Abstract
IL-25 has been shown to induce Th2 responses and airway hyperreactivity (AHR) in mice, but the mechanism of action is not understood and it is unclear which cells mediate this disease. In this study we show that the receptor for IL-25, IL-17RB, is highly expressed on a subset of naive and activated CD4+ invariant NKT (iNKT) cells, but not on activated T cells. IL-17RB+ iNKT cells produced large amounts of Th2 cytokines that were substantially increased by IL-25 stimulation. Furthermore, IL-17RB+ iNKT cells were capable of restoring AHR in iNKT cell-deficient mice, whereas IL-17RB− iNKT cells failed to reconstitute AHR and lung inflammation. Finally, IL-17RB+ iNKT cells were detected in the lungs of wild-type mice, and induction of AHR by intranasal administration of IL-25 was significantly impaired in iNKT cell-deficient mice. Overall, our data suggest a critical role for iNKT cells in IL-25-mediated AHR. These results may lead to novel therapeutic approaches to target IL-17RB+ iNKT cells for the treatment of allergic asthma.
Asthma is a major public health problem that has increased dramatically in prevalence over the past two decades (1). Asthma is an immunological disease that is caused by Th2-type inflammation and is characterized by increased mucus production in the bronchioles and by airway hyperreactivity (AHR).3 Although conventional CD4+ T cells play a major role in asthma by recognizing exogenous Ags and initiating allergic inflammation in the lungs, we and others have shown that invariant NKT (iNKT) cells are important effector cells in asthma (2–4). Thus, iNKT cell-deficient mice failed to develop AHR and have substantially reduced eosinophilia after sensitization and challenge with allergen, although Th2 responses developed normally (2–4). IL-25 (also known as IL-17E), a member of the IL-17 family, has been implicated in Th2-polarized immune responses (5, 6). IL-25 has been reported to induce IL-4, IL-5, and IL-13 production from undefined non-T/non-B cells and has been shown to promote eosinophilia and to elevate AHR (5–7). In addition, overexpression of IL-25 or administration of rIL-25 was shown to result in allergic pathologies (8, 9). Conversely, mice deficient in IL-25 exhibited reduced Th2 cytokine production in the draining lymph nodes (10, 11), and inhibition of IL-25 in wild-type (WT) mice prevented inflammation and hyperreactivity of the airways (12). IL-25R, which is also called IL-17RB, IL-17Rh1, or Evi27, is a 56-kDa single transmembrane protein with homology to IL-17R (6, 13, 14). IL-17RB was first identified as a receptor for IL-17B but has subsequently been shown to exhibit a higher affinity for IL-25 than for IL-17B.
In this report, we show that IL-17RB is selectively expressed on a subset of CD4+ iNKT cells. Upon stimulation with α-galactosylceramide (α-GalCer), IL-17RB+CD4+ iNKT cells secreted high levels of Th2 cytokines, which were further enhanced by the addition of IL-25. Moreover, we found that the adoptive transfer of IL-17RB+CD4+ iNKT cells restores AHR in iNKT cell-deficient mice. Finally, induction of AHR by IL-25 required the presence of iNKT cells, as intranasal (i.n.) application of IL-25 induced AHR in WT BALB/c but not in iNKT-deficient mice.
Materials and Methods
Mice
Female BALB/cByJ mice (6–8 wk old) and Jα18−/− mice (backcrossed to BALB/c) were a gift from M. Taniguchi (Chiba University, Chiba, Japan). CD1d−/− (BALB/c) mice were a gift from M. Grusby (Harvard School of Public Health, Boston, MA). Pathogen-free female BALB/c mice (Federal Institute for Health Protection of Consumers and Veterinary Medicine (BgVV), Berlin, Germany, and The Jackson Laboratory), 6–8 wk of age, were maintained under institutional approved guidelines (Charité University Medicine, Berlin, Germany, and Keck School of Medicine, University of Southern California, Los Angeles, CA).
In vivo treatments with IL-25
Mice were lightly anesthetized with isoflurane. Murine IL-25 (0.75 µg; R&D Systems) or saline was applied i.n. on three consecutive days. Six days after the last i.n. treatment, mice were analyzed for AHR and lung inflammation by histological staining.
Flow cytometry analysis
Cells were preincubated with anti-Fc blocking mAb (2.4G2) as well as normal rat serum and washed before staining. iNKT cells were identified using various Ab combinations that included PE-conjugated CD1d:PBS-57 loaded tetramer (National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) MHC Tetramer Core Facility, Atlanta GA), TCRb-PECy5 or allophycocyanin (clone H57–597, eBioscience, San Diego, California) and CD4 allophycocyanin or allophycocyanin-Alexa Fluor 750 (clone RM4-5) (eBioscience). Monoclonal IL-17RB Ab was purchased from Lifespan Biosciences. Cells were acquired on a FACSCanto flow cytometer (BD Biosciences). The data were analyzed with FlowJo software (Tree Star).
Purification of iNKT cells
For isolation of splenic iNKT cells, cells were labeled with a PE-conjugated CD1d tetramer followed by anti-PE microbeads and then sorted by autoMACS according to the manufacturer’s instruction (Miltenyi Biotec). Purity of the iNKT cells was >90%. For isolation of CD4+ and double negative (DN) iNKT cell subsets, cells were purified from spleens using FITC-PBS-57 (α-GalCer analog)-loaded CD1d tetramers (NIAID, NIH MHC Tetramer Core Facility) and using MACS anti-FITC MultiSort kit (Miltenyi Biotec). Anti-FITC microbeads bound to tetramer-positive cells were detached using a MACS MultiSort release reagent and a MACS MultiSort stop reagent according to the manufacturer’s instructions (Miltenyi Biotec). The resultant tetramer-positive cells were further enriched for CD4+ cells by positive selection on a MACS column after incubating with anti-CD4 microbeads. For detection of iNKT cells in lung, single cell suspensions from lung parenchyma were prepared as follows. Lungs were flushed in situ with 20 ml of cold PBS via cannulation of the heart to remove the intravascular blood pool. Minced lung tissue was incubated for 90 min at 37°C on a rocker in DMEM supplemented with 10% FCS, 50 U/ml DNase I (Boehringer Mannheim), and 250 U/ml collagenase I, type 4197 (Worthington). Passing the digested lung tissue through a nylon mesh and subsequent discontinuous Percoll gradient (Pharmacia) centrifugation (35–55%) yielded purified live lung parenchymal cells. For purification of IL-17RB+ and IL-17RB− NKT cells, cells were purified from spleens as described above using FITC-conjugated IL-17RB Ab. All preparations, cellular isolations, and staining were performed on ice. In some experiments, selected cells were further enriched using a MoFlo high-speed cell sorter (DakoCytomation). Tetramer-positive CD4+ and CD4− NKT cell preparations were confirmed to contain an average of >91 and >93% pure populations, respectively.
In vitro culture of NKT cells
CD4+ and DN NKT cells were magnetically enriched from spleens of BALB/c mice as described above. iNKT cells (25 × 103) were cultured in round-bottom, 96-well plates with 1 × 103 bone marrow-derived dendritic cells (BMDCs) from BALB/c mice prepared as previously described (15, 16) with some modifications (17, 18). As indicated, in some wells IL-17RB+ or IL-17RB− iNKT cells were treated with 20 µg/ml IL-25. Supernatants were collected after 48 h and measured for cumulative levels of cytokines by ELISA.
Real-time PCR for IL-17RB
For analysis of IL-17RB expression, total RNA was prepared from CD4+ and DN iNKT cells, lung iNKT cells, or BMDCs by TRIzol, and cDNA was generated. Real-time RT-PCR was performed as described before (13, 19).
Adoptive transfers
Total, IL-17RB+, or IL-17RB− iNKT cells (2 × 106) were isolated as described above and adoptively transferred into Jα18−/− mice that were sensitized to OVA (50 µg of OVA in alum administered i.p. 1 wk previously) and recipients were challenged with three consecutive doses of OVA i.n. (50 µg).
Cytokine ELISA
Cytokine secretion following in vitro challenge was determined by ELISA as previously described (2). The mAb pairs used were as follows (capture-detection): IFN-γ, R4–6A2-XMG1.2; IL-4, BVD4–BVD6; and IL-5, TRFK-5-TRFK-4. Reagents for mouse IL-13 were purchased from R&D Systems.
Induction of AHR and measurement of airway responsiveness
To induce AHR, mice were sensitized with 100 µg of OVA Ag (ICN Biomedical) in alum administered i.p. After 8 days, mice were exposed to i.n. OVA (50 µg) or PBS on three consecutive days. AHR responses were assessed by methacholine-induced airflow obstruction in conscious mice placed in a whole body plethysmograph (Buxco Electronics) as described previously (2, 20). In some experiments we assessed AHR by invasive measurement of airway resistance in which anesthetized and tracheostomized mice were mechanically ventilated using a modified version of a described method (21). Aerosolized methacholine was administered in increasing concentrations, and we continuously computed lung resistance and dynamic compliance by fitting flow, volume, and pressure to an equation of motion.
Bronchoalveolar lavage (BAL) fluid
Following measurement of AHR and euthanasia, the lungs were lavaged twice with 1 ml of PBS and 2% FCS and the fluid was pooled as described previously (2). Differential leukocyte counts were determined from slide preparations of BAL fluid stained with H&E.
Statistical analysis
The statistical significance of differences between indicated groups was determined by Student’s t test. Any difference with a value of p < 0.01 was considered significant (*, p < 0.01; **, p < 0.001).
Results
The IL-25 receptor IL-17RB is preferentially expressed on CD4+ iNKT cells
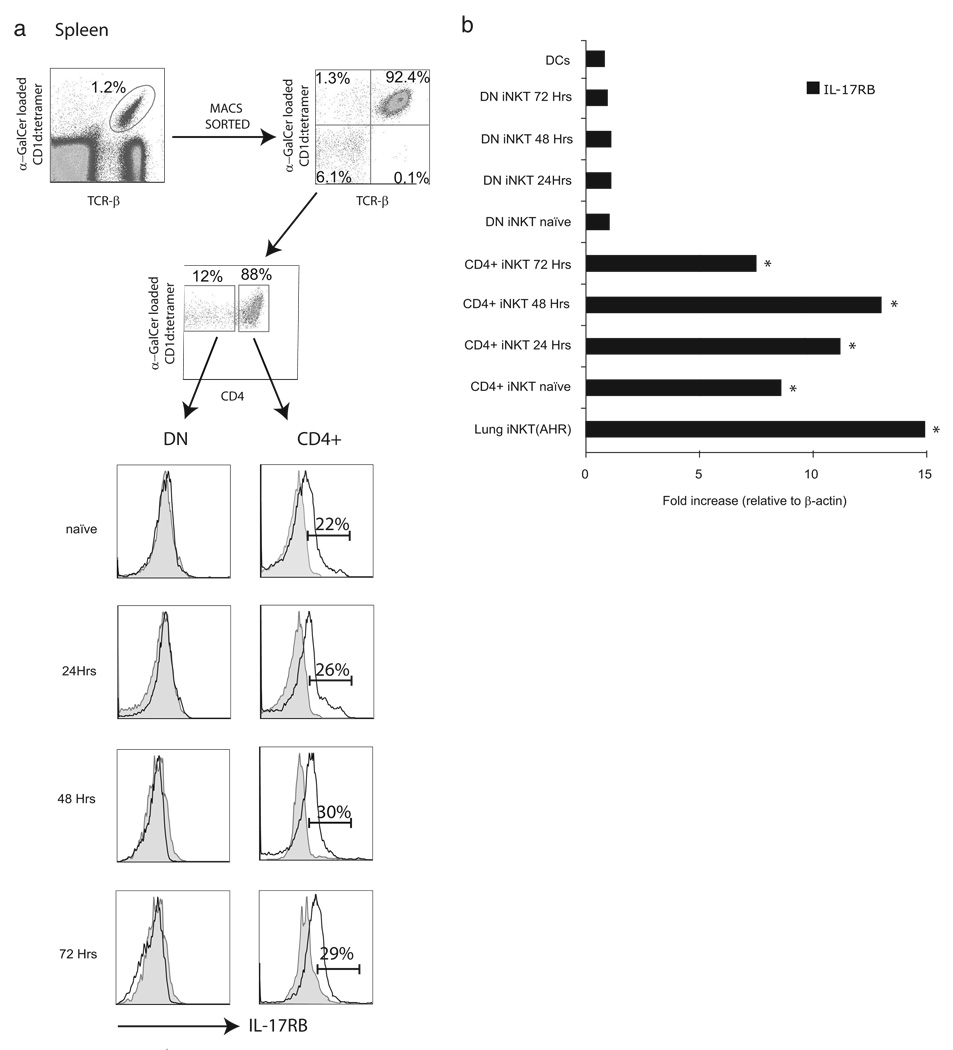
To define the expression levels of IL-17RB on iNKT cells, we isolated CD4+ and DN iNKT cells from the spleens of naive BALB/c mice as described in Material and Methods. Positively selected iNKT cells were activated in vitro by culturing them with BMDCs loaded with α-GalCer for various time points as indicated in Fig. 1a. Surface expression of IL-17RB on activated CD4+ and DN iNKT cells was assessed by flow cytometry and compared with isotype control at each time point. We found that IL-17RB was selectively expressed on the CD4+ subset of naive iNKT cells and the levels increased upon activation, showing peak expression after 48 h of culture. In contrast, neither naive nor activated DN iNKT cells expressed significant amounts of IL-17RB as compared with isotype controls.
FIGURE 1.
A subset of CD4+ iNKT cell expresses IL-17RB. iNKT cells were positively selected with a CD1d tetramer loaded with α-GalCer as described in Materials and Methods. iNKT cell subsets (25 × 103) were cultured with 1 × 103 BMDCs as described previously (24). a, Cells were stained with IL-17RB mAb and analyzed by flow cytometry gated on enriched CD4+ or DN iNKT cells. IL-17RB+ cells were quantified and the percentages of IL-17RB+CD4+ iNKT cells are indicated in the histograms. b, CD4+ and DN iNKT cells were enriched from the spleens of BALB/c mice as described in Materials and Methods. CD4+ or DN iNKT cells (25 × 103) were cultured with 103 BMDCs. Cells were collected at various time points after cell culture and analyzed for the expression of IL-17RB by quantitative real-time PCR. Also, 25 × 103 positively selected iNKT cells from the lungs of BALB/c mice with AHR were cultured with 103 BMDCs for 48 h and analyzed for the expression of IL-17RB. As a negative control, 1 × 106 BMDCs were also cultured for 48 h and analyzed for IL-17RB expression. Data are representative of three separate experiments. The IL-17RB expression levels in CD4+ and lung iNKT cells were compared with those in the appropriate DN iNKT cells *, p < 0.01.
To examine the expression of IL-17RB mRNA on the afore-mentioned iNKT cell subsets, cells were positively selected and activated as described above and gene expression levels were characterized by RT-PCR (Fig. 1b). We found that IL-17RB mRNA was substantially and exclusively expressed on CD4+ iNKT cells, and expression levels were induced upon activation with α-GalCer. We also found no expression of IL-17RB on the dendritic cells added to our culture. In concordance with our data regarding IL-17RB expression on the surface of iNKT cells, DN iNKT cells contained no significant levels of IL-17RB mRNA in comparison to CD4+ iNKT cells. In contrast, CD4+ T cells did not express IL-17RB at resting or activated conditions (data not shown).
Furthermore, we analyzed the number of iNKT cell subsets expressing IL-17RB obtained from total splenocytes of BALB/c mice (Table I). We found that ~25% of CD4+ iNKT cells from the spleens of naive mice express IL-17RB (974 ± 136 total CD4+ iNKT cells vs 235 ± 23 IL-17RB CD4+ iNKT cells). By contrast, expression of IL-17RB on DN iNKT cells was comparable to that on isotype controls (< 2%). The number of IL-17RB+ cells gradually increased upon activation and peaked after 72 h to ~30% of total CD4+ iNKT cells (1121 ± 141 total CD4+ iNKT cells vs 329 ± 29 IL-17RB CD4+ iNKT cells). Together, these data indicate that CD4+ iNKT cells express significant levels of IL-17RB and thus have the potential to respond to IL-25.
Table I.
Absolute number of IL-17RB+ iNKT cells per spleen (× 1000)a
| 0 h | 24 h | 48 h | 72 h | |
|---|---|---|---|---|
| Total CD4+iNKT | 974 ± 136 | 1018 ± 147 | 1214 ± 129 | 1121 ± 141 |
| Total DN iNKT | 251 ± 49 | 311 ± 61 | 363 ± 78 | 411 ± 112 |
| CD4+ iNKT IL-17RB+ | 235 ± 23 | 265 ± 34 | 359 ± 46 | 329 ± 29 |
| DN iNKT IL-17RB+ | 5.2 ± 0.32 | 7.1 ± 0.52 | 8.7 ± 0.64 | 7.3 ± 0.33 |
Mean absolute numbers of iNKT cell subsets isolated from spleens of BALB/c mice were quantified and analyzed for the expression of IL-17RB by flow cytometry. Cells were activated in vitro by culturing them with BMDCs loaded with α-GalCer for various time points as indicated. Data are mean of three separate experiments (n = 5) and are shown as mean ± SD.
IL-25 promotes the secretion of Th2 cytokines on IL-17RB+ iNKT cells
To analyze the effects of IL-25 on iNKT cells, we magnetically sorted IL-17RB+ and IL-17RB− iNKT cells from the spleens of naive BALB/c mice. The cells were stimulated with α-GalCer-loaded dendritic cells in the presence of either IL-25 or isotype controls in vitro. As demonstrated in (Fig. 2), IL-17RB+ iNKT cells secreted significantly higher amounts of IL-4, IL-5, and IL-13 and lower amounts of IFN-γ when compared with IL-17RB− iNKT cells. The addition of IL-25 to α-GalCer stimulation of iNKT cells further enhanced the production of Th2 cytokines, pre-dominantly in the IL-17RB+ iNKT cell subset. These data indicate that subsets of iNKT cells may respond to IL-25 to rapidly secrete Th2 cytokines.
FIGURE 2.
IL-17RB+ iNKT cells preferentially produce Th2 cytokines. IL-17RB+ or IL-17RB− iNKT cells were sorted as described in Materials and Methods. Cells were incubated with α-GalCer-loaded BMDCs in the presence or absence of IL-25 (50 ng/ml). Supernatants were collected after 48 h and cytokines were measured by ELISA. ELISA data are representative of three separate experiments and are shown as mean ± SD for triplicate samples. The cytokine levels from IL-25-treated IL-17RB+ cells were compared with IL-25-treated IL-17RB− cells; *, p < 0.01.
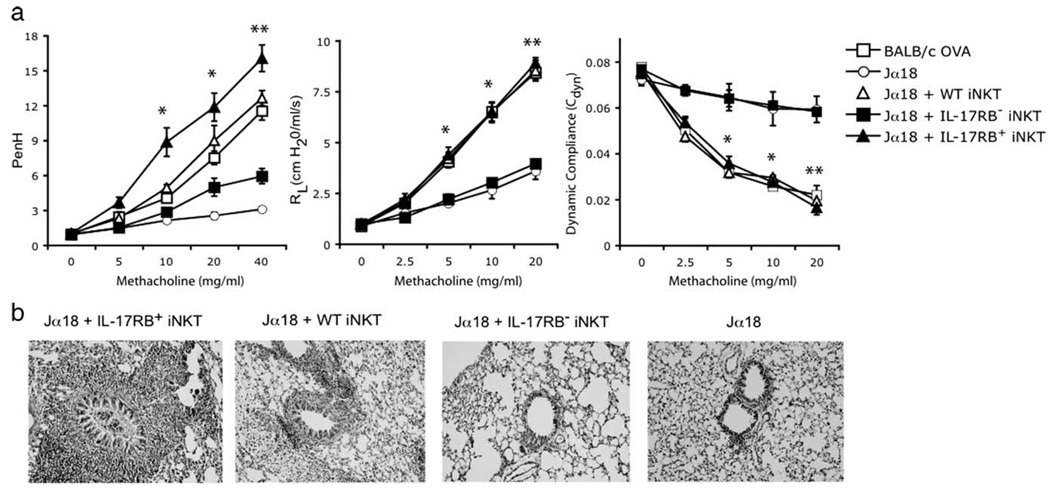
IL-25 and IL-17RB+ iNKT induce AHR in iNKT cell-deficient mice
iNKT cell deficient Jα18−/− mice do not develop AHR. Due to the fact that the expression of CD1d is unaffected in these mice, they were preferentially used for iNKT cell adoptive transfer studies. We and others have previously been able to demonstrate that the adoptive transfer of iNKT cells from WT mice restores the development of AHR in Jα18−/− mice (2–4). To determine whether the expression of IL-17RB on iNKT cells is required for the induction of AHR, IL-17RB+ and IL-17RB− iNKT cells were purified from the spleens of BALB/c mice and adoptively transferred into OVA-sensitized Jα18−/− mice, which were then challenged with OVA to induce AHR. Only the IL-17RB+ and not the IL-17RB− iNKT cells from WT BALB/c mice restored AHR in iNKT cell-deficient mice, as measured by airway resistance, dynamic compliance, and enhanced pause (Penh) (Fig. 3a). Moreover, examination of the lungs of iNKT cell-deficient mice that received IL-17RB+ iNKT cells from WT mice showed a significantly increased inflammation of the airways in comparison with recipients of IL-17RB− iNKT cells (Fig. 3b). In addition, the pulmonary inflammation of the recipients of IL-17RB+ iNKT cells was more severe than that of recipients of total iNKT cells. This may be explained by the fact that only 25% of total iNKT cells are positive for IL-17RB, whereas recipients of IL-17RB+ cells received enriched IL-17RB+ iNKT cells (purity > 80%; data not shown).
FIGURE 3.
IL-17RB+ iNKT cells restore airway hyperreactivity in Jα18−/− mice. a, IL-17RB+ or IL-17RB− iNKT cells were positively selected from the spleens of BALB/c mice and adoptively transferred (2 × 106 cells) into OVA-sensitized Jα18−/− mice that were then challenged with OVA (50 µg) i.n. on three consecutive days and assessed for AHR by measuring Penh (left panel), lung resistance (RL; middle panel), and dynamic compliance (Cdyn; right panel). As controls, nonrecipient WT BALB/c or Jα18−/− mice were sensitized (i.p.) and challenged (i.n.) with OVA. Data are the mean ± SD and are representative of three experiments (n = 5) with the following p values: *, p < 0.01; and **, p < 0.001 (recipients of IL-17RB+ vs recipients of IL-17RB− iNKT cells). b, Lung histopathology of Jα18−/− recipient mice from a. Lung tissue from an OVA-challenged Jα18−/− mouse shows normal airway and surrounding parenchyma (far right panel). Lung tissue from an OVA-treated Jα18−/− mouse that received 2 × 106 wild-type iNKT cells shows inflammatory cells surrounding the airways (second panel from left). Lung parenchyma of an OVA-sensitized Jα18−/− mouse that received 2 × 106 iNKT cells from IL-17RB−/− mice shows minimal mucus production and negligible cellular infiltration (third panel from left). However, lung tissue from OVA-treated Jα18−/− mice that received 2×106 IL-17RB+ iNKT cells, shows a significantly higher infiltration of inflammatory cells and mucus production (far left panel). All stainings are H&E (original magnification, ×200).
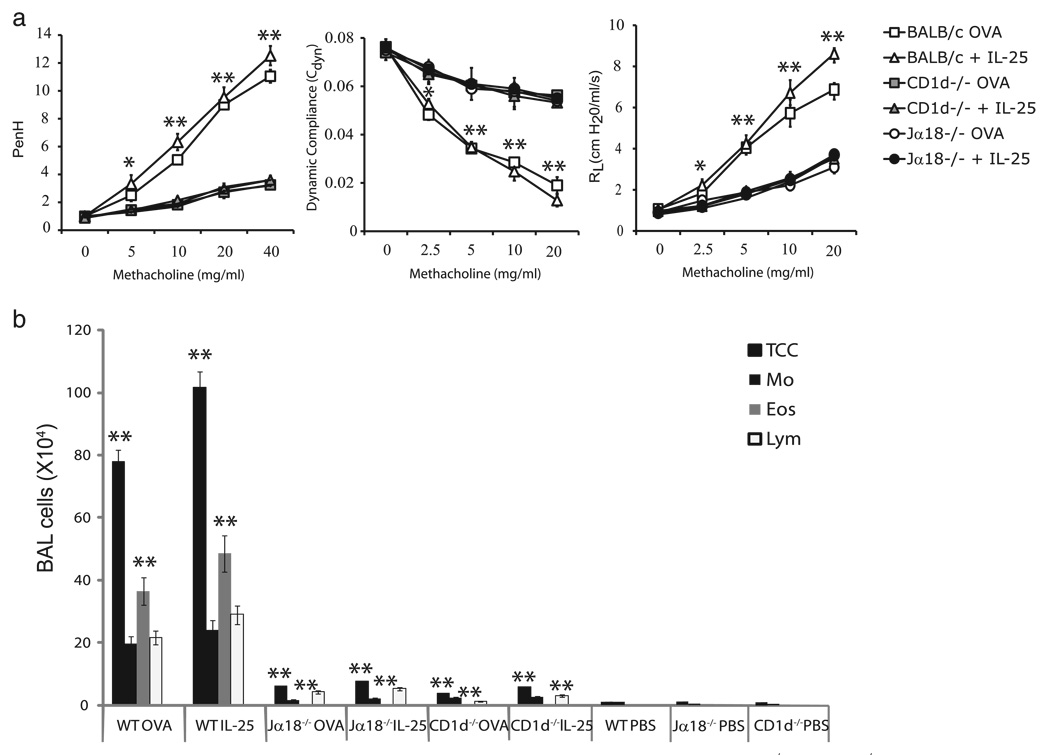
To gain a better understanding of the biological influence of IL-25 on iNKT cells in vivo, we administered IL-25 i.n. to naive WT BALB/c mice or both iNKT cell-deficient mice and Jα18−/− and CD1d−/− mice. As controls, WT BALB/c or CD1d−/− mice were systemically immunized (i.p.) and locally challenged (i.n.) with OVA. In all groups, AHR was measured by airway resistance, dynamic compliance, or Penh (Fig. 4a). Both Jα18−/− and CD1d−/− mice immunized and challenged with OVA did not develop AHR when compared with BALB/c controls, confirming previous results (2–4). Intranasal administration of IL-25, while inducing severe hyperreactivity of the airways in BALB/c mice, was unable to induce AHR in both iNKT cell-deficient mice and CD1d−/− and Jα18−/− mice. The defect in the CD1d−/− and Jα18−/− mice treated with IL-25 was associated with a substantial reduction in airway eosinophilia, as indicated by a reduction of eosinophils in BAL fluid as compared with BAL fluid from BALB/c mice sensitized and challenged with OVA or treated with IL-25 (Fig. 4b). No significant recruitment of T cell subsets (including iNKT cells) to the BAL fluid was observed in PBS-treated BALB/c, Jα18−/−, or CD1d−/− mice. Overall, these findings confirm that IL-25 may indeed function primarily via iNKT cells to induce Th2-polarized immune responses and AHR. In the absence of cells expressing the IL-25 receptor (IL-17RB+CD4+ iNKT cells), IL-25 had no effect and was unable to induce allergic airway disease in mice.
FIGURE 4.
iNKT cell-deficient mice are resistant to the induction of AHR by IL-25. a, Naive BALB/c, Jα18−/−, or CD1d−/− mice were treated with three consecutive doses of IL-25 (0.75 µg per dose) i.n. (day s1, 2, and 3). AHR was assessed by measuring Penh (left panel), lung resistance (RL; middle panel), and dynamic compliance (Cdyn; right panel) on day 4. As controls, mice were sensitized (i.p.) and challenged (i.n.) with OVA. Data are the mean ± SD and are representative of three experiments (n = 4) with the following p values: *, p < 0.01; and **, p < 0.001 (BALB/c plus IL-25 vs Jα18−/− plus IL-25 or CD1d−/− plus IL-25). b, BAL fluid from the mice in a was analyzed 24 h after AHR measurement. Results are shown as the number of cells per milliliter in BAL fluid. Eos, eosinophils; Lym, lymphocytes; Mo, monocytes/macrophages; TCC, total cell count. The total cell number and the eosinophils from the BAL of WT mice treated with OVA or IL-25 were compared with Jα18−/− or CD1d−/− mice treated with OVA or IL-25; *, p < 0.01; and **, p < 0.001.
Lung iNKT cells and the expression of IL-17RB
As previously demonstrated, induction of AHR in WT BALB/c mice with OVA caused activation and recruitment of iNKT cells to the lungs. These iNKT cells were CD4+ and secreted predominantly Th2 cytokines such as IL-4 and IL-13. To test whether iNKT cells infiltrating the lungs in this model express IL-17RB, we induced AHR in WT mice with either OVA or rIL-25. Subsequently, lungs were digested and analyzed by flow cytometry to assess the expression level of IL-17RB on iNKT cells. As shown in Fig. 5, ~12% of TCRβ+ cells stained positive for α-GalCer-loaded CD1d tetramer in the lungs of mice that had been immunized and challenged with OVA and thus resemble iNKT cells. IL-25 was almost twice as effective as OVA in recruiting iNKT cells to the lungs of mice (21.6% of TCRβ+ cells). Likewise, we found that 48% of iNKT cells in the lungs of IL-25 treated mice expressed IL-17RB. In contrast, only 23% of iNKT cells in the lungs of OVA-treated mice expressed the IL-25 receptor IL-17RB. These data indicate that iNKT cells respond to IL-25 and that IL-17RB-positive iNKT cells are recruited to the lungs in significant numbers upon activation with IL-25.
FIGURE 5.
Pulmonary iNKT cells express high levels of IL-17RB. Lymphoid cells purified from the lungs of BALB/c mice that had been immunized and challenged with OVA or IL-25 to induce AHR were stained with α-GalCer-loaded, PE-conjugated CD1d tetramers and anti-TCR Vβ after gating on total CD45+ cells. IL-17RB expression was analyzed by gating on a CD1d tetramer and TCR Vβ-positive iNKT cells. IL-17RB+ cells were quantified and the percentages of IL-17RB+ iNKT cells are indicated in the histograms. Filled histograms represent the isotype control.
Discussion
The IL-17 cytokine family consists of several isoforms (IL-17A to IL-17F). Five members of the IL-17R family act as receptors for the IL-17 isoforms (IL-17RA to IL-17RE) (22). Although IL-17RA is essential for most biologic activities of IL-17 and is expressed ubiquitously, IL-17RB has been reported to be specifically required for IL-25 activity, even though a recent report suggests that IL-25 requires both IL-17RB and IL-17RA (23).
We and others have already reported that iNKT cells are essential for the induction of allergic diseases like asthma (2–4); however, it was not clear whether particular subsets of iNKT cells are specifically required for the induction of AHR. We recently demonstrated that only the CD4+ and not the DN iNKT cells from WT BALB/c mice restored AHR in Jα18−/− recipients (24) and that CD4+ iNKT cells constitutively express ICOS. We have shown that CD4+ iNKT cells predominantly secrete Th2 cytokines when compared with DN iNKT cells. In this report, we show that CD4+ iNKT cells can be further differentiated regarding their expression of the IL-25 receptor IL-17RB (i.e., IL-17RB+ or IL-17RB− subsets). In the current study, we show that only IL-17RB+CD4+ iNKT cells are responsible for the induction of AHR in mice.
Several groups have already demonstrated that IL-25 promotes Th2 responses (5–7, 19, 25), and IL-25 was found to be crucial for the induction of AHR in mice (12, 26). However, it was unclear which cell type mediates these effects. Our findings that the IL-25 receptor IL-17RB is expressed on a subset of CD4+ iNKT cells and that only IL-17RB+CD4+ iNKT cells induce allergic airway disease in mice sheds new light onto those previous findings and suggest that IL-25 leads to allergic airway disease in mice by activating a subset of CD4+ iNKT cells.
Furthermore, we show that IL-25 increases the release of Th2 cytokine by IL-17RB+CD4+ iNKT cells in vitro (Fig. 2). As stated above, several studies have demonstrated that IL-25 promotes a Th2 polarization of the immune response (5, 6). It was also reported that IL-25-responsive cells aid in the further development of CD4+ Th2 cells that are fundamental for the development of asthma. In confirmation of those observations, a recent study showed that IL-25 is expressed by lung epithelial cells of mice as a result of innate immune responses to allergens (19). Furthermore, levels of IL-25 were increased in the lung and gut during pathogen infections and in the BAL fluids after the induction of AHR in mice (6, 7). In patients with asthma, IL-25 was also detected in significant amounts in lung samples (27). The fact that only the IL-25-responsive subset of iNKT cells (IL-17RB+ CD4+ iNKT) was capable of restoring allergic airway disease in iNKT cell-deficient mice confirms previous findings and strengthens the hypothesis that responsiveness to IL-25 is a key mechanism by which iNKT cells may induce Th2-polarized allergic diseases like asthma.
Our data also suggest that lung iNKT cells express a significant level of IL-17RB after the induction of AHR by OVA. The level of IL-17RB expression was increased even further after i.n. administration and induction of AHR by IL-25. These observations imply that local CD4+ iNKT cells in the lungs are the main targets for IL-25. The increase in the expression of IL-17RB after the administration of IL-25 indicates that CD4+ iNKT cells play an important role in the biologic effects of IL-25, potentially by using local IL-25 in lungs to secrete high levels of Th2 cytokines. Our data are in concordance with a recently published study indicating that IL-25 may act via a subset of iNKT cells to induce allergic airway disease in mice (28). These authors have elegantly shown that iNKT cell-deficient mice failed to develop IL-25-dependent AHR and that expression of IL-17RB on iNKT cells was essential for the induction of IL-25-induced inflammation and hyperreactivity of airways.
Undoubtedly, Th2 cytokines such as IL-13 can directly induce AHR (2, 29). In this line, IL-5 secreted by iNKT cells may be responsible for the infiltration and recruitment of eosinophils to the lungs of mice treated with IL-25, as we observed a substantial reduction in airway eosinophilia in the lungs of iNKT-deficient mice after treatment with IL-25 (Fig. 4b). Thus, we believe that the combination of IL-4, IL-5, and IL-13 from lung iNKT cells provides a microenvironment in the lungs that conditions local T cells for further Th2 polarization.
In conclusion, we have identified a subset of CD4+ iNKT cells that expresses IL-17RB and thus responds to IL-25. Our data suggest that IL-25 induces proallergic immune responses in mice by selectively activating IL-17RB+CD4+ iNKT cells to secrete Th2 cytokines. Therefore, IL-25 and IL-17RB+CD4+ iNKT cells appear to mediate allergic diseases and may provide novel therapeutic targets for Th2-driven immune pathologies like asthma.
Footnotes
This work was supported by National Institutes of Health Grant R01 AI066020 (to O.A.) and by German Research Foundation (DFG) Grant STO 467/4-2 (to P.S.).
Abbreviations used in this paper: AHR, airway hyperreactivity; BAL, bronchoalveolar lavage; BMDC, bone marrow-derived dendritic cell; DN, double negative; α-GalCer, α-galactosylceramide; i.n., intranasal(ly); iNKT, invariant NKT; Penh, enhanced pause; WT, wild type.
Disclosures
We have no financial conflict of interest.
References
- 1.Centers for Disease Control and Prevention. Forecasted state-specific estimates of self-reported asthma prevalence - - United States, 1998. Morbidity and Mortality Weekly Report. 1998;47:1022–1025. [PubMed] [Google Scholar]
- 2.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat. Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 3.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo L, Hachem P, Fourneau J, Sidobre S, Kronenberg M, Taniguchi M, et al. Cutting edge: invariant Vα14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J. Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 4.Fang L, Adkins B, Deyev V, Podack ER. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J. Exp. Med. 2008;205:1037–1048. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 6.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J. Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 7.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, Suto A, Suzuki K, Watanabe N, Saito Y, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J. Allergy Clin. Immunol. 2006;118:606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 8.Pan G, French D, Mao W, Maruoka M, Risser P, Lee J, Foster J, Aggarwal S, Nicholes K, Guillet S, et al. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multi-organ inflammation in mice. J. Immunol. 2001;167:6559–6567. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 9.Kim MR, Manoukian R, Yeh R, Silbiger SM, Danilenko DM, Scully S, Sun J, DeRose ML, Stolina M, Chang D, et al. Transgenic overexpression of human IL-17E results in eosinophilia, B-lymphocyte hyperplasia, and altered antibody production. Blood. 2002;100:2330–2340. doi: 10.1182/blood-2002-01-0012. [DOI] [PubMed] [Google Scholar]
- 10.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, McIlgorm A, Jolin HE, McKenzie AN. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 13.Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, Goddard AD, Yansura DG, Vandlen RL, Wood WI, Gurney AL. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J. Biol. Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 14.Tian E, Sawyer JR, Largaespada DA, Jenkins NA, Copeland NG, Shaughnessy JD., Jr Evi27 encodes a novel membrane protein with homology to the IL17 receptor. Oncogene. 2000;19:2098–2109. doi: 10.1038/sj.onc.1203577. [DOI] [PubMed] [Google Scholar]
- 15.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheicher C, Mehlig M, Zecher R, Reske K. Dendritic cells from mouse bone marrow: in vitro differentiation using low doses of recombinant granulocyte-macrophage colony-stimulating factor. J. Immunol. Methods. 1992;154:253–264. doi: 10.1016/0022-1759(92)90199-4. [DOI] [PubMed] [Google Scholar]
- 17.Stockinger B, Hausmann B. Functional recognition of in vivo processed self antigen. Int. Immunol. 1994;6:247–254. doi: 10.1093/intimm/6.2.247. [DOI] [PubMed] [Google Scholar]
- 18.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells secreting IL-10 mediate T cell tolerance induced by respiratory exposure to antigen. Nat. Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 19.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J. Clin. Invest. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J. Appl. Physiol. 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 22.Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang SK. IL-17 cytokine family. J. Allergy Clin. Immunol. 2004;114:1265–1273. doi: 10.1016/j.jaci.2004.10.019. quiz 1274. [DOI] [PubMed] [Google Scholar]
- 23.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, Anders PM, Tocker JE, Comeau MR, Budelsky AL. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J. Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 24.Akbari O, Stock P, Meyer EH, Freeman GJ, Sharpe AH, Umetsu DT, DeKruyff RH. ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival. J. Immunol. 2008;180:5448–5456. doi: 10.4049/jimmunol.180.8.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, Hippe A, Corrigan CJ, Dong C, Homey B, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharkhuu T, Matthaei KI, Forbes E, Mahalingam S, Hogan SP, Hansbro PM, Foster PS. Mechanism of interleukin-25 (IL-17E)-induced pulmonary inflammation and airways hyper-reactivity. Clin. Exp. Allergy. 2006;36:1575–1583. doi: 10.1111/j.1365-2222.2006.02595.x. [DOI] [PubMed] [Google Scholar]
- 27.Letuve S, Lajoie-Kadoch S, Audusseau S, Rothenberg ME, Fiset PO, Ludwig MS, Hamid Q. IL-17E upregulates the expression of proinflammatory cytokines in lung fibroblasts. J. Allergy Clin. Immunol. 2006;117:590–596. doi: 10.1016/j.jaci.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 28.Terashima A, Watarai H, Inoue S, Sekine E, Nakagawa R, Hase K, Iwamura C, Nakajima H, Nakayama T, Taniguchi M. A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J. Exp. Med. 2008;205:2727–2733. doi: 10.1084/jem.20080698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]