Summary
This study probed possible age-related changes in mitochondrial bioenergetics in naïve Fischer 344 rats. Synaptic and extrasynaptic mitochondria were isolated from the cortex of one hemisphere of young (3–5 mos), middle (12–14 mos), or aged (22–24 mos) rats. Respiration parameters were obtained using a Clarke-type electrode. Aged rats displayed no significant alterations in respiration, indicating mitochondria must be more resilient to the aging process than previously thought. Synaptic mitochondria displayed lower respiration capacities than the extrasynaptic fraction. Aged F344 rats appear capable of normal electron transport chain function without declines in ability to produce ATP. Markers of cortical oxidative damage (3-nitrotyrosine [3-NT], 4-hydroxynonenal [4-HNE], and protein carbonyls [PC]) were collected from the post mitochondrial supernatant (PMS) from the contralateral hemisphere, and from mitochondrial samples following respiration analysis. Age-related increases in PC and 3-NT levels were found in synaptic mitochondria, whereas significant extrasynaptic elevations were only found in middle aged rats. These findings support an age-related increase in oxidative damage in the cortex, while proposing the two fractions of mitochondria are differentially affected by the aging process. Levels of oxidative damage that accumulates in the cortex with age does not appear to significantly impair cortical mitochondrial respiration of F344 rats.
Keywords: aging, Clark-type electrode, respiration, cortex, mitochondria, oxidative damage
Introduction
The free-radical theory of aging (FRTA), first postulated by Harman (1956), gave a molecular mechanistic explanation for why physiological systems decline with aging. This theory maintains that critical cellular components are under constant attack by various types of free radicals (FR), and that endogenously produced oxidants overcome endogenous antioxidants. Mitochondria are a major source of FR generated during normal cellular respiration (Shigenaga, Hagen et al. 1994; Richter 1995; Kowaltowski and Vercesi 1999; Floyd and Hensley 2002).
The absolute dependency of mammalian systems on mitochondria and their inherent production of FR compelled Harman to modify his FRTA into the mitochondrial theory of aging (MTA) (Harman 1972). The MTA postulates that cellular aging is the product of mutations in the mitochondrial DNA (mtDNA) genome as a result of oxidative damage. As a result of accumulating damage to mtDNA, the mitochondrial blueprints are markedly altered perpetuating a production the aberrant ETC components. Mitochondrial dysfunction has been implicated as a primary contributor in many clinical problems (e.g. renal dysfunction, liver disease), and may be a part of the primary mechanisms associated with Parkinsonism and Alzheimer’s disease (Beal 2005; Atamna and Frey 2007; Leuner, Hauptmann et al. 2007). The gradual and perpetual cycle of accumulation of damaged cellular components necessary for energy production creates an energy crisis situation within the cell leading to its eventual dysfunction and demise (Rossignol, Faustin et al. 2003).
A brief review of brain aging in context of mitochondrial aging research is given to reveal how our study is unique and contributes to this field. Many studies have shown significant age-related declines in mitochondrial enzymatic activity, especially in complex 1 and 4, isolated from whole brains of mice (Navarro, Sanchez Del Pino et al. 2002; Navarro, Gomez et al. 2004; Navarro, Gomez et al. 2005; Navarro and Boveris 2007), rats (Davis, Whitely et al. 1997; Sandhu and Kaur 2003; Navarro and Boveris 2004; Navarro and Boveris 2008; Petrosillo, Fattoretti et al. 2008; Petrosillo, Matera et al. 2008; Long, Gao et al. 2009), and primates (Bowling, Mutisya et al. 1993). Conversely, there are a few groups reporting no age-related declines in the activity of these complexes in rodents (Kwong and Sohal 2000; Davies, Poljak et al. 2001). These studies do not address possible regional differences in the brain.
Others have analyzed a specific brain region and/or a particular mitochondrial fraction for declines in enzymatic activity. Desmukh et al (1980) reported no significant age-related changes in complex 1 activity in either synaptic or extrasynaptic mitochondria isolated from whole rat brain. A later study (Gorini, Arnaboldi et al. 1989) also reported no age-related declines in complex 4 activity of synaptic and extrasynaptic mitochondria in the frontal cortex, hippocampus, or striatum of rats. Synaptic mitochondria isolated from the whole brain of mice showed significant decreases in complex IV and V activities, while extrasynaptic mitochondria manifested significant declines in complexes I, II + III, and IV (Ferrandiz, Martinez et al. 1994). Subsequent studies by Genova et al (1997) and Cocco et al (2005) reported age-related declines in complex 1 activity in extrasynaptic mitochondria isolated from the cortex of rat but failed to analyze the synaptic fraction. Sharman and Bondy (2001), using mice, failed to find any age-related declines in enzymatic activities of cortical synaptic mitochondria. However, Navarro et al (2008) showed significant declines in mitochondrial enzymatic activity of complex I and IV in tissue isolated from the cortex and hippocampus of rats. Although several groups report declines in enzymatic activities of certain ETC complexes with age, it is unclear if these enzymatic changes translate into a functional decline in respiration. It has been reported that components of the ETC are present in excess and substantial inhibition of certain components is probably necessary before a decline in ATP production is realized (Van Remmen and Richardson 2001; Rossignol, Faustin et al. 2003; Lesnefsky and Hoppel 2006).
The present study evaluated possible age-related changes in bioenergetics of synaptic and extrasynaptic mitochondria separately, since several groups have reported heterogeneity in the these two fractions (Dennis, Lai et al. 1977; Lai, Walsh et al. 1977; Deshmukh, Owen et al. 1980; Deshmukh and Patel 1980; Villa, Gorini et al. 1989; Lai, Liang et al. 1994; Davey and Clark 1996; Davey, Peuchen et al. 1998; Brown, Sullivan et al. 2006; Naga, Sullivan et al. 2007). Several complex 1- and 2-driven respiration parameters were quantified from cortical synaptic and extrasynaptic mitochondria. Levels of oxidative damage were also determined from the same group rats in both mitochondrial fractions and in the post mitochondrial supernatant (PMS), structures outside mitochondria, to determine if levels of oxidative damage impact possible functional changes that occurs with age.
Materials and Methods
Adult male Fischer 344 rats (National Institute of Aging - Harlan Labs, Indianapolis, IN; n = 34) were used in this study and housed in group cages (2 per cage) on a 12-h light/dark cycle with free access to food and water. All experimental protocols involving animals were approved by the University of Kentucky Animal Use and Care Committee. All rats were naïve (not subjected to any type of operation, testing, or prior medication) and placed into one of three groups based on their age: (I). Young - 3–5 mos (n = 11); (II). Middle Aged - 12–14 mo (n = 13); and (III). Aged - 22–24 mo (n = 10).
Synaptic and extrasynaptic mitochondria isolation
Animals from multiple age groups were randomly selected each day to ensure homogeneity of procedures. Animals were briefly gassed with CO2 until flaccid, decapitated, and the brains rapidly removed. All subsequent procedures were performed on ice including the entire mitochondrial isolation protocol. Cortical tissue from one hemisphere was randomly chosen for measuring levels of oxidative damage in the PMS and the other was used for respiration analysis. Unused synaptic and extrasynaptic mitochondria samples leftover from respiration analysis were then probed for markers of oxidative damage.
The cortex used for respiration analysis was placed in an all-glass dounce homogenizer with 4 mL of isolation buffer with 1 mM EGTA (215 mM mannitol, 75 mM sucrose, 0.1% BSA, 20 mM HEPES, 1 mM EGTA, pH 7.2). Tissue was homogenized and mitochondria were extracted by differential centrifugation. The homogenate was spun twice at 1300 × g for 3 min in an Eppendorf microcentrifuge at 4°C and the supernatant was transferred to new tubes. The resulting supernatant was topped off with isolation buffer with EGTA and spun at 13,000 × g for 10 min. The supernatant was discarded; the pellet was resuspended with isolation buffer with EGTA to a total volume of 500 µL; Both fractions were washed with fresh buffer a second time to ensure that the Ficoll was eliminated. Synaptosomes were resuspended in isolation buffer with EGTA, and mitochondria released using a nitrogen cell disruption bomb made by Parr Instrument Company (Moline, IL) at 1,200 psi for 10 min. Disrupted synaptosomes were placed on a new Ficoll gradient and returned to the ultracentrifuge and spun at 32,000 rpm for 30 min at 4°C. and samples were place on a discontinuous Ficoll gradient (F5415 Ficoll solution Type 400, 20% in H2O, Sigma, St. Louis, MO), composed of 2 layers (2 mL of a 7.5% on top of 2 mL of 10% Ficoll cut with isolation buffer with EGTA). The final volume of the gradient was ~ 4.5 mL in the 7 mL Beckman tubes (344057, Fullerton, CA). Samples were placed in a Beckman SW 55 Ti swinging bucket rotor and centrifuged at 32,000 rpm for 30 min at 4°C. The gradient produced two separate mitochondrial fractions: the extrasynaptic mitochondrial pellet (bottom) and synaptic mitochondria trapped in synaptosomes located at the interphase of the two Ficoll layers. Synaptosomes were collected in 2.5 mL tubes, resuspended, and washed in isolation buffer with EGTA by centrifugation at 13,000 × g for 10 min to remove Ficoll from sample. The extrasynaptic mitochondrial pellet was collected in 500 µL tubes and also resuspended and washed using isolation buffer with EGTA. After removal of the Ficoll gradient, the synaptic mitochondria pellet was resuspended in isolation buffer with EGTA and centrifuged at 13,000 × g for 10 min. Both mitochondrial fractions (synaptic and extrasynaptic) were resuspended with isolation buffer without EGTA and centrifuged at 10,000 ×g for 10 min to wash out the calcium chelator (EGTA). The final mitochondrial pellets were resuspended in isolation buffer without EGTA to yield a concentration of 10 mg/mL or higher. Protein concentration was determined using the bicinchoninic acid protein assay kit from Pierce (Rockford, IL) by measuring absorbance at 560 nm with a Molecular Devices microplate reader (Sunnyvale, CA).
Mitochondrial respiration measurements
Mitochondrial functionality was assessed using an Oxytherm Clark-type oxygen electrode (OXYT1/ED, Hansatech Instruments, Norfolk, UK). Mitochondria (~60 µg) were placed in the sealed Oxytherm chamber containing respiration buffer (125 mM KCl, 0.1% BSA, 20 mM HEPES, 2 mM MgCl2, 2.5 mM KH2PO4, pH 7.2) and continuously stirred at 37° C. The rate of oxygen consumption was defined as the slope of the response of isolated mitochondria to the consecutive administrations of oxidative substrates as previously described (Gilmer, Roberts et al. 2009), with minor adjustments. One adjustment had to be made to the previous protocol due to the highly level of purity the Ficoll gradient offers compared to differential centrifugation alone. In this study ~60 µg of mitochondrial protein was used for each respiration analysis compared to using ~180 µg previously. The amount of oligomycin administered was cut in half, from 1 µL to 0.4 µL, because the original amount was too high and was poisoning the mitochondria. Two additions of adenosine diphosphate (ADP), instead of one addition, were administered to ensure accurate state III respiration measurements. The modified protocol of the substrates injected into the Oxytherm chamber sequentially as follows: 2.5 µL of pyruvate/malate (P/M; 2.5 mM); 1.25 µL of ADP (150 µM) added twice in 1-min intervals; 0.5 µL oligomycin (1 µM); 1µL carbonylcyanide-4-(trifluoromethoxy)-phenylhydrazone (FCCP; 1 µM); 0.2 µL rotenone (1 µM) added to shut down complex 1 activity resulting in cessation of oxygen utilization; and finally 2.5 µL succinate (10 mM).
Overall oxygen utilization rate was determined by measuring the amount of oxygen consumed throughout all states of respiration divided by the time elapsed and amount of mitochondrial protein used for the respiration assay. The oxygen utilization rate can detect alterations in the time mitochondria respond to substrate additions, which serve as an index of their overall respiration capacities. States II - V are mitochondrial respiration parameters driven by complex 1 (P/M, ADP, oligomycin, and FCCP), while complex 2-driven respiration was measured separately by shutting down complex 1 with rotenone, to produce state V respiration (succinate). Figure 1 is a schematic drawing of a typical mitochondria respiration trace depicting the sequence of substrate additions and subsequent oxygen utilization rates. The respiratory control ratio (RCR) was determined by dividing the rate of oxygen utilization for state III (ADP) by state IV (oligomycin). RCRs indicate how coupled the ETC is to ATP production. The states of respiration (III–V), overall oxygen utilization rates, and RCRs were all collected to further define respiration capabilities. All isolated mitochondrial fractions, regardless of age, were able to produce RCRs of ≥ 5, indicating that the mitochondria were not damaged during the isolation procedure. Actual respiration rates for all states of respiration, RCRs, and overall oxygen utilization rates were plotted as a function of mitochondrial fraction and age. Two mitochondrial samples were lost during isolation procedures (young – synaptic n=1; middle aged – synaptic n=1).
Fig. 1.

Typical oxygen utilization traces of synaptic and extrasynaptic mitochondria isolated from a naïve cortex of an aged animal. state I: no substrates for respiration added; no oxygen utilization apparent. state II: addition of P/M; basal rate of respiration. state III: two separate additions of ADP; each addition connects the ETC with oxidative phosphorylation; high level of oxygen utilization indicated ADP being converted to ATP. state IV: addition of oligomycin; electrons are blocked from returning into the matrix through the ATP synthase, the ETC slows only to maintain mitochondrial membrane potential that is lost through inner membrane into the matrix, and oxygen utilization is greatly reduced. state V: addition of FCCP; results in an uncoupling of the ETC to ATP synthesis, represents maximum rate of respiration since no bottle-necking occurs at ATP synthase - protons are allowed to rush back into the matrix. Rotenone is then added to shut down complex 1-driven respiration. state V (succinate): addition of succinate; maximum rate of respiration via complex 2 due to presence of FCCP still in the system.
Markers of oxidative damage quantification
When a sufficient amount of the mitochondria fraction following respiration analysis was available, it was probed for possible oxidative damage. These samples were frozen at −80°C until time of analyses. Some samples were lost due to temperature contamination (middle age synaptic n=6, extrasynaptic n=6) and some samples didn’t have sufficient quantities to carry out the analysis (young extrasynaptic n=1; middle age synaptic n=2, extrasynaptic n=1).
Estimation of protein carbonylation (PC)
Levels of PC were assessed as described previously (Ansari, Roberts et al. 2008) from the PMS, synaptic, and extrasynaptic mitochondria. All samples were normalized to 4mg/ml, then combined with sodium dodecyl sulfate (SDS) and dilute 2,4-dinitrophenylhydrazine (DNP). Samples were incubated at room temperature for 20 min then neutralized with 2 M Tris in 30% glycerol. The resulting samples were loaded on a nitrocellulose membrane and run on a slot-blot apparatus. Membranes were blocked in 3% bovine serum albumin and PBS/Tween for 1 h and incubated with a 1:100 dilution of anti-DNP polyclonal rabbit antibody in PBS/Tween for 1 h. Membranes were washed in PBS/Tween and incubated for 1 h with an anti-rabbit IgG alkaline phosphatase secondary antibody diluted in PBS/Tween in a 1:8000 ratio. Each membrane was washed in PBS/Tween and developed in Sigma Fast tablets. Blots were dried, scanned with Adobe PhotoShop, and quantified with Scion Image. Nonspecific binding of antibody to the membrane was not observed. The intensity of PC staining was quantified by arbitrary optical density and data reported as percent of the young age group values.
Estimation of 3-nitrotyrosine (3-NT; an index of protein nitration) and 4-hydroxynonenal (4-HNE; an index of lipid peroxidation)
The PMS, synaptic, and extrasynaptic mitochondria samples were normalized with 12% SDS and modified Laemmli buffer containing 0.125 M Tris base, pH 6.8, 4% (v/v) SDS, and 20% (v/v) glycerol were incubated for 20 min at room temperature. Samples (250ng) were loaded into a well on a nitrocellulose membrane in a slot-blot apparatus under vacuum. Membranes were blocked with 3% bovine serum albumin in PBS/Tween for 1 h, and then incubated with either a 1:2000 dilution of anti-3-NT polyclonal rabbit antibody or a 1:5000 dilution of anti-HNE polyclonal goat antibody in PBS/Tween for 1.5 h. Each membrane was washed in PBS/Tween for 5 min three times after incubation. Membranes were incubated for 1 h, after washing, with an anti-rabbit IgG alkaline phosphatase secondary antibody diluted in PBS/Tween in 1:8000 ratio. Membranes were washed three times in PBS/Tween for 5 min and developed in Sigma Fast tablets. Blots were dried, scanned with Adobe PhotoShop, and quantified with Scion Image as above. Nonspecific binding of antibody to the membrane was not observed. The intensities of 4-HNE and 3-NT staining were quantified by arbitrary optical density and data reported as percent of the young age group values.
Statistical analyses
All data are reported as group means ± SD. The states of respiration are reported as nmol O2 utilized / min / mg of mitochondrial protein. A two-way repeated measure ANOVA (age X mitochondrial fraction) was used to probe for possible differences in respiration data. Based on evidence that synaptic and extrasynaptic mitochondria have very different respiration properties (Davey and Clark 1996; Erecinska, Nelson et al. 1996; Davey, Canevari et al. 1997; Davey, Peuchen et al. 1998), we expected to find differences between the two fractions of mitochondria.
A two-way repeated measure ANOVA (age X mitochondrial fraction) was used to compare the levels of oxidative damage. Separate analysis were performed for each of the measured of oxidative damage.
A Pearson product-moment correlation coefficient was use to probe possible associations between the levels of each marker of oxidative damage and the overall oxygen utilization rate and RCR value in each subject.
When appropriate, all ANOVAs were followed by a Student-Newman-Keuls post-hoc analysis to determine individual group differences. The alpha level was set at 0.05 for significance.
Results
Age-related changes in mitochondria bioenergetics
Mitochondrial protein levels
The average amount of mitochondrial protein used to assess respiration capabilities was extrasynaptic: 59.5 µg ± 8.3 (young), 59.0 ± 12.6 (middle aged), and 65.5 ± 9.9 (aged), synaptic: 53.8 µg ± 13.5 (young), 62.2 ± 13.3 (middle aged), and 67.4 ± 10.2 (aged). The ANOVA failed to reveal a significant age effect [F (2, 29) = 2.413, p > 0.1] or a significant effect for mitochondrial fraction [F (1,29) = 0.619, p > 0.1]. In all experiments, the amounts of mitochondrial protein used produced respiration traces similar to the one depicted in Figure 1.
Overall oxygen utilization rate
There were no significant age-related changes in overall rate of oxygen utilization [F (2, 29) = 0.126, p > 0.1]. However, the analysis did reveal a significant main effect for mitochondrial fraction [F (1, 29) = 6.537, p < 0.01] (Figure 2). The mean overall oxygen utilization rates of extrasynaptic mitochondria were significantly higher than those from the synaptic fraction. There was no significant interaction between age and mitochondrial fraction (p > 0.1).
Fig. 2.
Overall oxygen utilization rate (nmol 02/min/mg). The overall oxygen utilization rate was not significantly different in either mitochondrial fraction as a function of age. Bars represent group means ± SD; numbers inside the bars represent sample size.
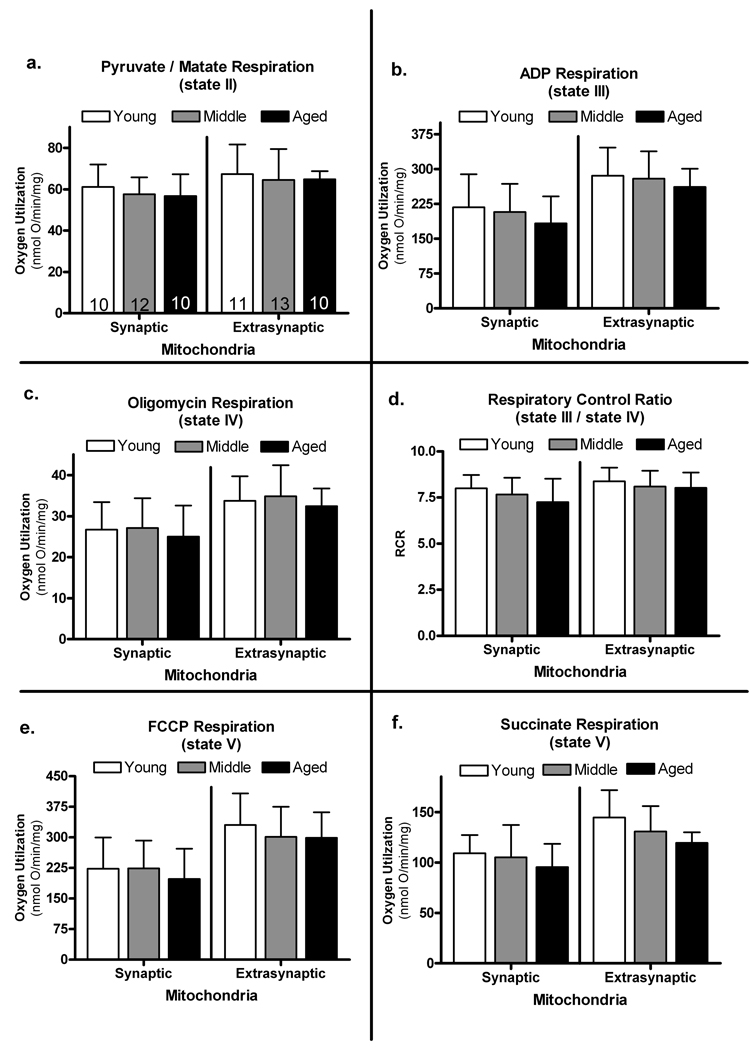
P/M (state II respiration via complex 1)
An ANOVA revealed no significant age-related differences in state II respiration [F (2, 29) = 0.288, p > 0.1] or a significant main effect for mitochondrial fraction [F (1, 29) = 1.724, p > 0.1] (Figure 3a). Since the amount of mitochondrial protein utilized for each assay and state II respiration rates were not significantly different, the data indicates that all mitochondrial groups contain the same ability to produce similar respiration traces.
Fig. 3.
Actual states of respiration for synaptic and extrasynaptic fractions. a. state II – P/M b. state III – ADP c. state IV – Oligomycin d. state V – FCCP e. state V – Succinate. f. respiratory control ratio (RCR) – which is an index of how coupled respiration is to ATP production and is calculated by dividing state III by state IV. Bars represent group means ± SD; numbers inside the bars represent sample size.
ADP (state III respiration)
Figure 3b depicts how efficiently ADP was utilized for ATP synthesis in both fractions of mitochondria across age groups. An ANOVA failed to reveal any significant age-related changes in state III respiration [F (2, 29) = 0.158, p > 0.1]. The analysis did reveal a significant main effect for mitochondrial fraction [F (1, 29) = 17.220, p < 0.0005]. Extrasynaptic mitochondria were significantly higher than those seen in the synaptic fraction.
Oligomycin (state IV respiration)
Possible age-related changes in state IV respiration were assessed by the addition of oligomycin. An ANOVA revealed no significant age-related changes [F (2, 29) = 0.8714, p > 0.1] but a significant main effect for mitochondrial fraction [F (1,29) = 13.007, p < 0.005] (Figure 3c). These data indicate aging does not deteriorate the inner membrane to the point where there are functional impairments in the membrane potential in either synaptic or extrasynaptic mitochondria. Regardless of age, mitochondria were able to maintain the proton gradient indicating preservation of inner membrane integrity. Extrasynaptic mitochondria had significantly higher values indicating an inherently higher control over respiration in the synaptic fraction. There was no significant age by mitochondrial fraction interaction.
RCR (state III / state IV respiration)
The RCR is an index of how coupled the ETC is to ATP production. No age-related changes in the overall condition of the mitochondria were apparent [F (2, 29) = 0..450, p > 0.1]. There was a significant main effect for mitochondrial fraction [F (1, 29) = 4.188, p > 0.05] (Figure 3d). Synaptic mitochondria displayed significantly lower RCRs than the extrasynaptic fraction.
FCCP (state V respiration)
Maximum respiration capabilities of both fractions of mitochondria were quantified by charging the ETC with the addition of all the necessary substrates (P/M and ADP) and subsequently bypassing the ATP synthase with the addition of FCCP. FCCP, a pure uncoupler that acts as a protonophore, allows the protons built up in the inner membrane space to freely pass back into the matrix at a rapid rate. The ETC quickly attempts to replenish this sudden decline in the electrochemical gradient, which results in a high level of oxygen consumption via complex 4 (Figure 3e). An ANOVA revealed no significant age-related change in maximum rates of respiration [F (2, 29) = 0.008, p > 0.1]. The repeated measure ANOVA detected a significant main effect for mitochondrial fraction [F (1,29) = 14.598, p < 0.001]. Extrasynaptic mitochondria had significantly higher levels of oxygen utilization than synaptic following addition of FCCP.
Succinate (state V respiration via complex 2)
Rotenone was added to specifically inhibit complex 1, allowing succinate driven respiration to be measured independently. Rotenone works by competitively inhibiting the transfer of electrons from iron-sulfur centers of complex 1 to ubiquinone. An ANOVA revealed no significant age-related changes in maximum complex 2-driven respiration [F (2, 29) = 0.359, p > 0.1] (Figure 3f). There was a significant main effect for mitochondrial fraction [F(1,29) = 12.771, p < 0.005] with extrasynaptic displaying significantly higher values than synaptic.
Markers of oxidative damage
Estimation of PC
The levels of oxidized proteins were significantly higher in the PMS fraction taken from the cortex [F (3, 21) = 13.462, p < 0.0005] (Figure 4a). Post hoc analysis revealed that the PMS of middle and aged rats contained higher levels of PC compared to young (p < 0.05). Synaptic and extrasynaptic fractions of mitochondria were also probed for levels of PC, quantified as a percent of the mean value for the young group, and compared across age groups using a two-way repeated measure ANOVA. Analysis revealed no significant difference in PC between mitochondrial fractions [F (2, 21) = 4.846, p > 0.1], but did reveal a significant difference across age groups [F (2, 21) = 4.846, p < 0.05] (Figure 4b). The level of PC in the synaptic fraction did not significantly correlate with the overall oxygen utilization rate (r = 0.014, p > 0.5) or the RCR values (r = 0.086, p > 0.5). In the extrasynaptic fraction there was positive significant correlation between the levels of PC with the overall oxygen utilization rate (r = 0.475, p < 0.01), but no significant correlation with RCR values (r = 0.004, p > 0.9).
Fig. 4.
Markers of oxidative damage are expressed as a percent of values observed in young animals. Relative optical densities of protein carbonyls (PC) were determined in the a. post mitochondrial supernatant (PMS) and in b. purified mitochondrial fractions (synaptic and extrasynaptic). Relative levels of 4-hydroxynonenal (4-HNE)-protein adducts, the most prevalent toxic peroxidation product formed during oxidant stress, were assessed in the c. PMS and d. both mitochondrial fractions. Relative levels of nitrotyrosine (3-NT), a biomarker of reactive nitrogen species formation, were measured in the e. PMS and f. both mitochondrial fractions. Bars represent group means ± SD; numbers inside bars represent the sample size. * p < 0.05; ** p < 0.01; ***p < 0.001 compared to young. # p < 0.01 compared to middle aged rats.
Estimation of 4-HNE
An ANOVA detected an age-related increase in 4-HNE in the cortical PMS [F (2, 21) = 23.2, p < 0.0001]. Post hoc analysis revealed a significant increase in 4-HNE in middle aged and aged animals compared to young (Figure 4c). The synaptic and extrasynaptic fractions of mitochondria were also probed for levels of 4-HNE and compared across age groups using two-way repeated measures ANOVA. Analysis revealed a significant difference in 4-HNE, measured as a percent of the mean value for the young group, between mitochondrial fractions [F (2, 21) = 7.662, p < 0.05], but were not significantly different when compared across age groups [F (2, 21) = 2.009, p > 0.1] (Figure 4d). Post-hoc analysis revealed synaptic mitochondria displayed significantly higher levels of 4-HNE compared to the extrasynaptic mitochondria. The level of 4-HNE in the synaptic fraction demonstrated no significant correlation with the overall oxygen utilization rate (r = 0.024, p > 0.9) or the RCR values (r = 0.014, p > 0.9). In the extrasynaptic fraction there was a positive significant correlation between the levels of PC with the overall oxygen utilization rate (r = 0.598, p < 0.001), but no correlation was apparent with RCR values (r = 0.250, p > 0.2). Synaptic mitochondria displayed levels of 4-HNE equivalent to that in the extrasynaptic fraction of young animals (p > 0.1).
Estimation of 3-NT
3-NT is used as a biomarker of reactive nitrogen species formation (Walker, York et al. 2001) specifically peroxynitrite-induced protein modification (Kaufmann, Bickford et al. 2001). An ANOVA detected an age-related change in 3-NT levels in the cortical PMS [F (2, 21) = 6.439, p < 0.01]. Post hoc analysis revealed a significant increase in aged animals compared to young but not to middle aged subjects (Figure 4e). A two-way repeated measures ANOVA revealed a significant difference in 3-NT levels between mitochondrial fractions [F (2, 21) = 5.59, p < 0.01] and when compared across age groups [F (2, 21) = 4.016, p < 0.05] (Figure 4f). Post-hoc analysis revealed a significant increase in middle aged animals in the extrasynaptic fraction (p < 0.05) and aged subjects in the synaptic fraction (p < 0.05) compared to young animals. The level of 3-NT in the synaptic fraction had no correlation with the overall oxygen utilization rate (r = 0.254, p > 0.2) or the RCR values (r = 0.033, p > 0.8). In the extrasynaptic fraction there was a significant positive correlation between the levels of 3-NT with the overall oxygen utilization rate (r = 0.420, p < 0.05), but no significant correlation was found with the RCR values (r = 0.096, p > 0.6).
Discussion
This study is the first to perform a comprehensive analysis of possible age-related changes in mitochondria respiration in the cortex of Fischer 344 (F344) rats. Two different fractions of mitochondrial were analyzed, synaptic and extrasynaptic, in rats representing three different stages of development. The analysis demonstrated robust mitochondrial respiration in the oldest subjects (22–24 mos) that was not significantly different from younger (3–5 mos) cohorts, in either mitochondrial fraction. Across all age groups, synaptic mitochondria displayed significantly lower respiration capacities compared to the extrasynaptic fraction. Age-related increases in oxidative damage were observed in the synaptic mitochondria and brain parenchyma.
The evidence for age-related functional decline in neuronal mitochondria is controversial. A relatively early study (Chiu and Richardson 1980) assessed total mitochondria in the whole brain from F344 rats and reported a significant decline only in state III respiration. RCR and state III respiration declines have also been reported in Swiss CD-1/UCadiz mice (Navarro, Gomez et al. 2005) and Wistar rats (Navarro and Boveris 2008; Petrosillo, Fattoretti et al. 2008; Petrosillo, Matera et al. 2008). However, Weindruch (1980) found no significant changes in these parameters in C57BL/6J mice and a more rigorous study (Meng, Wong et al. 2007) failed to observe these changes in F344 rats.
Several laboratories, using young adult animals, have reported differences in the bioenergetics of synaptic and extrasynaptic mitochondrial fractions (Leong, Lai et al. 1984; Davey and Clark 1996; Davey, Canevari et al. 1997; Davey, Peuchen et al. 1998). Examinations of age-related functional differences in synaptic and extrasynaptic mitochondrial fractions isolated from whole brain preparations have reported significant declines in both fractions (Deshmukh, Owen et al. 1980; Deshmukh and Patel 1980; Deshmukh and Patel 1982; Harmon, Nank et al. 1987). These studies are controversial since the results were obtained by pooling tissue from multiple animals and subsequently replicating findings from this common pool of tissue and treating the results as a large sample size. A study by Leslie (1985) using Long Evans rats failed to find a functional decline in extrasynaptic mitochondria.
Relatively few studies limited their evaluations to a specific region of the brain. Navarro et al (2008) reported a significant age-related decline in total cortical and hippocampal mitochondria, showing complex 1- and 2-driven respiration deficits (RCRs, state III, and state IV). Cocco et al (2005) reported a significant age-related decline in cortical state III respiration by both complex 1 (43%) and complex 2 (25%) in extrasynaptic mitochondria using Wistar rats. The present set of observations are unique in that the analyses were limited to the cortex and carefully evaluated both synaptic and extrasynaptic mitochondrial bioenergetics. While we failed to find any age-related differences in mitochondrial bioenergetics, we did observe significant differences between synaptic and extrasynaptic mitochondria in support of the previously mentioned studies.
Several possibilities may account for the lack of age-related bioenergetic changes in cortical mitochondria. Our study compared aged F344 rats 22–24 mos old to young (3–5 mos) and middle aged (12–14 mos) subjects. A previous study showed that naïve F344 rats begin to die spontaneously at around 20 months of age (Coleman, Barthold et al. 1977) as a result of aging. F344 rats 22–24 mos old have been frequently used in aging research, have been extensively characterized, and display many age-related motor and cognitive deficits (Coleman, Barthold et al. 1977; Spangler, Waggie et al. 1994; Shukitt-Hale, Mouzakis et al. 1998). Long et al (2009) reported that these rats display age-related mitochondrial enzymatic impairments and increased levels of oxidative damage at 24 mos of age. Meng et al (2007) reported no significant declines in mitochondrial membrane potential or membrane integrity in F344 rats 26 mos of age, while several groups report deficits in considerably older (28–32 mos) animals (Chiu and Richardson 1980; Leslie, Chandler et al. 1985; Cocco, Sgobbo et al. 2005). LaFrance et al (2005) reported no age-related declines in membrane potential or sensitivity to calcium of cortical mitochondria at 24 mos in F344 rats, but did find significant alterations in animals 33 mos of age. A lack of significant age-related respiration changes in aged F344 rats may indicate mitochondria are more resilient than previously thought and alterations in respiration may not occur until more advanced ages. Since 33 mos are well beyond the normal lifespan of F344 male rats, the findings may represent observations from a unique population and not representative of normal aging.
The quality of the mitochondrial preparation may explain the differences in our findings from those reported previously. While it is difficult to compare the quality of the mitochondrial preparation between studies, the RCR is one reasonable method. The RCR is a good indicator of overall mitochondrial function because it measures how quickly mitochondria can produce ATP (state III) as well as the ability to maintain the proton gradient when not making ATP (state IV). In intact cells of naïve tissues, the ETC is tightly coupled to ATP production (high RCR). Declines in RCR values indicate an uncoupling of the ETC from ATP production (low RCR). The RCR has a range of 0 (totally nonfunctional) to 10 (well-coupled and functional). Several studies have reported mitochondrial RCR values as being much higher than 5 in naïve and uninjured central nervous tissue, demonstrating that the mitochondria are tightly coupled with little damage from isolation procedures (Brown, Sullivan et al. 2006; Singh, Sullivan et al. 2006; Opii, Nukala et al. 2007; Mbye, Singh et al. 2008; Gilmer, Roberts et al. 2009; Patel, Sullivan et al. 2009). The mean RCR in the present study ranged from 7.2 to 8.4 for young and middle aged, and aged cohorts for both synaptic and extrasynaptic fraction, with no significant age-related declines. These values support the idea that the mitochondria used in our study were of high quality. Several other groups have reported no age-related decline in RCR values of rodents (Deshmukh, Owen et al. 1980; Deshmukh and Patel 1980; Weindruch, Cheung et al. 1980; Leslie, Chandler et al. 1985; Meng, Wong et al. 2007), while others report declines ranging from 7–33% (Deshmukh, Owen et al. 1980; Deshmukh and Patel 1980; Deshmukh and Patel 1982; Navarro, Gomez et al. 2005; Navarro, Lopez-Cepero et al. 2008). Almost all prior aging studies evaluating possible changes in mitochondrial bioenergetics had RCR values of 5 or lower in young animals (Chiu and Richardson 1980; Deshmukh and Patel 1980; Weindruch and Masoro 1991; Navarro, Gomez et al. 2005; Meng, Wong et al. 2007; Navarro, Lopez-Cepero et al. 2008). The range for RCR values in much of the aging literature among young, naive animals was 1.3 (Meng, Wong et al. 2007) to 6.1 (Deshmukh, Owen et al. 1980). Low RCR values (below 5) have been reported when mitochondria in young animals have sustained significant damage (Xiong, Gu et al. 1997; Singh, Sullivan et al. 2006; Gilmer, Roberts et al. 2009). Low RCR values not only signify uncoupling of the ETC but also possible disruption of the mitochondrial membranes. Considering that the animals are naïve and not subjected to any type of injury, low RCR values may indicate mitochondrial damage could have occurred during the isolation process. We were unable to detect any age-related changes in the RCR in either the synaptic or extrasynaptic fractions.
In addition to assessing possible age-related changes in respiration, levels of oxidative damage were quantified in the same animals. Three well-characterized markers of oxidative damage (4-HNE, 3-NT, PC) were quantified in the PMS and in purified synaptic and extrasynaptic mitochondria. In these experiments, the levels vary between the PMS and mitochondrial fractions, but the largest age-related increases were observed in synaptic mitochondria. All three markers of oxidative damage were elevated in the PMS with age indicates that reactive oxygen species, primarily formed in mitochondria, are capable to diffusion out from mitochondria and attack surrounding cell structures (Valko, Rhodes et al. 2006). Though mitochondria may be a main source for oxidative damage, it does exclude other possible sources of this oxidative damage (e.g. lysosomes, phagocytes, peroxisomes, etc). Synaptic mitochondria displayed significant age-related increases in levels of PC and 3-NT, while only middle aged animals showed increases in these markers in the extrasynaptic fraction. There were not significant age-related increases found in either mitochondrial fraction, but higher levels were seen in older groups.
Previous research supports an age-related increase in oxidative damage to a number of lipids, proteins, and enzymes in F344 rats (Baek, Kwon et al. 1999). Oxidative damage is likely to be responsible for alterations in mitochondrial respiration observed with aging (Shigenaga, Hagen et al. 1994; Ames, Shigenaga et al. 1995; O'Donnell and Lynch 1998) due to chemical-physical alteration in membranes (Armeni, Principato et al. 2003) and/or electron carriers (Benzi, Pastoris et al. 1992). Structural alterations to components of the ETC explain increased free radical generation with age, but the extent of oxidative damage needed to produce respiration declines remains unexplained.
PC are proteins that have been oxidized by reactive oxygen species and are chemically stable, making them fitting markers of oxidative stress. In the PMS, PC levels were increased 46% (middle) and 52% (aged) compared to young rats. Data indicates an age-related increase in protein oxidation to cellular components outside of mitochondria. Several other groups have reported significant elevations of PC in neuronal tissue (Tian, Cai et al. 1998; Navarro, Gomez et al. 2004; Navarro, Gomez et al. 2005; Navarro and Boveris 2007) (Navarro and Boveris 2004). Extrasynaptic mitochondria displayed a significant increase in PC levels in only middle aged animals (45%) which likely represents the heterogeneity of the middle aged population. Some rats in this middle aged group would survive to older ages and others would not, we may have sampled a disproportionate number of rats that would not have survived to old ages due to 4 to 6 samples not being available for oxidative damage markers since they were limited sample needed for respiration analysis (Figure 4). Extrasynaptic mitochondria from aged animals, on the other hand, contained only 10% higher PC isolated than young rats.
4-HNE is one of the most prevalent and toxic products generated during lipid peroxidation (LP) formed during oxidative stress (Renes, de Vries et al. 2000). 4-HNE exerts cytotoxic effects primarily by modifying intracellular proteins (Uchida, Szweda et al. 1993). LP can modify molecular membrane structure resulting in altered membrane asymmetry and fluidity (Joseph, Denisova et al. 2000). The levels of 4-HNE significantly increased in an age-dependent manner in the PMS (46% in middle aged and an 82% in aged rats). Some studies have reported no age-related alterations of LP in Wistar rats (Dogru-Abbasoglu, Tamer-Toptani et al. 1997), while others observed significant elevations in several distinct fractions of the cerebral cortex (Sahoo and Chainy 1997; O'Donnell and Lynch 1998; Navarro and Boveris 2004) of these aged rats. Tian et al (1998) observed no age-related increases of LP in aged F344 rats, while noting a significant elevation in PC, suggesting that LP may not be the most sensitive measure of oxidative stress.
Several reactive nitrogen species are derived from nitric oxide (NO), one of which is peroxynitrite (ONOO−). A number of oxidation and nitration products are produced from the reaction of ONOO− with cellular macromolecules. One such product, 3-NT, is routinely used as a marker of ONOO− formation in vivo (Beckman and Koppenol 1996). Our data show a significant increase in 3-NT levels in the PMS of aged animals (17%) compared to young levels. Only synaptic mitochondria from aged animals displayed significant increases of 3-NT levels (71%), but middle aged animals did have elevated levels (38%) compared to young rats. In the extrasynaptic fraction, only middle aged animals displayed significantly higher 3-NT levels (55%) alluding to the possibility that the middle aged animals represent an extremely heterogeneous population. The middle aged group may represent a subgroup of rats that were experiencing high levels of oxidative stress that would not survive to older ages, due several samples being limited for respiration analysis (Figure 4). Shin et al (2002) reported that several areas of the brain displayed increased levels of 3-NT in 24–29 mos old Sprague-Dawley rats, with the cerebral cortex having almost twice the levels of young animals (4–6 mos).
An interesting finding from this study was that the levels of oxidative damage (PC, 4-HNE, and 3-NT) did not significantly correlate with the overall functionality, measured by RCR values, in either fraction of mitochondria (Figure 5). On the other hand, extrasynaptic mitochondria showed a positive correlation between the levels of oxidative damage and the overall oxygen utilization rate, whereas this correlation was not apparent in the synaptic fraction (Figure 5). Data indicates that mitochondria with the highest respiration capacity also accumulating the most oxidative damage. Extrasynaptic mitochondria display significantly higher respiration than synaptic mitochondria, indicative of inherent differences in these two fractions of mitochondria.
Fig. 5.
The relationship between the levels of oxidative damage (PC, 4-HNE, and 3-NT) and the RCR values in a. synaptic mitochondria and c. extrasynaptic mitochondria. The relationship between the levels of oxidative damage and the overall oxygen utilization rates in b. synaptic mitochondria and d. extrasynaptic mitochondria. Symbols represent individual values. * p < 0.05, ** 0.01, *** 0.005.
Extrasynaptic mitochondria may be utilized heavily for ATP production and when they accumulate oxidative damage they are replaced or repaired quickly. One possible explanation for not observing significant increases in oxidative damage in extrasynaptic mitochondria from aged animals are these animals represent a group of rats that have maintained proper mitochondrial maintenance and turnover. The significant increase in damage observed in the middle aged animals may represent a crucial turning point in whether the animal will survive to older ages if they have the capacity to make necessary repairs or not. Synaptic mitochondria display a lower respiration, which may indicate that they are utilized more for calcium buffering than ATP production in the synapse. Perhaps synaptic mitochondria accumulate more oxidative damage as long as the damage is not significantly affecting the calcium buffering ability. The relatively large distance of the synapse from the cell body may also determine how often maintenance or replacement of mitochondria occurs.
Data in this report suggest that the mitochondria have not sustained sufficient amounts of damage that manifests into significant functional changes at 22–24 mos of age. Stuart et al (2009) supports this idea in that OGG1 mice, deficient in 8-oxodG removal, which allows high levels of mtDNA lesions to accumulate (20-fold higher than wild-type), did not show respiration deficits. Therefore it is possible mitochondria from 22–24 mos old F344 rats may be able to withstand a considerable amount of oxidative damage than previously thought before major functional respiration declines occur in a naïve state, as our data indicates. Having several compensatory mechanisms in place for increasing ATP production may offset minor deficiencies in oxidative phosphorylation (Rossignol, Faustin et al. 2003). Having an excess of active respiratory complexes can be used as reserves, direct chemical regulation of active complexes, or varying concentrations of intermediate metabolites, all of which can increase ATP production within mitochondria. Systemic approaches to increase ATP synthesis would be to promote glycolysis, enhance oxygen delivery by the circulatory system, or use of phosphocreatine if mitochondria are exhibiting minor deficits.
In conclusion, this study found no significant age-related declines in mitochondrial bioenergetics in the cortex. The aging rodent cortex accumulated higher levels of oxidative damage especially in the synaptic fraction. Data from this study suggest that the accumulation of oxidative damage with aging is not sufficient to result in significant bioenergetic changes in naïve rats 22–24 mos of age. Our data show that synaptic mitochondria display a high degree of respiratory control in agreement with other studies reporting a relatively large range (<25% up to 80%) of the maximal respiratory capacity depending on synaptic activity (Chance and Williams 1956; Scott and Nicholls 1980; Davey, Peuchen et al. 1998; Nicholls 2002). Synaptic mitochondria may maintain more of a basal respiration state in readiness for neurotransmission (e.g. calcium influx, ATP production for vesicle transportation and maintenance of synaptic membrane potential, etc); increasing the probability of free radical formation (Papa and Skulachev 1997) and explaining the elevation of oxidative damage apparent in the synaptic fraction of aged rats.
Acknowledgements
We thank Paula Thomason for carefully reading the manuscript. This work was supported by NIH grant AG21981 and training grant NIH-NIDA 1T32 DA 022738-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflict of interest.
References
- Ames BN, Shigenaga MK, et al. Mitochondrial decay in aging. Biochim Biophys Acta. 1995;1271(1):165–170. doi: 10.1016/0925-4439(95)00024-x. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Roberts KN, et al. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. J Neurotrauma. 2008;25(5):513–526. doi: 10.1089/neu.2007.0451. [DOI] [PubMed] [Google Scholar]
- Armeni T, Principato G, et al. Mitochondrial dysfunctions during aging: vitamin E deficiency or caloric restriction--two different ways of modulating stress. J Bioenerg Biomembr. 2003;35(2):181–191. doi: 10.1023/a:1023754305218. [DOI] [PubMed] [Google Scholar]
- Atamna H, Frey WH., 2nd Mechanisms of mitochondrial dysfunction and energy deficiency in Alzheimer's disease. Mitochondrion. 2007;7(5):297–310. doi: 10.1016/j.mito.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Baek BS, Kwon HJ, et al. Regional difference of ROS generation, lipid peroxidation, and antioxidant enzyme activity in rat brain and their dietary modulation. Arch Pharm Res. 1999;22(4):361–366. doi: 10.1007/BF02979058. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58(4):495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271(5 Pt 1):C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Benzi G, Pastoris O, et al. The mitochondrial electron transfer alteration as a factor involved in the brain aging. Neurobiol Aging. 1992;13(3):361–368. doi: 10.1016/0197-4580(92)90109-b. [DOI] [PubMed] [Google Scholar]
- Bowling AC, Mutisya EM, et al. Age-dependent impairment of mitochondrial function in primate brain. J Neurochem. 1993;60(5):1964–1967. doi: 10.1111/j.1471-4159.1993.tb13430.x. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, et al. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem. 2006;281(17):11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Chance B, Williams GR. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chiu YJ, Richardson A. Effect of age on the function of mitochondria isolated from brain and heart tissue. Exp Gerontol. 1980;15(6):511–517. doi: 10.1016/0531-5565(80)90003-0. [DOI] [PubMed] [Google Scholar]
- Cocco T, Sgobbo P, et al. Tissue-specific changes of mitochondrial functions in aged rats: effect of a long-term dietary treatment with N-acetylcysteine. Free Radic Biol Med. 2005;38(6):796–805. doi: 10.1016/j.freeradbiomed.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Coleman GL, Barthold W, et al. Pathological changes during aging in barrier-reared Fischer 344 male rats. J Gerontol. 1977;32(3):258–278. doi: 10.1093/geronj/32.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey GP, Canevari L, et al. Threshold effects in synaptosomal and nonsynaptic mitochondria from hippocampal CA1 and paramedian neocortex brain regions. J Neurochem. 1997;69(6):2564–2570. doi: 10.1046/j.1471-4159.1997.69062564.x. [DOI] [PubMed] [Google Scholar]
- Davey GP, Clark JB. Threshold effects and control of oxidative phosphorylation in nonsynaptic rat brain mitochondria. J Neurochem. 1996;66(4):1617–1624. doi: 10.1046/j.1471-4159.1996.66041617.x. [DOI] [PubMed] [Google Scholar]
- Davey GP, Peuchen S, et al. Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J Biol Chem. 1998;273(21):12753–12757. doi: 10.1074/jbc.273.21.12753. [DOI] [PubMed] [Google Scholar]
- Davies SM, Poljak A, et al. Measurements of protein carbonyls, ortho- and meta-tyrosine and oxidative phosphorylation complex activity in mitochondria from young and old rats. Free Radic Biol Med. 2001;31(2):181–190. doi: 10.1016/s0891-5849(01)00576-7. [DOI] [PubMed] [Google Scholar]
- Davis M, Whitely T, et al. Selective impairments of mitochondrial respiratory chain activity during aging and ischemic brain damage. Acta Neurochir Suppl. 1997;70:56–58. doi: 10.1007/978-3-7091-6837-0_17. [DOI] [PubMed] [Google Scholar]
- Dennis SC, Lai JC, et al. Comparative studies on glutamate metabolism in synpatic and non-synaptic rat brain mitochondria. Biochem J. 1977;164(3):727–736. doi: 10.1042/bj1640727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh DR, Owen OE, et al. Effect of aging on the metabolism of pyruvate and 3-hydroxybutyrate in nonsynaptic and synaptic mitochondria from rat brain. J Neurochem. 1980;34(5):1219–1224. doi: 10.1111/j.1471-4159.1980.tb09962.x. [DOI] [PubMed] [Google Scholar]
- Deshmukh DR, Patel MS. Age-dependent changes in glutamate oxidation by non-synaptic and synaptic mitochondria from rat brain. Mech Ageing Dev. 1980;13(1):75–81. doi: 10.1016/0047-6374(80)90131-1. [DOI] [PubMed] [Google Scholar]
- Deshmukh DR, Patel MS. Age-dependent changes in pyruvate uptake by nonsynaptic and synaptic mitochondria from rat brain. Mech Ageing Dev. 1982;20(4):343–351. doi: 10.1016/0047-6374(82)90101-4. [DOI] [PubMed] [Google Scholar]
- Dogru-Abbasoglu S, Tamer-Toptani S, et al. Lipid peroxidation and antioxidant enzymes in livers and brains of aged rats. Mech Ageing Dev. 1997;98(2):177–180. doi: 10.1016/s0047-6374(97)00082-1. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, et al. Metabolic and energetic properties of isolated nerve ending particles (synaptosomes) Biochim Biophys Acta. 1996;1277(1–2):13–34. doi: 10.1016/s0005-2728(96)00103-x. [DOI] [PubMed] [Google Scholar]
- Ferrandiz ML, Martinez M, et al. Impairment of mitochondrial oxidative phosphorylation in the brain of aged mice. Brain Res. 1994;644(2):335–338. doi: 10.1016/0006-8993(94)91699-3. [DOI] [PubMed] [Google Scholar]
- Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23(5):795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- Genova ML, Bovina C, et al. Decrease of rotenone inhibition is a sensitive parameter of complex I damage in brain non-synaptic mitochondria of aged rats. FEBS Lett. 1997;410(2–3):467–469. doi: 10.1016/s0014-5793(97)00638-8. [DOI] [PubMed] [Google Scholar]
- Gilmer LK, Roberts KN, et al. Early mitochondrial dysfunction following cortical contusion injury. J Neurotrauma. 2009 doi: 10.1089/neu.2008.0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorini A, Arnaboldi R, et al. Brain cytochrome oxidase activity of synaptic and nonsynaptic mitochondria during aging. Basic Appl Histochem. 1989;33(2):139–145. [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Harman D. The biologic clock: the mitochondria? J Am Geriatr Soc. 1972;20(4):145–147. doi: 10.1111/j.1532-5415.1972.tb00787.x. [DOI] [PubMed] [Google Scholar]
- Harmon HJ, Nank S, et al. Age-dependent changes in rat brain mitochondria of synaptic and non-synaptic origins. Mech Ageing Dev. 1987;38(2):167–177. doi: 10.1016/0047-6374(87)90076-5. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Denisova NA, et al. Oxidative stress protection and vulnerability in aging: putative nutritional implications for intervention. Mech Ageing Dev. 2000;116(2–3):141–153. doi: 10.1016/s0047-6374(00)00128-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann JA, Bickford PC, et al. Oxidative-stress-dependent up-regulation of Bcl-2 expression in the central nervous system of aged Fisher-344 rats. J Neurochem. 2001;76(4):1099–1108. doi: 10.1046/j.1471-4159.2001.00118.x. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med. 1999;26(3–4):463–471. doi: 10.1016/s0891-5849(98)00216-0. [DOI] [PubMed] [Google Scholar]
- Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys. 2000;373(1):16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- LaFrance R, Brustovetsky N, et al. Age-related changes in regional brain mitochondria from Fischer 344 rats. Aging Cell. 2005;4(3):139–145. doi: 10.1111/j.1474-9726.2005.00156.x. [DOI] [PubMed] [Google Scholar]
- Lai JC, Liang BB, et al. Brain mitochondrial citrate synthase and glutamate dehydrogenase: differential inhibition by fatty acyl coenzyme A derivatives. Metab Brain Dis. 1994;9(2):143–152. doi: 10.1007/BF01999767. [DOI] [PubMed] [Google Scholar]
- Lai JC, Walsh JM, et al. Synaptic and non-synaptic mitochondria from rat brain: isolation and characterization. J Neurochem. 1977;28(3):625–631. doi: 10.1111/j.1471-4159.1977.tb10434.x. [DOI] [PubMed] [Google Scholar]
- Leong SF, Lai JC, et al. The activities of some energy-metabolising enzymes in nonsynaptic (free) and synaptic mitochondria derived from selected brain regions. J Neurochem. 1984;42(5):1306–1312. doi: 10.1111/j.1471-4159.1984.tb02788.x. [DOI] [PubMed] [Google Scholar]
- Leslie SW, Chandler LJ, et al. Reduced calcium uptake by rat brain mitochondria and synaptosomes in response to aging. Brain Res. 1985;329(1–2):177–183. doi: 10.1016/0006-8993(85)90523-2. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Hoppel CL. Oxidative phosphorylation and aging. Ageing Res Rev. 2006;5(4):402–433. doi: 10.1016/j.arr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Leuner K, Hauptmann S, et al. Mitochondrial dysfunction: the first domino in brain aging and Alzheimer's disease? Antioxid Redox Signal. 2007;9(10):1659–1675. doi: 10.1089/ars.2007.1763. [DOI] [PubMed] [Google Scholar]
- Long J, Gao F, et al. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res. 2009;34(4):755–763. doi: 10.1007/s11064-008-9850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez de la Plata CD, Hart T, et al. Impact of age on long-term recovery from traumatic brain injury. Arch Phys Med Rehabil. 2008;89(5):896–903. doi: 10.1016/j.apmr.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbye LH, Singh IN, et al. Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a non-immunosuppressive cyclosporin A analog. Exp Neurol. 2008;209(1):243–253. doi: 10.1016/j.expneurol.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Meng Q, Wong YT, et al. Age-related changes in mitochondrial function and antioxidative enzyme activity in fischer 344 rats. Mech Ageing Dev. 2007;128(3):286–292. doi: 10.1016/j.mad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Naga KK, Sullivan PG, et al. High cyclophilin D content of synaptic mitochondria results in increased vulnerability to permeability transition. J Neurosci. 2007;27(28):7469–7475. doi: 10.1523/JNEUROSCI.0646-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol. 2004;287(5):R1244–R1249. doi: 10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Brain mitochondrial dysfunction in aging: conditions that improve survival, neurological performance and mitochondrial function. Front Biosci. 2007;12:1154–1163. doi: 10.2741/2133. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Mitochondrial nitric oxide synthase, mitochondrial brain dysfunction in aging, and mitochondria-targeted antioxidants. Adv Drug Deliv Rev. 2008 doi: 10.1016/j.addr.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, et al. Beneficial effects of moderate exercise on mice aging: survival, behavior, oxidative stress, and mitochondrial electron transfer. Am J Physiol Regul Integr Comp Physiol. 2004;286(3):R505–R511. doi: 10.1152/ajpregu.00208.2003. [DOI] [PubMed] [Google Scholar]
- Navarro A, Gomez C, et al. Vitamin E at high doses improves survival, neurological performance, and brain mitochondrial function in aging male mice. Am J Physiol Regul Integr Comp Physiol. 2005;289(5):R1392–R1399. doi: 10.1152/ajpregu.00834.2004. [DOI] [PubMed] [Google Scholar]
- Navarro A, Lopez-Cepero JM, et al. Hippocampal mitochondrial dysfunction in rat aging. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R501–R509. doi: 10.1152/ajpregu.00492.2007. [DOI] [PubMed] [Google Scholar]
- Navarro A, Sanchez Del Pino MJ, et al. Behavioral dysfunction, brain oxidative stress, and impaired mitochondrial electron transfer in aging mice. Am J Physiol Regul Integr Comp Physiol. 2002;282(4):R985–R992. doi: 10.1152/ajpregu.00537.2001. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial function and dysfunction in the cell: its relevance to aging and aging-related disease. Int J Biochem Cell Biol. 2002;34(11):1372–1381. doi: 10.1016/s1357-2725(02)00077-8. [DOI] [PubMed] [Google Scholar]
- O'Donnell E, Lynch MA. Dietary antioxidant supplementation reverses age-related neuronal changes. Neurobiol Aging. 1998;19(5):461–467. doi: 10.1016/s0197-4580(98)00082-7. [DOI] [PubMed] [Google Scholar]
- Opii WO, Nukala VN, et al. Proteomic identification of oxidized mitochondrial proteins following experimental traumatic brain injury. J Neurotrauma. 2007;24(5):772–789. doi: 10.1089/neu.2006.0229. [DOI] [PubMed] [Google Scholar]
- Papa S, Skulachev VP. Reactive oxygen species, mitochondria, apoptosis and aging. Mol Cell Biochem. 1997;174(1–2):305–319. [PubMed] [Google Scholar]
- Patel SP, Sullivan PG, et al. Differential effects of the mitochondrial uncoupling agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on synaptic or nonsynaptic mitochondria after spinal cord injury. J Neurosci Res. 2009;87(1):130–140. doi: 10.1002/jnr.21814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrosillo G, Fattoretti P, et al. Melatonin prevents age-related mitochondrial dysfunction in rat brain via cardiolipin protection. Rejuvenation Res. 2008;11(5):935–943. doi: 10.1089/rej.2008.0772. [DOI] [PubMed] [Google Scholar]
- Petrosillo G, Matera M, et al. Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem Int. 2008;53(5):126–131. doi: 10.1016/j.neuint.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Renes J, de Vries EE, et al. Multidrug resistance protein MRP1 protects against the toxicity of the major lipid peroxidation product 4-hydroxynonenal. Biochem J. 2000;350(Pt 2):555–561. [PMC free article] [PubMed] [Google Scholar]
- Richter C. Oxidative damage to mitochondrial DNA and its relationship to ageing. Int J Biochem Cell Biol. 1995;27(7):647–653. doi: 10.1016/1357-2725(95)00025-k. [DOI] [PubMed] [Google Scholar]
- Rossignol R, Faustin B, et al. Mitochondrial threshold effects. Biochem J. 2003;370(Pt 3):751–762. doi: 10.1042/BJ20021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo A, Chainy GB. Alterations in the activities of cerebral antioxidant enzymes of rat are related to aging. Int J Dev Neurosci. 1997;15(8):939–948. doi: 10.1016/s0736-5748(97)00049-x. [DOI] [PubMed] [Google Scholar]
- Sandhu SK, Kaur G. Mitochondrial electron transport chain complexes in aging rat brain and lymphocytes. Biogerontology. 2003;4(1):19–29. doi: 10.1023/a:1022473219044. [DOI] [PubMed] [Google Scholar]
- Scott ID, Nicholls DG. Energy transduction in intact synaptosomes. Influence of plasma-membrane depolarization on the respiration and membrane potential of internal mitochondria determined in situ. Biochem J. 1980;186(1):21–33. doi: 10.1042/bj1860021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman EH, Bondy SC. Effects of age and dietary antioxidants on cerebral electron transport chain activity. Neurobiol Aging. 2001;22(4):629–634. doi: 10.1016/s0197-4580(01)00226-3. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, et al. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A. 1994;91(23):10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CM, Chung YH, et al. Age-related changes in the distribution of nitrotyrosine in the cerebral cortex and hippocampus of rats. Brain Res. 2002;931(2):194–199. doi: 10.1016/s0006-8993(01)03391-1. [DOI] [PubMed] [Google Scholar]
- Shukitt-Hale B, Mouzakis G, et al. Psychomotor and spatial memory performance in aging male Fischer 344 rats. Exp Gerontol. 1998;33(6):615–624. doi: 10.1016/s0531-5565(98)00024-2. [DOI] [PubMed] [Google Scholar]
- Singh IN, Sullivan PG, et al. Time course of post-traumatic mitochondrial oxidative damage and dysfunction in a mouse model of focal traumatic brain injury: implications for neuroprotective therapy. J Cereb Blood Flow Metab. 2006;26(11):1407–1418. doi: 10.1038/sj.jcbfm.9600297. [DOI] [PubMed] [Google Scholar]
- Spangler EL, Waggie KS, et al. Behavioral assessment of aging in male Fischer 344 and brown Norway rat strains and their F1 hybrid. Neurobiol Aging. 1994;15(3):319–328. doi: 10.1016/0197-4580(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Tian L, Cai Q, et al. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic Biol Med. 1998;24(9):1477–1484. doi: 10.1016/s0891-5849(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Uchida K, Szweda LI, et al. Immunochemical detection of 4-hydroxynonenal protein adducts in oxidized hepatocytes. Proc Natl Acad Sci U S A. 1993;90(18):8742–8746. doi: 10.1073/pnas.90.18.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utomo WK, Gabbe BJ, et al. Predictors of in-hospital mortality and 6-month functional outcomes in older adults after moderate to severe traumatic brain injury. Injury. 2009 doi: 10.1016/j.injury.2009.05.034. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160(1):1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Richardson A. Oxidative damage to mitochondria and aging. Exp Gerontol. 2001;36(7):957–968. doi: 10.1016/s0531-5565(01)00093-6. [DOI] [PubMed] [Google Scholar]
- Villa RF, Gorini A, et al. Enzyme activities in perikaryal and synaptic mitochondrial fractions from rat hippocampus during development. Mech Ageing Dev. 1989;49(3):211–225. doi: 10.1016/0047-6374(89)90072-9. [DOI] [PubMed] [Google Scholar]
- Walker LM, York JL, et al. Oxidative stress and reactive nitrogen species generation during renal ischemia. Toxicol Sci. 2001;63(1):143–148. doi: 10.1093/toxsci/63.1.143. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Masoro EJ. Concerns about rodent models for aging research. J Gerontol. 1991;46(3):B87–B88. doi: 10.1093/geronj/46.3.b87. [DOI] [PubMed] [Google Scholar]
- Weindruch RH, Cheung MK, et al. Modification of mitochondrial respiration by aging and dietary restriction. Mech Ageing Dev. 1980;12(4):375–392. doi: 10.1016/0047-6374(80)90070-6. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Gu Q, et al. Mitochondrial dysfunction and calcium perturbation induced by traumatic brain injury. J Neurotrauma. 1997;14(1):23–34. doi: 10.1089/neu.1997.14.23. [DOI] [PubMed] [Google Scholar]