Abstract
Introduction
Prostaglandins (PGs) can act on both hematopoietic and osteoblastic lineages to enhance osteoclast formation.
Methods
We examined PGE2 stimulated osteoclastogenesis in RAW 264.7 cells and the role of endogenous PGE2 in lipopolysaccharide (LPS) stimulated osteoclastogenesis.
Results
RANKL (1–100 ng/ml) increased formation of osteoclasts, defined as tartrate resistant acid phosphatase multinucleated cells, with peak effects at 30 ng/ml. Addition of PGE2 (0.01–1.0 μM) to RANKL (30 ng/ml) dose dependently increased osteoclast number 30 to 150%. Use of NS-398 (0.1 μM) or indomethacin (Indo, 1.0 μM) to block endogenous PG synthesis had little effect on the response to RANKL alone but significantly decreased the response to PGE2. Addition of LPS (100 ng/ml) to RANKL increased osteoclast number 50%, and this response was significantly decreased by NS-398 and Indo. RANKL and PGE2 produced small, additive increases in COX-2 mRNA levels, while LPS produced a larger increase. PG release into the medium was not increased by RANKL and PGE2 but markedly increased by LPS.
Conclusion
We conclude that RANKL stimulated osteoclastogenesis can be enhanced by PGE2 and LPS though direct effects on the hematopoietic cell lineage and that these effects may be mediated in part by induction of COX-2 and enhanced intracellular PG production.
Keywords: Cyclooxygenase, RANKL
Introduction
Prostaglandin E2 (PGE2) and bacterial lipopolysaccharide (LPS) are known to promote osteoclast formation in marrow cultures and co-cultures of hematopoietic spleen cells and osteoblastic stromal cells [1,2,3,4]. The effect of LPS in these cultures may be dependent on stimulation of PGE2 production in osteoblastic cells [4]. In addition PGE2 can have a direct effect on the production of osteoclasts from hematopoietic precursors in spleen cell cultures treated with receptor activator of NFκB ligand (RANKL) and macrophage colony stimulating factor (M-CSF) [5]. Moreover both PGE2 and LPS have been shown to stimulate the inducible cyclooxygenase (COX-2) in a transformed cell line with macrophage characteristics, RAW 264.7 [6,7,8,9,10]. Cultured RAW 264.7 cells treated with RANKL can produce tartrate resistant acid phosphatase positive (+) multinucleated cells (TRAP+MNC) that have the characteristics of osteoclasts. RAW 264.7 cells express the PGE2 receptors EP2 and EP4, which are implicated in stimulation of osteoclastogenesis [11].
In the present study we confirmed that PGE2 could increase osteoclast formation in RANKL treated cultures and that LPS had similar effects. Osteoclast formation in response to these agonists was reduced by inhibitors of prostaglandin synthesis. RANKL and PGE2 produced small increases in COX-2 mRNA levels which were additive, while LPS produced a larger increase. The mRNA levels for the constitutive enzyme, COX-1, were not increased. Prostaglandin release into the medium before and after treatment with arachidonic acid to increase PGE2 production was not significantly increased by RANKL and PGE2 but markedly increased by LPS. These results indicate that osteoclastogenesis can be enhanced by PGE2 and LPS though direct effects on the hematopoietic cell lineage. These effects may be mediated in part by induction of COX-2 and enhanced intracellular prostaglandin production.
Material and Methods
Materials
Culture dishes and plates were from Corning (Corning, NY). PGE2 and NS-398 were obtained from Cayman Chemical (Ann Arbor, MI). LPS (from E. coli serotype 055:B5) was from Sigma (St Louis, MO). Recombinant murine RANKL was obtained from R & D Systems (Minneapolis, MN). alpha-Minimum essential medium (αMEM) and fetal calf serum were from Invitrogen (Carlsbad, CA). Leukocyte acid phosphatase A kit for TRAP staining was from Sigma (St. Louis, MO). PGE2 EIA kits were from Assay Designs (Ann Arbor, MI) and Cayman Chemicals (Ann Arbor, MI). Real-time PCR primers were from Applied Biosystems (Foster City, CA). Other chemicals and reagents were from analytical grade and obtained from Sigma.
Cell Culture
The RAW 264.7 monocyte/macrophage cell line was cultured at 37°C in 5% CO2 atmosphere in Dulbecco modified Eagles medium (DMEM) supplemented with 10% heat inactivated fetal calf serum (HIFCS). For osteoclast cultures, RAW cells were seeded in a 48 well plate at a density of 5 × 103 cells per well and cultured for 4 days in αMEM with 10% HIFCS in the presence of 30 ng/ml of RANKL unless otherwise stated. To examine the effects of PGE2 and LPS on osteoclastogenesis, cells were plated in 48 well dish and culture in αMEM and treated with PGE2 or LPS in the presence of RANKL. Half of the media was changed on the 3rd day. After 4 days (unless otherwise stated) cultures were fixed for 30 min with 2.5% gluteraldehyde and stained for TRAP. TRAP+MNC containing 3 or more nuclei were counted as osteoclasts. Total number of osteoclasts was counted in 4 wells.
Quantitative real time PCR (qPCR) analysis
For RNA extraction RAW 264.7 cells were plated in 6-well dishes at 6 × 104 cells per ml in αMEM with 10% HIFCS. Cells were grown for 2 days before they were treated for 1, 4 and 24 h with RANKL, PGE2, LPS or a combination. Total RNA was extracted using Tri-Reagent (Molecular Research Center, Cincinnati, Ohio) following the manufacturer’s instructions. Two to five μg of total RNA was converted to cDNA by the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) following company recommended protocol. qPCR was performed for different genes in separate wells (singleplex assay) of 96- well plate in a reaction volume of 20 μl. GAPDH served as the endogenous control for all experiments. Three replicates of each sample were amplified using Assays-on-Demand Gene Expression (Applied Biosystems, Foster City, CA) to give one measurement for a sample. Predesigned unlabeled gene-specific PCR primers and TaqMan dye-labeled probes were purchased from Applied Biosystems. Primers were tested for equal efficiency over a range of target gene concentration before use. All primers were designed to cross exon-exon boundaries. The PCR reaction mixture (including 2X TaqMan Universal PCR Master Mix, 20X Assays-on-Demand Gene Expression Assay Mix, 100 ng of cDNA) was run in Applied Biosystems ABI Prism 7300 Sequence Detection System instrument utilizing universal thermal cycling parameters. The relative quantification of target gene expression in a test sample to a control calibrator sample (ΔΔCt Method) was used for data analysis.
PGE2 measurements
RAW 264.7 cells were cultured for 2 days in αMEM with 10% HIFCS in a 24 well dish at 6 × 104 cells per ml. Treatments were pulsed in for 2 h; cells were washed well twice with serum-free media and then media were collected after 4 h. Arachdonic acid (10−5 M) was pulsed in for 15 min. and media was collected again. The PGE2 levels were measured in the media using the commercially available PGE2 enzyme immunoassay kits from Assay Designs (Ann Arbor, MI) and Cayman Chemicals (Ann Arbor, MI) according to the company’s recommended protocols.
Statistical analysis
The results are expressed as the mean ± SE. Statistical significance of differences among means was determined by analysis of variance (ANOVA) with post-hoc comparison of more than 2 means by the Bonferroni method using SigmaStat for osteoclast numbers or by t-test for mRNA induction (Jandel Scientific, San Rafael, CA).
Results
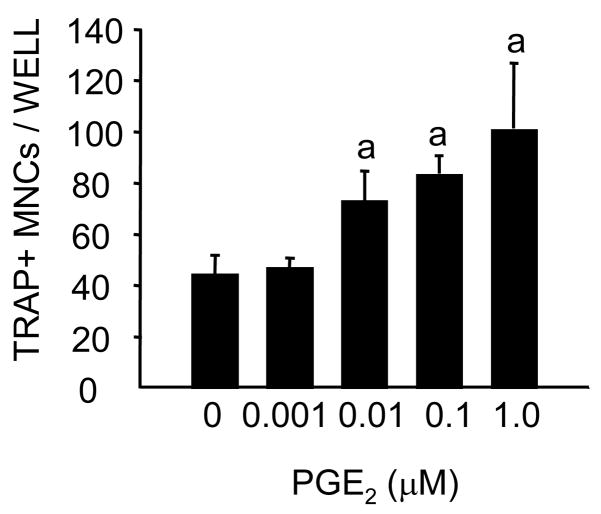
RANKL produced a dose related increase in osteoclast formation in RAW 264.7 cells (figure 1). The effects of 30 and 100 ng/ml were not significantly different. PGE2 at 10−6 M produced an increase in osteoclast number at all doses of RANKL. In the experiment illustrated in figure 2 this was significant at 3 and 30 ng/ml of RANKL. The effects of PGE2 were dose related with a significant increase at 0.01μM in some experiments and at 0.1 μM in all experiments (figure 3 and 4). The effects of 1 μM of PGE2 ranged from 30–150% in different experiments. The time course for osteoclast formation (figure 4A) showed a peak of osteoclast numbers at 4 d. In contrast to the previous studies using spleen cells [5], there was no early inhibition or prolongation of osteoclast survival and there was no difference in osteoclast size in cultures treated with PGE2 compared to those treated with RANKL alone (Fig. 4A). One day of treatment with PGE2 was sufficient to produce an increase in osteoclast number at 4 d and the effects were not significantly different from those of 4 d of continuous treatment (figure 4B). PGE2 alone without RANKL did not increase osteoclast number (data not shown).
Figure 1. Effect of RANKL on osteoclast formation in RAW 264.7 cell cultures.

RAW cells were treated with increasing doses of RANKL (1–100 ng/ml) for 4 days. Cells were fixed and stained for TRAP. TRAP+ cells with more than 3 nucleii were counted as osteoclasts. RANKL produced a dose related increase in the osteoclast number. Values are means +/− SEM. a significant effect of RANKL treatment, P<0.05, b significantly different from next lower dose by t-test.
Figure 2. Effect of PGE2 (1 μM) on osteoclastogenesis in RAW 267.4 cells treated with different concentrations of RANKL.
Conditions were the same as for figure 1. Cells were treated with 3–100 ng/ml of RANKL in the presence or absence of PGE2. Values are means +/− SEM. a Significant effects of PGE2, P<0.05.
Figure 3. Effect of increasing doses of PGE2 (0.001– 1.0 μM) in RAW 264.7 cells treated with RANKL (30 ng/ml) for.
Conditions were the same as for figure 1 Cells were treated with 30 ng/ml of RANKL alone or in the presence of increasing concentrations of PGE2. Values are means +/− SEM. aSignificant effects of PGE2, P<0.05.
Figure 4.
(A) Time course for the effect of different concentrations of PGE2 on osteoclastogenesis in RAW 264.7 cells treated with 30 ng/ml RANKL:Cells were treated with 0.01 μM, 0.1 μM and 1.0 μM of PGE2 for 3, 4 and 5 days. aSignificant effect of treatment, P<0.01; b P<0.05.
(B) Effect of PGE2 (1 μM) given for 1 d or continuously on RAW 264.7 cells treated with RANKL (30 ng/ml) for 4 d: Cells were treated with PGE2 and RANKL for 1 day and then with RANKL alone for 3 days or PGE2 was given continuously for 4 days in the presence of RANKL. aSignificantly effect of PGE2, P<0.01.
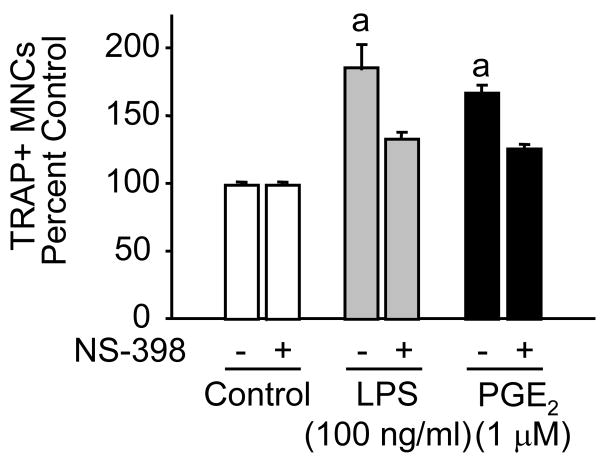
The effects of LPS were similar in magnitude to those of PGE2 in RANKL treated RAW cells. LPS at 100 ng/ml produced a significant increase in osteoclast number of 60–80% (figure 5 and 6). The effects of both LPS and PGE2 were reduced by the addition of NS398 (0.1 μM) at a concentration which blocks COX-2 mediated prostaglandin production but may also affect production from COX-1 [12] (figure 6 and 7). Indomethacin (1μM), which blocks both COX-2 and COX-1, also inhibited PGE2 stimulated osteoclastogenesis (figure 7). In this experiment the effect of PGE2, although reduced, was still significant in the presence of inhibitors of prostaglandin synthesis and there was a small non-significant reduction in osteoclast number in cultures treated with RANKL alone. Since there is evidence that the prostaglandin F pathway might be involved in induction of COX-2 [12], we tested PGF2α and the selective agonist fluprostenol (figure 8). PGF2α had a small stimulatory effect, which was reduced by NS-398. However, fluprostenol, at a concentration that activates the FP receptor, had no effect.
Figure 5. Effect of LPS on osteoclastogenesis in RAW 264.7 cells treated with 30 ng/ml RANKL.
Cells were treated with 1, 10 and 100 ng/ml of LPS and PGE2 (1 μM) in the presence of RANKL. a Significant effect of LPS or PGE2, P< 0.01, b P< 0.05.
Figure 6. Effect of NS398 (0.1 μM) on stimulation of osteoclastogenesis by PGE2 (1 μM) and LPS (100 ng/ml) in RAW 264.7 cells treated with RANKL (30 ng/ml).
Cells were treated with PGE2 and LPS in the presence of RANKL ± NS398 for 4 days. a Significantly different from RANKL alone, P<0.01.
Figure 7. Effects of PGE2 (1μM) on osteoclastogenesis in RAW 264.7 cells treated with RANKL (30 ng/ml), with or without NS-398 (0.1 μM) or indomethacin (1 μM).
Conditions were the same as for figure 1. a Significantly different from RANKL alone, P<0.01. b Significant effect of NS398 or Indomethacin, P<0.05.
Figure 8. Effects of PGF2a (1 μM) and fluprostenol (flup, 0.01 μM) compared with PGE2 (1 μM) on RAW 264.7 cells treated with RANKL (30 ng/ml) for 4 days, with and without NS-398 (0.1 μM).
Conditions were the same as for figure 1. aSignificantly different from RANKL alone, P<0.01; b Significant effect of NS 398, P<0.05.
COX-2 mRNA levels were significantly increased by RANKL and PGE2, while the effect of LPS was substantially greater (Table 1). The combination of RANKL and PGE2 appeared to be additive while RANKL did not further enhance the response to LPS. COX-1 levels were unaffected. A time course study showed that the effects of RANKL and PGE2 were transient, while LPS produced sustained increases in COX-2 levels at 24-hours (data not shown). Prostaglandin production was examined in cultures that were treated with RANKL, PGE2, LPS or combinations for 2 hours in serum free medium, washed, cultured for 4 hours and then treated with arachidonic acid (10 μM) for 15 minutes. PGE2 released into the medium before adding AA was markedly increased by LPS and slightly but not significantly increased by RANKL plus PGE2. After adding AA there was a further increase in PGE2 in all cultures, but there was no significant effect of RANKL, PGE2 or the combination compared to control, while the values were further increased in LPS treated cultures (Table 2). In another experiment in which the cultures were not serum deprived the results were similar (data not shown).
TABLE 1.
Changes in COX-2 mRNA levels after treatment with RANKL, PGE2, LPS, RANKL + PGE2 and RANKL + LPS and analyzed by qPCR. Cells were subjected to the treatments for 1h. Each RQ value for COX-2 was calculated as fold induction relative to the control. Single data values were pooled from 2 independent experiments.
| Treatment (Time 1 h) | COX-2 | COX-1 |
|---|---|---|
| Control (N=5) | 1.00 ± 0.19 | 1.00 ± 0.11 |
| RANKL (N=6) | 5.59 ± 1.47a | 0.77 ± 0.08 |
| PGE2 (N=5) | 2.98 ± 0.45a | 0.92 ± 0.21 |
| LPS (N=3) | 46.23 ± 2.04a | 0.79 ± 0.27 |
| RANKL + PGE2 (N=6) | 7.52 ± 2.02a | 0.88 ± 0.12 |
| RANKL + LPS (N=3) | 38.78 ± 3.34a | 0.88 ± 0.31 |
The data are the means ± SEM values from N Cultures from 2 independent experiments.
Significantly different from control, P<0.05.
TABLE 2.
Changes in medium PGE2 levels after treatment with RANKL, PGE2, LPS, RANKL + PGE2 and RANKL + LPS. Cells were treated for 2h, washed with serum-free media and cultured in serum free media. Media were collected after 4 h. Arachadonic acid (AA) (10−5 M) was pulsed in for 15 min and media were again collected.
| Treatment | PGE2 conc. before adding AA (nM) | PGE2 conc. after adding AA (nM) |
|---|---|---|
| Control | 10.2 ± 7.2 | 91.7 ± 15.9 |
| RANKL | 9.1 ± 4.7 | 105.4 ± 16.1 |
| PGE2 | 11.6 ± 4.9 | 101.0 ± 12.2 |
| LPS | 996.7 ± 74.3a | 1667.9 ± 89.2a |
| RANKL + PGE2 | 23.3 ± 12.7 | 99.6 ± 12.1 |
| RANKL + LPS | 1206.5 ±58.8a | 2636.5 ± 810.0a |
The data are the means ± SEM from 4 independent cultures.
Significantly different from control, P<0.05.
Discussion
The present study confirms and extends previous results on the effects of RANKL, PGE2 and LPS on osteoclastogenesis in RAW 264.7 cells. Enhancement of RANKL induced osteoclastogenesis by PGE2 and LPS was quite similar, although the induction of COX-2 and the production of PGE2 was far greater in LPS treated cultures. Moreover NS-398, a selective COX-2 inhibitor, and indomethacin, an inhibitor of both COX-2 and COX-1, both reduced the responses to PGE2 and LPS to a similar extent. Prostaglandin synthesis inhibitors had only a small and variable effect on the response to RANKL alone. These results suggest that endogenous PGE2 production may play a role in osteoclastogenesis in this cell line. Previous studies have shown that prostaglandins can enhance osteoclast production in non-transformed hematopoietic precursors [2], but have not defined a role for endogenous prostaglandins. On the other hand, prostaglandin production by osteoblasts in clearly associated with an increase in the production of RANKL and increased osteoclast formation by co-cultured hematopoietic cells [13]. There was a substantial difference in medium PGE2 between control, RANKL or PGE2 cultures and LPS treated cultures yet PGE2 and LPS produced similar prostaglandin dependent increases in osteoclastogenesis. This suggests that the intracrine effects of prostaglandins may be important in this stimulation. Prostaglandin receptors are present on the nuclear as well as the cell membrane and the synthetic enzymes are located near the nuclear membrane as well [14,15,16,17].
There are few studies of the specific pathways by which prostaglandin directly enhances osteoclastic differentiation. Protein kinase A and phosphorylation of a transforming growth factor β activated kinase 1 (TAK-1) have been implicated [2]. Based on this it is likely that the effects in RAW 264.7 cells are mediated by activation of the EP2 or EP4 receptors that increase cAMP [2,18]. Both receptors have been identified in these cells, although in osteoclast precursors from the spleen the EP2 receptor seems to play the key role in the effect of prostaglandin to increase osteoclastogenesis [5]. Further studies on the specific receptor and signal transduction pathways need to be carried out in non-transformed osteoclast precursors.
Acknowledgments
Supported by NIH grants AR18063 (LR) and DK48361 (CP).
Footnotes
Presented in part at the 26th annual meeting of the American Society of Bone and Mineral Research at Seattle, WA, 2004.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ono K, Akatsu T, Kugai N, Pilbeam CC, Raisz LG. The effect of deletion of cyclooxygenase-2, prostaglandin receptor EP2, or EP4 in bone marrow cells on osteoclasts induced by mouse mammary cancer cell lines. Bone. 2003;33:798–804. doi: 10.1016/s8756-3282(03)00264-3. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi Y, Mizoguchi T, Take I, Kurihara S, Udagawa N, Takahashi N. Prostaglandin E2 enhances osteoclastic differentiation of precursor cells through protein kinase A-dependent phosphorylation of TAK1. J Biol Chem. 2005;280:11395–11403. doi: 10.1074/jbc.M411189200. [DOI] [PubMed] [Google Scholar]
- 3.Suda K, Woo JT, Takami M, Sexton PM, Nagai K. Lipopolysaccharide supports survival and fusion of preosteoclasts independent of TNF-alpha, IL-1, and RANKL. J Cell Physiol. 2002;190:101–108. doi: 10.1002/jcp.10041. [DOI] [PubMed] [Google Scholar]
- 4.Suda K, Udagawa N, Sato N, et al. Suppression of osteoprotegerin expression by prostaglandin E2 is crucially involved in lipopolysaccharide-induced osteoclast formation. J Immunol. 2004;172:2504–2510. doi: 10.4049/jimmunol.172.4.2504. [DOI] [PubMed] [Google Scholar]
- 5.Ono K, Kaneko H, Choudhary S, et al. Biphasic effect of prostaglandin E2 on osteoclast formation in spleen cell cultures: role of the EP2 receptor. J Bone Miner Res. 2005;20:23–29. doi: 10.1080/14041040510033842. [DOI] [PubMed] [Google Scholar]
- 6.Hinz B, Brune K, Pahl A. Prostaglandin E(2) upregulates cyclooxygenase-2 expression in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem Biophys Res Commun. 2000;272:744–748. doi: 10.1006/bbrc.2000.2859. [DOI] [PubMed] [Google Scholar]
- 7.Wadleigh DJ, Reddy ST, Kopp E, Ghosh S, Herschman HR. Transcriptional activation of the cyclooxygenase-2 gene in endotoxin-treated RAW 264.7 macrophages. J Biol Chem. 2000;275:6259–6266. doi: 10.1074/jbc.275.9.6259. [DOI] [PubMed] [Google Scholar]
- 8.Cok SJ, Acton SJ, Sexton AE, Morrison AR. Identification of RNA-binding proteins in RAW 264.7 cells that recognize a lipopolysaccharide-responsive element in the 3-untranslated region of the murine cyclooxygenase-2 mRNA. J Biol Chem. 2004;279:8196–8205. doi: 10.1074/jbc.M308475200. [DOI] [PubMed] [Google Scholar]
- 9.Han SY, Lee NK, Kim KH, et al. Transcriptional induction of cyclooxygenase-2 in osteoclast precursors is involved in RANKL-induced osteoclastogenesis. Blood. 2005;106:1240–1245. doi: 10.1182/blood-2004-12-4975. [DOI] [PubMed] [Google Scholar]
- 10.Shoji M, Tanabe N, Mitsui N, et al. Lipopolysaccharide stimulates the production of prostaglandin E2 and the receptor Ep4 in osteoblasts. Life Sci. 2006;78:2012–2018. doi: 10.1016/j.lfs.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Arakawa T, Laneuville O, Miller CA, et al. Prostanoid receptors of murine NIH 3T3 and RAW 264.7 cells. Structure and expression of the murine prostaglandin EP4 receptor gene. J Biol Chem. 1996;271:29569–29575. doi: 10.1074/jbc.271.47.29569. [DOI] [PubMed] [Google Scholar]
- 12.Pilbeam CC, Fall PM, Alander CB, Raisz LG. Differential effects of nonsteroidal anti-inflammatory drugs on constitutive and inducible prostaglandin G/H synthase in cultured bone cells. J Bone Miner Res. 1997;12:1198–1203. doi: 10.1359/jbmr.1997.12.8.1198. [DOI] [PubMed] [Google Scholar]
- 13.Okada Y, Lorenzo JA, Freeman AM, et al. Prostaglandin G/H synthase-2 is required for maximal formation of osteoclast-like cells in culture. J Clin Invest. 2000;105:823–832. doi: 10.1172/JCI8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spencer AG, Woods JW, Arakawa T, Singer II, Smith WL. Subcellular localization of prostaglandin endoperoxide H synthases-1 and -2 by immunoelectron microscopy. J Biol Chem. 1998;273:9886–9893. doi: 10.1074/jbc.273.16.9886. [DOI] [PubMed] [Google Scholar]
- 15.Bhattacharya M, Peri K, Ribeiro-da-Silva A, et al. Localization of functional prostaglandin E2 receptors EP3 and EP4 in the nuclear envelope. J Biol Chem. 1999;274:15719–15724. doi: 10.1074/jbc.274.22.15719. [DOI] [PubMed] [Google Scholar]
- 16.Gobeil F, Jr, Vazquez-Tello A, Marrache AM, et al. Nuclear prostaglandin signaling system: biogenesis and actions via heptahelical receptors. Can J Physiol Pharmacol. 2003;81:196–204. doi: 10.1139/y02-163. [DOI] [PubMed] [Google Scholar]
- 17.Helliwell RJ, Berry EB, O’Carroll SJ, Mitchell MD. Nuclear prostaglandin receptors: role in pregnancy and parturition? Prostaglandins Leukot Essent Fatty Acids. 2004;70:149–165. doi: 10.1016/j.plefa.2003.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Sakuma Y, Li Z, Pilbeam CC, et al. Stimulation of cAMP production and cyclooxygenase-2 by prostaglandin E(2) and selective prostaglandin receptor agonists in murine osteoblastic cells. Bone. 2004;34:827–834. doi: 10.1016/j.bone.2003.12.007. [DOI] [PubMed] [Google Scholar]