Abstract
Mutations of the gene encoding unconventional myosin XVa are associated with sensorineural deafness in humans (DFNB3) and shaker (Myo15sh2) mice. In deaf Myo15sh2/sh2 mice, stereocilia are short, nearly equal in length, and lack myosin XVa immunoreactivity. We previously reported that myosin XVa mRNA and protein are expressed in cochlear hair cells. We now show that in the mouse, rat, and guinea pig, endogenous myosin XVa localizes to the tips of the stereocilia of the cochlear and vestibular hair cells. Myosin XVa localization overlaps with the barbed ends of actin filaments and extends to the apical plasma membrane of the stereocilia. Gene gun-mediated transfection of mouse inner ear sensory epithelia explants shows selective accumulation of myosin XVa-GFP at the tips of stereocilia, confirming the localization of native myosin XVa. Expression in COS7 cells also reveals targeting of myosin XVa-GFP to the dynamic actin region at the tips of filopodia. In a wild-type mouse, during auditory and vestibular hair cell development, myosin XVa appears at the tips of stereocilia at the time when the hair bundle begins to develop its characteristic staircase pattern. We propose that myosin XVa is essential for the graded elongation of stereocilia during their functional maturation.
Stereocilia are actin-filled mechanosensory organelles that project outward from the apical surfaces of each inner ear hair cell. They appear as thick long microvilli organized into a bundle. Adjacent parallel rows of stereocilia of graded height form a staircase-like pattern. The development of hair cell stereocilia in the chick (1) occurs in three main phases. Initially, thin immature stereocilia, indistinguishable from microvilli, emerge at the apical surface of the hair cells and elongate. During the second phase, stereocilia stop elongating and gradually acquire an adult-like thickness. In the third phase, stereocilia begin the process of differential elongation. In mammals, the overall sequence of events is similar, although the growth and thickening of stereocilia occur simultaneously (2).
The core of mature stereocilia consists of unidirectionally oriented actin filaments crosslinked in a paracrystalline array (3, 4). Their barbed ends (plus ends) are oriented toward the tips of stereocilia (3) where the addition of actin monomers occurs (5, 6). The tips of shorter stereocilia and the side of the neighboring longer stereocilia are interconnected by tiny filaments referred to as tip-links (7). The mechanically gated transduction channels (8) are postulated to be located at tip-link insertion sites at the tops of shorter and on the sides of the longer stereocilia (9, 10). When stereocilia deflect during sound stimulation, these elastic linkages stretch and thereby open the transduction channels (11). Additionally, there is an adaptation mechanism, which resets the tip-link tension and maintains a high level of sensitivity of the hair cell transduction machinery during periods of sustained acoustic stimulation (12). Because stereocilia are actin-enriched structures, and ATP analogs are capable of altering hair cell adaptation, it has been hypothesized that the adaptation process is based on the operation of a myosin motor, which moves the tip-link insertion plaque of the taller stereocilium toward the tip (13, 14). This change in position of the insertion plaque applies tension to the tip-link, which is mechanically coupled to the transduction channel (11, 15). Candidate proteins for this adaptation motor include unconventional myosin 1C (16–18) and myosin VIIA (19). Myosin 1C has been shown to be localized to the tips of stereocilia in the bullfrog (16, 17) but in mouse and rat is distributed evenly along the length of cochlear hair cell stereocilia and is concentrated toward the tips of vestibular hair cell stereocilia (20). Myosin VIIA is also found along the entire length of mouse hair cell stereocilia (21) and is thought to anchor the crosslinks between adjacent stereocilia to the actin cytoskeleton (22, 23).
Mutations of another unconventional myosin, myosin XVa, lead to deafness in humans (24) and mice (25). Homozygous Myo15sh2 deaf mice have stereocilia that are short and seem to lack tip-link connections (26), even though the spatial organization and orientation of the hair bundles are largely preserved (25, 27). Here we demonstrate that wild-type myosin XVa is localized at the tips of cochlear and vestibular hair cell stereocilia and is necessary for the formation of the staircase-like arrangement of stereocilia of mature hair bundles.
Methods
Construction of the [-N]Myo15a-GFP Expression Vector. A 6.9-kb cDNA encoding mouse myosin XVa (Myo15a) and containing the entire ORF of the short isoform (2,306-aa residues; National Center for Biotechnology Information accession no. AY331133; see Fig. 6, which is published as supporting information on the PNAS web site) was synthesized from 200 ng of mouse inner ear polyA+ RNA by using SuperScript II reverse transcriptase (Invitrogen) and specific primers in exon 1 and 66 (primer sequences are provided in Table 1, which is published as supporting information on the PNAS web site). Three overlapping cDNA fragments were synthesized by using Pfu Turbo (Stratagene) and sequenced (see primer sequences in Table 2, which is published as supporting information on the PNAS web site). These cDNA fragments were cloned individually into pGEM-3Zf(–) vector (Promega) and then transformed into XL10-Gold Ultracompetent Cells (Stratagene), excised with appropriate restriction endonucleases (see Supporting Methods, which is published as supporting information on the PNAS web site), gel purified, sequentially ligated into the EcoRI and SalI sites of the pEGFP-C2 vector (Clontech), and sequenced. This cDNA insert corresponds to an endogenously expressed isoform of Myo15a that lacks exon 2 encoding 1,187 amino acids preceding the motor domain (N-terminal extension) and exon 26, which encodes 18 amino acids between the light chain-binding motifs (IQs) and the first MyTh4 domain (Fig. 6). The construct was designated as [-N]Myo15a-GFP. Because mouse inner ear sensory epithelia explants transfected with the isoform containing the N-terminal extension exhibited a low efficiency of transfection, we focused this study on [-N]Myo15a-GFP. The pEGFP-C2 without an insert was used as a control.
Evaluation of the Specificity of Antimyosin XVa Antibodies. To confirm the specificity of the previously described TF1 and PB48 antibodies (Fig. 1A and ref. 28), a colocalization assay was performed by using [-N]Myo15a-GFP transfected COS7 cells, which lack endogenous expression of myosin XVa. The cells were plated on glass coverslips in DMEM media with 10% FBS (Invitrogen) at a density of 4 × 106 cells per cm2, cultured until 80% confluency, and transfected by using Lipofectamine 2000 (Invitrogen). After 36 h of incubation at 37°C and 5% CO2, transfected cells were fixed with 4% paraformaldehyde in PBS for 30 min, permeabilized for 15 min in PBS with 0.5% Triton X-100, blocked with 2% BSA and 5% goat serum in PBS for 30 min, and incubated with TF1 or PB48 antibodies (≈5 μg/ml) for 2 h followed by incubation with Alexa 594 anti-rabbit secondary antibody (Molecular Probes) for 30 min. After several washes in PBS, samples were mounted by using a ProLong Antifade Kit (Molecular Probes).
Fig. 1.
Evaluation of the specificity of antimyosin XVa antibodies in COS7 cells transiently transfected with [-N]Myo15a-GFP. (A) Domain structure of the chimeric protein encoded by [-N]Myo15a-GFP construct. Red bars depict the location of myosin XVa epitopes to which TF1 and PB48 antisera were raised. Shown are COS7 cells transfected with [-N]Myo15a-GFP (green; B) and stained with TF1 antibody (red; C). (D) Colocalization of the GFP signal of myosin XVa-GFP with TF1 antibody staining. (B–D Insets) Enlarged from the areas marked by the squares. Arrows point to myosin XVa-GFP at the tips of filopodia. (Bar = 10 μm.)
Immunofluorescence Study of Inner Ear Sensory Epithelia. The cochleae of adult C57BL/6 mice (Charles River Breeding Laboratories), adult (120–150 g) Sprague–Dawley rats (Taconic Farms), adult pigmented guinea pigs (Elm Hill Laboratories, Chelmsford, MA), postnatal day 21 (P21) shaker 2 mice, and C57BL/6 mice ranging from embryonic day 14.5 (E14.5) embryos to P21 pups were fixed with 4% paraformaldehyde in PBS for 2 h. Dissected vestibular and organ of Corti (OC) sensory epithelia were permeabilized in 0.5% Triton X-100 in PBS for 30 min followed by 3 × 10-min washes in PBS. Nonspecific binding sites were blocked by 5% goat serum and 2% BSA in PBS for 1 h at room temperature. Samples were incubated for 2 h with TF1 or PB48 antibodies (≈5 μg/ml) followed by several rinses in PBS and incubation with the anti-rabbit FITC-conjugated secondary antibody (Amersham Pharmacia Biosciences) for 30 min. F-actin was visualized by rhodamine–phalloidin staining (26).
Culture and Transfection of Inner Ear Sensory Epithelia. Inner ear sensory epithelium cultures were prepared from OC, saccule, utricle, and ampule of P3–P5 C57BL/6 mice and from OC of P3 rats. The OC spiral was dissected away from the modiolus in Leibowitz cell culture medium (Invitrogen). The apical and middle turns of the OC were separated from the basal turn and cut into four pieces. The sensory maculae of the utricle, saccule, and ampule were dissociated from the nonsensory tissue, and the otoconia layers were removed. Each piece of sensory epithelium was attached to a glass-bottom Petri dish (MatTek, Ashland, MA) coated with CellTak (Collaborative Biomedical Products, Bedford, MA) and maintained at 37°C and 5% CO2 in DMEM supplemented with 7% FBS for 1–3 days. Cultures were then transfected by using a Helios gene gun (Bio-Rad). Gold particles of 1.0-μm diameter (Bio-Rad) were coated with plasmid DNA at a ratio of 2 μg of plasmid DNA to 1 mg of gold particles and precipitated onto the inner wall of Tefzel tubing, which was cut into individual cartridges containing ≈1 μg of plasmid DNA. Cartridges containing the pEGFP-C2 vector with [-N]Myo15a insert and control cartridges containing the pEGFP-C2 vector alone were prepared separately. Samples were bombarded with the gold particles from one cartridge per culture by using 120 psi of helium. After an additional 8 h to 4 days in culture, samples were fixed in 4% paraformaldehyde, stained with rhodamine–phalloidin, and observed by using a Zeiss LSM510 confocal microscope equipped with a ×100, N.A. = 1.45 objective.
Results
Antimyosin XVa Antibody Staining Is Colocalized with [-N]Myo15a-GFP Fluorescence at the Tips of Filopodia in COS7 Cells. At 36 h after transfection with the [-N]Myo15a-GFP construct (Fig. 1 A), myosin XVa-GFP was detected in the cell bodies and at the tips of filopodia of COS7 cells (Fig. 1 B–D), which do not express a detectable level of endogenous myosin XVa (Fig. 7E, which is published as supporting information on the PNAS web site). Immunolabeling of transfected COS7 cells revealed that the TF1 antibody staining pattern overlapped with the green fluorescent signal of myosin XVa-GFP (Fig. 1 B–D). PB48 antibody staining produced similar results (Fig. 7). No fluorescent signal was detected at the tips of filopodia in nontransfected or COS7 cells transfected with empty pEGFP-C2 vector with either antibody (Fig. 7).
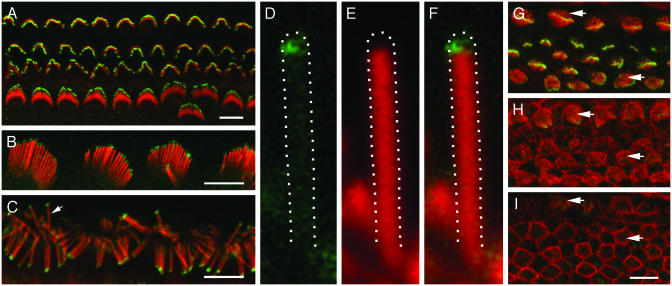
Myosin XVa Is Localized at the Tips of Stereocilia of Cochlear and Vestibular Hair Cells. In confocal fluorescence images of whole-mount preparations of adult mouse, rat, and guinea pig OCs, we observed myosin XVa immunoreactivity at the top of the stereocilia bundles (Figs. 2 B and C and 4D). In the mouse, OC hair cell stereocilia bundles acquire their mature staircase shape during late embryonic and early postnatal development. By using TF1 antibody, myosin XVa in the developing mouse OC hair cell stereocilia was first detected at E18.5 in the basal turn of the cochlea (Fig. 2G), where both inner hair cell (IHC) and outer hair cell (OHC) hair bundles begin to form a staircase-like pattern. In the middle turn, only the more mature IHC stereocilia were positive for myosin XVa (Fig. 2H). Within the apical turn, no developing hair cell stereocilia exhibited staining (Fig. 2I). Myosin XVa immunoreactivity was present at the tips of stereocilia in the cochlear hair cells during mouse postnatal development (Fig. 2 A) and in the adult (Fig. 4D). The brightest staining was consistently observed at the tips of the tallest stereocilia of both IHCs and OHCs. Myosin XVa-specific staining at the tips of short stereocilia was barely detectable by confocal fluorescence microscopy (Fig. 2 A). However, when the hair cell stereocilia were spread apart, it was possible to see that myosin XVa was indeed localized at the tip of each individual stereocilium (Fig. 2C). Myosin XVa localization extended ≈0.5 μm from the tip downward. Myosin XVa immunofluorescence did not completely overlap with F-actin, as revealed by rhodamine–phalloidin staining (Fig. 2 D–F), although we cannot rule out the possibility that there is a low level of F-actin at the top of the stereocilia core.
Fig. 2.
Localization of myosin XVa protein in OC hair cell stereocilia visualized by using TF1 antiserum (green) in rat, guinea pig, and mouse. The actin core of each stereocilium was counterstained with rhodamine–phalloidin (red). (A) Localization of myosin XVa at the tips of stereocilia of three rows of OHCs and one row of IHCs of P5 mouse. (B) Myosin XVa at the tips of OHC stereocilia of adult guinea pig OC. (C) IHC stereocilia of adult rat OC were splayed by pressing on the coverslip to better visualize the myosin XVa signal at each stereocilium tip. Arrow indicates stereocilium enlarged in D–F. (D) Myosin XVa staining is concentrated at the tip (green). No detectable staining of myosin XVa is evident along the length of a stereocilium. (E) Red channel of the same image. We were unable to detect F-actin at the tip. (F) Double staining reveals partial overlap of myosin XVa signal (green, D) with F-actin core of stereocilia (red, E). (G–I) Myosin XVa immunoreactivity (green) in the OC of E18.5 mouse at basal (G), middle (H), and apical (I) turns. Maturation of hair cells progresses from base to apex of the cochlea with the development of IHCs slightly preceding OHCs (28). Myosin XVa staining (green) is prominent at the tips of stereocilia of IHC and OHC in the basal turn, faint in the middle turn, and not detectable in apical turn. Arrows point to the immature stereocilia, which lack myosin XVa signal at the tips. (Bars = 5 μm.)
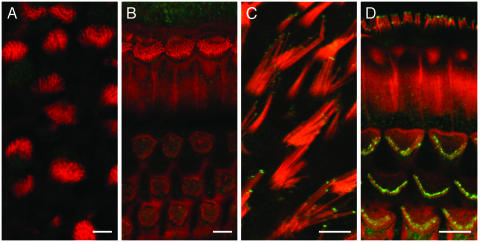
Fig. 4.
Myosin XVa is absent from stereocilia of P21 Myo15sh2/sh2 mice. Hair cell stereocilia of a P21 Myo15sh2/sh2 mouse utricle (A) and OC (B) lack myosin XVa immunoreactivity at the tips (TF1, green; rhodamine–phalloidin, red). Note that stereocilia have a similar length in the different rows within a bundle but are much shorter than corresponding mature hair bundles of a control C57BL/6 P19 mouse utricle (C) and OC (D) hair cells, which have myosin XVa at the stereocilia tips. (Bars = 5 μm.)
A similar distribution of myosin XVa staining was observed at the tips of all stereocilia of the vestibular hair cells of the three ampule (Fig. 3B), the utricle (Fig. 4C), and the saccule (Fig. 3C). The staining of hair cell stereocilia of ampule was detectable as early as E14.5 (Fig. 3A), corresponding to the time in development when stereocilia bundles of vestibular hair cells begin to exhibit a staircase-like arrangement (29). Myosin XVa also continued to be present at the tips of hair cell stereocilia of utricle and saccule from E14.5 onward. Independent of stereocilia length, the area of myosin XVa localization was ≈0.5 μm in length from the top of all stereocilia (Figs. 2 and 3). Both TF1 and PB48 antisera produced a similar pattern of staining in cochlear (Figs. 2 and 4D) and vestibular (Fig. 3 B and C) hair cell stereocilia.
Fig. 3.
Localization of myosin XVa (green) at the tips of stereocilia of mouse vestibular hair cells. (A) Myosin XVa, revealed by TF1 antibody, at the tips of stereocilia of E14.5 mouse hair cells of the ampulla, when the gradation in height of stereocilia is just beginning to appear. (B) Myosin XVa at the tips of stereocilia of hair cells of adult mouse ampulla stained with TF1 antiserum. (C) PB48 antibody also revealed myosin XVa at the tips of saccular hair cell stereocilia of adult mouse. (Bars = 5 μm.)
Shortened Hair Cell Stereocilia of Shaker 2 Mice Do Not Possess Myosin XVa at the Tips and Do Not Develop the Staircase Shape of a Mature Hair Bundle. No myosin XVa immunoreactivity was detected at the stereocilia tips of Myo15sh2/sh2 mice (Fig. 4 A and B). By counterstaining inner ear sensory epithelia from adult Myo15sh2/sh2 mice with rhodamine–phalloidin, we observed that their abnormally short hair cell stereocilia do not exhibit the characteristic staircase organization of hair bundles, observed in the wild type. This was particularly evident in the vestibular end organs. In the utricle of P21 Myo15sh2/sh2 mice, all hair cells had stereocilia of nearly equal length within and between the bundles (Fig. 4A).
Targeting of Endogenous Myosin XVa and Myosin XVa-GFP to the Tips of Stereocilia. Hair cell bundles in the OC of P0 wild-type mice already have a well developed staircase pattern (29) and generate a mechanotransduction current of an adult-like amplitude (30). To assess the ability of these hair cells to target myosin XVa to the tips of stereocilia, we transfected hair cells of P4 and P6 mouse inner ear sensory epithelia cultures with [-N]Myo15a-GFP. At 8–96 h after transfection, myosin XVa-GFP protein was concentrated at the tip of every stereocilium of a transfected cell (Fig. 5). From 40 inner ear sensory epithelia explants (four to eight explants per mouse), we observed 106 transfected hair cells. Some vestibular sensory epithelium cultures had up to seven transfected hair cells with slightly different levels of myosin XVa-GFP expression; possibly due to unequal binding of [-N]Myo15a-GFP to the gold bullets (Fig. 5C). Nontransfected cells were used as indicators of the extent to which preservation of the structural integrity of the hair cell bundles was maintained. Among the 40 explants, we observed no systematic differences in bundle morphology between transfected and adjacent non-transfected cells. Hair cells transfected with an empty pEGFP-C2 vector show uniformly distributed green fluorescence along the stereocilia length and in the cell body (data not shown).
Fig. 5.
Gene gun-mediated transfection of mouse vestibular and cochlear sensory epithelia explants with [-N]Myo15a-GFP (green). The actin core of stereocilia was counterstained with rhodamine–phalloidin (red). (A) Saccular hair cell 24 h after transfection. (B) Myosin XVa-GFP (green) at the tips of stereocilia 18 h after transfection of IHC from P5 OC explants. (C) The image of utricular explants illustrating that stereocilia bundles of three hair cells 48 h after transfection have slightly different amounts of myosin XVa-GFP at the tips of stereocilia. (D) Distension of the stereocilia tips of a vestibular hair cell due to an excessive accumulation of myosin XVa-GFP 96 h after transfection (see also Fig. 8). (E) Myosin XVa-GFP surrounds the top of the actin filaments of stereocilia but does not penetrate inside the actin core. Unstained spots corresponding to the actin core are visible in the center of patches of green fluorescence. (F) Rhodamine–phalloidin staining of the same stereocilia bundle. (Bars = 2 μm.)
A close-up view of the tip of a stereocilium revealed a reproducible difference between the immunostaining of the native myosin XVa and the localization of myosin XVa-GFP, overexpressed in hair cells. Native myosin XVa was localized precisely in the capping region of the stereocilium, between the upper end of the actin core and the apical plasma membrane, slightly overlapping the actin core (Fig. 2 D–F). In contrast, the myosin XVa-GFP appeared to surround the upper portion of the actin core (Fig. 5D). The images collected 24–96 h after transfection revealed that the tip of every stereocilium of transfected hair cells acquired a bulbous shape (Fig. 5 B–E; Fig. 8, which is published as supporting information on the PNAS web site). No patches of myosin XVa were observed along the length of stereocilia (Fig. 5).
Discussion
Our data show that myosin XVa is localized precisely to the tips of mammalian hair cell stereocilia, under the apical plasma membrane and overlapping with the barbed ends of the actin filaments of the stereocilia core (Figs. 2 and 3). This is the location of growth and remodeling of the actin core (6). We first detect myosin XVa at the tips of mouse E14.5 vestibular and E18.5 cochlear hair cell stereocilia, coinciding with the onset of the formation of a characteristic staircase arrangement of the stereocilia in the hair bundle. In homozygous shaker 2 mice (Myo15sh2/sh2), no myosin XVa was detected at the tips of the inner ear hair cell stereocilia, and bundle elongation seems to have been arrested at the stage when stereocilia are of approximately equal height. This finding is consistent with the timing of the appearance of myosin XVa within the tips of the stereocilia in the wild-type mouse (Fig. 3A). Taken together, these data suggest that myosin XVa is necessary for the differential growth of the stereocilia to form their staircase relationship.
Targeting and Tethering of Myosin XVa to the Tips of Stereocilia. Colocalization of myosin XVa-GFP with antimyosin XVa antibody staining in transfected COS7 cells not only confirmed the specificity of the two antibodies (TF1 and PB48) but also revealed targeting of myosin XVa to the tips of filopodia (Fig. 1 B–D). This is similar to the localization of unconventional myosin X in transfected HeLa cells (31, 32). In mammalian inner ear hair cells, myosin XVa seems to be the only molecular motor thus far shown to be concentrated exclusively at the tip of a stereocilium, which is one of the proposed locations of the hair cell transduction machinery.
The observation that myosin XVa only partially colocalizes with the most distal detectable rhodamine–phalloidin staining of filamentous actin at the tips of the stereocilia (Fig. 2D) suggests that domains of myosin XVa, other than the actin-binding site of the motor domain, may be important for its localization at the apex of stereocilia. This possibility is also supported by the observation of an apparently identical phenotype of Myo15sh2/sh2 and Myo15sh2j/sh2j mice (27), which results from a missense mutation in the motor domain and a deletion of the last six exons encoding the C-terminal 268 amino acids of the myosin XVa tail, respectively (27). In mice homozygous for either of the mutant shaker 2 alleles, myosin XVa is not present in the stereocilia but is localized ectopically under the cuticular plate in the hair cell body, suggesting that an intact motor and tail are both required for correct stereocilia tip localization of myosin XVa.
Previously elucidated structures and protein partners of other myosins (33–35) suggest the possibility that some myosin XVa tail domains could be important for targeting and tethering at the tips of stereocilia. The tail of myosin XVa contains an SH3 domain, which in myosin I was implicated in directed polymerization of actin (33, 36) and two pairs of tandemly arranged MyTh4 and FERM domains (Fig. 1 A). MyTh4 and FERM domains are also similarly grouped in the tail of myosins VIIA and X (31, 33), which were also found in actin-rich protrusions such as microvilli, filopodia, and stereocilia (21, 31). Moreover, both the long and the short isoforms of myosin XVa (Fig. 6) have predicted class 1 consensus PDZ-binding ligands (X-S/T-X-V/L) that are formed by the last four amino acids at the C terminus (I-T-L-L), suggesting the possible interaction of myosin XVa with a PDZ-containing protein such as whirlin (37) and in a manner similar to the interaction of myosin VIIA with harmonin in stereocilia (23).
Mutations of the motor and of various regions of the myosin XVa tail, including the MyTh4 and FERM domains, cause deafness in humans and mice (24, 25, 38). To date, no mutations that cause hearing loss have been identified in the alternatively spliced exon 2, which encodes ≈1,200-aa residues of an N-terminal extension preceding the motor domain. The exact function of the long proline/tyrosine rich N-terminal extension is currently unknown. Our transfection experiments using [-N]Myo15a-GFP show that the N-terminal extension of myosin XVa is not essential for this molecular motor to be specifically targeted and tethered to the tips of stereocilia.
Accumulation of Myosin XVa-GFP at the Tips of Stereocilia. When GFP-tagged β-actin monomers are added to the barbed apical ends of actin filaments, the existing actin core shifts toward the base of stereocilia. After 48 h, β-actin of the stereocilia core seems to be replaced by β-actin-GFP, and stereocilia become fluorescent along their length (6). In contrast, myosin XVa-GFP appears only at the tips of the stereocilia and remains concentrated there for at least 96 h after transfection (Fig. 5). Excessive accumulation of newly synthesized myosin XVa-GFP at the upper end of a stereocilium causes distension of the tip (Figs. 5 B–E and 8). We presume that in transiently transfected hair cells, the cytomegalovirus promoter driving [-N]Myo15a-GFP expression results in an abnormally high concentration of myosin XVa-GFP at the tips of stereocilia. In addition or alternatively, the GFP epitope tag may interfere with a steady-state recycling process that prevents excessive accumulation of endogenous myosin XVa at the tip of each stereocilium. Whatever the reason, this excessive accumulation of myosin XVa does not confound the demonstration by our data that native and GFP-tagged myosin XVa are both targeted and confined to the tip of each stereocilium. There appears to be only a slight overlap of endogenous myosin XVa with the barbed ends of actin filaments (Fig. 2 D–F), which may mean that a significant fraction of myosin XVa at the tip of a stereocilium is detached from the actin core and may not be entrained by the β-actin treadmill (32).
Myosin XVa and Mechanoelectrical Transduction. In our experiments, myosin XVa was not detected by confocal immunofluorescence microscopy near the insertion sites of the tip-link on the side of the stereocilium (Fig. 2 D–F), which is a proposed location of the adaptation motor (39). However, myosin XVa at the tip of the shorter stereocilium may influence the tension applied to the tip-link and, therefore, participate in the process of adaptation. Also, tip-links appear to be absent from stereocilia of Myo15sh2/sh2 mice (26), suggesting a possible requirement of myosin XVa for tip-link assembly and/or maintenance. The molecular identities of the tip-link, the mammalian hair cell mechanotransduction channel, and the additional elastic elements involved in the gating of this channel are still unknown. Some of these molecules may specifically bind to myosin XVa and may be transported by this motor to the tips of stereocilia, as was suggested for myosin molecular motors (11, 15).
Myosin XVa and the Formation of Hair Bundle Staircase Shape. The graded height of stereocilia in a bundle is a characteristic feature of auditory hair cells of the modern amniotes including reptiles, birds, and mammals (40). The structural integrity of this arrangement appears to be essential for auditory transduction, and the directional sensitivity of mechanotransduction has been shown to be associated with the gradation in height of the stereocilia (41). When the formation of a characteristic staircase pattern of the bundle is first evident, we are able to detect myosin XVa at the tips of stereocilia. In mouse, this occurs as early as E18.5 in cochlear hair cells and at E14.5 in the vestibular end organs (Figs. 2 G–I and 3A). The absence of functional myosin XVa at the tips of stereocilia in Myo15sh2/sh2 mice results in a failure of stereocilia bundles to form the expected staircase arrangement (Fig. 4 A and B), although there is no noticeable influence on the previous stages of bundle development. The stereocilia of Myo15sh2/sh2 mice are spatially well organized but short and of approximately equal height within the bundle. In summary, myosin XVa is localized precisely to the tips of inner ear hair cell stereocilia and is critical for their graded elongation during formation of the staircase arrangement of mammalian hair bundles.
Supplementary Material
Acknowledgments
We thank T. Ben-Yosef, D. Drayna, G. I. Frolenkov, A. Griffith, M. Kelly, R. Morell, J. Schultz, and J. Sellers for critical reading of our manuscript. We also thank Drs. S. Camper (Department of Internal Medicine, Division of Molecular Medicine and Genetics, University of Michigan, Ann Arbor) and Y. Raphael (Kresge Hearing Research Institute, University of Michigan Medical School, Ann Arbor) for providing shaker 2 mouse cochleae; and Drs. R. Chadwick and B. Shoelson (Laboratory of Cell Biology, Section on Auditory Mechanics, National Institute on Deafness and Other Communication Disorders, National Institutes of Health, Bethesda) for providing guinea pig cochleae.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: OC, organ of Corti; OHC, outer hair cell; IHC, inner hair cell; Myo15a, gene encoding mouse myosin XVa; En, embryonic day n; Pn, postnatal day n.
References
- 1.Tilney, L. G. & Tilney, M. S. (1986) Hear. Res. 22, 55–77. [DOI] [PubMed] [Google Scholar]
- 2.Kaltenbach, J. A., Falzarano, P. R. & Simpson, T. H. (1994) J. Comp. Neurol. 350, 187–198. [DOI] [PubMed] [Google Scholar]
- 3.Tilney, L. G., Derosier, D. J. & Mulroy, M. J. (1980) J. Cell Biol. 86, 244–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeRosier, D. J., Tilney, L. G. & Egelman, E. (1980) Nature 287, 291–296. [DOI] [PubMed] [Google Scholar]
- 5.Tilney, L. G., Bonder, E. M. & DeRosier, D. J. (1981) J. Cell Biol. 90, 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider, M. E., Belyantseva, I. A., Azevedo, R. B. & Kachar, B. (2002) Nature 418, 837–838. [DOI] [PubMed] [Google Scholar]
- 7.Pickles, J. O., Comis, S. D. & Osborne, M. P. (1984) Hear. Res. 15, 103–112. [DOI] [PubMed] [Google Scholar]
- 8.Hudspeth, A. J. & Corey, D. P. (1977) Proc. Natl. Acad. Sci. USA 74, 2407–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denk, W., Holt, J. R., Shepherd, G. M. & Corey, D. P. (1995) Neuron 15, 1311–1321. [DOI] [PubMed] [Google Scholar]
- 10.Lumpkin, E. A. & Hudspeth, A. J. (1995) Proc. Natl. Acad. Sci. USA 92, 10297–10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudspeth, A. J. & Gillespie, P. G. (1994) Neuron 12, 1–9. [DOI] [PubMed] [Google Scholar]
- 12.Howard, J. & Hudspeth, A. J. (1987) Proc. Natl. Acad. Sci. USA 84, 3064–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillespie, P. G. & Corey, D. P. (1997) Neuron 19, 955–958. [DOI] [PubMed] [Google Scholar]
- 14.Eatock, R. A. (2000) Annu. Rev. Neurosci. 23, 285–314. [DOI] [PubMed] [Google Scholar]
- 15.Howard, J. & Hudspeth, A. J. (1988) Neuron 1, 189–199. [DOI] [PubMed] [Google Scholar]
- 16.Steyger, P. S., Gillespie, P. G. & Baird, R. A. (1998) J. Neurosci. 18, 4603–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia, J. A., Yee, A. G., Gillespie, P. G. & Corey, D. P. (1998) J. Neurosci. 18, 8637–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holt, J. R., Gillespie, S. K., Provance, D. W., Shah, K., Shokat, K. M., Corey, D. P., Mercer, J. A. & Gillespie, P. G. (2002) Cell 108, 371–381. [DOI] [PubMed] [Google Scholar]
- 19.Kros, C. J., Marcotti, W., van Netten, S. M., Self, T. J., Libby, R. T., Brown, S. D., Richardson, G. P. & Steel, K. P. (2002) Nat. Neurosci. 5, 41–47. [DOI] [PubMed] [Google Scholar]
- 20.Dumont, R. A., Zhao, Y. D., Holt, J. R., Bahler, M. & Gillespie, P. G. (2002) J. Assoc. Res. Otolaryngol. 3, 375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolfrum, U., Liu, X., Schmitt, A., Udovichenko, I. P. & Williams, D. S. (1998) Cell Motil. Cytoskeleton 40, 261–271. [DOI] [PubMed] [Google Scholar]
- 22.Hasson, T., Gillespie, P. G., Garcia, J. A., MacDonald, R. B., Zhao, Y., Yee, A. G., Mooseker, M. S. & Corey, D. P. (1997) J. Cell Biol. 137, 1287–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boeda, B., El-Amraoui, A., Bahloul, A., Goodyear, R., Daviet, L., Blanchard, S., Perfettini, I., Fath, K. R., Shorte, S., Reiners, J., et al. (2002) EMBO J. 21, 6689–6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang, A., Liang, Y., Fridell, R. A., Probst, F. J., Wilcox, E. R., Touchman, J. W., Morton, C. C., Morell, R. J., Noben-Trauth, K., Camper, S. A., et al. (1998) Science 280, 1447–1451. [DOI] [PubMed] [Google Scholar]
- 25.Probst, F. J., Fridell, R. A., Raphael, Y., Saunders, T. L., Wang, A., Liang, Y., Morell, R. J., Touchman, J. W., Lyons, R. H., Noben-Trauth, K., et al. (1998) Science 280, 1444–1447. [DOI] [PubMed] [Google Scholar]
- 26.Beyer, L. A., Odeh, H., Probst, F. J., Lambert, E. H., Dolan, D. F., Camper, S. A., Kohrman, D. C. & Raphael, Y. (2000) J. Neurocytol. 29, 227–240. [DOI] [PubMed] [Google Scholar]
- 27.Anderson, D. W., Probst, F. J., Belyantseva, I. A., Fridell, R. A., Beyer, L., Martin, D. M., Wu, D., Kachar, B., Friedman, T. B., Raphael, Y., et al. (2000) Hum. Mol. Genet. 9, 1729–1738. [DOI] [PubMed] [Google Scholar]
- 28.Liang, Y., Wang, A., Belyantseva, I. A., Anderson, D. W., Probst, F. J., Barber, T. D., Miller, W., Touchman, J. W., Jin, L., Sullivan, S. L., et al. (1999) Genomics 61, 243–258. [DOI] [PubMed] [Google Scholar]
- 29.Lim, D. J. & Anniko, M. (1985) Acta Otolaryngol. Suppl. 422, 1–69. [PubMed] [Google Scholar]
- 30.Kros, C. J., Rusch, A. & Richardson, G. P. (1992) Proc. R. Soc. London Ser. B 249, 185–193. [DOI] [PubMed] [Google Scholar]
- 31.Berg, J. S., Derfler, B. H., Pennisi, C. M., Corey, D. P. & Cheney, R. E. (2000) J. Cell Sci. 113, 3439–3451. [DOI] [PubMed] [Google Scholar]
- 32.Berg, J. S. & Cheney, R. E. (2002) Nat. Cell Biol. 4, 246–250. [DOI] [PubMed] [Google Scholar]
- 33.Friedman, T. B., Sellers, J. R. & Avraham, K. B. (1999) Am. J. Med. Genet. 89, 147–157. [DOI] [PubMed] [Google Scholar]
- 34.Kierke, M. C. & Titus, M. A. (2002) in Molecular Motors, ed. Schuwa, M. (Wiley VCH, Weinheim, Germany), pp. 3–44.
- 35.Mermall, V., Post, P. L. & Mooseker, M. S. (1998) Science 279, 527–533. [DOI] [PubMed] [Google Scholar]
- 36.Geli, M. I., Lombardi, R., Schmelzl, B. & Riezman, H. (2000) EMBO J. 19, 4281–4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mburu, P., Mustapha, M., Varela, A., Weil, D., El-Amraoui, A., Holme, R. H., Rump, A., Hardisty, R. E., Blanchard, S., Coimbra, R. S., et al. (2003) Nat. Genet. 34, 421–428. [DOI] [PubMed] [Google Scholar]
- 38.Liburd, N., Ghosh, M., Riazuddin, S., Naz, S., Khan, S., Ahmed, Z., Riazuddin, S., Liang, Y., Menon, P. S., Smith, T., et al. (2001) Hum. Genet. 109, 535–541. [DOI] [PubMed] [Google Scholar]
- 39.Gillespie, P. G. & Walker, R. G. (2001) Nature 413, 194–202. [DOI] [PubMed] [Google Scholar]
- 40.Manley, G. A. (2000) Proc. Natl. Acad. Sci. USA 97, 11736–11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pickles, J. O., Rouse, G. W. & von Perger, M. (1991) Scanning Microsc. 5, 1115–11124. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.