onset Alzheimer’s disease from a genome-wide association study of putative functional variants. Hum Mol Genet. 2007;16:865–873. doi: 10.1093/hmg/ddm031. [DOI] [PubMed] [Google Scholar]
- 91.Holmans P, Hamshere M, Hollingworth P, et al. Genome screen for loci influencing age at onset and rate of decline in late onset Alzheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2005;135:24–32. doi: 10.1002/ajmg.b.30114. [DOI] [PubMed] [Google Scholar]
- 92.Liu F, Arias-Vasquez A, Sleegers K, et al. A genomewide screen for late-onset Alzheimer disease in a genetically isolated dutch population. Am J Hum Genet. 2007;81:17–31. doi: 10.1086/518720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Myers A, Wavrant De-Vrieze F, Holmans P, et al. Full genome screen for Alzheimer disease: stage II analysis. Am J Med Genet. 2002;114:235–244. doi: 10.1002/ajmg.10183. [DOI] [PubMed] [Google Scholar]
- 94.De Ferrari GV, Papassotiropoulos A, Biechele T, et al. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer’s disease. Proc Natl Acad Sci USA. 2007;104:9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reiman EM, Webster JA, Myers AJ, et al. GAB2 alleles modify Alzheimer’s risk in APOE epsilon4 carriers. Neuron. 2007;54:713–720. doi: 10.1016/j.neuron.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dreses-Werringloer U, Lambert JC, Vingtdeux V, et al. A polymorphism in CALHM1 influences Ca2+ homeostasis, Abeta levels, and Alzheimer’s disease risk. Cell. 2008;133:1149–1161. doi: 10.1016/j.cell.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Blacker D, Wilcox MA, Laird NM, et al. Alpha-2 macroglobulin is genetically associated with Alzheimer disease. Nat Genet. 1998;19:357–360. doi: 10.1038/1243. [DOI] [PubMed] [Google Scholar]
- 98.Giedraitis V, Hedlund M, Skoglund L, et al. New Alzheimer’s disease locus on chromosome 8. J Med Genet. 2006;43:931–935. doi: 10.1136/jmg.2006.043000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Y, Nowotny P, Holmans P, et al. Association of late-onset Alzheimer’s disease with genetic variation in multiple members of the GAPD gene family. Proc Natl Acad Sci USA. 2004;101:15688–15693. doi: 10.1073/pnas.0403535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ozturk A, Desai PP, Minster RL, et al. Three SNPs in the GSTO1, GSTO2 and PRSS11 genes on chromosome 10 are not associated with age at onset of Alzheimer’s disease. Neurobiol Aging. 2005;26:1161–1165. doi: 10.1016/j.neurobiolaging.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 101.Bertram L, Hiltunen M, Parkinson M, et al. Family-based association between Alzheimer’s disease and variants in UBQLN1. N Engl J Med. 2005;352:884–894. doi: 10.1056/NEJMoa042765. [DOI] [PubMed] [Google Scholar]
- 102.Blacker D, Bertram L, Saunders AJ, et al. Results of a high-resolution genome screen of 437 Alzheimer’s disease families. Hum Mol Genet. 2003;12:23–32. doi: 10.1093/hmg/ddg007. [DOI] [PubMed] [Google Scholar]
- 103.Farrer LA, Bowirrat A, Friedland RP, et al. Identification of multiple loci for Alzheimer disease in a consanguineous Israeli-Arab community. Hum Mol Genet. 2003;12:415–422. doi: 10.1093/hmg/ddg037. [DOI] [PubMed] [Google Scholar]
- 104.Hahs DW, McCauley JL, Crunk AE, et al. A genome-wide linkage analysis of dementia in the Amish. Am J Med Genet B Neuropsychiatr Genet. 2006;141:160–166. doi: 10.1002/ajmg.b.30257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lee JH, Cheng R, Santana V, et al. Expanded genomewide scan implicates a novel locus at 3q28 among Caribbean hispanics with familial Alzheimer disease. Arch Neurol. 2006;63:1591–1598. doi: 10.1001/archneur.63.11.1591. [DOI] [PubMed] [Google Scholar]
- 106.Pericak-Vance MA, Grubber J, Bailey LR, et al. Identification of novel genes in late-onset Alzheimer’s disease. Exp Gerontol. 2000;35:1343–1352. doi: 10.1016/s0531-5565(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 107.Rademakers R, Cruts M, Sleegers K, et al. Linkage and association studies identify a novel locus for Alzheimer disease at 7q36 in a Dutch population-based sample. Am J Hum Genet. 2005;77:643–652. doi: 10.1086/491749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scott WK, Hauser ER, Schmechel DE, et al. Ordered-subsets linkage analysis detects novel Alzheimer disease loci on chromosomes 2q34 and 15q22. Am J Hum Genet. 2003;73:1041–1051. doi: 10.1086/379083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li M, Atmaca-Sonmez P, Othman M, et al. CFH haplotypes without the Y402H coding variant show strong association with susceptibility to age-related macular degeneration. Nat Genet. 2006;38:1049–1054. doi: 10.1038/ng1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sweet RA, V, Nimgaonkar L, Devlin B, Jeste DV. Psychotic symptoms in Alzheimer disease: evidence for a distinct phenotype. Mol Psychiatry. 2003;8:383–392. doi: 10.1038/sj.mp.4001262. [DOI] [PubMed] [Google Scholar]
- 111.Wijsman EM, Daw EW, Yu CE, et al. Evidence for a novel late-onset Alzheimer disease locus on chromosome 19p13.2. Am J Hum Genet. 2004;75:398–409. doi: 10.1086/423393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Daw EW, Heath SC, Wijsman EM. Multipoint oligogenic analysis of age-at-onset data with applications to Alzheimer disease pedigrees. Am J Hum Genet. 1999;64:839–851. doi: 10.1086/302276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Daw EW, Payami H, Nemens EJ, et al. The number of trait loci in late-onset Alzheimer disease. Am J Hum Genet. 2000;66:196–204. doi: 10.1086/302710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 115.Blangero J, Williams JT, Almasy L. Novel family-based approaches to genetic risk in thrombosis. J Thromb Haemost. 2003;1:1391–1397. doi: 10.1046/j.1538-7836.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- 116.Ebrahim S, Davey Smith G. Mendelian randomization: can genetic epidemiology help redress the failures of observational epidemiology? Hum Genet. 2008;123:15–33. doi: 10.1007/s00439-007-0448-6. [DOI] [PubMed] [Google Scholar]
- 117.Lehtovirta M, Helisalmi S, Mannermaa A, et al. Apolipoprotein E polymorphism and Alzheimer’s disease in eastern Finland. Neurosci Lett. 1995;185:13–15. doi: 10.1016/0304-3940(94)11213-3. [DOI] [PubMed] [Google Scholar]
- 118.Geschwind DH, Miller BL, DeCarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proc Natl Acad Sci USA. 2002;99:3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]