Abstract
More than 300 human clinical trials utilize recombinant adenoviruses (rAds) as a gene transfer vector, confirming that rAds continue to be of high clinical interest. A primary weakness of rAds is their known propensity to trigger an innate, proinflammatory immune response rapidly after high-dose, systemic administration. In this study, we investigated what affects that pre-emptive treatment with anti-inflammatory glucocorticoids might have upon Ad vector-triggered inflammatory immune responses. We found that a simple pretreatment regimen with Dexamethasone (DEX) can significantly reduce most Ad-induced innate immune responses. DEX prevented rAd induction of systemic cytokine/chemokine releases in a dose-dependent fashion, with higher dosages preventing rAd induction of acute thrombocytopenia, endothelial cell activation, proinflammatory gene induction, and leukocyte infiltration into transduced organs. Transient glucocorticoid pretreatment also significantly reduced rAd-induced adaptive immune responses, including a decreased induction of Ad-neutralizing antibodies (NAbs). Importantly, use of DEX did not reduce the efficacy of rAd-mediated gene transduction nor rAd-derived transgene expression. Our results demonstrate that a simple, pre-emptive and transient glucocorticoid pretreatment is a viable approach to reduce rAd-associated acute toxicities that currently limit the use of Ad vectors in systemic clinical applications.
Introduction
Adenovirus (Ad)-based vectors continue to be the most commonly utilized gene transfer vector in a variety of potential applications. Ad vectors can be easily produced to high titers (scalability is a critical point when considering potential human applications) possess the ability to transduce dividing and nondividing cells without the need for chromosomal integration and have an extremely broad tropism. These advantages have resulted in the initiation of 342 human clinical trials utilizing Ad vectors since the first Ad clinical trial in 1993 (http://www.wiley.co.uk/wileychi/genmed/clinical/). Furthermore, first-generation Ad vectors have been repeatedly demonstrated to persist for long periods when transducing nonimmunogenic transgenes.1 Limitations to long-term persistence of first-generation Ads-transducing immunogenic transgenes have been largely overcome with the development of multiply deleted, helper-independent, or fully deleted helper virus-dependent, advanced-generation, Ad-based vectoring systems.2 Despite these encouraging facts, safety concerns regarding Ad vector-associated innate toxicities, responses that often prime subsequent adaptive immune responses, have severely limited progress in the use of this important vector class for systemic applications, such as gene transfer to the liver.
Several approaches have been studied to minimize the inflammatory responses acutely induced by systemic exposure to Ad vectors. These approaches include genetic modification of the Ad capsid to alter the tropism of the vector for liver cells, pre-emptive depletion (or blockade) of Ad sequestration by liver macrophages to minimize induction of macrophage-dependent inflammatory responses, use of immunosuppressive drugs (such as tumor necrosis factor blockers, Toll-like receptor 9 (TLR-9) inhibitors, extracellular signal-regulated kinase inhibitors, and others) to transiently block acute inflammatory responses, as well surgical isolation procedures to minimize systemic distribution of recombinant Ads (rAds).3,4,5,6,7,8,9 All of these approaches have an ability to reduce portions of the multifaceted Ad-induced innate immune response, but their ability to impact upon the full inflammatory response induced by rAds is either limited or has not been fully determined. Furthermore, many of these approaches have inherent problems as well. For example, nonspecific toxicities are noted with use of clodronated liposomes; Ad capsid changes may result in significantly altered tropisms and bio-distribution patterns that can correlate with an enhanced innate toxicity, and technical difficulties may be associated with moderate to significantly invasive surgical procedures.4,5,10 What is needed is a safe, simple, transient, inexpensive, and widely accepted method for the reduction and/or elimination of the myriad Ad vector-induced inflammatory responses induced after systemic administration.
It is noteworthy that the synthetic anti-inflammatory glucocorticoid Dexamethasone (DEX) is an FDA-approved drug widely used to treat a number of transient and/or chronic inflammatory conditions.11,12 Importantly, mechanisms of action of DEX include preventing the activation of nuclear factor κB (NF-κB) and AP1 transcription factors, as well as mitogen-activated protein kinases, all of which have been shown by us and others to also be important mediators of the Ad-induced inflammatory response complex.13 Interleukin (IL)-8 and interferon β production have also been shown to be inhibited by DEX.13 As a result, the maturation of mast cell progenitors, as well as granulocyte–macrophage colony–stimulating factor and tumor necrosis factor-α secretion by mast cells, are also altered by DEX treatment.14
Very limited data are available regarding the effects that DEX treatment has on animals treated with gene transfer agents. Use of glucocorticoids has been shown to increase the efficacy of gene transfer in vitro; in vivo studies showed that DEX could improve nonviral gene and virus-derived gene expression in experimental animal models.15,16,17 Relative to Ad-mediated gene transfer, DEX can improve Ad vector-derived gene expression when these vectors are directly administered into the spinal cord, nasal mucosa, inner ear, or lungs.17,18,19 Pulmonary delivery of Ad vectors into mice pretreated with DEX/spermine resulted in a reduced activation of limited portions of the lung-specific innate and adaptive immune response to the Ad vector utilized.19
These results prompted us to evaluate the efficacy of DEX to prevent the numerous innate toxicities induced by systemic administration of Ad vectors. In our study, we intravenously injected high doses of a first-generation (E1-deleted) Ad5 vector encoding a highly immunogenic transgene (Bacterial B-galactosidase (LacZ)) into either control or DEX pretreated C57BL/6 mice, and investigated the subsequent innate and adaptive immune responses to the Ad and the LacZ protein expressed by the vector. Our results demonstrate that multiple aspects of the systemic inflammatory response typically induced rapidly after systemic delivery of Ads can be minimized or obliterated by simple, transient pretreatment with a potent glucocorticoid, in a dose-dependent fashion.
Results
DEX blocks Ad-mediated cytokine and chemokine release in C57BL/6 mice in a dose-dependent manner
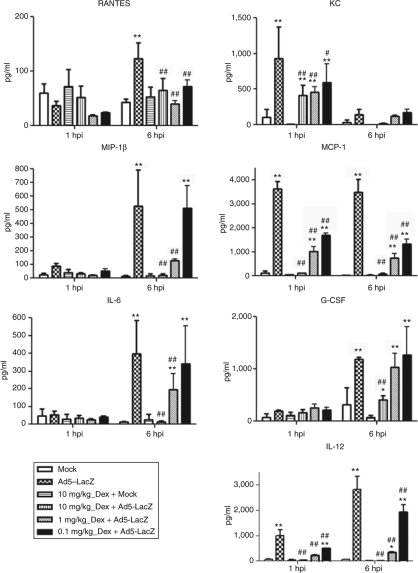
We quantified the release of inflammatory cytokines and chemokines in murine plasma at 1 and 6 hours after systemic Ad delivery utilizing a multiplexed, bead array–based detection system. Based on our previous publications, as well those of others, we focused upon seven cytokines and chemokines that are known to be rapidly (within 1–6 hours) induced following systemic injection of Ad vectors.4,20 Keratinocyte derived chemokine (chemokine (C–X–C motif) ligand 1) and monocyte chemoattractant protein 1 are the first inflammatory chemokines to be induced (within 1 hour of Ad injection). These murine chemokines are known to recruit neutrophils and monocytes to the sites of tissue damage, comprising one of the first defensive barriers to Ad vectors.4,20 At 6 hpi, plasma levels of IL-6, IL-12p40, granulocyte colony–stimulating factor, macrophage inflammatory protein 1β, and normal T-cell Expressed and Secreted (RANTES) also reach a peak, further activating the cellular arm of the innate immune system.4,20 As expected, these seven cytokines and chemokines were induced after Ad injection into wild-type (WT) mice (Figure 1). To investigate the impact of DEX pretreatment (15 hours and 2 hours prior to intravenous Ad5-LacZ injection) in the induction of these proinflammatory factors, we completed a dose–response study where mice were pretreated with 10 mg/kg, 1 mg/kg, or 0.1 mg/kg of DEX. At 1 hour postinjection of Ad5-LacZ, the level of keratinocyte derived chemokine in the serum of DEX pretreated mice (all dosages) was significantly lower compared to mice identically injected with Ad. RANTES levels at 6 hpi were also equivalently reduced by DEX. Interestingly, we did not observe a dose-dependent reduction of these chemokines, as the 0.1 mg/kg dose was as efficacious as the 10 mg/kg dose in modulating the induction of both keratinocyte derived chemokine and RANTES.
Figure 1.
Dexamethasone (DEX) blocks Ad-mediated systemic cytokine and chemokine release in C57BL/6 mice in a dose-dependent manner. C57BL/6 mice were intravenously injected with 0.75 × 1011 vp/mouse of Ad5-LacZ vector. DEX pretreatment was performed via intraperitoneal injection at 15 and 2 hours before virus injection. Plasma samples were collected at 1 and 6 hours postvirus injection (hpi). Plasma samples were analyzed using a multiplexed bead array–based system. The bars represent mean ± SD. *,**Plasma cytokine values that are statistically different from those in mock-injected animals of the same treatment at the same time point (i.e., WT_DEX_Ad5-LacZ groups from WT-DEX-Mock group), P < 0.05 and P < 0.001, respectively. #,##Statistically different values in WT_DEX_Ad5-LacZ groups compared to WT_Ad5-LacZ group at the same time point, P < 0.05 and P < 0.001, respectively. Statistical analysis was completed using two-way analysis of variance with a Bonferroni post hoc test. N = 4 for Mock (phosphate buffered saline)–injected animals, N = 6 for virus-injected mice. MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; IL, interleukin; WT, wild type.
At a dose of 10 mg/kg, DEX completely blocked the release of IL-6 and macrophage inflammatory protein 1β (P < 0.001) at 6 hpi, and monocyte chemoattractant protein 1 and IL-12(p40) at both 1 and 6 hpi (P < 0.001). The levels of IL-12(p40) and monocyte chemoattractant protein 1, at both time points, were also significantly reduced when mice were pretreated with either 1.0 or 0.1 mg/kg DEX. Serum levels of macrophage inflammatory protein 1β and IL-6 were also significantly lower in mice pretreated with 10.0 or 1 mg/kg DEX, but were not significantly reduced in Ad-injected mice pretreated with 0.1 mg/kg DEX. Finally, plasma levels of granulocyte colony–stimulating factor were only significantly reduced when Ad-injected mice were pr-treated with 10 mg/kg DEX (Figure 1). These results demonstrate that DEX pretreatment of animals can abrogate Ad induction of the “cytokine storm” noted after systemic delivery of Ad5-based vectors.21 Because pretreatment of mice with 10 mg/kg DEX maximally suppressed these responses, we evaluated the ability of this dose to further prevent the induction of other detrimental responses typically observed following systemic Ad vector administrations.
DEX prevents Ad induction of thrombocytopenia in C57BL/6 mice
Next, we determined whether DEX treatment could prevent the acute, consumptive thrombocytopenia typically observed within 24 hours after systemic Ad injection, an event that we have shown to be due to Ad activation of the alternative complement pathway.20,21,22 As expected, thrombocytopenia developed in WT mice treated with Ad5-LacZ (Figure 2). DEX pretreated mice did not develop thrombocytopenia after Ad injection, as their platelet levels were not different from mock-injected animals (Figure 2).
Figure 2.
Dexamethasone (DEX) prevents Ad-mediated thrombocytopenia in C57BL/6 mice. C57BL/6 mice were intravenously injected with 0.75 × 1011 vp/mouse of Ad5-LacZ vector. DEX pretreatment and platelets enumerations were performed as described in Materials and Methods. Four groups of wild-type mice were analyzed: WT_Mock (N = 4), WT_DEX_Mock (N = 4), WT_Ad5-LacZ (N = 5), and WT_DEX_Ad5-LacZ (N = 5). The bars represent mean ± SD. Statistical analysis was completed using one-way analysis of variance with a Student–Newman–Keuls post hoc test; P < 0.05 was deemed a statistically significant difference. *,**Statistically different from those in WT_Mock–injected animals, P < 0.05 and P < 0.001, respectively. Note: Normal range levels were adapted from Jackson laboratories studies on C57BL/6 mice (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&sym=Peters3). WT, wild type.
DEX minimizes Ad-dependent activation of endothelial cells in C57BL/6 mice
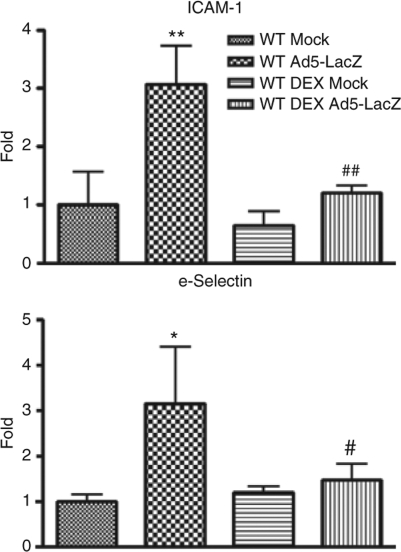
Endothelial cell activation facilitates the infiltration of inflammatory cells, including macrophages, granulocytes and mast cells, into the parenchyma of inflamed tissues and organs. When endothelial cells become activated, they overexpress surface as well as soluble forms of adhesion molecules such as e-Selectin, intercellular adhesion molecule 1 (ICAM-1), and vascular cell adhesion molecule 1 (VCAM-1).23,24 Intravenous injection of Ad vectors is known to induce endothelial cell activation, an event that is mediated by Ad interactions with Kupffer cells that can result in significant hypotension.25 We have measured plasma levels of soluble ICAM-1 and e-Selectin molecules in Ad-injected mice at 6 hpi. Ad-treated WT mice had at least threefold higher levels of soluble ICAM-1 and e-Selectin molecules than levels measured in mock-injected animals (Figure 3). DEX pretreatment completely blocked Ad-dependent activation of synthesis of these two molecules, indirectly demonstrating that DEX pretreatment can reduce the Ad-dependent activation of endothelial cells (Figure 3).
Figure 3.
Dexamethasone (DEX) minimizes Ad-dependent activation of endothelial cells in C57BL/6 mice. DEX pretreated or nontreated C57BL/6 mice were intravenously injected with 0.75 × 1011 vp/mouse of Ad5-LacZ vector. Plasma samples, collected at 6 hpi (N = 6 for virus-treated groups, N = 4 for mock-injected groups) were analyzed using a multiplexed bead array–based quantitative system. The bars represent mean ± SD. Statistical analysis was completed using one-way analysis of variance with a Student–Newman–Keuls post hoc test. Fold difference over WT_Mock group is shown. *,**Statistically different from those in mock-injected animals of the same treatment, P < 0.05 and P < 0.001, respectively. #,##Statistically different values in any group compared to WT_Mock group, P < 0.05 and P < 0.001, respectively. ICAM-1, intercellular adhesion molecule 1; WT, wild type.
DEX pretreatment minimizes the induction of proinflammatory genes in livers of Ad-treated C57BL/6 mice
We have previously and extensively characterized the cell or tissue-specific transcriptome changes rapidly induced after transduction by Ad vectors both in vitro and in vivo.20,26,27 These studies revealed that Ads induce the expression of genes involved in innate immune responses, such as pattern recognition receptors (TLRs, nucleotide-binding oligomerization domains), TLR signaling pathways (myeloid differentiation factor-88, TIR-domain-containing adapter-inducing interferon-β, tumor necrosis factor receptor associated factor 6, tumor necrosis factor receptor associated factor 2bp, TANK-binding kinase 1), markers of endothelial cells activation (e-Selectin, ICAM-1, VCAM-1), interferon responsive genes (2′–5′ oligoadenylate synthetase, interferon regulatory factor 7, interferon regulatory factor 8), negative regulators of cytokine signaling (suppressor of cytokine signalling-1, suppressor of cytokine signalling-3), and dsRNA-editing enzymes (adenosine deaminase–RNA-specific).26,27 We have also previously shown that in C57BL/6 mice, systemically administered Ads mediate induction of maximal liver transcription dysregulation at 6 hpi.26 Therefore, this time point was selected to determine whether pretreatment with DEX might block and/or minimize Ad vector induction of transcription of these proinflammatory genes (Table 1). Besides the genes mentioned above, we interrogated the expression of several additional genes: decay accelerating factor, potent membrane-bound complement inhibitor; NF-κB-RelA, subunit of transcription factor; Janus kinase 1 and Janus kinase 3, molecules involved in cytokine signaling in this assay. Thus, a total 23 genes were tested by quantitative reverse transcriptase (qRT)-PCR–based methods. Our results demonstrate that all of the genes tested (except interferon α) were significantly induced at 6 hpi in the livers of C57BL/6 mice systemically treated with Ad5-LacZ, as compared to mock-injected animals (P < 0.05), whereas only adenosine deaminase–RNA-specific and TLR3 were induced in DEX pretreated Ad5-LacZ–injected mice, as compared to mock-injected animals. Importantly, the level of transcription induction of all the genes except one was significantly reduced in virus-injected DEX pretreated mice, compared to the virus-treated group (P < 0.05, in vast majority of cases P < 0.001). Some of the genes in the DEX-treated, mock-infected groups of mice also had lower levels of transcription relative to mock-infected mice not treated with DEX, but in only five genes did this reach statistically significant levels: Janus kinase 1, Janus kinase 3, VCAM-1, nucleotide-binding oligomerization domain-1, and nucleotide-binding oligomerization domain-2, which indicates that several genes nonspecifically respond to DEX treatment. These data strongly indicate that despite the huge redundancy inherent to the Ad-induced host–transcriptome response mechanism, DEX can efficiently block all of these responses. Possibly, DEX can block transcription activation pathways and/or directly downregulate the transcription of proinflammatory genes typically responsive to glucocorticoid receptor (GR) activation, although post-transcriptional modifications altering RNA stability is a legitimate alternative explanation of DEX-mediated blockage of gene induction.28
Table 1.
Ad5-LacZ induced gene expression in a liver of C57BL/6 mice (fold over Mock, 6 hpi)
DEX treatment causes an increase in lymphopenia and neutrophilia in C57BL/6 mice, an effect not related to Ad treatment
Besides acute thrombocytopenia, high-dose, systemic Ad injections can induce lymphopenia and neutrophilia.29 At the dose of Ad we used (0.75 × 1011 vp/mouse) we did not detect any increase in neutrophils and/or decrease in lymphocytes in C57BL/6 mice at 24 hpi as compared to mock-injected mice (Supplementary Figure S1). Interestingly, both groups of mice treated with DEX experienced neutrophilia and lymphopenia when compared with nontreated WT mice (Supplementary Figure S1). These phenomena were not related to Ad injection, as there were no significant differences between the DEX pretreated, mock-, or Ad5-LacZ–injected groups. DEX has been shown to induce lymphopenia and neutrophilia in several animal models, including C57BL/6 mice30 and rabbits.31
DEX pretreatment preserves the efficacy of Ad-mediated gene transduction of the liver of C57BL/6 mice
It is known that systemic injection of high-dose Ad vectors results in over 90% of an Ad vector bolus becoming sequestered in liver Kupffer cells, with the remaining virus primarily infecting hepatocytes, as the 100 nm Ad capsid can pass through the fenestrated liver endothelium.4,32,33 Having determined that DEX pretreatment efficiently blocked the vast majority of the acute toxicities typically elicited rapidly after systemic Ad administrations, it was critical to confirm whether DEX pretreatment impacted upon the efficacy of Ad transduction of the murine liver. With this information, we analyzed the level of Ad-derived transgene expression (β-Gal) in the liver hepatocytes of Ad vector–treated C57BL/6 mice at 6 hpi, 24 hpi, and 28 dpi (Supplementary Figure S2a,b). The liver activity of Ad vector-derived β-Gal was not different between Ad5-LacZ groups DEX at 24 hpi and 28 dpi, suggesting similar transductional efficacy. Although transgene-derived enzyme activity was highest at 24 hpi, enzyme activity was still present at significant levels at 28 dpi.
We noted significant differences (P < 0.001) in initial transgene expression (at 6 hpi) between virus-injected DEX pretreated mice and mice treated with virus alone (Supplementary Figure S2b), which were not due to different levels of liver transduction by the Ad in these two experimental groups (Supplementary Figure S2c). DEX did not diminish the efficacy of liver transduction by Ads, as Ad genome content in the liver was not different in all Ad5-LacZ treatment groups at all time points tested (Supplementary Figure S2c). Moreover, we noted a mild though significant increase in the number of Ad vector genomes per liver cell at 24 hpi in DEX pretreated group (P < 0.05), which possibly suggests a somewhat reduced level of clearance of Ad vectors by the host innate immune system at initial time points. Despite this finding, an increased number of Ad genomes at 24 hpi in DEX pretreated, Ad5-LacZ–treated mice did not result in significant increases in enzyme activity, suggesting that LacZ expression was already at maximal levels.
DEX treatment does not change Kupffer cell loss in livers of Ad-injected mice
Resident liver macrophages, Kupffer cells, comprise a primary innate defensive system, a system known to nonspecifically engulf, sequester, and destroy Ads prior to hepatocyte encounter.32 It is known that systemic injection of high-dose Ad vectors results in Kupffer cell–dependent increases in plasma cytokine and chemokine responses (as well endothelial cell activation), which is quickly followed by a rapid Kupffer cells necrosis and loss from the liver.4,25,34 Therefore, it was important to determine whether DEX pretreatment altered Kupffer cell responses to systemic Ad. We tested whether DEX prevented Ad-induced Kupffer cell destruction by staining frozen liver sections with the macrophage-specific F4/80 antibody at 24 hpi (Supplementary Figure S3). As expected, a significant decrease of Kupffer cell numbers occurred in the livers of Ad5-LacZ–treated mice as compared to mock-infected mice. Similarly, DEX pretreated, Ad-injected mice also had Kupffer cells destroyed at 24 hpi at rates no different from Ad5-LacZ–injected mice. Finally, DEX treatment by itself did not have any significant effect on Kupffer cell populations in the murine liver based on this assay. Therefore, although it is known that the induction by Ads of a number of proinflammatory cytokines, chemokines, and markers of endothelial cell activation after systemic Ad injection is Kupffer cell dependent, Kupffer cell destruction by Ads is not directly responsible for many of these responses per se.34
DEX treatment significantly reduces leukocyte infiltration into the liver of Ad-treated mice
To test whether DEX pretreatment can impact upon the known propensity of systemically administered Ads to induce leukocyte infiltration into the murine liver, we quantified leukocyte infiltration in liver sections collected at 28 dpi from control and experimental animal groups (Figure 4a,b).35,36 Treatment of mice with only Ad5-LacZ–induced significant infiltration of macrophages and lymphocytes into the liver (Figure 4a,b). DEX pretreatment significantly (P < 0.05) reduced portal inflammation in virus-injected animals, and trended to lower periportal and lobular inflammation at this time point as well. Furthermore, the total inflammation index score was significantly (P < 0.01) lower in DEX-treated, virus-injected mice, as compared to mice receiving only the virus. We have also evaluated leukocyte infiltration in livers of Ad-treated mice at 6 and 24 hours after Ad injection. We did not detect any significant infiltrations of inflammatory cells by this assay in any of the groups tested (data not shown).
Figure 4.
Dexamethasone (DEX) treatment results in significantly reduced Ad-mediated leukocytes infiltration to the liver of C57BL/6 mice at 28 dpi. Mice injections and morphometric evaluation of liver sections was performed as described in Materials and Methods. (a) Representative H&E–stained liver sections obtained at 28 dpi from three groups of mice: WT_Mock (N = 4), WT_Ad5-LacZ (N = 5), and WT_DEX_Ad5-LacZ (N = 5) are shown. Note the lack of any inflammation in WT_Mock, the large number of inflammatory cells in WT_Ad5-LacZ and moderate infiltration in WT_DEX_Ad5-LacZ. (b) Representative sections from each treated animal were analyzed, scored and averaged for the levels of portal, periportal, and lobular inflammation, as described in Materials and Methods. The sum of averages for each category was computed to obtain a total inflammation index score. The error bars represent ± SD. Statistical analysis was completed using two-tailed Student's t-test to compare two groups of virus-injected animals. #,##Statistically different values in WT_DEX_Ad5-LacZ group compared to WT_Ad5-LacZ group, P < 0.05 and P < 0.001, respectively. H&E, hematoxilin and eosin; WT, wild type.
DEX pretreatment significantly alters Ad vector and transgene-specific adaptive immune responses
With the knowledge that pretreatment with DEX minimizes and/or abrogates multiple Ad-triggered innate immune responses, we next determined how these blunted innate responses impacted upon subsequent adaptive immune responses. We and others have previously shown that anti-Ad–specific primary humoral responses can reach their peak at 7–14 days postvirus treatment, whereas antitransgene responses peak at 23–28 days.7,21 We have performed a series of ELISAs to determine the levels of total Ad virus- or LacZ-specific IgM, IgA, IgG, and IgG isotype (IgG1, 2a, 2b, and 3) antibody levels at 14 and 28 dpi. Pretreatment of Ad5-LacZ–injected mice with DEX significantly reduced the levels of all antibodies generated against the Ad capsid (Figure 5a). Specifically, IgG1 and IgG3 were significantly reduced at 14 dpi, whereas IgM, IgA, total IgG, IgG2a, and IgG2b were all significantly reduced both at 14 and 28 dpi relative to Ad5-LacZ–treated mice not treated with DEX (Figure 5a).
Figure 5.
Dexamethasone (DEX) treatment significantly reduced Ad vector capsid–specific humoral immune responses, including capsid-neutralizing antibodies. (a) Three groups of mice were treated as described in Materials and Methods: WT_Mock (N = 4), WT_Ad5-LacZ (N = 5), WT_DEX_Ad5-LacZ (N = 5). Plasma samples, collected at 14 dpi and 28 dpi, were analyzed for anti–Ad-capsid-specific total IgM, IgA, and IgG antibodies and various IgG subclasses. The error bars represent ± SD. Statistical analysis was completed using two-tailed Student's t-test to compare two groups of virus-injected animals. #,##Statistically different values in WT_DEX_Ad5-LacZ group compared to WT_Ad5-LacZ group, P < 0.05 and P < 0.001, respectively. *,**Statistically different from animals of the same group at different time point, P < 0.05 and P < 0.001, respectively. (b) IgG2a/IgG1 ratio, indicative of Th1/Th2 response were calculated based on subclass titering. (c) Three groups of mice were treated as described in Materials and Methods: WT_Mock (N = 4), WT_Ad5-LacZ (N = 5), WT_DEX_Ad5-LacZ (N = 5). Plasma samples were collected at 28 dpi and assayed for neutralizing antibodies using successive dilutions (see Materials and Methods). The error bars represent ± SD. Statistical analysis was completed using one-way analysis of variance with a Student–Newman–Keuls post hoc test; P < 0.05 was deemed a statistically significant difference. *,**Statistically different from those in WT_Mock–injected animals, P < 0.05 and P < 0.001, respectively. #,##Statistically different values in WT_DEX_Ad5-LacZ group compared to WT_Ad5-LacZ group, P < 0.05 and P < 0.001, respectively. WT, wild type.
The IgG2a/IgG1 ratio is considered to reflect the relative contribution of the Th1/Th2 response. DEX treatment noticeably altered the Th1/Th2 ratio from 1.21 in Ad-injected mice to 0.74 in DEX pretreated, Ad-injected mice at 28 dpi. Similar reductions were observed at 14 dpi (Figure 5b). Previous reports suggest that glucocorticoids such as DEX can suppress Th1 responses while exaggerating Th2 responses.37 In an attempt to clarify the altered Th1/Th2 balance, we also investigated the systemic induction of Th1 (interferon γ) and Th2 (IL-4 and IL-5)-specific cytokines at both 6 and 24 hpi; however, we were unable to detect significant inductions of any of these factors in the plasma of the treated mice (data not shown). We next determined whether these differences in primary humoral immune responses resulted in any significant changes in Ad-neutralizing antibody (NAb) titers at 28 dpi. We found that at several plasma dilutions, Ad5-LacZ–injected mice had significant amounts of anti-Ad NAb titers, whereas DEX pretreated, virus-injected mice had significant lower NAb titers (Figure 5c).
DEX pretreatment also reduced antitransgene (β-Gal) humoral immune responses because LacZ-specific IgM and IgA antibodies titers were significantly lower in DEX pretreated, Ad5-LacZ–treated animals relative to Ad-LacZ–treated mice. However, similar levels of LacZ-specific IgG antibody isotypes were induced in Ad-injected mice, regardless of DEX pretreatment status. The LacZ-specific Th1/Th2 ratio was slightly reduced in DEX-treated animals as compared to WT mice not treated with DEX (Figure 6). Overall, several anti transgene-specific humoral immune responses were reduced with DEX treatment, although less significantly than the DEX-induced reductions in anticapsid responses. Importantly, we have shown that DEX treatment did not interfere with antibody production in C57BL/6 mice, as levels of total nonspecific mouse IgG measure in mock-injected animals were identical in DEX pretreated mice (Supplementary Figure S4).
Figure 6.
Dexamethasone (DEX) treatment significantly reduced Ad vector-derived transgene-specific humoral immune responses. (a) There groups of mice were treated as described in Materials and Methods: WT_Mock (N = 4), WT_Ad5-LacZ (N = 5), WT_DEX_Ad5-LacZ (N = 5). Plasma samples, collected at 14 dpi and 28 dpi, were analyzed for anti β-Gal (Ad-derived transgene)-specific total IgM, IgA, and IgG antibodies and various IgG subclasses. The error bars represent ± SD. Statistical analysis was completed using two-tailed Student's t-test to compare two groups of virus-injected animals. #,##Statistically different values in WT_DEX_Ad5-LacZ group compared to WT_Ad5-LacZ group, P < 0.05, P < 0.001 respectively. *,**Statistically different from animals of the same group at different time point, P < 0.05 and P < 0.001, respectively. (b) IgG2a/IgG1 ratio, indicative of Th1/Th2 response were calculated based on subclass titering. WT, wild type.
Discussion
Toxicities that rapidly develop after intravenous injection of Ad vectors is a major problem, limiting the usage of Ads in gene therapy applications requiring systemic administration, that is, for high-level liver transduction.4 To improve the risk/benefit ratio of systemic administration of Ad vectors requires simultaneous blockade of several Ad-induced innate immune responses, such as acute thrombocytopenia,22 cytokines/chemokine release,20,38 induction of proinflammatory gene expression,4,20,26,39 and activation of endothelial cells.23,25 In this study, we attempted to block all of these responses with a simple pretreatment with a clinically convenient and the widely utilized glucocorticoid, in this instance, DEX. Our results confirmed that transient pretreatments of mice with several doses of DEX can abrogate most innate toxicities attributable to systemic delivery of Ad-based vectors, improving the risk/benefit ratio of this vector class for numerous applications such as liver gene therapy. The drug dosages used in our studies parallel doses routinely utilized in humans, which range from those attempting to capitalize upon the anti-inflammatory effects of DEX to treat mild conditions, to doses used to treat complications of sepsis and clinical shock.40
The mechanism of action of glucocorticoids is relatively well known. Glucocorticoid hormones bind to their respective GRs, which dimerize and translocate to the nucleus, where they bind to specific DNA sequences, the glucocorticoid response elements and either activate transcription of anti-inflammatory genes (IL-10, IL-1_RA) or indirectly downregulate transcription of a number of proinflammatory genes (cytokines, adhesion molecules) through a variety of mechanisms.12 Glucocorticoids are known to change chromatin structure allowing for increased (or decreased) accessibility for transcriptional machinery. A number of reports confirm the role of high-dose glucocorticoids in reducing transcription of proinflammatory genes mediated by GRs,12 in particular in lipopolysaccharide-induced models.41 Systemic activation of the innate immune system can also be minimized by high-dose DEX pretreatment (or other glucocorticoids) at least in part by GR-mediated blocking of proinflammatory genes transcription.41,42
Based upon this analysis, we felt that many of the same innate immune response pathways that are induced by Ad vectors may also be impacted upon by glucocorticoids. Clearly, our results confirm this notion, as DEX pretreatment can minimize or prevent numerous Ad vector-induced innate immune responses. In Figure 7, we have attempted to summarize some of the known innate immune response pathways induced by Ad that appear to be responsive to DEX pretreatment (Figure 7).
Figure 7.
Dexamethasone (DEX) pretreatment mediates blockage of Ad-induced innate immune responses: model of action. Ad interactions with the complement system, as Ad capsid–triggered TLR signaling results in nuclear translocation of inflammatory transcription factors, such as NF-κB, IRF7, and others. The expression of a number of genes involved in innate immune responses becomes upregulated in response to systemic injection of Ads. This leads to cytokine and chemokine release and signal amplification by autocrine and paracrine cytokine signaling. DEX, when injected prior to Ad treatment, causes glucocorticoid receptor dimerization and nuclear translocation, where GR interacts with genes containing the target DNA sequence (GRE). Thus, GR activation by DEX pretreatment interferes with induction of transcription of proinflammatory genes, to block Ad-induced transcription of adhesion molecules (ICAM-1, VCAM-1) minimize systemic cytokine release and, therefore, in lack of induction of IFN responsive genes, such as 2′–5′ oligoadenylate synthase 1a or adenosine deaminase–RNA-specific. See refs. 7,12,20,25,26,27,39,40,41 for further details. GR, glucocorticoid receptor; GRE, glucocorticoid response elements; ICAM-1, intercellular adhesion molecule 1; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; NF-κB, nuclear factor κB; TLR, Toll-like receptor; VCAM-1, vascular cell adhesion molecule 1.
We found that DEX pretreatment significantly diminished the Ad-dependent activation of most innate immune responses noted after systemic delivery of Ads.4,22,23,25,38 Specifically, the systemic release of proinflammatory cytokines/chemokines within 6 hours of Ad injection was partially or completely blocked by pretreatment of mice with escalating doses of DEX. At maximal DEX dosages, Ad induction of thrombocytopenia was prevented; the transcriptional activation of multiple, proinflammatory liver genes at 6 hpi was completely blocked; and instances of endothelial cell activation were all mitigated by transient DEX pretreatment of Ad-injected mice. DEX pretreatment also blocked leukocyte infiltration into the Ad-transduced liver at 28 dpi. Our previous studies and those of others suggests that this effect was likely mediated by significantly altering Ad interactions with the TLR and complement systems, as these systems are known to mediate Ad interactions with macrophages, dendritic cells, mast cells, endothelial cells, and/or granulocytes.4,5,7,20,21,26,27,39,43
Cellular infiltration of the liver by elements of the mammalian innate immune response is dependent on activation of endothelial cells; elevated expression of adhesion molecules on their surface, such as the P and E selectins, mediate tethering and rolling of leukocytes, whereas increased expression of ICAM-1, ICAM-2, and VCAM-1 mediate firm adhesion of leukocytes. It is known that high-dose Ad vectors cause rapid activation of endothelial cells both in vitro and in vivo.23,25 We have shown that plasma levels of endothelial cell–derived activation markers were induced at least threefold in Ad-injected mice as compared to mock-injected animals (e-Selectin, ICAM-1). This induction was completely blocked with DEX pretreatment of Ad-injected mice, a result verified by qRT-PCR analysis of ICAM-1 and VCAM-1 genes transcripts. We confirmed that systemic Ad injection into DEX pretreated mice still resulted in Kupffer cell necrosis, we have now confirmed that Kupffer cell–dependent innate immune responses induced by Ads are likely consequent to immediate interactions with Kupffer cells, rather than due to Ad-induced Kupffer cell necrosis per se.7,25
We further confirmed that pretreatment with DEX did not prevent Ad vectors from transducing the murine liver with transgenes. Interestingly, DEX pretreatment increased the detectable number of Ad genomes in the murine liver early after injection, but this did not elevate Ad-derived β-Gal expression levels in murine liver at 24 hpi and 28 dpi. This paradox may be due to the fact that in the vectors utilized in this study the LacZ gene was expressed under control of the cytomegalovirus promoter/enhancer element. This enhancer is known to contain NF-κB response elements.44 NF-κB is a key transcription factor activating proinflammatory gene expression and is induced over twofold in the livers of Ad5-LacZ–treated C57BL/6 mice at 6 hpi compared to mock-injected animals. In contrast, DEX pretreated virus-injected animals had NF-κB transcript levels no different from mock-injected animals; in fact they had 1.6-fold less NF-κB transcripts compared to WT_Mock group. This >3.5-fold difference between DEX-treated and nontreated Ad5-LacZ–injected animals may explain why cytomegalovirus promoter/enhancer-derived LacZ gene transcription did not correlate with Ad vector genome copy numbers.
DEX pretreatment of mice minimized Ad-induced innate immune responses, an effect that also resulted in blunted adaptive immune responses. NAb titers, anti-Ad, and anti-LacZ antibody titers were significantly reduced in DEX pretreated Ad-injected mice relative to non–DEX-treated, Ad vector–treated mice. The fact that DEX pretreatment diminished the generation of anti-Ad NAb may have significant applicability in Ad readministration scenarios.45
In summary, results obtained in this study confirm that the simple pre-emptive and transient use of a potent glucocorticoid prior to systemic delivery of an Ad vector can allow for safer systemic Ad-mediated gene transfer. This approach avoids the complications associated with long-term glucocorticoid usage, whereas simultaneously significantly improving the safety profile of systemic Ad administration for use in gene therapy strategies requiring widespread liver transduction. Furthermore, this method can be simply applied in conjunction with use of either advanced-generation Ad vectors, and/or other pre-emptive strategies previously shown to impact on Ad-induced innate immune responses to further improve the safety profile of this important gene transfer platform. These benefits, coupled with DEX-dependent diminishment of generation of Ad-specific NAbs further highlights the importance of this study.
Materials and Methods
Ad vector production and characterization. A first-generation, human Ad type 5–derived replication-deficient vector (deleted for the E1 genes) encoding β-galactosidase (LacZ) as a transgene (Ad5-LacZ) was used in this study. Virus construction, propagation, and purification was performed as previously described.38,46 Briefly, a number of serial passages on HEK293 cells allowed high titer purification of Ad5-LacZ by sequential, cesium chloride density–gradient centrifugations. Purified virus was dialyzed against 10 mmol/l Tris (pH 8.0) and stored in 1% sucrose, 1× phosphate buffered saline (PBS) at −80 °C until use. Viral particle (vp) and transducing unit titers (bfu/ml) were determined as previously described, and were 2.6 × 1012 vp/ml and 1.8 × 1011 bfu/ml, respectively.20,47 The vp to bfu ration was ~14:1. Virus was found to be RCA free both by RCA PCR (E1 region amplification) and direct sequencing, methods as previously described.26
Animal procedures. Adult C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). The Ad vector was injected intravenously (via the retro-orbital sinus) into 8–10-week-old male C57BL/6 mice after performing proper anesthesia with isofluorane. A total of 0.75 × 1011 vp in 200 µl of PBS was injected per mouse. DEX (America Pharmaceutical Partners, Schauburg, IL) was administered by intraperitoneal injection (10 mg/kg, 1 mg/kg, 0.1 mg/kg), at 15 hours and 2 hours prior to Ad vector administration. Note that 0.1 mg/kg and 1 mg/kg DEX was used only in measuring Ad-induced cytokines/chemokines release. The highest dose of DEX was selected based upon current dose regimens utilized to treat bacterial sepsis and shock human clinical applications,40 Ad vector–induced inflammatory responses closely resemble these conditions. These dosages have been widely used in a number of studies on animal models.42,48 Four groups of mice were analyzed in this study: WT mice mock injected with PBS, WT mice pretreated with DEX and then mock injected with PBS (to identify DEX-mediated responses not directly attributed to Ad), WT mice injected only with Ad5-LacZ, and WT mice pretreated with DEX and subsequently injected with Ad5-LacZ. Control and experimental mice were sacrificed at different times after mock or virus treatment: 6 hours postinjection (hpi), (N = 6 for virus-injected groups, N = 4 for mock-injected groups), 24 hpi (N = 4 for all groups), and 28 dpi (N = 5 for all groups). Plasma and tissue samples were collected and processed at the indicated time points in accordance with Michigan State University Institutional Animal Care and Use Committee. All procedures with rAds were performed under BSL-2, and all vector-treated animals were maintained in ABSL-2 conditions. All animal procedures were reviewed and approved by the Michigan State University ORCBS and IACUC. Care for mice was provided in accordance with PHS and AAALAC standards.
Cytokine/chemokine/endothelial cells activation markers release measurement. To determine whether DEX has any effect on Ad-mediated release of cytokines/chemokines, Ad-induced systemic release of proinflammatory cytokines/chemokines in murine plasma was measured in all groups of mice utilizing a multiplex bead array system. Plasma samples were collected at 1 and 6 hpi using heparinized capillary tubes and EDTA-coated microvettes (Sarstedt, Nümbrecht, Germany) and centrifuged at 3,400 rpm for 10 minutes to retrieve plasma samples. Samples were assayed for seven independent cytokines/chemokines, which we have previously shown to be rapidly induced by systemically injected Ad vectors (monocyte chemoattractant protein 1, keratinocyte derived chemokine, macrophage inflammatory protein 1β, IL-6, IL-12p40, G-SCF, RANTES).20,26,39 All procedures were performed exactly as previously described according to manufacturer's instructions (Bio-Rad, Hercules, CA) via Luminex 100 technology (Luminex, Austin, TX).26 The measurement of soluble ICAM-1 and e-Selectin molecules (endothelial cells activation markers) in murine plasma (collected at 6 hpi) was performed utilizing mouse cardiovascular disease panel LINCOplex kit (Millipore, Billerica, MA) as per manufacturer's instructions.
Complete blood count analysis and cell-type differentiation. Total blood (0.3–0.4 ml) was collected into EDTA-coated 1.0 ml Lavender tubes (BD Microtainer, Franklin Lakes, NJ) at 24 hpi. For complete blood counts, blood was analyzed on an Advia 120 Hematology System (Bayer, New York) by the Clinical Pathology Laboratory of the Diagnostic Center for Population and Animal Health at Michigan State University (East Lansing, MI). In addition, all blood samples were examined microscopically and underwent manual differential count.
β-Galactosidase enzyme activity and in situ X-gal staining. Ad-mediated expression of the transgene LacZ was measured both qualitatively and quantitatively. Liver sections from animals scarified at 6 hpi, 24 hpi, and 28 dpi were embedded in optimal cutting temperature (OCT) compound, frozen and stored at −80 °C until use. Frozen samples were sectioned (7 µm sections) on a Leica cryostat and were fixed and in situ stained for LacZ expression using 5-bromo-4-chloro-3-indolyl-b-D-galactopyranoside (X-Gal, 1 mg/ml) as previously described.35 For quantitative assay, enzyme β-Galactosidase (β-gal) activity was measured in snap frozen liver samples. Liver samples (<0.1 g) were homogenized and total protein concentration was determined by bicinchoninic acid assay, Pierce (Rockford, IL). β-gal activity was quantified by use of a β-gal activity detection kit (Stratagene, La Jolla, CA) according to manufacturers instructions and as previously described.36 Data were reported as units of β-gal activity per µg of total protein.
NAb assay. 2 × 103 HEK293 cells were seeded in microwells in 125 µl of complete media (DMEM, 10% fetal bovine serum, 1% penicillin + streptomycin + fungizone). Cells were cultured overnight in a 37 °C, 5% CO2 incubator. To inactivate complement, plasma was heat inactivated for 60 minutes at 56 °C and brought to room temperature (RT). Dilutions were made as indicated in complete media in a total volume of 100 µl for each well. 1.3 × 106 viral particles of Ad5-LacZ (~650 vp/cell) was next added to each dilution, mixed well, and incubated at RT for 1 hour. 100 µl of the medium/plasma/virus mixture was applied to cells and incubated for 2–3 days. Control samples were incubated with either virus alone or complete media alone. CELLTITER 96 AQueous One solution (Promega, Madison, WI) was added to each well and incubated for 2 hours in a 37 °C, 5% CO2 incubator. 150 µl of media from each well was removed into a new microtiter plate and read at 492 nm in a microplate spectrophotometer. Blank subtracted OD492 values are reported.
Antibody titering assay. ELISA-based titering experiments were essentially completed as previously described.49 Briefly, 5 × 108 vp/well or 0.2 µg recombinant LacZ protein/well (each diluted in PBS) was used to coat wells of a 96-well plate overnight at 4 °C. Plates were washed with PBS-Tween (0.05%) solution, and blocking buffer (3% bovine serum albumin in PBS) was added to each well and incubated for 1–3 hours at RT. For titering of total IgG antibodies, plasma was diluted in 1:800 in blocking buffer, added to the wells, and incubated at RT for 1 h. Wells were washed using PBS-Tween (0.05%) and HRP-conjugated rabbit antimouse antibody (Bio-Rad, Hercules, CA) was added at a 1:4000 dilution in PBS-Tween. TMB (Sigma-Aldrich, St Louis, MO) substrate was added to each well, and the reaction was stopped with 1 N phosphoric acid. Plates were read at 450 nm in a microplate spectrophotometer. Subisotype titering was completed using a hybridoma subisotyping kit (Calbiochem, La Jolla, CA) using plasma at a dilution of 1:200 per manufacturer's recommendations.
qRT-PCR analysis. To determine relative levels of a specific, liver-derived RNA transcript, liver tissues were snap frozen in liquid nitrogen and RNA was harvested from ≈100 mg of frozen tissue using TRIzol reagent (Invitrogen, Carlsbad, CA) per the manufacturer's protocol. Following RNA isolation, reverse transcription was performed on 180 ng of total RNA using SuperScript II (Invitrogen, Carlsbad, CA) reverse transcriptase and random hexamers (Applied Biosystems, Foster City, CA) per manufacturer's protocol. RT reactions were diluted to a total volume of 60 µl and 2 µl were used as the template in the subsequent PCRs. Primers were designed using Primer Bank Web-based software (http://pga.mgh.harvard.edu/primerbank/). Some primers used for amplification have been previously described.4,20 Complete list of primers utilized in this study is available in Supplementary Table S1. Q-PCR was carried out on an ABI 7900HT Fast Real-Time PCR System using SYBR Green PCR Mastermix (Applied Biosystems, Foster City, CA) in a 15 µl reaction. All PCRs were subjected to the following procedure: 95.0 °C for 10 minutes followed by 40 cycles of 95.0 °C for 15 seconds followed by 60.0 °C for 1 minute. The comparative Ct method was used to determine relative gene expression using GAPDH to standardize expression levels across all samples. Relative expression changes were calculated based on comparing experimental levels of a respective liver transcript to those quantified in liver samples derived from mock-injected animals.
Kupffer cell staining. Liver blocks, preserved in OCT compound at −80 °C, were sliced into 7 µmol/l sections using a cryostat, placed on glass slides and frozen at −80 °C for future use. Slides were thawed, fixed in 100% ethanol for 15 min, and washed in potassium phosphate buffered saline containing 0.2% gelatin and 0.05% tween-20. Sections were permeabilized in 0.1% triton-X100 and blocked with potassium phosphate buffered saline containing 0.1% gelatin, 1% tween-20, and 5% bovine serum albumin for 60 minutes at RT. To prevent nonspecific binding of the secondary antibody, 5% rabbit serum in wash buffer was added and incubated at RT for 30 minutes. Rat antimouse F4/80 antibody (Invitrogen, Carlsbad, CA) diluted to 5.2 µg/ml in wash buffer containing 2% rabbit serum was added to the sections and incubated overnight at 4 °C. Sections were washed and incubated at RT for 45 minutes with rabbit antirat Alexafluor-488 (Invitrogen, Carlsbad, CA) secondary antibody diluted 1:25,000 in wash buffer containing 2% rabbit serum. Sections were then washed, stained with DAPI (Invitrogen, Carlsbad, CA) for 5 minutes at RT, and mounted using VECTASHIELD (Vector Laboratories, Burlingame, CA). Images were obtained using a Leica DMLB microscope, and captured using SPOT software v.3.5.9. Both DAPI and F4/80 stained images were transferred to Adobe Photoshop and converted to grayscale. The background threshold was set based on mock-injected livers and pixels were quantified. F4/80 fluorescence values were normalized to DAPI fluorescence values to control for cell number differences. Values are reported as percent relative to number of Kupffer cells identically enumerated in liver tissues derived from mock-injected control mice.
Hematoxilin and eosin staining. To study the effects of DEX treatment on Ad vector–mediated hepatic inflammation at 28 dpi, hematoxilin and eosin staining of mouse liver samples was performed as previously described.36 Briefly, tissues were fixed in 10% neutral formalin for 12 hours, washed in 70% ethanol, embedded in paraffin and 6-µm sectioned, and stained with hematoxilin and eosin. We have adapted a previously developed semiquantitative scoring system, which allows the level of hepatic pathology between different liver sections to be quantified and statistically compared.36 For every mouse, 10 liver sections obtained at different portions of the liver (0–1000 µm from liver surface) were analyzed and given a numerical score (0–3) for three different categories of liver pathology:
1. Portal inflammation:
0—no portal inflammation
1—low to moderate number of inflammatory cells (macrophages, lymphocytes) evident in <1/3 of portal tracts
2—moderate number of inflammatory cells in 1/3–2/3 of portal tracts
3—high number of inflammatory cells in >2/3 of portal tracts
2. Periportal inflammation:
0—no inflammation
1—low to moderate number of inflammatory cells infiltration evident around <1/3 of portal tracts
2—moderate number of inflammatory cells infiltrated through 1/3–2/3 of portal tracts, in majority of which they take less that 50% of circumference. Minimum hepato-cellular necrosis observed
3—moderate to high number of inflammatory cells infiltrated through over 2/3 of portal tracts or infiltrated through over 1/3 of tracts but occupy over 50% of circumference in at least 50% of them. Significant hepato-cellular necrosis observed
3. Lobular inflammation:
0—no inflammation
1—minimum to moderate necrosis observed in <1/3 of lobules
2—moderate hepato-cellular necrosis observed in 1/3–2/3 of lobules
3—moderate to severe hepato-cellular necrosis observed in over 2/3 of lobules.
Two independent researchers have scored all the slides in a blind manner and the averages of their scores were taken. The sum of scores (10 slides) for each mouse was taken and individual category scores were averaged for each group. Total inflammation index was computed by averaging the sum of all three individual category scores for each mouse.
Ad genome copy number per liver cell. To determine the number of Ad genome copies per liver cell at different time points post-transduction liver tissues (<0.1 g) were snap frozen in liquid nitrogen, crushed to a fine powder using a mortar and pestle, and total DNA was extracted from as previously described.50 Ad genome copy numbers were assessed using Real-Time PCR-based quantification. PCRs were performed on an ABI 7900HT Fast Real-Time PCR System using the SYBR Green PCR Mastermix as described for qRT-PCR technique. Primers generated against the Ad5 Hexon gene have been previously described.4 As an internal control for ensuring adequate DNA amplification, liver DNA was quantified using primers spanning the GAPDH gene. Standard curves were run in duplicate and consisted of 6 half-log dilutions using total genomic DNA, or DNA extracted from the purified Ad5-LacZ virus. These standard curves were used to determine the number of viral genomes present per liver cell. Melting curve analysis confirmed the quality and specificity of the PCR (data not shown).
Statistical analysis. For every experiment, pilot trials were performed with 3 mice per group. This allowed us to determine effect size and sample variance so that Power Analysis could be performed to correctly determine the number of subjects per group required to achieve a statistical power > 0.8 at the 95% confidence level. Statistically significant differences in toxicities associated with innate immune response (i.e., platelet counts, gene induction, etc.) were determined using one-way analysis of variance with a Student–Newman–Keuls post hoc test (P value <0.05). Furthermore, a two-way analysis of variance with a Bonferroni post hoc test was used to analyze the levels of cytokines at 1 and 6 hpi to determine significant differences (P value <0.05) between groups. For antibody titering assays, liver hematoxilin and eosin stains, β-Gal activity, and Ad genomes in mouse liver, a two-tailed Student's t-test was used to compare two groups of virus-injected animals (P < 0.05). All graphs in this paper are presented as mean of the average ± SD. GraphPad Prism software was utilized for statistical analysis.
Supplementary MaterialFigure S1. Dexamethasone treatment causes an increase in lymphopenia and neutrophilia in a blood of C57BL/6 mice, which is not related to Ad treatment.Figure S2. Dexamethasone treatment preserves the efficacy of Ad Ad-derived transgene expression and Ads genomes persistency in the livers of C57BL/6 mice.Figure S3. Dexamethasone treatment does not change Ad- dependent Kupfer cells degradation in a liver of C57BL/6 mice.Figure S4. Dexamethasone treatment does not change natural levels of total nonspecific IgG antibodies in a blood of C57BL/6 mice.Table S1. A pair of forward (For) and reverse (Rev) primers are provided for every transcript tested by qRTPCR-based methods. The primers were designed as described in Materials and Methods section, the length of the resulted PCR products was 100–160 nucleotides.
Supplementary Material
Dexamethasone treatment causes an increase in lymphopenia and neutrophilia in a blood of C57BL/6 mice, which is not related to Ad treatment. C57BL/6 mice were intravenously injected with 0.75x1011 vp/mouse of Ad5-LacZ vector. DEX pre-treatment and CBC differential count was performed as described in Materials and Methods. Total four groups of mice (N=4) analyzed: WT_Mock, WT_DEX_Mock, WT_Ad5-LacZ, WT_DEX_Ad5-LacZ. The bars represent Mean ± SD. Statistical analysis was completed using One Way ANOVA with a Student-Newman-Keuls post-hoc test, p<0.05 was deemed a statistically significant difference. #, ## - indicate statistically different values in WT_DEX_Ad5-LacZ group compared to WT_Ad5-LacZ group, p<0.05, p<0.001 respectively. Normal ranges for Neutrophils (A) and Lymphocytes (B) count in male C57BL/6 mice are indicated by light grey shaded boxes. Note: Normal range levels were adapted from Jackson laboratories studies on C57BL/6 mice (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&sym=Peters3).
Dexamethasone treatment preserves the efficacy of Ad Ad-derived transgene expression and Ads genomes persistency in the livers of C57BL/6 mice. A) In situ visualization of bacterial β-galactosidase in liver of Ad5-LacZ treated C57BL/6 mice. Cryosections of liver from all groups of mice were stained for β-Gal in situ as described in Materials and Methods. Representative sections for each of the groups are shown. Total magnification of 200X was used to obtain images. N=6 for all virus injected groups at 6 hpi, N=4 for all virus injected groups at 24 hpi, N=5 for all virus injected groups at 28 dpi, N=4 for all Mock injected groups at all time points. B) Bacterial β-galactosidase activity levels were analyzed in liver protein homogenates prepared at 6 hpi, 24 hpi and 28 dpi from four groups of C57BL/6 mice: WT_Mock, WT_DEX_Mock, WT_Ad5-LacZ, WT_DEX_Ad5-LacZ. Activity levels were presented as Units per mg of total protein (see Materials and Methods). The bars represent Mean ± SD. Statistical analysis was completed using two-tailed Student t-test to compare 2 groups of virus injected animals. #, ## - indicate statistically different values in WT_DEX_Ad5-LacZ group compared to WT_Ad5-LacZ group, p<0.05, p<0.001 respectively. C) qPCR based quantification of Ad5-LacZ genomes in livers harvested from C57BL/6 mice at 6 hpi, 24 hpi, 28 dpi. The bars represent Mean ± SD. Statistical analysis was completed using two-tailed Student t-test to compare 2 groups of virus injected animals. #, ## - indicate statistically different values in WT_DEX_Ad5-LacZ group compared to WT_Ad5-LacZ group, p<0.05, p<0.001 respectively. Note the difference in scale for different time points.
Dexamethasone treatment does not change Ad- dependent Kupfer cells degradation in a liver of C57BL/6 mice. 7 μm liver sections obtained at 6 and 24 hpi were stained with macrophage specific F4/80 antibody. Pixel density of both Kupffer cell staining and DNA staining (DAPI) was quantified. Kupffer cells values were normalized to DAPI values to control for cell density variation. Values were subsequently divided by WT_Mock average values to give percent difference. Error bars indicate ±SD. N=4 for all groups tested, pictures represent one of at least 12 sections derived from 4 mice in each group: WT_Mock, WT_DEX_Mock, WT_Ad5-LacZ, WT_DEX_Ad5-LacZ. Statistical analysis was completed using One Way ANOVA with a Student-Newman-Keuls post-hoc test, p<0.05 was deemed a statistically significant difference. *, ** - indicate values, statistically different from those in WT_Mock injected animals, p<0.05, p<0.001 respectively.
Dexamethasone treatment does not change natural levels of total nonspecific IgG antibodies in a blood of C57BL/6 mice. Plasma samples from Mock injected mice (WT_Mock and WT_DEX_Mock, N=4 for each group) were analyzed for total non-specific IgG antibodies. The bars represent Mean ± SD. Statistical analysis was completed using two-tailed Student t-test to compare 2 groups of Mock injected animals, p<0.05 was deemed a statistically significant difference. No significant difference was found.
A pair of forward (For) and reverse (Rev) primers are provided for every transcript tested by qRTPCR-based methods. The primers were designed as described in Materials and Methods section, the length of the resulted PCR products was 100–160 nucleotides.
Acknowledgments
We thank Michigan State University Laboratory Animal support facility for their assistance in the humane care and maintenance of the animals utilized in this work. S.S.S. was supported by American Heart Association Midwest Affiliate Fellowship 0815660G. A.A. was supported by the National Institutes of Health grants RO1DK-069884, P01 CA078673, the MSU Foundation, as well as the Osteopathic Heritage Foundation.
REFERENCES
- Maelandsmo GM, Ross PJ, Pavliv M, Meulenbroek RA, Evelegh C, Muruve DA, et al. Use of a murine secreted alkaline phosphatase as a non-immunogenic reporter gene in mice. J Gene Med. 2005;7:307–315. doi: 10.1002/jgm.666. [DOI] [PubMed] [Google Scholar]
- Amalfitano A., and , Parks RJ. Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr Gene Ther. 2002;2:111–133. doi: 10.2174/1566523024605618. [DOI] [PubMed] [Google Scholar]
- Stone D, Liu Y, Li ZY, Tuve S, Strauss R., and , Lieber A. Comparison of adenoviruses from species B, C, E, and F after intravenous delivery. Mol Ther. 2007;15:2146–2153. doi: 10.1038/sj.mt.6300319. [DOI] [PubMed] [Google Scholar]
- Appledorn DM, Kiang A, McBride A, Jiang H, Seregin S, Scott JM, et al. Wild-type adenoviruses from groups A-F evoke unique innate immune responses, of which HAd3 and SAd23 are partially complement dependent. Gene Ther. 2008;15:885–901. doi: 10.1038/gt.2008.18. [DOI] [PubMed] [Google Scholar]
- Hartman ZC, Appledorn DM, Serra D, Glass O, Mendelson TB, Clay TM, et al. Replication-attenuated Human Adenoviral Type 4 vectors elicit capsid dependent enhanced innate immune responses that are partially dependent upon interactions with the complement system. Virology. 2008;374:453–467. doi: 10.1016/j.virol.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama S, Tominaga K, Mitoro A, Tsujinoue H, Nakatani T, Yamazaki M, et al. Immunomodulation with FK506 around the time of intravenous re-administration of an adenoviral vector facilitates gene transfer into primed rat liver. Int J Cancer. 2000;85:839–844. doi: 10.1002/(sici)1097-0215(20000315)85:6<839::aid-ijc17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Appledorn DM, Patial S, McBride A, Godbehere S, Van Rooijen N, Parameswaran N, et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008;181:2134–2144. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- Cerullo V, Seiler MP, Mane V, Brunetti-Pierri N, Clarke C, Bertin TK, et al. Toll-like receptor 9 triggers an innate immune response to helper-dependent adenoviral vectors. Mol Ther. 2007;15:378–385. doi: 10.1038/sj.mt.6300031. [DOI] [PubMed] [Google Scholar]
- Brunetti-Pierri N, Stapleton GE, Palmer DJ, Zuo Y, Mane VP, Finegold MJ, et al. Pseudo-hydrodynamic delivery of helper-dependent adenoviral vectors into non-human primates for liver-directed gene therapy. Mol Ther. 2007;15:732–740. doi: 10.1038/sj.mt.6300102. [DOI] [PubMed] [Google Scholar]
- Shayakhmetov DM, Eberly AM, Li ZY., and , Lieber A. Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. J Virol. 2005;79:1053–1061. doi: 10.1128/JVI.79.2.1053-1061.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardash KJ, Sarrazin F, Tessler MJ., and , Velly AM.Single-dose dexamethasone reduces dynamic pain after total hip arthroplasty Anesth Analg 20081061253–1257.table of contents [DOI] [PubMed] [Google Scholar]
- Pace TW, Hu F., and , Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen TH, Berg RS, Paludan SR., and , Ostergaard L. Mechanisms of dexamethasone-mediated inhibition of Toll-like receptor signaling induced by Neisseria meningitidis and Streptococcus pneumoniae. Infect Immun. 2008;76:189–197. doi: 10.1128/IAI.00856-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJ, Piliponsky AM, Rosenhead F, Elchalal U, Nagler A., and , Levi-Schaffer F. Dexamethasone inhibits maturation, cytokine production and Fc epsilon RI expression of human cord blood-derived mast cells. Clin Exp Allergy. 2002;32:906–913. doi: 10.1046/j.1365-2745.2002.01418.x. [DOI] [PubMed] [Google Scholar]
- Braun S, Jenny C, Thioudellet C, Perraud F, Claudepierre MC, Langle-Rouault F, et al. In vitro and in vivo effects of glucocorticoids on gene transfer to skeletal muscle. FEBS Lett. 1999;454:277–282. doi: 10.1016/s0014-5793(99)00818-2. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liggitt HD, Dow S, Handumrongkul C, Heath TD., and , Debs RJ. Strain-based genetic differences regulate the efficiency of systemic gene delivery as well as expression. J Biol Chem. 2002;277:4966–4972. doi: 10.1074/jbc.M110285200. [DOI] [PubMed] [Google Scholar]
- Ishimoto S, Kawamoto K, Stover T, Kanzaki S, Yamasoba T., and , Raphael Y. A glucocorticoid reduces adverse effects of adenovirus vectors in the cochlea. Audiol Neurootol. 2003;8:70–79. doi: 10.1159/000069000. [DOI] [PubMed] [Google Scholar]
- Otake K, Ennist DL, Harrod K., and , Trapnell BC. Nonspecific inflammation inhibits adenovirus-mediated pulmonary gene transfer and expression independent of specific acquired immune responses. Hum Gene Ther. 1998;9:2207–2222. doi: 10.1089/hum.1998.9.15-2207. [DOI] [PubMed] [Google Scholar]
- Price AR, Limberis MP, Wilson JM., and , Diamond SL. Pulmonary delivery of adenovirus vector formulated with dexamethasone-spermine facilitates homologous vector re-administration. Gene Ther. 2007;14:1594–1604. doi: 10.1038/sj.gt.3303031. [DOI] [PubMed] [Google Scholar]
- Kiang A, Hartman ZC, Everett RS, Serra D, Jiang H, Frank MM, et al. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol Ther. 2006;14:588–598. doi: 10.1016/j.ymthe.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Appledorn DM, McBride A, Seregin S, Scott JM, Schuldt N, Kiang A, et al. Complex interactions with several arms of the complement system dictate innate and humoral immunity to adenoviral vectors. Gene Ther. 2008;15:1606–1617. doi: 10.1038/gt.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolins N, Lozier J, Eggerman TL, Jones E, Aguilar-Cordova E., and , Vostal JG. Intravenous administration of replication-incompetent adenovirus to rhesus monkeys induces thrombocytopenia by increasing in vivo platelet clearance. Br J Haematol. 2003;123:903–905. doi: 10.1046/j.1365-2141.2003.04719.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Muruve DA, Collins RG, Lee SS., and , Kubes P. The role of selectins and integrins in adenovirus vector-induced neutrophil recruitment to the liver. Eur J Immunol. 2002;32:3443–3452. doi: 10.1002/1521-4141(200212)32:12<3443::AID-IMMU3443>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Hertel S., and , Kochanek S. Efficient transformation of primary human amniocytes by E1 functions of Ad5: generation of new cell lines for adenoviral vector production. Hum Gene Ther. 2000;11:2105–2116. doi: 10.1089/104303400750001417. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Bloch W, Hertel S, Johnston M, Molojavyi A, Dries V, et al. A hemodynamic response to intravenous adenovirus vector particles is caused by systemic Kupffer cell-mediated activation of endothelial cells. Hum Gene Ther. 2003;14:1631–1641. doi: 10.1089/104303403322542275. [DOI] [PubMed] [Google Scholar]
- Hartman ZC, Kiang A, Everett RS, Serra D, Yang XY, Clay TM, et al. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J Virol. 2007;81:1796–1812. doi: 10.1128/JVI.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman ZC, Black EP., and , Amalfitano A. Adenoviral infection induces a multi-faceted innate cellular immune response that is mediated by the toll-like receptor pathway in A549 cells. Virology. 2007;358:357–372. doi: 10.1016/j.virol.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Huang HW, Bi W, Jenkins GN., and , Alcorn JL. Glucocorticoid regulation of human pulmonary surfactant protein-B mRNA stability involves the 3'-untranslated region. Am J Respir Cell Mol Biol. 2008;38:473–482. doi: 10.1165/rcmb.2007-0303OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell MA, Zhang Y, Tazelaar J, Gao GP, Yu QC, Qian R, et al. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol Ther. 2001;3:708–722. doi: 10.1006/mthe.2001.0330. [DOI] [PubMed] [Google Scholar]
- Miller TA., and , Schaefer FW., 3rd Changes in mouse circulating leukocyte numbers in C57BL/6 mice immunosuppressed with dexamethasone for Cryptosporidium parvum oocyst production. Vet Parasitol. 2007;149:147–157. doi: 10.1016/j.vetpar.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Jeklova E, Leva L, Jaglic Z., and , Faldyna M. Dexamethasone-induced immunosuppression: a rabbit model. Vet Immunol Immunopathol. 2008;122:231–240. doi: 10.1016/j.vetimm.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Lieber A, He CY, Meuse L, Schowalter D, Kirillova I, Winther B, et al. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J Virol. 1997;71:8798–8807. doi: 10.1128/jvi.71.11.8798-8807.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone D, Liu Y, Shayakhmetov D, Li ZY, Ni S., and , Lieber A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol. 2007;81:4866–4871. doi: 10.1128/JVI.02819-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manickan E, Smith JS, Tian J, Eggerman TL, Lozier JN, Muller J, et al. Rapid Kupffer cell death after intravenous injection of adenovirus vectors. Mol Ther. 2006;13:108–117. doi: 10.1016/j.ymthe.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Everett RS, Hodges BL, Ding EY, Xu F, Serra D., and , Amalfitano A. Liver toxicities typically induced by first-generation adenoviral vectors can be reduced by use of E1, E2b-deleted adenoviral vectors. Hum Gene Ther. 2003;14:1715–1726. doi: 10.1089/104303403322611737. [DOI] [PubMed] [Google Scholar]
- Hu H, Serra D., and , Amalfitano A. Persistence of an [E1-, polymerase-] adenovirus vector despite transduction of a neoantigen into immune-competent mice. Hum Gene Ther. 1999;10:355–364. doi: 10.1089/10430349950018805. [DOI] [PubMed] [Google Scholar]
- Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, et al. Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J Immunol. 2000;164:1768–1774. doi: 10.4049/jimmunol.164.4.1768. [DOI] [PubMed] [Google Scholar]
- Hodges BL, Evans HK, Everett RS, Ding EY, Serra D., and , Amalfitano A. Adenovirus vectors with the 100K gene deleted and their potential for multiple gene therapy applications. J Virol. 2001;75:5913–5920. doi: 10.1128/JVI.75.13.5913-5920.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman ZC, Appledorn DM., and , Amalfitano A. Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 2008;132:1–14. doi: 10.1016/j.virusres.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefering R., and , Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23:1294–1303. doi: 10.1097/00003246-199507000-00021. [DOI] [PubMed] [Google Scholar]
- Wang XQ, Zhou X, Zhou Y, Rong L, Gao L., and , Xu W. Low-dose dexamethasone alleviates lipopolysaccharide-induced acute lung injury in rats and upregulates pulmonary glucocorticoid receptors. Respirology. 2008;13:772–780. doi: 10.1111/j.1440-1843.2008.01344.x. [DOI] [PubMed] [Google Scholar]
- Kumahara K, Nagata H, Watanabe K, Shimizu N, Arimoto Y, Isoyama K, et al. Suppression of inflammation by dexamethasone prolongs adenoviral vector-mediated transgene expression in murine nasal mucosa. Acta Otolaryngol. 2005;125:1301–1306. doi: 10.1080/00016480410018160. [DOI] [PubMed] [Google Scholar]
- Schiedner G, Hertel S, Johnston M, Dries V, van Rooijen N., and , Kochanek S. Selective depletion or blockade of Kupffer cells leads to enhanced and prolonged hepatic transgene expression using high-capacity adenoviral vectors. Mol Ther. 2003;7:35–43. doi: 10.1016/s1525-0016(02)00017-5. [DOI] [PubMed] [Google Scholar]
- Everett RS, Evans HK, Hodges BL, Ding EY, Serra DM., and , Amalfitano A. Strain-specific rate of shutdown of CMV enhancer activity in murine liver confirmed by use of persistent [E1(-), E2b(-)] adenoviral vectors. Virology. 2004;325:96–105. doi: 10.1016/j.virol.2004.04.032. [DOI] [PubMed] [Google Scholar]
- Yang TC, Millar JB, Grinshtein N, Bassett J, Finn J., and , Bramson JL. T-cell immunity generated by recombinant adenovirus vaccines. Expert Rev Vaccines. 2007;6:347–356. doi: 10.1586/14760584.6.3.347. [DOI] [PubMed] [Google Scholar]
- Ng P., and , Graham FL. Construction of first-generation adenoviral vectors. Methods Mol Med. 2002;69:389–414. doi: 10.1385/1-59259-141-8:389. [DOI] [PubMed] [Google Scholar]
- Amalfitano A, Hauser MA, Hu H, Serra D, Begy CR., and , Chamberlain JS. Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J Virol. 1998;72:926–933. doi: 10.1128/jvi.72.2.926-933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner D., and , Germann PG. Dexamethasone enhances the activity of rSP-C surfactant but not of exosurf in a rat model of the acute lung injury. J Pharmacol Toxicol Methods. 1999;42:39–48. doi: 10.1016/s1056-8719(99)00048-9. [DOI] [PubMed] [Google Scholar]
- Hensley SE, Cun AS, Giles-Davis W, Li Y, Xiang Z, Lasaro MO, et al. Type I interferon inhibits antibody responses induced by a chimpanzee adenovirus vector. Mol Ther. 2007;15:393–403. doi: 10.1038/sj.mt.6300024. [DOI] [PubMed] [Google Scholar]
- Hofstetter JR, Zhang A, Mayeda AR, Guscar T, Nurnberger JI., Jr., and , Lahiri DK. Genomic DNA from mice: a comparison of recovery methods and tissue sources. Biochem Mol Med. 1997;62:197–202. doi: 10.1006/bmme.1997.2637. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Dexamethasone treatment causes an increase in lymphopenia and neutrophilia in a blood of C57BL/6 mice, which is not related to Ad treatment. C57BL/6 mice were intravenously injected with 0.75x1011 vp/mouse of Ad5-LacZ vector. DEX pre-treatment and CBC differential count was performed as described in Materials and Methods. Total four groups of mice (N=4) analyzed: WT_Mock, WT_DEX_Mock, WT_Ad5-LacZ, WT_DEX_Ad5-LacZ. The bars represent Mean ± SD. Statistical analysis was completed using One Way ANOVA with a Student-Newman-Keuls post-hoc test, p<0.05 was deemed a statistically significant difference. #, ## - indicate statistically different values in WT_DEX_Ad5-LacZ group compared to WT_Ad5-LacZ group, p<0.05, p<0.001 respectively. Normal ranges for Neutrophils (A) and Lymphocytes (B) count in male C57BL/6 mice are indicated by light grey shaded boxes. Note: Normal range levels were adapted from Jackson laboratories studies on C57BL/6 mice (http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&sym=Peters3).
Dexamethasone treatment preserves the efficacy of Ad Ad-derived transgene expression and Ads genomes persistency in the livers of C57BL/6 mice. A) In situ visualization of bacterial β-galactosidase in liver of Ad5-LacZ treated C57BL/6 mice. Cryosections of liver from all groups of mice were stained for β-Gal in situ as described in Materials and Methods. Representative sections for each of the groups are shown. Total magnification of 200X was used to obtain images. N=6 for all virus injected groups at 6 hpi, N=4 for all virus injected groups at 24 hpi, N=5 for all virus injected groups at 28 dpi, N=4 for all Mock injected groups at all time points. B) Bacterial β-galactosidase activity levels were analyzed in liver protein homogenates prepared at 6 hpi, 24 hpi and 28 dpi from four groups of C57BL/6 mice: WT_Mock, WT_DEX_Mock, WT_Ad5-LacZ, WT_DEX_Ad5-LacZ. Activity levels were presented as Units per mg of total protein (see Materials and Methods). The bars represent Mean ± SD. Statistical analysis was completed using two-tailed Student t-test to compare 2 groups of virus injected animals. #, ## - indicate statistically different values in WT_DEX_Ad5-LacZ group compared to WT_Ad5-LacZ group, p<0.05, p<0.001 respectively. C) qPCR based quantification of Ad5-LacZ genomes in livers harvested from C57BL/6 mice at 6 hpi, 24 hpi, 28 dpi. The bars represent Mean ± SD. Statistical analysis was completed using two-tailed Student t-test to compare 2 groups of virus injected animals. #, ## - indicate statistically different values in WT_DEX_Ad5-LacZ group compared to WT_Ad5-LacZ group, p<0.05, p<0.001 respectively. Note the difference in scale for different time points.
Dexamethasone treatment does not change Ad- dependent Kupfer cells degradation in a liver of C57BL/6 mice. 7 μm liver sections obtained at 6 and 24 hpi were stained with macrophage specific F4/80 antibody. Pixel density of both Kupffer cell staining and DNA staining (DAPI) was quantified. Kupffer cells values were normalized to DAPI values to control for cell density variation. Values were subsequently divided by WT_Mock average values to give percent difference. Error bars indicate ±SD. N=4 for all groups tested, pictures represent one of at least 12 sections derived from 4 mice in each group: WT_Mock, WT_DEX_Mock, WT_Ad5-LacZ, WT_DEX_Ad5-LacZ. Statistical analysis was completed using One Way ANOVA with a Student-Newman-Keuls post-hoc test, p<0.05 was deemed a statistically significant difference. *, ** - indicate values, statistically different from those in WT_Mock injected animals, p<0.05, p<0.001 respectively.
Dexamethasone treatment does not change natural levels of total nonspecific IgG antibodies in a blood of C57BL/6 mice. Plasma samples from Mock injected mice (WT_Mock and WT_DEX_Mock, N=4 for each group) were analyzed for total non-specific IgG antibodies. The bars represent Mean ± SD. Statistical analysis was completed using two-tailed Student t-test to compare 2 groups of Mock injected animals, p<0.05 was deemed a statistically significant difference. No significant difference was found.
A pair of forward (For) and reverse (Rev) primers are provided for every transcript tested by qRTPCR-based methods. The primers were designed as described in Materials and Methods section, the length of the resulted PCR products was 100–160 nucleotides.