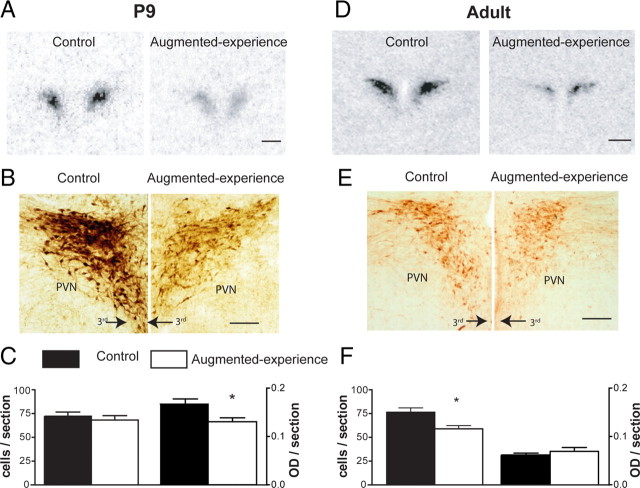
Figure 2.
Augmented early-life experience leads to early onset and persistent reduction in CRH expression in parvocellular PVN at both mRNA and protein levels. A, Representative bright-field photomicrographs of coronal sections at the level of the PVN from control and experience-augmented rats. The sections were subjected to ISH for CRH mRNA. CRH mRNA expression was reduced by 52% in P9 experience-augmented rats compared with undisturbed controls. B, Bright-field photomicrographs, taken under similar viewing parameters, showing CRH immunohistochemistry in PVN of control and experience-augmented rats. C, Quantitative analysis of the numbers of CRH-ir neurons and intensity of the immunoreactivity. The changes observed for mRNA expression were translated to protein levels as apparent from the ∼20% reduction in the intensity of CRH expression in experience-augmented rats. D, Representative autoradiographs after CRH mRNA ISH of CRH mRNA signal in adult control and experience-augmented rat PVN. CRH expression in experience-augmented adult rats was 50% lower compared with controls, indicating that repressed CRH expression, found on P9, was long-lasting. E, F, Bright-field photomicrographs and quantitative analysis of CRH immunohistochemistry in adult PVN. The enduring suppression of CRH expression observed at the mRNA level was translated to the protein level as evident from the ∼21% reduction in CRH-ir cells in the experience-augmented rats. 3rd, Third ventricle. Scale bars: A, D, 500 μm; B, E, 200 μm. Data are represented as mean ± SEM. *p < 0.05.