Abstract
In recent years, various rna-based technologies have been under evaluation as potential next-generation cancer therapeutics. Micrornas (mirnas), known to regulate the cell cycle and development, are deregulated in various cancers. Thus, they might serve as good targets or candidates in an exploration of anticancer therapeutics. One attractive candidate for this purpose is let-7 (“lethal-7”).
Let-7 is underexpressed in various cancers, and restoration of its normal expression is found to inhibit cancer growth by targeting various oncogenes and inhibiting key regulators of several mitogenic pathways. In vivo, let-7 administration was found effective against mouse-model lung and breast cancers, and our computational prediction supports the possible effectiveness of let-7 in estrogen receptor (er)–positive metastatic breast cancer. Data also suggest that let-7 regulates apoptosis and cancer stem cell (csc) differentiation and can therefore be tested as a potential therapeutic in cancer treatment. However, the exact role of let-7 in cancer is not yet fully understood. There is a need to understand the causative molecular basis of let-7 alterations in cancer and to develop proper delivery systems before proceeding to therapeutic applications. This article attempts to highlight certain critical aspects of let-7’s therapeutic potential in cancer.
Keywords: Let-7, microrna, cancer therapy, let-7 regulation, future medicine
1. INTRODUCTION
Micrornas (mirnas) are natural non-coding rnas of approximately 22 nucleotides (nt) in size. They regulate genes post-transcriptionally by binding to a site in the 3′ untranslated region (utr) of target messenger rnas (mrnas). Identification of an mirna target involves base pairing with the target site, which is mostly imperfect in the case of animals. However, a perfect pairing in a 7-nt region at the 5′ end of mirna, called the seed region, is essential for target identification 1.
The mirnas are known to regulate cellular processes such as stem-cell differentiation, heart development 2–4, insulin secretion 5, apoptosis 6,7, aging 8,9, and immunity 10,11, among other processes. It is therefore not surprising that mirnas are differentially expressed in several pathophysiologic conditions including, for instance, Alzheimer disease 12,13, Parkinson disease 14, cardiovascular diseases 4,15,16, the Cowden and Down syndromes 17,18, and various cancers 19.
Let-7 was first discovered and well studied in Caenorhabditis elegans, in which it regulates developmental timing 20–23 (larval stage 4–to–adult transition 20,24) and stage-specific neuromuscular tissue development 25. Let-7 has orthologs in various species. In Drosophila, let-7 plays a role in determining the timing for cell-cycle exit, metamorphosis, neuromuscular Junction development, juvenile-to-adult-stage transition, and adult behaviour 26,27. The zebrafish ortholog of let-7 is prominently expressed in nervous tissue, indicating its certain role in neural development 28. In the adult newt, let-7 regulates transdifferentiation and regeneration of lens and inner ear-hair cells 29.
Little is known about the function of let-7 in mammalian development and normal physiology. In the mouse, let-7 is involved in neural lineage specificity of embryonic stem cells, brain development 30, and mammary epithelial progenitor cell maintenance by induction of loss of self-renewal 31. In humans, 12 genomic loci encode the let-7 family members (let-7a-1, -2, -3; let-7b; let-7c; let-7d; let-7e; let-7f-1, -2; let-7g; let-7i; mir98). Human let-7 is upregulated during embryonic cell differentiation 32, but the roles it plays in normal physiology are mostly unknown.
Human let-7 family members are found to be downregulated in several cancers, with a few exceptions (Table I); restoration of normal expression prevents tumorigenesis 37,44,45,52. Let-7 therefore acts as a tumour suppressor and a regulator of terminal differentiation and apoptosis. This finding implies that let-7 can possibly be used as a next-generation cancer therapeutic. But, to date, the mechanism of let-7 deregulation, and its precise role in tumorigenesis, is not fully understood, creating a hurdle to effectively using this mirna in cancer therapy.
TABLE I.
Deregulation of microrna let-7 family members in various cancers
| Cancers | Microrna let-7 family members | References |
|---|---|---|
| Cancers that exhibit downregulation of specific let-7 family members | ||
| Acute lymphoblastic leukemia | let-7b | Mi et al., 2007 33 |
| Bladder cancer | let-7b, let-7d, let-7e, let-7f | Nam et al., 2008 34 |
| Breast cancer | let-7, let-7a | Sempere et al., 2007 35 Yu et al., 2007 36 |
| Bronchioloalveolar cancer | let-7 | Inamura et al., 2007 37 |
| Burkitt lymphoma | let-7a | Sampson et al., 2007 38 |
| Colon cancer | let-7 | Michael et al., 2003 39 Akao et al., 2006 40 Fang et al., 2007 41 |
| Gastric cancer | let-7 | Zhang et al., 2007 42 Motoyama et al., 2008 43 |
| Hepatocellular cancer | let-7 | Johnson et al., 2007 44 |
| Kidney cancer | let-7a, let-7c, let-7d, let-7e, let-7f, let-7g | Nam et al., 2008 34 |
| Lung cancer | let-7 | Johnson et al., 2007 44 Takamizawa et al., 2004 45 Johnson et al., 2005 46 |
| Malignant melanoma | let-7b | Schultz et al., 2008 47 |
| Ovarian cancer | let-7a-3 | Lu et al., 2007 48 |
| Pancreatic cancer | let-7 | Jérôme et al., 2007 49 |
| Prostate cancer | let-7c | Jiang et al., 2005 50 |
| Cancers that exhibit upregulation of specific let-7 family members | ||
| Acute myeloid leukemia | let-7 | Garzon et al., 2008 51 |
| Breast cancer | let-7b | Nam et al., 2008 34 |
| Colon cancer | let-7a, let-7g | Nam et al., 2008 34 |
| Lung cancer | let-7a | Nam et al., 2008 34 |
| Retinoblastoma | let-7a, let-7b, let-7c | Nam et al., 2008 34 |
| Uterine cancer | let-7i | Nam et al., 2008 34 |
This article presents an overview of let-7 and discusses the critical issues that must be explored to develop a let-7–based therapeutic strategy against various cancers.
2. DISCUSSION
2.1. Biogenesis and Mechanism of Action
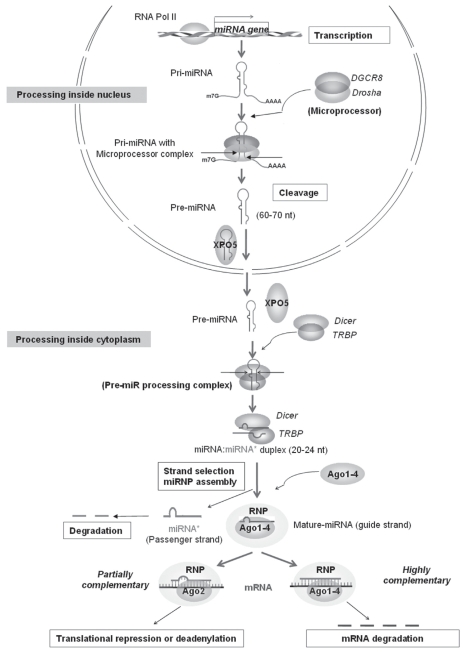
The biogenesis of let-7 is similar to that of other mirnas. The first step in mirna biogenesis is transcription from the mirna transcription unit by rna polymerase ii to produce a primary transcript called pri-mirna. The pri-mirna is processed by the microprocessor complex containing an rnase iii–like enzyme, Drosha, and its cofactor, a double-stranded rna binding protein, Dgcr8, to produce an approximately 60–70 nt pre-mirna (precursor mirna). The pre-mirna is then transported to cytoplasm by exportin 5 (XPO5), in a Rangtp (ras-related nuclear protein–guanosine triphosphate complex)–dependent way, where it is cleaved by Dicer (a cytoplasmic rnase iii), to generate an imperfect mirna:mirna* duplex of approximately 21–24 nt. One of the strands (the “guide strand”) from the duplex is then incorporated into Argonaute (Ago)–containing ribonucleoprotein (rnp) complex; the other strand (the “passenger strand”) is degraded. However, there are cases in which both strands of the duplex are detected in the cell 53. The mirna–Ago rnp complex causes posttranscriptional regulation of genes, in which mirna is used as a tether to guide the complex to the specific mrna. The exact mechanism by which the mirnp complex regulates expression of the target remains unclear. Various models try to explain this mechanism 1. Figure 1 shows a general model.
FIGURE 1.
The most-accepted model of microrna (mirna) biogenesis and its mechanism of action. For detail, see text. rna Pol ii = rna polymerase ii; Pri-mirna = primary transcripts of mirna; DGCR8 = DiGeorge syndrome critical region gene 8; Drosha = class 2 rnase iii enzyme; XPO5 = exportin 5; Dicer = formal symbol DICER1 (dicer 1, ribonuclease type iii); TRBP = now labelled TARBP2P [tar (hiv-1) rna binding protein 2 pseudogene]; Ago1–4 = Argonaute-1 to -4 [symbol EIF2C1, 2, 3, 4 (eukaryotic translation initiation factor 2C, 1–4)]; rnp = ribonucleoprotein; mrna = messenger rna.
2.2. Regulation of Let-7
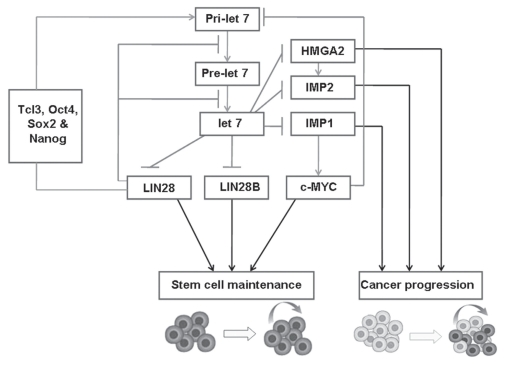
Expression of let-7 is regulated at various stages of its biogenesis and also depending on cell type. Similarly, let-7 regulates many transcription factors that play important roles in regulation of the cell cycle, cell differentiation, and apoptosis. Many of the factors controlling the expression of let-7 form regulatory circuits with the factors being regulated by such expression. These regulatory circuits—such as double-negative feedback loops and so on—are salient network motifs in development and differentiation. LIN28, POU5F1, SOX2, NANOG, TLX1, HMGA2, MYC, and IMPs are known to form such regulatory loops (Figure 2).
FIGURE 2.
Regulatory circuits of microrna (mirna) let-7. The loop consists of pluripotency promoting factors {LIN28 [lin-28 homolog (Caenorhabditis elegans)], OCT4 [now labelled POU5F1 (pou class 5 homeobox 1)], SOX2 [sry (sex determining region Y)–box 2], NANOG [Nanog homeobox], and TCL3 [now labelled TLX1 (T-cell leukemia homeobox 1)]}, oncofetal genes [HMGA2 (high mobility group at–hook 2) and imps (insulin-like growth factor 2 mrna-binding proteins)], and oncogene MYC. For detail, see text. Pri-let 7 = primary transcripts of let-7; LIN28B = lin-28 homolog B (C. elegans).
2.2.1. Regulation of Let-7 by Pluripotency-Promoting Factors in Embryonic and Cancer Stem Cells
LIN28, which maintains the undifferentiated state of embryonic cells, is a well-known target of let-7 and is downregulated by let-7 during developmental commitment 54,55. Lin28 was recently shown to act as a posttranscriptional repressor of let-7 biogenesis, binding to the loop portion of the pri–let-7 hairpin and the stem part of pre–let-7 and thereby inhibiting its processing. Lin28 and Lin28B also inhibit processing of let-7 by mediating terminal uridylation of let-7 precursors 56. What is unclear is whether the regulation by Lin28 occurs at the Drosha or Dicer processing step 55,57–59. Lin28 induces pri–let-7 expression through induction of other pluripotency-promoting factors such as Pou5F1, Sox2, Nanog, and Tlx1 60, thus regulating let-7 expression at multiple levels.
The early embryonic oncofetal gene HMGA2 is involved in the self-renewal and maintenance of adult stem cells. It is highly expressed in hematopoietic and fetal neuronal stem cells 61,62, and the low levels of let-7 in stem cells inversely correlate with HMGA2 expression. Thus, the undifferentiated state is maintained 63. In differentiated tissues, HMGA2 is downregulated because of the high expression of let-7 61, and during induced differentiation, ectopic expression of let-7 reduces ras and HMGA2 expression, leading to inhibition of cell proliferation and induction of apoptosis. Therefore, HMGA2 is a direct target of let-7 64.
Like normal stem cells, cancer stem cells (slowly dividing tumour-initiating cells) exhibit low levels of let-7 and possess unlimited self-renewal capability and pluripotency, allowing them to repopulate and metastasize 65,66. It has been proposed that, during carcinogenesis, the let-7–targeted embryonic genes, which are otherwise not expressed in adult tissues, are re-expressed because of loss of let-7 control. This reprogramming promotes de-differentiation and cancer progression 67. A good example is that of HMGA2, which is undetectable in most differentiated tissues, but highly expressed in various cancers, including neuroblastoma and pancreatic, lung, and thyroid cancers 68–71. Breast cancer stem cells are also devoid of let-7, but abundantly express HMGA2 and ras 36 (Figure 2).
2.2.2. Regulatory Circuit Between Myc and Let-7
IMP1 is another oncofetal gene that is expressed only during early fetal life 72,73 and is re-expressed in several cancers 74. It is selectively expressed in young, but not in old, hematopoietic stem cells 75. IMP1 regulates stem cell functions by stabilizing insulin-like growth factor 2 and C-myc mrnas 76,77, and the phenotype of stem cells from the IMP1 knockout mouse resembles that of cells from the HMGA2-deficient mouse 73,78. Let-7 targets IMP1, and therefore indirectly acts as a negative regulator of MYC expression 64,79,80. It has been shown that Myc binds directly to let-7 promoter and downregulates its transcription 81. Thus, an indirect feedback circuit exists between let-7 and Myc (Figure 2).
2.3. Let-7 Targets Multiple Oncogenes and Components of Cell Cycle, Cell Proliferation, and Apoptosis
Apart from targeting oncogenes (ras, MYC, HMGA2, and so on) as already discussed, let-7 regulates several key components of the cell cycle and cell proliferation. Microarray analysis of hepatocellular carcinoma (HepG2) and lung cancer (A549) cell lines revealed that let-7 inhibits multiple cell-cycle- and proliferation-associated genes, including cyclin A2 (CCNA2), CDC34, Aurora A [AURKA (formerly STK6)] and B [AURKB (formerly SKT12)] kinases, E2F5, CDK8, and PLAGL2, among others 46. In HepG2 cells, let-7 directly represses CCNA2, CDC25A, SKP2, AURKA, CDC16, CCND1, and CDK6, among others. Let-7 also inhibits several dna replication machinery components (ORC1L; RRM1, 2; and so on) and transcription factors [E2F6, CBFB, PLAGL2, SOX9, GZF1 (formerly ZNF336), YAP1, GTF2I, ARID3A, and so on]. Surprisingly, that study also showed that let-7 represses several tumour suppressor genes (BRCA1, BRCA2, FANCD2, and PLAGL1, among others) and checkpoint regulators (CHEK1, BUB1, BUB1B, MAD2L1, and CDC23, among others). Our recent in silico analysis shows that let-7 may potentially target er signalling and angiogenic pathways by targeting key molecules of these cascades 82. Various targets of let-7 are listed in Table II and shown in Figure 3.
Table II.
Microrna let-7 targets in various cancers
| Cancer | Microrna let-7 |
Model used | References | ||
|---|---|---|---|---|---|
| Expression | Targets | Effect on targets | |||
| Breast cancer | let-7 ↓ | ANG; CCND1, 2; CDC25A; CDK4, 6;CYP19A1; dna polymerases; E2F5, 6; ESR1, 2; FGF11; fgfr; GRB2; HMGB2; IGF1, 1R; IL6; ITGB3; MAPK4, 6; MMP2; MMP8; MYC; ras; RB1; SKP2; TGFB1, BR1; TP53 | Transcription | In silico | Barh et al., 2008 82 |
| let-7 ↓ | HMGA2, H-ras | Transcription | Cell line, mouse model | Sempere et al., 2007 35 Yu et al., 2h007 36 |
|
| Burkitt lymphoma | let-7a ↓ | MYC | Transcription/translation | Cell line | Sampson et al., 2007 38 |
| Colon cancer | let-7 ↓ | ras, MYC | Translation | Cell line | Akao et al., 2006 40 |
| Hepatocellular cancer | let-7 ↓ | AURKA; BRCA1, 2; BUB1; CCNA2, B1, E2, F, J; CDC2, 6, 20, 23, 25A, 34, 45L; NUF2; CBX2; CDCA2, 3, 4, 5, 7, 8; CDK8; CHEK1; CKS1B; DBF4; DICER1; E2F5, 6, 8;FANCD2; GMNN; CDT1; HMGA2; LIN28B; MAD2L1; NRAS; ORC1L; PLAGL1, 2; RRM1, 2; SKP2; SOX9; ARUKB (formerly STK12) | Transcription | Cell line | Johnson et al., 2007 44 |
| Lung cancer | let-7 ↓ | MYC, ras | Transcription/translation | Cell line | Johnson et al., 2005 46 Kumar et al., 2008 52 |
| let-7 ↓ | AURKA; CCNA2; CDC34; CDK8; DBF4; DICER1; E2F5; GMNN; HMGA2; LIN28B; NRAS; PLAGL1, 2; ARUKB (formerly STK12) | Transcription | A549 lung cancer cells | Johnson et al., 2007 44 | |
| let-7 ↓ | HMGA2 | Transcription | Cell line | Kumar et al., 2008 52 Lee and Dutta, 2007 83 |
|
| Malignant melanoma | let-7b ↓ | CDK4; cyclins A, D1, D3 | Translation | Cell line | Schultz et al., 2008 47 |
| Uterine leiomyoma | let-7 ↓ | HMGA2 | Transcription | Tumour sample, cell line | Peng et al., 2008 84 |
fgfr = fibroblast growth factor receptor; ↓ = downregulation.
FIGURE 3.
Let-7 targets various key components of mitogenic and tumorigenic pathways to exert its tumour suppressor activity. Pathways include cell cycle, cell division, cell proliferation, dna replication, angiogenesis, and apoptosis. PLAGL1, 2 = pleomorphic adenoma gene-like 1, 2; CKS1B = cdc28 protein kinase regulatory subunit 1B; SKP2 = S-phase kinase-associated protein 2 (p45); fgf, fgfr = fibroblast growth factor and fibroblast growth factor receptor; igf = insulin-like growth factor; il-s = interleukin S; tgfb = transforming growth factor β; GRB2 = growth factor receptor-bound protein 2; mapk = mitogen-activated protein kinase; CYP19A1 = cytochrome P450, family 19, subfamily A, polypeptide 1; ESR1 = estrogen receptor 1; MMP2, 8 = matrix metallopeptidases 2, 8; ITGB3 = integrin β3; ANG = angiogenin; RRM1, 2 = ribonucleotide reductases M1 and M2; CDC6 = cell division cycle 6 homolog (Saccharomyces cerevisiae); ORC1L = origin recognition complex, subunit 1-like (yeast); MCM2 = minichromosome maintenance complex component 2; RFC2–5 = replication factor C (activator 1) 2–5; GMNN = geminin, dna replication inhibitor; E2F5, 6, 8 = e2f transcription factors 5, 6, 8; CDK8 = cyclin-dependent kinase 8; CDC16 = cell division cycle 16 homolog (S. cerevisiae); AURKA = aurora kinase A; CDC25A = cell division cycle 25 homolog A (Schizosaccharomyces pombe); CCNA2 = cyclin A2; CDC20, 23 = cell division cycle 20 and 23 homologs (S. cerevisiae); CDCA1 = (now labelled NUF2) NDC80 kinetochore complex component, homolog (S. cerevisiae); CHEK1 = chk1 checkpoint homolog (S. pombe); BUB1, 1B = budding uninhibited by benzimidazoles 1 and 1 β homologs (yeast); CCNB1, D1, D2, E2, F, J = cyclins B1, D1, D2, E2, F, J; CDC2 = cell division cycle 2, G1 to S and G2 to M; CDK2, 4, 6 = cyclin-dependent kinases 2, 4, 6; mrna = messenger rna.
Apoptosis regulatory functions of let-7 have recently been reported in both human and mouse. Let-7 targets Casp3 in the A431 and HepG2 cell lines, and inhibits doxorubicin- and paclitaxel-induced apoptosis 85. In NIH3T3 mouse fibroblast cells, let-7 is involved in ultraviolet B–induced apoptosis by modulating Casp3, Bcl2, Map3k1, and Cdk5 86.
2.4. Emerging Role of Let-7 in Cancer Diagnosis and Therapy
The facts discussed here indicate that let-7 acts as a tumour suppressor by targeting various oncogenes and key components of the cell cycle and developmental pathways. Most reports reveal that let-7 is frequently underexpressed (Table I) and that the chromosomal region of human let-7 is frequently deleted in many cancers 87. Similarly, in more differentiated tumour cells, let-7 is expressed at higher levels, and its target oncogenes (HMGA2 and ras) are downregulated. Thus, loss of let-7 expression is a marker for less differentiated cancer 88, and expression levels are also found to be effective prognostic markers in several cancers 40,46,88. In lung cancer, reduced let-7 expression was also found to significantly correlate with shortened postoperative survival regardless of disease stage 45.
From the therapeutic viewpoint, let-7 is attractive molecule for preventing tumorigenesis and angiogenesis 89; it is a potential therapeutic in several cancers that underexpress let-7. Let-7 replacement was found to inhibit anchorage-independent growth and cell-cycle progression in melanoma cells by repressing regulators of the cell cycle and cell proliferation such as cyclins A, D1, and D3 and CDK4 47. Together with TP53, ras and MYC have been implicated as key oncogenes in lung cancer. The reduced expression of let-7 in lung cancer directly correlates with upregulation of oncogene ras; introduction of let-7 represses ras and MYC translation by targeting the related mrnas 45,46. In both lung and hepatocellular carcinomas, replacement or restoration of normal expression levels of let-7 inhibits cancer growth by repressing multiple cell-cycle and proliferation pathways, together with ras and MYC 37,44,45,52 (Table II). Intranasal let-7 administration was found effective in reducing tumour growth in a K-ras mutant mouse model of lung cancer 90. Similarly, restoration of let-7 restrains the growth and proliferation of colon and hepatic cancers 40,80. Transfection of let-7 in a Burkitt lymphoma cell line downregulates MYC and reverts MYC-induced cell growth 38. Ectopic expression of let-7 inhibits cell proliferation by directly repressing the HMGA2 oncogene in lung cancers 52,83 and uterine leiomyoma 84.
Induced expression of let-7 in breast cancer cells targets HMGA2 and H-ras 36, and in a mouse model of breast cancer, exogenous let-7 delivery suppresses cell proliferation, mammosphere formation, and the population of undifferentiated cells by downregulating both of the foregoing oncogenes 35,36. In our in silico analysis, we recently showed that, apart from repressing MYC, ras, and HMGA2, let-7 may also target CYP19A1, ESR1, and ESR2, thereby potentially blocking estrogen signalling in er-positive breast cancers. Similarly, by repressing angiogenin, fibroblast growth factor, transforming growth factor, interleukin 6, and matrix metallopeptidase 2, let-7 may prevent growth, angiogenesis, and metastasis in breast cancer 82 (Table II).
2.5. Limitations of Let-7–Based Therapy
2.5.1. Limitations Because of Limited Knowledge of Let-7 Biology
Although restoration of normal let-7 expression proves beneficial, limited knowledge concerning its transcriptional and processing control during biogenesis and its exact role in tumorigenesis make it difficult to directly apply let-7 as a therapeutic. It is necessary to know whether downregulation of let-7 in tumours is a primary or secondary phenomenon during tumorigenesis. Supporting the csc hypothesis, we agree with the opinion that epigenetic downregulation of let-7 in cscs leads to upregulation of oncofetal genes (HMGA2 and LIN28, among others) and, thereby, to loss of differentiation and tumorigenesis. In that scenario, downregulation of let-7 is the primary event, a view that can be supported by observation of where in ovarian cancer let-7 is hypermethylated 48.
Because mirnas act on the 3′ utr of target mrnas, it is important to determine how efficiently let-7 will work as a therapeutic, because 3′ utr truncated oncogenes may be prevalent in neoplasia. Grimm et al. 91 reported that delivery of adeno-associated virus (aav)–mediated recombinant pre-mirnas causes death in mice from severe liver cytotoxicity. Details of the immunogenic and cytotoxic effects of let-7 therefore need to be explored so that such side effects can be minimized in an effective treatment strategy. Similarly, we proposed that let-7 may be involved in an as-yet-unknown regulatory network of mirnas that resembles the gene regulatory network involving transcription factors. Therefore, anti-mirna oligo-based knockdown of let-7 inhibitory mirnas is not currently possible.
2.5.2. Limitations in Delivery Methods and Systems
Lack of an appropriate, safe, and effective delivery method for let-7 is another drawback of possible therapy. Biological vectors such as aav and lentivirus may be used for targeted delivery 92, but standardization of the method is required to prevent non-targeted site introduction. Also, brain-specific mirna delivery is not yet successful 93, and effective neuron-specific delivery methods have to be developed to tackle brain and neuronal tumours. As discussed earlier, aav- and lentivirus-mediated delivery of let-7 in a mouse model of lung cancer 52,90 was found to be inefficient in pre-existing tumours because of the resistance to let-7 developed by the tumour over time 52. A strategy for let-7–mediated therapy for pre-existing tumours therefore also has to be developed.
2.6. Strategies to Overcome the Limitations
The optimal or normal level of let-7 may be restored in cancer cells either by administering exogenous let-7 in situ with a vector overexpressing let-7, or by repressing let-7 repressors. Recent mirna technologies are, in general, designed to use complementary or chemically modified single-stranded rna analogs (or both) to repress the specific mirnas responsible for a given disease or cancer. These analogs, including asos (antisense oligonucleotides), amos (anti-mirna asos called “antagomirs”), locked nucleic acids, and antisense-technology-based small interfering rnas, are widely and effectively used in regulation of mirna expression 92,94–99. But direct information is not available on the mirnas that regulate let-7 expression; this aspect limits the scope for such a strategy. Instead, technologies are required that can effectively upregulate let-7 expression. Hence, either vector-mediated overexpression of let-7 or transient transfection of double-stranded let-7 will be the choice.
Introduction of double-stranded let-7 duplex may produce mature let-7, equivalent to the endogenous version, during Dicer processing, potentially rescuing a downregulated let-7 level. This strategy has already been successfully used 83. Vectors containing pre–let-7–like synthetic short hairpin rnas, driven by highly inducible Pol iii promoters such as H1 and U6 100,101 may provide high expression of let-7 from predefined transcription start and termination sites 102. But instead of designing artificial hairpins, direct cloning of the entire natural pri–let-7 hairpin with flanking sequences into the expression vector may be a better approach— assuming that natural pre–let-7 will be a better substrate for generating mature let-7 during Dicer processing 103–107. A pri-mir–Pol ii transgene system has been successfully used to overexpress mir155 104, mir30 108, and mir122 109. This system was also found useful in expressing multiple mirnas from a single transcript 104 and can therefore be adopted for let-7 expression too.
High-density lipoprotein conjugated sirna has been reported to increase delivery efficacy in certain specific organs such as liver, gut, kidney, and steroid secreting organs 110. A similar approach may therefore have the possibility to be effective in let-7 delivery as well. But the synthesis and purification of therapeutic-grade let-7 is difficult. A nanoparticle-based delivery system may prove beneficial.
Other delivery methods that have been found promising in both in vitro and in vivo conditions include lentivirus-mediated pre–let-7 oligonucleotides 36, adenovirus-mediated delivery of hairpin sequences of mature let-7 90, cationic liposome–mediated delivery of pre–let-7 40, and electroporation of synthetic let-7 90. Although such methods are at the bench level, they might be translated into therapeutic approaches in the near future.
2.7. Current Industry Status of Let-7 Therapy
Because of its potential as a cancer therapeutic, let-7 has been filed for patent protection (Australia: 2007/333109 A1; United States: 20090163430). While diagnostic companies are developing let-7–based tests for various diseases, including several cancers, pharma giants are working toward development of effective delivery systems. But let-7 restoration methods are not yet satisfactory. Asuragen (www.asuragen.com), the rna-based therapeutic and diagnostics major with a core focus on mirna through its subsidiary Mirna Therapeutics (www.mirnatherapeutics.com), is developing mirna-based diagnostics and therapeutics for non-small-cell lung cancer, metastatic prostate cancer, and acute myeloid leukemia—all currently in preclinical trials. For lung cancer and acute myeloid leukemia, their main focus is let-7. Similarly, Regulus Therapeutics LLC (www.regulusrx.com) is using more than 60 mirnas, including let-7, to develop mirna therapeutics to treat several diseases (including cancers). Their main focus is on delivery systems and enhancement of treatment efficacy.
3. SUMMARY
Let-7 exerts its tumour suppressor and antiproliferative activities by repressing several oncogenes and by regulating key regulators of the cell cycle, cell differentiation, and apoptotic pathways. Downregulation of let-7 is a common phenomenon in several cancers, and restoration of normal let-7 expression has been found to prevent cancer growth. As a result, let-7 is a molecular marker in certain cancers and a potential therapeutic in cancer therapy. However, efficient delivery strategies have to be developed if this molecule is to be used as a therapeutic in vivo. Use of viral vectors, artificial virus-like particles, and nano materials may be a promising way to realize this goal, but optimization is needed. Also, a better understanding of let-7 biology and its regulatory networks is required to exploit the curative benefits of let-7 and to reduce off-target side effects.
4. ACKNOWLEDGMENTS
We acknowledge the support of all members of the Institute of Integrative Omics and Applied Biotechnology, India, and we especially thank Dr. Souvik Maiti (Scientist E-1, Institute of Genomics and Integrative Biology, India) for his valuable suggestions regarding the writing of this article.
5. REFERENCES
- 1.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by micrornas: are the answers in sight? Nat Rev Genet. 2008;9:102–14. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 2.Ivey KN, Muth A, Arnold J, et al. Microrna regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008;2:219–29. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen JF, Mandel EM, Thomson JM, et al. The role of microrna-1 and microrna-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–33. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao Y, Ransom JF, Li A, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking mirna-1–2. Cell. 2007;129:303–17. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Plaisance V, Abderrahmani A, Perret–Menoud V, Jacquemin P, Lemaigre F, Regazzi R. Microrna-9 controls the expression of Granuphilin/Slp4 and the secretory response of insulin-producing cells. J Biol Chem. 2006;281:26932–42. doi: 10.1074/jbc.M601225200. [DOI] [PubMed] [Google Scholar]
- 6.Lukiw WJ, Pogue AI. Induction of specific micro rna (mirna) species by ros-generating metal sulfates in primary human brain cells. J Inorg Biochem. 2007;101:1265–9. doi: 10.1016/j.jinorgbio.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarasov V, Jung P, Verdoodt B, et al. Differential regulation of micrornas by p53 revealed by massively parallel sequencing: mir-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586–93. doi: 10.4161/cc.6.13.4436. [DOI] [PubMed] [Google Scholar]
- 8.Kumamoto K, Spillare EA, Fujita K, et al. Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir-34b, and mir-34c expression, and induce senescence. Cancer Res. 2008;68:3193–203. doi: 10.1158/0008-5472.CAN-07-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maes OC, An J, Sarojini H, Wang E. Murine micrornas implicated in liver functions and aging process. Mech Ageing Dev. 2008;129:534–41. doi: 10.1016/j.mad.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Chen CZ, Li L, Lodish HF, Bartel DP. Micrornas modulate hematopoietic lineage differentiation. Science. 2004;303:83–6. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez A, Vigorito E, Clare S, et al. Requirement of Bic/microrna-155 for normal immune function. Science. 2007;316:608–11. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hébert SS, Horré K, Nicolaï L, et al. Loss of microrna cluster mir-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/β-secretase expression. Proc Natl Acad Sci U S A. 2008;105:6415–20. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang WX, Rajeev BW, Stromberg AJ, et al. The expression of microrna mir-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of beta-site amyloid precursor protein-cleaving enzyme 1. J Neurosci. 2008;28:1213–23. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang G, van der Walt JM, Mayhew G, et al. Variation in the mirna-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of alpha-synuclein. Am J Hum Genet. 2008;82:283–9. doi: 10.1016/j.ajhg.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. Micrornas play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–24. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 16.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microrna that targets Hand2 during cardiogenesis. Nature. 2005;436:214–20. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 17.Pezzolesi MG, Platzer P, Waite KA, Eng C. Differential expression of PTEN-targeting micrornas mir-19a and mir-21 in Cowden syndrome. Am J Hum Genet. 2008;82:1141–9. doi: 10.1016/j.ajhg.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn DE, Nuovo GJ, Martin MM, et al. Human chromosome 21–derived mirnas are overexpressed in Down syndrome brains and hearts. Biochem Biophys Res Commun. 2008;370:473–7. doi: 10.1016/j.bbrc.2008.03.120. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Blenkiron C, Miska EA. mirnas in cancer: approaches, aetiology, diagnostics and therapy. Human Mol Genet. 2007;16:R106–13. doi: 10.1093/hmg/ddm056. [DOI] [PubMed] [Google Scholar]
- 20.Reinhart BJ, Slack FJ, Basson M, et al. The 21-nucleotide let-7 rna regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–6. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 21.Grishok A, Pasquinelli AE, Conte D, et al. Genes and mechanisms related to rna interference regulate expression of the small temporal rnas that control C. elegans developmental timing. Cell. 2001;6:23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 22.Abbott AL, Alvarez–Saavedra E, Miska EA, et al. The let-7 microrna family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Dev Cell. 2005;9:403–14. doi: 10.1016/j.devcel.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Jones–Rhoades MW, Lau NC, Bartel DP, Rougvie AE. Regulatory mutations of mir-48, a C. elegans let-7 family microrna, cause developmental timing defects. Dev Cell. 2005;9:415–22. doi: 10.1016/j.devcel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Grosshans H, Johnson T, Reinert KL, Gerstein M, Slack FJ. The temporal patterning microrna let-7 regulates several transcription factors at the larval to adult transition in C. elegans. Dev Cell. 2005;8:321–30. doi: 10.1016/j.devcel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 25.Frasch M. A matter of timing: microrna-controlled temporal identities in worms and flies. Genes Dev. 2008;22:1572–6. doi: 10.1101/gad.1690608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caygill EE, Johnston LA. Temporal regulation of metamorphic processes in Drosophila by the let-7 and mir-125 heterochronic micrornas. Curr Biol. 2008;18:943–50. doi: 10.1016/j.cub.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sokol NS, Xu P, Jan YN, Ambros V. Drosophila let-7 microrna is required for remodeling of the neuromusculature during metamorphosis. Genes Dev. 2008;22:1591–6. doi: 10.1101/gad.1671708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wienholds E, Kloosterman WP, Miska E, et al. Microrna expression in zebrafish embryonic development. Science. 2005;309:310–11. doi: 10.1126/science.1114519. [DOI] [PubMed] [Google Scholar]
- 29.Tsonis PA, Call MK, Grogg MW, et al. Micrornas and regeneration: let-7 members as potential regulators of dedifferentiation in lens and inner ear hair cell regeneration of the adult newt. Biochem Biophys Res Commun. 2007;362:940–5. doi: 10.1016/j.bbrc.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wulczyn FG, Smirnova L, Rybak A, et al. Post-transcriptional regulation of the let-7 microrna during neural cell specification. FASEB J. 2006;21:415–26. doi: 10.1096/fj.06-6130com. [DOI] [PubMed] [Google Scholar]
- 31.Ibarra I, Erlich Y, Muthuswamy SK, Sachidanandam R, Hannon GJ. A role for micrornas in maintenance of mouse mammary epithelial progenitor cells. Genes Dev. 2007;21:3238–43. doi: 10.1101/gad.1616307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bar M, Wyman SK, Fritz BR, et al. Microrna discovery and profiling in human embryonic stem cells by deep sequencing of small rna libraries. Stem Cells. 2008;26:2496–505. doi: 10.1634/stemcells.2008-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mi S, Lu J, Sun M, et al. Microrna expression signatures accurately discriminate acute lymphoblastic leukemia from acute myeloid leukemia. Proc Natl Acad Sci U S A. 2007;104:19971–6. doi: 10.1073/pnas.0709313104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nam S, Kim B, Shin S, Lee S. mirGator: an integrated system for functional annotation of micrornas. Nucleic Acids Res. 2008;36:D159–64. doi: 10.1093/nar/gkm829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sempere LF, Christensen M, Silahtaroglu A, et al. Altered microrna expression confined to specific epithelial cell sub-populations in breast cancer. Cancer Res. 2007;67:11612–20. doi: 10.1158/0008-5472.CAN-07-5019. [DOI] [PubMed] [Google Scholar]
- 36.Yu F, Yao H, Zhu P, et al. Let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131:1109–23. doi: 10.1016/j.cell.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 37.Inamura K, Togashi Y, Nomura K, et al. Let-7 microrna expression is reduced in bronchioloalveolar carcinoma, a non-invasive carcinoma, and is not correlated with prognosis. Lung Cancer. 2007;58:392–6. doi: 10.1016/j.lungcan.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 38.Sampson VB, Rong NH, Han J, et al. Microrna let-7a downregulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762–70. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- 39.Michael MZ, O’Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific micrornas in colorectal neoplasia. Mol Cancer Res. 2003;1:882–91. [PubMed] [Google Scholar]
- 40.Akao Y, Nakagawa Y, Naoe T. Let-7 microrna functions as a potential growth suppressor in human colon cancer cells. Biol Pharm Bull. 2006;29:903–6. doi: 10.1248/bpb.29.903. [DOI] [PubMed] [Google Scholar]
- 41.Fang WJ, Lin CZ, Zhang HH, Qian J, Zhong L, Xu N. Detection of let-7a microrna by real-time pcr in colorectal cancer: a single-centre experience from China. J Int Med Res. 2007;35:716–23. doi: 10.1177/147323000703500518. [DOI] [PubMed] [Google Scholar]
- 42.Zhang HH, Wang XJ, Li GX, Yang E, Yang NM. Detection of let-7a microrna by real-time pcr in gastric carcinoma. World J Gastroenterol. 2007;13:2883–8. doi: 10.3748/wjg.v13.i20.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motoyama K, Inoue H, Nakamura Y, Uetake H, Sugihara K, Mori M. Clinical significance of high mobility group A2 in human gastric cancer and its relationship to let-7 microrna family. Clin Cancer Res. 2008;14:2334–40. doi: 10.1158/1078-0432.CCR-07-4667. [DOI] [PubMed] [Google Scholar]
- 44.Johnson CD, Esquela–Kerscher A, Stefani G, et al. The let-7 microrna represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–22. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 45.Takamizawa J, Konishi H, Yanagisawa K, et al. Reduced expression of the let-7 micrornas in human lung cancers in association with shortened postoperative survival. Cancer Res. 2004;64:3753–6. doi: 10.1158/0008-5472.CAN-04-0637. [DOI] [PubMed] [Google Scholar]
- 46.Johnson SM, Grosshans H, Shingara J, et al. Ras is regulated by the let-7 microrna family. Cell. 2005;120:635–47. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Schultz J, Lorenz P, Gross G, Ibrahim S, Kunz M. Microrna let-7b targets important cell cycle molecules in malignant melanoma cells and interferes with anchorage-independent growth. Cell Res. 2008;18:549–57. doi: 10.1038/cr.2008.45. [DOI] [PubMed] [Google Scholar]
- 48.Lu L, Katsaros D, de la Longrais IA, Sochirca O, Yu H. Hyper-methylation of let-7a-3 in epithelial ovarian cancer is associated with low insulin-like growth factor-ii expression and favorable prognosis. Cancer Res. 2007;67:10117–22. doi: 10.1158/0008-5472.CAN-07-2544. [DOI] [PubMed] [Google Scholar]
- 49.Jérôme T, Laurie P, Louis B, Pierre C. Enjoy the silence: the story of let-7 microrna and cancer. Curr Genomics. 2007;8:229–33. doi: 10.2174/138920207781386933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang J, Lee EJ, Gusev Y, Schmittgen TD. Real-time expression profiling of microrna precursors in human cancer cell lines. Nucleic Acids Res. 2005;33:5394–403. doi: 10.1093/nar/gki863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garzon R, Garofalo M, Martelli MP, et al. Distinctive microrna signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin. Proc Natl Acad Sci U S A. 2008;105:3945–50. doi: 10.1073/pnas.0800135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar MS, Erkeland SJ, Pester RE, et al. Suppression of non-small cell lung tumor development by the let-7 microrna family. Proc Natl Acad Sci U S A. 2008;105:3903–8. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okamura K, Phillips MD, Tyler DM, Duan H, Chou YT, Lai EC. The regulatory activity of microrna* species has substantial influence on microrna and 3′ utr evolution. Nat Struct Mol Biol. 2008;15:354–63. doi: 10.1038/nsmb.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richards M, Tan SP, Tan JH, Chan WK, Bongso A. The transcriptome profile of human embryonic stem cells as defined by SAGE. Stem Cells. 2004;22:51–64. doi: 10.1634/stemcells.22-1-51. [DOI] [PubMed] [Google Scholar]
- 55.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microrna processing by Lin-28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor microrna. Mol Cell. 2008;32:276–84. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Newman MA, Thomson JM, Hammond SM. Lin-28 interaction with the let-7 precursor loop mediates regulated microrna processing. RNA. 2008;14:1539–49. doi: 10.1261/rna.1155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piskounova E, Viswanathan SR, Janas M, et al. Determinants of microrna processing inhibition by the developmentally regulated rna-binding protein Lin28. J Biol Chem. 2008;283:21310–14. doi: 10.1074/jbc.C800108200. [DOI] [PubMed] [Google Scholar]
- 59.Rybak A, Fuchs H, Smirnova L, et al. A feedback loop comprising lin-28 and let-7 controls pre–let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10:987–93. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 60.Peter ME. Let-7 and mir-200 micrornas: guardians against pluripotency and cancer progression. Cell Cycle. 2009;8:843–52. doi: 10.4161/cc.8.6.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishino J, Kim I, Chada K, Morrison SJ. Hmga2 promotes neural stem cell self-renewal in young, but not old, mice by reducing p16Ink4a and p19Arf expression. Cell. 2008;135:227–39. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lengner CJ, Camargo FD, Hochedlinger K, et al. OCT4 expression is not required for mouse somatic stem cell self-renewal. Cell Stem Cell. 2007;1:403–15. doi: 10.1016/j.stem.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Droge P, Davey CA. Do cells let-7 determine stemness? Cell Stem Cell. 2008;2:8–9. doi: 10.1016/j.stem.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Boyerinas B, Park SM, Shomron N, et al. Identification of let-7–regulated oncofetal genes. Cancer Res. 2008;68:2587–91. doi: 10.1158/0008-5472.CAN-08-0264. [DOI] [PubMed] [Google Scholar]
- 65.Dalerba P, Cho RW, Clarke MF. Cancer stem cells: models and concepts. Annu Rev Med. 2007;58:267–84. doi: 10.1146/annurev.med.58.062105.204854. [DOI] [PubMed] [Google Scholar]
- 66.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–99. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 67.Park SM, Shell S, Radjabi AR, et al. Let-7 prevents early cancer progression by suppressing expression of the embryonic gene HMGA2. Cell Cycle. 2007;6:2585–90. doi: 10.4161/cc.6.21.4845. [DOI] [PubMed] [Google Scholar]
- 68.Giannini G, Kim CJ, Di Marcotullio L, et al. Expression of the HMGI(Y) gene products in human neuroblastic tumours correlates with differentiation status. Br J Cancer. 2000;83:1503–9. doi: 10.1054/bjoc.2000.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiappetta G, Bandiera A, Berlingieri MT, et al. The expression of the high mobility group HMGI (Y) proteins correlates with the malignant phenotype of human thyroid neoplasias. Oncogene. 1995;10:1307–14. [PubMed] [Google Scholar]
- 70.Abe N, Watanabe T, Suzuki Y, et al. An increased high-mobility group A2 expression level is associated with malignant phenotype in pancreatic exocrine tissue. Br J Cancer. 2003;89:2104–9. doi: 10.1038/sj.bjc.6601391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sarhadi VK, Wikman H, Salmenkivi K, et al. Increased expression of high mobility group A proteins in lung cancer. J Pathol. 2006;209:206–12. doi: 10.1002/path.1960. [DOI] [PubMed] [Google Scholar]
- 72.Nielsen J, Christiansen J, Lykke–Andersen J, Johnsen AH, Wewer UM, Nielsen FC. A family of insulin-like growth factor ii mrna-binding proteins represses translation in late development. Mol Cell Biol. 1999;19:1262–70. doi: 10.1128/mcb.19.2.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hansen TV, Hammer NA, Nielsen J, et al. Dwarfism and impaired gut development in insulin-like growth factor ii mrna-binding protein 1–deficient mice. Mol Cell Biol. 2004;24:4448–64. doi: 10.1128/MCB.24.10.4448-4464.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yisraeli JK. vickz proteins: a multi-talented family of regulatory rna-binding proteins. Biol Cell. 2005;97:87–96. doi: 10.1042/BC20040151. [DOI] [PubMed] [Google Scholar]
- 75.Kiel MJ, Iwashita T, Yilmaz OH, Morrison SJ. Spatial differences in hematopoiesis but not in stem cells indicate a lack of regional patterning in definitive hematopoietic stem cells. Dev Biol. 2005;283:29–39. doi: 10.1016/j.ydbio.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 76.Sun Y, Li H, Liu Y, Mattson MP, Rao MS, Zhan M. Evolutionarily conserved transcriptional co-expression guiding embryonic stem cell differentiation. PLoS ONE. 2008;3:3406. doi: 10.1371/journal.pone.0003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Knoepfler PS. Why Myc? An unexpected ingredient in the stem cell cocktail. Cell Stem Cell. 2008;2:18–21. doi: 10.1016/j.stem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 78.Zhou X, Benson KF, Ashar HR, Chada K. Mutation responsible for the mouse pygmy phenotype in the developmentally regulated factor Hmgi-C. Nature. 1995;376:771–4. doi: 10.1038/376771a0. [DOI] [PubMed] [Google Scholar]
- 79.Ioannidis P, Mahaira LG, Perez SA, et al. CRD-BP/IMP1 expression characterizes cord blood CD34+ stem cells and affects C-myc and igf-ii expression in MCF-7 cancer cells. J Biol Chem. 2005;280:20086–93. doi: 10.1074/jbc.M410036200. [DOI] [PubMed] [Google Scholar]
- 80.Shah YM, Morimura K, Yang Q, Tanabe T, Takagi M, Gonzalez FJ. ppara regulates an mirna-mediated signaling cascade responsible for hepatocellular proliferation. Mol Cell Biol. 2007;27:4238–47. doi: 10.1128/MCB.00317-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chang TC, Yu D, Lee YS, et al. Widespread microrna repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barh D, Parida S, Parida BP, Viswanathan G. Let-7, mir-125, mir-205, and mir-296 are prospective therapeutic agents in breast cancer molecular medicine. Gene Ther Mol Biol. 2008;12:189–206. [Google Scholar]
- 83.Lee YS, Dutta A. The tumor suppressor microrna let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21:1025–30. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng Y, Laser J, Shi G, et al. Antiproliferative effects by let-7 repression of high-mobility group A2 in uterine leiomyoma. Mol Cancer Res. 2008;6:663–73. doi: 10.1158/1541-7786.MCR-07-0370. [DOI] [PubMed] [Google Scholar]
- 85.Tsang WP, Kwok TT. Let-7a microrna suppresses therapeutics-induced cancer cell death by targeting caspase-3. Apoptosis. 2008;13:1215–22. doi: 10.1007/s10495-008-0256-z. [DOI] [PubMed] [Google Scholar]
- 86.He YJ, Guo L, D ZH. Let-7 and mir-24 in uvb-induced apoptosis [Chinese] Zhonghua Fang She Yi Xue Yu Fang Hu Za Zhi. 2009;29:234–6. [Google Scholar]
- 87.Calin GA, Sevignani C, Dumitru CD, et al. Human microrna genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shell S, Park SM, Radjabi AR, et al. Let-7 expression defines two differentiation stages of cancer. Proc Natl Acad Sci U S A. 2007;104:11400–5. doi: 10.1073/pnas.0704372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microrna expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 90.Esquela–Kerscher A, Trang P, Wiggins JF, et al. The let-7 microrna reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–64. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- 91.Grimm D, Streetz KL, Jopling CL, et al. Fatality in mice due to oversaturation of cellular microrna/short hairpin rna pathways. Nature. 2006;441:537–41. doi: 10.1038/nature04791. [DOI] [PubMed] [Google Scholar]
- 92.Krutzfeldt J, Rajewsky N, Braich R, et al. Silencing of micrornas in vivo with “antagomirs. Nature. 2005;438:685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 93.Krutzfeldt J, Kuwajima S, Braich R, et al. Specificity, duplex degradation and subcellular localization of antagomirs. Nucleic Acids Res. 2007;35:2885–92. doi: 10.1093/nar/gkm024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valoczi A, Hornyik C, Varga N, Burgyan J, Kauppinen S, Havelda Z. Sensitive and specific detection of micrornas by northern blot analysis using lna-modified oligonucleotide probes. Nucleic Acids Res. 2004;32:175. doi: 10.1093/nar/gnh171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davis S, Lollo B, Freier S, Esau C. Improved targeting of mirna with antisense oligonucleotides. Nucleic Acids Res. 2006;34:2294–304. doi: 10.1093/nar/gkl183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Esau C, Davis S, Murray SF, et al. mir-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 97.Orom UA, Kauppinen S, Lund AH. lna-modified oligonucleotides mediate specific inhibition of microrna function. Gene. 2006;372:137–41. doi: 10.1016/j.gene.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 98.Weiler J, Hunziker J, Hall J. Anti-mirna oligonucleotides (amos): ammunition to target mirnas implicated in human disease? Gene Ther. 2006;13:496–502. doi: 10.1038/sj.gt.3302654. [DOI] [PubMed] [Google Scholar]
- 99.Esau CC, Monia BP. Therapeutic potential for micrornas. Adv Drug Deliv Rev. 2007;59:101–14. doi: 10.1016/j.addr.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 100.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering rnas in mammalian cells. Science. 2002;296:550–3. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 101.Miyagishi M, Taira K. U6 promoter-driven sirnas with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol. 2002;20:497–500. doi: 10.1038/nbt0502-497. [DOI] [PubMed] [Google Scholar]
- 102.Soifer HS, Rossi JJ, Saetrom P. Micrornas in disease and potential therapeutic applications. Mol Ther. 2007;15:2070–9. doi: 10.1038/sj.mt.6300311. [DOI] [PubMed] [Google Scholar]
- 103.Boden D, Pusch O, Silbermann R, Lee F, Tucker L, Ramratnam B. Enhanced gene silencing of hiv-1 specific sirna using microrna designed hairpins. Nucl Acids Res. 2004;32:1154–8. doi: 10.1093/nar/gkh278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chung KH, Hart CC, Al-Bassam S, et al. Polycistronic rna polymerase ii expression vectors for rna interference based on Bic/mir-155. Nucl Acids Res. 2006;34:e53. doi: 10.1093/nar/gkl143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeng Y, Cai X, Cullen BR. Use of rna polymerase ii to transcribe artificial micrornas. Methods Enzymol. 2005;392:371–80. doi: 10.1016/S0076-6879(04)92022-8. [DOI] [PubMed] [Google Scholar]
- 106.Zeng Y, Wagner EJ, Cullen BR. Both natural and designed micro rnas can inhibit the expression of cognate mrnas when expressed in human cells. Mol Cell. 2002;9:1327–33. doi: 10.1016/s1097-2765(02)00541-5. [DOI] [PubMed] [Google Scholar]
- 107.Zhou H, Xia XG, Xu Z. An rna polymerase ii construct synthesizes short-hairpin rna with a quantitative indicator and mediates highly efficient rnai. Nucl Acids Res. 2005;33:e62. doi: 10.1093/nar/gni061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stegmeier F, Hu G, Rickles RJ, Hannon GJ, Elledge SJ. A lentiviral microrna-based system for single-copy polymerase ii–regulated rna interference in mammalian cells. Proc Natl Acad Sci U S A. 2005;102:13212–17. doi: 10.1073/pnas.0506306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen S, Ni M, Yu B, Lv T, Lu M, Gong F. Construction and identification of a human liver specific microrna eukaryotic expression vector. Cell Mol Immunol. 2007;4:473–7. [PubMed] [Google Scholar]
- 110.Wolfrum C, Shi S, Jayaprakash KN, et al. Mechanisms and optimization of in vivo delivery of lipophilic sirnas. Nat Biotechnol. 2007;25:1149–57. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]