Abstract
Beta-adrenergic receptors (β-ARs) critically modulate long-lasting synaptic plasticity and long-term memory storage in the mammalian brain. Synaptic plasticity is widely believed to mediate memory storage at the cellular level. Long-term potentiation (LTP) is one type of synaptic plasticity that has been linked to memory storage. Activation of β-ARs can enhance LTP and facilitate long-term memory storage. Interestingly, many of the molecular signaling pathways that are critical for β-adrenergic modulation of LTP mirror those required for the persistence of memory. In this article, we review the roles of signaling cascades and translation regulation in enabling β-ARs to control expression of long-lasting LTP in the rodent hippocampus. These include the cyclic-AMP/protein kinase-A (cAMP-PKA) and extracellular signal-regulated protein kinase cascades, two key pathways known to link transmitter receptors with translation regulation. Future research directions are discussed, with emphasis on defining the roles of signaling complexes (e.g. PSD-95) and glutamatergic receptors in controlling the efficacy of β-AR modulation of LTP.
Keywords: beta-adrenergic receptor, synaptic plasticity, long-term potentiation (LTP), hippocampus
1. Introduction
A major goal of neuroscience research is to determine how enduring memories are made. Such knowledge would enhance therapies for memory disorders and illuminate many key brain functions that rely on enduring memories. Activity-induced changes in synaptic strength (“synaptic plasticity”) are widely believed to underlie memory storage at the cellular level [1,2]. Research has established the principle that synaptic plasticity is critical for associative learning and long-term memory [3,4]. In the mammalian hippocampus, a brain structure critical for making new memories, one form of synaptic plasticity, called “long-term potentiation” (LTP) [5], has been linked to spatial and contextual learning and memory [1,6]. LTP is an activity-dependent increase in excitatory synaptic strength that can last for several hours in isolated brain slice preparations and up to a year in intact animals [7,8]. Many key signaling requirements for LTP (e.g. NMDA receptors, protein kinase-A, calcium-dependent protein kinases) mirror those needed for memory storage in the mammalian brain [6,9-15] . Many manipulations that modify LTP also alter memory expression. Importantly, LTP-like changes in synaptic efficacy can occur in behaving animals as they learn [4]. Since its discovery by Tim Bliss and Terje Lomo [5], LTP has become the leading candidate synaptic mechanism for memory storage in the mammalian brain.
Many neuromodulatory transmitters control the endurance of LTP and memory. One neuromodulator that can significantly enhance hippocampal LTP stability is noradrenaline (NA). NA fibres originate mainly in the locus coeruleus (LC) [16]. LC projects widely throughout the forebrain, providing dense innervation to the hippocampus, amygdala, and thalamus [16]. As such, NA can influence many key brain functions such as attention, arousal, sleep, learning, and memory. The hippocampus is richly innervated by noradrenergic fibres from the LC, and endogenous release of NA can induce persistent synaptic plasticity [17,18]. Interestingly, hippocampus-dependent memory is impaired following reduction of NA or after blockade of β-ARs [19,20]. In the hippocampus, NA binds to β-ARs to enhance the endurance of LTP and promote stability of memories. However, the signaling mechanisms that enable β-ARs to enhance the longevity of LTP are unclear. Because LTP has been strongly correlated with memory storage, understanding how β-ARs modulate the persistence of LTP will shed light on how these receptors regulate memory storage. In this review, we highlight several signaling mechanisms believed to enable β-ARs to enhance the expression of long-lasting forms of hippocampal LTP. We focus on hippocampal β-ARs because of the important roles of these receptors in enhancing LTP and memory. α-ARs are not covered here; their roles in hippocampal synaptic plasticity and memory storage are reviewed elsewhere [21].
2. β-AR Signaling: Importance for Synaptic Plasticity in the Mammalian Hippocampus
NA binds to noradrenergic receptors coupled to G-proteins that initiate intracellular signaling. These can be broadly classified as α1-, α2-, β1-, and β2-ARs [21]. In the hippocampus, all four of these receptor subtypes are expressed in pyramidal neurons and dentate granule cells [22,23]. β-ARs are also expressed outside of the hippocampus, mainly in the cortex, thalamus, and cerebellum [22,24]. Interneurons apparently express very few or no β-ARs [22,23], and glia in area CA1 express mainly β2-ARs [25]. Both β1- and β2-ARs are strongly activated by the noradrenergic agonist, isoproterenol [16]. Stimulation of β-ARs, either with isoproterenol or NA, generally increases intracellular cAMP through Gs-mediated activation of adenylyl cyclase [26].
It has been shown that β-ARs importantly modulate numerous processes involved in synaptic plasticity through cAMP-mediated activation of cAMP-dependent protein kinase (PKA) [29]. cAMP-dependent activation of PKA also recruits other key signaling pathways in hippocampal cells, including the extracellular signal-regulated protein kinase (ERK) pathway via Rap1 (a GTPase) and B-Raf (a protein kinase) [30]. Specifically, Rap1 is involved in cAMP-dependent ERK activation by β2-ARs [30].
Other more recently discovered mechanisms may diversify signaling targets downstream of β-ARs. Interestingly, studies of non-neuronal cells have revealed that, under certain conditions, β2- [27] and β1-ARs [28] may switch their signaling mode from Gs to Gi. This switch appears to be mediated by Gβγ subunits, Src, Ras, and c-Raf1 [31]. It also requires previous phosphorylation of the β-AR by PKA, suggesting that desensitization of the receptor to Gs-dependent signaling may enable Gi signaling. β-AR-dependent Gi signaling in cell lines and in vivo can activate ERK [32-34]. Furthermore, the signaling mode of β-ARs is dynamically regulated, as recruitment of specific phosphodiesterases to the β-AR signaling complex can decrease receptor phosphorylation and subsequently prevent further signaling through the Gi pathway [35]. It is unclear whether such switching can occur in hippocampal neurons, and if so, how it might impact synaptic plasticity.
3. Regulatory control of β-AR signalling
Intracellular signaling events engaged by β-AR activation have several regulatory feedback mechanisms that serve to both prevent activation of downstream effectors in the absence of β-AR agonists and amplify β-AR-dependent responses by compartmentalizing second messenger signalling [36]. Phosphodiesterases (PDEs) are the primary enzymes for cAMP degradation and can constrain β-AR -dependent cellular responses. The primary PDE isoform in the CNS is PDE4 and regulation of β-AR desensitization is mediated through various PDE4 isoforms, which in turn are regulated through phosphorylation by kinases including PKA and ERK [36,37]. PKA phosphorylates PDE4, which hydrolyzes cAMP, thereby restricting cAMP activity in a negative feedback mechanism [36,37]]. However, particular PDE4 isoforms mediate their effects through the presence of particular regulatory domains known as upstream conserved regions (UCRs) which contain PKA and ERK binding sites [36]. Long isoforms of PDE4 contain both UCR1 and UCR2 . UCR1 contains a PKA binding site which allows for the activation of PDE4 in the presence of PKA and subsequent downregulation of cAMP [36]. Conversely, ERK binding to the UCR1 inhibits PDE4 thereby preventing PDE4-dependent β-AR desensitization. ERK activates and has no effect respectively, on the so-called short (lacks UCR1, contains UCR2) and super-short (lacks UCR1, truncated UCR2) PDE4 isoforms [36,37]. Thus, activation of β-AR s and subsequent induction of PKA and ERK can both facilitate and limit cellular responses depending on the presence of specific PDE4 isoforms which play dynamic regulatory roles in β-AR -dependent signaling cascades.
As excessive cAMP signaling may contribute to the pathogenesis of several disease states, PDE inhibitors are potentially useful therapeutic agents for the treatment of conditions such as cancer, inflammatory (asthma, chronic obstructive pulmonary disease, arthritis) diseases, depression and neurodegenerative disorders [36-39]. Interestingly, increased expression of PDE4 has been observed following chronic administration of antidepressants, with the high-affinity conformer involved in the effects of antidepressants that target the NA and 5-HT systems [38]. Furthermore, sleep deprivation, which has been implicated in the pathogenesis of depression and cognitive dysfunction, increases the activity of PDE4, which correlates with impaired cAMP- and PKA-dependent synaptic plasticity and memory formation in sleep-deprived mice[40]. These results suggest that cellular mechanisms required for normal cognitive function and synaptic plasticity are influenced by PDE4 activity and that PDEs may regulate information processing in the CNS.
Inhibition of PDEs enhances LTP, learning and memory processes [40-42]. Application of rolipram, a selective inhibitor of PDE4, to hippocampal slices facilitates the induction of late-LTP by subthreshold stimulation paired with forskolin activation [43]. Rolipram also facilitates heterosynaptic long-term depression [41]. Additionally, synaptic plasticity and context-specific memory can be restored by rolipram treatment following sleep deprivation [40], and long-term contextual memory formation is improved in mice if rolipram is administered prior to training in a hippocampus-dependent task [43]. Results from animal models and preclinical studies indicate that Alzheimer's disease symptoms, such as dementia and memory loss , can be alleviated by PDE inhibitor treatments [39].
β-arrestin, a multifunctional adapter protein, can also initiate intracellular signaling events downstream of the β-AR in a G-protein-independent manner [44,45]. Although β-arrestin mediates termination of G-protein dependent receptor signaling by physically uncoupling the receptor from G-proteins, it generates a second wave of signaling that has a distinct temporal profile and subcellular downstream targets compared to the original receptor response [44,46]. In HEK cell culture, stimulation of β1-ARs initiates β-arrestin-dependent signaling and consequent sustained ERK activation that is restricted to the cytosol [45]. This subcellular targeting may be mediated by ubiquitination of β-arrestin, which leads to rapid internalization of β-ARs and formation of a signaling complex (‘signalosome’) [47]. As such, diverse signaling mechanisms can be recruited downstream of β-ARs, and the specifics of these interactions are still being elucidated. The role of such signaling mechanisms in hippocampal neurons is unknown, but it is likely that similar mechanisms exist in neurons to facilitate compartmentalization and temporal restriction of signaling.
PDEs interact with membrane-bound scaffolding proteins such as β-arrestin, AKAPs and RACK1, which can compete for access to PDE4 isoforms, thus modulating β-AR signaling [36,48]. Importantly, PDEs can interact with β-arrestin in a β-AR -subtype specific manner [49,50]. In cardiomyocytes, inactive β 1ARs complex directly with PDE4D8 which dissociates in the presence of β-AR agonists, whereas stimulation of β2ARs recruits a β-arrestin-PDE45 complex to the β2AR [49]. Sequestering this complex prevents β2ARs from switching to Gi signaling, thereby preventing β2AR desensitization [35,50]. Furthermore, β -arrestin is necessary for recruiting PDE4D5, as selective knockdown of β -arrestin in HEK cells diminishes PDE4D5 sequestration thereby enhancing β -AR desensitization [35]. The ability of β -arrestin to influence receptor conformation and G protein interactions provides potential targets for therapeutic intervention.
Dysfunction of the dopaminergic (DA) system is involved in the pathogenesis of schizophrenia. Several antipsychotics have been found to mediate their effects through β-arrestin-2 which regulates DA signal transduction [51]. Recent evidence suggests that the D2R agonist quinpirole may act by blocking D2R-β-arrestin-2 interactions, thus facilitating Gi/o coupling [52]. β-arrestin-2 also plays a role in another DA-linked pathology, drug addiction. β-arrestin-2 knock-out mice displayed increased striatal extracellular DA release in response morphine administration, which correlated with enhanced conditioned place preference, indicative of enhanced reward experience[42]. These results suggest that β -arrestins provide another mechanism in the regulation of GPCRs which can significantly impact cellular responses and behavioural output by modulating intracellular signaling.
Despite the multitude of signaling mechanisms potentially activated by β-ARs, PKA and ERK are frequently identified as key downstream signaling kinases. PKA and ERK are critical for establishing enduring memories and long-term synaptic plasticity in numerous species, including mammals [3,29,53]. The coupling of β-ARs to these critical signaling pathways may explain the enhancing effects of NA on hippocampal synaptic plasticity and memory. In contrast, α-ARs mediate the inhibitory effects of NA on hippocampal neurons; activation of α-ARs has mixed effects on memory [21]. Thus, to understand how NA enhances synaptic plasticity and memory storage, attention must be primarily paid to β-ARs.
4. Modulation of Excitability by Hippocampal β-ARs
Neuromodulators can affect neuronal ability to undergo synaptic plasticity by changing cellular excitability. Activation of β-ARs generally increases the excitability of principal neurons in the dentate gyrus, area CA3, and area CA1 of the rodent hippocampus. In the dentate gyrus, application of either NA or a β-adrenergic agonist such as isoproterenol induces pathway-specific changes in cellular excitability. β-AR-mediated enhancement of the population spike is observed in the medial perforant path, whereas β-AR-mediated depression is seen in the lateral perforant path [54]. These pathways are histochemically and anatomically distinct, suggesting that differential effects of NA in this subregion may be important for selective information processing [54,55]. β1-ARs also enhance potentiation of the pyramidal cell population spike in areas CA3 and CA1 [56-59]. This increased cellular excitability amplifies the frequency of spontaneous firing in area CA3 [59], potentially facilitating the auto-associative properties of this hippocampal subregion [60].
5. β-AR Modulation of Hippocampal LTP
Highly significant events are easily remembered, often for an entire lifetime. There is evidence to suggest that the physiologic mechanism underlying this retention is related to activation of the brain's noradrenergic system, which promotes plasticity in brain structures that mediate enduring behavioral adaptations[61,62]. A plausible cellular mechanism for enhancement of hippocampal memory by β-ARs is facilitation of enduring LTP by β-ARs. This phenomenon can be studied in vitro by inducing LTP in the presence of β-AR agonists. Long-lasting forms of LTP are induced in hippocampal slices by repeated high-frequency stimulation (HFS, usually 3-4 100-Hz trains), they can last 6-12 hrs, and require translation (protein synthesis) for their stability [3,29,63]. Shorter-lasting LTP is commonly induced by weaker HFS (usually one 100-Hz train), lasts about 2 hrs, and does not require translation [3]. The effect of β-AR activation on LTP is different depending on the hippocampal subregion examined.
5.1 Dentate Gyrus
In the dentate gyrus, β-AR activation is required for LTP generated by HFS [64,65], and blockade of these receptors prevents induction of LTP by electrical stimulation in the medial and lateral perforant paths [65]. However, β-AR blockade inhibits only HFS-induced potentiation of the excitatory postsynaptic potential (EPSP), without affecting potentiation of the population spike (i.e. cellular excitability) [64]. Distinct mechanisms likely underlie potentiation of synaptic strength and cellular excitability.
Interestingly, application of NA or β-AR agonists without electrical stimulation induces long-lasting potentiation of EPSPs in the medial perforant path, and long-lasting depression of EPSPs in the lateral perforant path [54,66]. This plasticity requires activation of N-methyl-D-aspartate (NMDA) receptors, but not electrical activation of afferent neurons [66]. Taken together, these findings suggest that the role of β-ARs on synaptic plasticity in vitro is affected by the type of stimulation applied.
In vivo studies were therefore performed to clarify the role of β-ARs in the dentate gyrus during physiologic stimulation patterns. Initial in vivo studies did not find alterations in synaptic strength in response to NA or LC activation [67-69], in contrast to the marked effects of NA on EPSPs in vitro. However, it was subsequently found that stimulation of the LC potentiates EPSPs in the dentate gyrus at 24 hours, but not 3 hours, after stimulation [18]. NA may therefore selectively enhance long-term, but not short-term plasticity in vivo. Similarly, activation of the basolateral amygdala causes a β-AR-mediated increase in LTP maintenance in the dentate gyrus [17]. This enduring potentiation requires new protein synthesis [17,18], a key characteristic of stable forms of LTP and long-term memory [70-72].
5.2 Area CA3
LTP in area CA3 is β-AR-dependent. Blockade of β-receptors during HFS prevents early and late phases of LTP [73], and stimulation of β-ARs generates a frequency-dependent increase in the magnitude, duration and induction probability of LTP [74,75]. β-AR activation elicits long-lasting LTP when paired with stimulation protocols that normally induce short-lasting LTP [73]. However, activation of β-ARs during weaker, low-frequency electrical stimulation (LFS) of mossy fibre synapses has little effect on synaptic strength [74,75]. Similarly, pairing β-AR activation with LFS at associational-commissural CA3 synapses does not induce plasticity [76]. In this hippocampal subregion, β-AR activation can modulate properties of LTP, but cannot increase synaptic strength without concurrent HFS.
The mechanism for this modulation of LTP is thought to be presynaptic [73], consistent with studies demonstrating that HFS-induced LTP and forskolin-induced LTP are also presynaptically mediated in area CA3 [77-79]. Endogenous NA could increase excitatory transmitter release from mossy fibre presynaptic terminals to enhance initial expression of LTP [73].
5.3 Area CA1
Unlike other hippocampal subregions, β-ARs in area CA1 are not required for the induction of LTP by HFS [20,80-82]. Activation of β-ARs by application of the agonist, isoproterenol, during multiple trains of strong HFS generates long-lasting LTP that does not differ in either induction or maintenance properties from LTP elicited by HFS alone [83]. Similarly, activation of β-ARs alone does not persistently alter basal synaptic strength in area CA1 [84,85]. However, β-AR activation significantly modulates synaptic strength when coupled with various patterns of weaker electrical stimulation. The signaling pathways underlying this modulation in area CA1 are beginning to be elucidated.
LFS applied to area CA1 produces a transient depression of synaptic strength, whereas pairing LFS with β-AR activation generates robust LTP (termed “β-LTP”) [76,84-86]. Induction of β-LTP requires activation of multiple signaling cascades, including PKA, mammalian target of rapamycin (mTOR), PI3-kinase, and ERK [85,87-90]. These signaling cascades may independently contribute to β-LTP induction, but their specific mechanisms of action in this process are unknown. Growing evidence suggests, however, that ERK and PKA-mediated changes in CA1 pyramidal cell excitability are likely to play an important role in the enhancement of LFS-induced LTP by β-AR activation [90].
The induction of LTP by low-frequency patterns of presynaptic fiber stimulation is critically dependent on postsynaptic complex spike bursting [91], a characteristic form of action potential generation seen in CA1 pyramidal cells in vivo. This suggests that postsynaptic action potentials triggered in the soma and backpropagating into dendrites provide the postsynaptic depolarization needed for NMDA receptor activation and LTP induction. The ability of postsynaptic action potentials to provide the membrane depolarization needed for NMDA receptor activation is limited, however, by the progressive attenuation of backpropagating action potentials that occurs as spikes propagate away from the soma and through the dendrite [92-94]. In large part this appears to result from the progressive increase in the density of A-type potassium channels (Kv4.2) with distance from the soma [94]. Interestingly, ERK activation downstream of β-ARs increases phosphorylation of Kv4.2 potassium channels, inhibits A-type potassium channel activity, and strongly facilitates the amplitude of backpropagating action potentials in pyramidal cell dendrites [95,96]. Together, these findings suggest that increases in dendritic excitability due to decreased A-type potassium channel activity in pyramidal cell dendrites play a crucial role in the enhancement of LFS-induced LTP by β-AR activation. Consistent with this, β-AR activation enhances complex spike bursting in CA1 pyramidal cells during low frequency trains of presynaptic fiber stimulation in an ERK-dependent manner [87,90].
Recent studies suggest, however, that the ability of β-AR activation to enhance LTP induction not only arises through modulation of A-type K+ channels but may also importantly involve alterations in the activity of other types of dendritic K+ channels. For example, small conductance, Ca2+-activated K+ channels (SK2 channels) are present in dendritic spines of CA1 pyramidal cells where they can exert a powerful influence on spine depolarization and NMDA receptor activation [97] (see [98] for review). Dendritic spine SK channels appear to be primarily activated by increases in spine calcium following activation of voltage-dependent, CaV2.3 (R-type) calcium channels that are also present in dendritic spines [99]; this is a remarkable example of the importance of microdomains in spine calcium signaling. The resulting increase in K+ conductance limits spine depolarization during synaptic transmission and thus inhibits NMDA receptor activation by opposing the voltage-dependent relief of the Mg2+ block of NMDA receptor channels. PKA activation strongly reduces cell surface expression of SK2 channels [100,101] suggesting that activation of PKA and Inhibition of SK2 channel activity may be a mechanism whereby β-AR agonists facilitate the induction of LTP. Consistent with this, β-AR activation elicits a PKA-mediated loss of SK2 channels in dendritic spines of amygdalar neurons, suggesting that this may be an important mechanism underlying the ability of β-AR activation to enhance induction of LTP at excitatory synapses on principal cells in the amygdala [102]. It remains to be determined, however, whether a similar mechanism might contribute to the modulatory effects of β-AR activation on LTP induction in the hippocampal CA1 region.
In addition to effects mediated by modulation of voltage-activated channels, β-AR activation may also enhance LTP through modulation of ligand-gated ion channels for glutamate. NMDA and AMPA-type glutamate receptors are phosphorylated by PKA (see [103,104] for review) and thus are potential targets for modulation by β-AR activation. Although the potential role of β-AR modulation of NMDA receptors in LTP has not been investigated, PKA phosphorylation of NMDA receptors enhances calcium influx through these receptors [105], suggesting that β-AR activation could facilitate LTP through direct effects on NMDA receptor activity. In contrast, a growing number of studies indicate that modulation of AMPA receptor function may have a central role in the facilitatory effects of β-AR activation on LTP induction.
AMPA-type glutamate receptors are heteromeric proteins comprised of four subunits named GluR1-GluR4. The GluR1 subunit, which is thought to have a crucial role in LTP [106-108], is phosphorylated by a number of different protein kinases, including PKA [109-111]. Phosphorylation of GluR1 at its PKA site (serine 845 in the intracellular c-terminal domain of the subunit) not only enhances the mean open time of AMPA receptor ion channels [112] but it also has potent effects on AMPAR trafficking [113,114] and facilitates the insertion of GluR1 subunit-containing AMPA receptors into extrasynaptic sites [115]. Once inserted, these extrasynaptic AMPA receptors appear to be primed for synaptic insertion during LTP induction [115,116]. Thus, a key component of the downstream signaling effects underlying the enhancement of LTP by β-AR activation may involve PKA-mediated alterations in AMPA receptor trafficking that increase the size of the pool of AMPA receptors competent for insertion into synapses during LTP induction. Consistent with this notion, β-AR activation induces large increases in GluR1 phosphorylation at S845 in hippocampal neurons [89,117-120]. Moreover, a recent study found that the enhancement of LTP by β-AR activation is abolished at cortical synapses of “knock-in” mutant mice expressing GluR1 subunits where serine 845 is converted to a nonphosphorylatable alanine (S845A) [121]. Noradrenergic enhancement of LFS-induced LTP in the hippocampal CA1 region is also reduced in mice expressing mutant GluR1 subunits that can no longer be phosphorylated at S845 and S831 (a CaMKII and PKC phosphorylation site in GluR1: [120]). Notably, not only is the β-AR modulation of LTP disrupted in these mutants but there is also a striking impairment in the ability of norepinephrine to enhance contextual fear conditioning [120]. This suggests that phosphorylation of AMPA receptor GluR1 subunits has a key role in the ability of β-AR activation to enhance both LTP and behavioral learning.
Importantly, β-AR activation not only facilitates the induction of LTP by modulating the activity of voltage-activated and ligand-gated ion channels, but it also engages downstream mechanisms that modulate key components of the signaling pathways important for LTP induction. For example, low-frequency trains of presynaptic fiber stimulation can not only activate the protein kinases needed for LTP induction but they also appear to activate protein phosphatases, such as protein phosphatase 1 (PP1), that can oppose kinase activity and inhibit LTP induction [85,122]. The activity of PP1 is strongly modulated by PKA phosphorylation of the PP1 regulatory protein inhibitor-1, which when phosphorylated, binds to and inhibits PP1 activity [123]. This provides a potential key point of convergence where β-AR signaling through cAMP and PKA can modulate the calcium-dependent activation of protein phosphatases that normally act to suppress LTP induction. Consistent with this, there is evidence to suggest that pairing activation of β-ARs with LFS overcomes the activation of protein phosphatases (elicited by LFS alone) that can oppose LTP induction [84,85,87]. Moreover, biochemical experiments have demonstrated that inhibitor-1 phosphorylation is increased following β-AR activation in the hippocampal CA1 region [122]. cAMP signaling is implicated in this inhibition of phosphatase activity [122], but the specific kinases involved downstream have not been identified.
Activation of β-AR signaling can also alter the properties of LTP through crosstalk with other intracellular signaling pathways important for the maintenance of LTP. For example, weak HFS protocols generate LTP that is short-lasting and protein synthesis-independent when delivered alone, but they can induce persistent LTP that requires dendritic protein synthesis when paired with β-AR activation [84, 144]. The maintenance of this LTP requires ERK and mTOR, and may be related to the ability of these signaling cascades to upregulate translation initiation at dendrites (see below for further details; [21,84,144]). LTP induced by pairing stimulation of β-ARs with weak HFS does not require PKA signaling, in contrast to the PKA-dependence of LTP induced by pairing β-AR stimulation with LFS [90]. Therefore, activated β-ARs demonstrate differential recruitment of signaling cascades based on specific patterns of electrical stimulation. Furthermore, pairing β-AR activation with a different form of HFS known as theta-burst does not enhance the maintenance of LTP [82]. It is possible that a different combination of signaling pathways is engaged downstream of the β-AR in this case, exemplifying the diverse signaling potential of β-ARs in synaptic plasticity.
The ability of β-AR activation to recruit various signaling pathways that can enhance induction and maintenance of synaptic plasticity may compensate for impairments of LTP generated by other mechanisms. For instance, genetic inhibition of hippocampal PKA activity in transgenic mice leads to impaired LTP maintenance that can be abolished by activation of β-ARs [90]. Several inbred mouse strains that display impaired maintenance of LTP also generate robust LTP when electrical stimulation is paired with activation of β-ARs [83]. The specific mechanism underlying this β-AR-dependent rescue of LTP remains unclear, and may depend on the etiology of the original LTP impairment. As these PKA transgenic, and inbred, mouse strains exhibit impaired hippocampal memory function [6,83], it is possible that β-AR activation in vivo may similarly alleviate these deficits. Thus, β-AR activation holds promise as a pharmacologic strategy for enhancing synaptic plasticity, and possibly, memory function.
Neuromodulators such as NA can influence the ‘state’ of a synapse, altering its response to future stimulation, a process known as “metaplasticity” [124,125]. Application of a β-adrenergic agonist can reduce inhibitory forms of metaplasticity that would normally prevent further LTP induction at previously activated synapses [126,127]. Activation of β-ARs can also lengthen the time window within which independent synaptic inputs can induce associative LTP [128]. Furthermore, concurrent activation of α- and β-ARs prevents the activity-dependent reversal, or depotentiation, of LTP [86]. Taken together, these studies suggest that NA acting through β-ARs can engage metaplastic processes to modulate induction parameters of synaptic plasticity.
6. PKA-Independent β-AR Signalling
Traditionally, cAMP-dependent signaling in hippocampal neurons has been thought to be mediated primarily through PKA (for review, see 29). However, novel cAMP receptors (Epac 1 and 2) also participate in neuronal cAMP-dependent signaling. Epac-dependent, PKA-independent modulation of cellular processes has been demonstrated at the crayfish neuromuscular junction [129], the calyx of Held [130], and in cortical neurons [131]. In the hippocampus, Epac contributes to the forskolin response in cell culture [132], and plays a role in both long-term potentiation [90] and long-term depression [133]. Given the emerging role of Epac as a neuronal signaling molecule that operates alongside PKA to generate a multitude of cAMP-dependent cellular effects, it is possible that β-AR signaling also recruits the Epac pathway.
Indeed, Epac signaling is required for hippocampus-dependent memory retrieval, a process that also requires β1-AR signaling [134,135]. In parallel with the enhancement of long-term memory observed following application of noradrenaline to rodent hippocampus [136], Epac has been found to facilitate long-term memory formation [137]. The details of how the Epac and PKA signaling pathways may interact to generate β-AR-dependent effects on synaptic plasticity and memory remain to be elucidated.
7. Roles of Translational Regulation in β-AR Modulation of LTP
The inhibitory effects of translation inhibitors on LTP can be detected as early as 20 minutes after induction [137-139]. Thus, plasticity-related proteins can be produced rapidly and locally in dendrites after electrical stimulation. Indeed, dendritic expression of some proteins is increased within 5 min. after LTP induction [138]. LTP induced by pairing isoproterenol with one 100-Hz train of HFS (“β-LTP”) requires dendritic translation, but not transcription [84]. Other forms of translation-dependent LTP can be induced by application of brain-derived neurotrophic factor (BDNF), neurotrophin-3, or dopamine to hippocampal slices [140]. Also, pharmacological activation of group-1 metabotropic glutamate receptors (mGluRs) in area CA1 induces translation-dependent long-term depression (LTD) [141]. Thus, activation of multiple receptors, including β-ARs, can induce translation-dependent, long-lasting synaptic plasticity. Because such plasticity may underlie the formation of enduring memories, elucidating how transmitter receptors are coupled to translation is critical for grasping the molecular bases of long-lasting synaptic plasticity and long-term memory.
Cells respond to external stimuli by regulating the translational efficiencies of specific mRNAs. Translation initiation is rate-limiting, and most translational control mechanisms act on initiation [142]. These mechanisms predominantly involve the phosphorylation of eukaryotic initiation factors (eIFs) that help assemble initiation complexes to promote ribosomal binding to mRNAs, a required step for translation initiation [142]. Phosphorylation of many eIFs correlates positively with translation initiation; levels of expression of specific phospho-eIFs are used as measures of translation initiation [142].
One key rate-limiting step during translation initiation for most species of mRNA is formation of the eIF4F initiation complex, which consists of the translation initiation factors eIF4A, 4E, and 4G [142]. The eIF4F complex facilitates binding of the 5’ mRNA cap structure to the ribosome to initiate translation. Formation of the eIF4F complex is possible when 4E is released from its basal state of sequestration by the inhibitory binding protein, 4EBP, and couples with 4G (Fig. 1). Release of eIF4E occurs when 4EBP is phosphorylated by upstream signaling kinases. When 4E is bound to 4G, it can then be phosphorylated by Mnk1 to further upregulate translation initiation. The physiologic significance of this translational control mechanism has been demonstrated by genetic deletion of the predominant neuronal 4EBP isoform (4EBP2) in a mouse model. These mice display increased 4F complex formation in the basal state, and electrical stimuli that generated short-lasting, translation-independent LTP in wildtypes instead induced long-lasting, translation-dependent LTP in the 4EBP2 knockouts [143]. However, a stimulation protocol that generated enduring LTP in wildtype mice failed to elicit long-lasting LTP in 4EBP2 knockout mice. These alterations in synaptic plasticity were paralleled by behavioural observations that 4EBP2 knockout mice have intact short-term, but impaired long-term, memory for hippocampus-dependent memory tasks [143]. Thus, translational control at the level of eIF4F complex formation significantly affects both synaptic plasticity and memory.
Figure 1.
Beta-AR-dependent translation initiation. Synaptic stimulation paired with beta-AR activation (by noradrenaline or isoproterenol) promotes translation initiation through mTOR and ERK signaling pathways. Increased cAMP may activate both PKA and EPAC to recruit the ERK pathway via Rap-1 and B-Raf [21]. ERK activation may engage the Akt-mTOR pathway through RSK. Activation of mTOR phosphorylates and suppresses the eIF4E repressor, 4E-BP. This releases eIF4E, which is then free to bind with eIF4G, which binds to eIF4A. Together, they form the initiation complex eIF4F which initiates cap-dependent translation [140]. ERK increases translation rates through phosphorylation of MnK1 which causes eIF4E to dissociate from the eIF4F initiation complex, allowing eIF4E to engage in further rounds of translation[140]. AC, adenylate cyclase; eIF, eukaryotic initiation factor; EPAC, exchange protein activated by cAMP; MnK1, mitogen-activated protein kinase-interacting kinase-1; RSK, ribosomal protein S6 kinase.
Interestingly, β-LTP involves translational upregulation at the level of the eIF4F complex. Increased levels of 4E, 4EBP and Mnk1 phosphorylation are observed when β-LTP is induced, and manipulations that blocked these specific phosphorylations reduced the persistence of β-LTP [144]. 4F complex formation (measured as 4E-4G binding) was also increased during β-LTP [144]. Increased levels of phosphorylated 4EBP were evident in CA1 pyramidal cell dendrites during β-LTP [144], consistent with a dendritic localization of translational control, which may permit rapid, synapse-specific induction of enduring plasticity. HFS-induced LTP and mGluR-LTD involve enhanced 4EBP phosphorylation as well [140], suggesting that increased translation initiation may allow numerous signaling pathways to induce and modulate persistent synaptic plasticity. HFS-induced LTP is associated with ERK-dependent increases in translational capacity [145], evident as increased expression of components of the translational machinery. It is unclear whether β-LTP similarly increases translational capacity.
The roles of signaling pathways involved in translational control in hippocampal neurons are just beginning to be defined [146,147]. Two key signaling kinases involved in translation initiation via the eIF4F complex have been found to have physiologic significance in the hippocampus – mTOR and ERK (Fig. 1). Activation of the mTOR pathway results in phosphorylation of 4EBP, promoting formation of the 4F initiation complex [148]. The ERK pathway similarly facilitates translation by activating Mnk1, a kinase that phosphorylates 4E once it is bound to 4G. Because ERK-dependent 4E phosphorylation occurs when 4E is released from 4EBP, a process that requires mTOR, these two signaling cascades independently converge at regulation of 4E. Both β-LTP and mGluR-LTD require concomitant ERK and mTOR signaling for upregulation of translation initiation and expression of synaptic plasticity. Recruitment of mTOR and ERK signaling pathways may therefore facilitate precise regulation of translation-dependent synaptic plasticity downstream of various neuromodulatory receptors.
In summary, multiple forms of long-lasting synaptic plasticity, including β-LTP, are associated with regulation of a critical translation factor, eIF4E, by ERK and mTOR. Because similar cascades are also activated during long-term plasticity in the marine snail Aplysia [149,150], these pathways may represent evolutionarily conserved mechanisms for translational control of enduring forms of synaptic plasticity.
8. Prospects for Future Research: Signaling Complexes and Glutamate Receptors
An important principle in intracellular signaling is the key role played by adaptor or scaffolding proteins that couple protein kinases and other signaling molecules near their upstream activators and downstream targets, thereby forming signaling complexes that enable fast, efficient, and highly localized signaling. A prominent family of scaffolding proteins that serves this function for the cAMP/PKA signaling pathway are the A-kinase anchoring proteins (AKAPs), a family of more than 50 proteins that contain a binding domain for the type-II regulatory subunits of PKA as well as protein binding domains that couple PKA-bound AKAPs to different target proteins [151].
One AKAP, known as AKAP79/150, not only binds PKA but also contains binding sites for protein kinase C and the Ca2+-activated protein phosphatase, calcineurin (PP2B), and is now known to have a crucial role in cAMP/PKA signaling at excitatory synapses in the hippocampal CA1 region. AKAP79/150 is localized at excitatory synapses through interactions with the membrane-associated guanylate kinases (MAGUKs) PSD-95 and SAP97 [152], scaffolding proteins that bind to NMDA and AMPA type glutamate receptors, respectively (Fig. 2) [153]. A number of studies using different manipulations to disrupt this AKAP signaling complex have found significant effects on both synaptic transmission and synaptic plasticity that highlight the important role of AKAPs in cAMP signaling at excitatory synapses. For example, disrupting PKA binding to AKAPs with a peptide that blocks RII binding to AKAPs (Ht31 peptide) induces a rundown of AMPA receptor-mediated currents [154,155] and reduces the numbers of AMPA receptors at the cell surface [156]. Moreover, infusion of the Ht31 peptide into postsynaptic CA1 pyramidal cells leads to a depression of excitatory synaptic transmission in CA1 pyramidal cells that “occludes” the induction of LTD by synaptic stimulation [156]. This suggests that disrupting the association of PKA with AKAP79/150 induces a depression of synaptic strength that shares properties with LTD. Indeed, recent studies indicate that AKAP/PSD-95 interactions are crucial for the induction of LTD, most likely because of the ability of AKAP79/150 and PSD-95 to couple synaptic NMDA receptors to PP2B [157]. Although many of these examples highlight the functional significance of AKAP-mediated association of PP2B with NMDA receptors in regulating AMPA receptor function, PKA-dependent forms of LTP are strongly disrupted in AKAP150 mutant mice, as are forskolin-induced increases in GluR1 phosphorylation at S845 [158]. This indicates that AKAP79/150 has a crucial role in cAMP/PKA signaling at excitatory synapses.
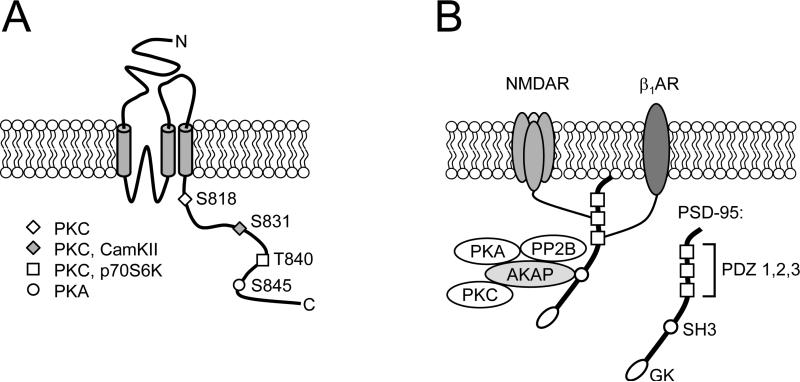
Figure 2.
A. Membrane topology and phosphorylation sites in the intracellular C-terminal domain of AMPA receptor GluR1 subunits. As indicated, S818, S831, and T840 are all phosphorylated by PKC as well as other kinases such as CamKII and p70S6-kinase, whereas S845 is phosphorylated by PKA [103,119,166]. B. Putative signaling complex formed by the scaffolding/adaptor proteins PSD-95 and AKAP79/150. The c-terminus of β1-ARs can interact with the 3rd PDZ domain in PSD-95 [167] whereas the remaining protein binding domains in PSD-95 can couple β1-ARs to NMDA receptors and via AKAPs to PKA, PKC, and PP2B. The inset shows the PSD-95/discs large/zona occludens-1 (PDZ), Src homology 3 (SH3), and guanylate kinase-like (GK) protein binding domains in PSD-95. Note that β1-ARs can also potentially integrate into signaling complexes with AMPA-type glutamate receptors via interactions mediated by AKAP binding to the AMPA receptor-associated MAGUK, SAP97 [152,160].
Are AKAPs equally important for β-AR modulation of LTP? β1-ARs are highly localized at excitatory synapses in the hippocampal CA1 region [22,23,25] and yeast two-hybrid screens have shown that the c-terminus of β1-ARs can bind to one of the three PDZ domains in PSD-95 [159]. This suggests that at excitatory synapses, β1-ARs may exist as part of a larger signaling complex containing PSD-95, NMDA receptors, AKAPs, and associated signaling molecules (Fig. 2). In addition, it also appears likely that β1-ARs can form signaling complexes with AMPA receptors, AKAPs, and PKA, mediated by the MAGUK, SAP97 [160]. PSD-95 can also bind to β2-ARs and, via interactions mediated by the transmembrane AMPA receptor regulatory proteins (TARPs), couple these β-ARs to synaptic AMPA receptors [161]. Although the potential role of these signaling complexes in β-AR modulation of LTP has not yet been investigated, recent findings suggest that they have a crucial role in the enhancement of synaptic transmission induced by β-AR activation [161]. Thus, it will be interesting to see whether β-AR modulation of LTP is altered in AKAP and MAGUK mutant mice, as would be expected if AKAPs and MAGUKs are essential components of β-AR signaling complexes at excitatory synapses.
Importantly, recent evidence suggests that the composition of these receptor signaling complexes is not static but instead can be dynamically altered in an activity-dependent manner. For example, NMDA receptor activation rapidly redistributes AKAP 79/150 away from dendritic spines in hippocampal neurons [162,163]; this is associated with a decrease in synaptic levels of PKA RII subunits and a decrease in GluR1 phosphorylation at S845 [163]. The molecular basis for this effect is still unclear, although some evidence suggests that it is dependent on activation of PP2B and involves alterations in spine actin dynamics [162,163]. In any event, translocation of AKAPs and PKA away from dendritic spines following NMDA receptor activation could significantly affect the ability of β-AR activation to facilitate LTP induction. Indeed, prior activation of NMDA receptors induces a long-lasting and robust inhibition of the ability of β-AR activation to increase GluR1 phosphorylation at S845 in the hippocampal CA1 region [117,118]. Prior NMDA receptor activation also strongly inhibits increases in GluR1 phosphorylation at S845 induced by directly activating adenylate cyclase with forskolin [118]. This suggests that NMDA receptor-dependent changes in β-AR signaling are likely due to downstream components of the signaling pathway (such as AKAP and PKA localization) rather than a direct effect on β-ARs. If true, NMDA receptor activation may also disrupt other aspects of β-AR signaling that may be involved in enhancing LTP induction, such as modulation of Kv4.2 and SK3 type potassium channels. Thus, β-AR modulation of LTP may act like a modulatory switch – strongly facilitating the induction of LTP at naïve synapses but having no effect on plasticity at synapses where prior patterns of synaptic activity produced sufficient NMDA receptor activation to activate PP2B. Although the physiological significance of this phenomenon is unclear, it may act as a mechanism for protecting the storage of information encoded by decreases in synaptic strength induced by NMDA receptor-dependent LTD from erasure by the robust LTP-enhancing effects of β-AR activation.
It is clear that release of noradrenaline and subsequent β-AR activation can facilitate the storage of information that may not normally be encoded or retained. Such a mechanism could explain the increased clarity and strength of memories formed during times of intense emotions when noradrenaline release is increased [164]. Additionally, because memory enhancement is a key goal of many cognitive rehabilitation programs, the finding that β-AR activation can gate the dependence of synaptic plasticity on PKA, which is a key requirement for making new long-term memories [165], may provide novel insights on potential molecular drug targets for reducing memory deficits resulting from neurodegenerative diseases.
Acknowledgements
T.J.O. was supported by National Institute of Mental Health grant MH609197. S.A.C. was supported by a Postgraduate Scholarship from the Natural Sciences and Engineering Research Council of Canada. P.V.N. is a Scientist of the Alberta Heritage Foundation for Medical Research, and received research support from the Canadian Institutes of Health Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin SJ, Grimwood PD, Morris RG. Annu. Rev. Neurosci. 2000;23:649. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 2.Lynch MA. Physiol. Rev. 2004;84(1):87. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- 3.Kandel ER. Science. 2001;294(5544):1030. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Science. 2006;313(5790):1093. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 5.Bliss TV, Lomo T. J.Physiol. 1973;232(2):331. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Cell. 1997;88(5):615. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 7.Andersen P, Sundberg SH, Sveen O, Wigstrom H. Nature. 1977;266(5604):736. doi: 10.1038/266736a0. [DOI] [PubMed] [Google Scholar]
- 8.Abraham WC, Logan B, Greenwood JM, Dragunow M. J. Neurosci. 2002;22(21):9626. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collingridge GL, Kehl SJ, McLennan H. J. Physiol. 1983;334:33. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris RG, Anderson E, Lynch GS, Baudry M. Nature. 1986;319(6056):774. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 11.Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN. Nature. 1989;340(6234):554. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- 12.Malinow R, Schulman H, Tsien RW. Science. 1989;245(4920):862. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- 13.Frankland PW, O'Brien C, Ohno M, Kirkwood A, Silva AJ. Nature. 2001;411(6835):309. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- 14.Mayford M, Wang J, Kandel ER, O'Dell TJ. Cell. 1995;81(6):891. doi: 10.1016/0092-8674(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 15.Citri A, Malenka RC. Neuropsychopharmacology. 2008;33(1):18. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 16.Cooper J, Roth R, Bloom F. The Biochemical Basis of Neuropharmacology. Oxford University Press; New York: 2003. [Google Scholar]
- 17.Frey S, Bergado-Rosado J, Seidenbecher T, Pape HC, Frey JU. J. Neurosci. 2001;21(10):3697. doi: 10.1523/JNEUROSCI.21-10-03697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walling SG, Harley CW. J. Neurosci. 2004;24(3):598. doi: 10.1523/JNEUROSCI.4426-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji JZ, Zhang XH, Li BM. Behav. Neurosci. 2003;117(6):1378. doi: 10.1037/0735-7044.117.6.1378. [DOI] [PubMed] [Google Scholar]
- 20.Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee M, Thomas SA. Cell. 2004;117(1):131. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- 21.Gelinas JN, Nguyen PV. CNS agents in Med. Chem. 2007;7:17. [Google Scholar]
- 22.Nicholas AP, Pieribone VA, Hokfelt T. Neuroscience. 1993;56(4):1023. doi: 10.1016/0306-4522(93)90148-9. [DOI] [PubMed] [Google Scholar]
- 23.Hillman KL, Knudson CA, Carr PA, Doze VA, Porter JE. Brain Res. Mol. Brain Res. 2005;139(2):267. doi: 10.1016/j.molbrainres.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 24.Wanaka A, Kiyama H, Murakami T, Matsumoto M, Kamada T, Malbon CC, Tohyama M. Brain Res. 1989;485(1):125. doi: 10.1016/0006-8993(89)90674-4. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Kimelberg HK. Dev. Brain Res. 2004;148(1):77. doi: 10.1016/j.devbrainres.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Siegel GJ. Basic Neurochemistry. 7th ed. Elsevier; Elsevier; Sand Diego: 2006. [Google Scholar]
- 27.Baillie GS, Sood A, Mcphee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. Proc. Natl. Acad. Sci. U.S.A. 2003;100(3):940. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Martin NP, Whalen EJ, Zamah MA, Pierce KL, Lefkowitz RJ. Cell Signal. 2004;16(12):1397. doi: 10.1016/j.cellsig.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen PV, Woo NH. Prog. Neurobiol. 2003;71(6):401. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt JM, Stork PJS. J. Biol. Chem. 2000;275(33):25342. doi: 10.1074/jbc.M003213200. [DOI] [PubMed] [Google Scholar]
- 31.Lefkowitz RJ, Pierce LKL, Luttrell LM. Mol. Pharmacol. 2002;62(5):971. doi: 10.1124/mol.62.5.971. [DOI] [PubMed] [Google Scholar]
- 32.Luo X, Zeng W, Xu X, Popov S, Davignon I, Wilkie TM, Mumby SM, Muallem S. J. Biol. Chem. 1999;274:17684. doi: 10.1074/jbc.274.25.17684. [DOI] [PubMed] [Google Scholar]
- 33.Zamah AM, Delahunty M, Luttrell LM, Lefkowitz RJ. J. Biol. Chem. 2002;277:31249. doi: 10.1074/jbc.M202753200. [DOI] [PubMed] [Google Scholar]
- 34.Hasseldine ARG, Harper EA, Black JW. Pharamcologist. 2002;44(Suppl 1):A199. [Google Scholar]
- 35.Lynch MJ, Baillie GS, Mohamed A, Li X, Maisonneuve C, Klussman E, van Heeke G, Houslay MD. J. Biol. Chem. 2005;280(39):33178. doi: 10.1074/jbc.M414316200. [DOI] [PubMed] [Google Scholar]
- 36.Houslay MD, Schafer P, Zhang KYJ. Drug Discov. Today. 2005;10(22):1503. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- 37.Houslay MD, Baillie GS, Maurice DH. Circ. Res. 2009;100:950. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell JM, Zhang H-T. Trends pharamacol. sci. 2004;25(3):158. doi: 10.1016/j.tips.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Ghavami A, Hirst WD, Novak TJ. Drugs R. D. 2006;7(2):63. doi: 10.2165/00126839-200607020-00001. [DOI] [PubMed] [Google Scholar]
- 40.Vecsey GC, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li X-Y, Descalzi G, Kim SS, Chen T, S. Y-Z, Zhuo M, Houslay MD, Abel T. Nature. 2009;461:1122. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navakkode S, Sajikumar S, Frey JU. J. Neurosci. 2005;25(46):10664. doi: 10.1523/JNEUROSCI.2443-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bohn LM, Gainetdinov RR, Sotnikova TD, Medvedev IO, Lefkowitz RJ, Dykstra LA, Caron MG. J. Neurosci. 2003;23(32):10265. doi: 10.1523/JNEUROSCI.23-32-10265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Proc. Natl. Acad. Sci. U S A. 1998;95:15020. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel PA, Tilley DG, Rockman HA. Circ. J. 2008;72(11):1725. doi: 10.1253/circj.cj-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tilley DG, Kim I-M, Patel PA, Violin JD, Rockman HA. J. Biol. Chem. 2009;284(30):20375. doi: 10.1074/jbc.M109.005793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luttrell LM, Roudabush FL, Choy EW, Miller WE, Field ME, Pierce KL, Lefkowitz RJ. Proc. Natl. Acad. Sci. 2001;98(5):2449. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shenoy SK, Barak LS, Xiao K, Ahn S, Berthouze M, Shukla AK, Luttrell LM, Lefkowitz RJ. J. Biol. Chem. 2007;282(40):29549. doi: 10.1074/jbc.M700852200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bolger GB, Baillie GS, Li X, Lynch MJ, Herzyk P, Mohamed A, High Mitchell L, McCahill A, Hundsrucker C, Klussmann E, Adams DR, Houslay MD. J. Biol. Chem. 2006;398:23. doi: 10.1042/BJ20060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richter W, Day P, Agrawal R, Bruss MD, Granier S, Wang YL, Rasmussen SGF, Horner K, Wang P, Lei T, Patterson AJ, Kobilka B, Conti M. EMBO J. 2008;27(2):384. doi: 10.1038/sj.emboj.7601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD. Proc. Natl. Acad. Sci. USA. 2003;100(3):940. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Masri B, Salahpour A, Didriksen M, Ghisi V, Beaulieu JM, Gainetdinov RR, Caron MG. Proc. Natl. Acad. Sci. U.S.A. 2008;105:13656. doi: 10.1073/pnas.0803522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Houslay MD. Sci. Signal. 2009;2(66):1. doi: 10.1126/scisignal.266pe22. [DOI] [PubMed] [Google Scholar]
- 53.Sweatt JD. Curr. Opin. Neurobiol. 2004;14(3):311. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 54.Dahl D, Sarvey JM. Proc. Natl. Acad. Sci. U.S.A. 1989;86(12):4776. doi: 10.1073/pnas.86.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lanthorn TH, Cotman CW. Brain Res. 1981;225(1):171. doi: 10.1016/0006-8993(81)90326-7. [DOI] [PubMed] [Google Scholar]
- 56.Mueller AL, Hoffer BJ, Dunwiddie TV. Brain Res. 1981;214(1):113. doi: 10.1016/0006-8993(81)90442-x. [DOI] [PubMed] [Google Scholar]
- 57.Dunwiddie TV, Taylor M, Heginbotham LR, Proctor WR. J. Neurosci. 1992;12(2):506. doi: 10.1523/JNEUROSCI.12-02-00506.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heginbotham LR, Dunwiddie TV. J. Neurosci. 1991;11(8):2519. doi: 10.1523/JNEUROSCI.11-08-02519.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jurgens CW, Rau KE, Knudson CA, King JD, Carr PA, Porter JE, Doze VA. J. Pharmacol. Exp. Ther. 2005;314(2):552. doi: 10.1124/jpet.105.085332. [DOI] [PubMed] [Google Scholar]
- 60.Haselmo ME. Behav. Brain Res. 1995;67(1):1. [Google Scholar]
- 61.Harley C. Prog. Brain Res. 1991;88:307. doi: 10.1016/s0079-6123(08)63818-2. [DOI] [PubMed] [Google Scholar]
- 62.Bouret S, Sara SJ. Trends Neurosci. 2005;28(11):574. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Sutton MA, Schuman EM. Cell. 2005;127:49. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 64.Munro CA, Walling SG, Evans JH, Harley CW. Hippocampus. 2001;11(3):322. doi: 10.1002/hipo.1046. [DOI] [PubMed] [Google Scholar]
- 65.Bramham CR, Bacher-Svendsen K, Sarvey JM. Neuroreport. 1997;8(3):719. doi: 10.1097/00001756-199702100-00028. [DOI] [PubMed] [Google Scholar]
- 66.Dahl D, Sarvey JM. Brain Res. 1990;526(2):347. doi: 10.1016/0006-8993(90)91245-c. [DOI] [PubMed] [Google Scholar]
- 67.Neuman RS, Harley CW. Brain Res. 1983;273(1):162. doi: 10.1016/0006-8993(83)91106-x. [DOI] [PubMed] [Google Scholar]
- 68.Harley CW, Milway JS. Exp. Brain Res. 1986;63(1):143. doi: 10.1007/BF00235656. [DOI] [PubMed] [Google Scholar]
- 69.Harley C, Milway JS, Lacaille JC. Brain Res. Bull. 1989;22(4):643. doi: 10.1016/0361-9230(89)90084-1. [DOI] [PubMed] [Google Scholar]
- 70.Davis HP, Squire LR. Psychol. Bull. 1984;96(3):518. [PubMed] [Google Scholar]
- 71.Huang YY, Nguyen PV, Abel T, Kandel ER. Learm. Mem. 1996;3(23):74. doi: 10.1101/lm.3.2-3.74. [DOI] [PubMed] [Google Scholar]
- 72.Squire LR, Barondes SH. Nature. 1970;225(5233):649. doi: 10.1038/225649a0. [DOI] [PubMed] [Google Scholar]
- 73.Huang YY, Kandel ER. Neuron. 1996;16(3):611. doi: 10.1016/s0896-6273(00)80080-x. [DOI] [PubMed] [Google Scholar]
- 74.Hopkins WF, Johnston D. Science. 1984;226(4672):350. doi: 10.1126/science.6091272. [DOI] [PubMed] [Google Scholar]
- 75.Hopkins WF, Johnston D. J. Neurophysiol. 1988;59(2):667. doi: 10.1152/jn.1988.59.2.667. [DOI] [PubMed] [Google Scholar]
- 76.Moody TD, Thomas MJ, Makhinson M, O'Dell TJ. Brain Res. 1998;794(1):75. doi: 10.1016/s0006-8993(98)00217-0. [DOI] [PubMed] [Google Scholar]
- 77.Reid CA, Dixon DB, Takahashi M, Bliss TV, Fine A. J. Neuorsci. 2004;24(14):3618. doi: 10.1523/JNEUROSCI.3567-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zalutsky RA, Nicoll RA. Science. 1990;248(4963):1619. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- 79.Huang YY, Li XC, Kandel ER. Cell. 1994;79(1):69. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 80.Dunwiddie TV, Roberson NL, Worth T. Pharmacol. Biochem. Behav. 1982;17(6):1257. doi: 10.1016/0091-3057(82)90130-7. [DOI] [PubMed] [Google Scholar]
- 81.Sarvey JM, Burgard EC, Decker G. J. Neurosci. Methods. 1989;28:109. doi: 10.1016/0165-0270(89)90016-2. [DOI] [PubMed] [Google Scholar]
- 82.Swanson-Park JL, Coussens CM, Mason-Parker SE, Hargreaves CR, Dragunow EL, Cohen AS, Abraham WC. Neuroscience. 1999;92(2):485. doi: 10.1016/s0306-4522(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 83.Schimanski LA, Ali DW, Baker GB, Nguyen PV. Eur. J. Neurosci. 2007;25(5):1589. doi: 10.1111/j.1460-9568.2007.05376.x. [DOI] [PubMed] [Google Scholar]
- 84.Gelinas JN, Nguyen PV. J. Neurosci. 2005;25(13):3294. doi: 10.1523/JNEUROSCI.4175-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomas MJ, Moody TD, Makhinson M, O'Dell TJ. Neuron. 1996;17(3):475. doi: 10.1016/s0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- 86.Katsuki H, Izumi Y, Zorumski CF. J. Neurophysiol. 1997;77(6):3013. doi: 10.1152/jn.1997.77.6.3013. [DOI] [PubMed] [Google Scholar]
- 87.Winder DG, Martin KC, Muzzio IA, Rohrer D, Chruscinski A, Kobilka B, Kandel ER. Neuron. 1999;24(3):715. doi: 10.1016/s0896-6273(00)81124-1. [DOI] [PubMed] [Google Scholar]
- 88.Giovannini MG, Blitzer RD, Wong T, Asoma K, Tsokas P, Morrison JH, Iyengar R, Landau EM. J. Neurosci. 2001;21(18):7053. doi: 10.1523/JNEUROSCI.21-18-07053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Opazo P, Watabe AM, Grant SG, O'Dell TJ. J. Neurosci. 2003;23(9):3679. doi: 10.1523/JNEUROSCI.23-09-03679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gelinas JN, Tenorio G, Lemon N, Abel T, Nguyen PV. Learn. Mem. 2008;15(5):281. doi: 10.1101/lm.829208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas MJ, Watabe AM, Moody TD, Makhinson M, O'Dell TJ. J. Neurosci. 1998;18(18):7118. doi: 10.1523/JNEUROSCI.18-18-07118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Spruston N, Schiller Y, Stuart G, Sakmann B. Science. 1995;268(5208):297. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- 93.Stuart G, Schiller J, Sakmann B. J. Physiol. 1997;505:617. doi: 10.1111/j.1469-7793.1997.617ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hoffman DA, Magee JC, Colbert CM, Johnston D. Nature. 1997;387(6636):869. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 95.Hoffman DA, Johnston D. J. Neurophysiol. 1999;81(1):408. doi: 10.1152/jn.1999.81.1.408. [DOI] [PubMed] [Google Scholar]
- 96.Yuan L-L, Adams JP, Swank M, Sweatt JD, Johnston D. J.Neurosci. 2002;22(12):4860. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. Nat. Neurosci. 2005;8(5):642. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- 98.Bloodgood BL, Sabatini RL. J. Physiol. 2008;586(6):1475. doi: 10.1113/jphysiol.2007.148353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bloodgood BL, Sabatini RL. Neuron. 2007;53(2):249. doi: 10.1016/j.neuron.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 100.Ren Y, Barnwell LF, Alexander JC, Lubin FD, Adelman JP, Pfaffinger PJ, Schrader LA, Anderson AE. J. Biol. Chem. 2006;281(17):11769. doi: 10.1074/jbc.M513125200. [DOI] [PubMed] [Google Scholar]
- 101.Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. Nat. Neurosci. 2008;11(2):170. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Faber ESL, Delaney AJ, Power JM, Sedlak PL, Crane JW, Sah P. J. Neurosci. 2008;28(43):10803. doi: 10.1523/JNEUROSCI.1796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee HK. Pharmcol Ther. 2006;112:810. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen B-S, Roche KW. Neuropharmacology. 2007;53(3):362. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Skeberdis VA, Chevaleyre V, Lau CG, Goldber JH, Pettit DL, Suadicani SO, Lin Y, Bennett MV, Yuste R, Castillo PE, Zukin S. Nat. Neurosci. 2006;9(4):501. doi: 10.1038/nn1664. [DOI] [PubMed] [Google Scholar]
- 106.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Science. 2000;287(5461):2262. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 107.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Science. 1999;284(5421):1811. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 108.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster JH, Borchardt T, Worley P, Lubke J, Frotsher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Science. 1999;284(5421):1805. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- 109.Blackstone C, Murphy TH, Moss SJ, Baraban JM, Huganir RL. J.Neurosci. 1994;14(12):7585. doi: 10.1523/JNEUROSCI.14-12-07585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Neuron. 1996;16(6):1179. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- 111.Mammen AL, Kameyama K, Roche KW, Huganir RL. J. Biol. Chem. 1997;272(51):32528. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- 112.Banke TG, Bowie D, Lee H-K, Huganir RL, Schousboe A, Traynelis SF. J. Neurosci. 2000;20(1):89. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ehlers MD. Neuron. 2000;28(2):511. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- 114.Man H-Y, Sekine-Aizawa Y, Huganir RL. Proc. Natl. Acad. Sci. U.S.A. 2007;104(9):3579. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oh MC, Derkach VA, Guire ES, Soderling TR. J. Biol. Chem. 2006;281(2):752. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- 116.Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. Nat. Neurosci. 2003;6(2):136. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- 117.Vanhoose AM, Winder DG. J. Neurosci. 2003;23(13):5827. doi: 10.1523/JNEUROSCI.23-13-05827.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vanhoose AM, Clements JM, Winder DG. J. Neurosci. 2006;26(4):1138. doi: 10.1523/JNEUROSCI.3572-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Delgado JY, Coba M, Anderson CNG, Thompson KR, Gray EE, Heusner CL, Martin KC, Grant SNG, O'Dell TJ. J. Neurosci. 2007;27(48):13210. doi: 10.1523/JNEUROSCI.3056-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu H, Real E, Takamiya K, Kang M-G, LeDoux J, Huganir RL, Malinow R. Cell. 2007;131(1):160. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 121.Seol GH, Ziburkus J, Huang S, Song L, Kim IT, Takamiya K, Huganir RL, Lee H-K, Kirkwood A. Neuron. 2007;55(6):919. doi: 10.1016/j.neuron.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brown GP, Blitzer RD, Connor JH, Wong T, Shenolikar S, Iyengar R, Landau EM. J. Neurosci. 2000;20(21):7880. doi: 10.1523/JNEUROSCI.20-21-07880.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ingebritsen TS, Cohen P. Science. 1983;221(4608):331. doi: 10.1126/science.6306765. [DOI] [PubMed] [Google Scholar]
- 124.Abraham WC. News Physiol. Sci. 1999;14:85. doi: 10.1152/physiologyonline.1999.14.2.85. [DOI] [PubMed] [Google Scholar]
- 125.Abraham WC. Nat. Rev. Neurosci. 2008;9(5):387. doi: 10.1038/nrn2356. [DOI] [PubMed] [Google Scholar]
- 126.Abraham WC, Tate WP. Prog. Neurobiol. 1997;52(4):303. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 127.Frey U, Schollmeier K, Reymann KG, Seidenbecher T. Neuroscience. 1995;67(4):799. doi: 10.1016/0306-4522(95)00117-2. [DOI] [PubMed] [Google Scholar]
- 128.Lin YW, Min MY, Chiu TH, Yang HW. J. Neurosci. 2003;23(10):4173. doi: 10.1523/JNEUROSCI.23-10-04173.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhong N, Zucker RS. J. Neurosci. 2005;25(1):208. doi: 10.1523/JNEUROSCI.3703-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaneko M, Takahashi T. J. Neurosci. 2004;24(22):5202. doi: 10.1523/JNEUROSCI.0999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huang CC, Hsu KS. Mol. Pharmacol. 2006;69(3):846. doi: 10.1124/mol.105.018093. [DOI] [PubMed] [Google Scholar]
- 132.Gekel I, Neher E. J. Neurosci. 2008;28(32):7991. doi: 10.1523/JNEUROSCI.0268-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ster J, de Bock F, Bertaso F, Abitol K, Daniel H, Bockaert J, Fagni L. J. Physiol. 2008;587(Pt 1):101. doi: 10.1113/jphysiol.2008.157461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ostroveanu A, van der Zee EA, Eisel UL, Schmidt M, Nijholt IM. Hippocampus. 2009 doi: 10.1002/hipo.20700. Epub. [DOI] [PubMed] [Google Scholar]
- 135.Ouyang Y, Rosenstein A, Kreiman G, Schuman EM, Kennedy MB. J.Neurosci. 1999;19(18):7823. doi: 10.1523/JNEUROSCI.19-18-07823.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Izquierdo I, Medina JH, Izquierdo LA, Barros DM, de Souza MM, Mello e Souza T. Neurobiol. Learn. Mem. 1998;69(3):219. doi: 10.1006/nlme.1998.3825. [DOI] [PubMed] [Google Scholar]
- 137.Ma N, Abel T, Hernandez PJ. Learn. Mem. 2009;16(6):367. doi: 10.1101/lm.1231009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, Landau EM, Blitzer RD. J. Neurosci. 2005;25(24):5833. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kelleher RJ, III, Govindarajan A, Jung HY, Kang H, Tonegawa S. Cell. 2004;116(3):467. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 140.Klann E, Richter ED. In: Translational Control in Biology and Medicine. Matthews MB, Sonenberg N, Hershey JWB, editors. CSHL Press; New York: 2007. pp. 485–506. [Google Scholar]
- 141.Huber KM, Kayser MS, Bear MF. Science. 2000;288(5469):1254. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 142.Matthews MB, Sonenberg N, Hershey JWB. Translational Control in Biology and Medicine. CSHL Press; New York: 2007. [Google Scholar]
- 143.Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. J. Neurosci. 2005;25(42):9581. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. J. Biol. Chem. 2007;282(37):27527. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- 145.Tsokas P, Ma T, Landau EM, Blitzer RD. J. Neurosci. 2007;27(22):5885. doi: 10.1523/JNEUROSCI.4548-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kellerher RJ, III, Govindarajan A, Tonegawa S. Neuron. 2004;44(1):59. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 147.Klann E, Antion MD, Banko JL, Hou L. Learn. Mem. 2004;11(4):365. doi: 10.1101/lm.79004. [DOI] [PubMed] [Google Scholar]
- 148.Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. Genes Dev. 1998;12(4):502. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dyer JR, Michel S, Lee W, Castellucci VF, Wayne NL, Sossin WS. Nat. Neurosci. 2003;6(3):219. doi: 10.1038/nn1018. [DOI] [PubMed] [Google Scholar]
- 150.Carroll M, Dyer J, Sossin WS. Mol. Cell Biol. 2006;26(22):8586. doi: 10.1128/MCB.00955-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wong W, Scott JD. Nat. Rev. Mol. Cell Biol. 2004;5:959. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 152.Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Neuron. 2000;27(1):107. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 153.Funke L, Dakoji S, Bredt DS. Ann. Rev. Biochem. 2005;74:219. doi: 10.1146/annurev.biochem.74.082803.133339. [DOI] [PubMed] [Google Scholar]
- 154.Rosenmund C, Carr DW, Bergeson SE, Nilaver G, Scott JD, Westbrook GL. Nature. 1994;368(6474):853. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 155.Tavalin SJ, Colledge M, Hell JW, Langeberg LK, Huganir RL, Scott JD. J. Neurosci. 2002;22(8):3044. doi: 10.1523/JNEUROSCI.22-08-03044.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Snyder EM, Colledge M, Crozier RA, Chen WS, Scott JD, Bear MF. J. Biol. Chem. 2005;280(17):16962. doi: 10.1074/jbc.M409693200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bhattacharyya S, Biou V, Xu W, Schluter O, Malenka RC. Nat. Neurosci. 2009;12(2):172. doi: 10.1038/nn.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lu Y, Allen M, Halt AR, Weisenhaus M, Dallapiazza RF, Hall DD, Usacheve YM, McKnight GS, Hell JW. EMBO J. 2007;26(23):4879. doi: 10.1038/sj.emboj.7601884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hu LA, Tang Y, Miller WE, Cong M, Lau AG, Lefkowitz RJ, Hall RA. J. Biol. Chem. 2000;275(49):38659. doi: 10.1074/jbc.M005938200. [DOI] [PubMed] [Google Scholar]
- 160.Gardner LA, Naren A, Bahouth S. J. Biol. Chem. 2007;282(7):5085. doi: 10.1074/jbc.M608871200. [DOI] [PubMed] [Google Scholar]
- 161.Joiner MA, Lise' MF, Yuen EY, Kam AYF, Zhang M, Hall DD, Malik ZA, Qian H, Chen Y, Ulrich JD, Burette AC, Weinberg RJ, Law P-Y, El-Husseini A, Yan Z, Hell JW. EMBO J. 2009 doi: 10.1038/emboj.2009.344. doi:10.1038/emboj.2009.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gomez LL, Alam S, Smith KE, Horne E, Dell'Acqua ML. J. Neurosci. 2002;22(16):7027. doi: 10.1523/JNEUROSCI.22-16-07027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Smith KE, Gibson ES, Dell'Acqua ML. J. Neurosci. 2006;26(9):2391. doi: 10.1523/JNEUROSCI.3092-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Cahill L, Prins B, Weber M, McGaugh JL. Nature. 1994;371(6499):702. doi: 10.1038/371702a0. [DOI] [PubMed] [Google Scholar]
- 165.Abel T, Nguyen PV. Prog. Brain Res. 2008;169:97. doi: 10.1016/S0079-6123(07)00006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Neuron. 2006;51(2):213. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 167.Hu LA, Chen W, Premont RT, Cong M, Lefkowitz RJ. J. Biol. Chem. 2002;277(2):1607. doi: 10.1074/jbc.M107297200. [DOI] [PubMed] [Google Scholar]