Abstract
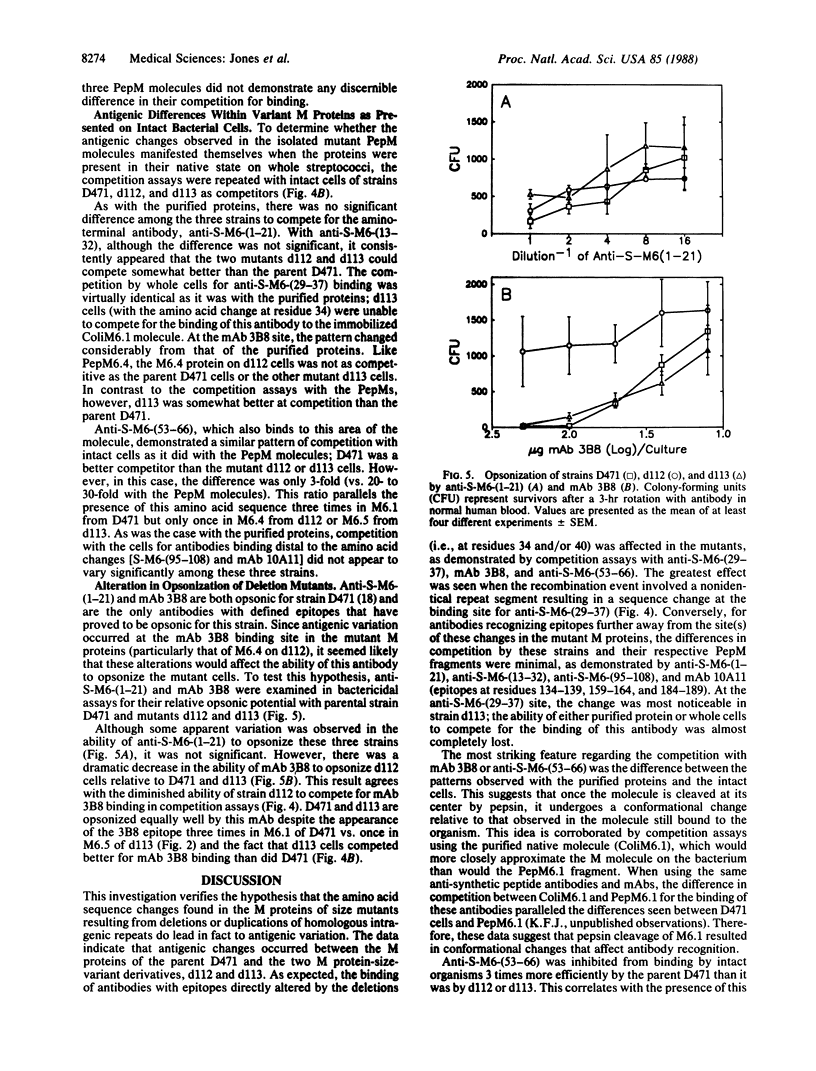
Deletions of highly, but not entirely, homologous intragenic sequence repeats result in amino acid sequence and conformational changes in the M proteins of spontaneous M protein-size variants of group A streptococci. To determine if antigenic changes occurred as a result of these deletion mutations, monoclonal and polyclonal antibodies with defined epitopes were used in competition assays. Competing antigens were either purified pepsin-derived fragments (representing the amino-terminal half of the molecule) of parent and mutant M proteins or were intact bacterial cells. These assays showed that antigenic variation occurred at the site(s) of these deletions but not at adjacent or distant epitopes. Once cleaved from the bacterium by pepsin, the M molecules also underwent conformational changes, which were reflected in their ability to compete. A monoclonal antibody opsonic for M6 streptococci lost its ability to completely opsonize one of the size mutants in this study. Therefore, spontaneous intragenic events between repeats within emm-6, the structural gene for the M6 protein, do result in structural variations within the mutant M molecules. This variation alters the ability of certain antibodies, originally produced in response to sequences in the parental M molecule, to bind to the mutant M molecules or opsonize the mutant organisms. Group A streptococci have evolved a mechanism for generating antigenic diversity that differs from currently known mechanisms in other bacterial species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H., Seyer J. M., Kang A. H. Primary structure of protective antigens of type 24 streptococcal M protein. J Biol Chem. 1980 Jul 10;255(13):6284–6289. [PubMed] [Google Scholar]
- Beachey E. H., Seyer J. M. Protective and nonprotective epitopes of chemically synthesized peptides of the NH2-terminal region of type 6 streptococcal M protein. J Immunol. 1986 Mar 15;136(6):2287–2292. [PubMed] [Google Scholar]
- Beachey E. H., Stollerman G. H., Chiang E. Y., Chiang T. M., Seyer J. M., Kang A. H. Purification and properties of M protein extracted from group A streptococci with pepsin: covalent structure of the amino terminal region of type 24 M antigen. J Exp Med. 1977 Jun 1;145(6):1469–1483. doi: 10.1084/jem.145.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell T. D., Black W. J., Kawula T. H., Barritt D. S., Dempsey J. A., Kverneland K., Jr, Stephenson A., Schepart B. S., Murphy G. L., Cannon J. G. Recombination among protein II genes of Neisseria gonorrhoeae generates new coding sequences and increases structural variability in the protein II family. Mol Microbiol. 1988 Mar;2(2):227–236. doi: 10.1111/j.1365-2958.1988.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Gotschlich E. C., Siviglia G., Zabriskie J. B. Streptococcal M protein: an antiphagocytic molecule assembled on the cell wall. J Infect Dis. 1977 Aug;136 (Suppl):S222–S233. doi: 10.1093/infdis/136.supplement.s222. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Jarymowycz M., Jones K. F., Scott J. R. Streptococcal M protein size mutants occur at high frequency within a single strain. J Exp Med. 1986 Oct 1;164(4):971–980. doi: 10.1084/jem.164.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Manjula B. N., Scott J. R. Streptococcal M6 protein expressed in Escherichia coli. Localization, purification, and comparison with streptococcal-derived M protein. J Exp Med. 1984 Apr 1;159(4):1083–1095. doi: 10.1084/jem.159.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Jones K. F., Scott J. R. Size variation of the M protein in group A streptococci. J Exp Med. 1985 Jun 1;161(6):1384–1401. doi: 10.1084/jem.161.6.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Parry D. A., Trus B. L., Hollingshead S. K., Scott J. R., Manjula B. N. Conformational characteristics of the complete sequence of group A streptococcal M6 protein. Proteins. 1988;3(1):60–69. doi: 10.1002/prot.340030106. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Size variation in group A streptococcal M protein is generated by homologous recombination between intragenic repeats. Mol Gen Genet. 1987 May;207(2-3):196–203. doi: 10.1007/BF00331578. [DOI] [PubMed] [Google Scholar]
- Jones K. F., Fischetti V. A. The importance of the location of antibody binding on the M6 protein for opsonization and phagocytosis of group A M6 streptococci. J Exp Med. 1988 Mar 1;167(3):1114–1123. doi: 10.1084/jem.167.3.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. F., Khan S. A., Erickson B. W., Hollingshead S. K., Scott J. R., Fischetti V. A. Immunochemical localization and amino acid sequences of crossreactive epitopes within the group A streptococcal M6 protein. J Exp Med. 1986 Oct 1;164(4):1226–1238. doi: 10.1084/jem.164.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. F., Manjula B. N., Johnston K. H., Hollingshead S. K., Scott J. R., Fischetti V. A. Location of variable and conserved epitopes among the multiple serotypes of streptococcal M protein. J Exp Med. 1985 Mar 1;161(3):623–628. doi: 10.1084/jem.161.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandke K. M., Fairwell T., Acharya A. S., Trus B. L., Manjula B. N. Complete amino acid sequence of streptococcal PepM49 protein, a nephritis-associated serotype. Conserved conformational design among sequentially distinct M protein serotypes. J Biol Chem. 1988 Apr 15;263(11):5075–5082. [PubMed] [Google Scholar]
- Koomey M., Gotschlich E. C., Robbins K., Bergström S., Swanson J. Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics. 1987 Nov;117(3):391–398. doi: 10.1093/genetics/117.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANCEFIELD R. C. Current knowledge of type-specific M antigens of group A streptococci. J Immunol. 1962 Sep;89:307–313. [PubMed] [Google Scholar]
- LANCEFIELD R. C. Persistence of type-specific antibodies in man following infection with group A streptococci. J Exp Med. 1959 Aug 1;110(2):271–292. doi: 10.1084/jem.110.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula B. N., Acharya A. S., Mische S. M., Fairwell T., Fischetti V. A. The complete amino acid sequence of a biologically active 197-residue fragment of M protein isolated from type 5 group A streptococci. J Biol Chem. 1984 Mar 25;259(6):3686–3693. [PubMed] [Google Scholar]
- Manjula B. N., Fischetti V. A. Studies on group A streptococcal M-proteins: purification of type 5 M-protein and comparison of its amino terminal sequence with two immunologically unrelated M-protein molecules. J Immunol. 1980 Jan;124(1):261–267. [PubMed] [Google Scholar]
- Mouw A. R., Beachey E. H., Burdett V. Molecular evolution of streptococcal M protein: cloning and nucleotide sequence of the type 24 M protein gene and relation to other genes of Streptococcus pyogenes. J Bacteriol. 1988 Feb;170(2):676–684. doi: 10.1128/jb.170.2.676-684.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips G. N., Jr, Flicker P. F., Cohen C., Manjula B. N., Fischetti V. A. Streptococcal M protein: alpha-helical coiled-coil structure and arrangement on the cell surface. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent S. J., Beachey E. H., Corbett C. E., Dale J. B. Sequence of protective epitopes of streptococcal M proteins shared with cardiac sarcolemmal membranes. J Immunol. 1987 Aug 15;139(4):1285–1290. [PubMed] [Google Scholar]
- Scott J. R., Pulliam W. M., Hollingshead S. K., Fischetti V. A. Relationship of M protein genes in group A streptococci. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1822–1826. doi: 10.1073/pnas.82.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Hsu K. C., Gotschlich E. C. Electron microscopic studies on streptococci. I. M antigen. J Exp Med. 1969 Nov 1;130(5):1063–1091. doi: 10.1084/jem.130.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Robbins K., Barrera O., Corwin D., Boslego J., Ciak J., Blake M., Koomey J. M. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987 May 1;165(5):1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]


