Abstract
We previously reported that pericontusional, extracellular chondroitin sulphate proteoglycans (CSPGs) are profoundly reduced for 3 weeks after experimental traumatic brain injury (TBI) indicating a potential growth-permissive window for plasticity. Here, we investigate the extracellular environment of sprouting neurons after controlled cortical impact injury in adult rats to determine the spatial and temporal arrangement of inhibitory and growth-promoting molecules in relation to growth-associated-protein 43-positive (GAP43+) neurons. Spontaneous cortical sprouting was maximal in pericontused regions at 7 and 14 days after injury but absent by 28 days. Perineuronal nets (PNNs) containing CSPGs were reduced at 7 days after injury in the pericontused region (p < 0.05), which was commensurate with a reduction in extracellular CSPGs. Sprouting was restricted to the PNNs and CSPG-deficient regions at 7 days, indicating that the pericontused region is temporarily and spatially permissive to new growth. At this time point, GAP43+ neurons were associated with brain regions containing cells positive for poly-sialic acid neural cell adhesion molecule but not with fibronectin-positive cells. Brain-derived neurotrophic factor was reduced in the immediate pericontused region at 7 days. Along with prior Western blot evidence, these data suggest that a lowered intrinsic growth stimulus together with a later return of growth-inhibitory CSPGs may contribute to the ultimate disappearance of sprouting neurons after TBI.
Keywords: Axon sprouting, Brain-derived neurotrophic factor (BDNF), Brain trauma, Chondroitin sulphate proteoglycan, Fibronectin, GAP43, Neuroplasticity, Perineuronal nets
INTRODUCTION
The prevailing dogma that the adult brain has limited morphological plasticity is largely based on the idea that either the extrinsic environment is non-permissive to growth or that central nervous system (CNS) neurons lack an intrinsic ability to respond to growth cues. In the immature CNS there are higher levels of regeneration-associated genes and lower levels of growth-inhibitory substrates that likely contribute to greater growth than in the adult CNS (1). There is, however, ample evidence of cortical plasticity in the normal adult brain (2–5); spontaneous neuroplasticity in the adult brain has been shown to occur following a variety of CNS insults, including cortical lesion (6), stroke (7), and spinal cord injury (8).
Compared to other types of CNS injury, spontaneous neuroplasticity after experimental traumatic brain injury (TBI) has been only sparsely studied. Although deafferentation-induced sprouting or reactive synaptogenesis would seem to occur after all types of injury, including TBI, differences in lesion induction can drastically affect the potential for axon plasticity (9). Spontaneous structural plasticity after TBI can be inferred from an upregulation of growth-promoting genes (10, 11) and a transient increase in neurotrophic receptors (12). Axonal sprouting has been demonstrated after TBI (13–15), and some studies suggest that axonal reorganization correlates with functional outcome (14, 16) or with novel ipsilesional metabolic activation after forelimb stimulation at chronic times after injury (17). Other studies have documented synaptogenesis in cortical and hippocampal regions after TBI (18) or after TBI combined with bilateral entorhinal cortex lesions (19, 20). On the other hand, disparity between the magnitude of synaptogenesis in the hippocampus and cognitive recovery has been reported in experimental TBI (21).
Despite some spontaneous neuroplasticity and the eventual resolution of the major pathophysiologic deficits, TBI ultimately results in enduring functional deficits. There are likely multiple reasons for this but the CNS does not appear to support long distance axon regeneration (22) and sprouting axons generally become dystrophic (23). Failure of axon regeneration after TBI may occur through extrinsically mediated effects. For example, growth cone collapse can be attributed to upregulation of growth-inhibitory molecules, such as myelin-associated glycoproteins within the extracellular environment (24–26), or to the eventual development of a physical barrier via the glial scar (27). The failure of activation and support of an intrinsic cellular growth state also likely contributes to failed axon regeneration. For example, experimental TBI is known to induce deficits in brain-derived neurotrophic factor (BDNF) (28), a growth-promoting neurotrophin that supports sprouting and reduces death of axotomized spinal neurons (29). The pathway involving cyclic adenosine monophosphate (30), a second messenger molecule associated with sprouting, is also impaired (31).
We recently identified a temporary reduction in the transcription and translation of the growth-inhibitory chondroitin sulphate proteoglycans (CSPGs) versican, neurocan, phosphacan and aggrecan after TBI, suggesting that there is a temporary window of growth permissiveness that exists within pericontusional regions after cortical trauma (26). Therefore, we hypothesized that spontaneous axonal sprouting coincides with this growth permissive period, whereas spatially, evidence of axonal growth is confined to the pericontusional regions that contain reduced CSPGs and/or increased levels of growth-promoting molecules. Therefore, we investigated the temporal and spatial arrangement of inhibitory and growth-promoting molecules in relation to sprouting neurons after TBI as the basis for future strategies to widen the temporal window of growth permissiveness.
MATERIALS AND METHODS
Experimental Groups
Groups of adult male, Sprague Dawley rats (250–275 g) were randomly assigned as follows: (i) immunohistochemistry (IHC) analysis at post-injury day 7, 14 and 28 (n = 6, 6, and 3, respectively, for each time-point for both controlled cortical impact injury [CCI] or sham injury groups); (ii) growth-associated protein 43 (GAP43) in situ hybridization at post-injury day 7 (n = 5 and 3 per CCI and sham injury group, respectively); and (iii) for BDNF Western blot analysis at post-injury days 7 (n = 3, 5, 5 per naïve, CCI and sham injury group, respectively). All procedures were approved by the UCLA Chancellor’s Committee for Animal Research.
Surgery and Controlled Cortical Impact Injury
A unilateral CCI to the left hemisphere model was used as previously characterized (27, 32, 33). Animals were anesthetized with isoflurane (3% in 100% O2 [1.5–2.0 l/minute flow rate] for induction; 1.5–2% for maintenance) and received an application of ophthalmic ointment to both eyes when they were under a surgical level of anesthesia. During all surgical procedures, body temperature was maintained at 37 ± 0.5°C using a thermostatically controlled heating pad (Harvard Apparatus, Edenbridge, KY). After shaving and sterilizing the scalp, the anesthetized animal was placed into a stereotaxic frame, a midline incision was made over the skull, and the skin, fascia and temporal muscles were reflected bilaterally. Using a microscope and intermittent saline-cooled perfusion of the skull area, a 6-mm-diameter craniotomy was made over the left sensorimotor cortex, centered at Bregma and 3.5 mm lateral to midline. An electronically controlled pneumatic piston (Hydraulics Control, Inc, Emeryville, CA) mounted on a stereotaxic micro-manipulator (Kopf Instruments, Tujunga, CA) and angled at 22.5° to the vertical to allow the rounded 4-mm-diameter tip to make contact perpendicular to the surface of the brain, was used to create the CCI. The injury (20 psi; 2 mm dural compression) was induced by depression and rapid contraction of the pneumatic piston. Hemostat material (Surgicel™, Johnson & Johnson, Langhorne, PA) was placed over the injury site before the bone flap was replaced and sealed in place with cyanoacrylate glue, used sparingly to prevent excess from running onto the hemostat. Sham injury control rats were subjected to all surgical procedures, but did not experience induction of the CCI. Scalp incisions were sutured closed and Bupivacaine (0.05–0.07 mg/kg, s.c.) was infiltrated into this wound margin. After removal from anesthesia rats were maintained in a warm recovery cage for ~30 minutes before being returned to home cages. Additional control rats that did not experience any anesthesia or surgical procedures were used in the Western blot experiment.
Immunohistochemistry
Following overdose with sodium pentobarbital (100 mg/kg, i.p.) rats with CCI or sham injury were transcardially-perfused with 0.1 M phosphate-buffered saline (PBS) at approximately mean arterial blood pressure followed by 4% paraformaldehyde in PBS. Following cryoprotection in 25% sucrose in PBS until the brain sank, the brain was frozen and 50-μm-thick coronal sections were cut. Standard free-floating, single label IHC was performed on one series of sections. In brief, antigen retrieval was carried out using citrate buffer (1 M, pH = 6.0, incubation overnight at 4°C) followed by two rounds of 10 minutes heating to 95°C and 10 minutes cooling. Following TRIS-buffered saline washes (TBS, 0.2M) single label IHC was performed for stereologic analysis of cell density. All sections were quenched in a solution of 10% methanol and 10% H2O2 in distilled water for 60 minutes before washing 3X in TBS. Sections were then blocked for 60 minutes in TXTBS (TBS containing 0.2% Triton X-100; Sigma, St. Louis, MO) with 10% normal horse serum (NHS; Vector Labs, Burlingame, CA), 1% bovine serum albumin (BSA, Vector Labs). Adjacent sections were then incubated over-night at room temperature (RT) on a shaker in either GAP43 primary antibody (1:3000; #MAB347, Chemicon/Millipore, Billerica, MA) in TBS containing 5% NHS, or the biotin-conjugated lectin Wisteria floribunda (WFA; 1:1000; #L-1766, Sigma) in TBS containing 2% BSA. After washing three times in TBS, sections were incubated with biotinylated, rat-adsorbed anti-mouse IgG (1:500; Vector) in TBS with 5% NHS for 1 hour followed by three washes in TBS. Signal amplification was achieved using the Vectastain elite ABC kit (Vector) for 30 minutes following the manufacturer’s instructions, followed by 3 washes in TBS. Antibody binding was then visualized with diaminobenzidine in distilled water containing 0.03% H2O2 and excess stain was removed by washing in TBS 3X. Sections were then mounted on gel-coated slides, dehydrated, and cover-slipped with DPX mounting medium.
For double label IHC, sections were processed for antigen retrieval as above and then blocked for 15 minutes each in streptavidin and then biotin solution (Vector) and in 10% NHS in PBS for 1 hour, followed by overnight incubation in either anti-GAP43 (1:1500) or WFA, as described above. Following rinsing in PBS, GAP43 sections were incubated in rat-adsorbed, biotinylated secondary antibody for 2 hours (1:500 dilution in 5% NHS, Vector), followed by 3 washes in PBS. Antigens were visualized with streptavidin-conjugated fluorescent Alexa-488 and -555 probes (Molecular Probes/Invitrogen, Carlsbad, CA, 1.25 μg/ml in PBS for 60 minutes). Double labeling was then achieved using another biotin blocking step followed by mouse-on-mouse blocking reagent when required (MKB-2213, Vector) and a serum blocking step as before, all of which facilitated the use of additional mouse-host primary antibodies on the same preparation for GAP43 IHC. Sections were incubated overnight at RT on a shaker in either mouse monoclonal antibodies: anti-NeuN, (1:5,000; #MAB377, Chemicon/Millipore), or poly-sialic acid neural cell adhesion molecule (PSA-NCAM, 1:300; #MAB5622, Millipore), or rabbit antibodies: glutathione S-Transferase pi (GSTpi, 1:500; #MSA-102, Stressgen, Ann Arbor, MI), anti-MAP2 (1:500; #AB5622, Millipore), fibronectin (1:400; #F3648, Sigma), or BDNF (1:50; #SC-546, Santa Cruz Biotechnology Inc, Santa Cruz, CA). Following 1-hour incubation in primary host-specific biotinylated secondary antibody, antigens were visualized with a different colored Alexa Fluoro-streptavidin conjugate as before. Finally, all sections were either stained with the nucleic acid marker TOPRO-3 (TPR3; 1:1000 in PBS, Invitrogen) or DAPI (Vectashield, Vector). Sections were mounted on slides and cover-slipped with anti-bleaching medium (Vector).
Multi-IHC-labeled sections were imaged by confocal microscopy (LSM Pascal System, Zeiss, Thornwood, NY) in single channel acquisition mode to prevent crosstalk and with fluorophore-appropriate laser excitation and filter-sets configured for optimal emission. Montage (X-Y) data were acquired with a 12-μm optical section thickness at x40 magnification over 4 to 8 fields of view in automatic mode. Z-axis data were acquired at x63 at 1 μm optical thickness. These data are shown to demonstrate that the positive staining is more widespread compared to that typically shown in a x40 or x63-objective field-of-view. Optimal primary and secondary antibody concentrations were obtained using standard dilutions as single labels and test sections were run without primary antibody to confirm the specificity of the labeling for each secondary antibody isotype. Double-label test sections were run exhaustively without one of the primary antibodies to confirm the specificity of antibody binding, especially when using 2 mouse primary antibodies. At least 3 injured brains were double-labeled (1 section every 600 μm) for each doublet of IHC markers to assess reproducibility and to summarize trends.
Cell Density Counts
GAP43+ cell profile counts were made on 3 to 5 single label DAB-stained IHC sections/brain that contained the injured/ipsilateral sensorimotor or parietal cortex using sections spaced at least 600 μm apart. The total number of GAP43+ profiles, defined as either a stained cell body or neurite/fibril-like segment, were counted in the entire ipsilateral grey and white matter encompassing the region from the midline to the rhinal fissure (Fig. 1A). Large, tangentially lying GAP43+ processes were counted once. The counts were expressed as average density of GAP43+ profiles in the area of evaluated tissue
Figure 1.
Pericontusional sprouting. Immunostained cortical GAP43+ cells and processes were quantified at 7 to 28 days after controlled cortical impact injury (3–5 coronal sections/rat, >600 μm apart in sensorimotor and parietal cortex). (A–E) Injury resulted in the appearance of GAP43+ cellular profiles at 7 days for distances up to 1 to 2 mm from the injury site, shown in overview (A), montage (B) and at higher power from a different animal (C –E). No staining was present in homotypic regions of the contralateral or in the sham-injured sensorimotor cortex. Both perikarya and neuronal processes were stained throughout the pericontusion cortical gray and white matter (C, D, inset-D) as were numerous “blebs” (i.e. presumed neuronal growth cones) on the contusion edge (E, inset E). (F) Pericontusional sprouting was maximal at 7 and 14 days after injury and was not present by 28 days; the difference between 7 and 14 days was not significant (p = 0.09).
Due to the high density of WFA stained cells in uninjured brain, counts of WFA+ cells were performed using the optical fractionator probe technique with StereoInvestigator software (MicroBrightfield, Williston, VT) on alternate sections adjacent to those used for the GAP43 analysis. To reduce effects of minor variation in location of contusion boundaries with respect to the cell counting area, the anatomical boundaries of the counting area at each time-point were first defined on 3 to 5 single label DAB-stained IHC sections/brain (600 μm apart) containing the injured sensorimotor or parietal cortex. This was achieved by determining the average distance between the midline and the most medial-lying hypointense (contused tissue) cortical grey matter region, and a tangential line from the rhinal fissure to the white matter and the most ventral-lying hypointense cortical grey matter. These medial and lateral distances were averaged for each brain and then for all injured brains at each time-point. The resulting medial and lateral average measurements/time-point were then used to draw the contour boundaries of the counting area on each of the 3 to 5 sections/brain for both injured and sham-injured groups. This resulted in a conservative lesion boundary in all injured brains that encompassed both edges of some contused tissue as well as normal-appearing tissue containing WFA+ cells. Counts were made at x200 magnification with a counting frame size of 550 μm2 and between 15 to 25 sampling sites of 175 μm2/section. Only WFA+ cells with a characteristic ring-shaped, stained soma and well-stained soma-initial processes were counted. Small, round cells with intracellular staining but with no processes were not counted. Data are expressed as area density counts rather than estimated cell numbers.
In Situ Hybridization
Riboprobe Isolation and Amplification
Primers encompassing a 512 bp sequence of GAP43 (sense: 5′-CTGTGCTGTATGAGAAGAAC-3′, anti-sense 5′-GAGAGAAATAGAGAGGAAAGTG3′, Genebank accession # M16228) were used to amplify cDNA obtained from post-natal day 21 rat cortical tissue using reverse transcription polymerase chain reaction. The size and gene location of the riboprobe was based upon work done in adult rat (34). The purity and size of the cDNA generated was confirmed by agarose gel electrophoresis. Following purification using Miniprep kit (Qiagen, Valencia, CA) the cDNA was ligated into a plasmid (PGEM-T Easy, Promega, Madison, WI) using T4 DNA ligase enzyme and then amplified by transfection into E. Coli competent cells overnight at 37°C. Ampicillin-resistant clones were selected and then grown-up in liquid medium for 2 days after which plasmids were extracted and purified using the Miniprep kit. Recombinant clones were sequenced at the UCLA sequencing core with the M13 forward and reverse primers against the plasmid DNA including the GAP43 insert to confirm amplification of the correct region and probe orientation.
Probe Labeling
Both sense and antisense probes were radiolabeled with 35S-dUTP (MP Biochemicals, Solon, OH) using the riboprobe combo system (Promega, Madison, WI). Briefly, probe cDNA was transcribed back to the 521 bp RNA using T7 and SP6 primers specific for the promoter regions that flank the probe insert on the PGEM-T Easy plasmid. 35S-labeled RNA formed by this in vitro synthesis was then extracted using a standard phenol/chloroform extraction and concentrated by precipitation. After repurification, probe labeling efficiency was checked on a gamma counter to determine the correct amount to add to the hybridization solution.
Hybridization
Rats were perfused-fixed with autoclaved PBS and 4% paraformaldehyde in PBS and the brains sectioned coronally at 20 μm onto RNAase-free slides. Following post-fixation in 4% paraformaldehyde/PBS, sections were washed in glycine, acetylated, dehydrated, defatted, force-air-dried and then hybridized with sense and antisense cRNA probes at 60°C overnight. Sections were then washed in formamide and sodium chloride/citrate solutions, RNAse-treated and then washed again in formamide and sodium chloride/citrate solutions (Sigma).
Analysis
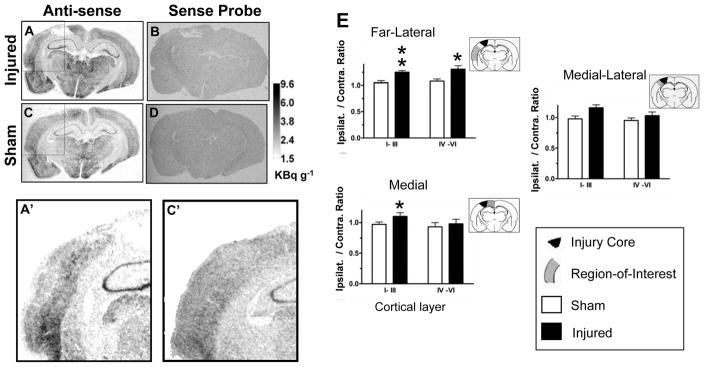
Sections were opposed to Kodak film for 5 days together with 14C standards (Amersham, Piscataway, NJ). There is a simple linear relationship between 14C and 35S at 15 hours to 6 days exposure using Kodak film, presumably due to similar energies of the emitted β-particles (156, 167keV) (35). This precludes the requirement for construction of 35S brain paste standards. Films were scanned into a PC computer at 1200 DPI and images were calibrated against 14C standards. Region of interest (ROI) optical density measurements were obtained using ImageJ (36) by placing ~2 × 1 mm ROI encompassing either cortical layers I-III or IV-VI within medial, medial-lateral and far-lateral ipsilateral cortex on at least 5 to 6 sections spaced at 80 μm apart from sensorimotor regions of the injured cortex. Measurements were obtained from contralateral cortex and in bilateral cortex of sham-injured rats at the same approximate locations. Data were averaged within each region for both superficial and deep cortex and plotted as a ratio of ipsilateral to contralateral optical density.
Western Blots
On post-surgery day 7, rats were briefly anesthetized with isofluorane, decapitated and the brains quickly removed and placed on ice for dissection. Tissue blocks of ~3 to 4 mm3 were taken from the injury zone encompassing both the contusion core and bordering regions in the left injured cortex and from a similar region in the left/ipsilateral cortex of sham injury and naïve rats, as previously described (26). Tissue was stored at −80°C before being harvested by homogenization in lysis buffer containing Tris-HCl (0.05M, pH = 7.5), NaCl (0.15M), protease inhibitors (Complete,™, Roche, Basel, Switzerland) and centrifuged at 13,000 rpm for 30 minutes at 4°C. Protein concentration was measured using the RC-DC Protein Assay Kit (BioRad, Hercules, CA). Protein (25 μg) was mixed with 2× Laemmli buffer and boiled for 5 minutes and proteins were resolved on a 15% SDS-PAGE gel (BioRad) running at 33 mA for 60 minutes with a set of molecular weight standards –Precision Plus Protein Standards 10–250 kD (BioRad). Proteins were electro-transferred to polyvinylidene diflurodide membrane (BioRad, Hercules, CA) and blots were stained for total protein with Sypro Ruby (BioRad) and imaged under ultraviolet light (Fluoromax system, BioRad) for visual confirmation of equal lane loading. After washing in a solution of TRIS-buffered saline, Tween-20 (TTBS) and blocking in 5% milk for 1 hour, membranes were probed overnight with BDNF primary antibody (1:1000; #SC-546, Santa Cruz Biotechnology). Membranes were washed in TTBS for 3× 10 minutes and then incubated with anti-rabbit HRP-conjugated secondary antibody (1:10,000 in 1% milk) for 60 minutes. Following washing, antigen detection was achieved using an enhanced chemiluminescent substrate kit (SuperSignal West Femto Substrate, Thermo, Fisher Scientific, Waltham, MA) and images of the membranes were acquired using a camera system sensitive to chemiluminescence (Fluoromax system, BioRad). Blots were stripped and re-probed for β-actin (1:500; SC-1616, Santa Cruz Biotechnology) and re-imaged. The protein band corresponding to the mature form of BDNF at 14 kD was determined by reference to the molecular weight standards and quantified by integrated optical density measurement using ImageJ (36) and normalized to the corresponding integrated optical density. All gels were run with naïve control samples that were used to normalize the data for all samples from each gel to control for minor changes in experimental conditions.
Statistics
For all data the Kolmogorov-Smirnov with Lillie correction and Levene median tests were used to describe the distribution of the data and to determine the equality of the variances, respectively. Having passed these normality tests, group numerical data were expressed as the means ± standard error of the mean. For GAP43 IHC data, a one-way ANOVA was used to test for an overall difference between the groups and a post hoc test (Dunnett test) was used to determine individual group differences. GAP43 mRNA ROI data were not corrected for multiple comparisons since a priori evidence suggests that primary and secondary cortices differ in GAP43 mRNA expression (34) and can thus be treated as independent parameters. An initial 2-way ANOVA was used to test for overall group and time effects in the WFA data. Since there were no significant differences between sham groups the data were pooled and used in a 1-way ANOVA to test for group differences across time. Statistical significance (α) was set at p < 0.05 for all comparisons.
RESULTS
The injury model produces a cortical contusion over the forelimb area, extending from the frontal to anterior parietal cortex and to cortical layer V at its deepest extent within the sensorimotor cortex. We refer to the central, severely injured tissue as the contusion ‘core’ and the surrounding, less severely injured tissue as ‘pericontusion’ regions. Previous studies have shown that the model produces early cortical neuronal apoptosis and necrosis, but these are largely confined to the first few days after trauma (33, 37, 38).
Pericontusional GAP43+ Neuronal Sprouting Is Maximal at 7 Days Post-injury
We first investigated the extent of cortical neuronal sprouting after TBI using GAP43 IHC. GAP43+ cellular staining in the hippocampus in both sham and injured rats was used as a positive control for antibody staining. At 7 days post-injury GAP43+ profiles were noted in most sections that contained contused parenchyma and were visible for several millimeters from the contusion edge (Fig. 1A). GAP43+ perikarya, often with a stained process (Figs. 1C, 2B), were distributed around the vertical edges of the contusion, underneath the contusion core within layer V and within the corpus callosum (Fig. 1A–C). GAP43+ perikarya and processes were seen in the gray matter on either side of the contusion core and often extended from the deeper to more superficial cortical layers (Fig. 1D), and there were growth cone-like GAP43+ blebs noted around the contusion edges (Fig. 1E). No GAP43+ processes were seen in the contralateral cortex or in any cortical region of the sham-injured brains. There were a few GAP43+ profiles in the caudate in a few brains, but no other brain region showed the marked changes seen in the cortex ipsilateral to the contusion.
Figure 2.
GAP43+ cell phenotype. (A–C) Representative confocal montages of layer V sensorimotor cerebral cortex adjacent to the injury site 7 days after injury illustrate neuronal fiber and perikarya- GAP43+ profiles (A, GAP43 and merge). The pial surface is at the top of the image; the injury site is the left. Perikarya express the neuronal marker NeuN (A, inset A′ and z stack in B) and MAP2 (C). No GAP43+ profiles display the oligodendrocyte marker glutathione S-Transferase pi (GSTPi) in gray or white matter (D). Confocal data trends are based on an analysis of 3 injured brains. TPR3 = nucleic acid marker TOPRO-3.
Temporal analysis of GAP43+ process density showed that sprouting peaked at 7 to 14 days and that by 28 days, no GAP43+ staining was observed (Fig. 1F). The density of GAP43+ processes was higher at 7 than 14 days after injury, although this did not reach significance (p = 0.09). Since there are reports that GAP43 can be expressed by astrocytes (39, 40) or immature oligodendrocytes (41), we validated the phenotype of GAP43 expression using double-labeled IHC. No GAP43+ cells expressed the mature neuronal somatic marker NeuN, the somato-dendritic marker MAP2 (Fig. 2A–C), or the mature oligodendrocyte marker GSTpi (Fig. 2D).
Since protein synthesis may be reduced early after fluid percussion brain injury (42), we wanted to exclude the possibility that we had underestimated the sprouting response due to potential effects of trauma on protein synthesis after CCI. Therefore, we mapped cortical GAP43 mRNA using in situ hybridization with a riboprobe similar to that used previously to determine GAP43 distribution in the adult rodent brain (34). We performed the analysis at 7 days after injury, i.e. the peak time of the IHC response in ipsilateral sensorimotor and parietal cortex regions. Data showed the expected high hippocampal anti-sense probe binding in both injured and sham-injured rats (Fig. 3A, C) and no significant binding in any region with the sense probe (Fig. 3B, D). Cortical ROI analysis showed that GAP43 mRNA, expressed as the ipsilateral/contralateral ratio, was significantly increased above sham values in the cortex of injured rats, in both far lateral regions in all cortical layers and in medial superficial layers (Fig. 3A′ versus C′, E). The increase in GAP43 mRNA was more widespread compared to the IHC data, wherein increases were largely limited to pericontusional tissue, suggesting that translation into protein is affected by CCI.
Figure 3.
GAP43 mRNA: (A–D) In situ hybridization for GAP43 mRNA from sham and injured rats 7 days after injury in sensorimotor/parietal cortex showed regional hyperintensity with the GAP43 mRNA anti-sense probe (A, C) but uniform intensity with the sense probe (B, D). Region of interest (ROI) analysis showed increases in cortical GAP43 anti-sense probe intensity in injured cortex compared to sham (A′, C′- inset of A, C), which was significant in both far lateral regions and more medial ROIs (E). Data are plotted as ipsilateral/contralateral optical density ROI; **p < 0.01, *p < 0.05.
Pericontusional Perineuronal Nets Are Reduced by Injury at 7 Days and Begin to Repopulate by 28 Days
Our previous work had shown qualitative decreases in perineuronal net (PNN)-associated CSPGs after injury (26) and CSPGs in their normal arrangement are likely to present a significant barrier to sprouting axons and/or new synaptic connections. Therefore, we used the lectin WFA to label the CSPG-rich PNNs of cortical cells through binding of the cell surface molecule N-acetylgalactosamine (43). WFA+ cells were almost completely absent from the pericontused cortical regions despite the presence of normal, intact tissue at 7 days; this absence occurred up to several mm from the contusion edge (Fig. 4A, B). Within the cystic contusion cavity there were numerous, highly intense WFA+ cells that appeared to be condensed dead cells, infiltrating glia or fibroblasts (Fig 5A). Density counts of the pericontused cortex revealed that there was a significant group but no time effect of injury on WFA+ cell density (Fig. 4D, p < 0.001), and that the decrease in cell density due to injury was significant at all times (p < 0.05). At 28 days some normal appearing, branched WFA+ cells were often noted within the pericontusional area (Fig 4E), but overall the cell numbers was still significantly depleted compared to sham injured animals (Fig 4F).
Figure 4.
Perineuronal Nets: Numbers of Wisteria floribunda-positive (WFA+) cells 7 days after injury were markedly reduced for up to 1 to 2 mm from the contusion edge, shown in layer V of the injured sensorimotor cortex (A, B), compared to sham-injured rats (C); this was significant at 7, 14 and 28 days (D, *p < 0.05). The number of perineuronal nets was reduced at 28 days (D, E) compared to sham (D, F), but some WFA+ cells (white arrowheads, E) are present closer to the contusion edge in layer V of the cortex (dashed line, E).
Figure 5.
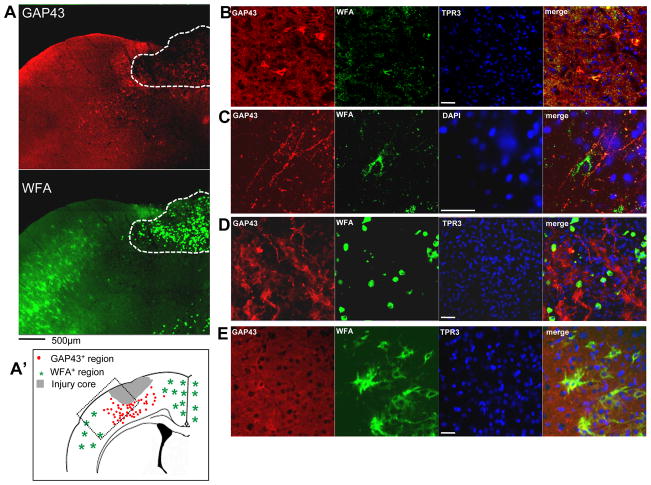
Neuronal sprouting and perineuronal nets. (A) Representative confocal montage of pericontused, ipsilateral sensorimotor cortex 7 days after injury showing that the majority of GAP43+ cells occur within regions devoid of Wisteria floribunda-positive (WFA+) cells. The hatched region shows the cystic cavity of the core injured region that contains ovoid, dead or infiltrating cells completely filled intracellularly with very intense WFA+ staining. (A′) Summary drawing based on observations from at least 3 injured brains showing the presence of GAP43+ neurons around the contusion site within a region containing no or few WFA+ cells (dotted box region represents approximate position of panel A). (B–E) Higher power figures at 7 days post-injury (different section from that in A) showing pericontusional GAP43+ perikarya (B) and GAP43+ fibers from cortical layer V containing very few cells double-labeled with WFA. (D) GAP43+ fibers and numerous blebs are present within the cystic contusion cavity, even within a field of highly intense ovoid WFA+ cells; none are double-labeled. (E) No GAP43+ sprouting neurons are observed contralaterally; normal-appearing WFA+ cells are stained only around the cell body and proximal fiber segments. Scale bar = 50 μm.
Sprouting Neurons Occur in Regions of Low Perineuronal Nets and Low Extracellular Chondroitin Sulphate Proteoglycans
We hypothesized that spontaneous neuronal sprouting would occur only in brain regions in which either PNN or CSPG or both were reduced after TBI. Data showing an absence of IHC double-labeling for sprouting neurons and PNNs appear to support this hypothesis (Fig. 5A, B), since GAP43+ neurons generally appeared only in pericontused regions that are deficient in WFA+ cells at 7 days post-injury, and no sprouting appeared contralateral within the normally distributed WFA+ cells (Fig. 5E). In some cases, pericontused GAP43+ neurons were partially WFA+ (Fig. 5B). Cortical GAP43+ fibers were noted in the gray matter in layers II to V (Fig 5C) and in the contusion cavity among highly intense, WFA+, dead or infiltrating cells (Fig. 5D), although none were co-labeled.
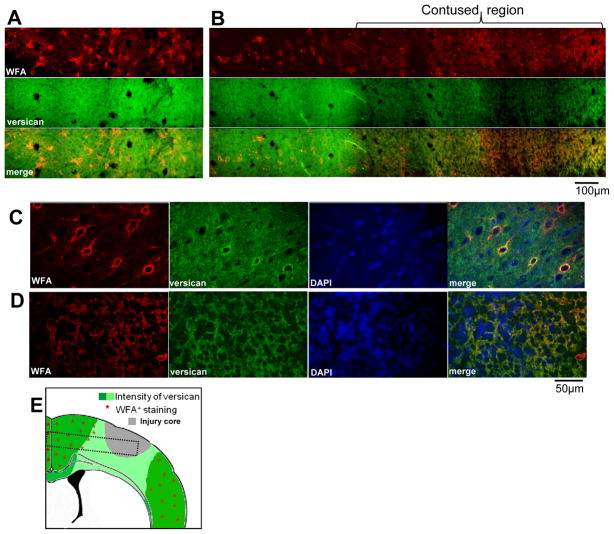
Since TBI results in a temporary reduction in extracellular CSPG proteins (26), we suspected that reduction in CSPGs contained within PNNs would occur in similar brain regions, i.e. that the injured brain environment is temporarily less inhibitory to sprouting than previously thought. Our current data appear to support this supposition for day 7 post-injury. In contralateral cortex, densely arranged WFA+ cells were associated with intense versican staining (Fig. 6A, B left, 6C), but in cortex ipsilateral to contusion there was a sharply demarcated zone where both the incidence of WFA+ cells and versican intensity were reduced (Fig. 6B, D).
Figure 6.
Perineuronal nets and chondroitin sulphate proteoglycans (CSPGs). (A, B) Representative confocal montages of contralateral (A) and ipsilateral (B) sensorimotor layer IV–V cortex 7 days after injury showing that the growth-inhibitory CSPG versican is markedly reduced within the pericontusional region sparsely populated with Wisteria floribunda-positive (WFA+) cells. (C, D) Higher power images from different sections acquired with different confocal acquisition settings to highlight the perineuronal nets from the high extracellular versican of contralateral cortex showing normal appearing WFA+ perineuronal nets, many of which are versican+ (C). In the pericontusional ipsilateral cortex containing low amounts of extracellular versican, there are no WFA+ or versican+ perineuronal nets (D). (E) Stylized summary diagram of changes in versican and WFA+ cells in injured cortex based on observations from at least 3 injured brains (dotted box corresponds to the approximate location of panels A and B).
We also looked at the spatial arrangement of sprouting neurons relative to regions of reduced extracellular CSPGs by staining for neurocan and aggrecan (Supplementary Fig. 1). As anticipated from results with versican, GAP43+ neurons were found in regions where extracellular neurocan was low, such as within some of the pericontused tissue regions compared to contralateral cortex (Supplementary Fig. 1A, top of image, vs. 1B) and compared to some pericontused regions that were hyperintense for extracellular neurocan, consistent with the development of a glial scar in this model (Supplementary Fig. 1A). Most GAP43+ perikarya were neurocan−, but some were neurocan+ (Supplementary Fig. 1A inset). Unlike in the contralateral cortex where only the outer rims of perikarya were positively stained for neurocan, however, staining was intracellular within perikarya of the pericontused cortex; this likely indicates that the neurons are actively synthesizing neurocan for extracellular deposition. GAP43+ neurons did not appear to be arranged in a similar fashion in sections stained for aggrecan, i.e. sprouting neurons were observed in regions containing aggrecan+ hypertrophied fibers, presumed to be glial cells, and the GAP43+ cells were often surrounded by an aggrecan+ PNN (Supplementary Fig. 1C, D).
Sprouting Neurons Occur Within Regions of Poly-Sialic Acid Neural Cell Adhesion Molecule+ Cells But Are Not Restricted to Regions Containing Positive Effectors of Sprouting
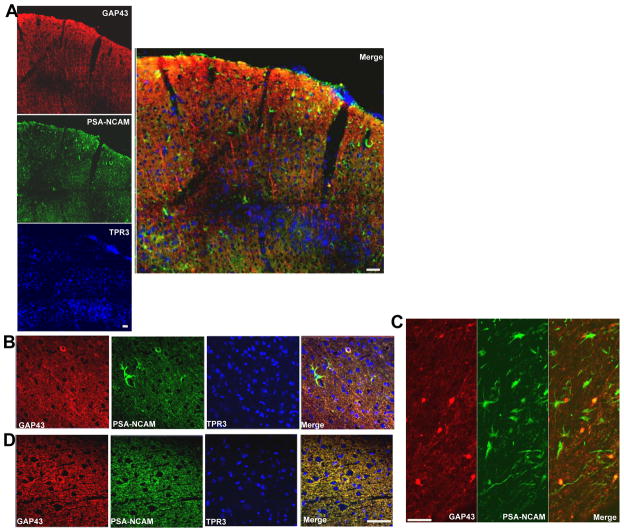
Double-staining was conducted for GAP43 and the growth-associated protein PSA-NCAM), a protein often associated with sprouting neurons but also with actively proliferating cells. There was robust upregulation of PSA-NCAM+ cells throughout the pericontusional cortex at 7 days (Fig. 7A), many of which appeared to be immature and/or glial cells, although none were seen contralaterally (Fig. 7B vs. D). GAP43+ perikarya and processes were associated with gray and white matter regions containing PSA-NCAM+ cells (Fig. 7A merge, 7C); however, on the cellular level there was only occasional overlap between these processes (Fig. 7B merge).
Figure 7.
Poly-sialic acid neural cell adhesion molecule (PSA-NCAM) and GAP43. Representative confocal montages of the sensorimotor cortical region 7 days after injury within a pericontused region anterior to the core injured tissue (in A the pial surface is shown at the top of the image). GAP43+ processes occur within a region containing numerous PSA-NCAM+ cells (A, merge). Most of the GAP43+ processes are not PSA-NCAM+ (A, Merge), but there are occasional double-labeled processes within the cortical gray and white matter underlying the contusion (B, C, respectively). The significant enhancement in the number of ipsilateral PSA-NCAM+ cells (A) does not extend to the contralateral cortex (D). Scale bars = 50 μm.
Fibronectin is a reparative molecule that also promotes cellular growth and has been reported to be enhanced after TBI (44). Therefore, we sought to determine whether it is enhanced in regions with sprouting neurons. By double-labeled IHC, fibronectin+ cells and blood vessels were limited to the most severely contused injury core regions, with only very minor incursions within the pericontused tissue (Supplementary Fig. 2A, B). GAP43+ neurons often overlapped with fibronectin+ regions at the contusion edge (Supplementary Fig. 2C), but they never co-labeled with fibronectin+ cellular structures; GAP43+ axonal profiles were often separated spatially from fibronectin+ regions (Supplementary Fig. 2C, E). Fibronectin immunostaining was never seen in other, more remote, ipsilateral regions or in the contralateral cortex.
Enhanced BDNF levels are associated with increased plasticity and promotion of a growth-permissive environment (45). We reasoned, therefore, that BDNF might be enhanced within pericontused regions containing sprouting neurons. By 7 days post-injury, the ipsilateral cortex remote from the contusion injury showed similar BDNF fluorescent intensity to the contralateral cortex (Fig. 8A, B); many GAP43+ neurons were intensely stained with BDNF (Fig. 8A2). Within pericontused regions, often the site of a significant number of densely arranged GAP43+ processes; however, BDNF staining intensity was markedly reduced (Fig. 8A-insets, 8A merge and 8A3). GAP43+/BDNF− fibers were seen a short distance from the contusion edge (Fig 8A1). In parallel, Western blot analysis of tissue from around the contusion core showed an almost 2-fold reduction in BDNF protein compared to sham-injured levels (Fig. 8C; p = 0.06). This occurred despite the likely inclusion of normal tissue within the sample due to the limited ability to discriminate between injured and non-injured brain when sampling for Western blots.
Figure 8.
Neuronal sprouting and brain-derived neurotrophic factor (BDNF). (A, B) Representative confocal montages of the pericontused cortical region 7 days after injury (A, pial surface is at top, contusion core to right) and contralateral cortex (B). Intensity of BDNF staining was reduced along the border of the contusion (A, insets and merge) compared to more remote ipsilateral tissue and the contralateral cortex (B). GAP43+ neurons were also BDNF+ within the pericontusional ipsilateral cortical tissue (A, A2) but most of the GAP43+ fibers at the contusion edge were BDNF- (A, A3), as were most of the GAP43+ fibers in intact tissue (A1). (C) Western blot analysis graph shows mean BDNF protein concentration and individual data (black symbols) in ipsilateral injured and sham-injured cortex as a percent of uninjured controls 7 days after injury (p = 0.06). (D) Stylized summary diagram of the spatial relationship in ipsilateral cortex between GAP43+ processes and BDNF immunostaining intensity 7 days after injury.
DISCUSSION
The current data show that experimental TBI results in spontaneous GAP43+ neuronal sprouting that is maximal in pericontused cortical regions at 7 and 14 days after injury but absent by 28 days. PNN-containing CSPGs were also maximally reduced at this time in the pericontused region, commensurate with a reduction in immunostaining of the extracellular CSPGs versican and neurocan. Neuronal sprouting was more robust within these same regions that were deficient in PNNs and extracellular CSPGs at 7 days, indicating that the pericontused cortical region after TBI is temporarily and spatially permissive to new growth. At the same time there were GAP43+ neurons in white and grey matter regions containing PSA-NCAM+ cells but not with fibronectin+ regions. BDNF was reduced in the pericontused region at 7 days, likely indicating that there is a lowered intrinsic growth stimulus occurring concomitantly with the period of reduced CSPGs in the same regions. PNN density remained decreased at 28 days, suggesting a continued positive environment for plasticity. The normalization of extracellular growth-inhibitory CSPGs at 14 days reported previously (26) suggests that CSPGs that are not associated with PNNs may contribute to the ultimate disappearance of sprouting neurons at 28 days post-TBI.
We previously found that CSPGs are temporarily markedly decreased within the pericontused regions and that large areas adjacent to the site of injury have reduced extracellular and PNN/CSPG protein expression after TBI (26). These data suggest a temporary window of growth-permissiveness after trauma that might provide the optimal environment to drive the spontaneous plasticity that occurs after TBI (13–16, 18–20). The time-course of GAP43 enhanced sprouting described herein suggests that this might indeed be the case, i.e. most sprouting neurons were distributed in pericontusional cortical regions that also exhibited reduced extracellular CSPGs and/or PNNs. Optimal conditions for spontaneous sprouting axons are generally thought to arise from both the extracellular environment (extrinsic control) and from a switch to growth promoting mode by the cell (intrinsic control) (46–49).
GAP43 protein or mRNA expression has been the marker of choice characterizing spontaneous sprouting after CNS injury because it plays a key role in guiding the growth of axons and modulating the formation of new connections (49) and its peak developmental expression is coincident with periods of axonal growth, synaptic organization and activity-dependent plastic reorganization (50–52). GAP43 is normally limited to presynaptic terminals and unmyelinated axons and absent from neuronal somata (51) but optic nerve lesion experiments have demonstrated that a somato-centric expression is causally associated with axon elongation (53). The intense soma labeling we observed after TBI suggests marked intrinsic changes in the growth status of the neuron, consistent with GAP43 being a prime determinant of plasticity (49). The length of time over which GAP43 or expression of other growth-related molecules is enhanced has been suggested to influence the degree and extent of axon growth (47); thus, approximately 2 weeks of GAP43 expression after trauma may represent the time at which rehabilitative strategies would be most beneficial. On the other hand, the sustained decrease in PNNs to 28 days after injury shown herein, together with the idea that PNNs are involved in synapse formation and stabilization, would suggest that the brain is relatively plastic for longer periods. This requires further study with additional markers of plasticity or techniques that fully determine the temporal extent to which the brain remains plastic.
New synthesis of GAP43 required for efficient growth cone regeneration (54) would be affected by deficits in both blood flow and metabolic compromise after CCI (55–57). Indeed, evidence of reduced protein synthesis has been reported up to 3 days after lateral fluid percussion brain injury (FPI) via increased phosphorylation of the α-subunit of eukaryotic translation initiation factor 2 (42). This might explain the more widespread enhancement of GAP43 mRNA expression that we identified by in situ hybridization compared to protein IHC at 7 days. Thus, the GAP43 protein response at 7 and 14 days may be sub-maximal after CCI injury, since although protein synthesis was found to have normalized by 7 days after FPI (42), earlier deficits in synthesis would have blunted the response.
PSA-NCAM is expressed highly in development and is maintained in mature parts of the CNS that retain some degree of structural plasticity (58, 59). Co-expression of GAP43/PSA-NCAM has been shown after hypothalamic lesions in regions demonstrating considerable morphological plasticity and in cells not actively dividing (60). It is notable, therefore, that despite the growth of GAP43+ fibers within regions of PSA-NCAM+ cells in the present study, sprouting remained relatively subtle and limited to the cortical pericontused tissue. One possible reason for this is that the “net” cortical environment is still unfavorable to new growth, so that cells require a greater intrinsic stimulus to tip the balance in favor of growth rather than inhibition.
The loss of targets through axotomy is a powerful stimulus for promoting neuritic growth (48) and is likely to be a major reason for the localized sprouting in the pericontused region after TBI. Indeed, in the lateral FPI model perisomatic axonal injury does not result in cell death, and ultrastructural and Western blot evidence suggests that axotomized cells make a concerted effort at repair and reorganization at 7 days (42). Further evidence to explain the lesion-centric sprouting response comes from studies in the optic nerve and the spinal cord, in which only proximal axonal damage close to the cell body rather than distal axon injury results in a regenerative response (53, 61). Although we did not look earlier than 7 days after CCI injury, repair processes do begin earlier, as indicated by a lengthening of non-swollen axons 2 days after FPI (42) and early increases in gene expression of GAP43, CAP23 and cJun after stroke (62).
Our finding that neuronal sprouting is largely limited to pericontused regions in which extracellular and PNN-associated CSPGs are reduced provides correlative evidence that the traumatically injured cortical environment is extrinsically permissive to growth. PNNs are mainly composed of CSPGs that bind to hyaluronic acid (63) and are anchored to the cell surface of GABAergic neurons and diffusely around glutamatergic neurons (64). Lying in the perisynaptic space (65), they form a protective barrier ensheathing the presynaptic bouton, initial axon segment and glial processes (66), and their gradual accumulation postnatally suggests an involvement in the termination of synaptogenesis (67). The observed reduction in PNNs combined with a reduced extracellular burden of CSPGs after trauma shown herein by IHC and previously by Western blot (26) indicates there is a decline in the growth inhibition that is normally present to restrict inappropriate neuronal connections. As a result, there may be some potential for new connectivity via activation of either new or alternative circuits within the injured cortex. Indeed, functional blood flow autoradiography data from this contusion model has shown the existence of novel ipsilateral regions of activation at chronic times after injury (17).
In addition to the reduction in growth-inhibitory molecules that can enable sprouting, there is also undisputable evidence of the efficacy of growth-promoting molecules and neurotrophins in providing the growth stimulus for plastic changes. Fibronectin is a growth-promoting molecule found mainly around blood vessels in the adult brain. It is present in cortex at higher levels developmentally coincident with the formation of cortical afferents, suggesting a role in axon growth and pathfinding (68). It is increased after TBI (44), its presence after stroke is neuroprotective (69), and it can provide a substrate that is permissive for robust oriented axonal growth in the damaged spinal cord (70). When it is associated with astrocytes, fibronectin enables dorsal root ganglion cells to regenerate in heavily myelinated regions of corpus callosum in slice culture (71) and the pericontused region is rich in astrocytes after trauma (27). Therefore, we thought that fibronectin might be growth-promoting within the pericontused region after trauma but the present results do not support this contention. Although tissue within the center of the contusion had abundant fibronectin+ structures, presumably reflecting its plasma origin and involvement in wound healing (69, 72), sprouting neurons were not spatially consistent with this pattern since few GAP43+ profiles were co-labeled with fibronectin.
Exogenously applied BDNF enhances survival of axotomized spinal neurons and promotes sprouting after spinal cord injury (73). BDNF is widespread in the normal cerebral cortex, but its levels drop off considerably within pericontused tissue after CNS injury (28, 74). This is surprising since activated microglia and macrophages, which peak at 4 to 7 days after CCI injury (27), express BDNF after injury (75). The present IHC data clearly show normal BDNF immunostaining intensity around sprouting neurons after TBI. This probably enables new sprouts to grow, even across regions containing CSPGs (76). Interventions with exogenous BDNF to enhance the functional outcome after TBI have, however, been unsuccessful (77). Thus, while it is apparent that the injured cortex is deficient in BDNF, the current data suggests that a multifaceted intervention aimed at promoting growth, reducing growth inhibition and/or prolonging the window for spontaneous sprouting would likely be required to enhance functional outcome. Demonstrating the specificity of this outcome to axons in the forebrain will likely be an important, but difficult task.
We are uncertain as to why GAP43+ fibers occur within the contusion cavity, an area that is replete with highly intense, dead or infiltrating WFA+ cells. This is, however, not without precedent (76). One possibility is that it might reflect a localized, temporary imbalance between trophic factors released from accumulating glial cells, and the net reduction in CSPG proteins that occurs prior to 14 days (26). Another possibility is that the WFA epitope, which appears completely confined to the intracellular space of cells within this region, does not exert a marked growth-inhibitory effect here.
We observed aggrecan+ PNNs around sprouting neurons in pericontusion regions at 7 days after injury. This may be evidence of an attempt of the injured brain at re-directing the balance toward growth inhibition, since aggrecan is one of the first molecules upregulated in developing WFA+ PNNs in the immature brain (79). In addition, the injury-induced reduction in pericontusional extracellular CSPGs also begins to normalize towards control levels at 14 days post-injury (26); we observed that some neurons were neurocan+ at 7 days (Supplementary Fig. 1A). This might indicate either a limited potential for plasticity or the closing of a period of plasticity for that particular neuron or region of the cortex. Within a similar time period there is maturation of the glial scar and invasion by immature NG2+ oligodendrocytes in this model (27); both of these can potentiate the number of growth inhibitory molecules to hasten the end of a temporary window for plasticity. Furthermore, matrix metalloproteinase activities that can digest CSPGs (80) are normalized by 2 weeks after injury (81). As a result, the release of matrix-bound trophic factors would be curtailed, thereby further reducing the potential for growth. Given this evidence, it is surprising that we did not observe the normalization of cortical PNNs back to control levels by 28 days after injury. Developmentally, the age-related increase in visual cortex PNNs marks the end of a critical period for plasticity (78) and we had anticipated that this would occur after trauma. Since reconstitution of PNNs can take up to 5 months following digestion with exogenously applied enzyme (82), however, this may indicate that the brain remains somewhat plastic for longer. On the other hand, the presence of enduring functional deficits after trauma would suggest that any contribution of axonal sprouting to recovery is hampered by those regions that remain with, and/or regain a significant burden of extracellular CSPGs not associated with PNNs.
Compared to other brain regions, the sensory motor cortex is normally densely populated with PNNs (83) and GAP43 expression in sensory motor cortex and in the relay nuclei of the thalamus is low (51), implying that this system might be hard-wired for function and inherently less plastic. One future direction to overcome this is the exogenous application of chondroitinase enzyme to digest CSPG inhibitory side-chains. This has been shown to be beneficial after spinal cord injury by enhanced sprouting, commensurate with an electrophysiological and behavioral improvement (84, 85). We also have preliminary evidence that similar treatment after brain trauma enhances sprouting within sensory motor cortex (86). Future studies will be required to tailor these types of interventions together with rehabilitative strategies to enhance functional outcome following brain trauma.
In summary, we have shown that spontaneously sprouting neurons appear in the pericontused region after cortical trauma, coincident both spatially and temporally with deficits in inhibitory CSPGs that confer a period of permissive growth. Deficits in BDNF occur within the pericontused region and a critical period of axon sprouting appears to end as inhibitory CSPGs accumulate and reassert the balance in favor of growth inhibition.
Supplementary Material
Acknowledgments
This work was supported by the UCLA Brain Injury Research Center and Award Number R01 NS055910 from the National Institute of Neurological Disorders and Stroke (NINDS).
We wish to thank Mr. Andres Paucar for technical advice on riboprobe construction and Ms. Yan Cai, Sierra Lin, Johanna Aguilar and Mr. Louie Cruz for technical assistance. The content is the sole responsibility of the authors and does not necessarily represent official views of the NINDS or the National Institutes of Health.
References
- 1.Hsu JC, Stein SA, Xu X. Temporal and spatial distribution of growth-associated molecules and astroglial cells in the rat corticospinal tract during development. J Neurosc Res. 2005;80:330–40. doi: 10.1002/jnr.20472. [DOI] [PubMed] [Google Scholar]
- 2.Greenough WT, Larson JR, Withers GS. Effects of unilateral and bilateral training in a reaching task on dendritic branching of neurons in the rat motor-sensory forelimb cortex. Behav Neural Biol. 1985;44:301–14. doi: 10.1016/s0163-1047(85)90310-3. [DOI] [PubMed] [Google Scholar]
- 3.Darian-Smith C, Gilbert CD. Topographic reorganization in the striate cortex of the adult cat and monkey is cortically mediated. J Neurosci. 1995;15:1631–47. doi: 10.1523/JNEUROSCI.15-03-01631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleim JA, Lussnig E, Schwarz ER, et al. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–35. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gogolla N, Galimberti I, Caroni P. Structural plasticity of axon terminals in the adult. Curr Opin Neurobiol. 2007;17:516–24. doi: 10.1016/j.conb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J Neurosci. 1994;14:2140–52. doi: 10.1523/JNEUROSCI.14-04-02140.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmichael ST, Wei L, Rovainen CM, et al. New patterns of intracortical projections after focal cortical stroke. Neurobiol Dis. 2001;8:910–22. doi: 10.1006/nbdi.2001.0425. [DOI] [PubMed] [Google Scholar]
- 8.Weidner N, Ner A, Salimi N, et al. Spontaneous corticospinal axonal plasticity and functional recovery after adult central nervous system injury. Proc Natl Acad Sci U S A. 2001;98:3513–18. doi: 10.1073/pnas.051626798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uryu K, MacKenzie L, Chesselet M-F. Ultrastructural evidence for differential axonal sprouting in the striatum after thermocoagulatory and aspiration lesions of the cerebral cortex in adult rats. Neurosci. 2001;105:307–16. doi: 10.1016/s0306-4522(01)00203-2. [DOI] [PubMed] [Google Scholar]
- 10.Kobori N, Clifton G, Dash P. Altered expression of novel genes in the cerebral cortex following experimental brain injury. Brain Res Mol Brain Res. 2002;104:148. doi: 10.1016/s0169-328x(02)00331-5. [DOI] [PubMed] [Google Scholar]
- 11.Li HH, Lee SM, Cai Y, et al. Differential gene expression in hippocampus following experimental brain trauma reveals distinct features of moderate and severe injuries. J Neurotrauma. 2004;21:1141–53. doi: 10.1089/neu.2004.21.1141. [DOI] [PubMed] [Google Scholar]
- 12.Oyesiku NM, Evans CO, Houston S, et al. Regional changes in the expression of neurotrophic factors and their receptors following acute traumatic brain injury in the adult rat brain. Brain Res. 1999;833:161–72. doi: 10.1016/s0006-8993(99)01501-2. [DOI] [PubMed] [Google Scholar]
- 13.Christman CW, Salvant JB, Walker SA, et al. Characterization of a prolonged regenerative attempt by diffusely injured axons following traumatic brain injury in adult cat: A light and electron microscopic immunocytochemical study. Acta Neuropathol (Berl) 1997;94:329–37. doi: 10.1007/s004010050715. [DOI] [PubMed] [Google Scholar]
- 14.Hulsebosch CE, DeWitt DS, Jenkins LW, et al. Traumatic brain injury in rats results in increased expression of Gap-43 that correlates with behavioral recovery. Neurosci Lett. 1998;255:83–86. doi: 10.1016/s0304-3940(98)00712-5. [DOI] [PubMed] [Google Scholar]
- 15.Emery DL, Raghupathi R, Saatman KE, et al. Bilateral growth-related protein expression suggests a transient increase in regenerative potential following brain trauma. J Comp Neurol. 2000;424:521–31. [PubMed] [Google Scholar]
- 16.Dunn-Meynell AA, Levin BE. Lateralized effect of unilateral somatosensory cortex contusion on behavior and cortical reorganization. Brain Res. 1995;675:143–56. doi: 10.1016/0006-8993(95)00050-z. [DOI] [PubMed] [Google Scholar]
- 17.Harris NG, Chen SF, Pickard JD. Reorganisation after experimental traumatic brain injury: A functional autoradiography study (Abstract) J Cerebral Metab Blood Flow. 2003;21:1318. [Google Scholar]
- 18.Jorgensen OS, Hansen LI, Hoffman SW, et al. Synaptic remodeling and free radical formation after brain contusion injury in the rat. Exp Neurol. 1997;144:326–38. doi: 10.1006/exnr.1996.6372. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Fillmore HL, Reeves TM, et al. Elevation of hippocampal MMP-3 expression and activity during trauma-induced synaptogenesis. Exp Neurol. 2005;192:60–72. doi: 10.1016/j.expneurol.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Phillips LL, Reeves TM. Interactive pathology following traumatic brain injury modifies hippocampal plasticity. Restor Neurol Neurosci. 2001;19:213–35. [PubMed] [Google Scholar]
- 21.Scheff SW, Price DA, Hicks RR, et al. Synaptogenesis in the hippocampal CA1 field following traumatic brain injury. J Neurotrauma. 2005;22:719–32. doi: 10.1089/neu.2005.22.719. [DOI] [PubMed] [Google Scholar]
- 22.Kerschensteiner M, Schwab ME, Lichtman JW, et al. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–77. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Raisman G. Sprouts from cut corticospinal axons persist in the presence of astrocytic scarring in long-term lesions of the adult rat spinal cord. Exp Neurol. 1995;134:102–11. doi: 10.1006/exnr.1995.1041. [DOI] [PubMed] [Google Scholar]
- 24.Huber AB, Weinmann O, Brosamle C, et al. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after cns lesions. J Neurosci. 2002;22:3553–67. doi: 10.1523/JNEUROSCI.22-09-03553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thompson HJ, Marklund N, LeBold DG, et al. Tissue sparing and functional recovery following experimental traumatic brain injury is provided by treatment with an anti-myelin-associated glycoprotein antibody. Eur J Neurosci. 2006;24:3063–72. doi: 10.1111/j.1460-9568.2006.05197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris NG, Carmichael ST, Hovda DA, et al. Traumatic brain injury results in disparate regions of chondroitin sulphate proteoglycan expression that are temporally limited. J Neurosci Res. 2009;87:2937–50. doi: 10.1002/jnr.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S, Pickard JD, Harris NG. Time course of cellular pathology after controlled cortical impact injury. Exp Neurol. 2003;182:87–102. doi: 10.1016/s0014-4886(03)00002-5. [DOI] [PubMed] [Google Scholar]
- 28.Hicks RR, Li C, Zhang L, et al. Alterations in BDNF and trkB mRNA levels in the cerebral cortex following experimental brain trauma in rats. J Neurotrauma. 1999;16:501–10. doi: 10.1089/neu.1999.16.501. [DOI] [PubMed] [Google Scholar]
- 29.Tobias CA, Shumsky JS, Shibata M, et al. Delayed grafting of BDNF and NT-3 producing fibroblasts into the injured spinal cord stimulates sprouting, partially rescues axotomized red nucleus neurons from loss and atrophy, and provides limited regeneration. Exp Neurol. 2003;184:97–113. doi: 10.1016/s0014-4886(03)00394-7. [DOI] [PubMed] [Google Scholar]
- 30.Atkins CM, Oliva J, Alonso OF, et al. Modulation of the cAMP signaling pathway after traumatic brain injury. Exp Neurol. 2007;208:145–58. doi: 10.1016/j.expneurol.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai D, Shen Y, De Bellard M, et al. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- 32.Dixon CE, Clifton GL, Lighthall JW, et al. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–62. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- 33.Sutton RL, Lescaudron L, Stein DG. Unilateral cortical contusion injury in the rat: vascular disruption and temporal development of cortical necrosis. J Neurotrauma. 1993;10:135–49. doi: 10.1089/neu.1993.10.135. [DOI] [PubMed] [Google Scholar]
- 34.Feig SL. Corticothalamic cells in layers 5 and 6 of primary and secondary sensory cortex express GAP-43 mRNA in the adult rat. J Comp Neurol. 2004;468:96–111. doi: 10.1002/cne.10969. [DOI] [PubMed] [Google Scholar]
- 35.Miller JA. The calibration of 35S or 32P with 14C-labeled brain paste or 14C-plastic standards for quantitative autoradiography using LKB Ultrofilm or Amersham Hyperfilm. Neurosci Lett. 1991;121:211–14. doi: 10.1016/0304-3940(91)90687-o. [DOI] [PubMed] [Google Scholar]
- 36.Rasband WS. ImageJ. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2008. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 37.Newcomb JK, Zhao X, Pike BR, et al. Temporal profile of apoptotic-like changes in neurons and astrocytes following controlled cortical impact injury in the rat. Exp Neurol. 1999;158:76–88. doi: 10.1006/exnr.1999.7071. [DOI] [PubMed] [Google Scholar]
- 38.Dunn-Meynell AA, Levin BE. Histological markers of neuronal, axonal and astrocytic changes after lateral rigid impact traumatic brain injury. Brain Res. 1997;761:25–41. doi: 10.1016/s0006-8993(97)00210-2. [DOI] [PubMed] [Google Scholar]
- 39.Vitkovic L, Steisslinger HW, Aloyo VJ, et al. The 43-kDa neuronal growth-associated protein (GAP-43) is present in plasma membranes of rat astrocytes. Proc Natl Acad Sci U S A. 1988;85:8296–300. doi: 10.1073/pnas.85.21.8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamada K, Goto S, Oyama T, et al. In vivo induction of the growth associated protein GAP43/B-50 in rat astrocytes following transient middle cerebral artery occlusion. Acta Neuropathol (Berl) 1994;88:553–57. doi: 10.1007/BF00296492. [DOI] [PubMed] [Google Scholar]
- 41.Deloulme JC, Janet T, Au D, et al. Neuromodulin (GAP43): A neuronal protein kinase C substrate is also present in 0–2A glial cell lineage. Characterization of neuromodulin in secondary cultures of oligodendrocytes and comparison with the neuronal antigen. J Cell Biol. 1990;111:1559–69. doi: 10.1083/jcb.111.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singleton RH, Zhu J, Stone JR, et al. Traumatically induced axotomy adjacent to the soma does not result in acute neuronal death. J Neurosci. 2002;22:791–802. doi: 10.1523/JNEUROSCI.22-03-00791.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurokawa T, Tsuda M, Sugino Y. Purification and characterization of a lectin from Wistaria floribunda seeds. J Biol Chem. 1976;251:5686–93. [PubMed] [Google Scholar]
- 44.Tate CC, Tate MC, LaPlaca MC. Fibronectin and laminin increase in the mouse brain after controlled cortical impact injury. J Neurotrauma. 2007;24:226–30. doi: 10.1089/neu.2006.0043. [DOI] [PubMed] [Google Scholar]
- 45.Ghiani CA, Ying Z, de Vellis J, et al. Exercise decreases myelin-associated glycoprotein expression in the spinal cord and positively modulates neuronal growth. SO: Glia. 2007;55:966–75. doi: 10.1002/glia.20521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caroni P. Intrinsic neuronal determinants that promote axonal sprouting and elongation. Bioessays. 1997;19:767–75. doi: 10.1002/bies.950190906. [DOI] [PubMed] [Google Scholar]
- 47.Fawcett JW. Intrinsic neuronal determinants of regeneration. Trends Neurosci. 1992;15:5–8. doi: 10.1016/0166-2236(92)90338-9. [DOI] [PubMed] [Google Scholar]
- 48.Rossi F, Gianola S, Corvetti L. Regulation of intrinsic neuronal properties for axon growth and regeneration. Prog Neurobiol. 2007;81:1–28. doi: 10.1016/j.pneurobio.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 49.Benowitz LI, Routtenberg A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 50.Lovinger DM, Colley PA, Akers RF, et al. Direct relation of long-term synaptic potentiation to phosphorylation of membrane protein F1, a substrate for membrane protein kinase C. Brain Res. 1986;399:205–11. doi: 10.1016/0006-8993(86)91510-6. [DOI] [PubMed] [Google Scholar]
- 51.Dani JW, Armstrong DM, Benowitz LI. Mapping the development of the rat brain by GAP-43 immunocytochemistry. Neurosci. 1991;40:277–87. doi: 10.1016/0306-4522(91)90190-y. [DOI] [PubMed] [Google Scholar]
- 52.Neve RL, Bear MF. Visual experience regulates gene expression in the developing striate cortex. Proc Natl Acad Sci U S A. 1989;86:4781–84. doi: 10.1073/pnas.86.12.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doster SK, Lozano AM, Aguayo AJ, et al. Expression of the growth-associated protein GAP-43 in adult rat retinal ganglion cells following axon injury. Neuron. 1991;6:635–47. doi: 10.1016/0896-6273(91)90066-9. [DOI] [PubMed] [Google Scholar]
- 54.Verma P, Chierzi S, Codd AM, et al. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–42. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen SF, Richards HK, Smielewski P, et al. Relationship between flow-metabolism uncoupling and evolving axonal injury after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2004;24:1025–36. doi: 10.1097/01.WCB.0000129415.34520.47. [DOI] [PubMed] [Google Scholar]
- 56.Sutton RL, Hovda DA, Adelson PD, et al. Metabolic changes following cortical contusion: Relationships to edema and morphological changes. Acta Neurochir Suppl (Wien ) 1994;60:446–48. doi: 10.1007/978-3-7091-9334-1_122. [DOI] [PubMed] [Google Scholar]
- 57.Chen S, Richards HK, Smielewski P, et al. Preventing flow-metabolism uncoupling acutely reduces evolving axonal injury after traumatic brain injury (Abstract) J Neurotrauma. 2002;19:1300. doi: 10.1089/neu.2011.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonfanti L. PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol. 2006;80:129–64. doi: 10.1016/j.pneurobio.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 59.Gascon E, Vutskits L, Kiss JZ. Polysialic acid-neural cell adhesion molecule in brain plasticity: From synapses to integration of new neurons. Brain Res Rev. 2007;56:101–18. doi: 10.1016/j.brainresrev.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Alonso G, Prieto M, Legrand A, et al. PSA-NCAM and B-50/GAP-43 are coexpressed by specific neuronal systems of the adult rat mediobasal hypothalamus that exhibit remarkable capacities for morphological plasticity. J Comp Neurol. 1997;384:181–99. [PubMed] [Google Scholar]
- 61.Tetzlaff W, Kobayashi NR, Giehl KM, et al. Response of rubrospinal and corticospinal neurons to injury and neurotrophins. Prog Brain Res. 1994;103:271–86. doi: 10.1016/s0079-6123(08)61142-5. [DOI] [PubMed] [Google Scholar]
- 62.Carmichael ST, Archibeque I, Luke L, et al. Growth-associated gene expression after stroke: Evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193:291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Rauch U, Gao P, Janetzko A, et al. Isolation and characterization of developmentally regulated chondroitin sulfate and chondroitin/keratan sulfate proteoglycans of brain identified with monoclonal antibodies. J Biol Chem. 1991;266:14785–801. [PubMed] [Google Scholar]
- 64.Wegner F, Hartig W, Bringmann A, et al. Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABAA receptor [alpha]1 subunit form a unique entity in rat cerebral cortex. Exp Neurol. 2003;184:705–14. doi: 10.1016/S0014-4886(03)00313-3. [DOI] [PubMed] [Google Scholar]
- 65.Murakami T, Ohtsuka A, Taguchi T. Neurons with intensely negatively charged extracellular matrix in the human visual cortex. Arch Histol Cytol. 1994;57:509–22. doi: 10.1679/aohc.57.509. [DOI] [PubMed] [Google Scholar]
- 66.Bruckner G, Hartig W, Kacza J, et al. Extracellular matrix organization in various regions of rat brain grey matter. J Neurocytol. 1996;25:333–46. doi: 10.1007/BF02284806. [DOI] [PubMed] [Google Scholar]
- 67.Hockfield S, Kalb RG, Zaremba S, et al. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harbor Symp Quant Biol. 1990;55:505–14. doi: 10.1101/sqb.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- 68.Stewart GR, Pearlman AL. Fibronectin-like immunoreactivity in the developing cerebral cortex. J Neurosci. 1987;7:3325–33. doi: 10.1523/JNEUROSCI.07-10-03325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakai T, Johnson KJ, Murozono M, et al. Plasma fibronectin supports neuronal survival and reduces brain injury following transient focal cerebral ischemia but is not essential for skin-wound healing and hemostasis. Nat Med. 2001;7:324–30. doi: 10.1038/85471. [DOI] [PubMed] [Google Scholar]
- 70.King VR, Henseler M, Brown RA, et al. Mats made from fibronectin support oriented growth of axons in the damaged spinal cord of the adult rat. Exp Neurol. 2003;182:383–98. doi: 10.1016/s0014-4886(03)00033-5. [DOI] [PubMed] [Google Scholar]
- 71.Tom VJ, Doller CM, Malouf AT, et al. Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J Neurosci. 2004;24:9282–90. doi: 10.1523/JNEUROSCI.2120-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moretti FA, Chauhan AK, Iaconcig A, et al. A major fraction of fibronectin present in the extracellular matrix of tissues is plasma-derived. J Biol Chem. 2007;282:28057–62. doi: 10.1074/jbc.M611315200. [DOI] [PubMed] [Google Scholar]
- 73.Plunet W, Kwon BK, Tetzlaff W. Promoting axonal regeneration in the central nervous system by enhancing the cell body response to axotomy. J Neurosci Res. 2002;68:1–6. doi: 10.1002/jnr.10176. [DOI] [PubMed] [Google Scholar]
- 74.Batchelor PE, Liberatore GT, Porritt MJ, et al. Inhibition of brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor expression reduces dopaminergic sprouting in the injured striatum. Eur J Neurosci. 2000;12:3462–68. doi: 10.1046/j.1460-9568.2000.00239.x. [DOI] [PubMed] [Google Scholar]
- 75.Batchelor PE, Porritt MJ, Martinello P, et al. Macrophages and microglia produce local trophic gradients that stimulate axonal sprouting toward but not beyond the wound edge. Mol Cell Neurosci. 2002;21:436–53. doi: 10.1006/mcne.2002.1185. [DOI] [PubMed] [Google Scholar]
- 76.Batchelor PE, Wills TE, Hewa AP, et al. Stimulation of axonal sprouting by trophic factors immobilized within the wound core. Brain Res. 2008;1209:49–56. doi: 10.1016/j.brainres.2008.02.098. [DOI] [PubMed] [Google Scholar]
- 77.Blaha GR, Raghupathi R, Saatman KE, et al. Brain-derived neurotrophic factor administration after traumatic brain injury in the rat does not protect against behavioral or histological deficits. Neurosci. 2000;99:483–93. doi: 10.1016/s0306-4522(00)00214-1. [DOI] [PubMed] [Google Scholar]
- 78.Pizzorusso T, Medini P, Berardi N, et al. Reactivation of ocular dominance plasticity in the adult visual cortex. Sci. 2002;298:1248–51. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 79.Carulli D, Rhodes KE, Fawcett JW. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J Comp Neurol. 2007;501:83–94. doi: 10.1002/cne.21231. [DOI] [PubMed] [Google Scholar]
- 80.Muir EM, Adcock KH, Morgenstern DA, et al. Matrix metalloproteases and their inhibitors are produced by overlapping populations of activated astrocytes. Mol Brain Res. 2002;100:103–17. doi: 10.1016/s0169-328x(02)00132-8. [DOI] [PubMed] [Google Scholar]
- 81.von Gertten C, Holmin S, Mathiesen T, et al. Increases in matrix metalloproteinase-9 and tissue inhibitor of matrix metalloproteinase-1 mRNA after cerebral contusion and depolarisation. J Neurosci Res. 2003;73:803–10. doi: 10.1002/jnr.10729. [DOI] [PubMed] [Google Scholar]
- 82.Bruckner G, Bringmann A, Hartig W, et al. Acute and long-lasting changes in extracellular-matrix chondroitin-sulphate proteoglycans induced by injection of chondroitinase ABC in the adult rat brain. Exp Brain Res. 1998;121:300–10. doi: 10.1007/s002210050463. [DOI] [PubMed] [Google Scholar]
- 83.Bruckner G, Seeger G, Brauer K, et al. Cortical areas are revealed by distribution patterns of proteoglycan components and parvalbumin in the Mongolian gerbil and rat. Brain Res. 1994;658:67–86. doi: 10.1016/s0006-8993(09)90012-9. [DOI] [PubMed] [Google Scholar]
- 84.Massey JM, Hubscher CH, Wagoner MR, et al. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–14. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–40. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 86.Mironova Y, Hovda DA, Sutton RL, et al. Reduction in growth-inhibitory cspgs enhances cortical pericontusional sprouting but does not confer any behavioral improvement after cci brain injury (Abstract) J Neurotrauma. 2009;26:43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.