Abstract
Aggregation of α-synuclein (α-syn) is believed to play a critical role in the pathogenesis of disorders such as dementia with Lewy bodies and Parkinson’s disease. The function of α-syn remains unclear, although several lines of evidence suggest that α-syn is involved in synaptic vesicle trafficking probably via lipid binding. Moreover, interactions with cholesterol and lipids have been shown to be involved in α-syn aggregation. In this context, the main objective of this study was to determine if statins – cholesterol synthesis inhibitors – might interfere with α-syn accumulation in cellular models. For this purpose, we studied the effects of lovastatin, simvastatin, and pravastatin on the accumulation of α-syn in a stably transfected neuronal cell line and in primary human neurons. Statins reduced the levels of α-syn accumulation in the detergent insoluble fraction of the transfected cells. This was accompanied by a redistribution of α-syn in caveolar fractions, a reduction in oxidized α-syn, and enhanced neurite outgrowth. In contrast, supplementation of the media with cholesterol increased α-syn aggregation in detergent insoluble fractions of transfected cells and was accompanied by reduced neurite outgrowth. Taken together, these results suggest that regulation of cholesterol levels with cholesterol inhibitors might be a novel approach for the treatment of Parkinson’s disease.
Keywords: cholesterol, cholesterol inhibitors, Parkinson’s disease, protein aggregation, statins, synuclein
In Parkinson’s disease (PD), aggregation of α-synuclein (α-syn) appears to play a critical role in the neurodegenerative process (Iwai et al. 1994; Polymeropoulos et al. 1997; Spillantini et al. 1997; Wakabayashi et al. 1997; Kruger et al. 1998; Takeda et al. 1998; Trojanowski and Lee 1998; Masliah et al. 2000; Lee et al. 2002, 2004b; Singleton et al. 2003). Although the number of individuals with these conditions continues to increase, only few therapeutic treatments are currently available. Recent studies have suggested that as cholesterol is considered a risk factor for Alzheimer’s disease (AD) (Jarvik et al. 1995; Notkola et al. 1998) and probably for PD (Huang et al. 2007), then use of cholesterol inhibitors such as statins might have therapeutic potential (Selley 2005; Rajanikant et al. 2007).
Statins reduce cholesterol levels by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (Corsini et al. 1995), the enzyme that converts HMG-CoA to mevalonate, which is the rate-limiting step in the biosynthesis of cholesterol. While the role of cholesterol and cholesterol synthesis inhibitors in AD is well documented, less is known about these factors in PD. Recent studies have suggested that statins might lower the risk of PD (Huang et al. 2007; Wolozin et al. 2007). Moreover, previous studies have shown that cholesterol and α-syn might interact in lipid rafts (Fortin et al. 2004) and that lipid intake in the diet might be a risk factor for PD (Johnson et al. 1999); however, other studies have reported a less significant effect of hyperlipidemia in PD (Chen et al. 2003; de Lau et al. 2005; Huang et al. 2007). Recent studies have shown that concentrations of oxidized cholesterol metabolites are elevated in the brains of PD patients and accelerate α-syn aggregation (Bieschke et al. 2006; Bosco et al. 2006). Toxic α-syn species are represented by sodium dodecyl sulfate (SDS)-resistant soluble oligomers and not by fibrils (Cappai et al. 2005). This is of importance because accumulation of α-syn oligomers plays an important role in the pathogenesis of PD (Uversky et al. 2001; Lashuel et al. 2002; Lee et al. 2004a; Walsh and Selkoe 2004; Tsigelny et al. 2007).
α-Synuclein is an abundant nerve terminal protein that might play a role in dopaminergic synapse release and plasticity (Murphy et al. 2000). This molecule contains 11 lipid-binding domains associated with a number of membranes such as synaptic vesicles, lipid droplets, and yeast plasma membrane (Maroteaux et al. 1988; Jensen et al. 1998; Outeiro and Lindquist 2003), and has been shown to bind vesicles containing acidic phospholipids (Perrin et al. 2000) and polyunsaturated fatty acids (Perrin et al. 2001; Sharon et al. 2003a,b). Moreover, cholesterol in the membrane might modulate the conformational state of α-syn (Davidson et al. 1998).
We have recently shown that the cholesterol-extracting agent methyl-β-cyclodextrin (MβCD) reduces α-syn aggregation in cell lines and transgenic animal models (Bar-On et al. 2006). However, the use of cyclodextrins in vivo is controversial and will require further investigation (Monnaert et al. 2004; Binkowski-Machut et al. 2006). For this reason, we decided to investigate the potential effects of alternative cholesterol-reducing agents such as statins in models of α-syn accumulation and to investigate the role of cholesterol in α-syn aggregation. These studies showed that cholesterol-reducing agents such as lovastatin reduced the accumulation of α-syn and ameliorated the associated neuronal deficits, suggesting a potential role in the treatment of PD.
Experimental procedures
Cell cultures
B103 neuroblastoma cells transfected with human-α-syn or empty vector (pCEP4; Invitrogen, Carlsbad, CA, USA) were grown as previously described (Takenouchi et al. 2001). These cells were routinely cultured in high glucose Dulbecco’s modified Eagle’s medium containing 10% v/v fetal calf serum (Irvine Scientific, Santa Ana, CA, USA) supplemented with 50 µg/mL hygromycin B (Calbiochem, San Diego, CA, USA), 5% v/v sodium pyruvate (Gibco-BRL, Grand Island, NY, USA), and 1% v/v gentamycin (10 mg/mL, Invitrogen) in a 5% CO2, 95% air atmosphere. This neuronal cell line, derived from rat neuroblastoma, was selected because of its ability to express under basal conditions molecules associated with lipid rafts, such as flotillin (Eckert et al. 2003). Moreover, α-syn over-expression in this line results in the formation of discrete aggregates in the cell body and processes accompanied by reduced neurite outgrowth (Takenouchi et al. 2001), mimicking another important aspect of PD, namely compromised axonal plasticity.
Primary fetal human neurons (generously provided by Dr G. Chana, University of California, San Diego, CA, USA) were prepared essentially as previously described (Trillo-Pazos et al. 2000). Briefly, clinically anonymous human fetal brain tissue of 13–18 weeks gestation was collected by Advanced Biosciences (Almada, CA, USA) under UCSD IRB approval. The tissue was disaggregated by micropipetting into a single cell suspension and seeded at 1 × 105 cells in Neurobasal Medium (Gibco-BRL), with 2% v/v B27 supplement (Gibco-BRL), 0.5% v/v l-glutamine (200 mM; Gibco-BRL) and 0.2% v/v gentamycin (10 mg/mL; Invitrogen). The media was changed every 3 days to ensure cellular enrichment and cells were grown for 1 month to reach maturity. Cells are considered to have a mature phenotype at this age because of their expression of NMDA receptors (Everall et al. 2001).
Upon maturity, primary human neurons were infected with lentiviral (lenti) vectors expressing α-syn or green fluorescent protein (GFP) and incubated for 72 h. Lentiviral vectors were prepared as previously described (Marr et al. 2003). Briefly, vector plasmids were constructed for the production of third generation lenti-vectors and the human cytomegalovirus promoter was used to drive expression of the transgenes. Lentiviral vectors were produced using a four-plasmid transfection system, as described previously (Dull et al. 1998; Miyoshi et al. 1998). Titers were estimated by measuring the amount of human immunodeficiency virus p24 gag antigen with an ELISA kit (Perkin-Elmer Life Science, Boston, MA, USA) (100 000 transducing units per ng of p24).
Cell treatments
Cells were cultured in complete media until 50–60% confluent and then cultured in lipid-deficient fetal bovine serum media (Intracel, Royston Herts, UK) for 6–24 h. Cells were treated for 24 h with 10 µM lovastatin or simvastatin (Calbiochem) dissolved in dimethylsulfoxide or with 10 µM pravastatin (Calbiochem) dissolved in water. Treatment with cholesterol–EtOH or with lipid-enriched cholesterol (Sigma-Aldrich, St. Louis, MO, USA) was for 6 h. Mature human neurons were treated with 10 µM lovastatin for 24 h following the 72 h lentivirus infection described above.
Cell viability assay and analysis of neurite outgrowth
To study the fate of neuronal cells treated with cholesterol or statins and to find the optimum time course for treating cells with these agents, cell viability was measured with the CytoTox 96-Non-Radioactive Cytotoxicity (lactate dehydrogenase (LDH)) kit (Promega, San Luis Obispo, CA, USA) and with the Cell Proliferation Kit I (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Roche, Palo Alto, CA, USA) according to the manufacturers’ protocols for each assay. In addition, neurite outgrowth was evaluated as previously described (Takenouchi et al. 2001). For this purpose, averages of 10 images per condition of neuronal cells in culture were captured for subsequent analysis with the ImagePro Plus program (Media Cybernetic, Silver Spring, MO, USA). A neurite was defined as a cellular process longer than the diameter of one cell body. Neurite length was analyzed in at least 100 cells for each experimental group.
Determination of cholesterol levels
Quantification of total cholesterol (measuring both cholesterol and cholesteryl ester) was performed using the Cholesterol/Cholesteryl Ester Quantification Kit (BioVision, Mountain View, CA, USA), which is a colorimetric method. Briefly, for each condition, cells were first extracted with 200 µL of hexane–isopropanol, centrifuged for 5 min at 16 000 g in a microcentrifuge, and the supernatant was collected, vacuumed dry and then the lipids were dissolved in 200 µL 2-propanol containing 10% v/v Triton X-100, followed by analysis according to the manufacturer’s instructions.
Fractionation of neuronal cell lysates
As previous studies have shown that α-syn is more abundant in the cytosolic than in the particulate fraction and that interactions with lipids might favor the translocation of this protein to the membrane (Hashimoto et al. 2003; Ulmer et al. 2005), immunoblot analysis was performed with neuronal cells fractionated into detergent soluble and insoluble fractions, as previously described with minor modifications (Petrucelli et al. 2004; Ho et al. 2005). Briefly, cells were centrifuged for 10 min at 5000 g at 4°C and lysed in TNE buffer containing 1% v/v Triton X-100 (Sigma-Aldrich). Cells were sonicated for 30 s and ultracentrifuged (274 000 g, 1 h, 4°C), and the detergent soluble proteins were collected in the supernatant fraction. The pelleted detergent insoluble proteins were dissolved in Tris-NaCl-EDTA buffer containing 1% v/v Triton X-100 and 1% v/v SDS. Total protein concentration of each sample was determined using the BCA protein assay kit (Pierce, Rockford, IL, USA).
Preparation of fractions in sucrose gradients
Isolation of sucrose fractions was performed as previously described with some modifications (Morishima-Kawashima and Ihara 1998; Bar-On et al. 2006). Briefly, cells (1 × 107 in two confluent 100 mm dishes) were rinsed in phosphate-buffered saline (PBS), harvested, and homogenized in 800 µL of 2-N-morpholino-ethane-sulfonic acid-buffered saline (MBS; 25 mM 2-N-morpholino-ethane-sulfonic acid, pH 6.5, and 150 mM NaCl) containing 1% v/v Triton X-100 with protease inhibitors and phosphatase inhibitors (Chemicon International, Temecula, CA, USA). After treatment with DnaseI (10 U/mL) for 1 h, cell extracts were combined with 800 µL of 80% w/v sucrose in MBS and placed at the bottoms of ultracentrifuge tubes, and overlaid with a 35%, 5% discontinuous sucrose gradient (1.6 mL each) in MBS. The gradients were then centrifuged at 40 000 g for 23 h in a Tli rotor (Beckman, Fullerton, CA, USA) at 4°C. After centrifugation, each fraction (400 µL) was collected from the top of the gradient to yield a total of 12 fractions (fractions are numbered from the top of the gradient). Fractions 4–8 represent the lipid raft-like, insoluble components, while soluble components are located in fractions 9–12. Fractions were stored at −80°C until used. Equal volumes (20 µL) of each fraction were subjected to electrophoresis. This experiment was performed in duplicate to ensure accuracy of the results.
Western blot analysis
Samples from detergent soluble and insoluble fractions (20 µg protein), and fractions from the sucrose gradients (20 µL volume) were separated on 4–12% SDS–polyacrylamide gel electrophoresis (NuPAGE; Invitrogen) and transferred onto 0.22-µm nitrocellulose membranes (Schleicher & Schunell, Keene, NH, USA) using 1× 3-[cyclohexylamino]-1-propaneosulfonic acid transfer buffer containing 20% v/v methanol. Membranes were blocked with 3% w/v milk in PBS containing 0.1% v/v Tween-20 (Sigma-Aldrich), followed by incubation in primary antibody (1 : 1000) in PBS containing 0.1% v/v Tween-20 overnight at 4°C. The primary antibodies used were as follows: anti-flotillin-1 (mouse monoclonal) and anti-α-syn (Syn-1 clone, mouse monoclonal) from BD Transduction Laboratories (Newington, NH, USA); anti-actin (mouse monoclonal, C4 clone, pan-actin) and anti-α-syn (rabbit polyclonal) from Chemicon; anti-human-α-syn (LB509 clone) from Zymed (San Francisco, CA, USA), anti-phosphorylated (S129) α-syn (rabbit polyclonal, courtesy of Dr T. Iwatsubo; Fujiwara et al. 2002), antibodies against nitrated α-syn (Tyr39 or Tyr125, 136, mouse monoclonal antibodies) from Upstate (Lake Placid, NY, USA), and antioxidized α-syn (mouse monoclonal, Clone 4H316) from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Membranes were further incubated with goat anti-mouse or anti-rabbit IgG secondary antibodies conjugated to horseradish peroxidase (1 : 5000; American Qualex, San Clemente, CA, USA), visualized by enhanced chemiluminescence (NEN Life Sciences, Boston, MA, USA) and exposed to film or developed on the VersaDoc gel imaging system (Bio-Rad, Hercules, CA, USA). For determination of levels of immunoreactivity, images from enhanced chemiluminescence-treated membranes were analyzed using the Quantity One software (Bio-Rad). In some cases levels of α-syn were expressed as a ratio to the actin levels to account for loading of the samples.
Immunocytochemical analysis and confocal microscopy
Immunohistochemistry of neuronal cells treated with statins or cholesterol was performed as previously described (Takenouchi et al. 2001). Cells were seeded onto poly-l-lysine-coated glass coverslips, grown to 60% confluence, fixed in 4% v/v p-formaldehyde for 30 min, and pre-treated for 20 min with 0.1% v/v Triton X-100 in Tris-buffered saline (ScyTek Laboratories, Logan, UT, USA). The coverslips were first incubated overnight at 4°C either with the antibody against human-α-syn (72–10, 1 : 5000; Masliah et al. 2000) or anti-flotillin (1 : 1000). The next day, antibodies were detected with the Tyramide Signal Amplification-Direct (Red) system (NEN Life Sciences), followed by an overnight incubation with the mouse monoclonal anti-microtubule-associated protein 2 (1 : 50; Roche Molecular Biochemicals, Indianapolis, IN, USA) primary antibody. This antibody was then detected with the FITC conjugated anti-mouse secondary antibody (Vector Laboratories, Burlingame, CA, USA). Vector transfected cells were run in parallel as controls. Other control experiments included immunolabeling in the absence of primary antibodies or with antibodies adsorbed for 48 h with 20-fold excess peptide. Coverslips were air dried overnight, mounted on slides with anti-fading media (Vectashield; Vector), and imaged with the laser scanning confocal microscope (MRC1024; Bio-Rad). All images were obtained using the same gain settings and laser intensities on the laser scanning confocal microscopy. Analysis was performed with blind coded samples and was performed in duplicate to assure reproducibility of results.
Statistical analysis
All values in the figures are expressed as mean ± SEM. To determine statistical significance, the values were compared by Student’s t-test or one-way anova with post hoc Dunnett’s test using the Statview II statistical program (SAS Institute, Cary, NC, USA). The differences were considered significant if p < 0.05.
Results
Statins reduce α-syn levels in the detergent insoluble fraction of a stably transfected neuronal cell line
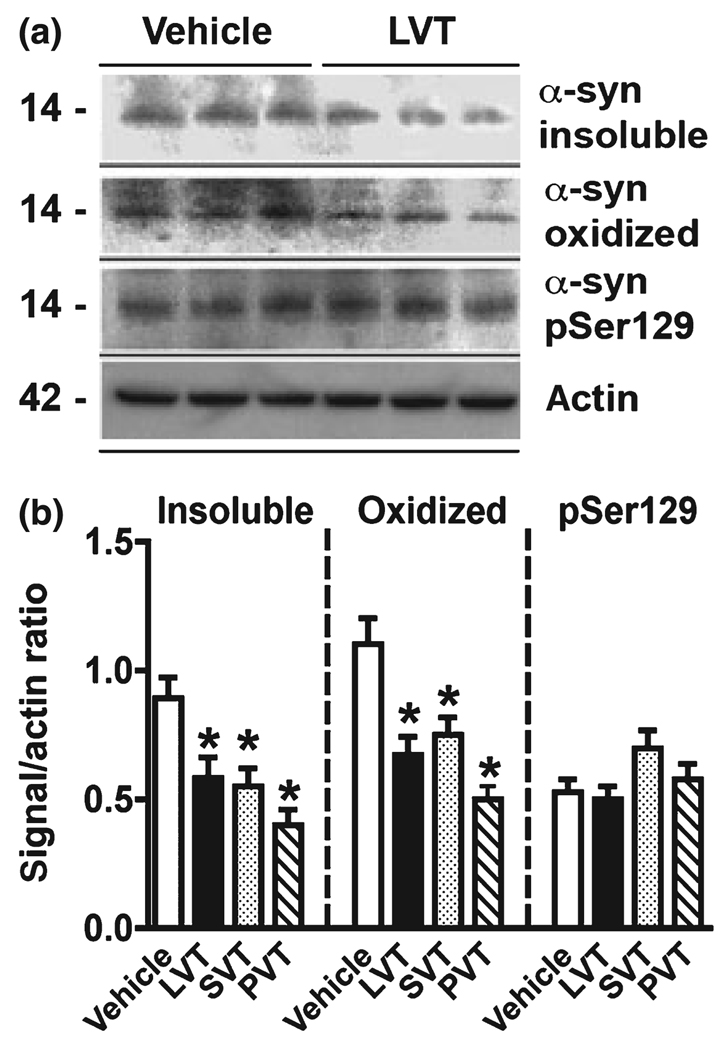
Studies have shown that oxidized cholesterol metabolites are elevated in PD (Bieschke et al. 2006; Bosco et al. 2006), and we showed in our previous study that the cholesterol-extracting agent MβCD reduced α-syn levels in vitro and in vivo (Bar-On et al. 2006). However, it is not clear whether inhibiting cholesterol synthesis would have similar effects. Therefore, we investigated the effect of cholesterol synthesis inhibitors such as statins – HMG-CoA reductase inhibitors, which inhibit cholesterol synthesis in its rate-limiting step – on α-syn aggregation. We used two hydrophobic statins, lovastatin and simvastain, and one hydrophilic compound, pravastatin. In order to determine the optimum time course for treatment, cultures from a neuronal cell line (B103 clone) stably transfected with α-syn were exposed to a 10 µM concentration of each of the statins, and cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and LDH assays. These studies showed that transfected B103 cells were tolerant of all three statins at a 10 µM concentration after 24 h treatment (Fig. 1a and b). To confirm that the statins were active at reducing the cholesterol levels in this in vitro model, cholesterol levels in both the media and in the cell lysates were measured. After 24 h, the cholesterol levels were significantly reduced in cell lysates prepared from statin-treated cells (Fig. 1c). Based on these results, cells were treated with the statins at 10 µM for 24 h and western blot analysis was performed. Compared with vehicle-treated controls (water or dimethylsulfoxide), treatment with statins resulted in a significant reduction in the levels of α-syn immunoreactivity in the detergent insoluble fraction (Fig. 1d and f), with pravastatin being the most effective at reducing α-syn levels. No significant effects were observed in the levels of α-syn immunoreactivity in the detergent soluble fraction (Fig. 1d and e). Untransfected cells did not display detectable levels of α-syn immunoreactivity (not shown).
Fig. 1.
Effects of statins on cell viability and α-syn accumulation in stably transfected B103 neuronal cells. (a) Analysis of cell viability by MTT assay in α-syn transfected B103 cells treated with lovastatin (LVT), simvastatin (SVT), or pravastatin (PVT). (b) Analysis of levels of cytotoxicity by LDH assay in α-syn transfected B103 cells treated with LVT, SVT, or PVT. LDH was measured in aliquots taken from the media of treated samples and values are expressed as percentage cell death in treated samples compared with total levels of LDH in a lysed control sample. (c) Cellular cholesterol levels in α-syn transfected B103 cells treated with LVT, SVT, or PVT. (d) Immunoblot analysis was performed with detergent soluble and insoluble cell lysate fractions (20 µg total protein per sample). Blots were probed with a rabbit polyclonal anti-α-syn antibody (Chemicon). Immunoblot analysis of fractions from α-syn transfected B103 cells treated with LVT, SVT, or PVT. Levels of α-syn immunoreactivity are expressed as a ratio compared with levels of actin. (e) Quantitative analysis of α-syn levels in detergent soluble fraction. (f) Quantitative analysis of α-syn levels in detergent insoluble fraction showing reduced α-syn accumulation in cells treated with statins. *p < 0.05 compared with vehicle-treated α-syn transfected controls; one-way anova with post hoc Dunnett’s test.
Consistent with previous studies (Takenouchi et al. 2001), immunocytochemical and confocal microscopy analysis showed increased expression of α-syn in the transfected B103 cells compared with untransfected controls (Fig. 2a–f). In contrast, all three statins reduced the immunoreactivity of α-syn in the cytoplasm and neuritic processes of the α-syn transfected neuronal cells (Fig. 2g–o) compared with vehicle-treated controls (Fig. 2d–f). The effects of the statins on the levels of α-syn (Fig. 2p) were accompanied by a recovery in the neurite extension deficits to lengths similar to vehicle-treated control untransfected cells (Fig. 2q). In contrast, α-syn-expressing B103 cells treated with vehicle alone displayed reduced neurite lengths compared with control vehicle-treated untransfected cells (Fig. 2q).
Fig. 2.
Immunocytochemical analysis of α-syn immunoreactivity in stably transfected B103 neuronal cells treated with statins. Cells were double-labeled with antibodies against microtubule-associated protein 2 (MAP2) (green channel) and α-syn (red channel) and analyzed by laser scanning confocal microscopy. (a–c) Control vehicle-treated, untransfected B103 cells. (d–f) Vehicle-treated α-syn transfected B103 cells. (g–o) Reduced α-syn immunoreactivity in α-syn transfected B103 cells treated with lovastatin (LVT; g–i), simvastatin (SVT; j–l) or pravastatin (PVT; m–o). (p) Levels of α-syn immunoreactivity in α-syn transfected B103 cells treated with vehicle (veh), LVT, SVT, or PVT. (q) Measurements of neurite lengths in control untransfected and α-syn transfected B103 cells treated with vehicle (veh), LVT, SVT, or PVT. *p < 0.05 compared with vehicle-treated α-syn transfected controls; **p < 0.05 compared with vehicle-treated untransfected controls; one-way anova with post hoc Dunnett’s test. Scale bar: 10 µm (a–o).
Lovastatin reduces α-syn in the insoluble fraction of lentivirus-infected mature human neurons
To confirm that statins have similar effects on α-syn accumulation in primary neurons, fetal cortical human cells were infected with lenti-GFP or lenti-α-syn and treated with lovastatin. This statin was selected because it is extensively used in the clinic; it crosses the blood–brain barrier, and is less toxic than other statins. Infection with lenti-GFP showed that the efficiency of infection was as high as 90% (Fig. 3a and b). Compared with cells infected with lenti-vector control (Fig. 3c), infection with lenti-α-syn gave rise to efficient and sustained α-syn immunoreactivity in primary neurons (Fig 3d). Lovastatin treatment of mature human neurons infected with lenti-α-syn resulted in a reduction of α-syn in the insoluble fraction of the cells when compared with untreated α-syn-infected cells (Fig. 3e and f). There were no significant changes in the soluble fractions of the cells (Fig. 3e and f). Moreover, lovastatin treatment of α-syn-expressing primary neurons ameliorated the alterations in neurite outgrowth in these cells (Fig. 3g–i). Lovastatin treatment of un-infected primary neurons did not significantly affect endogenous α-syn levels in either the soluble or insoluble fraction (Fig. 3j and k), nor did lovastatin treatment result in any alterations in neurite outgrowth in GFP-expressing cells (Fig. 3i) or un-infected neurons (Fig. 3l–n).
Fig. 3.
Lovastatin reduces α-syn accumulation in primary fetal cortical neuronal cultures. (a and b) Over 80% of the neuronal cells infected with lentiviral (lenti)-GFP expressed the marker. (c) Endogenous levels of α-syn expression in primary neuronal cultures. (d) Abundant α-syn accumulation in neuronal cell bodies and neurites of primary neurons infected with lenti-α-syn. (e and f) Immunoblot analysis was performed with detergent soluble and insoluble cell lysate fractions (20 µg total protein per sample). Blots were probed with a rabbit polyclonal anti-α-syn antibody (Chemicon). There was reduced α-syn accumulation in the detergent insoluble fraction of lenti-α-syn infected primary neuronal cells treated with lovastatin (LVT). (g–i) Treatment with LVT ameliorated the neurite outgrowth deficits in primary neuronal cells infected with lenti-α-syn. No changes in neurite length were detected in primary neuronal cells infected with lenti-GFP and treated with LVT. (j and k) Immunoblot analysis showing unchanged levels of endogenous α-syn in detergent soluble and insoluble fractions of un-infected primary neuronal cells treated with LVT. (l–n) Treatment with LVT had no significant effects on neurite outgrowth in un-infected primary neuronal cells. *p < 0.05 compared with lenti-α-syn infected, vehicle-treated controls; **p < 0.05 compared with lenti-GFP infected, vehicle-treated controls; Student’s t-test. Scale bar: 50 µm (a–d, g, h, l, and m).
Effects of statins on α-syn distribution in sucrose gradients
Recent studies have suggested that lipid rafts mediate the synaptic localization of α-syn (Fortin et al. 2004). It has been shown that α-syn colocalizes with lipid raft components in cells, and fractionation with detergent-resistant membranes on density gradients suggests that α-syn associates with lipid rafts under physiological conditions. In addition, disruption of the rafts by extracting cholesterol using MβCD significantly reduced the proportion of α-syn in the synapses (Fortin et al. 2004). In view of these findings, and to further investigate the effects of statins on α-syn redistribution and compartmentalization, immunoblot analysis was performed with sucrose gradient fractions from B103 stably transfected cells treated with each of the three statins at 10 µM for 24 h. The cholesterol distribution among the fractions displayed a significant difference between the treated and the untreated samples. Compared with the untreated samples, in the treated samples there was a significant reduction in the cholesterol level in the caveolar fractions, where most of the cholesterol is concentrated (Fig. 4a). Total levels of cholesterol from all of the fractions combined were reduced in statin-treated cells (Fig. 4b), which confirms that the statins are active in our model system and indeed inhibit cholesterol synthesis in these cells. As expected, the protein distribution pattern both in the treated and in the untreated samples was similar; most of the proteins were detected in the cytosolic fractions (fractions 9–12, Fig. 4c), whereas much lower levels of total protein were detected in the caveolar fractions (fractions 4–8, Fig. 4c) and almost no proteins were in the heavy fractions in the upper part of the gradient (fractions 1–3, Fig. 4c). No significant differences were detected between treatment groups in the total protein concentrations from all of the fractions combined (Fig. 4d). In the transfected neuronal cells, abundant α-syn accumulation was detected in fractions 5–9 that includes the caveolar fraction (Fig. 4e and f). Compared with vehicle-treated controls (Fig. 4e and f), lovastatin treatment resulted in a reduction of insoluble α-syn in the caveolar fractions, with a redistribution of α-syn from the caveolar fractions to all fractions, including cytosolic fractions (Fig. 4g and h). Similar effects were observed with simvastatin and pravastatin (not shown).
Fig. 4.
Effects of lovastatin on α-syn distribution in sucrose gradient fractions from stably transfected B103 neuronal cells. Fractions 1–3 contain little protein content, while fractions 4–8 represent the caveolar lipid raft-like, insoluble components, and soluble components are located in fractions 9–12. (a) Representative cholesterol levels in the sucrose gradient fractions of α-syn transfected cells treated with lovastatin (LVT), simvastatin (SVT), or pravastatin (PVT). (b) Reduced total cholesterol levels among all fractions in statin-treated cells. (c) Representative total protein levels in the sucrose gradient fractions. (d) Total protein levels among all fractions. (e and f) Immunoblot analysis was performed with sucrose gradient fractions prepared from cell lysates (20 µL volume per sample). Blots were probed with a rabbit polyclonal anti-α-syn antibody (Chemicon). Immunoblot analysis of levels of α-syn and flotillin in sucrose gradient fractions prepared from α-syn transfected, vehicle-treated B103 cells. (g and h) Immunoblot analysis of levels of α-syn and flotillin in sucrose gradient fractions prepared from α-syn transfected, LVT-treated B103 cells. *p < 0.05 compared with vehicle-treated α-syn transfected controls; one-way anova with post hoc Dunnett’s test.
Statins reduce levels of oxidized α-syn in α-syn-expressing neuronal cells
We showed that the effects of statins on cholesterol levels might reduce α-syn in the caveolar fraction (Fig. 4). In addition, statins have been shown to have antioxidant effects (Selley 2005) and might reduce α-syn accumulation by blocking post-translational modifications. To this end, α-syn-expressing B103 neuronal cells were treated with statins at 10 µM for 24 h and western blot analysis was performed. This study showed that statins reduced the levels of insoluble and oxidized α-syn but had no effects on phosphorylated α-syn (Fig. 5a and b). Comparable effects were observed with the three statins (Fig. 5b). With the antibodies against nitrated α-syn (Tyr39 and Tyr125,136), only a faint band was detected (not shown).
Fig. 5.
Immunoblot analysis of the effects of statins on post-translational modifications of α-syn. B103 neuronal cells expressing α-syn were treated for 24 h with lovastatin (LVT), simvastatin (SVT), or pravastatin (PVT). (a) Representative western blot illustrating the effects of LVT on insoluble, oxidized, and phosphorylated (pSer129) α-syn. (b) Levels of insoluble and oxidized α-syn but not phosphorylated α-syn were reduced with statins. *p < 0.05 compared with vehicle-treated α-syn transfected controls; one-way anova with post hoc Dunnett’s test.
Cholesterol induces α-syn aggregation in a-syn-expressing neuronal cells
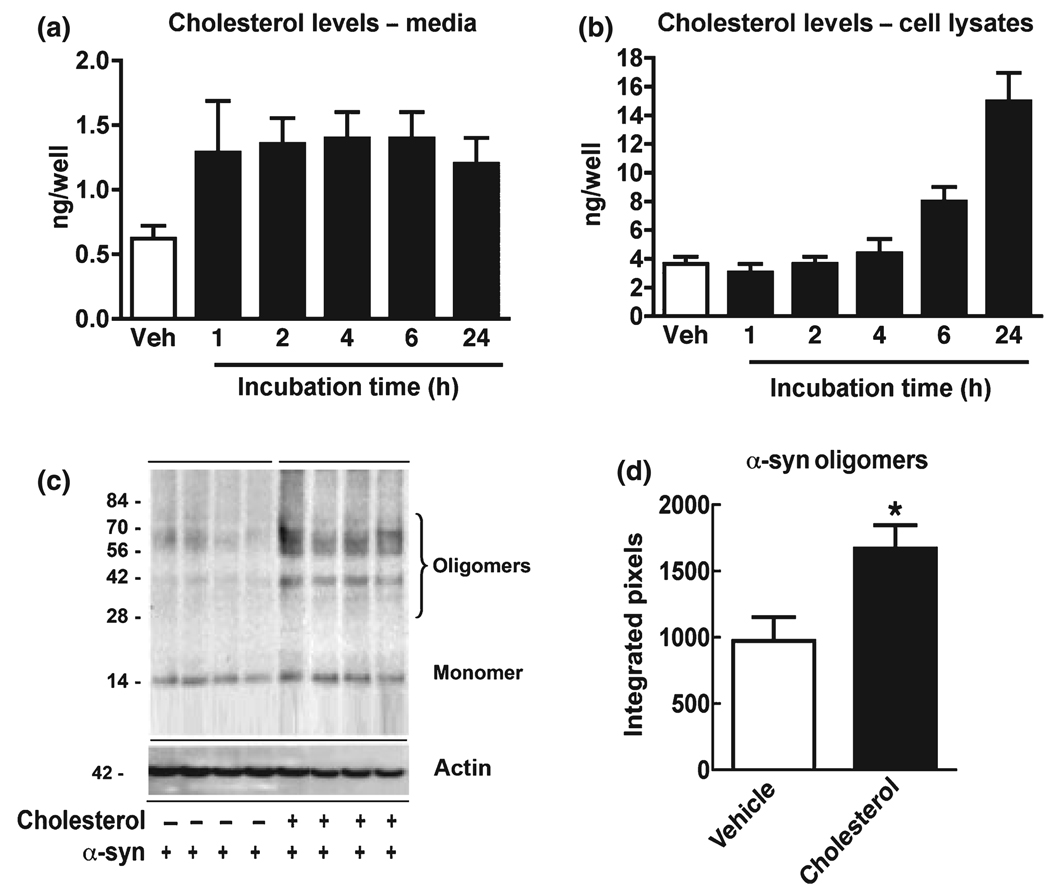
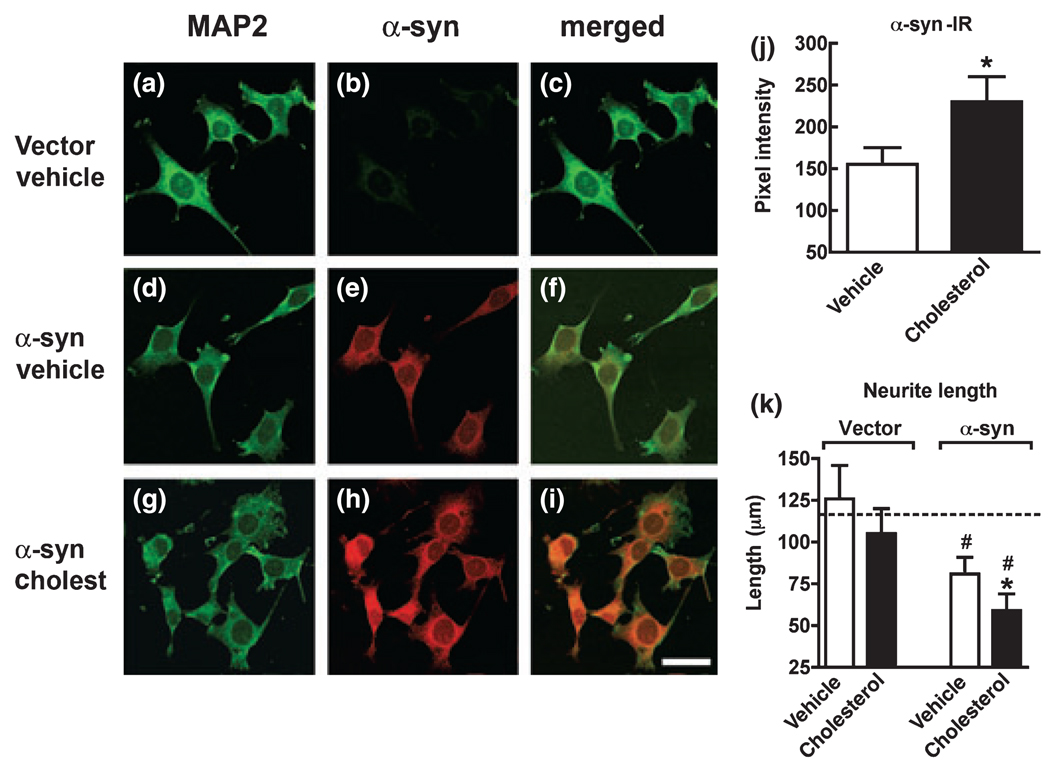
We have shown that reducing the endogenous cholesterol levels with statins decreases α-syn accumulation in the detergent insoluble fraction. However, it is unclear if increasing cholesterol over basal levels in transfected cells enhances α-syn accumulation. In order to determine the optimum time course for treatment in vitro, stably transfected neuronal cell lines were exposed to 25 µM cholesterol–EtOH for up to 24 h and cell viability was determined by LDH. These studies showed that transfected B103 cells were tolerant to cholesterol–EtOH at a 25 µM concentration for up to 24 h. To confirm that the cholesterol was active in the in vitro model, cholesterol levels were measured. This study showed that in both the media (Fig. 6a) and in total cell lysates after 6 h, the levels of cholesterol were increased (Fig. 6b). Based on these results, cells were treated with 25 µM cholesterol–EtOH for 6 h and western blot analysis was performed. Compared with vehicle-treated controls (EtOH only), treatment with cholesterol–EtOH resulted in increased levels of α-syn immunoreactivity in the detergent insoluble fraction (Fig. 6c and d). To determine the effect of cholesterol–EtOH on the cellular distribution of α-syn, double-labeling immunocytochemistry, and confocal analysis was performed. Compared with cells transfected with the vector control (Fig. 7a–c), in the α-syn transfected cells, abundant α-syn immunoreactivity was detected in the neuronal cell bodies and extended to the neurites (Fig. 7d– f). In α-syn transfected cells treated with cholesterol–EtOH, α-syn immunoreactivity was increased in the neuritic processes and to some extent in the cell bodies of neurons (Fig. 7g–i). Total levels of α-syn immunoreactivity were significantly increased (Fig. 7j). Consistent with the accumulation of insoluble α-syn with cholesterol, neurite out-growth was further reduced in these cells compared with control α-syn transfected cells (Fig. 7k). Cholesterol treatment of cells transfected with vector control (Fig. 7k) or untransfected cells did not significantly affect neurite outgrowth. Taken together, these studies support the notion that elevated cholesterol levels might play a role in promoting α-syn aggregation and that statins ameliorate these effects.
Fig. 6.
Analysis of the effects of cholesterol on α-syn accumulation in stably transfected B103 neuronal cells. (a and b) Total cholesterol levels in the media and cell lysates from α-syn transfected B103 cells exposed to 25 µM cholesterol. (c and d) Immunoblot analysis was performed with detergent insoluble fractions (20 µg total protein per sample). Blots were probed with a rabbit polyclonal anti-α-syn antibody (Chemicon). Immunoblot analysis demonstrating increasing levels of α-syn oligomers in α-syn transfected B103 cells exposed to 25 µM cholesterol. *p < 0.05 compared with vehicle-treated α-syn transfected controls; Student’s t-test.
Fig. 7.
Immunocytochemical analysis of the effects of cholesterol on α-syn accumulation and neurite extension in stably transfected B103 neuronal cells. Cells were double-labeled with antibodies against microtubule-associated protein 2 (MAP2) (green channel) and α-syn (red channel) and analyzed by laser scanning confocal microscopy. (a–c) Control vehicle-treated, vector transfected B103 cells. (d–f) Vehicle-treated α-syn transfected B103 cells. (g–i) Increased α-syn immunoreactivity in α-syn transfected B103 cells treated with cholesterol (Cholest). (j) Levels of α-syn immunoreactivity in α-syn transfected B103 cells treated with vehicle or cholesterol. (k) Measurements of neurite lengths in vector or α-syn transfected B103 cells treated with vehicle or cholesterol. Dashed line represents average neurite lengths in control untransfected cells. Experiments were performed in triplicate; *p < 0.05 compared with vehicle-treated α-syn transfected controls; #p < 0.05 compared with vehicle-treated vector transfected controls; Student’s t-test. Scale bar: 15 µm (a–i).
Discussion
The present study showed that cholesterol synthesis inhibitors such as lovastatin, simvastatin, and pravastatin decreased the accumulation of α-syn in the detergent insoluble fractions of α-syn-expressing neuronal cells and ameliorated the associated effects of α-syn on neuronal morphology. These findings are consistent with a previous study showing that MβCD reduces α-syn accumulation in a transfected neuronal cell line expressing α-syn and in transgenic mice (Bar-On et al. 2006) and support the possibility that cholesterol-reducing agents might be useful in the treatment of PD. Lovastatin is of particular interest because of its prevalence as a treatment in vivo and in vitro in other neurodegenerative disorders. Lovastatin is also lipophilic and crosses the blood–brain barrier better than hydrophilic statins such as pravastatin (Saheki et al. 1994).
In recent years, there has been an increased interest in the role of cholesterol and statins in neurodegenerative disorders (Menge et al. 2005; Rajanikant et al. 2007). Cholesterol has been associated with higher risk of AD (Jarvik et al. 1995; Notkola et al. 1998), and there is a reduced incidence of AD among patients taking statins (Jick et al. 2000;Wolozin et al. 2000). In addition to the therapeutic potential of statins in AD, these compounds have shown promise as therapies in other neurodegenerative disorders including PD (Menge et al. 2005; Rajanikant et al. 2007). Supporting this view, a recent epidemiological study with the US Veterans Affairs database showed that statins reduced the incidence of dementia and PD (Wolozin et al. 2007). In this study, simvastatin was associated with a significant reduction in the incidence of dementia in subjects over 65 years of age and also demonstrated a reduced hazard ratio for newly acquired PD. Lovastatin was not associated with a reduction in the incidence of dementia, although it is possible that it might have effects on parkinsonism. Another study in a smaller population suggests a potential neuroprotective effect of statins in PD (Huang et al. 2007), however the potential beneficial effects of statins in this neurodegenerative disorder are controversial (Huang et al. 2007). For example, an additional effect of statins is the reduction in levels of coenzyme Q10 (CoQ10) (Folkers et al. 1990), a strong antioxidant and a component of the mitochondrial electron transport chain that is currently being considered as a candidate drug for treatment of PD (Beal 2004). As oxidative stress is an important contributing factor to the pathogenesis of PD, and CoQ10 may play a role in PD (Beal 2004), statin treatment for PD might require CoQ10 supplementation. Nonetheless, it should be noted that statins do not exacerbate PD symptoms (Lieberman et al. 2005) and other studies have shown no effects on CoQ10 levels (Hargreaves et al. 2005). This may reflect the doses of statins used, since many have been used at doses below those recommended for their maximum therapeutic effects. However, further in vivo studies are necessary to clarify the precise nature of the interplay between statins and CoQ10 in the pathogenesis of PD.
The mechanisms through which statins might reduce α-syn accumulation in models of PD are not completely clear. One possibility is that decreasing the levels of cholesterol synthesis might change the composition of the plasma membrane and fluidity that are necessary for α-syn aggregation. Cholesterol is an important component of the caveolae and interaction between cholesterol and α-syn has been shown to occur in the caveolae (Fortin et al. 2004). In the present study, we showed that upon inhibition of cholesterol synthesis with statins, there is a shift in the distribution of α-syn among sucrose gradient fractions. In our model of neuronal cells over-expressing α-syn, under control conditions α-syn is mainly associated with lipid rafts in the caveolar fractions whereas in the presence of lovastatin there is a shift of α-syn from the higher density fractions to the cytosolic fractions (fractions 8–12). These findings are consistent with recent studies showing that lipid rafts mediate the synaptic localization of α-syn, and this association with lipid rafts is sensitive to the effects of cholesterol-extracting agents such as MβCD (Fortin et al. 2004; Bar-On et al. 2006). Accumulation of misfolded proteins in the caveolar fractions has been previously shown to be an important step in the neurodegenerative process (Russelakis-Carneiro et al. 2004). Reducing cholesterol with statins might also be important in reducing the pathological interactions with αsyn because elevated levels of oxidized cholesterol metabolites have been shown to accelerate α-syn aggregation and toxicity (Bosco et al. 2006). In support of this possibility, our western blot analysis showed that statins reduced oxidized (but not phosphorylated) α-syn. Alternatively, statins might have a more general antioxidant effect (Selley 2005). This is important because in PD, radical oxygen species have been shown to play a role in the mechanisms of neurodegeneration (Rockenstein et al. 2007) and α-syn aggregation (Hashimoto et al. 1999; Ischiropoulos 2003; Ischiropoulos and Beckman 2003).
Finally, in the present study, we showed that cholesterol promotes α-syn aggregation in a cell-free system and in neuronal cells expressing α-syn. In contrast, regulating cholesterol levels with statins reduces insoluble α-syn accumulation but does not affect levels of soluble α-syn. Moreover, recent studies have shown that both of these findings are relevant to the role of oxidative stress (Cappai et al. 2005). Supporting this view, a recent study demonstrated elevated concentrations of oxidative cholesterol metabolites in the cortices of individuals with dementia with Lewy bodies compared with those of age-matched controls, and showed that these oxidized metabolites accelerated α-syn aggregation in vitro (Bieschke et al. 2006; Bosco et al. 2006). In PD it has been proposed that changes in the levels of cholesterol and other lipids can lead to misfolded (toxic) accumulation of α-syn oligomers in the membrane (Volles et al. 2001; Ding et al. 2002; Tsigelny et al. 2007). Similarly, in the MPTP model of PD, there is an increased generation of radical oxygen species and cholesterol oxidation (Selley 2005) that might lead to α-syn accumulation. This is of interest because a previous study showed that simvastatin prevents dopamine depletion and oxidative stress in an MPTP model (Selley 2005). Taken together, these studies suggest that statins might have beneficial effects in PD through a variety of mechanisms including reducing cholesterol that could associate with α-syn, antioxidant effects, and probably an anti-inflammatory role by decreasing tumor necrosis factor-α (Selley 2005).
In conclusion, the present study has shown that cholesterol synthesis inhibitors, such as statins, decrease the levels of detergent insoluble α-syn in neuronal cells and that cholesterol might promote α-syn aggregation, providing additional evidence in favor of the role of statins in the management of PD. However, further in vivo studies are necessary to confirm the potential beneficial effects of statins in disorders with α-syn aggregation.
Acknowledgements
This work was supported by NIH Grants AG18440, AG022074, and AG10435 and by the Don and Marilyn Short SIRA Research Fellowship in Parkinson’s Disease.
Abbreviations used
- α-syn
α-synuclein
- AD
Alzheimer’s disease
- CoQ10
coenzyme Q10
- GFP
green fluorescent protein
- HMG-CoA
3-hydroxy-3-methylglutaryl-coenzyme A
- LDH
lactate dehydrogenase
- lenti
lentiviral
- MBS
2-β-morpholino-ethane-sulfonic acid-buffered saline
- MβCD
methyl-β cyclodextrin
- PBS
phosphate-buffered saline
- PD
Parkinson’s disease
- SDS
sodium dodecyl sulfate
References
- Bar-On P, Rockenstein E, Adame A, Ho G, Hashimoto M, Masliah E. Effects of the cholesterol-lowering compound methyl-beta-cyclodextrin in models of alpha-synucleinopathy. J. Neurochem. 2006;98:1032–1045. doi: 10.1111/j.1471-4159.2006.04017.x. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondrial dysfunction and oxidative damage in Alzheimer’s and Parkinson’s diseases and coenzyme Q10 as a potential treatment. J. Bioenerg. Biomembr. 2004;36:381–386. doi: 10.1023/B:JOBB.0000041772.74810.92. [DOI] [PubMed] [Google Scholar]
- Bieschke J, Zhang Q, Bosco DA, Lerner RA, Powers ET, Wentworth P, Jr, Kelly JW. Small molecule oxidation products trigger disease-associated protein misfolding. Acc. Chem. Res. 2006;39:611–619. doi: 10.1021/ar0500766. [DOI] [PubMed] [Google Scholar]
- Binkowski-Machut C, Hapiot F, Martin P, Cecchelli R, Monflier E. How cyclodextrins can mask their toxic effect on the blood-brain barrier. Bioorg. Med. Chem. Lett. 2006;16:1784–1787. doi: 10.1016/j.bmcl.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Bosco DA, Fowler DM, Zhang Q, Nieva J, Powers ET, Wentworth P, Jr, Lerner RA, Kelly JW. Elevated levels of oxidized cholesterol metabolites in Lewy body disease brains accelerate alpha-synuclein fibrilization. Nat. Chem. Biol. 2006;2:249–253. doi: 10.1038/nchembio782. [DOI] [PubMed] [Google Scholar]
- Cappai R, Leck SL, Tew DJ, et al. Dopamine promotes alpha-synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J. 2005;19:1377–1379. doi: 10.1096/fj.04-3437fje. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Hernan MA, Willett WC, Ascherio A. Dietary intakes of fat and risk of Parkinson’s disease. Am. J. Epidemiol. 2003;157:1007–1014. doi: 10.1093/aje/kwg073. [DOI] [PubMed] [Google Scholar]
- Corsini A, Maggi FM, Catapano AL. Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol. Res. 1995;31:9–27. doi: 10.1016/1043-6618(95)80042-5. [DOI] [PubMed] [Google Scholar]
- Davidson W, Jonas A, Clayton D, George J. Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 1998;273:9443–9449. doi: 10.1074/jbc.273.16.9443. [DOI] [PubMed] [Google Scholar]
- Ding TT, Lee SJ, Rochet JC, Lansbury PT., Jr Annular alpha-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes. Biochemistry. 2002;41:10209–10217. doi: 10.1021/bi020139h. [DOI] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert GP, Igbavboa U, Muller WE, Wood WG. Lipid rafts of purified mouse brain synaptosomes prepared with or without detergent reveal different lipid and protein domains. Brain Res. 2003;962:144–150. doi: 10.1016/s0006-8993(02)03986-0. [DOI] [PubMed] [Google Scholar]
- Everall IP, Trillo-Pazos G, Bell C, Mallory M, Sanders V, Masliah E. Amelioration of neurotoxic effects of HIV envelope protein gp120 by fibroblast growth factor: a strategy for neuroprotection. J. Neuropathol. Exp. Neurol. 2001;60:293–301. doi: 10.1093/jnen/60.3.293. [DOI] [PubMed] [Google Scholar]
- Folkers K, Langsjoen P, Willis R, Richardson P, Xia LJ, Ye CQ, Tamagawa H. Lovastatin decreases coenzyme Q levels in humans. Proc. Natl Acad. Sci. USA. 1990;87:8931–8934. doi: 10.1073/pnas.87.22.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DL, Troyer MD, Nakamura K, Kubo S, Anthony MD, Edwards RH. Lipid rafts mediate the synaptic localization of alpha-synuclein. J. Neurosci. 2004;24:6715–6723. doi: 10.1523/JNEUROSCI.1594-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Hargreaves IP, Duncan AJ, Heales SJ, Land JM. The effect of HMG-CoA reductase inhibitors on coenzyme Q10: possible biochemical/clinical implications. Drug Saf. 2005;28:659–676. doi: 10.2165/00002018-200528080-00002. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Hsu L, Xia Y, Takeda A, Sundsmo M, Masliah E. Oxidative stress induces amyloid-like aggregate formation of NACP/a-synuclein in vitro. Neuroreport. 1999;10:717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Crews L, Masliah E. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromol. Med. 2003;4:21–36. doi: 10.1385/NMM:4:1-2:21. [DOI] [PubMed] [Google Scholar]
- Ho GJ, Hashimoto M, Adame A, Izu M, Alford MF, Thal LJ, Hansen LA, Masliah E. Altered p59Fyn kinase expression accompanies disease progression in Alzheimer’s disease: implications for its functional role. Neurobiol. Aging. 2005;26:625–635. doi: 10.1016/j.neurobiolaging.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Huang X, Chen H, Miller WC, Mailman RB, Woodard JL, Chen PC, Xiang D, Murrow RW, Wang YZ, Poole C. Lower low-density lipoprotein cholesterol levels are associated with Parkinson’s disease. Mov. Disord. 2007;22:377–381. doi: 10.1002/mds.21290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ischiropoulos H. Oxidative modifications of alpha-synuclein. Ann. NY Acad. Sci. 2003;991:93–100. doi: 10.1111/j.1749-6632.2003.tb07466.x. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J. Clin. Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai A, Masliah E, Yoshimoto M, De Silva R, Ge N, Kittel A, Saitoh T. The precursor protein of non-Ab component of Alzheimer’s disease amyloid (NACP) is a presynaptic protein of the central nervous system. Neuron. 1994;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- Jarvik GP, Wijsman EM, Kukull WA, Schellenberg GD, Yu C, Larson EB. Interactions of apolipoprotein E genotype, total cholesterol level, age, and sex in prediction of Alzheimer’s disease: a case-control study. Neurology. 1995;45:1092–1096. doi: 10.1212/wnl.45.6.1092. [DOI] [PubMed] [Google Scholar]
- Jensen PH, Nielsen MS, Jakes R, Dotti CG, Goedert M. Binding of alpha-synuclein to brain vesicles is abolished by familial Parkinson’s disease mutation. J. Biol. Chem. 1998;273 doi: 10.1074/jbc.273.41.26292. 000. [DOI] [PubMed] [Google Scholar]
- Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- Johnson CC, Gorell JM, Rybicki BA, Sanders K, Peterson EL. Adult nutrient intake as a risk factor for Parkinson’s disease. Int. J. Epidemiol. 1999;28:1102–1109. doi: 10.1093/ije/28.6.1102. [DOI] [PubMed] [Google Scholar]
- Kruger R, Kuhn W, Muller T, Woitalla D, Graeber M, Kosel S, Przuntek H, Epplen J, Schols L, Reiss O. Ala30Pro mutation in the gene encoding a-synuclein in Parkinsons’s disease. Nat. Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, Lansbury PT., Jr Alpha-synuclein, especially the Parkinson’s disease-associated mutants, forms pore-like annular and tubular protofibrils. J. Mol. Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- de Lau LM, Bornebroek M, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Dietary fatty acids and the risk of Parkinson disease: the Rotterdam study. Neurology. 2005;64:2040–2045. doi: 10.1212/01.WNL.0000166038.67153.9F. [DOI] [PubMed] [Google Scholar]
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson’s disease-linked Ala-53 → Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc. Natl Acad. Sci. USA. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Khoshaghideh F, Patel S, Lee SJ. Clearance of alpha-synuclein oligomeric intermediates via the lysosomal degradation pathway. J. Neurosci. 2004a;24:1888–1896. doi: 10.1523/JNEUROSCI.3809-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee VM, Giasson BI, Trojanowski JQ. More than just two peas in a pod: common amyloidogenic properties of tau and alpha-synuclein in neurodegenerative diseases. Trends Neurosci. 2004b;27:129–134. doi: 10.1016/j.tins.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Lieberman A, Lyons K, Levine J, Myerburg R. Statins, cholesterol, co-enzyme Q10, and Parkinson’s disease. Parkinsonism Relat. Disord. 2005;11:81–84. doi: 10.1016/j.parkreldis.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Maroteaux L, Campanelli J, Scheller R. Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr RA, Rockenstein E, Mukherjee A, Kindy MS, Hersh LB, Gage FH, Verma IM, Masliah E. Neprilysin gene transfer reduces human amyloid pathology in transgenic mice. J. Neurosci. 2003;23:1992–1996. doi: 10.1523/JNEUROSCI.23-06-01992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Menge T, Hartung HP, Stuve O. Statins – a cure-all for the brain? Nat. Rev. Neurosci. 2005;6:325–331. doi: 10.1038/nrn1652. [DOI] [PubMed] [Google Scholar]
- Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J. Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnaert V, Tilloy S, Bricout H, Fenart L, Cecchelli R, Monflier E. Behavior of alpha-, beta-, and gamma-cyclodextrins and their derivatives on an in vitro model of blood-brain barrier. J. Pharmacol. Exp. Ther. 2004;310:745–751. doi: 10.1124/jpet.104.067512. [DOI] [PubMed] [Google Scholar]
- Morishima-Kawashima M, Ihara Y. The presence of amyloid β-protein in the detergent-soluble membrane compartment of human neuroblastoma cells. Biochemistry. 1998;37:15247–15253. doi: 10.1021/bi981843u. [DOI] [PubMed] [Google Scholar]
- Murphy D, Reuter S, Trojanowski J, Lee V-Y. Synucleins are developmentally expressed, and a-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, Tuomilehto J, Nissinen A. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology. 1998;17:14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- Outeiro TF, Lindquist S. Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science. 2003;302:1772–1775. doi: 10.1126/science.1090439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin R, Woods W, Clayton D, George J. Interaction of human alpha-synuclein and Parkinson’s disease variants with phospholipids: structural analysis using site0directed mutagenesis. J. Biol. Chem. 2000;275:34393–34398. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- Perrin RJ, Woods WS, Clayton DF, George JM. Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J. Biol. Chem. 2001;276:41958–41962. doi: 10.1074/jbc.M105022200. [DOI] [PubMed] [Google Scholar]
- Petrucelli L, Dickson D, Kehoe K, et al. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M, Lavedan C, Leroy E, et al. Mutation in the a-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Rajanikant GK, Zemke D, Kassab M, Majid A. The therapeutic potential of statins in neurological disorders. Curr. Med. Chem. 2007;14:103–112. doi: 10.2174/092986707779313462. [DOI] [PubMed] [Google Scholar]
- Rockenstein E, Crews L, Masliah E. Transgenic animal models of neurodegenerative diseases and their application to treatment development. Adv. Drug. Deliv. Rev. 2007;59:1093–1102. doi: 10.1016/j.addr.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Russelakis-Carneiro M, Hetz C, Maundrell K, Soto C. Prion replication alters the distribution of synaptophysin and caveolin 1 in neuronal lipid rafts. Am. J. Pathol. 2004;165:1839–1848. doi: 10.1016/S0002-9440(10)63439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saheki A, Terasaki T, Tamai I, Tsuji A. In vivo and in vitro blood-brain barrier transport of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors. Pharm. Res. 1994;11:305–311. doi: 10.1023/a:1018975928974. [DOI] [PubMed] [Google Scholar]
- Selley ML. Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res. 2005;1037:1–6. doi: 10.1016/j.brainres.2004.02.083. [DOI] [PubMed] [Google Scholar]
- Sharon R, Bar-Josef I, Mirick G, Serhan C, Selkoe D. Altered fatty acid composition of dopaminergic neurons expressing alpha-synuclein and human brains with alpha-synucleinopathies. J. Biol. Chem. 2003a;278:49874–49881. doi: 10.1074/jbc.M309127200. [DOI] [PubMed] [Google Scholar]
- Sharon R, Bar-Joseph I, Frosch MP, Walsh DM, Hamilton JA, Selkoe DJ. The formation of highly soluble oligomers of alpha-synuclein is regulated by fatty acids and enhanced in Parkinson’s disease. Neuron. 2003b;37:583–595. doi: 10.1016/s0896-6273(03)00024-2. [DOI] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J. Alpha-synuclein locus triplication causes Parkinson’s disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Spillantini M, Schmidt M, Lee V-Y, Trojanowski J, Jakes R, Goedert M. α-Synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Takeda A, Mallory M, Sundsmo M, Honer W, Hansen L, Masliah E. Abnormal accumulation of NACP/a-synuclein in neurodegenerative disorders. Am. J. Pathol. 1998;152:367–372. [PMC free article] [PubMed] [Google Scholar]
- Takenouchi T, Hashimoto M, Hsu L, Mackowski B, Rockenstein E, Mallory M, Masliah E. Reduced neuritic outgrowth and cell adhesion in neuronal cells transfected with human a-synuclein. Mol. Cell. Neurosci. 2001;17:141–150. doi: 10.1006/mcne.2000.0923. [DOI] [PubMed] [Google Scholar]
- Trillo-Pazos G, McFarlane-Abdulla E, Campbell IC, Pilkington GJ, Everall IP. Recombinant nef HIV-IIIB protein is toxic to human neurons in culture. Brain Res. 2000;864:315–326. doi: 10.1016/s0006-8993(00)02213-7. [DOI] [PubMed] [Google Scholar]
- Trojanowski J, Lee V. Aggregation of neurofilament and alpha-synuclein proteins in Lewy bodies: implications for pathogenesis of Parkinson disease and Lewy body dementia. Arch. Neurol. 1998;55:151–152. doi: 10.1001/archneur.55.2.151. [DOI] [PubMed] [Google Scholar]
- Tsigelny IF, Bar-On P, Sharikov Y, Crews L, Hashimoto M, Miller MA, Keller SH, Platoshyn O, Yuan JX, Masliah E. Dynamics of alpha-synuclein aggregation and inhibition of pore-like oligomer development by beta-synuclein. FEBS J. 2007;274:1862–1877. doi: 10.1111/j.1742-4658.2007.05733.x. [DOI] [PubMed] [Google Scholar]
- Ulmer TS, Bax A, Cole NB, Nussbaum RL. Structure and dynamics of micelle-bound human alpha-synuclein. J. Biol. Chem. 2005;280:9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- Uversky VN, Lee HJ, Li J, Fink AL, Lee SJ. Stabilization of partially folded conformation during alpha-synuclein oligomerization in both purified and cytosolic preparations. J. Biol. Chem. 2001;276:43495–43498. doi: 10.1074/jbc.C100551200. [DOI] [PubMed] [Google Scholar]
- Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein: implications for the pathogenesis and treatment of Parkinson’s disease. Biochemistry. 2001;40:7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Matsumoto K, Takayama K, Yoshimoto M, Takahashi H. NACP, a presynaptic protein, immunoreactivity in Lewy bodies in Parkinson’s disease. Neurosci. Lett. 1997;239:45–48. doi: 10.1016/s0304-3940(97)00891-4. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Oligomers on the brain: the emerging role of soluble protein aggregates in neurodegeneration. Protein Pept. Lett. 2004;11:213–228. doi: 10.2174/0929866043407174. [DOI] [PubMed] [Google Scholar]
- Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007;5:20. doi: 10.1186/1741-7015-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]