Abstract
Transport of mRNA is an efficient mechanism to target proteins to specific regions of a cell. Although it is well documented that mRNAs are transported in ribonucleoprotein (RNP) complexes, several of the mechanisms involved in complex formation and localization are poorly understood. Staufen (Stau) 1, a double-stranded RNA-binding protein, is a well accepted marker of mRNA transport complexes. In this manuscript, we provide evidence that Stau1 self-associates in live cells using immunoprecipitation and bioluminescence resonance energy transfer (BRET) assays. The double-stranded RNA-binding domains dsRBD3 and dsRBD4 contributed about half of the signal, suggesting that Stau1 RNA-binding activity is involved in Stau1 self-association. Protein–protein interaction also occurred, via dsRBD5 and dsRBD2, as shown by in vitro pull-down, yeast two-hybrid, and BRET assays in live cells. Interestingly, Stau1 self-association contributes to the formation of oligomeric complexes as evidenced by the coexpression of split Renilla luciferase halves covalently linked to Stau1 in a protein complementation assay (PCA) combined with a BRET assay with Stau1-YFP. Moreover, we showed that these higher-order Stau1-containing complexes carry RNAs when the RNA stain SYTO 14 was used as the energy acceptor in the PCA/BRET assay. The oligomeric composition of Stau1-containing complexes and the presence of specific mRNAs have been confirmed by biochemical approaches involving two successive immunoprecipitations of Stau1-tagged molecules followed by qRT-PCR amplification. Altogether, these results indicate that Stau1 self-associates in mRNPs via its multiple functional domains that can select mRNAs to be transported and establish protein–protein interaction.
Keywords: Staufen, ribonucleoprotein, mRNA transport, mRNA granule, mRNA particle
INTRODUCTION
Cytoplasmic mRNA transport and local translation to defined subcellular domains allow efficient spatial and temporal restriction of genetic expression (Sossin and DesGroseillers 2006; Rodriguez et al. 2008; Martin and Ephrussi 2009). This phenomenon is observed in a large variety of cell types and organisms and has been shown to play an important role in processes such as learning and memory, synaptic transmission, axis formation during development, cell motility, and asymmetric cell division. Evidence supports a model in which the differential delivery of new mRNAs to subcellular domains occurs in motile ribonucleoprotein (RNP) structures. A compelling framework was suggested about how mRNA localization could be achieved in an ordered, multi-step pathway. It predicted (1) the formation of mRNPs as a functional complex, (2) the transport of these particles to their destination via cytoskeletal elements and motor proteins, and (3) the derepression of translation of the localized mRNAs. RNA-binding proteins (RBP) become associated with mRNAs either in the nucleus or during remodeling of the complex in the cytoplasm, forming a heterogeneous population of transport complexes. While the composition of mRNA transport complexes begins to be elucidated, less is known about the role(s) of these various RBPs in the mechanism of transport.
Staufen is a member of the double-stranded RNA-binding protein (dsRBP) family. It was first described in Drosophila melanogaster where it is required during development for the localization of the oskar and bicoid transcripts at the posterior and anterior poles of the oocytes, respectively (St Johnston et al. 1991). Staufen was also shown to be required for translational derepression of osk mRNA once properly localized (Kim-Ha et al. 1995; Micklem et al. 2000). In mammals, two homologous Staufen genes, Stau1 and Stau2, were identified (DesGroseillers and Lemieux 1996; Buchner et al. 1999; Kiebler et al. 1999; Marion et al. 1999; Wickham et al. 1999; Duchaine et al. 2002). Stau1 is a multifunctional protein involved in different pathways of RNA metabolism. In neurons, Stau1 is required for the transport and localization of specific mRNAs to dendrites (Kiebler et al. 1999; Kanai et al. 2004; Vessey et al. 2008). Disruption of Staufen blocks transcription-dependent forms of synaptic plasticity/memory in Drosophila (Dubnau et al. 2003), Aplysia (Liu et al. 2006), and rodents (Lebeau et al. 2008). In addition to its role in mRNA transport, Stau1 plays additional roles in the regulation of post-transcriptional mRNA expression. When bound to the 3′ UTR of mRNAs, Stau1 recruits the nonsense mediated decay (NMD) factor Upf1 and triggers degradation of the bound mRNAs (Kim et al. 2005, 2007). In contrast to the NMD pathway that recognizes and degrades mRNAs with a premature termination codon, Stau1-mediated mRNA decay is not a quality control mechanism but rather a bona fide mechanism of regulation of gene expression modulating the stability of natural mRNAs. In contrast, when bound to the 5′ UTR of mRNAs, Stau1 enhances their translational efficiency (Dugre-Brisson et al. 2005).
Stau1 is a modular protein characterized by the presence of two conserved consensus amino acid sequences that fold into a domain involved in double-stranded RNA binding (dsRBD3, dsRBD4) (Marion et al. 1999; Wickham et al. 1999). The α−β−β−β−α structure of this domain interacts with the phosphate backbone of the double helix RNA in its minor and major grooves (Ryter and Schultz 1998; Ramos et al. 2000). Stau1 also contains two truncated dsRBDs (dsRBD2, dsRBD5) in which amino acid sequence conservation is restricted to the C-terminal half of the consensus (Lunde et al. 2007). These domains are unable to bind RNA. A tubulin-binding domain (TBD) is located between dsRBD4 and dsRBD5 and is believed to be involved in mRNA transport on the cytoskeleton (Wickham et al. 1999). Proteomic and cell biological experiments have clearly established that Stau1 is a component of ribonucleoprotein complexes in several cell types (Krichevsky and Kosik 2001; Mallardo et al. 2003; Brendel et al. 2004; Villace et al. 2004).
Although it is well accepted that Stau1 is a marker of mRNA transport complexes and a crucial player for mRNA transport (Kanai et al. 2004; Sossin and DesGroseillers 2006; Vessey et al. 2008), its exact roles within RNPs are still unclear. It is known that several proteins of the dsRBP family such as TRBP2, XlrbpA, ADAR2, PACT, and Rnt1p have the capacity to dimerize through their dsRBDs (Daher et al. 2001; Peters et al. 2001; Valente and Nishikura 2007). For many of these proteins, structural rearrangements resulting from homo- and/or heterodimerization are required for functional activation, and, therefore, dimerization may serve as a molecular regulator. Therefore, we tested whether Stau1, in addition to its other roles in mRNA processing, organizes Stau1-containing mRNPs through self-multimerization. In this manuscript, we provide evidence that Stau1 self-interacts in live cells to form multimeric structures and identify the molecular determinants involved in this property. We further show that these complexes contain specific RNAs.
RESULTS
Differentially tagged Stau1 are found in common complexes in vivo
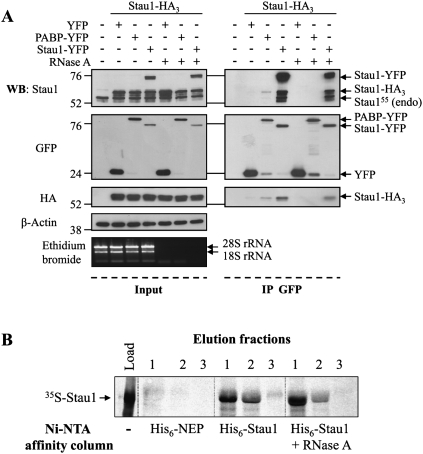
To determine whether Stau1 forms complexes in mammalian cells, we used immunoprecipitation of differentially tagged Stau1 proteins. HEK293T cells were co-transfected with plasmids coding for Stau1-HA3 and either poly(A)-binding protein yellow fluorescent protein (PABP-YFP), Stau1-YFP, or YFP. Western blot experiments indicated that transfected proteins were well expressed and that the levels of expression of Stau1-HA3 and Stau1-YFP were close to that of endogenous Stau1 (Fig. 1A). Proteins in the cell extracts were then immunoprecipitated using anti-GFP antibody, and co-immunoprecipitated proteins were analyzed by Western blotting (Fig. 1A). In these conditions, Stau1-HA3 was co-immunoprecipitated with Stau1-YFP and to a lesser extent with PABP-YFP. In contrast, Stau1-HA3 was not co-immunoprecipitated when co-expressed with YFP. Interestingly, endogenous Stau155 was also found in the precipitates, revealing the biological relevance of these interactions. Treatment of the cell extracts with RNase A before immunoprecipitation did not abolish Stau1/Stau1 interactions but prevented PABP-YFP/Stau1-HA3 interaction. These results show that endogenous and differentially tagged Stau1 are found in common complexes and that, if they are bound to RNA, their association is close enough to be protected from RNase degradation, in contrast to that of Stau1-PABP.
FIGURE 1.
Differentially tagged-Stau1s are found in common complexes. (A) HEK293T cells were mock transfected or co-transfected with Stau1-HA3 expressor and either YFP, PABP-YFP, or Stau1-YFP expressors in the absence or presence of RNase A as indicated. An aliquot of the proteins in the cell extracts was separated by SDS-PAGE (left panels, “Input”) and the remaining extracts were immunoprecipitated using anti-GFP antibody (right panels, “IP GFP”). Proteins were analyzed by Western blotting using anti-Stau1, anti-GFP, and anti-HA antibodies, as indicated. Anti-β-actin antibody was used as a loading control. Molecular weight markers are indicated on the left side of the gels. As control for RNase A digestion, total RNAs were isolated and separated on agarose gel. Ribosomal RNAs (rRNA) were detected by ethidium bromide staining. (B) Bacterially expressed his6-Stau1 or his6-NEP (as control) fusion proteins were fixed on a Ni-NTA column and extensively washed. In vitro synthesized 35S-Stau1 was loaded onto the columns. Columns were washed and then his6-Stau1 and his6-NEP were eluted and collected in three successively fractions (lanes 1–3). The co-eluted 35S-Stau1 protein was separated by SDS-PAGE and visualized by autoradiography. In parallel, his6-Stau1 and 35S-Stau1 preparations were treated with RNase A before loading onto the columns. An aliquot of 35S-Stau1 was also loaded on the gel (lane, “Load”) to control for its synthesis and size.
To determine if Stau1 monomers can self-associate in these complexes, we used an in vitro pull-down assay. Bacterially expressed and purified his6-Stau1 and his6-NEP, used as negative controls, were loaded on Ni-NTA columns. In vitro synthesized 35S-labeled Stau1 was loaded on the his6-Stau1 and his6-NEP columns. After several washing steps, his6-Stau1 and his6-NEP were eluted from the columns, and the co-eluted 35S-labeled Stau1 was detected by SDS-PAGE and autoradiography (Fig. 1B). Whereas 35S-labeled Stau1 was retained on the his6-Stau1 column, it was absent from the his6-NEP column, showing that Stau1 interacts with itself in this assay. The Stau1–Stau1 interaction experiment was done in the presence of RNase A (50 μg/mL). In these conditions, 35S-labeled Stau1 still interacted with his6-Stau1. These results indicate that Stau1 has the capacity to dimerize if not oligomerize and that protein–protein interactions contribute at least in part to Stau1 self-interaction.
Stau1 self-associates in live cells
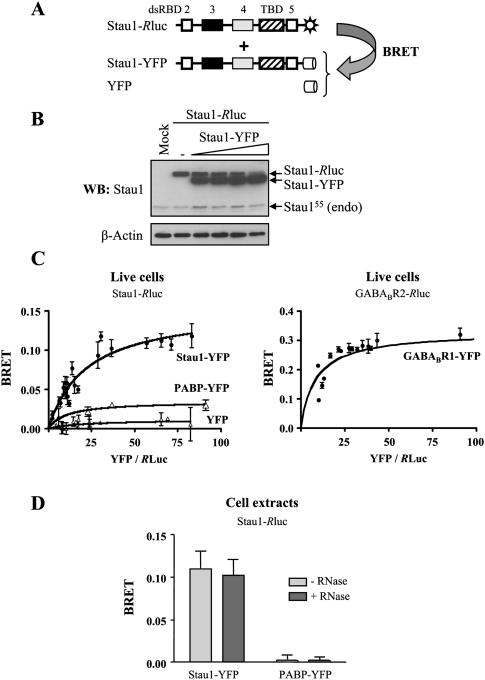
Our results indicate that several Stau1 monomers are found in common complexes in vivo and that direct interaction may contribute to complex formation. In order to confirm the proximity (or interaction) of Stau1 molecules within mRNPs in live cells, bioluminescence resonance energy transfer (BRET) assays were used (Fig. 2A). In this technique, one candidate interacting protein is fused to Renilla luciferase, a luminescent energy donor, and the other to YFP, a fluorescent energy acceptor. The two fusion proteins are then co-expressed in the same cells. If the two proteins interact, their close proximity allows nonradiative energy transfer (BRET) between the luciferase and the YFP. BRET does not occur if the two proteins are separated by >100 Å, making the technique ideal for monitoring protein–protein interactions in biological systems. Therefore, Rluc and YFP were fused at the C terminus of Stau1. Fixed amounts of Stau1-Rluc were transfected in combination with increasing amounts of Stau1-YFP (Fig. 2B), PABP-YFP, or YFP to generate BRET saturation curves (Fig. 2C). Co-expression of Stau1-Rluc and Stau1-YFP produced a strong BRET signal in live cells comparable to that of proteins known to form obligatory dimers (Fig. 2C, GABAB receptors), suggesting a close proximity between these two proteins. In contrast, Stau1-Rluc only produced a negligible BRET signal when co-expressed with PABP-YFP or YFP, confirming the specificity of the Stau1-Rluc/Stau1-YFP BRET signal and suggesting that the binding of two RNA-binding proteins on RNA molecules is not sufficient to induce a strong BRET signal. The BRET assay was then performed with cell extracts, allowing us to determine whether the interaction is sensitive to RNase treatment or not. Cells were lysed and the resulting cell extracts were incubated in the presence or absence of RNase A for 15 min at room temperature before fluorescence measurement. There was no significant difference in Stau1-Rluc/Stau1-YFP BRET signals between RNase A-treated and untreated extracts (Fig. 2D).
FIGURE 2.
Stau1 self-associates in live cells as measured by BRET. (A) Schematic representation of the BRET assay. (Black and gray boxes) Double-stranded RNA-binding domains with major (dsRBD3) and minor (dsRBD4) dsRNA-binding activity, respectively; (white boxes) domains with no demonstrated RNA-binding activity (dsRBD2 and dsRBD5); (hatched boxes) tubulin-binding domain (TBD). (Sun) Rluc, (cylinder) YFP. (B,C) Plasmids coding for Stau1-Rluc were co-transfected in HEK293T cells with various amounts of Stau1-YFP, PABP, or YFP expressors. (B) Expression of Stau1-Rluc and Stau1-YFP proteins using Western blotting with anti-Stau1 antibody. β-actin antibody was used as a loading control. (C) BRET saturation curves were generated to provide evidence of specific interactions between the proteins in live cells. BRET ratios were defined as described in the Materials and Methods and plotted as a function of the excited YFP activity to total Renilla luciferase activity ratio. (Left) Comparison of BRET saturation curves when Stau1-Rluc was co-expressed with Stau1-YFP, PABP-YFP, or YFP. Error bars correspond to standard deviation. (Right) GABAB receptor 2-Rluc and GABAB receptor 1-YFP, two proteins known to form strong functional dimer, were used as a BRET-positive control. (D) HEK293T cells were co-transfected with Stau1-Rluc and either Stau1-YFP or PABP-YFP expressors as described in B. Cells were lysed and cell extracts were incubated in the presence or absence of RNase A for 15 min at room temperature. BRET ratios at saturation in the presence (+) or absence (−) of RNase are shown. n = 3. Error bars correspond to standard deviation.
RNA-binding activity contributes to Stau1 self-association in live cells
Although Stau1 self-association is resistant to RNase treatment and can occur with purified proteins in vitro, it is possible that an RNA molecule may be required during an initial step that triggers Stau1 self-association or that a linker RNA is protected from RNase A degradation by bound Stau1 molecules. To determine whether Stau1 RNA-binding activity is involved in Stau1 self-association, RNA-binding-defective Stau1 mutants were fused to YFP and used in the BRET assay described above to determine their ability to interact with the wild-type Stau1-Rluc. Mutations were introduced in Stau1 dsRBD3, a domain shown to carry the major Stau1 RNA-binding activity, and in dsRBD4, a domain carrying a weak RNA-binding activity in vitro (Fig. 3A; Wickham et al. 1999). Four mutants were generated by either deletion of the complete dsRBD3 (Stau1Δ3-YFP) or dsRBD4 (Stau1Δ4-YFP) or through point mutations in amino acids required for RNA binding in dsRBD3 (Stau1KK-YFP) or in both dsRBD3 and dsRBD4 (Stau14K-YFP). In these mutants, lysines 153 and 154 in dsRBD3 and lysines 256 and 257 in dsRBD4 were mutated to alanines. HEK293T cells were co-transfected with plasmids coding for Stau1-Rluc and either one of the mutated fusion proteins (Fig. 3B). BRET signals were calculated in live cells 24 h post-transfection. Following expression of mutants in dsRBD3 alone (Stau1KK and Stau1Δ3), the BRET ratio was weakly affected, being only reduced by ∼20% (77% ± 10% and 77.5 ± 0.9%, respectively) as compared with the BRET activity generated with the wild-type Stau1-YFP (Fig. 3C). When mutations were introduced in both dsRBD3 and dsRBD4 (Stau14K), the BRET ratio was further reduced to 51.8% (± 0.4%) of the one obtained with Stau1-YFP. These results indicate that Stau1 RNA-binding activity contributes to Stau1 self-association and may be required to select mRNAs in Stau1-containing mRNPs and/or help to initiate or stabilize protein–protein interaction. Nevertheless, protein–protein interaction seems to also contribute to Stau1 self-association in live cells.
FIGURE 3.
Stau1 RNA-binding activity is involved in complex formation. (A) Schematic representation of Stau1 mutants. Symbols are described in the legend for Figure 2. (Stau1KK) Mutations K153A and K154A in dsRBD3; (Stau14K mutant) mutations K153A and K154A in dsRBD3 and K256A and K257A in dsRBD4. (B) Plasmids coding for Stau1-YFP and Stau1-YFP mutants were transfected in HEK293T cells and protein expression was monitored by Western blotting using anti-GFP antibody. Anti-β-actin antibody was used as loading control. Numbers above each lane correspond to those in A. (C) HEK293T cells were co-transfected with Stau1-Rluc and either Stau1-YFP, Stau1Δ3-YFP, StaulΔ4-YFP, Stau14K-YFP, or Stau1KK-YFP expressors. BRET ratios of wild-type Stau1 and RNA-binding defective Stau1 mutants were calculated as in Figure 2, and relative BRET ratios were generated with the Stau1-Rluc/Stau1-YFP BRET ratio arbitrarily fixed to 1. n = 3. Error bars correspond to standard deviation.
Characterization of the protein–protein determinants required for Stau1 self-association
To identify the molecular determinants involved in protein interaction, we first expressed different combinations of wild-type and Stau1 deletion mutants (Fig. 4A,B) fused to either Rluc or YFP and measured their BRET activity in live cells (Fig. 4C–E). When co-expressed with wild-type Stau1, Stau1Δ2 or Stau1Δ5 expression reduced the BRET activity by ∼20% and 50%, respectively, as compared with that obtained with wild-type Stau1 (Fig. 4C). These results suggest that dsRBD5 and dsRBD2 are major and minor determinants involved in the protein–protein aspect of Stau1 self-association, respectively. To further characterize these interactions, we then calculated BRET activity following co-expression of these two fusion proteins: Stau1Δ5-Rluc/Stau1Δ2-YFP, Stau1Δ2-Rluc/Stau1Δ2-YFP, Stau1Δ5-Rluc/Stau1Δ5-YFP, and Stau1Δ2-Rluc/Stau1Δ5-YFP. As shown in Figure 4D, co-expression of Stau1Δ5-Rluc and Stau1Δ5-YFP showed a BRET activity that was reduced to 51.3% (± 0.1%), consistent with a significant role of dsRBD5 in Stau1 association. In contrast, co-expression of Stau1Δ2-Rluc and Stau1Δ2-YFP generated a BRET signal that was similar to that obtained with Stau1-Rluc and Stau1-YFP. Finally, BRET signals generated by the co-expression of the Stau1Δ5-Rluc/Stau1Δ2-YFP or Stau1Δ2-Rluc/Stau1Δ5-YFP pairs were highly reduced, being only ∼25% (32.8% ± 0.2% and 24.3% ± 0.4%, respectively) of the BRET activity generated by the Stau1 wild-type pair (Fig. 4D). Consistently, a Stau1 mutant that carried only the dsRBD4 and tubulin-binding domains (RBD4/TBD) did not generate BRET signal when expressed with wild-type Stau1 (Fig. 4E). Altogether, these results indicate that dsRBD5 is clearly involved in Stau1 self-interaction in live cells, whereas the role of dsRBD2 is less clear.
FIGURE 4.
Characterization of Stau1 protein–protein interaction in live cells. (A) Schematic representation of Stau1 and mutants. Symbols are described in the legend of Figure 2. (B) Plasmids coding for Stau1-YFP and Stau1-YFP mutants were transfected in HEK293T cells and protein expression was monitored by Western blotting using anti-GFP antibody. Anti-β-actin antibody was used as loading control. Numbers above each lane correspond to those in A. (C–E) Cells were co-transfected with different combinations of Stau1 and Stau1 deletion mutants fused to either Rluc or YFP as indicated. BRET ratios were calculated as in Figure 2, and relative BRET ratios were generated with the Stau1-Rluc/Stau1-YFP BRET ratio arbitrarily fixed to 1. n = 3. Error bars correspond to standard deviation. (F) Yeast cells were co-transformed with a plasmid coding for Stau1-B42 and different expressors of Stau1 domains fused to LexA, as indicated. Cells were screened for growth on selective medium and β-galactosidase expression. Stau1-B42 interacts (+) with Stau1 dsRBD2 and dsRBD5 but not (−) with dsRBD3, dsRBD4, or TBD fused to LexA in a two-hybrid assay.
To confirm that dsRBD5 and dsRBD2 are the molecular determinants involved in Stau1–Stau1 protein interaction, the yeast two-hybrid system was used. The full-length Stau1 protein was fused to the B42 transcription activator, whereas each of the four dsRBDs of Stau1 and the tubulin-binding domain (TBD) were individually fused to the LexA DNA-binding domain. After selection for the presence of both plasmids in the yeast strains, cells were plated on a Ura−, His−, Trp−, Leu− medium with galactose and X-Gal. Our results showed that only yeast cells expressing the dsRBD2- and dsRBD5-LexA fusion proteins grew on the selective medium and produced β-galactosidase activity (Fig. 4F). Therefore, both dsRBD2 and dsRBD5 can interact with the full-length Stau1 protein in this assay.
Subcellular distribution of Stau1 and Stau1 mutants
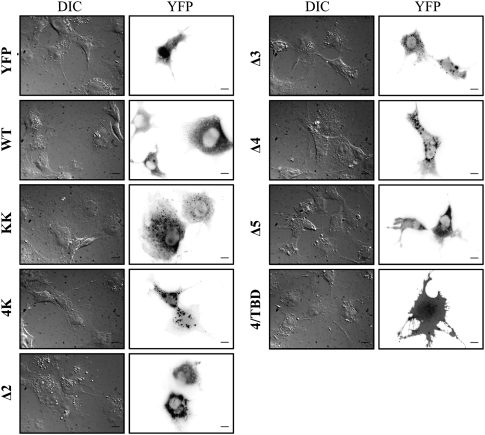
To determine whether the differential BRET responses obtained with the Stau1 mutants may be explained by major defects in the subcellular distribution of these proteins, Stau1-YFP and Stau1-YFP mutants were expressed in COS-7 cells and their subcellular distribution was observed by fluorescence microscopy. HEK293T cells were not used in this assay because of the small size of their cytoplasm. As previously shown (Marion et al. 1999; Wickham et al. 1999), Stau1-YFP showed a granular distribution in the cytoplasm and nuclear exclusion. Stau1Δ5-YFP, a mutant that produced weak BRET signals (Fig. 4), had a subcellular distribution similar to that of Stau1-YFP (Fig. 5). As compared with Stau1-YFP, Stau1KK-YFP showed an additional weak nucleolar distribution, and mutants in the RNA-binding domains dsRBD3 and dsRBD4 showed cytosolic granules that are slightly bigger than those observed with Stau1-YFP. However, this cannot explain the difference in the BRET response of Stau14K-YFP as compared with the others (Fig. 3C). The distribution of 4/TBD-YFP was random and similar to that of YFP alone as previously described (Luo et al. 2002).
FIGURE 5.
Subcellular distribution of Stau1 and Stau1 mutants. COS-7 cells were transfected with plasmids coding for Stau1-YFP or Stau1-YFP mutants as indicated. YFP expression was observed 24 h later by fluorescence microscopy. Both differential interference contrast microscopy (DIC) and the YFP constructs are shown.
Stau1 forms oligomers in live cells
Knowing that Stau1 is present in large mRNP complexes, we assessed the capacity of Stau1 to form higher order structures in live cells. To this end, we combined protein complementation (PCA) and BRET assays (Fig. 6A). For the PCA assay, Rluc is cut into two nonfunctional halves (RlucF1 and RlucF2). Each half of the split luciferase protein cannot by itself generate light when expressed in cells. However, their fusion to proteins that interact via protein–protein interactions allows the reconstitution of a functional luciferase and light emission. Therefore, the N-terminal half (Stau1-RlucF1) and the C-terminal half (Stau1-RlucF2) of a split Rluc protein were fused at the C terminus of Stau1. Consistent with the BRET assay, co-expression of Stau1-RlucF1 and Stau1-RlucF2 reconstituted a functional luciferase that produced light (8293 ± 1341 arbitrary units [AU]) via Stau1–Stau1 interaction, whereas expression of either fusion protein alone did not (580 ± 87 and 529 ± 41 AU, respectively). Then, to determine whether additional Stau1 molecules can interact with this complex in live cells, the PCA assay with Stau1-RlucF1 and Stau1-RlucF2 was repeated in the presence of an increasing concentration of Stau1-YFP for BRET measurement. If Stau1 induces the formation of large complexes, a complete Stau1-Stau1-Rluc will be reconstituted and will transfer energy to the Stau1-YFP that will be present in the same complex. As shown in Figure 6B, co-expression of Stau1-RlucF1, Stau1-RlucF2, and Stau1-YFP produced a strong BRET saturation curve, suggesting that at least three Stau1 molecules associate in these complexes. These interactions between Stau1 molecules were resistant to RNase treatment (Fig. 6C), consistent with results shown in Figure 2D. In contrast, Stau1-RlucF1 and Stau1-RlucF2 did not produce BRET activity when co-expressed with YFP or PABP-YFP (Fig. 6B). Other controls were done with CXCR4, a protein that does not interact with Stau1. Stau1-RlucF1 and CXCR4-RlucF2 as well as Stau1-RlucF2 and CXCR4-RlucF1 did not produce significant luciferase activity when co-expressed (533 ± 59 and 613 ± 19 AU, respectively); CXCR4-RlucF1 and CXCR4-RlucF2, two proteins known to interact in live cells (Hamdan et al. 2006), did not transfer energy to Stau1-YFP in the BRET assay (data not shown).
FIGURE 6.
Stau1 forms oligomers in live cells. (A) Schematic representation of the BRET-PCA assay. Stau1 was fused to either the N-terminal (RlucF1) or the C-terminal (RlucF2) of Rluc. Formation of Stau1 dimers will bring together the two Rluc halves through protein complementation (PCA) and reconstitute a functional luciferase. This enzyme will transfer energy and generate BRET signal if Stau1-YFP is also present in close proximity, suggesting the formation of oligomers. (B) HEK293T cells were co-transfected with constant amounts of plasmids coding for Stau1-RlucF1 and Stau1-RlucF2 and increasing amounts of plasmids coding for either Stau1-YFP, PABP-YFP, or YFP. The saturation curves provide evidence of specific interactions between at least three Stau1 proteins in live cells. BRET ratios were calculated as in Figure 2. Error bars correspond to standard deviation. (C) Plasmids were transfected as in B. Cells were lysed and cell extracts were treated with RNase (+RNase) or the buffer alone (−RNase) before BRET calculation.
Stau1-containing oligomeric complexes contain RNAs
To determine whether Stau1-containing oligomeric complexes contain RNAs, we modified the live cells assay that combined PCA and BRET to detect RNA–protein interaction (Fig. 7A). In this assay, Stau1-RlucF1 and Stau1-RlucF2 were co-expressed to reconstitute luciferase activity while SYTO 14, a dye that stains RNA, was used as the energy acceptor. If Stau1-containing oligomers associate with RNA, BRET signal will be generated as a consequence of the close proximity between the reconstituted Rluc and SYTO 14. As shown in Figure 7B, expression of both Stau1-Rluc and the split Stau1-RlucF1 and Stau1-RlucF2 generated BRET signals when RNA was stained with SYTO 14, indicating that RNAs are present in the Stau1-containing complex. In contrast, Rluc activity resulting from interaction of the split protein kinase A (PKA) regulatory subunit-RlucF1 and PKA catalytic subunit-RlucF2 did not induce BRET signal in the presence of SYTO 14. Whereas luciferase activity generated by interaction of the split Rluc fusion proteins was not impaired by RNase treatment (data not shown; Fig. 6C), the observed Stau1-Stau1-Rluc/SYTO 14 BRET signal was sensitive to RNase treatment, indicating that Stau1-containing complexes indeed contain RNAs (Fig. 7C).
FIGURE 7.
Stau1 oligomers contain RNA in live cells. (A) Schematic representation of the PCA-BRET assay for protein–RNA interaction. As described in Figure 6, Stau1-RlucF1 and Stau1-RlucF2 were transfected in HEK293T cells to reconstitute a functional luciferase as a consequence of Stau1–Stau1 interaction. Then cells were treated with SYTO 14, a fluorescent dye that stains RNA. Energy transfer can occur from the reconstituted Rluc to SYTO 14 if in close proximity and generate BRET signal. (B) BRET ratios at saturation are indicated for each association between monomeric (Rluc, Stau1-Rluc) or split (Stau1-RlucF1 + Stau1-RlucF2, PKA reg-RlucF1 + PKA cat-RlucF2) Rluc and SYTO 14. Error bars are standard deviation. (**) P < 0.01 (ANOVA and post-test Dunnett). (C) Transfected HEK293T cells were lysed and cell extracts were treated with RNase (+RNase) or the buffer alone (Ctl) before adding SYTO 14 for the BRET assay. (***) P < 0.001 (t-test). Note that the BRET values are higher in cell extracts than those in live cells probably due to the better availability of SYTO 14 in cell extracts.
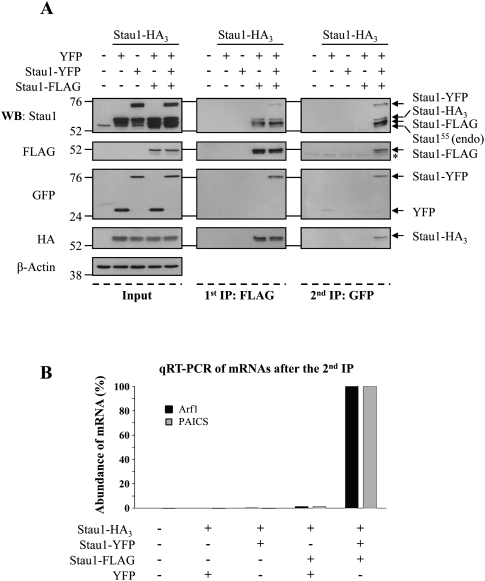
To confirm the formation of RNA-containing Stau1-oligomers, a biochemical approach involving two successive immunoprecipitation steps was used. HEK293T cells were transfected with plasmids coding for Stau1-HA3 and either YFP, Stau1-YFP, and/or Stau1-Flag (Fig. 8A). The level of expression of these proteins was evaluated by Western blotting. First, Stau1-containing complexes were immunoprecipitated with anti-Flag antibodies and eluted with the Flag peptide. The resulting complexes contained Stau1-HA3 and Stau1-YFP when co-expressed. Then, Stau1-Flag-containing complexes were re-immunoprecipitated using anti-GFP antibody. The resulting pellet also contained Stau1-HA3 and Stau1-Flag, indicating that the three tagged-Stau1 proteins were present in the same complexes. As controls, other combinations of transfected Stau1-tagged molecules lacking either Stau1-Flag or Stau1-GFP did not bring down Stau1-HA3.
FIGURE 8.
Stau1 forms RNA-containing higher-order complexes. (A) HEK293T cells were mock transfected or co-transfected with Stau1-HA3 expressor and either YFP, Stau1-YFP, or Stau1-Flag expressors as indicated. Proteins in the cell extracts were separated by SDS-PAGE (left panels, Input) or successively immunoprecipitated by first using anti-Flag antibody (middle panels, 1st IP: FLAG) and then using anti-GFP antibody (right panels, 2nd IP: GFP). Proteins were analyzed by Western blotting using anti-Stau1, anti-Flag, anti-GFP, and anti-HA antibodies, as indicated. Anti-β-actin antibody was used as a loading control. Molecular weight markers are indicated on the left side of the gels. (B) Immunoprecipitates obtained after the second round of immunoprecipitation were treated with TRIzol reagent to isolate putative Stau1-bound RNAs. RNAs were qRT-PCR amplified using oligonucleotide primers for ARF1 and PAICS mRNAs, two known Stau1-bound mRNAs (Kim et al. 2005). This figure is representative of three independent experiments.
To determine whether the Stau1-containing oligomeric complexes contain RNAs, immunoprecipitates obtained from the successive-IPs were treated with TRIzol reagent to isolate putative RNAs, and the resulting material was analyzed by qRT-PCR. Arf1 and PAICS mRNAs, two known Stau1-bound mRNAs (Kim et al. 2005), were specifically amplified from samples of the two successive IPs and were absent from other control IPs (Fig. 8B). To discriminate between specific Stau1-mRNA association and nonphysiological aggregates of mRNPs, we calculated the ratio between the amount of Arf1 and PAICS mRNAs in the successive immunoprecipitates and their amount in the cells before IPs (IP/input). As a control for specificity, we also calculated the ratio of an abundant non-Stau1-interacting mRNA coding for the ribosomal protein RPL22. When the ratio obtained for RPL22 mRNA was arbitrarily normalized to 1, an enrichment ranging from 13- to 20-fold was observed for Arf1 mRNA and from 32- to 43-fold for PAICS mRNA. Altogether, these results indicate that Arf1 and PAICS mRNAs are highly enriched in the multimeric complexes as compared with non-Stau1-interacting controls.
DISCUSSION
Many proteins self-associate to generate dimers and/or oligomers, and this structural reorganization is essential to control their function, structure, and/or regulation (Marianayagam et al. 2004). Enzymatic regulation, cell signaling, DNA binding, and regulation of gene expression are a few examples among all the molecular activities that are regulated by protein self-interaction. Especially, proteins of the dsRBP family such as PKR, TRBP2, XlrbpA, ADAR2, PACT, and Rnt1p are known to form homo- and/or heterodimers (Lamontagne et al. 2000; Daher et al. 2001; Peters et al. 2001; Hitti et al. 2004; Lemaire et al. 2005; Valente and Nishikura 2007). The structural rearrangement resulting from dimerization is required for their functional activation, and therefore dimerization serves as a molecular regulator (Daher et al. 2001; Peters et al. 2001; Valente and Nishikura 2007). In this study, we show that Stau1, like other members of the dsRBP family, self-associates. Using novel approaches in live cells, we further showed that Stau1 generates multimeric complexes through both RNA-binding activity and protein–protein self-interaction and that these complexes contain specific RNAs. Our results suggest that Stau1 has the potential to act as a scaffolding protein that organizes mRNAs into functional mRNPs.
Molecular determinants for mRNP formation
Our BRET assays indicate that Stau1 RNA-binding activity or intact dsRBD3 and dsRBD4 contributes for ∼50% of the Stau1 self-association (Fig. 3). Several biochemical studies (Nanduri et al. 2000) as well as NMR and crystallographic structures of bacterially expressed dsRBDs (Bycroft et al. 1995; Kharrat et al. 1995; Ryter and Schultz 1998; Nanduri et al. 2000; Ramos et al. 2000) indicate that RNA-binding competent dsRBDs behave as monomeric forms. Therefore, it is likely that the contribution of these domains to Stau1 self-interaction depends on their capability to bind RNA and not on putative protein–protein interaction. In addition, our observation that protein interactions occur via other domains that do not bind RNAs in vitro (Wickham et al. 1999) supports a model in which dsRBD3 and dsRBD4 are free to interact with mRNAs. Stau1 RNA-binding activity may then be used to recognize and select mRNAs to be included in mRNPs. Subsequently, it may facilitate the association of Stau1 monomers on a dsRNA structure and then facilitate the recognition, formation, and/or maintenance of protein–protein interaction mediated by dsRBD5 and dsRBD2.
The RNA-binding activity is nevertheless not essential for subsequent protein–protein interaction. Indeed, ∼50% of the BRET activity is still detectable when RNA-binding deficient Stau1 mutants are expressed in vivo (Fig. 3). Accordingly, Stau1 self-association can be detected in vitro using purified proteins in the presence of RNase (Fig. 1B), revealing the intrinsic property of Stau1 to self-associate. This is consistent with previous studies in which dsRBPs form dimers through both dsRBDs and other domains not involved in RNA binding (Lamontagne et al. 2000; Daher et al. 2001; Peters et al. 2001; Hitti et al. 2004; Valente and Nishikura 2007). Our results identified dsRBD5 as the major determinant for protein–protein interaction, contributing to ∼50% of the Stau1 self-interaction (Fig. 4C). Homophilic interaction between dsRBD5s may contribute to stabilize particle formation on mRNAs. The role of dsRBD2 is less clear. Although it can interact with Stau1 in the yeast assay (Fig. 4F), its deletion has no impact on the BRET signal in live cells (Fig. 4C). Interestingly, heterophilic interactions between dsRBD5 and the N-terminal end of Stau1 (including dsRBD2) were observed in the yeast two-hybrid assay (data not shown). This observation would permit generating large multimeric complexes through homo- and heterophilic interactions between dsRBDs. Moreover, it opens the additional possibility that Stau1 structure and/or functions may be regulated by intramolecular interaction between the N-terminal domain and dsRBD5. A similar regulatory mechanism has been described for Rnt1p, a member of the dsRBP family in yeast, in which an N-terminal domain established intramolecular interaction with the C-terminal dsRBD (Lamontagne et al. 2000). Competition between intramolecular Nter-dsRBD5 interaction and intermolecular homophilic binding of dsRBD5 may control Stau1 self-association and, as a consequence, Stau1 structure/functions. Alternatively, deletion of dsRBD2 may somehow destabilize dsRBD3-mediated RNA-binding activity. This may not be crucial in the presence of functional dsRBD5 (Fig. 4C,D, Stau1-Stau1Δ2, Stau1Δ2-Stau1Δ2), but it may explain why Stau1Δ2 and Stau1Δ5 only poorly interact in live cells (Fig. 4D) when dsRBD5-mediated protein–protein interactions are prevented.
Stau1 and the organization of mRNP complexes
The involvement of both Stau1 RNA-binding activity and protein–protein interaction for Stau1 self-multimerization suggests that Stau1 may act as an organizer of mRNP formation. In this model, Stau1 recognizes and binds specific mRNAs to be transported via its RNA-binding domains and organizes these mRNAs into functional mRNP complexes through protein–protein interaction involving dsRBD5 and likely dsRBD2. Interestingly, two of the known Stau1 RNA substrates are known to form homodimers: bicoid mRNAs in Drosophila (Ferrandon et al. 1997) and HIV-1 genomic RNA in humans (Darlix et al. 1990). Therefore, complexes may be formed through both Stau1 and RNA self-association. This novel function of Stau1 in complex formation would be complementary to other known downstream functions of Stau1 in mRNA transport, translation, and decay. Moreover, Stau1 was shown to make direct protein–protein interaction with tubulin (Wickham et al. 1999), ribosomes (Luo et al. 2002; Brendel et al. 2004), the nonsense mediated factor Upf1 (Kim et al. 2005; Gong et al. 2009), and protein phosphatase I (Monshausen et al. 2002), proteins known to be present in Stau1-containing mRNPs. Therefore, not only Stau1 can organize mRNPs through self-association, it can also attract additional RNP-associated proteins through direct interaction. Interestingly, all these interactions were mapped within the Stau1 TBD domain, a region that is not involved in RNA binding or Stau1 self-association. Therefore, Stau1 has the capability to select mRNAs, organize multimeric mRNA–protein structures, and attract additional proteins to form functional mRNPs.
Several data in the literature are consistent with this model. First, Stau1 is known to co-localize with mRNAs in mRNP complexes (Kiebler et al. 1999; Duchaine et al. 2000; Monshausen et al. 2001; Mallardo et al. 2003; Kanai et al. 2004; Furic et al. 2008) and directly bind mRNAs through a specific cis-acting element (Wickham et al. 1999; Kim et al. 2007), indicating that Stau1 has the capability to select mRNAs to be transported and form functional mRNPs. Second, biochemical and proteomic studies have shown that Stau1 is a component of large complexes that contain mRNA and protein cofactors (Krichevsky and Kosik 2001; Mallardo et al. 2003; Brendel et al. 2004; Villace et al. 2004). Its capacity to bind both mRNAs and specific mRNP-associated proteins via protein–protein interaction (see above) will facilitate the coordination between the fate of Stau1-bound mRNAs and proteins required to fulfill the functions. Third, relatively few Stau1 molecules were detected as monomers or dimers (Mallardo et al. 2003), indicating that Stau1 forms higher-order structures. The biochemical and BRET/PCA results (Figs. 6–8) that show the ability of Stau1 to form oligomers in live cells support this conclusion. Fourth, in situ hybridization using Stau1-bound mRNA sequences as probes revealed that, as a consequence of Stau1 down-regulation, the amount of granules containing these specific mRNAs is reduced in dendrites (Kanai et al. 2004; Vessey et al. 2008). Although these data can be interpreted as an indication that Stau1 down-regulation impairs mRNA transport, they may also indicate that Stau1 down-regulation also impairs mRNPs formation. Finally, the oligomeric nature of Stau1-containing particles in dendrites was exemplified in mouse with a loss-of-function allele for Stau1 (Stau1tm1Apa). Whereas a transport-defective ΔStau1-YFP was not properly transported in dendrites following transfection in hippocampal neurons derived from Stau1tm1Apa mouse, it was normally transported in neurons from wild-type mouse (Vessey et al. 2008). This result indicates that wild-type Stau1 can rescue the transport of ΔStau1, likely through an interaction that forms functional mRNPs to be transported in dendrites. A similar mechanism can be used to control the functions of mRNP complexes. Of particular interest is Stau1i, a Stau1 isoform that contains an insertion of six amino acids in dsRBD3 that impairs its ability to bind RNA and causes the formation of RNA-binding-defective particles (Duchaine et al. 2000; Monshausen et al. 2001). Its association with Stau1 in mRNPs modulates the RNA content of Stau1-containing mRNPs and their functions.
In conclusion, our results suggest that the formation of large Stau1-containing mRNP complexes may be initiated by Stau1 itself through both RNA-binding and homophilic and heterophilic protein–protein interactions. This mechanism may represent a first step for the regulation of downstream Stau1 functions such as mRNA transport and localization (Kanai et al. 2004), nuclear transit (Martel et al. 2006), translation regulation (Dugre-Brisson et al. 2005), interaction with the cytoskeleton (Wickham et al. 1999), and Stau1-mediated mRNA decay (Kim et al. 2005, 2007). Using sensitive and novel approaches, we were able for the first time to show that Stau1 dimers and most likely oligomers are associated with mRNAs. These techniques will contribute to our understanding of mRNA particle formation and transport and of how their misregulation may impair Stau1-mediated cellular functions.
MATERIALS AND METHODS
Antibodies and reagents
Goat polyclonal and mouse monoclonal anti-GFP antibodies were obtained from Rockland and Roche Applied Science, respectively. Mouse monoclonal anti-β-actin clone AC-74 and mouse monoclonal anti-Flag M2 were obtained from Sigma-Aldrich. The mouse monoclonal anti-Stau1 (clone 11C6) and anti-HA (clone 12CA5) antibodies were previously described (Dugre-Brisson et al. 2005). Anti-mouse and anti-goat polyclonal antibodies conjugated to horseradish peroxidase were obtained from DakoCytomation. X-gal and imidazole were purchased from Bioshop. Western blot signals were detected using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences).
cDNA construction
YFP-fused Stau1 mutants (Luo et al. 2002), Stau1-Rluc (Chatel-Chaix et al. 2004), Stau1-HA3, Stau1Δ2-his6 plasmids (Wickham et al. 1999), GABABR1-YFP, GABABR2-Rluc (Perroy et al. 2003), and PKAreg-RlucF1 and PKAcatRlucF2 (Stefan et al. 2007) were previously described. Stau1Δ2-Rluc and Stau1Δ5-Rluc were constructed from Stau1-Rluc by the all-around technique. Oligonucleotides used to generate these constructs are listed in Table 1. The 4K mutant was constructed from the 2K mutant (Dugre-Brisson et al. 2005) by the all-around technique (Table 1). The PCR fragment corresponding to PABP was digested with BamHI and cloned in pCMV-YFP-topaz (Packard Bioscience/PerkinElmer LifeSciences).
TABLE 1.
Primers used for PCR amplification
PCR fragments corresponding to dsRBD2, dsRBD3, dsRBD4, and dsRBD5 of Stau1 (Table 1) were digested with EcoRI and XhoI enzymes and cloned into pLexA pre-digested with EcoRI and XhoI. The PCR fragment corresponding to the TBD domain was digested with MunI and XhoI and cloned into pLexA pre-digested with EcoRI and XhoI. Full-length Stau1 was obtained by PCR amplification (Table 1) and digested with XhoI. This fragment was cloned into pB42AD pre-digested with XhoI.
Recombinant protein production and purification
Bacterially expressed his6-Stau1 and neutral endopeptidase his6-NEP were purified as previously described (Wickham et al. 1999). Aliquots of the purified proteins were stored at −80°C until use. Protein concentration was determined by the Bio-Rad dye reagent and BSA as a standard. For the pull-down assays, his6-Stau1 and his6-NEP (as control) were immobilized on a Ni-NTA-agarose matrix and in vitro translated 35S-labeled Staul was loaded onto the column. After extensive washings, bound proteins were eluted with 300 mM imidazole, and three successive elution fractions were analyzed by SDS-PAGE and autoradiography. In parallel, the in vitro translated 35S-Stau1 extract was treated with 20 pg/mL RNase A for 1 h prior to loading.
Yeast two-hybrid assay
The EGY48 strain was transformed with the pLexA or pB42 plasmids containing the Stau1 fragments of interest. Yeast cells were grown in either synthetic growth media lacking the nutrients indicated or rich media.
Cell culture, transfection, and BRET assay
Human embryonic kidney (HEK) 293T cells were cultured in Dulbecco's Modified Eagle Medium (Invitrogen) supplemented with 10% cosmic calf serum (HyClone). Transfections were carried out with the Lipofectamine 2000 reagent (Invitrogen). BRET experiments were carried out as described (Chatel-Chaix et al. 2004, 2007). Briefly, cells were plated into 12-well plates at 120,000 cells per well. Twenty-four hours later, cells were transfected with constant amounts of plasmids coding for Rluc-tagged fusion protein (10 ng) and increasing amounts of plasmids coding for YFP-tagged proteins (0–500 ng) as indicated. BRET assays were performed 24 h post-transfection in live cells. Cells were washed twice with phosphate buffered saline (PBS) at room temperature and diluted to 106 cells per mL. Coelenterazine H (NanoLight Technology) was added to 90 μL of cells at a final concentration of 5 μM. A saturation curve can be drawn when the Rluc-tagged protein becomes saturated by YFP-tagged proteins, and an optimal BRET ratio can be calculated at saturation for a specific protein–protein interaction. Luminescence (440–500-nm) as well as total and transmitted fluorescence (510–590-nm) emissions were measured using a Fusion α-FP apparatus (PerkinElmer). The BRET ratio was defined as [(emission at 510 to 590 nm) − (emission at 440 to 500 nm) × Cf]/(emission at 440–500 nm), where Cf corresponds to (emission at 510 to 590 nm)/(emission at 440 to 500 nm) when Rluc-fused protein is expressed alone. The total YFP activity/Rluc activity ratio reflects the relative levels of activity of the two fusion proteins in the cells. When BRET was carried out in cell extracts, cells were lysed in 200 μL of lysis buffer (0.1 M Tris-Cl at pH 7.9, 0.5% Nonidet P40, 1 mM DTT) and incubated for 15 min at room temperature with or without 30 μg/mL of RNase A (Fermentas).
For BRET between Stau1-Rluc and RNA, 1 μL of SYTO 14 (Invitrogen), an RNA selective fluorescence dye used as energy acceptor, was added to 100,000 cells or to cell extracts and was incubated for 20 min at room temperature. Because the emission of SYTO14 is shifted to the red compared with that of YFP, a 550 long-pass filter (Chroma) was used as acceptor filter. The BRET ratio was calculated as described before by making the ratio of the acceptor filter over the donor filter.
Microscopy
COS-7 cells were transfected with plasmids (500 ng) coding for different Stau1 mutants using PEI as previously described (Guerra-Crespo et al. 2003). Cells were fixed in a freshly made 4% paraformaldehyde solution in PBS for 20 min at room temperature and permeabilized with 0.1% Triton X-100 in PBS. Coverslips were mounted on microscope slides using DAKO fluorescent medium and images were taken on a Nikon TE2000U equipped with a CoolSnap fx CCD camera, a 60× objective (Pan Apo, N.A. 1.4), and a YFP filter cube (Chroma #41028). Images were treated in ImageJ 1.43d. The YFP channels were gamma corrected (0.5), the black level was subtracted (modal value), and the brightness and contrast were set to 0.5% saturation.
Immunoprecipitation and qRT-PCR
HEK293T cells transfected with the indicated plasmids were harvested 24 h post-transfection, washed three times in ice-cold PBS, and lysed in buffer (50 mM Tris-Cl at pH 7.5, 1% Triton X-100, 15 mM EGTA, 150 mM NaCl, 1 mM DTT, Complete EDTA-free protease inhibitor cocktail [Roche Applied Science]) supplemented or not with RNaseOUT RNase inhibitor (Invitrogen). Lysates were cleared by centrifugation at 17,000g for 15 min. The supernatant was incubated with or without 30 μg/mL RNase A for 10 min at 37°C and then pre-cleared with protein G-sepharose (GE Healthcare) for 45 min at 4°C. After centrifugation, the supernatant was incubated with goat polyclonal anti-GFP antibodies for 2 h at 4°C and then with protein G-sepharose for 90 min at 4°C. Immune complexes were washed three times with the lysis buffer and eluted from the resin by heating for 5 min at 95°C in 2× protein loading buffer (125 mM Tris-Cl at pH 6.8, 10% glycerol, 3.3% SDS, 0.2 M DTT, and 0.04% bromophenol blue). Proteins were analyzed by SDS-PAGE and Western blotting using monoclonal antibodies against the indicated proteins.
For the successive immunoprecipitation experiments, lysates were prepared as described above except that DTT and RNase inhibitor were omitted. The first immunoprecipitation was carried out with anti-Flag M2 affinity gel (Sigma-Aldrich) and eluted with the Flag peptide (Sigma-Aldrich) as described previously (Chatel-Chaix et al. 2008). The eluate was then pre-cleared with protein G-sepharose and the second immunoprecipitation was performed with polyclonal anti-GFP as described above. One-third of the immune complex was analyzed by SDS-PAGE and Western blotting and two-thirds was used for qRT-PRC analysis. To this end, RNA was isolated from the immunoprecipitates using the TRIzol Reagent (Invitrogen) according to the manufacturer's procedure. RNA was resuspended in 50 μL of water and digested with DNase using the TURBO DNA-free kit (Ambion). Reverse transcription reactions were done with 4 μL of RNA using the MuLV RT enzyme from the GeneAmp RNA PCR kit (Applied Biosystems), according to the manufacturer's procedure. Specific antisense primers used to produce the cDNAs were 5′-AGATCAGGCCTTGTGTGTTCTGGA-3′ for Arf1 and 5′-TTGAACAGCCAAGACCACTGGGTA-3′ for PAICS. Resulting cDNAs were qPCR amplified using the LightCycler 480 SYBR Green I Master kit (Roche) and the LightCycler 480 instrument (Roche). Sense and antisense primers are described in Table 1.
ACKNOWLEDGMENTS
We thank Francis Goyette, Phu Vinh On, Monique Vasseur, Louise Cournoyer, and Linda Huang for technical assistance. C.M., T.D., V.T., and S.D.-B. were supported by studentships from the Natural Sciences and Engineering Research Council of Canada (NSERC), and K.B. received studentships from the Fonds de Recherche en Santé du Québec. This work was supported by a grant from NSERC to L.D.G.
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.1664210.
REFERENCES
- Brendel C, Rehbein M, Kreienkamp HJ, Buck F, Richter D, Kindler S. Characterization of Staufen 1 ribonucleoprotein complexes. Biochem J. 2004;384:239–246. doi: 10.1042/BJ20040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner G, Bassi MT, Andolfi G, Ballabio A, Franco B. Identification of a novel homolog of the Drosophila staufen protein in the chromosome 8q13-q21.1 region. Genomics. 1999;62:113–118. doi: 10.1006/geno.1999.6015. [DOI] [PubMed] [Google Scholar]
- Bycroft M, Grunert S, Murzin AG, Proctor M, St Johnston D. NMR solution structure of a dsRNA binding domain from Drosophila staufen protein reveals homology to the N-terminal domain of ribosomal protein S5. EMBO J. 1995;14:3563–3571. doi: 10.1002/j.1460-2075.1995.tb07362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatel-Chaix L, Clement JF, Martel C, Beriault V, Gatignol A, DesGroseillers L, Mouland AJ. Identification of Staufen in the human immunodeficiency virus type 1 Gag ribonucleoprotein complex and a role in generating infectious viral particles. Mol Cell Biol. 2004;24:2637–2648. doi: 10.1128/MCB.24.7.2637-2648.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatel-Chaix L, Abrahamyan L, Frechina C, Mouland AJ, DesGroseillers L. The host protein Staufen1 participates in HIV-1 assembly in live cells by influencing pr55Gag multimerization. J Virol. 2007;81:6216–6230. doi: 10.1128/JVI.00284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatel-Chaix L, Boulay K, Mouland AJ, DesGroseillers L. The host protein Staufen1 interacts with the Pr55Gag zinc fingers and regulates HIV-1 assembly via its N-terminus. Retrovirology. 2008;5:41. doi: 10.1186/1742-4690-5-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher A, Longuet M, Dorin D, Bois F, Segeral E, Bannwarth S, Battisti PL, Purcell DF, Benarous R, Vaquero C, et al. Two dimerization domains in the trans-activation response RNA-binding protein (TRBP) individually reverse the protein kinase R inhibition of HIV-1 long terminal repeat expression. J Biol Chem. 2001;276:33899–33905. doi: 10.1074/jbc.M103584200. [DOI] [PubMed] [Google Scholar]
- Darlix JL, Gabus C, Nugeyre MT, Clavel F, Barre-Sinoussi F. Cis elements and trans-acting factors involved in the RNA dimerization of the human immunodeficiency virus HIV-1. J Mol Biol. 1990;216:689–699. doi: 10.1016/0022-2836(90)90392-Y. [DOI] [PubMed] [Google Scholar]
- DesGroseillers L, Lemieux N. Localization of a human double-stranded RNA-binding protein gene (STAU) to band 20q13.1 by fluorescence in situ hybridization. Genomics. 1996;36:527–529. doi: 10.1006/geno.1996.0499. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, et al. The Staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003;13:286–296. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Duchaine T, Wang HJ, Luo M, Steinberg SV, Nabi IR, DesGroseillers L. A novel murine Staufen isoform modulates the RNA content of Staufen complexes. Mol Cell Biol. 2000;20:5592–5601. doi: 10.1128/mcb.20.15.5592-5601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaine TF, Hemraj I, Furic L, Deitinghoff A, Kiebler MA, DesGroseillers L. Staufen2 isoforms localize to the somatodendritic domain of neurons and interact with different organelles. J Cell Sci. 2002;115:3285–3295. doi: 10.1242/jcs.115.16.3285. [DOI] [PubMed] [Google Scholar]
- Dugre-Brisson S, Elvira G, Boulay K, Chatel-Chaix L, Mouland AJ, DesGroseillers L. Interaction of Staufen1 with the 5′ end of mRNA facilitates translation of these RNAs. Nucleic Acids Res. 2005;33:4797–4812. doi: 10.1093/nar/gki794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandon D, Koch I, Westhof E, Nusslein-Volhard C. RNA-RNA interaction is required for the formation of specific bicoid mRNA 3′ UTR-STAUFEN ribonucleoprotein particles. EMBO J. 1997;16:1751–1758. doi: 10.1093/emboj/16.7.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furic L, Maher-Laporte M, DesGroseillers L. A genome-wide approach identifies distinct but overlapping subsets of cellular mRNAs associated with Staufen1- and Staufen2-containing ribonucleoprotein complexes. RNA. 2008;14:324–335. doi: 10.1261/rna.720308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C, Kim YK, Woeller CF, Tang Y, Maquat LE. SMD and NMD are competitive pathways that contribute to myogenesis: Effects on PAX3 and myogenin mRNAs. Genes & Dev. 2009;23:54–66. doi: 10.1101/gad.1717309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Crespo M, Charli JL, Rosales-Garcia VH, Pedraza-Alva G, Perez-Martinez L. Polyethylenimine improves the transfection efficiency of primary cultures of post-mitotic rat fetal hypothalamic neurons. J Neurosci Methods. 2003;127:179–192. doi: 10.1016/s0165-0270(03)00125-0. [DOI] [PubMed] [Google Scholar]
- Hamdan FF, Percherancier Y, Breton B, Bouvier M. Monitoring protein-protein interactions in living cells by bioluminescence resonance energy transfer (BRET) In: Sibley D, editor. Current protocols in neuroscience. Wiley; New York: 2006. Chap. 5, Unit 5.23. [DOI] [PubMed] [Google Scholar]
- Hitti EG, Sallacz NB, Schoft VK, Jantsch MF. Oligomerization activity of a double-stranded RNA-binding domain. FEBS Lett. 2004;574:25–30. doi: 10.1016/j.febslet.2004.07.080. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Dohmae N, Hirokawa N. Kinesin transports RNA: Isolation and characterization of an RNA-transporting granule. Neuron. 2004;43:513–525. doi: 10.1016/j.neuron.2004.07.022. [DOI] [PubMed] [Google Scholar]
- Kharrat A, Macias MJ, Gibson TJ, Nilges M, Pastore A. Structure of the dsRNA binding domain of E. coli RNase III. EMBO J. 1995;14:3572–3584. doi: 10.1002/j.1460-2075.1995.tb07363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebler MA, Hemraj I, Verkade P, Kohrmann M, Fortes P, Marion RM, Ortin J, Dotti CG. The mammalian staufen protein localizes to the somatodendritic domain of cultured hippocampal neurons: Implications for its involvement in mRNA transport. J Neurosci. 1999;19:288–297. doi: 10.1523/JNEUROSCI.19-01-00288.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Furic L, DesGroseillers L, Maquat LE. Mammalian Staufen1 recruits Upf1 to specific mRNA 3′UTRs so as to elicit mRNA decay. Cell. 2005;120:195–208. doi: 10.1016/j.cell.2004.11.050. [DOI] [PubMed] [Google Scholar]
- Kim YK, Furic L, Parisien M, Major F, DesGroseillers L, Maquat LE. Staufen1 regulates diverse classes of mammalian transcripts. EMBO J. 2007;26:2670–2681. doi: 10.1038/sj.emboj.7601712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Ha J, Kerr K, Macdonald PM. Translational regulation of oskar mRNA by bruno, an ovarian RNA-binding protein, is essential. Cell. 1995;81:403–412. doi: 10.1016/0092-8674(95)90393-3. [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, Kosik KS. Neuronal RNA granules: A link between RNA localization and stimulation-dependent translation. Neuron. 2001;32:683–696. doi: 10.1016/s0896-6273(01)00508-6. [DOI] [PubMed] [Google Scholar]
- Lamontagne B, Tremblay A, Abou Elela S. The N-terminal domain that distinguishes yeast from bacterial RNase III contains a dimerization signal required for efficient double-stranded RNA cleavage. Mol Cell Biol. 2000;20:1104–1115. doi: 10.1128/mcb.20.4.1104-1115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau G, Maher-Laporte M, Topolnik L, Laurent CE, Sossin W, DesGroseillers L, Lacaille JC. Staufen1 regulation of protein synthesis-dependent long-term potentiation and synaptic function in hippocampal pyramidal cells. Mol Cell Biol. 2008;28:2896–2907. doi: 10.1128/MCB.01844-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire PA, Lary J, Cole JL. Mechanism of PKR activation: Dimerization and kinase activation in the absence of double-stranded RNA. J Mol Biol. 2005;345:81–90. doi: 10.1016/j.jmb.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Liu J, Hu JY, Wu F, Schwartz JH, Schacher S. Two mRNA-binding proteins regulate the distribution of syntaxin mRNA in Aplysia sensory neurons. J Neurosci. 2006;26:5204–5214. doi: 10.1523/JNEUROSCI.4917-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde BM, Moore C, Varani G. RNA-binding proteins: Modular design for efficient function. Nat Rev Mol Cell Biol. 2007;8:479–490. doi: 10.1038/nrm2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Duchaine TF, DesGroseillers L. Molecular mapping of the determinants involved in human Staufen-ribosome association. Biochem J. 2002;365:817–824. doi: 10.1042/bj20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallardo M, Deitinghoff A, Muller J, Goetze B, Macchi P, Peters C, Kiebler MA. Isolation and characterization of Staufen-containing ribonucleoprotein particles from rat brain. Proc Natl Acad Sci. 2003;100:2100–2105. doi: 10.1073/pnas.0334355100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marianayagam NJ, Sunde M, Matthews JM. The power of two: Protein dimerization in biology. Trends Biochem Sci. 2004;29:618–625. doi: 10.1016/j.tibs.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Marion RM, Fortes P, Beloso A, Dotti C, Ortin J. A human sequence homologue of Staufen is an RNA-binding protein that is associated with polysomes and localizes to the rough endoplasmic reticulum. Mol Cell Biol. 1999;19:2212–2219. doi: 10.1128/mcb.19.3.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel C, Macchi P, Furic L, Kiebler MA, DesGroseillers L. Staufen1 is imported into the nucleolus via a bipartite nuclear localization signal and several modulatory determinants. Biochem J. 2006;393:245–254. doi: 10.1042/BJ20050694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Ephrussi A. mRNA localization: Gene expression in the spatial dimension. Cell. 2009;136:719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micklem DR, Adams J, Grunert S, St Johnston D. Distinct roles of two conserved Staufen domains in oskar mRNA localization and translation. EMBO J. 2000;19:1366–1377. doi: 10.1093/emboj/19.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen M, Putz U, Rehbein M, Schweizer M, DesGroseillers L, Kuhl D, Richter D, Kindler S. Two rat brain staufen isoforms differentially bind RNA. J Neurochem. 2001;76:155–165. doi: 10.1046/j.1471-4159.2001.00061.x. [DOI] [PubMed] [Google Scholar]
- Monshausen M, Rehbein M, Richter D, Kindler S. The RNA-binding protein Staufen from rat brain interacts with protein phosphatase-1. J Neurochem. 2002;81:557–564. doi: 10.1046/j.1471-4159.2002.00887.x. [DOI] [PubMed] [Google Scholar]
- Nanduri S, Rahman F, Williams BR, Qin J. A dynamically tuned double-stranded RNA binding mechanism for the activation of antiviral kinase PKR. EMBO J. 2000;19:5567–5574. doi: 10.1093/emboj/19.20.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perroy J, Adam L, Qanbar R, Chenier S, Bouvier M. Phosphorylation-independent desensitization of GABA(B) receptor by GRK4. EMBO J. 2003;22:3816–3824. doi: 10.1093/emboj/cdg383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters GA, Hartmann R, Qin J, Sen GC. Modular structure of PACT: Distinct domains for binding and activating PKR. Mol Cell Biol. 2001;21:1908–1920. doi: 10.1128/MCB.21.6.1908-1920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A, Grunert S, Adams J, Micklem DR, Proctor MR, Freund S, Bycroft M, St Johnston D, Varani G. RNA recognition by a Staufen double-stranded RNA-binding domain. EMBO J. 2000;19:997–1009. doi: 10.1093/emboj/19.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AJ, Czaplinski K, Condeelis JS, Singer RH. Mechanisms and cellular roles of local protein synthesis in mammalian cells. Curr Opin Cell Biol. 2008;20:144–149. doi: 10.1016/j.ceb.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter JM, Schultz SC. Molecular basis of double-stranded RNA-protein interactions: Structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17:7505–7513. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossin WS, DesGroseillers L. Intracellular trafficking of RNA in neurons. Traffic. 2006;7:1581–1589. doi: 10.1111/j.1600-0854.2006.00500.x. [DOI] [PubMed] [Google Scholar]
- St Johnston D, Beuchle D, Nusslein-Volhard C. Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell. 1991;66:51–63. doi: 10.1016/0092-8674(91)90138-o. [DOI] [PubMed] [Google Scholar]
- Stefan E, Aquin S, Berger N, Landry CR, Nyfeler B, Bouvier M, Michnick SW. Quantification of dynamic protein complexes using Renilla luciferase fragment complementation applied to protein kinase A activities in vivo. Proc Natl Acad Sci. 2007;104:16916–16921. doi: 10.1073/pnas.0704257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente L, Nishikura K. RNA binding-independent dimerization of adenosine deaminases acting on RNA and dominant negative effects of nonfunctional subunits on dimer functions. J Biol Chem. 2007;282:16054–16061. doi: 10.1074/jbc.M611392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JP, Macchi P, Stein JM, Mikl M, Hawker KN, Vogelsang P, Wieczorek K, Vendra G, Riefler J, Tubing F, et al. A loss of function allele for murine Staufen1 leads to impairment of dendritic Staufen1-RNP delivery and dendritic spine morphogenesis. Proc Natl Acad Sci. 2008;105:16374–16379. doi: 10.1073/pnas.0804583105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villace P, Marion RM, Ortin J. The composition of Staufen-containing RNA granules from human cells indicates their role in the regulated transport and translation of messenger RNAs. Nucleic Acids Res. 2004;32:2411–2420. doi: 10.1093/nar/gkh552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham L, Duchaine T, Luo M, Nabi IR, DesGroseillers L. Mammalian staufen is a double-stranded-RNA- and tubulin-binding protein which localizes to the rough endoplasmic reticulum. Mol Cell Biol. 1999;19:2220–2230. doi: 10.1128/mcb.19.3.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]