Abstract
The NRF2 transcription factor regulates a major environmental and oxidative stress response. NRF2 is itself negatively regulated by KEAP1, the adaptor of a Cul3-ubiquitin ligase complex that marks NRF2 for proteasomal degradation by ubiquitination. Electrophilic compounds activate NRF2 primarily by inhibiting KEAP1-dependent NRF2 degradation, through alkylation of specific cysteines. We have examined the impact on KEAP1 of reactive oxygen and nitrogen species, which are also NRF2 inducers. We found that in untreated cells, a fraction of KEAP1 carried a long range disulfide linking Cys226 and Cys613. Exposing cells to hydrogen peroxide, to the nitric oxide donor spermine NONOate, to hypochlorous acid, or to S-nitrosocysteine further increased this disulfide and promoted formation of a disulfide linking two KEAP1 molecules via Cys151. None of these oxidants, except S-nitrocysteine, caused KEAP1 S-nitrosylation. A cysteine mutant preventing KEAP1 intermolecular disulfide formation also prevented NRF2 stabilization in response to oxidants, whereas those preventing intramolecular disulfide formation were functionally silent. Further, simultaneously inactivating the thioredoxin and glutathione pathways led both to major constitutive KEAP1 oxidation and NRF2 stabilization. We propose that KEAP1 intermolecular disulfide formation via Cys151 underlies the activation of NRF2 by reactive oxygen and nitrogen species.
Keywords: Enzymes/Oxidation Reduction, Protein/Disulfide, Protein/Post-translational Modification, Regulation Covalent Modification, Signal Transduction/Nitric Oxide, Sulfhydryls/Disulfide, KEAP1, NRF2, Hydrogen Peroxide, Nitric Oxide
Introduction
The Cap'n'collar bZip transcription factor NRF2 regulates an environmental and oxidative stress response of major physiological importance in mammals. NRF2 is activated by reactive oxygen and nitrogen species, electrophilic xenobiotics, and heavy metals and promotes cytoprotection and survival toward these stresses (for a review, see Refs. 1 and 2). Activation of NRF2 is intricate, engaging controls at the level of subcellular distribution, interaction with other proteins, phosphorylation, and protein stability (reviewed in Ref. 2). Among these, protein stability is a major control determinant, involving KEAP1, the adaptor of a Cul3-ubiquitin ligase complex that ubiquitinates NRF2 and marks it for proteasomal degradation (3–6). Stress signals that activate NRF2, herein named NRF2 inducers, are primarily sensed at the level of KEAP1, causing NRF2 protein stabilization (7–9) by inhibiting KEAP1-mediated NRF2 ubiquitination (10, 11).
The large number of NRF2 inducers and their quite different chemical nature have raised the question of how they are specifically sensed by KEAP1. Although NRF2 inducers are chemically very different, they all have electrophilic properties, which has led to the proposal that they must operate by alkylation and/or oxidation of KEAP1 Cys residues (12). The 624-amino acid-long KEAP1 protein has 25 (mouse) or 27 (human) Cys residues and carries a Broad complex, Tramtrack, Bric-à-Brac (BTB)2 dimerization domain, an intervening region (IVR), and a six-Kelch repeat domain (Kelch) (see Fig. 2). It also binds zinc with a 1:1 stoichiometry, possibly through the IVR residues Cys254, Cys273, Cys288, and Cys293, as suggested by the 100-fold lower zinc affinity of mutants lacking these residues (13).
FIGURE 2.
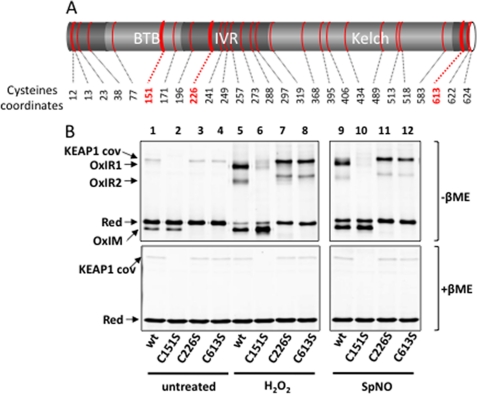
Identification of oxidized Keap1 cysteine residues. A, domain organization of the human KEAP1 protein. Positions of the KEAP1 cysteine residues are mapped in red. B, HeLa cells transfected with pcDNA expressing wild type (wt) HA-KEAP1 or the cysteine substitution derivatives C151S, C226S, or C613S were left untreated or exposed to H2O2 (0.2 mm) for 5 min, as indicated. The lysates were processed as described for Fig. 1. The arrowed band denoted cov corresponds to a noninducible, redox-insensitive KEAP1 modification (see text).
Several laboratories have sought to identify in vitro which of the KEAP1 Cys residues are modified by NRF2 inducers, each identifying a different set of Cys residues, with most residues identified at least once (summarized in Refs. 14 and 15). Still the BTB domain Cys151 and IVR Cys288 came out as the most frequently identified and the most reactive residues. In vivo proofs of the modification of KEAP1 at Cys residues have also been obtained from cells treated with oxidized lipids (16–18), a carnosic acid derivative (19), nitric oxide and 8-nitro-cGMP (20, 21), and N-iodoacetyl-N-biotinylhexylenediamine (22), thus corroborating the hypothesis of a Cys residue-based mechanism in KEAP1 regulation. The latter study also mapped modified residues that included IVR cysteines and Cys151 (22).
A third and very informative approach to the KEAP1 redox mechanism has been to evaluate the effect of Cys residue substitution on KEAP1 function. In cells expressing KEAP1 mutants that lack Cys273 or Cys288, NRF2 is constitutively active (11, 17, 23). Because these residues might contribute to zinc coordination, their substitution (or modification) could alter KEAP1 function through the loss of a structural or a redox regulatory zinc motif. In cells that express a KEAP1 mutant lacking Cys151, NRF2 basal activity is in contrast low and cannot be induced by tert-butylhydroquinone (t-BHQ) and many other NRF2 inducers (6, 11, 24–26). Mice transgenic complementation rescue experiments have confirmed the functional importance of KEAP1 Cys273, Cys288, and Cys151 (26). Further, a recent systematic study in zebra fish classified NRF2 inducers into at least two classes, one requiring KEAP1 Cys151 and the other requiring Cys273, thus re-emphasizing the functional importance of these residues (27). By concluding that different NRF2 inducers trigger different Cys-based regulatory mechanisms, this study may also explain at least in part why the in vitro searches for reactive Cys residues, which have been conducted by different laboratories using different inducers, have yielded different results.
In the present study, we have examined the mechanism of KEAP1 regulation by H2O2, NO, and HOCl. These compounds are NRF2 inducers (20, 28–32) and are physiologically important as endogenously produced. A negative NRF2 regulation by H2O2 has been described though (33). Although strongly electrophilic, these compounds differ chemically from the NRF2 inducers studied so far, because they are oxidants and not alkylating agents and are thus anticipated to modify Cys residue by oxidation. Despite the many studies of the KEAP1 redox mechanism, it is not known whether its Cys residues could undergo oxidation in vivo. We have thus carefully monitored the effect of H2O2, the NO releasing agent spermine NONOate (SpNO), and HOCl on the KEAP1 redox state and found that these compounds similarly oxidize KEAP1 with formation of intra- and intermolecular disulfides. Evaluation of the role of the oxidized residues on KEAP1 function suggests that the intermolecular disulfide is important for activation, whereas the intramolecular disulfide could have a structural role. We also show that simultaneous inactivation of the thioredoxin and glutathione systems leads to constitutive KEAP1 oxidation and strong NRF2 stabilization.
EXPERIMENTAL PROCEDURES
Cell Culture and Reagents
HeLa cells were grown at 37 °C, 5% CO2, in Dulbecco's modified Eagle's medium containing 1 g/liter of glucose, 110 mg/ml sodium pyruvate, 4 mm GlutaMAX (Invitrogen), complemented with 10% fetal calf serum (Sigma). For plasmid transfection, ∼5.5 105 cells were incubated 5 h with 3 μg of DNA and Lipofectamine 2000 (Invitrogen) following the supplier's recommendations, washed, and incubated in fresh medium. The cells were treated as described in the text 24 h after transfection. For the TRxR1 knockdown, ∼2.5 105 cells were transfected with 3 μg of DNA, by the same protocol. After 24 h, hygromycin B was added (250 μg/ml) to the culture medium twice every 2 days. The cells were then expanded and kept in the presence of hygromycin B (125 μg/ml). The selection was withdrawn before experiments. N-Ethylmaleimide (NEM), t-BHQ, H2O2, and cycloheximide were purchased from Sigma, and SpNO was from Cayman Chemical. S-nitrosocysteine (Cys-NO) was prepared by mixing stoichiometric amounts of l-cysteine and sodium nitrite at pH 4, followed by the measure of its concentration by recording the absorbance at 334 nm.
Plasmids
Plasmid pcDNA3-HA-mKEAP1 was a gift from Dr. M. Yamamoto, pCI-HA-mNRF2 from was J. A. Diehl, and peYFP-N1 was purchased from Clontech. Plasmid pcDNA3- Myc-His-mKEAP1 was constructed by PCR-mediated replacement of the HA tag sequence of pcDNA3-HA-mKEAP1 with three Myc tag sequences followed by a stretch of eight His codons. Mutagenesis was done with the Stratagene QuikChange multi kit following the manufacturer's instructions. For the TrxR1 knockdown, we used TrxR1-specific small hairpin RNA (shRNA) that targeted the following sequences within the open reading frame: GGATTAAGGCAACAAATAA (sh1), GCATCAAGCAGCTTTGTTA (sh2), and GCAAGACTCTCGAAATTAT (sh3). shRNA sequences were designed with the DSIR program that also operates an exact similarity search algorithm for potential off target detection (34). These shRNA were expressed under the control of the H1 promoter from the Epstein-Barr virus-based replicative plasmid that contains an oriP, the EBNA open reading frame, and a hygromycin B selection cassette (35). Control lines expressed a nonfunctional shRNA as reported (35).
Redox Western
The cells were washed on ice with 40 mm NEM in phosphate-buffered saline and lysed in lysis buffer (0.1 m Tris-HCl, pH 8.0, 120 mm NaCl, 0.2% deoxycholic acid, 5% Nonidet P-40, 0.2 mm NaF, 0.2 mm EGTA, 0.1 mm phenylmethylsulfonyl fluoride, Roche-Complete mini protease inhibitor mixture, 40 mm NEM). Centrifuged-cleared lysates were diluted with 2 volumes of 3× loading buffer (0.2 m Tris-HCl, pH 6.8, 45% glycerol, 6% SDS, 0.03% bromphenol blue). Half of the samples were reduced by the addition of β-mercaptoethanol (6% v/v). After heat denaturation, the proteins were separated by SDS-PAGE, transferred onto a nitrocellulose membrane, and immunostained with anti-NRF2 (H300; Santa Cruz), anti-HA (HA11; Covance), anti-Myc (9E10; a kind gift from G. Clément and C. Créminon, France), anti-TrxR1 (a kind gift from A Holmgren, Sweden), or anti-YFP (JL8; Clontech) specific antibodies. Detection was performed after chromophore-coupled secondary antibody staining, using the LICOR Odyssey infrared imager.
Protein Pulldown Assays
For pulling down His tag-containing polypeptides, the cells (106/sample) were washed in ice-cold phosphate-buffered saline containing NEM (40 mm) and lysed in precipitation buffer (0.1 m Tris-HCl, pH 8, 1% Nonidet P-40, 2% glycerol, 0.3 m NaCl, 0.2 mm phenylmethylsulfonyl fluoride, Roche-Complete mini protease Inhibitor mixture, 40 mm NEM). 10 mm imidazole was then added, and 90% of the sample (250 μl) was incubated with 50 μl of a nickel-nitrilotriacetic acid resin (Qiagen) for 1 h. After extensive washing with wash buffer (0.1 m Tris-HCl, pH 8, 1% Nonidet P-40, 2% glycerol, 0.3 m NaCl, 0.2 mm phenylmethylsulfonyl fluoride, 10 mm NEM, 10 mm imidazole), the proteins were eluted with 2× protein-loading buffer containing 10 mm NEM and separated by SDS-PAGE.
The Biotin-switch Technique
The biotin-switch method was performed as described in Ref. 36, with the difference that cells were directly lysed in HEN buffer (250 mm HEPES, pH 7.7, 1 mm EDTA, 0.1 mm neocuproine) containing SDS (2.5%) and the thiol-reactive compound S-methyl methanethiosulfonate (0.1%) to block thiol modification during and after the lysis. Briefly, the proteins were precipitated and washed twice with acetone, then the pellets were dissolved in HEN buffer containing SDS (1%), and reduction and labeling of S-nitrosothiols were achieved by the addition of sodium ascorbate (100 μm) and biotin-HPDP (0.25 mg/ml) upon incubation in the dark at room temperature. For the specific detection of S-nitroso-KEAP1, the samples were precipitated with acetone, washed with acetone, dissolved in HEN buffer 0.1× containing SDS (1%). 3 volumes of (v/v) neutralization buffer (25 mm HEPES, pH 7.5, 100 mm NaCl, 1 mm ETDA, 1% Triton X-100) was added before incubation with a streptavidin-agarose slurry. Elution was performed in 0.1× HEN buffer containing β-mercaptoethanol (1%). Western blots were then performed using anti-Myc antibodies.
RESULTS
Oxidation of KEAP1 in Cells Exposed to H2O2
We evaluated whether H2O2 could oxidize KEAP1 at Cys residues by analyzing the redox state of HA-KEAP1 ectopically expressed in HeLa cells. To prevent Cys residue oxidation, free sulfhydryls were blocked with NEM during sample preparation. HA-KEAP1 from untreated cell lysates that had been reduced by β-mercaptoethanol migrated in SDS-PAGE as a single band with an apparent molecular mass of ∼70 kDa (Fig. 1A, lower panel). When reduction was omitted, HA-KEAP1 from the same lysates still migrated as a major band of same molecular mass (denoted Red for reduced), but now a second less intense band of faster mobility was observed (denoted OxIM for oxidized intramolecular; see below) (Fig. 1A, upper panel). A 5-min exposure of cells to H2O2 (200 μm) further altered migration of KEAP1 under nonreducing conditions (Fig. 1A, lane 2); the reduced KEAP1 70-kDa band decreased in intensity, whereas OxIM increased and two new bands of slower migration appeared (denoted OxIR1 and OxIR2 for oxidized intermolecular; see below; lane 2). OxIM, OxIR1, and OxIR2 were absent under reducing conditions, indicating that they probably result from disulfide formation. OxIM might correspond to an intramolecular disulfide (see below), the formation of which is predicted to increase SDS-PAGE mobility because of a decrease in the hydrodynamic radius of the SDS-bound polypeptide, especially if its two constitutive Cys residues are far apart in the primary sequence. OxIR1/2 could correspond to intermolecular disulfides between KEAP1 and itself or with another polypeptide. Such oxidative KEAP1 modifications were not seen upon cell exposure to t-BHQ (supplemental Fig. S1A). Time course analysis showed that KEAP1 was maximally oxidized 5 min after exposure to H2O2 and then returned to the redox state of untreated cells 40 min after this treatment (Fig. 1A, lanes 2–7). Such kinetics presumably reflects KEAP1 reduction by endogenous reductases and not de novo protein synthesis, because it was similar in cells treated with the protein synthesis inhibitor cycloheximide (supplemental Fig. S1B). H2O2 dose-response analysis (Fig. 1B) indicated that the basal KEAP1 oxidation seen in lysates from untreated cells started to increase at a concentration of 100 μm and was maximal at 200 μm. Higher doses were not tested because of potential toxicity.
FIGURE 1.
KEAP1 becomes oxidized in cells treated with oxidants. A–C, HeLa cells transfected with pcDNA-HA-KEAP1 were exposed to H2O2 (0.2 mm) for the indicated time (A) or for 5 min in the presence of different concentrations of H2O2 as indicated (B), or to SpNO (1 mm) for the indicated time (C). The cells were lysed in the presence of NEM (40 mm) to block free sulfhydryls (see “Experimental Procedures”). The lysates were separated by nonreducing (upper panels) or reducing (lower panels) SDS-PAGE, and KEAP1 was revealed by Western blot using an anti-HA antibody. The arrows indicate the oxidized slow (OxIR1 and OxIR2) and fast (OxIM) and reduced (Red) KEAP1 species. D, HeLa cells expressing pcDNA-HA-KEAP1were either left untreated or were exposed to H2O2 (0.2 mm for 5 min), to CysNO (0.5 mm) for 10 min, or to SpNO (2 mm) for 45 min. KEAP1 S-nitrosylation was evaluated by the biotin-switch method as described under “Experimental Procedures.” KEAP1 was revealed by anti-HA Western blot of the streptavidin eluate (upper panel) and as control of the corresponding whole cell extracts (lower panel). βME, β-mercaptoethanol.
KEAP1 Disulfide Bond Formation Induced by NO Derivatives and HOCl
We were interested to see whether other oxidants known to activate NRF2, such as NO and derivatives or the potent thiol oxidant hypochlorous acid (HOCl) could also lead to KEAP1 Cys residue oxidation. We used the NO donor SpNO that decomposes in culture medium into two NO equivalents with an approximate half-life of 2 h (not shown). In lysates from HA-KEAP1-expressing HeLa cells exposed for 15 min to SpNO (1 mm), KEAP1 appeared oxidized in nonreduced SDS-PAGE, with a migration very similar to that of H2O2-oxidized KEAP1 (Fig. 1C, compare lanes 1–3). Oxidation was not as important but was maintained up to 3 h versus 40 min with H2O2 (Fig. 1A). Such a prolonged response probably reflects the 2-h half-life of SpNO-released NO, in contrast to a bolus of H2O2 that is rapidly degraded by cellular consumption. KEAP1 also became oxidized upon cell exposure to HOCl at the low dose of 1 mm, with the formation of oxidized species similar to those seen upon exposure to H2O2 and SpNO (supplemental Fig. S2A). NO can cause protein S-nitrosylation, but we could not detect any modification of KEAP1 by S-nitrosylation when cells were exposed to SpNO (2 mm) for 45 min or to H2O2 for 5 min (Fig. 1D). In contrast, and as a positive control, a 30-min cell exposure to the trans-nitrosylating agent Cys-NO (0.5 mm) led to potent KEAP1 S-nitrosylation. Interestingly, Cys-NO also led to KEAP1 disulfide bond formation (supplemental Fig. S2B).
Identification of the KEAP1 Cys Residues Engaged in Disulfide Bonds
We used a mutagenesis approach to identify the KEAP1 Cys residues whose oxidation caused alteration of protein migration. 23 of the 25 Cys residues of murine KEAP1 (Fig. 2A) were substituted by a serine, generating 19 single and two double-Cys residues mutants (C513S/C518S and C622S/C624S). Cys368 and Cys489 were not tested because, as predicted by a KEAP1 Kelch crystallographic structure (37), they are unlikely to participate in redox regulation, as not being solvent-exposed and lacking a proximal Cys residue. These mutants were each expressed in HeLa cells and tested for their oxidation upon a 5-min exposure to H2O2 (200 μm) or to SpNO (2 mm) (Fig. 2B and supplemental Fig. S3). Except KEAP1 Cys151, Cys226, and Cys613, all of the mutants had nonreducing SDS-PAGE migrations similar to that of wild type HA-KEAP1.
KEAPC151S still formed OxIM, but not OxIR1 and 2 (Fig. 2B, compare lanes 5 and 6 with lanes 9 and 10). A faint and fuzzy band just below OxIR1 was present instead. Note also here a β-mercaptoethanol-insensitive KEAP1 band just above OxIR1 already present in lysates from untreated cells that also disappeared in KEAPC151S (Fig. 2B) (denoted cov for covalent). This KEAP1 band, which varied in intensity between experiments, was resistant to all reducing agents tested (not shown) and therefore corresponds to a covalent modification of unknown nature engaging Cys151. Disappearance of OxIR1 and 2 in KEAPC151S indicates that KEAP1 engages Cys151 in an intermolecular disulfide with itself and/or with another protein to account for the two slow migrating β-mercaptoethanol-sensitive species. In the absence of Cys151, illegitimate unstable disulfides might form to account for the faint slow migrating band seen with KEAP1C151S.
Substituting Cys226 or Cys613 had the same effect on KEAP1 migration (Fig. 2B, lanes 3, 4, 7, 8, 11, and 12). These mutants were both unable to form OxIM but still formed the OxIR1 and 2 bands, although their migration was slightly up-shifted. These data indicate that Cys226 and Cys613 are engaged in an intramolecular disulfide linkage spanning across the Kelch domain (Fig. 2A). The presence of this disulfide in the wild type KEAP1 OxIR1/2 complexes would then explain the migration up-shift of these two bands when either Cys226 or Cys613 are mutated. Similarly, the higher intensity of OxIM in KEAP1Cys151 in oxidant-treated cells reflects the fact that all of the KEAP1 species that carry the intramolecular disulfide are now shifted down to the size of this band.
Thus, a fraction of KEAP1 carries an intramolecular Cys226–Cys613 disulfide. H2O2 and SpNO further increase this disulfide, also causing formation of an intermolecular KEAP1 disulfide(s). These two disulfides are independent, because one can form in the absence of the other(s).
KEAP1 Homodimerizes through a Cys151-dependent Disulfide Bond
We tested whether either one or both of the two KEAP1 intermolecular disulfide-linked complexes (OxIR1/2) could be the result of KEAP1 dimerization by co-immunoprecipitation assays, using two versions of KEAP1 that differed by both their size and tag. We thus co-expressed in HeLa cells HA-KEAP1 (molecular mass, 71 kDa) and a version of KEAP1 containing a three-Myc epitope tag fused to an eight-His tag (Myc-His-KEAP1) (molecular mass, 76 kDa).
When individually expressed, HA-KEAP1 from cells exposed to H2O2 displayed the characteristic migration of oxidized protein (Fig. 3, lane 5). Note here that reduced KEAP1 was absent, indicating almost full oxidation. When HA-KEAP1 was co-expressed with Myc-His-KEAP1, two new HA-immunoreactive faint bands could now be seen that migrated just above the HA-KEAP1 OxIR1/2 bands (Fig. 3, lane 6, see red arrows). In these lysates anti-Myc antibodies revealed Myc-His-KEAP1, which had the characteristic migration of oxidized protein but was slightly slower than HA-KEAP1 because of its extra 5 kDa (lane 2). However, migration of Myc-His-KEAP1 was not affected by co-expressed HA-KEAP1 in contrast to the latter, probably because of a higher level of expression of the former. Western blots were then performed with eluates of a nickel column on which lysates of cells co-expressing the two KEAP1 derivatives had been adsorbed to pull down Myc-His-KEAP1. In these eluates, the anti-Myc antibody revealed enriched oxidized Myc-His-KEAP1 (lane 4), the anti-HA antibody HA-KEAP1 OxIM, and two slow migrating bands of the size of the extra HA-immunoreactive faint bands seen in crude extracts (Fig. 3, lane 8, see the red arrows). When the same experiment was performed with the Cys151-substituted versions of the two KEAP1 derivatives, HA-KEAP1 OxIM1 was still pulled down with Myc-His-KEAP1, but no slower oxidized band could be seen (supplemental Fig. S4). We conclude that these two HA-immunoreactive bands pulled down with Myc-His-KEAP1, each corresponding to a Cys151-Cys151 mixed disulfide between one molecule of HA-KEAP1 and one of Myc-His-KEAP1. Such a conclusion is compatible with both the sizes of these two bands, which are intermediate between those of the KEAP1-derivatives OxIR1/2 bands, and with the ∼140-kDa apparent molecular mass of OxIR1 that equals two KEAP1 molecules. The faster OxIR2 band, always much fainter, might be redox conformation-dependent but is not the result of a N-terminal protein cleavage (not shown). Importantly, whereas the monomeric intramolecular disulfide form of HA-KEAP1 (HA-OxIM) co-precipitated with Myc-His-KEAP1 (see Fig. 3, lane 8 and supplemental Fig. S4), most probably by virtue of noncovalent dimerization imparted by the BTB domain (4, 5, 38), the intermolecular HA-KEAP1-HA-KEAP1 disulfide was not co-precipitated, which suggests that dimerization of KEAP1 through the BTB and through the intermolecular disulfide might exclude each other.
FIGURE 3.
KEAP1 forms disulfide-linked homodimers. HeLa cells transfected with pcDNA-HA-KEAP1 and pcDNA-Myc-His-KEAP1 as indicated, were exposed to H2O2 (0.2 mm) for 5 min. Whole cell lysates (WCE) or the eluate of lysates adsorbed onto a nickel column (Ni2+ p.d.) were separated by nonreducing (upper panel) or reducing (lower panel) SDS-PAGE. HA-KEAP1 and Myc-His-KEAP1 were then revealed by Western blot (WB) with anti-HA or anti-Myc antibodies, as indicated. The black arrows indicate the bands corresponding to the disulfide-based homodimers of HA-KEAP1 or Myc-His-KEAP1 (OxIR1 and OxIR2) and intramolecular disulfide forms of these proteins (OxIM). The red arrows indicate the bands corresponding to the disulfide-based HA-KEAP1-Myc-His-KEAP1 heterodimer. Lane M, molecular mass markers; βME, β-mercaptoethanol.
Functional Consequences of KEAP1 Disulfide Bond Formation
Stress signals activate NRF2 by inhibiting KEAP1-dependent NRF2 degradation. To verify the functional significance of KEAP1 oxidation and of identified Cys residues, we monitored NRF2 protein abundance as readout of KEAP1 activity in HeLa cells co-expressing HA-NRF2, Myc-His-KEAP1 or its Cys mutant derivatives, and YFP as transfection control.
H2O2 induced a slight but reproducible and transient stabilization of NRF2 that closely paralleled KEAP1 oxidation (Fig. 4A). Such a marginal effect of H2O2 might relate to the transient nature of KEAP1 modification induced by this very short-lived inducer that denied NRF2 build up by neo-synthesis. HOCl, as a short-lived oxidant, also slightly stabilized NRF2, with kinetics that paralleled KEAP1 oxidation (supplemental Fig. S2A), whereas Cys-NO, which had a very potent and extended effect on KEAP1 oxidation, stabilized NRF2 up to 3 h (supplemental Fig. S2B). SpNO was also kinetically different, because stabilization of NRF2 was not detected early in the kinetics (supplemental Fig. S2C) but was significant when measured at 5 h (Fig. 4B, lane 8), consistent with the long half-life of SpNO causing lower but prolonged KEAP1 oxidation (Fig. 1C) and therefore steady NRF2 accumulation with time.
FIGURE 4.
Oxidation of KEAP1 parallels NRF2 stabilization. HeLa cells transfected with pcDNA-Myc-His-KEAP1 or Cys-mutants derivatives (1 μg), pCI-HA-NRF2 (1.5 μg), and peYFP-N1 (0.3 μg), as indicated, were treated with H2O2 (A, 0.2 mm) for the indicated time or left untreated or treated with t-BHQ (80 μm) or SpNO (2 mm) as indicated (B). The lysates were processed as for Fig. 1. KEAP1, NRF2, and YFP were revealed by Western blot using anti-Myc, anti-NRF2, or anti-YFP antibodies, as indicated. NRF2 abundance was quantified and normalized by the value of the YFP signal (lower panel). βME, β-mercaptoethanol.
We took advantage of the potent stabilization of NRF2 by SpNO to evaluate the effect of mutations that affect KEAP1 oxidation. As shown in Fig. 4B, NRF2 levels were elevated in the absence of KEAP1 and decreased upon KEAP1 co-expression (compare lanes 1 and 2). Under basal conditions, substitution of neither Cys151 nor Cys226 altered KEAP1 influence on NRF2 levels (Fig. 4B, lanes 3 and 4). As a control experiment, we checked the response to t-BHQ, which as previously shown relieved KEAP1-mediated NRF2 repression in the presence of wild type KEAP1 but not of KEAP1Cys151S (Fig. 4B, lanes 5 and 6) (6, 11). However, KEAP1Cys226S still responded to t-BHQ (Fig. 4B, lane 7). Checking the response to SpNO showed that it was similar to t-BHQ; the potent repression relief caused by SpNO at 5 h in the presence of wild type KEAP1 was not observed with KEAP1Cys151S (Fig. 4B, lanes 8 and 9); however, KEAP1Cys226S had a wild type response to SpNO-induced derepression of NRF2 (Fig. 4B, lane 10). Therefore, intermolecular disulfide formation via Cys151 is important for relieving KEAP1-mediated NRF2 degradation.
The Effect of Inactivating Thiol Redox Control on KEAP1 Oxidation and Function
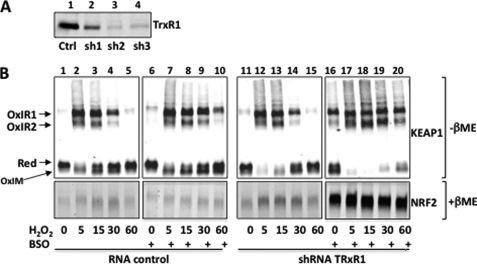
The transient nature of KEAP1 oxidation by H2O2 or SpNO (Figs. 1 and 4) suggests that cellular thiol reductases recycle the protein back to its reduced form. We sought to evaluate the redox state and activity of KEAP1 upon inactivating either one or both of the two thiol-redox control system. To inactivate the GSH pathway, we used buthionine sulfoximine, a specific inhibitor of the GSH rate-limiting biosynthetic enzyme γ-glutamyl cysteine synthase. To inactivate the thioredoxin pathway, we stably expressed in HeLa cells shRNAs targeting the TrxR1 mRNA. Two of the three tested TrxR1 shRNA caused protein level reduction of ∼90%, as compared with a TrxR1 unrelated small nonhairpin RNA (Fig. 5A). Buthionine sulfoximine treatment (0.1 mm for 24 h) lead to an 80% decrease of the total GSH cellular content as measured by the 5,5′-dithiobis (2-nitrobenzoic acid)-GSSG reductase recycling assay (not shown) but altered neither KEAP1 oxidation nor NRF2 stabilization, only slightly delaying protein reduction (Fig. 5B, compare lanes 1–5 with lanes 6–10). Knockdown of TrxR1 moderately increased the effect of H2O2 on KEAP1 oxidation and on NRF2 stabilization but did not delay reduction (compare lanes 1–5 with lanes 11–15). In contrast, buthionine sulfoximine treatment of TrxR1 knockdown cells caused constitutive KEAP1 oxidation and major NRF2 stabilization. In these cells, H2O2 further increased KEAP1 oxidation up to shifting all of the protein to the OxIR1 and 2 bands but did not further stabilize NRF2 because its levels might have already reached a plateau. Surprisingly 30 min after H2O2 treatment, some reduction of KEAP1 started to occur back to the levels of untreated cells, which indicate the presence of a remnant thiol reductase activity in these cells.
FIGURE 5.
The effect of inactivating thiol-reductase pathways on KEAP1 oxidation. A, TRxR1 protein abundance in HeLa cells stably expressing a control (Ctrl) nonhairpin RNA (RNA control) or shRNAs targeting the TRxR1 message (sh1, sh2, and sh3), evaluated by Western blot using an anti-TrxR1 antibody. B, HeLa cells stably expressing sh2 were transfected with pcDNA3-Myc-His-mKEAP1 (1 μg) and pCI-HA-mNRF2 (1.5 μg). The cells were incubated or not with buthionine sulfoximine (BSO, 0.1 mm) for 24 h as indicated and were then treated or not with H2O2 (0.1 mm) for the indicated time. The redox state of KEAP1 was evaluated by redox Western blot as in Fig. 1, and the abundance of NRF2 by Western blot was determined.
Thus, upon inactivating both thiol redox pathways, low endogenous reactive oxygen species levels cause a build up of KEAP1 oxidation and NRF2 levels with time, further indicating a correlation between KEAP1 oxidation and NRF2 stabilization. These data could also indicate redundancy of the two pathways in KEAP1 reduction, but partial inactivation of these pathways, as indicated by the residual KEAP1 reduction, does not allow such a conclusion.
DISCUSSION
In this study we have questioned whether regulation of KEAP1, the inhibitor of the electrophilic and oxidative stress response regulator NRF2, involves disulfide bond formation, a post-translational control mechanism often used by oxidative stress regulators (39). We addressed this question by studying the KEAP1 response to H2O2, the nitric oxide donor SpNO, and HOCl, three chemicals that differ from other inducers by not being alkylating agents but oxidants that modify Cys residues by oxidation or S-nitrosylation. We now show that in untreated cells, a fraction of KEAP1 carried a long range intramolecular disulfide linking Cys226 and Cys613. Exposing cells to H2O2, SpNO, or HOCl further induced this intramolecular disulfide and also triggered formation of an independent intermolecular disulfide linking two KEAP1 molecules via Cys151. Oxidation was transient, with KEAP1 returning to the redox state of untreated cells by endogenous reduction, and this was after variable periods of time, depending on the oxidant. None of these oxidants caused detectable KEAP1 S-nitrosylation, in contrast to the prototypical trans-nitrosative agent S-nitrosocysteine. Other KEAP1 Cys modifications such as a short range disulfide might have formed but cannot be detected by the techniques used here. Oxidation of KEAP1 at Cys residues in cells exposed to Cys-NO has been reported (40), but neither its nature nor the identity of the Cys residues involved were revealed. Similarly, in vitro reaction of KEAP1 with oxidized GSH caused disulfide formation that included a Cys319-based intermolecular disulfide (14). We could not detect this intermolecular disulfide, nor could this study identify the disulfides characterized here, emphasizing the difficulty of extrapolating in vivo the results of in vitro studies and vice versa.
Proof for a cause-and-effect relationship between Cys residues modification and regulation is always a difficult task and cannot be definitely established here. Nevertheless, the parallel observed between the kinetics of KEAP1 oxidation and NRF2 stabilization is suggestive of the functional significance of KEAP1 disulfide formation. SpNO, which releases NO with a half-life of ∼2 h, had a modest but long lasting effect on KEAP1 oxidation that resulted in a significant increase in NRF2 abundance late in the kinetics, whereas H2O2 and HOCl, which are short-lived, had a temporary effect on oxidation that caused only moderate NRF2 stabilization. Simultaneous impairment of the GSH and thioredoxin pathways caused both constitutive KEAP1 oxidation and NRF2 stabilization, which is further suggestive of a cause-and-effect relationship between the two phenomena, also re-emphasizing that the half-life of the oxidized form, which was in this case perpetuated by defective reduction, is important for efficient NRF2 stabilization.
Significance of disulfide formation was also evaluated by the effect of Cys residue substitution on KEAP1 function. Mutants lacking either Cys226 or Cys613 were still able to repress NRF2 and to respond to SpNO, indicating that under the experimental conditions used the Cys226–Cys613 intramolecular disulfide is dispensable for KEAP1 function and regulation. Further studies will be needed to elucidate the role of this long range intramolecular disulfide that spans the entire Kelch domain. Nonetheless, our data suggest that in the fully folded state Cys226 and Cys613 are close to each other, an unexpected finding based on the current knowledge of KEAP1 structure. In contrast, the mutant lacking Cys151 could not be derepressed by SpNO, which indicates that KEAP1 Cys151 disulfide-linked homodimer formation is important for derepression in response to oxidants.
Hannink and coworkers (6, 11) initially established the importance of Cys151 by showing that cells expressing a KEAP1 mutant lacking this amino acid have low NRF2 basal activity that cannot be derepressed by t-BHQ and sulforaphane. This result was confirmed with many other NRF2 inducers, across species (24, 25, 27), and in mouse transgenic complementation rescue experiments (26). Further, Cys151 becomes covalently modified in vivo by biotinylated derivatives of iodoacetamide (22) and has been shown to be consistently modified in vitro using different electrophiles (compiled in Ref. 14 and 15). The data shown here now indicate that KEAP1 Cys151 is also required for NRF2 activation by oxidants such as NO and H2O2, which instead of operating by alkylation engage this residue in a disulfide-linked KEAP1 homodimer. How Cys151 modification inhibits function is at the heart of KEAP1 regulation.
Several mechanisms have been proposed for how chemical inducers inhibit KEAP1-mediated NRF2 ubiquitination. The initial notion that regulation involves a disruption of the KEAP1-NRF2 interaction (12, 41) was later invalidated by several studies (6, 42, 43). A two-site recognition model has been proposed that predicts a KEAP1-NRF2 2:1 stoichiometry with one of the two Kelch domains of dimeric KEAP1 contacting NRF2 through a strong binding ETGE motif and the other contacting it through a weak binding DLG motif (44, 45). This model suggests that stress signals modify KEAP1 conformation in a way that loosens its interaction at the DLG and places NRF2 in a position unfavorable for ubiquitination but does not decipher the role of Cys151. As another regulatory mechanism, electrophiles have been shown to disrupt the KEAP1-Cul3 interaction by modifying Cys151, thus also ceasing NRF2 ubiquitination (6, 22, 46). In support of this mechanism, evaluation of a series of substitutions at position 151 of human KEAP1 elegantly established that residues with increasing partial molar volume decrease the protein affinity for Cul3 and its ability to target NRF2 for ubiquitination and predicted that bulky modification at this position imposes structural effects at the BTB that alter KEAP1-Cul3 binding (47). As shown by Eggler et al. (47) and also in zebrafish KEAP1 by Kobayashi et al. (27), a Cys151 substitution by a tryptophan, which has the highest partial molar volume, caused constitutive NRF2 activation.
To gain insight into how Cys151 disulfide-linked KEAP1 homodimerization could alter protein function, we have modeled the KEAP1 BTB domain from the Bach1 BTB structure and have positioned Cys151 with regard to the BTB dimerization interface and to Cul3 using human LRF1 (48) and the Skp1-Cul1 complex (49) structures (Fig. 6). In this model Cys151 appears remote from both the BTB dimerization interface and Cul3. This residue is also buried by four positively charged amino acids, which restrict its accessibility but might also favor its reactivity toward electrophiles and oxidants by stabilization of the deprotonated form. Because of the apparent buried nature of Cys151, modification of this residue to the sulfenic acid or S-nitrosylated forms upon reaction with H2O2 or NO-derived reactive nitrogen species, respectively, should necessarily cause some structural editing that might be further augmented upon disulfide formation between the Cys151 of two KEAP1 monomers. Such local structural editing could impact the BTB canonical dimerization and Cul3 interaction domains at distance. Co-precipitation experiments (Fig. 3) indeed suggest that nonredox, presumably though the BTB, and disulfide-mediated KEAP1 dimerization are exclusive, supporting the idea that disulfide formation disrupts the BTB dimerization interface. Although we were not able to assay the interaction of KEAP1 with Cul3, such interaction might also be affected by formation of the Cys151-intermolecular disulfide, as is the case in the presence of a bulky modification of Cys151 (47). The idea of a major conformational editing of KEAP1 following Cys151 modification has experimental support. Circular dichroism analysis of recombinant KEAP1 revealed that biotinylated iodoacetamide, which S-alkylates KEAP1 Cys151, causes a conformational rearrangement of the protein, an effect that is lost by serine substitution of Cys151 (22). Further studies will be needed to establish the impact of KEAP1 intermolecular disulfide formation on its dimerization and association with Cul3, which in both cases, if abrogated, would explain cessation of KEAP1-mediated NRF2 degradation.
FIGURE 6.
Prediction structure of the KEAP1 BTB domain indicating the location of Cys151. The KEAP1 BTB domain structure (delimitations 44–183) was modeled using the Bach1 BTB domain structure (Protein Data Bank code 2Z8H) as template (51). These two BTB sequences share 30% identity with no sequence insertions surrounding KEAP1 Cys151 within the alignment, thus ensuring the reliability of the structural model in this region. In this model the Cys151 side chain (magenta stick) appears buried by four surrounding positively charged amino acids (labeled in blue; Lys131, Arg135, Lys150, and His154). These four residues side chains can be modeled under different conformers, but irrespective of them, the accessibility of Cys151 never exceeds 15%, emphasizing the buried character of this residue. The position of Cys151 with regard to Cul3 was modeled using the structural similarity between Skp1 and BTB domains and the structure of the Skp1-Cul1 complex (Protein Data Bank code 1LDK) (49). These models show that Cys151 is remote from both Cul3 and the BTB interface.
Whether oxidation of KEAP1 involves a direct reaction with oxidants or is somehow catalyzed is not known. If direct, both H2O2 and HOCl are expected to proceed via Cys residue sulfenic acid formation, the condensation of which with a proximal Cys residue leads to disulfide formation. The chemistry behind NO-induced disulfide formation is not well understood but is presumably a multistep reactions process involving oxygen- dependent generation of reactive nitrogen species from NO, such as NO2 or N2O3; Cys-NO formation; release of a thiyl radical (R-S·) via S–N bond homolytic scission or through Cu2+-mediated catalysis; and generation of a disulfide with a proximal Cys residue (50). An alternative pathway involving the NO-dependent formation of 8-nitro-cGMP and S-guanylation of KEAP1 has also been suggested (21).
In summary, we have shown here that H2O2, SpNO, and HOCl lead to the oxidation of KEAP1 into two disulfides, one intramolecular and the other intermolecular. We propose that the Cys151-based intermolecular disulfide bridging two monomers of KEAP1 represents a novel modification of this Cys residue, which, as proposed for the alkylation of this residue by electrophilic compounds, leads to KEAP1 inactivation and stabilization of NRF2.
Supplementary Material
Acknowledgments
We acknowledge and thank A. Delaunay-Moisan and members of the Toledano lab for discussions and comments on the manuscript and Aied Igbaria, Gael Palais, and Stéphanie Luriau for help with experiments. We greatly appreciate the gifts of reagents from J. A. Diehl, A. Holmgren, M. Yamamoto, and G. Clément and C. Créminon.
This work was supported by funds from ARC, ANR, and Région Ile-de-France DIM SEnT (to M. B. T.) and by a Programme Toxicologie Nucléaire fellowship (to S. F.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
- BTB
- Broad complex, Tramtrack, Bric-à-Brac
- Cys-NO
- S-nitrocysteine
- NEM
- N-ethylmaleimide
- SpNO
- spermine NONOate
- t-BHQ
- tert-butyl hydroquinone
- IVR
- intervening region
- HA
- hemagglutinin
- shRNA
- small hairpin RNA
- YFP
- yellow fluorescent protein
- TrxR1
- thioredoxin reductase 1.
REFERENCES
- 1.Dinkova-Kostova A. T., Holtzclaw W. D., Kensler T. W. (2005) Chem. Res. Toxicol. 18, 1779–1791 [DOI] [PubMed] [Google Scholar]
- 2.Hayes J. D., McMahon M. (2009) Trends Biochem. Sci. 34, 176–188 [DOI] [PubMed] [Google Scholar]
- 3.Cullinan S. B., Gordan J. D., Jin J., Harper J. W., Diehl J. A. (2004) Mol. Cell Biol. 24, 8477–8486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Furukawa M., Xiong Y. (2005) Mol. Cell Biol. 25, 162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi A., Kang M. I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. (2004) Mol. Cell Biol. 24, 7130–7139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang D. D., Lo S. C., Cross J. V., Templeton D. J., Hannink M. (2004) Mol. Cell Biol. 24, 10941–10953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMahon M., Itoh K., Yamamoto M., Hayes J. D. (2003) J. Biol. Chem. 278, 21592–21600 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen T., Sherratt P. J., Huang H. C., Yang C. S., Pickett C. B. (2003) J. Biol. Chem. 278, 4536–4541 [DOI] [PubMed] [Google Scholar]
- 9.Stewart D., Killeen E., Naquin R., Alam S., Alam J. (2003) J. Biol. Chem. 278, 2396–2402 [DOI] [PubMed] [Google Scholar]
- 10.Itoh K., Wakabayashi N., Katoh Y., Ishii T., O'Connor T., Yamamoto M. (2003) Genes Cells 8, 379–391 [DOI] [PubMed] [Google Scholar]
- 11.Zhang D. D., Hannink M. (2003) Mol. Cell Biol. 23, 8137–8151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinkova-Kostova A. T., Holtzclaw W. D., Cole R. N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11908–11913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinkova-Kostova A. T., Holtzclaw W. D., Wakabayashi N. (2005) Biochemistry 44, 6889–6899 [DOI] [PubMed] [Google Scholar]
- 14.Holland R., Hawkins A. E., Eggler A. L., Mesecar A. D., Fabris D., Fishbein J. C. (2008) Chem. Res. Toxicol. 21, 2051–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sekhar K. R., Rachakonda G., Freeman M. L. (June26, 2009) Toxicol. Appl. Pharmacol. 10.1016/j.taap.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Itoh K., Mochizuki M., Ishii Y., Ishii T., Shibata T., Kawamoto Y., Kelly V., Sekizawa K., Uchida K., Yamamoto M. (2004) Mol. Cell Biol. 24, 36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levonen A. L., Landar A., Ramachandran A., Ceaser E. K., Dickinson D. A., Zanoni G., Morrow J. D., Darley-Usmar V. M. (2004) Biochem. J. 378, 373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh J. Y., Giles N., Landar A., Darley-Usmar V. (2008) Biochem. J. 411, 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satoh T., Kosaka K., Itoh K., Kobayashi A., Yamamoto M., Shimojo Y., Kitajima C., Cui J., Kamins J., Okamoto S., Izumi M., Shirasawa T., Lipton S. A. (2008) J. Neurochem. 104, 1116–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckley B. J., Marshall Z. M., Whorton A. R. (2003) Biochem. Biophys. Res. Commun. 307, 973–979 [DOI] [PubMed] [Google Scholar]
- 21.Sawa T., Zaki M. H., Okamoto T., Akuta T., Tokutomi Y., Kim- Mitsuyama S., Ihara H., Kobayashi A., Yamamoto M., Fujii S., Arimoto H., Akaike T. (2007) Nat. Chem. Biol. 3, 727–735 [DOI] [PubMed] [Google Scholar]
- 22.Rachakonda G., Xiong Y., Sekhar K. R., Stamer S. L., Liebler D. C., Freeman M. L. (2008) Chem. Res. Toxicol. 21, 705–710 [DOI] [PubMed] [Google Scholar]
- 23.Wakabayashi N., Dinkova-Kostova A. T., Holtzclaw W. D., Kang M. I., Kobayashi A., Yamamoto M., Kensler T. W., Talalay P. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 2040–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakurai T., Kanayama M., Shibata T., Itoh K., Kobayashi A., Yamamoto M., Uchida K. (2006) Chem. Res. Toxicol. 19, 1196–1204 [DOI] [PubMed] [Google Scholar]
- 25.Satoh T., Okamoto S. I., Cui J., Watanabe Y., Furuta K., Suzuki M., Tohyama K., Lipton S. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 768–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto T., Suzuki T., Kobayashi A., Wakabayashi J., Maher J., Motohashi H., Yamamoto M. (2008) Mol. Cell Biol. 28, 2758–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., Yamamoto M. (2009) Mol. Cell Biol. 29, 493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashino T., Yamanaka R., Yamamoto M., Shimokawa H., Sekikawa K., Iwakura Y., Shioda S., Numazawa S., Yoshida T. (2008) Mol. Immunol. 45, 2106–2115 [DOI] [PubMed] [Google Scholar]
- 29.Dhakshinamoorthy S., Porter A. G. (2004) J. Biol. Chem. 279, 20096–20107 [DOI] [PubMed] [Google Scholar]
- 30.Ishii T., Itoh K., Takahashi S., Sato H., Yanagawa T., Katoh Y., Bannai S., Yamamoto M. (2000) J. Biol. Chem. 275, 16023–16029 [DOI] [PubMed] [Google Scholar]
- 31.Wilson L. A., Gemin A., Espiritu R., Singh G. (2005) FASEB J. 19, 2085–2087 [DOI] [PubMed] [Google Scholar]
- 32.Zhu L., Pi J., Wachi S., Andersen M. E., Wu R., Chen Y. (2008) Am. J. Physiol. Lung Cell Mol. Physiol. 294, L469–L477 [DOI] [PubMed] [Google Scholar]
- 33.Jain A. K., Jaiswal A. K. (2006) J. Biol. Chem. 281, 12132–12142 [DOI] [PubMed] [Google Scholar]
- 34.Vert J. P., Foveau N., Lajaunie C., Vandenbrouck Y. (2006) BMC Bioinformatics 7, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biard D. S., Despras E., Sarasin A., Angulo J. F. (2005) Mol. Cancer Res. 3, 519–529 [DOI] [PubMed] [Google Scholar]
- 36.Forrester M. T., Foster M. W., Stamler J. S. (2007) J. Biol. Chem. 282, 13977–13983 [DOI] [PubMed] [Google Scholar]
- 37.Padmanabhan B., Tong K. I., Ohta T., Nakamura Y., Scharlock M., Ohtsuji M., Kang M. I., Kobayashi A., Yokoyama S., Yamamoto M. (2006) Mol. Cell 21, 689–700 [DOI] [PubMed] [Google Scholar]
- 38.Zipper L. M., Mulcahy R. T. (2002) J. Biol. Chem. 277, 36544–36552 [DOI] [PubMed] [Google Scholar]
- 39.D'Autréaux B., Toledano M. B. (2007) Nat. Rev. Mol. Cell Biol. 8, 813–824 [DOI] [PubMed] [Google Scholar]
- 40.Buckley B. J., Li S., Whorton A. R. (2008) Free Radic. Biol. Med. 44, 692–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J. D., Yamamoto M. (1999) Genes Dev. 13, 76–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eggler A. L., Liu G., Pezzuto J. M., van Breemen R. B., Mesecar A. D. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 10070–10075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi M., Yamamoto M. (2006) Adv. Enzyme Regul. 46, 113–140 [DOI] [PubMed] [Google Scholar]
- 44.McMahon M., Thomas N., Itoh K., Yamamoto M., Hayes J. D. (2006) J. Biol. Chem. 281, 24756–24768 [DOI] [PubMed] [Google Scholar]
- 45.Tong K. I., Katoh Y., Kusunoki H., Itoh K., Tanaka T., Yamamoto M. (2006) Mol. Cell Biol. 26, 2887–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao L., Wang J., Sekhar K. R., Yin H., Yared N. F., Schneider S. N., Sasi S., Dalton T. P., Anderson M. E., Chan J. Y., Morrow J. D., Freeman M. L. (2007) J. Biol. Chem. 282, 2529–2537 [DOI] [PubMed] [Google Scholar]
- 47.Eggler A. L., Small E., Hannink M., Mesecar A. D. (2009) Biochem. J. 422, 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schubot F. D., Tropea J. E., Waugh D. S. (2006) Biochem. Biophys. Res. Commun. 351, 1–6 [DOI] [PubMed] [Google Scholar]
- 49.Zheng N., Schulman B. A., Song L., Miller J. J., Jeffrey P. D., Wang P., Chu C., Koepp D. M., Elledge S. J., Pagano M., Conaway R. C., Conaway J. W., Harper J. W., Pavletich N. P. (2002) Nature 416, 703–709 [DOI] [PubMed] [Google Scholar]
- 50.Stamler J. S., Toone E. J. (2002) Curr. Opin. Chem. Biol. 6, 779–785 [DOI] [PubMed] [Google Scholar]
- 51.Ito N., Watanabe-Matsui M., Igarashi K., Murayama K. (2009) Genes Cells 14, 167–178 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.