Abstract
Neuromyelitis optica is an inflammatory demyelinating disease of the central nervous system associated with autoantibodies against the glial water channel protein aquaporin-4. It has recently been reported that immunoglobulin from neuromyelitis optica patients injected peripherally does not cause lesions in naive rats, but only when pre-existing central nervous system inflammation is present. Here, we investigated whether immunoglobulin G from aquaporin-4-autoantibody-positive neuromyelitis optica patients has the potential to damage the central nervous system either alone or in the presence of human complement. Immunoglobulin G from neuromyelitis optica patients did not activate mouse complement and was not pathogenic when injected into mouse brain. However, co-injection of immunoglobulin G from neuromyelitis optica patients with human complement produced neuromyelitis optica-like lesions in mice. Within 12 h of co-injecting immunoglobulin G from neuromyelitis optica patients and human complement, there was a striking loss of aquaporin-4 expression, glial cell oedema, myelin breakdown and axonal injury, but little intra-parenchymal inflammation. At 7 days, there was extensive inflammatory cell infiltration, perivascular deposition of activated complement components, extensive demyelination, loss of aquaporin-4 expression, loss of reactive astrocytes and neuronal cell death. In behavioural studies, mice injected with immunoglobulin G from neuromyelitis optica patients and human complement into the right hemisphere preferentially turned to the right at 7 days. No brain inflammation, demyelination or right-turning behaviour was seen in wild-type mice that received immunoglobulin G from non-neuromyelitis optica patients with human complement, or in aquaporin-4-null mice that received immunoglobulin G from neuromyelitis optica patients with human complement. We conclude that co-injection of immunoglobulin G from neuromyelitis optica patients with human complement reproduces the key histological features of neuromyelitis optica and that aquaporin-4 is necessary and sufficient for immunoglobulin G from neuromyelitis optica patients to exert its effect. In our mouse model, immunoglobulin G from neuromyelitis optica patients does not require pre-existing central nervous system inflammation to produce lesions.
Keywords: autoantibody, immunoglobulin, Devic’s syndrome, mouse model, water channel
Introduction
Neuromyelitis optica is an inflammatory demyelinating disease of the CNS that can cause severe disability including blindness and paralysis (Wingerchuk et al., 2007; Jarius et al., 2008b). Most patients with neuromyelitis optica have immunoglobulin (Ig)-G autoantibodies in their serum that bind to extracellular epitope(s) of the water channel protein aquaporin-4 (AQP4) (Lennon et al., 2005; Hinson et al., 2007). AQP4, the most abundantly expressed water channel in the CNS, is found in perimicrovessel astrocyte foot processes, glia limitans and ependyma (Tait et al., 2008). AQP4 facilitates brain oedema formation and elimination, astrocyte migration and neuronal excitability (Tait et al., 2008).
Although detection of AQP4-binding autoantibody (AQP4-Ab) in the serum is widely used to diagnose neuromyelitis optica (Weinshenker et al., 2006; Waters and Vincent, 2008), it remains unclear whether AQP4-Ab is a bystander antibody or whether it plays an active role in the pathogenesis of neuromyelitis optica. Indirect evidence suggests that AQP4-Ab may be pathogenic. AQP4-Ab is found in most neuromyelitis optica patients and is highly specific for neuromyelitis optica (Lennon et al., 2005; Hinson et al., 2007). Neuromyelitis optica exacerbations are preceded by rising levels of serum AQP4-Ab (Jarius et al., 2008a). In vitro, AQP4-Ab binds AQP4-expressing cells, activates human and rabbit complement, and causes plasma cell membrane lysis (Hinson et al., 2007; Waters and Vincent, 2008; Kinoshita et al., 2009b). However, there is also evidence that AQP4-Ab may not be pathogenic. Neuromyelitis optica is typically associated with demyelination, although oligodendrocytes do not express AQP4 and cannot, therefore, bind AQP4-Ab. Neuromyelitis optica lesions primarily occur in the optic nerves and spinal cord even though AQP4 is expressed throughout the CNS (Lennon et al., 2005; Hinson et al., 2007). Another intriguing feature of neuromyelitis optica is that it spares peripheral organs that express AQP4 such as the kidney, stomach and skeletal muscle and only causes CNS lesions, even though AQP4-Ab levels are 500 times higher in the blood compared with the cerebrospinal fluid (Lennon et al., 2005; Hinson et al., 2007; Takahashi et al., 2007). Moreover, it was recently reported that IgG from neuromyelitis optica patients does not cause neuromyelitis optica lesions when injected peripherally in naive rats, but abolishes astrocyte AQP4 expression and exacerbates CNS inflammation when injected in rats with experimental allergic encephalomyelitis (Bennett et al., 2009; Bradl et al., 2009; Kinoshita et al., 2009a).
Defining the precise role of AQP4-Ab in neuromyelitis optica is important for understanding the pathogenesis, for creating an animal model, and for developing novel treatments. Here, we show that co-injection of IgG obtained from neuromyelitis optica patient serum with human complement into mouse brain is sufficient to produce the characteristic histological features of neuromyelitis optica including loss of AQP4 and glial fibrillary acidic protein (GFAP) expression, vasculocentric deposition of activated complement components, inflammatory cell infiltration and demyelination.
Methods
Mice
Wild-type and AQP4 null mice on a CD1 genetic background were used. AQP4 null mice were generated as described (Ma et al., 1997) and experiments were done at the University of California at San Francisco and at St George’s, University of London, using weight-matched mice (30–35 g), typically 8- to 12-weeks-old. Protocols were approved by the University of California at San Francisco Committee on Animal Research and the British Home Office, as appropriate. Investigators were unaware of mouse genotype or whether IgG from neuromyelitis optica patients or non-neuromyelitis optica subjects was used during the experiments.
Isolating IgG from patient serum
Serum was obtained from five patients (P1–P5) with an established diagnosis of neuromyelitis optica and strong AQP4-Ab serum positivity. We also used three different pooled non-neuromyelitis optica control sera (C1–C3). Clinical details of the neuromyelitis optica patients are shown in Supplementary Table S1. Five millilitres of each serum (P1–P5) or pooled serum (C1–C3) was diluted 1:4 in phosphate buffered saline (PBS) and loaded onto a Protein-A column (Sigma, Poole, UK). After washing with PBS, the bound IgG was eluted with glycine pH 2.3 and immediately neutralized in 1 M Tris pH 8.0. The positive fractions were pooled and dialyzed against Hartmann’s solution. They were concentrated by dialysis against polyethylene glycol, dialysed again against Hartmann’s solution and stored at 4°C. IgG concentration in the samples were 6–38 mg/ml. We term IgGNMO as the total IgG isolated from the serum of neuromyelitis optica patients (that contains AQP4-Ab) and IgGCON as the total IgG isolated from the serum of non-neuromyelitis optica subjects. The presence or absence of AQP4-Ab in each sample was confirmed by immunocytochemistry on cultured Chinese hamster ovary (CHO) cells expressing AQP4 or AQP1 (as control). AQP4-Ab titres were independently measured by fluoroimmunoprecipitation and cell-based assays (Supplementary Table S2). Each mouse was injected with IgG from a single sample (P1–P5 or C1–C3). In experiments involving several mice, we used at least three different IgGNMO samples (from P1 to P5) and at least two different IgGCON samples (from C1 to C3).
Human and mouse complement
Non-haemolysed blood was collected from human volunteers or CD1 mice in a plain glass tubes and allowed to clot at room temperature for 30 min. The samples were centrifuged at 1000 r.p.m. and the serum supernatant was collected, aliquoted and stored at −80°C. Serum collected in this way preserves complement activity.
Brain injections
Mice were anaesthetized using 2,2,2-tribromoethanol (125 mg/kg intraperitoneally, Sigma) and mounted onto a stereotactic frame (Benchmark, Neurolab, St Louis, MO, USA). Rectal temperature was kept at 37–38°C using a heating lamp. A midline scalp incision to expose the bregma and lambda was made. For intra-parenchymal injections, four burrholes were made on the right side using a high speed drill (0.7 mm burr, Foredom, Bethel, CT) at the following coordinates in millimetres from the bregma (lateral, anterior): (1, 0), (1, −1), (1, −2), (2, −1). For intra-ventricular injection, one burrhole was made on the right at (0.5, −0.5). A 30 g needle attached to 50 µl gas-tight glass syringe (Hamilton, Reno, NV) was inserted 3 mm deep to infuse 28 µl IgGNMO, or 16.8 µl IgGNMO + 11.2 µl human complement (hC), or 16.8 µl IgGCON + 11.2 µl hC, or 16.8 µl IgGNMO + 11.2 µl hC + 5 µl C1 inhibitor (equal volume per burrhole, 1 µl/min). C1 inhibitor was purchased from Biopur (Bubendorf, Switzerland). The IgG concentration in IgGNMO and IgGCON was 6–38 mg/ml (see Supplementary Table S2). C1 inhibitor was 1 mg/ml. The scalp was closed with 5/0 Vicryl suture, mice were given 0.5 ml 0.9% saline subcutaneously and returned to their cage once they recovered their righting reflex. In one experiment the effect of infusion was studied at 12 h, but in most the infusion was repeated at Days 3 and 5.
Cell culture
CHO K1 cells stably transfected with plasmids encoding M23 AQP4 (CHO-AQP4) or AQP1 (CHO-AQP1) were grown on coverslips in F12 medium with 10% foetal bovine serum (Invitrogen, Paisley, UK). More than 95% of cells expressed the respective proteins in their plasma membranes (Saadoun et al., 2005).
Immunocytochemistry
For AQP1, AQP4 and C5b-9 immunostaining, CHO cells were washed in PBS, fixed in 4% neutral buffered formaldehyde (Sigma) for 5 min followed by rabbit anti-AQP1 (1 : 200, Chemicon, Millipore, Livingstone, UK) or rabbit anti-AQP4 (1 : 200, Chemicon) or rabbit anti-C5b-9 (1 : 100, Abcam, Cambridge, UK) primary antibody for 1 h at 25°C. Cells were washed with PBS, and incubated with AlexaFluor-linked goat anti-rabbit secondary antibody (1 : 200, Invitrogen). For AQP4 immunostaining with IgGNMO, live cells were washed with PBS, exposed to IgGNMO (1 : 200, 15 min, 4°C in PBS + 5 mM dextrose), washed with PBS, post-fixed in 4% neutral buffered formaldehyde (Sigma), washed with PBS, and incubated with Texas-red-linked anti-human IgG secondary antibody (Vector Laboratories, Peterborough, UK). After secondary antibody incubation, the coverslips were washed with PBS and mounted in Aquamount medium with 4',6-diamidino-2-phenylindole (Vector Laboratories). Coverslips were examined using a BX-51 Olympus epifluorescence microscope.
Complement activation and cell viability assays
CHO cells on coverslips were exposed at 37°C to F12 medium without serum containing (by volume) 5% IgGNMO or IgGCON and 5% human or mouse complement, and where stated 0.25 mg/ml C1 inhibitor (Biopur, Bubendorf, Switzerland) final concentration. After 2 h, some coverslips were immunostained for C5b-9 and some coverslips were stained with a LIVE/DEAD® cell viability kit (Molecular Probes—Invitrogen) according to the manufacturer’s instructions. Live cells stain fluorescent green and dead cells with damaged plasma membranes stain fluorescent red. Coverslips were examined using a BX-51 Olympus epifluorescence microscope and the number of red and green cells was counted.
Immunohistochemistry
At 12 h or 7 days after the start of brain injections, mice were anaesthetized using 2,2,2-tribromoethanol and perfused fixed through the left cardiac ventricle with 0.9% saline followed by 4% neutral buffered formaldehyde. Brains were removed and post-fixed in 4% neutral buffered formaldehyde, dehydrated in serial alcohols, immersed in Histoclear® and processed into paraffin. Tissue sections (7 µm thick) were unmasked in citric acid and immunostained with the following primary antibodies at room temperature for 1 h: rabbit anti-AQP4 (1 : 100, Chemicon), mouse anti-GFAP (1 : 200, Chemicon), mouse anti-CD45 (1:10, Pharmingen, BD Biosciences, Oxford, UK), rat anti-macrophage (1 : 10, eBioscience, Hatfield, UK), mouse anti-human β-amyloid precursor protein (βAPP, 1 : 100, Chemicon) or rabbit anti-C5b-9 (1 : 100, Abcam, Cambridge, UK) followed by the appropriate species biotinylated secondary antibody (1 : 500, Vector Laboratories). Immunostaining was visualized brown using the Vectastain horseradish peroxidase kit (Vector Laboratories) followed by diaminobenzidine/H2O2. Nuclei were counterstained blue with haematoxylin. The tissue sections were examined with an Olympus BX-51 light microscope.
Luxol fast blue stain
Brain sections were stained using the myelin-specific dye Luxol fast blue as previously described (Saadoun et al., 2008, 2009). Briefly, the brain sections were deparaffinized, rehydrated and incubated in 0.1% Luxol fast blue (in 95% alcohol and 0.5% acetic acid) (Sigma, Poole, UK) solution at 40°C overnight. After rinsing excess stain with 95% ethanol and washing in distilled water, slides were immersed in 0.05% lithium carbonate solution for 30 s. Sections were then dehydrated in serial alcohols and mounted in VectaMount mounting medium (Vector Laboratories).
Fluoro Jade-C staining
Coronal brain sections through the injection tract were deparaffinized, rehydrated, incubated in 0.06% potassium permanganate solution for 10 min, water-rinsed and transferred for 10 min to a 0.0001% solution of Fluoro Jade-C (FJ-C) (Chemicon) dissolved in 0.1% acetic acid. Slides were then rinsed with distilled water, air-dried at 50°C and coverslipped with VectaMount® mounting medium (Vector Laboratories).
Image processing and quantification
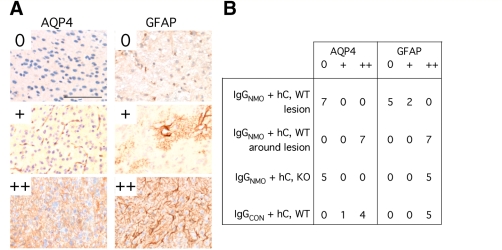
For qualitative examination, coronal brain sections were made 7 µm thick to include the injection tract. To quantify neuronal cell death, we counted the density of FJ-C-positive cells in coronal sections through the injection tract as shown in Fig. 11A. To quantify inflammatory cell infiltration and demyelination, we used adjacent axial cuts 7 µm thick at 2 mm from the inferior brain surface. One section was immunostained for CD45 and the other was stained with Luxol fast blue. Twenty overlapping images were obtained at 4× magnification to cover each axial brain section. The 20 images were combined using CellF software (Olympus, UK) to obtain a view of the entire brain section. Combined images, saved in .jpg format, were imported into ImageJ (v.1.40, http://rsb.info.nih.gov/ij/). The CD45 immunopositive area was outlined by hand and measured. The lengths of myelinated and demyelinated external capsule in the injected hemisphere were measured and the percentage of demyelinated length was calculated. AQP4 immunoreactivity was graded as: 0, nil; +, perivascular; ++, parenchymal. GFAP immunoreactivity was graded as: 0, nil; +, perivascular and/or occasional reactive astrocyte; ++, high density of reactive astrocytes. In wild-type mice injected with IgGNMO + human complement, AQP4 and GFAP immunoreactivities were graded in the injected (right) hemisphere within lesions and in a 100 µm zone around lesions (perilesional). We also graded AQP4 and GFAP immunoreactivities within 100 µm of the needle tract in the right hemisphere of AQP4-null mice injected with IgGNMO + human complement and in wild-type mice injected with IgGCON + human complement.
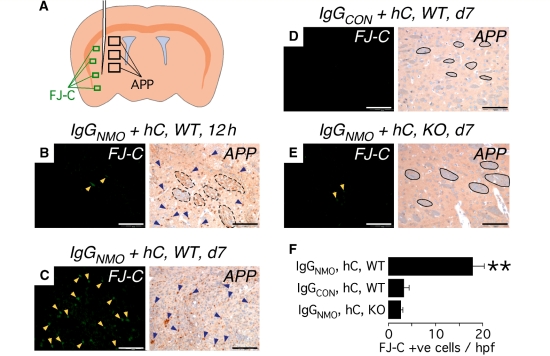
Figure 11.
Intra-cerebral injection of IgGNMO + hC causes axonal injury within 12 h and neuronal cell death within 7 days. (A) Schematic showing needle and areas examined for FJ-C stain (green rectangles) and βAPP immunostain (black rectangles). (B–E) Representative areas stained with FJ-C (left) or immunostained with βAPP (right). Yellow arrowheads indicate FJ-C-positive cells, blue arrowheads show βAPP immunopositivity, dashed black lines demarcate degenerating white matter tracts and continuous black lines demarcate intact white matter tracts. (F) Data summary of FJ-C-positive cells/high power field at Day 7. Mean ± SEM. N = 6 (IgGNMO, hC, WT), 5 (IgGCON, hC, WT), 5 (IgGNMO, hC, KO); four high-power fields per mouse. Bar 50 µm (B–E, left), 100 µm (B–E, right). **P < 0.005 compared with IgGCON, hC, WT and with IgGNMO, hC, KO.
Behavioural assessment
A Y-shaped tunnel 4 cm wide was cut in a 2-inch-thick polystyrene block. At Day 7, each mouse was positioned at the Y-shaped intersection and allowed to take a right or left turn. The experiment was repeated 20 times to calculate the percentage of right turns per mouse. In preliminary experiments, five wild-type and five AQP4-null mice turned right or left equally often.
Statistics
Data are presented as mean ± SEM. Statistical comparisons were made using the Student's t-test when comparing two groups or ANOVA with Student–Newman–Keuls post hoc analysis for pairwise comparing multiple groups. Significance is indicated with *P < 0.05 and **P < 0.005.
Results
Intra-cerebral IgGNMO injection does not cause neuromyelitis optica lesions in mice
IgGNMO (28 µl) was injected into the right cerebral hemisphere at Days 0, 3 and 5. At Day 7, sections through the needle tracts and adjacent brain were examined (Fig. 1). A little inflammation was seen, which was accounted for by the trauma of needle insertion, but there was no perivascular inflammation (Fig. 1A–C). There were few CD45-positive cells, mainly located within the needle tract. Near the injection site, AQP4 immunoreactivity was seen around the blood vessels and in the brain parenchyma (Fig. 1D). GFAP immunostaining revealed reactive astrocytes next to the injection site (Fig. 1E), where AQP4 expression was high. Luxol fast blue staining showed no loss of myelin next to the needle tract (Fig. 1F). Similarly, no loss of AQP4 or GFAP, no demyelination and no complement activation were found when a larger volume of IgGNMO (50 µl), or when IgGNMO or IgGCON from different patients were injected into other mice (data not shown). Therefore, IgGNMO and IgGCON do not produce neuromyelitis optica-like lesions when injected into mouse brain.
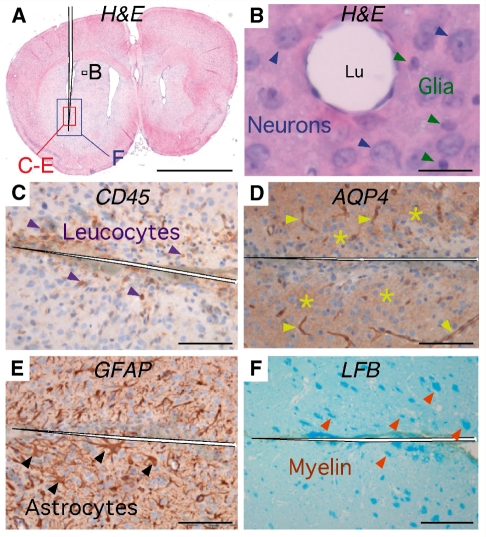
Figure 1.
Intra-cerebral injections of IgGNMO at Days 0, 3 and 5 do not cause neuromyelitis optica lesions at Day 7. (A) Haematoxylin and eosin stain: coronal brain section through the needle tract. Black rectangle corresponds to section B, red rectangle to C–E and blue rectangle to F. (B) Haematoxylin and eosin stain: capillary 250 µm from needle tract surrounded by normal endothelial, neuronal (blue arrowheads) and glial (green arrowheads) cell nuclei without perivascular inflammation. (C) CD45 immunostain: a few leucocytes (purple arrowheads) were seen around the needle tract. (D) AQP4 immunostain: AQP4 around capillaries (yellow arrowheads) and in brain parenchyma (yellow stars) next to the needle tract. (E) GFAP immunostain: intact astrocytes, including reactive astrocytes (black arrowheads) next to the needle tract. (F) Luxol fast blue stain. Intact white matter tracts (orange arrowheads) next to the needle tract. Bar 2 mm (A), 25 µm (B), 100 µm (C–E), 200 µm (F).
IgGNMO lysis of AQP4-expressing cultured cells requires human complement
To investigate why IgGNMO did not cause inflammatory demyelination, we used CHO cells stably expressing AQP4 (CHO-AQP4) or AQP1 (CHO-AQP1). We confirmed plasma membrane AQP4 and AQP1 protein expression by immunocytochemistry (Fig. 2A, top row). As expected, IgGNMO-labelled non-permeabilized CHO-AQP4 cells, but did not label CHO-AQP1 cells (Fig. 2A, middle row). IgGCON did not label CHO-AQP4 or CHO-AQP1 (Fig. 2A, bottom row). Exposure to IgGNMO with human complement caused C5b-9 deposition on the cell plasma membrane in CHO-AQP4 but not in CHO-AQP1 cells (Fig. 2B). After 2 h exposure, IgGNMO with human complement lysed about half of the CHO-AQP4 cells, but there was little lysis of CHO-AQP1 cells (Fig. 2B and C). IgGCON plus human complement did not lyse CHO-AQP4 cells. Notably, IgGNMO with mouse complement did not lyse CHO-AQP4 cells, suggesting that IgGNMO activates human complement but not mouse complement. The complement inhibitor C1 inhibitor markedly protected CHO-AQP4 cells from lysis produced by IgGNMO and human complement (Fig. 2C).
Figure 2.
IgGNMO activates human but not mouse complement. (A) CHO cells expressing AQP4 (left column, CHO-AQP4) or AQP1 (right column, CHO-AQP1). Top row: AQP visualized red using anti-AQP4 or anti-AQP1 antibody. Middle row: live cells incubated with IgGNMO followed by Texas Red linked anti-human IgG. Bottom row: live cells incubated with control IgGCON followed by Texas Red linked anti-human IgG. (B) CHO-AQP4 (top) and CHO-AQP1 (bottom) cells were exposed to IgGNMO + hC. At 2 h, dead cells were labelled red and live cells green (left). At 2 h, some cells were immunostained to detect C5b-9 deposition (red, arrowheads) in the plasma cell membrane with 4',6-diamidino-2-phenylindole (blue nuclei) counterstain (right). (C) Plot shows percentage of dead CHO cells [=100 × red/(red + green)] after 2 h incubation. C1inh = C1 inhibitor, hC = human complement, mC = mouse complement. Eight coverslips per condition, mean ± SEM. **P < 0.005 for IgGNMO + hC, CHO-AQP4 compared with each other condition. Bar 50 µm (A), 100 µm (B, left), 25 µm (B, right).
Intra-cerebral injection of IgGNMO and human complement causes marked inflammation
To overcome the inability of the human IgGNMO to activate mouse complement, we injected mouse brains at Days 0, 3 and 5 with a mixture of 16.8 µl IgGNMO plus 11.2 µl human complement and examined the brains at Day 7 (Fig. 3). There was extensive inflammation in the right hemisphere including marked perivascular inflammation within 1 mm of the needle tract (Fig. 3A and B). There was no inflammation in the contralateral (non-injected) hemisphere, which had normal neuronal, glial and endothelial cells (Fig. 3A and C). Haematoxylin and eosin staining revealed that most (>90%) of the leucocytes within the lesion were mononuclear with a few polymorphonuclear cells in or around blood vessels (Fig. 3D). Selective immunostaining confirmed macrophages with few or no neutrophils (Fig. 3E and F). There was perivascular deposition of activated complement within 1 mm of the injection tract, with two patterns of C9neo immunoreactivity, rosette (Fig. 3G) and linear (Fig. 3H). No complement deposition was seen in the non-injected hemisphere (data not shown).
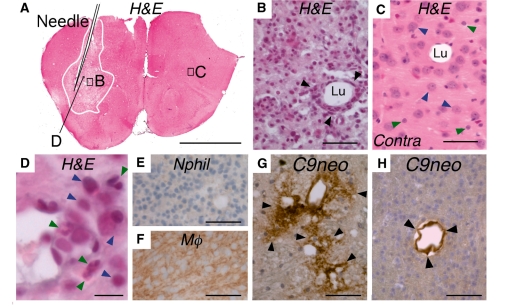
Figure 3.
Intra-cerebral injections of IgGNMO with hC at Days 0, 3 and 5 produce extensive inflammation at Day 7. (A–D) Haematoxylin and eosin stain. (A) Coronal brain section shows the needle and the inflamed area (within the white line). (B) Magnified view of area B (from A) shows a vessel ∼200 µm from the needle. Arrowheads indicate inflammatory cells. (C) Magnified view of area C (from A) shows a capillary surrounded by normal endothelium, neuronal (blue arrowheads) and glial (green arrowheads) nuclei without inflammation. (D) Vessel ∼100 µm from the needle with mononuclear (blue arrowheads) and polymorphonuclear (green arrowheads). (E) Neutrophil (Nphil) immunostain and (F) macrophage (Mϕ) immunostain of the CD45+ area in A. (G and H) C9neo immunostain: vasculocentric deposition of C9neo (arrowheads) in rosette (G) and linear (H) pattern. Contra = contralateral (non-injected) hemisphere, Lu = capillary lumen. Bar 2 mm (A), 50 µm (B–C and E–H), 10 µm (D).
Immunostaining with the leucocyte marker CD45 showed the CD45-positive area in relation to the needle tract of a mouse brain that was injected with IgGNMO and human complement (Fig. 4A). High magnification revealed strongly CD45-immunopositive cells throughout the lesion. Inflammation was more marked in major myelinated tracts, including the corpus callosum and external capsules. Only a few isolated CD45-positive cells, mainly located within the needle tract, were seen when IgGNMO with human complement was injected into AQP4-null mouse brain (Fig. 4B) or when IgGCON with human complement was injected (Fig. 4C). To quantify the inflammation, we measured the CD45-positive area in axial brain sections at 2 mm from the inferior brain surface (Fig. 4D). IgGNMO from different patients and human complement caused extensive inflammation in wild-type mice, but little inflammation in AQP4-null mice. IgGCON with human complement also caused little inflammation. Moreover, the complement inhibitor, C1 inhibitor, markedly reduced the inflammation produced by IgGNMO and human complement. Our findings suggest that IgGNMO activates human complement at sites of high AQP4 expression and causes leucocyte infiltration. To demonstrate the crucial importance of human complement, we performed the same experiment with mouse complement; there was no inflammation or demyelination at Day 7 in three mouse brains, after co-injecting each mouse brain with 16.8 µl IgGNMO from a different patient and 11.2 µl mouse complement (Supplementary Fig. S1).
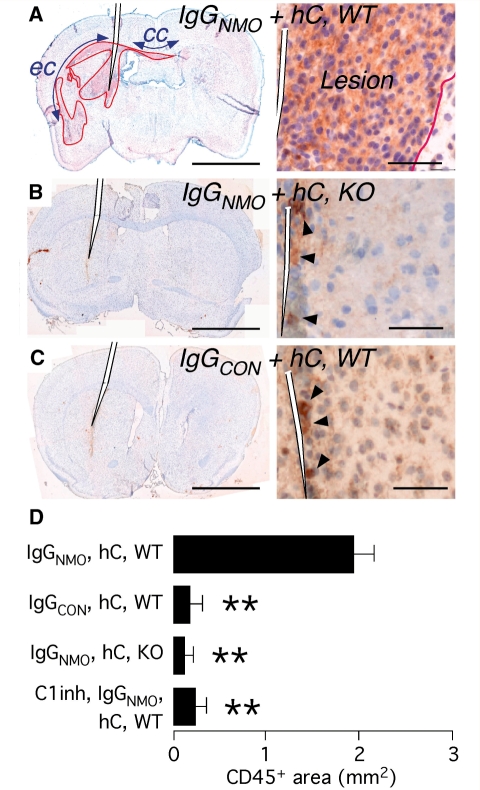
Figure 4.
Brain leucocyte infiltration quantified by CD45 immunostain. (A) Wild-type mouse (WT) brain 7 days after injection of IgGNMO + hC. Left: coronal section with the CD45+ area outlined. cc = corpus callosum; ec = external capsule. Right: border (red line) between CD45+ area (Lesion) and surrounding brain. (B) Left: AQP4-null mouse (KO) brain 7 days after injection of IgGNMO + hC. Right: brain next to needle tract. (C) Left: WT brain 7 days after injection of IgGCON + hC. Right: brain next to needle tract. Arrowheads in (B) and (C) indicate leucocytes in the needle tract. (D) CD45+ area (square millimetres) measurements at Day 7 in axial sections 2 mm from the inferior brain surface. Mean ± SEM. n = 7 (IgGNMO, hC, WT), 5 (IgGCON, hC, WT), 5 (IgGNMO, hC, KO), 4 (C1 inhibitor, IgGNMO hC, WT). Haematoxylin counterstain (A–D). **P < 0.005 compared with IgGNMO hC. Bar 2 mm (A–C, Left), 50 µm (A–C, Right).
Intra-cerebral injection of IgGNMO and human complement causes demyelination
Injection of 16.8 µl IgGNMO and 11.2 µl human complement from different patients also produced extensive demyelination in the injected hemisphere, seen as loss of Luxol fast blue staining in the corpus callosum, external and internal capsules and lesser white matter tracts (Fig. 5A, D and E). We used the corpus callosum and external capsule to quantify demyelination in axial cuts 2 mm from the inferior brain surface (Fig. 5D and E). Normal myelination was evident in the non-injected contralateral hemisphere (Fig. 5A and D). Injection of IgGNMO plus human complement in AQP4-null mice (Fig. 5B and E) or injection of IgGCON plus human complement (Fig. 5C and E) caused little or no demyelination. C1 inhibitor markedly protected wildtype mice from demyelination produced by IgGNMO and human complement (Fig. 5E). These results suggest that, in the presence of human complement and AQP4, IgGNMO causes demyelination.
Figure 5.
Intra-cerebral injection of IgGNMO + hC at Days 0, 3 and 5 causes demyelination at Day 7. (A–C) Coronal and (D) axial brain sections stained with Luxol fast blue. (A) Wild-type (WT) mouse injected with IgGNMO + hC. Green arrowheads indicate demyelinated external capsule and green line demarcates demyelinated tracts in the injected hemisphere. Red arrowheads show normally myelinated external capsule and red line demarcates normally myelinated tracts in the non-injected side. (B) AQP4-null mouse (KO) brain injected with IgGNMO + hC. (C) WT brain injected with IgGCON + hC. (D) Section at 2.0 mm from the inferior brain surface. a = length of demyelinated external capsule in injected hemisphere; b = length of myelinated external capsule in non-injected hemisphere. (E) Summary of demyelination (a/b) data. Mean ± SEM. n = 7 (IgGNMO + hC, WT), 5 (IgGCON + hC, WT), 5 (IgGNMO + hC, KO), 4 (IgGNMO + hC + C1 inhibitor, WT). **P < 0.005 compared with IgGNMO + hC, WT. Bar 2 mm (A–D).
Intra-cerebral injection of IgGNMO and human complement causes loss of AQP4 and GFAP
IgGNMO and human complement injection caused marked gliosis in the perilesional area as evidenced by strong GFAP (Fig. 6A) and AQP4 (Fig. 6B) immunoreactivities. Many cells around the lesions had characteristic features of reactive astrocytes including high GFAP expression and a star-like shape with multiple processes (Fig. 6A, Inset). Within lesions, however, there were only few, scattered GFAP-positive cell processes and a striking loss of AQP4 expression. In the non-injected hemisphere, GFAP expression was weak and mostly perivascular (Fig. 6C). AQP4 immunoreactivity was also perivascular with little expression in the brain parenchyma (Fig. 6D). In control experiments, IgGNMO and human complement injection in AQP4-null mouse brain produced reactive gliosis next to the needle tract (Fig. 6E, left). IgGCON and human complement injection also caused reactive gliosis (Fig. 6F, left) and increased AQP4 immunoreactivity (Fig. 6F, right) adjacent to the needle tract. Expression studies from several mice, quantified as shown in Fig. 7A and summarized in Fig. 7B, confirm loss of AQP4 and GFAP immunoreactivities within the neuromyelitis optica lesions and increased expression around the lesions. Injection of IgGNMO with human complement thus ablates the astrocytes within the lesions and causes gliosis around the lesions.
Figure 6.
Intra-cerebral injection of IgGNMO + hC at Days 0, 3, and 5 causes loss of GFAP and AQP4 expression at Day 7. (A–D) Wild-type (WT) brain 7 days after injection of IgGNMO + hC. (A) GFAP and (B) AQP4 immunostain of lesion and perilesional area. (A) and (B) are sequential sections. Blue and green arrowheads mark corresponding vessels. Black arrowheads indicate fragmented GFAP+ processes within the lesion. Inset: reactive astrocyte. (C) GFAP and (D) AQP4 immunostain of contralateral (non-injected) hemisphere. Arrowheads indicate microvessels. (E) AQP4-null mouse (KO) brain injected with IgGNMO + hC immunostained for (left) GFAP and (right) AQP4. (F) WT mouse brain injected with IgGCON + hC immunostained for (left) GFAP and (right) AQP4. Bar 100 µm (A and B), 50 µm (C–F), 20 µm (A, Inset).
Figure 7.
Quantification of AQP4 and GFAP immunoreactivities 7 days after intra-cerebral injection of IgGNMO + hC or IgGCON + hC. (A) AQP4 expression: 0 = nil; += perivascular; ++ = parenchymal. GFAP expression: 0 = nil; + = perivascular and/or occasional reactive astrocyte; ++ = high density of reactive astrocytes. (B) Summary of AQP4 and GFAP immunoreactivities in and around lesions of WT mice injected with IgGNMO + hC, and next to the needle tracts of AQP4-null (KO) mice injected with IgGNMO + hC or WT mice injected with IgGCON + hC. Bar 100 µm (all panels).
Behavioural studies
Mice injected with IgGNMO and human complement in the right hemisphere were observed to turn to the right more often than to the left. To quantify this behaviour, we counted the number of right versus left turns when mice were placed in a Y-shaped tunnel (Fig. 8A). Wild-type mice injected with IgGCON and human complement, or AQP4-null mice injected with IgGNMO and human complement, showed no significant right versus left preference (Fig. 8B). Wild-type mice that received IgGNMO with human complement were 2.5 times more likely to turn to the right than to the left, and inhibiting human complement activation with C1 inhibitor abolished this right-turning behaviour (Fig. 8B). Therefore, in the presence of AQP4 and human complement, IgGNMO causes sufficient damage to the function of the right hemisphere, which results in the right-turning behaviour.
Figure 8.
Injection of IgGNMO + hC into the right hemisphere at Days 0, 3 and 5 causes right-turning behaviour at Day 7. (A) Y-shaped tunnel for quantifying mouse turning behaviour. The mouse was placed at the Y-shaped intersection 20 times. Each time we noted whether the mouse turned right or left. (B) Summary of behavioural data (percentage of right turns). n = 7 (IgGNMO + hC, WT), 6 (IgGCON + hC, WT), 7 (IgGNMO + hC, KO), 4 (IgGNMO + hC + C1 inhibitor, WT). *P < 0.05, **P < 0.005 compared with IgGNMO + hC WT. Gray line indicates percentage of right turns in uninjected WT and AQP4-null mice.
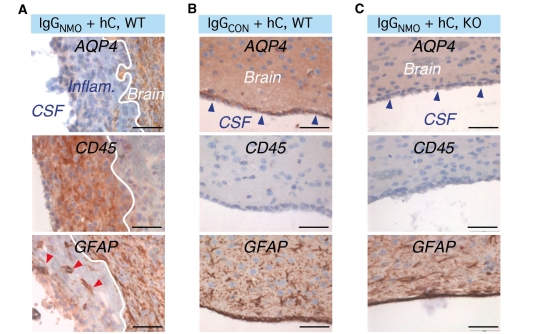
Intra-ventricular injection of IgGNMO and human complement causes destruction of the ependyma
AQP4 is strongly expressed in the ependyma lining the ventricles (Rash and Yasumura, 1999). To find out whether the ependyma can also be damaged by IgGNMO, we injected IgGNMO with human complement intraventricularly at Days 0, 3 and 5. At Day 7, the ependyma appeared destroyed and was replaced by a thick layer of CD45-immunopositive cells (Fig. 9A). There was strong AQP4 immunostaining in the brain next to the lesion with no AQP4 expression within the inflammatory lesion (Fig. 9A). GFAP immunostaining showed reactive astrocytes next to the lesion, corresponding to the region of high AQP4 expression, with a few scattered GFAP-positive but AQP4-negative cells within the inflammatory lesion (Fig. 9A). Similar findings were observed in three mice that received intra-ventricular injections of human complement with IgGNMO obtained from three different patients. Intra-ventricular injection of IgGCON with human complement in two wild-type mice (Fig. 9B) or IgGNMO with human complement in two AQP4-null mice (Fig. 9C) produced no ependymal damage or inflammation. Taken together, these experiments suggest that IgGNMO and human complement can destroy AQP4-expressing cells and cause leucocyte infiltration at different sites within the brain.
Figure 9.
Intra-ventricular injection of IgGNMO + hC at Days 0, 3 and 5 causes ependymal destruction at Day 7. Sections of brain after intra-ventricular injection of (A) IgGNMO + hC into WT mouse, (B) IgGCON + hC into WT mouse and (C) IgGNMO + hC into AQP4-null (KO) mouse. Top panel: AQP4 immunostain of the border between cerebrospinal fluid (CSF) and brain parenchyma (Brain). Blue arrowheads in (B) and (C) indicate intact ependyma. In (A) the ependyma is replaced by inflammatory cell infiltrate (Inflam.). White line marks the border between inflammation and brain. Middle pictures: CD45 immunostain. Bottom panel: GFAP immunostain. Red arrowheads in (A) show reactive astrocyte processes within the inflamed region. Bar 50 µm (A–C).
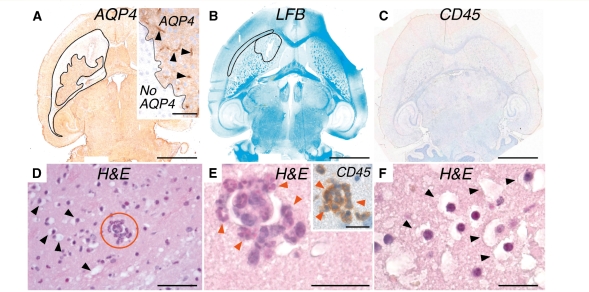
Early effects of intra-cerebral IgGNMO and human complement injection
Established human neuromyelitis optica lesions are characterized by loss of AQP4 expression, demyelination and inflammation (Misu et al., 2007; Roemer et al., 2007). To determine the temporal sequence of these key pathological features, we injected IgGNMO and human complement intra-cerebrally and killed mice at 12 h. In the injected hemisphere, there was striking loss of AQP4 immunoreactivity (Fig. 10A) as well as myelin breakdown (Fig. 10B), but little or no inflammatory cell infiltration into the brain parenchyma (Fig. 10C). Inflammatory cells, mostly polymorphonuclear leucocytes, were only evident inside blood vessels (Fig. 10D and E). Glial cells appeared swollen and their cytoplasm was vacuolated (Fig. 10F). These findings were confirmed in three mice injected with IgGNMO and human complement from three different patients. No loss of AQP4 expression, myelin breakdown, intra-vascular inflammation or glial oedema was found in the non-injected hemisphere or in three mice injected with IgGCON and human complement. There was no glial cell swelling or demyelination in three AQP4-null mice that received IgGNMO and human complement. Taken together, these findings suggest that loss of AQP4 and myelin breakdown are early events in neuromyelitis optica, whereas inflammatory cell infiltration occurs later.
Figure 10.
Intra-cerebral injection of IgGNMO + hC causes loss of AQP4 expression and myelin breakdown within 12 h. Wildtype mouse brain 12 h after injection of IgGNMO + hC. (A) AQP4 immunostain, (B) Luxol fast blue stain and (C) CD45 immunostain. Non-stained area in (A) and (B) is outlined. Inset in (A) shows border between AQP4-immunopositive and -immunonegative area and arrowheads indicate capillaries. (D–F). Haematoxylin and eosin stain of brain 0.5 mm from injection site. (D) Arrowheads mark swollen glia. Circled area shows neutrophils within microvessel lumen. (E) Magnified view of circled area in (D). Arrowheads indicate neutrophils. Inset: CD45 immunostain. (F) Magnified view of swollen glia 0.5 mm from the injection site. Bar 2 mm (A–C), 50 µm (D), 20 µm (E, E Inset, F).
Intra-cerebral injection of IgGNMO and human complement causes axonal injury at 12 h and neuronal cell death by Day 7
To test whether IgGNMO and human complement damage the neurons, we immunostained mouse brain sections for βAPP and stained with FJ-C. βAPP immunopositivity is an early marker of axonal injury, whereas FJ-C selectively stains degenerating neuronal cell bodies fluorescent green regardless of the mechanism of cell death. Four mice received intra-cerebral injection of IgGNMO and human complement. Within 12 h, there was prominent βAPP immunoreactivity throughout the injected hemispheres in all mice. Most βAPP immunoreactivity was found in patches corresponding to white matter tracts, with some individual βAPP-immunopositive axons between the patches (Fig. 11B, right). Six mice received intra-cerebral injection of IgGNMO and human complement at Days 0, 3 and 5. At Day 7, there was βAPP immunostaining of individual axons with no patchy βAPP immunoreactivity, indicating loss of white matter tracts (Fig. 11C, right). There was little or no βAPP immunoreactivity in five wild-type mice injected with IgGCON and human complement or in five AQP4-null mice injected with IgGNMO and human complement (Fig. 11D and E, right). Few (<5 per high-power field) FJ-C-positive cells were seen at 12 h after injecting IgGNMO and human complement (Fig. 11B), but by Day 7 several (>15 per high power field) dying neurons were evident (Fig. 11C). The FJ-C data are summarized in Fig. 11F.
Discussion
We found that injection of IgGNMO together with human complement into mouse brain produced lesions with characteristic histological features of human neuromyelitis optica lesions, including inflammatory cell infiltration, demyelination, loss of AQP4 and GFAP expression, and perivascular deposition of activated complement components (Misu et al., 2007; Roemer et al., 2007). At Day 7, the infiltrating leucocytes were primarily macrophages, with a few granulocytes, as found in established human neuromyelitis optica lesions (Misu et al., 2007). IgGNMO did not activate mouse complement or produce CNS injury when co-injected with mouse complement into mouse brain, confirming the importance of using complement from the same species as the autoantibody in passive transfer (Lassmann et al., 1983). Importantly, IgGNMO with human complement did not cause neuromyelitis optica-like lesions in AQP4-null mice indicating that it is the antibodies to AQP4, rather than some other autoimmune component of the IgG preparations that is responsible for the lesions. Control (non-neuromyelitis optica) IgG with human complement did not cause lesions in wild-type mice. Taken together, our experiments show that AQP4-Ab, human complement and AQP4 are each essential for the neuromyelitis optica lesions to form. We, therefore, suggest that AQP4-Ab plays a key role in the pathogenesis of human neuromyelitis optica disease by binding to AQP4 on astrocytes, activating human complement and lysing the astrocytes. Death of the AQP4-expressing astrocytes explains the lack of AQP4 and GFAP immunoreactivities within the human (Misu et al., 2007; Roemer et al., 2007) and mouse lesions.
Others have recently developed animal models of neuromyelitis optica (Bennett et al., 2009; Bradl et al., 2009; Kinoshita et al., 2009a), based on modifications of the rat experimental allergic encephalomyelitis model. In their experiments, experimental allergic encephalomyelitis was produced by active immunization against myelin basic protein (Kinoshita et al., 2009a), myelin basic protein peptide (Bennett et al., 2009) or passive transfer of myelin basic protein-reactive T cells (Bradl et al., 2009). Rats that subsequently received systemic injection of IgGNMO developed CNS lesions with some features typical of neuromyelitis optica lesions including inflammation, loss of AQP4 and GFAP expression and perivascular complement activation, although myelin was largely intact (Bennett et al., 2009; Bradl et al., 2009; Kinoshita et al., 2009a). Rats that received IgGCON also developed CNS lesions, but with no loss of AQP4 and GFAP and no complement activation. Moreover, naive rats injected with IgGNMO alone did not develop neuromyelitis optica lesions (Bradl et al., 2009) demonstrating the importance of the experimental allergic encephalomyelitis-induced inflammation in these models. It was thus concluded that AQP4-Ab alone does not cause neuromyelitis optica, but augments CNS damage produced by experimental allergic encephalomyelitis. In contrast, our mouse model does not require prior inflammation and suggests that, in the presence of human complement, AQP4-Ab is the key player that produces the neuromyelitis optica lesions.
Our observations in the mouse demonstrate a requirement for AQP4-Ab, human complement and AQP4 in the formation of neuromyelitis optica lesions, which faithfully reproduce the main components of human neuromyelitis optica lesions (Misu et al., 2007; Roemer et al., 2007). The perivascular deposition of activated complement components, loss of GFAP and AQP4 expression, inflammatory cell infiltration and demyelination are characteristic features of human neuromyelitis optica lesions (Misu et al., 2007; Roemer et al., 2007), suggesting that our model closely mimics the human disease. The model is easy to set up and requires only small amounts of IgGNMO. A major advantage is that the inflammation, demyelination and right-turning behaviour, produced by injecting IgGNMO with human complement into the right hemisphere, are quantifiable, thus allowing the model to be used for evaluating novel treatments.
The mouse model allowed us to define the pathological processes that take place as neuromyelitis optica lesions begin to form. Within 12 h of injecting IgGNMO and human complement into mouse brain, there was evidence of astrocyte injury including loss of AQP4 expression and glial cell oedema. Surprisingly, myelin breakdown was also an early event that preceded (and therefore was not initiated by) intra-parenchymal inflammatory cell infiltration. The model also revealed early axonal injury that progressed to neuronal death within a week. Our model thus reproduces the acute axonal injury and delayed neuronal cell death reported in human neuromyelitis optica lesions (Lucchinetti et al., 2002). Since oligodendrocytes and neurons do not express AQP4, they cannot be directly damaged by AQP4-Ab. It is therefore unclear how AQP4-Ab-mediated astrocyte injury rapidly causes myelin breakdown and whether the axonal injury occurs secondary to demyelination or as an independent process. Taken together, our data suggest that neuromyelitis optica lesions begin with astrocyte injury and myelin degradation, followed by inflammatory cell infiltration, extensive demyelination and neuronal death.
Since AQP4 is ubiquitously expressed in the CNS, in theory, any part of the CNS should be susceptible to AQP4-Ab-mediated damage. In agreement with this idea, we observed primarily parenchymal injury and inflammation when IgGNMO with human complement was injected intra-parenchymally, but primarily ependymal injury and inflammation when IgGNMO with human complement was injected intraventricularly. Human neuromyelitis optica disease was initially thought to predominate in the circumventricular organs, optic nerves and spinal cord (Wingerchuk et al., 2007; Jarius et al., 2008b). However, recent evidence indicates that the brain is also involved in ∼90% of patients (Ito et al., 2009). This clinical observation supports our experimental finding that all parts of the CNS parenchyma that express AQP4 are vulnerable to neuromyelitis optica.
We conclude that AQP4-Ab is pathogenic in neuromyelitis optica and that AQP4 is the target antigen. Our experiments show that neuromyelitis optica lesions are initiated by AQP4-Ab binding AQP4 and activating human complement, through the classical pathway, which destroys the astrocytes. The inflammation and demyelination, which are characteristics of neuromyelitis optica, are secondary to classical complement activation. We therefore suggest that complement inhibitors, such as C1 inhibitor, may effectively limit CNS injury in human neuromyelitis optica, as observed in our mouse neuromyelitis optica model. The mouse model described should be useful for studying the different stages of neuromyelitis optica pathogenesis and for evaluating novel treatments.
Funding
Guthy-Jackson Charitable Foundation (Grant to M.C.P. and A.S.V.); National Institutes of Health (grant DK35124 to A.S.V.). Oxford Biomedical Research Centre (to P.W.); Neurosciences Research Foundation / Harrison Fund (to S.S.).
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
We thank Dr Jacqueline Palace for providing sera and Dr M. Isabel Leite for measuring AQP4 antibody titre using a cell-based assay.
Glossary
Abbreviations
- AQP4
aquaporin-4
- AQP4-Ab
AQP4-binding autoantibody
- βAPP
β-amyloid precursor protein
- CHO
Chinese hamster ovary
- FJ-C
Fluoro-Jade C
- GFAP
glial fibrillary acidic protein
- hC
human complement
- IgGCON
total immunoglobulin G from serum of non-neuromyelitis optica subjects
- IgGNMO
total immunoglobulin G from serum of neuromyelitis optica patients
- PBS
phosphate buffered saline
References
- Bennett JL, Lam C, Kalluri SR, Saikali P, Bautista K, Dupree C, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol. 2009 doi: 10.1002/ana.21802. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradl M, Misu T, Takahashi T, Watanabe M, Mader S, Reindl M, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol. 2009 doi: 10.1002/ana.21837. in press. [DOI] [PubMed] [Google Scholar]
- Hinson SR, Pittock SJ, Lucchinetti CF, Roemer SF, Fryer JP, Kryzer TJ, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology. 2007;69:2221–31. doi: 10.1212/01.WNL.0000289761.64862.ce. [DOI] [PubMed] [Google Scholar]
- Ito S, Mori M, Makino T, Hayakawa S, Kuwabara S. ‘Cloud-like enhancement’ is a magnetic resonance imaging abnormality specific to neuromyelitis optica. Ann Neurol. 2009;66:425–8. doi: 10.1002/ana.21753. [DOI] [PubMed] [Google Scholar]
- Jarius S, Aboul-Enein F, Waters P, Kuenz B, Hauser A, Berger T, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008a;131:3072–80. doi: 10.1093/brain/awn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S, Paul F, Franciotta D, Waters P, Zipp F, Hohlfeld R, et al. Mechanisms of disease: aquaporin-4 antibodies in neuromyelitis optica. Nat Clin Pract Neurol. 2008b;4:202–14. doi: 10.1038/ncpneuro0764. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Nakatsuji Y, Kimura T, Moriya M, Takata K, Okuno T, et al. Neuromyelitis optica: passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun. 2009a;386:623–7. doi: 10.1016/j.bbrc.2009.06.085. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Nakatsuji Y, Moriya M, Okuno T, Kumanogoh A, Nakano M, et al. Astrocytic necrosis is induced by anti-aquaporin-4 antibody-positive serum. Neuroreport. 2009b;20:508–12. doi: 10.1097/wnr.0b013e32832776f4. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Stemberger H, Kitz K, Wisniewski HM. In vivo demyelinating activity of sera from animals with chronic experimental allergic encephalomyelitis. Antibody nature of the demyelinating factor and the role of complement. J Neurol Sci. 1983;59:123–37. doi: 10.1016/0022-510x(83)90086-2. [DOI] [PubMed] [Google Scholar]
- Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–7. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti CF, Mandler RN, McGavern D, Bruck W, Gleich G, Ransohoff RM, et al. A role for humoral mechanisms in the pathogenesis of Devic’s neuromyelitis optica. Brain. 2002;125:1450–61. doi: 10.1093/brain/awf151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest. 1997;100:957–62. doi: 10.1172/JCI231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misu T, Fujihara K, Kakita A, Konno H, Nakamura M, Watanabe S, et al. Loss of aquaporin-4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain. 2007;130:1224–34. doi: 10.1093/brain/awm047. [DOI] [PubMed] [Google Scholar]
- Rash JE, Yasumura T. Direct immunogold labeling of connexins and aquaporin-4 in freeze-fracture replicas of liver, brain, and spinal cord: factors limiting quantitative analysis. Cell Tissue Res. 1999;296:307–21. doi: 10.1007/s004410051291. [DOI] [PubMed] [Google Scholar]
- Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130:1194–205. doi: 10.1093/brain/awl371. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Bell BA, Verkman AS, Papadopoulos MC. Greatly improved neurological outcome after spinal cord compression injury in AQP4-deficient mice. Brain. 2008;131:1087–98. doi: 10.1093/brain/awn014. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–92. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Tait MJ, Reza A, Davies DC, Bell BA, Verkman AS, et al. AQP4 gene deletion in mice does not alter blood-brain barrier integrity or brain morphology. Neuroscience. 2009;161:764–72. doi: 10.1016/j.neuroscience.2009.03.069. [DOI] [PubMed] [Google Scholar]
- Tait MJ, Saadoun S, Bell BA, Papadopoulos MC. Water movements in the brain: role of aquaporins. Trends Neurosci. 2008;31:37–43. doi: 10.1016/j.tins.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fujihara K, Nakashima I, Misu T, Miyazawa I, Nakamura M, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130:1235–43. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- Waters P, Vincent A. Detection of anti-aquaporin-4 antibodies in neuromyelitis optica: current status of the assays. Int MS J. 2008;15:99–105. [PubMed] [Google Scholar]
- Weinshenker BG, Wingerchuk DM, Pittock SJ, Lucchinetti CF, Lennon VA. NMO-IgG: a specific biomarker for neuromyelitis optica. Dis Markers. 2006;22:197–206. doi: 10.1155/2006/586306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol. 2007;6:805–15. doi: 10.1016/S1474-4422(07)70216-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.