Abstract
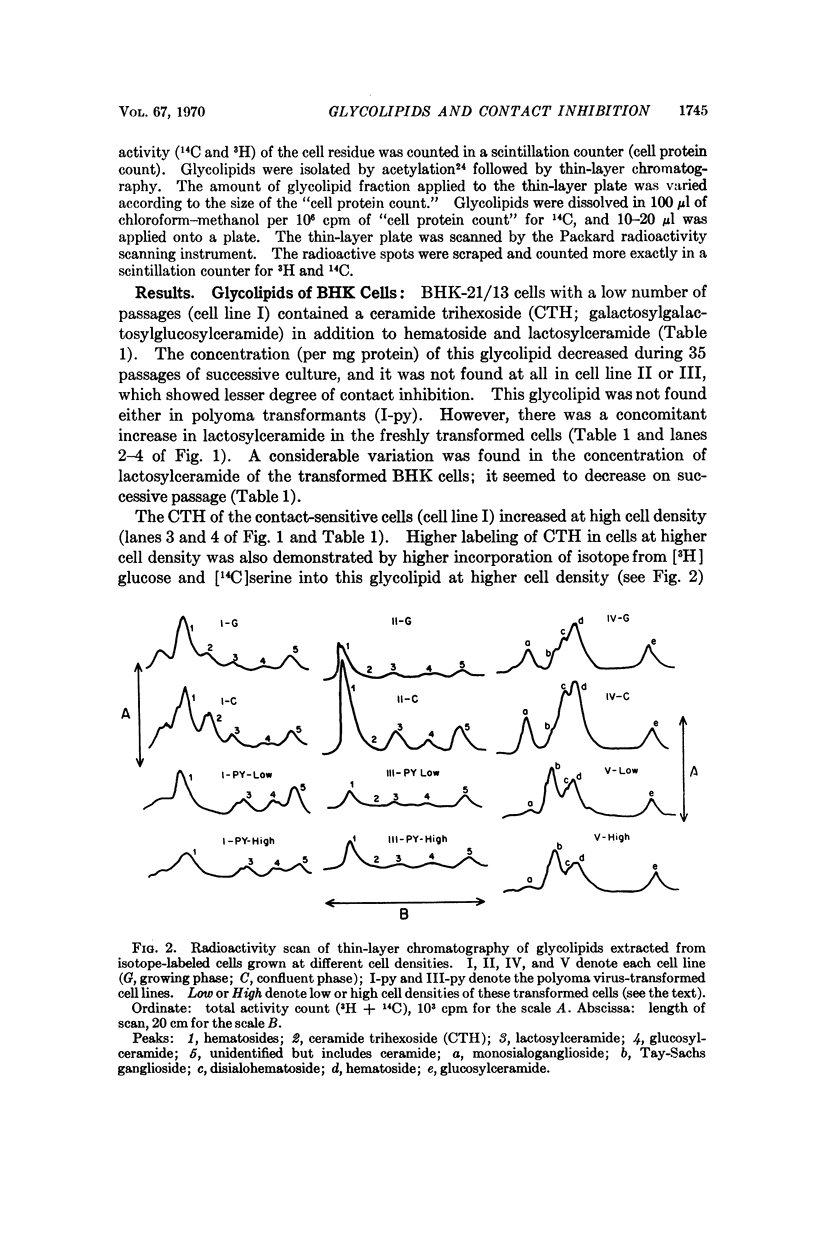
The glycolipids of normal fibroblasts cells at different stages of growth and with differing degrees of susceptibility to contact inhibition have been analyzed, as well as the glycolipids of virally transformed cells. The concentrations (per mg protein) of certain glycolipids, including galactosylgalactosyl-glucosyl-, N-acetylneuraminosylgalactosylglucosyl-, and N-acetylneuraminosyl-(N-acetylneuraminosyl)galactosylglucosyl-c eramide, increase on cell-to-cell contact of susceptible cells. The concentrations of these glycolipids decrease when susceptibility to contact inhibition is lost as a result of extensive passage in vitro or transformation by simian virus 40 or polyoma virus, and in all the malignant cells examined, the concentrations of these glycolipids were independent of cell density.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., AMBROSE E. J. The surface properties of cancer cells: a review. Cancer Res. 1962 Jun;22:525–548. [PubMed] [Google Scholar]
- Brady R. O., Borek C., Bradley R. M. Composition and synthesis of gangliosides in rat hepatocyte and hepatoma cell lines. J Biol Chem. 1969 Dec 10;244(23):6552–6554. [PubMed] [Google Scholar]
- Burger M. M. A difference in the architecture of the surface membrane of normal and virally transformed cells. Proc Natl Acad Sci U S A. 1969 Mar;62(3):994–1001. doi: 10.1073/pnas.62.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEFENDI V., GASIC G. SURFACE MUCOPOLYSACCHARIDES OF POLYOMA VIRUS TRANSFORMED CELLS. J Cell Physiol. 1963 Aug;62:23–32. doi: 10.1002/jcp.1030620105. [DOI] [PubMed] [Google Scholar]
- Hakomori S. I., Murakami W. T. Glycolipids of hamster fibroblasts and derived malignant-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Jan;59(1):254–261. doi: 10.1073/pnas.59.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakomori S. I., Teather C., Andrews H. Organizational difference of cell surface "hematoside" in normal and virally transformed cells. Biochem Biophys Res Commun. 1968 Nov 25;33(4):563–568. doi: 10.1016/0006-291x(68)90332-x. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Strycharz G. D. Investigations on cellular blood-group substances. I. Isolation and chemical composition of blood-group ABH and Le-b isoantigens of sphingoglycolipid nature. Biochemistry. 1968 Apr;7(4):1279–1286. doi: 10.1021/bi00844a005. [DOI] [PubMed] [Google Scholar]
- Häyry P., Defendi V. Surface antigen(s) of SV40-transformed tumor cells. Virology. 1970 May;41(1):22–29. doi: 10.1016/0042-6822(70)90050-4. [DOI] [PubMed] [Google Scholar]
- Inbar M., Sachs L. Structural difference in sites on the surface membrane of normal and transformed cells. Nature. 1969 Aug 16;223(5207):710–712. doi: 10.1038/223710a0. [DOI] [PubMed] [Google Scholar]
- JAMES A. M., AMBROSE E. J., LOWICK J. H. Differences between the electrical charge carried by normal and homologous tumour cells. Nature. 1956 Mar 24;177(4508):576–577. doi: 10.1038/177576a0. [DOI] [PubMed] [Google Scholar]
- Meezan E., Wu H. C., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by sephadex chromatography. Biochemistry. 1969 Jun;8(6):2518–2524. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- Mora P. T., Brady R. O., Bradley R. M., McFarland V. W. Gangliosides in DNA virus-transformed and spontaneously transformed tumorigenic mouse cell lines. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1290–1296. doi: 10.1073/pnas.63.4.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIOUS D. A., HAMBURGER R. N., MILLS S. E. CLONAL GROWTH OF PRIMARY HUMAN CELL CULTURES. Exp Cell Res. 1964 Feb;33:495–507. doi: 10.1016/0014-4827(64)90014-x. [DOI] [PubMed] [Google Scholar]
- Penick R. J., Meisler M. H., McCluer R. H. Thin-layer chromatographic studies of human brain gangliosides. Biochim Biophys Acta. 1966 Apr 4;116(2):279–287. doi: 10.1016/0005-2760(66)90010-5. [DOI] [PubMed] [Google Scholar]
- Robinson E. A., Kalckar H. M., Troedsson H., Sanford K. Metabolic inhibition of mammalian uridine diphosphate galactose 4-epimerase in cell cultures and in tumor cells. J Biol Chem. 1966 Jun 25;241(12):2737–2745. [PubMed] [Google Scholar]
- TODARO G. J., WOLMAN S. R., GREEN H. RAPID TRANSFORMATION OF HUMAN FIBROBLASTS WITH LOW GROWTH POTENTIAL INTO ESTABLISHED CELL LINES BY SV40. J Cell Physiol. 1963 Dec;62:257–265. doi: 10.1002/jcp.1030620305. [DOI] [PubMed] [Google Scholar]
- VOGT P. K. THE CELL SURFACE IN TUMOR VIRUS INFECTION. Cancer Res. 1963 Oct;23:1519–1527. [PubMed] [Google Scholar]
- Warren L., Glick M. C. Membranes of animal cells. II. The metabolism and turnover of the surface membrane. J Cell Biol. 1968 Jun;37(3):729–746. doi: 10.1083/jcb.37.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Meezan E., Black P. H., Robbins P. W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry. 1969 Jun;8(6):2509–2517. doi: 10.1021/bi00834a038. [DOI] [PubMed] [Google Scholar]