Abstract
The importance of H2O2 as a cellular signaling molecule has been demonstrated in a number of cell types and pathways. Here we explore a positive feedback mechanism of H2O2-mediated regulation of the phagocyte respiratory burst NADPH oxidase (NOX2). H2O2 induced a dose-dependent stimulation of superoxide production in human neutrophils, as well as in K562 leukemia cells over-expressing NOX2 system components. Stimulation was abrogated by addition of catalase, the extracellular Ca2+ chelator BAPTA, the T-type Ca2+ channel inhibitor mibefradil, the PKCδ inhibitor rottlerin, or the c-Abl non-receptor tyrosine kinase inhibitor imatinib mesylate, or by over-expression of a dominant-negative form of c-Abl. H2O2 induced phosphorylation of tyrosine311 on PKCδ and this activating phosphorylation was blocked by treatment with rottlerin, imatinib mesylate, or BAPTA. Rac GTPase activation in response to H2O2 was abrogated by BAPTA, imatinib mesylate, or rottlerin. In conclusion, H2O2 stimulates NOX2-mediated superoxide generation in neutrophils and K562/NOX2 cells via a signaling pathway involving Ca2+ influx and c-Abl tyrosine kinase acting upstream of PKCδ. This positive feedback regulatory pathway has important implications for amplifying the innate immune response and contributing to oxidative stress in inflammatory disorders.
Keywords: NADPH oxidase; NOX2; Neutrophil; Superoxide; Hydrogen peroxide; Signaling; c-Abl, Calcium; Free radicals
Introduction
Much of our understanding of the mechanisms of generation and functions of reactive oxygen species (ROS) is based on studies of peripheral blood neutrophils, wherein microbicidal oxidants released during the phagocytosis of microorganisms serve as a first line of host defense. In phagocytic cells, the major biochemical pathway for ROS formation proceeds through superoxide anion, which is generated through the single-electron reduction of molecular oxygen by an NADPH oxidase complex. Phagocyte NADPH oxidase is composed of the membrane-bound flavocytochrome b558 (a heterodimer of NOX2/gp91phox and p22phox), several modular cytosolic regulators (p47phox, p67phox, p40phox), and a small GTPase, Rac1 or Rac2 [1, 2]. The stimulus-dependent assembly of an active oxidase is tightly regulated, requiring translocation of the cytosolic components and their physical association with the membrane-bound flavocytochrome. Following cell stimulation by phagocytosis or soluble mediators, p47phox undergoes extensive serine phosphorylation by a number of kinases and recruits p67phox to the nascent membrane-associated complex. Rac, activated by GDP-GTP exchange, associates with the forming complex where, together with p67phox, it regulates electron flow through the NOX2 catalytic subunit [3, 4].
We recently demonstrated that NOX5, a Ca2+-regulated NOX isoform, is activated by H2O2 through a novel signaling pathway requiring Ca2+ influx and c-Abl tyrosine kinase activation [5]. Whereas NOX5 activation ordinarily requires the direct binding of Ca2+ to the EF-hand domains present in its cytosolic N-terminal extension [6], the assembly of NOX2 features translocation of the cytosolic factors to the membrane-bound flavocytochrome, a process that involves Ca2+ only in proximal signaling events, rather than for direct binding to NOX [7, 8]. In the present work, we investigated the hypothesis that NOX2, the index member of the NOX family, is regulated by H2O2 and explored the signaling mechanisms involved. In human blood neutrophils, as well as in K562 human leukemia cells over-expressing the NOX2 system, we found that H2O2 stimulates NOX2-catalyzed superoxide generation and that this regulation involves Ca2+ and c-Abl tyrosine kinase pathways upstream of the classical protein kinase C (PKC) pathway. Furthermore, H2O2 enhances and accelerates the oxidative burst in response to phorbol myristate acetate (PMA).
Experimental procedures
Isolation of neutrophils
Human neutrophils >90% pure were isolated from heparinized venous blood by dextran sedimentation, centrifugation on Hypaque-Ficoll, and hypotonic lysis of erythrocytes as described previously [9].
Expression of the NOX2 NADPH oxidase system in K562 cells
The clonal K562 human leukemia cell line stably expressing human NOX2 (gp91phox) has been described previously [10]. The cells were maintained in complete RPMI medium (RPMI-1640 supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin). For the expression of p47phox and p67phox, cells in the logarithmic stage of growth were washed once, and resuspended in complete medium to 15×106 cells/ml. The cDNAs for human p67phox and p47phox (25 µg total) in the episomal vector pREP4 (Invitrogen, Carlsbad, CA), were added to 375 µl of the cells and 325 µl aliquots electroporated at 250 volts/960 µFd. Stable expressing cells were selected in medium containing 250 µg/ml of hygromycin for 7 days, then subsequently maintained in medium with 150 µg/ml of hygromycin. Expression of the p67phox/p47phox proteins in K562/NOX2 cells was confirmed by western blot analysis and by measuring superoxide generation (see below). These cells are referred to as K562/NOX2 cells. When the over-expression of a protein in addition to the phox proteins was required, we used transient transfection and pcDNA3.1 vectors containing the cDNAs of interest.
Stable expression of GFP-c-Abl fusion proteins
The expression plasmid pcDNA3.1/Zeo (+) containing cDNAs for either wild-type c-Abl (GFP-c-Abl) or kinase-dead c-Abl (GFP-KD-c-Abl) was linearized and transfected into K562 cells as described above. Stable expressing cells were selected in 250 µg/ml of zeocin for 14 days. Single-cell clones were established by limiting dilution in 96-well plates. The expression of GFP-c-Abl and GFP-KD-c-Abl in the selected clones was determined by fluorescence microscopy. These cells were then transiently transfected with pcDNA3.1 vectors encoding NOX2, p67phox, and p47phox.
Cell culture, inhibitors, and subcellular fractionation
Transfected K562 cells grown in complete RPMI medium, or freshly isolated neutrophils were treated as indicated in the text with inhibitors of PKC (staurosporine 1 µM, 30 min), PKCδ (rottlerin 20 µM, 3h), Src family kinases (genistein 10 µM, 30 min), c-Abl tyrosine kinase (imatinib mesylate 10 µM, overnight), SERCA (thapsigargin [TG] 100 nM, 30 min), T-type Ca2+ channels (mibefradil 15 µM, 1h), G-proteins (pertussis toxin [PTX] 10 µg/ml, 1h) and RhoGTPase (Clostridium difficile toxin B 1 nM, overnight). Cells were also treated, where indicated, with PMA 1µg/ml or the extracellular Ca2+ chelator BAPTA (50 µM). Control cells were treated with vehicle dimethyl sulfoxide or phosphate-buffered saline (PBS) plus 10 mM glucose (PBS-G). At the end of the treatment the cells were washed in PBS-G and treated with H2O2 (100 µM) for 10 min at 37°C. Cell lysis was carried out in buffer A (20 mM HEPES, pH 7.9; 350 mM NaCl; 0.5 mM EDTA; 0.5 mM EGTA; 1 mM MgCl2) plus 10% glycerol, 1% Nonidet P-40, 10 mM NaF, 0.1 mM NaVO4, 8 mM β-glycerophosphate, phosphatase inhibitor cocktail I and II (Sigma), and a protease inhibitor cocktail (Roche, Mannheim, Germany; 1 tablet/50 ml buffer). Lysates were cleared by centrifugation, and when required, the total protein extracts were centrifuged at 100,000 g for 1 h to separate crude membranes from cytosolic proteins. Protein content was estimated as described [11].
Superoxide assay in whole cells
Superoxide generation was measured using a luminol-based chemiluminescence assay (Diogenes, National Diagnostics). Cells were collected by centrifugation, washed once in PBS, resuspended at 5×106/ml in PBS-G, and kept on ice until assayed. For the assay, 100 µl of the luminol reagent were mixed with 0.25 to 0.5×106 cells and incubated at 37°C for 2–4 min. Superoxide generation was stimulated by the addition of PMA 1 µg/ml in PBS-G, H2O2 0–500 µM, or glucose oxidase (0.25 mU/ml) in the presence of glucose (5 mM) or the addition of formyl-methionyl-leucyl-phenylalanine (fMLF; 100 nM). Chemiluminescence was measured every 30–60 s using a Turner Designs 20/20 luminometer and a 5 s integration time.
Broken-cell NADPH oxidase assay
Neutrophils were disrupted by sonication in buffer B (100 mM KCI, 3 mM NaCI, 3.5 mM MgCI2 1.25 mM EGTA, 10 mM PMSF, 10 mM NaF, 0.1 mM, NaVO4, 8 mM β-glycerophosphate, phosphatase inhibitor cocktail I and II [Sigma], and a protease inhibitor cocktail [Roche, Mannheim, Germany; 1 tablet/50 ml buffer]). Lysates were cleared by centrifugation. Crude membranes were separated from cytosolic proteins by centrifugation at 100,000 g for 1 h. Protein content was estimated as described [11]. Mixtures containing PBS, pH 7.5, 2 mM MgCl2, 20 mM GTPγS, 200 µM ATP, 10 nM arachidonic acid/µg of protein and 5 µg of membrane protein mixed with 45 µg of cytosolic proteins in a final volume of 100 µl were used to reconstitute NADPH oxidase activity. Superoxide production was initiated with the addition of 200 µM NADPH and determined by measuring the SOD-inhibitable reduction of cytochrome C (100 µM) and quantitated using Δε 550 = 21,000 M−1 cm−1 [12] blanked against identical wells containing 400 U/ml SOD. Average rates of superoxide generation were calculated from the linear region of increase in absorbance at 550 nm and were expressed as nmol of O2•/min/mg of membrane protein.
Rac activation assay (GST-PAK-CRIB pull-down assay)
Cell lysates prepared as above in buffer A were pre-cleared with GST-bound GSH-Sepharose, then incubated at 4°C for 30 min with 10 µg of GST-Cdc42/Rac interactive binding (CRIB) region (amino acids 54–165) of PAK1 (p21-activated kinase 1) protein (GST-PAK-CRIB) immobilized on glutathione-Sepharose beads [13]. The bead pellet was thrice-washed with the lysis buffer and resuspended in 40 µl of SDS-PAGE sample buffer. The samples were separated on 12% SDS-PAGE gels, transferred to a nitrocellulose membrane, and blotted with a monoclonal antibody to Rac1/2 (Upstate Biotechnology). Parallel immunoblots were carried out on 20 µg of total cell lysate to quantitate the total amount of Rac1/2 protein. The antigen-antibody complexes were visualized by enhanced chemiluminescence (ECL, Amersham Corp.).
Immunoblotting
Protein extracts (20–50 µg) were separated on 4–20% SDS polyacrylamide gradient gels and transferred to polyvinylidene difluoride membranes. The membranes were then incubated with antibodies against c-Abl (Sigma, cat # A5844), phosphotyrosine-245 c-Abl (Cell Signaling, cat # 2861), PKCδ (Santa Cruz Biotechnology, cat # sc937), phosphotyrosine-311 PKCδ (Cell Signaling, cat # 2055), p47phox or p67phox (William M. Nauseef, University of Iowa), or NOX2 [14]. Actin (Sigma) was used as a loading control. The antigen-antibody complexes were visualized by enhanced chemiluminescence (ECL, Amersham Corp.).
Live cell imaging
Transfected K562 cells were washed twice in PBS-G and plated on poly-L-lysine-coated-glass coverslips for 60 min (3×105 cells/ml) in complete RPMI medium without phenol red. The cells were loaded with 6 µM fluo-4-AM and 300 nM dihydroethidium (DHE; Molecular Probes). Ca2+ influx and superoxide production were recorded using a confocal microscope (LSM 510; Carl Zeiss MicroImaging, Inc.) with a 63X/1.4 NA plan-apochromat objective. Fluo-4 and DHE fluorescence were excited with dual laser at 488/543 nm, attenuated to avoid photobleaching and saturation. Detection was through a 545 nm long-pass dichroic mirror and a band-pass filter at 500–530 nm for fluo-4 fluorescence and LP560 for DHE fluorescence. Image acquisition was performed with the Zeiss LSM510 Software 3.2 SP2.
Statistical analysis
Data are presented as the mean ± SEM of the values and were normalized to controls. Statistical analysis was performed using the Dunnett multiple comparisons test to adjust for multiple testing when comparing several means against the mean for a common control sample. A value of P < 0.05 was accepted as significant.
Results
H2O2 induces superoxide generation by NOX2 NADPH oxidase
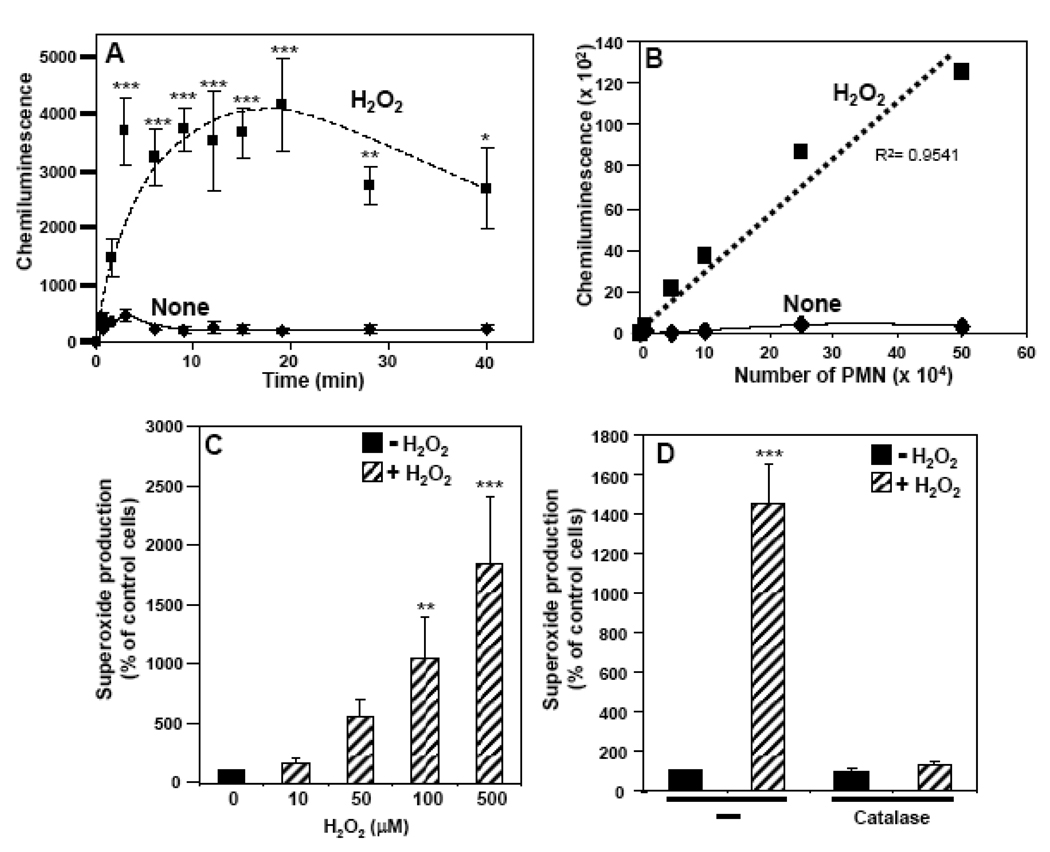
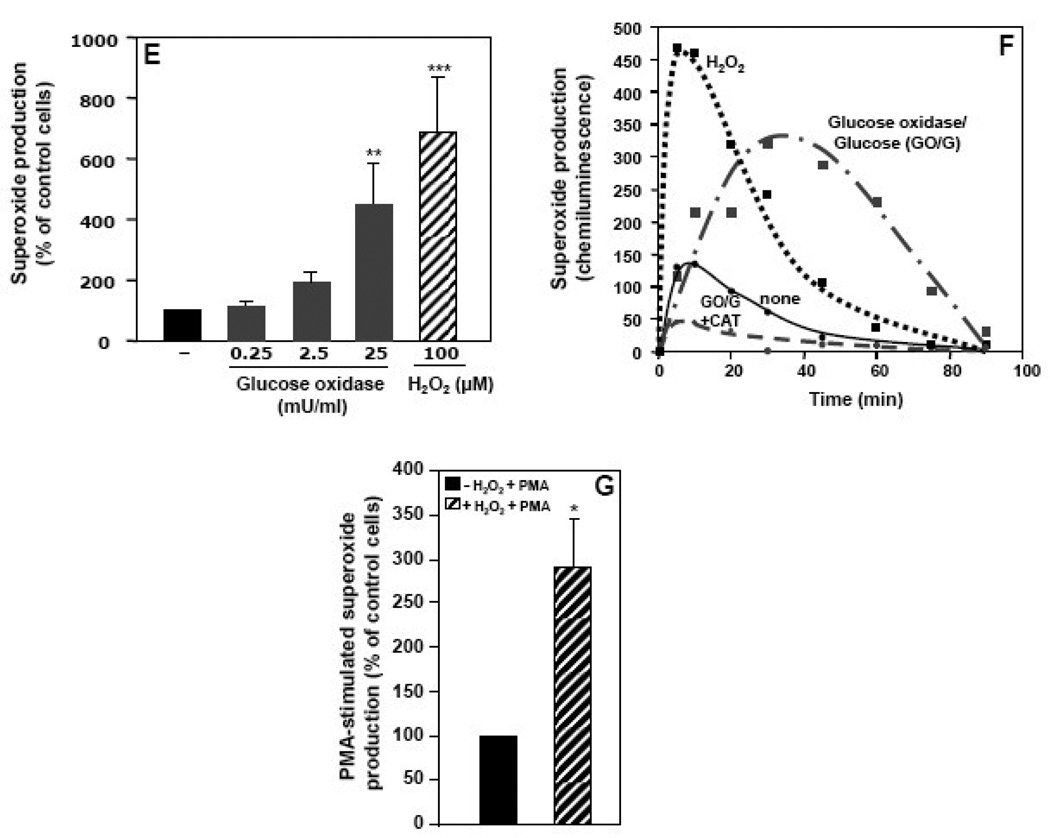
Addition of 100 µM H2O2 to freshly isolated neutrophils induced a rapid and significant increase in chemiluminescence (via the Diogenes assay) that reached maximum values within 2 to 5 min and persisted at high levels for 20 to 40 min (Fig. 1A). Very little chemiluminescence was observed in the absence of H2O2 (Fig. 1A), cells, or Diogenes reagent (data not shown). Furthermore, the induction of chemiluminescence by H2O2 was completely abrogated by the prior addition of superoxide dismutase (400 U/ml) or diphenylene iodonium (10 µM), but not by azide (1 mM) (data not shown). Thus, H2O2 induced a marked and sustained production of superoxide by neutrophils. The level of superoxide generated was directly dependent on the number of neutrophils (Fig. 1B) and the concentration of H2O2 (Fig. 1C) and was largely blocked by the addition of catalase (Fig. 1D). A combination of glucose oxidase 25 mU/ml and 5 mM glucose mimicked the effect observed with H2O2 (Fig. 1E), albeit with slower kinetics (Fig. 1F). Using this system, a period of about thirty min was required to reach the level of superoxide produced with 100 µM H2O2 in 5–10 min. Analogous to H2O2, the effect was dependent on the concentration of glucose oxidase (Fig. 1F) and was inhibited by catalase (Fig. 1F). In comparison with fMLF, H2O2 was less potent in stimulating peak superoxide generation, but its effect was more sustained (data not shown). Furthermore, H2O2 pre-treatment increased superoxide production stimulated by PMA (Fig. 1G). These results show that not only does H2O2 serve as a primary activator of NOX2, but it also acts synergistically with PMA to enhance superoxide production.
Fig. 1. Effects of H2O2 on superoxide production by human neutrophils.
Superoxide production by neutrophils was measured using a whole cell assay with the Diogenes reagent. (A) Chemiluminescence generated by 0.25×106 neutrophils incubated with 100 µM H2O2 (broken line) or PBS-G (solid line) was measured for the indicated times. (B) Chemiluminescence generated by increasing numbers of neutrophils incubated with 100 µM H2O2 (broken line) or PBS-G (solid line) was measured over 10 min, and after subtraction of the zero-time values the area under the curve was calculated (arbitrary units) as the measure of total superoxide production. (C) Superoxide production was determined in neutrophils stimulated with increasing concentrations of H2O2 and after subtraction of the zero-time values the area under the curve was calculated and expressed as a percent of the control cells without H2O2. (D) Neutrophils, either non-treated (−) or pre-treated with catalase (900 U/ml), were stimulated with 100 µM H2O2 and superoxide production was measured and expressed as in Fig. 1C. (E) Neutrophils were incubated with increasing concentrations of glucose oxidase and 5 mM glucose, or with 100 µ M H2O2 and superoxide production was measured and expressed as in Fig. 1C. (G) Neutrophils were incubated with 25 mU/ml of glucose oxidase and 5 mM glucose in the presence or absence of catalase (900 U/ml), or with 100 µM H2O2 and superoxide production was measured for the indicated times. (G) Neutrophils were pre-incubated with or without 100 µM H2O2 for 10 min, then stimulated with PMA (1 µg/ml), and superoxide production was measured and expressed as in Fig. 1C. The data are the means +/− SEM of 3–6 independent experiments. Asterisks indicate statistical significance versus control cells without H2O2. *P<0.05, **P<0.01, ***P<0.001.
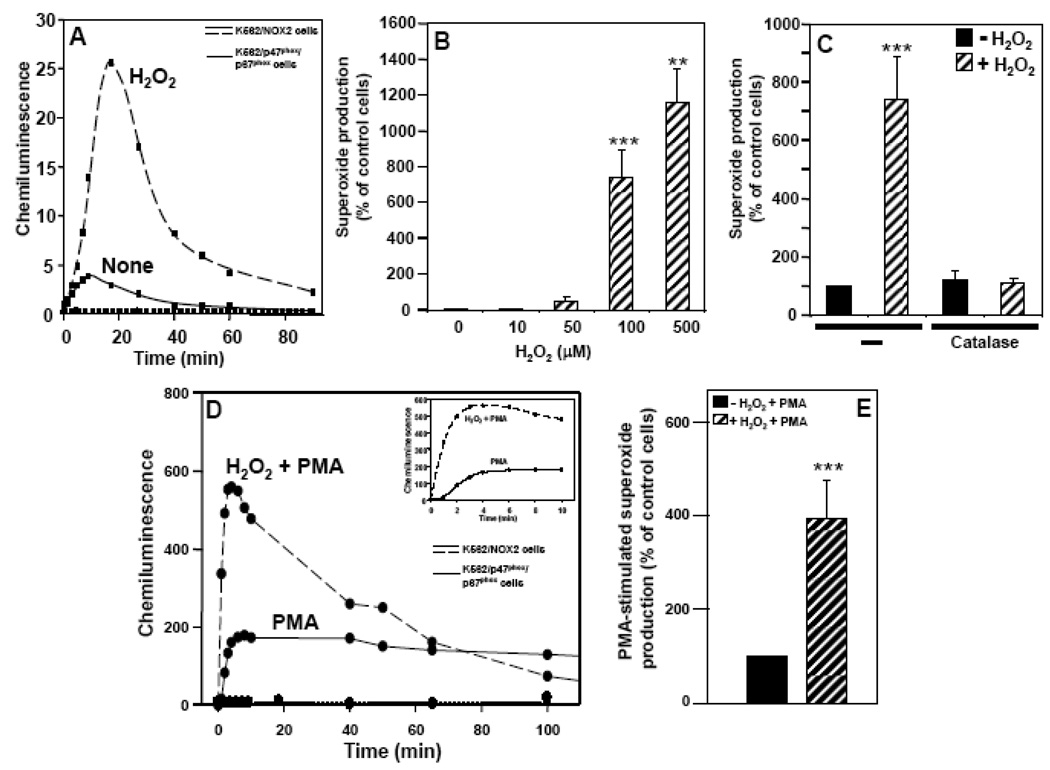
Since neutrophils are short-lived cells and therefore only modestly amenable to molecular interventions in vitro, further studies of the mechanisms underlying the regulation of NOX2 by H2O2 were carried out using the human K562 cell line. Untransfected K562 cells express Rac and the p22phox component of NADPH oxidase, but not NOX2, p67phox, p47phox or p40phox. Therefore, to study the NOX2 system, the essential components NOX2, p67phox, and p47phox were transfected into K562 cells. H2O2 induced a substantial increase in superoxide production in these K562/NOX2 cells, with maximum activity observed 10–20 min after addition of 100 µM H2O2 (Fig. 2A). To confirm that the chemiluminescence detected was due to the activity of the NOX2 system, we compared superoxide generation in these cells with K562 cells expressing only the p67phox and p47phox cytosolic factors. Under these conditions there was little or no induction of superoxide generation by either H2O2 or PMA (Fig. 2A and 2D, respectively). The activation of NOX2 was dose-dependent from 10 to 500 µM H2O2 (Fig. 2B), over which range there was no effect on cell viability as determined by trypan blue exclusion (data not shown). The response to H2O2 was abrogated by the addition of catalase (Fig. 2C). Pre-incubation of K562/NOX2 cells with H2O2 enhanced their response to PMA (Fig. 2D and 2E). Relative to the effect of PMA alone, H2O2 pre-incubation resulted in increases in both the rate (6- to 10-fold) and total amount (3-fold) of superoxide produced. This effect gradually decreased and was undetectable after 1 h (Fig. 2D).
Fig. 2. Effect of H2O2 on superoxide production by K562/NOX2 cells.
K562 cells over-expressing NOX2 plus p47phox/p67phox or only p47phox/p67phox were assayed for superoxide production as described in Fig. 1. (A) Chemiluminescence output was measured from 0.5x106 K562/NOX2 cells incubated with (broken line) or without (solid line) 100 µM H2O2 and from K562 cells expressing only p47phox and p67phox (dotted line) incubated with H2O2 for the indicated times. (B) Superoxide production by K562/NOX2 cells incubated with increasing concentrations of H2O2 (0–500 µM) was measured for 10 min and expressed as in Fig. 1C. (C) K562/NOX2 cells, either non-treated (−) or pre-treated with catalase (900 U/ml), were stimulated with 100 µM H2O2 and superoxide production was measured and expressed as in Fig. 1C. (D) K562/NOX2 cells were pre-incubated with or without 100 µM H2O2 for 10 min, then stimulated with PMA (1 µg/ml), and chemiluminescence was measured and expressed as in Fig. 1A. Chemiluminescence was also measured in K562 cells expressing only p47phox and p67phox pre-incubated with H2O2 and then stimulated by PMA (dotted line). (E) K562/NOX2 cells were pre-incubated with or without 100 µM H2O2 for 10 min, then stimulated with PMA (1 µg/ml), and superoxide production was measured and expressed as in Fig. 1C. The data shown are the mean +/− SEM of 3–9 independent experiments. Asterisks indicate statistical significance versus non-treated cells without H2O2. **P <0.01, ***P < 0.001.
Role of Ca2+ influx in NOX2 activation by H2O2
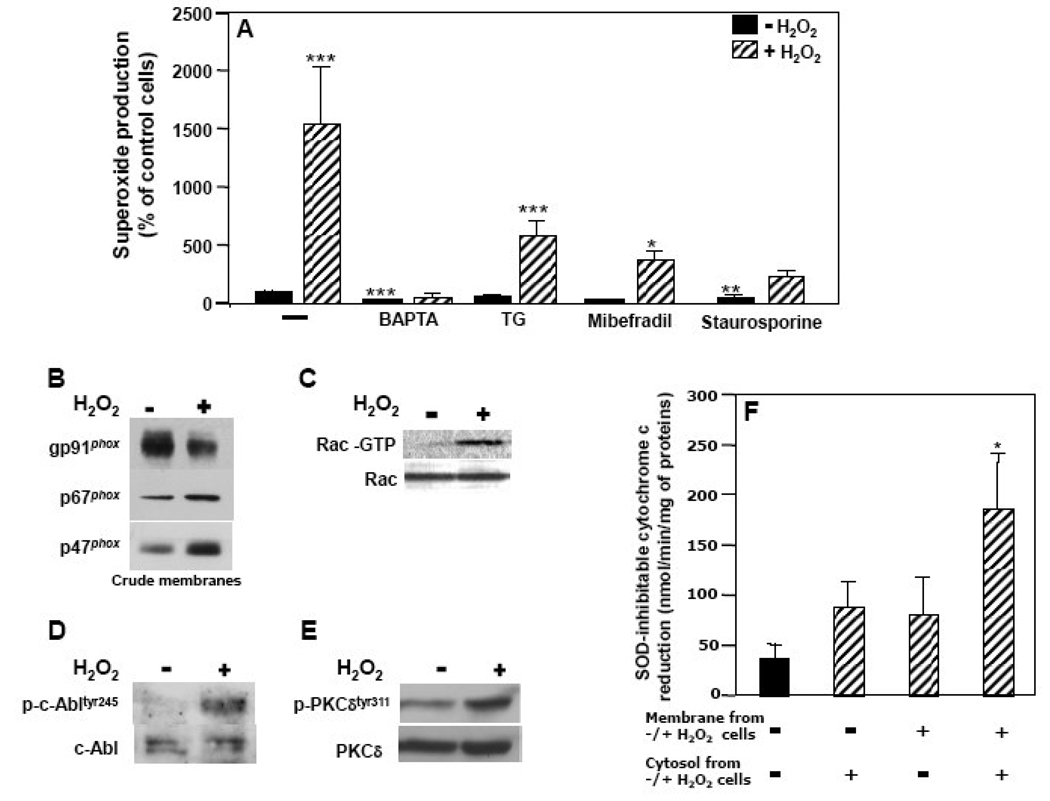
Based on our previous work on H2O2-induced NOX5-mediated superoxide generation [5], we investigated whether Ca2+ influx is involved in H2O2-induced NOX2 activity. The induction of superoxide generation in K562/NOX2 cells by H2O2 was abrogated by treatment with either the Ca2+ chelator BAPTA or the T-type Ca2+-channel blocker mibefradil (Fig. 3A). In contrast, depletion of intracellular Ca2+ stores by thapsigargin had no significant effect on H2O2-induced superoxide generation, whereas it increased somewhat the basal level of superoxide production. The synergistic effect of H2O2 on PMA-stimulated superoxide generation was significantly reduced by pre-treatment with either BAPTA or mibefradil, but not thapsigargin (Fig. 3B). These results suggest that H2O2 activation of NOX2 as well as its synergistic effect on PMA stimulation requires an increase in cytosolic Ca2+ derived largely by influx from the extracellular pool.
Fig. 3. Role of Ca2+ in H2O2-NOX2 regulation.
(A, B) K562/NOX2 cells were pre-treated with the indicated inhibitors: BAPTA (50 µM, 5 min), thapsigargin (TG; 100 nM, 30 min), mibefradil (15 µM, 1h). The cells were then tested for superoxide production with (hatched bars) or without (solid bars) 100 µM H2O2 for 10 min (A) or after further stimulation with PMA (1 µg/ml) for an additional 10 min (B). Superoxide production was expressed as in Fig. 1C as a percent of control cells (−) in the absence of H2O2. The data shown are the means +/- SEM of 5–10 independent experiments. Asterisks indicate statistical significance versus non-treated cells without H2O2. *P < 0.05; ***P < 0.001. (C) Confocal microscopy of H2O2-induced superoxide generation and Ca2+ influx was performed using either K562 cells expressing NOX2, p67phox, and p47phox (right panels) or only p67phox and p47phox (left panels). The cells were loaded with fluo-4 (green fluorescence, Ca2+) and DHE (red fluorescence, superoxide) and stimulated with 100 µM H2O2. Confocal images using a Zeiss microscope were recorded for up to 10 min using dual excitation at 488/543 nm. Each set of four photomicrographic panels corresponds to superoxide (top left), Ca2+ (top right), the differential interference contrast image (bottom left), and a merge of the two fluorescence images (bottom right). The scale bar indicates 10 µm.
The role of Ca2+ in the activation of NOX2 by H2O2 was also investigated by confocal imaging using the Ca2+-sensitive probe fluo-4 and the superoxide-sensitive probe dihydroethidium (DHE) (Fig. 3C). Only K562 cells expressing the complete NOX2 system demonstrated superoxide formation (red fluorescence) following treatment with H2O2, whereas Ca2+ influx (green fluorescence) was induced by H2O2 both in K562 cells expressing the complete NOX2 system and in those expressing p47phox and p67phox only. Notably, Ca2+ influx in response to H2O2 treatment was accentuated in cells expressing the complete NOX2 system, suggesting a positive feedback effect of NOX2 products on H2O2 signaling, as we have described for NOX5 [5].
Role of c-Abl in NOX2 activation by H2O2
To investigate the role of c-Abl in H2O2-NOX2 regulation, we first treated K562/NOX2 cells with imatinib mesylate (10 µM, overnight), an inhibitor of Abl tyrosine kinase. This agent completely blocked NOX2 stimulation by H2O2 (Fig. 4A) and significantly reduced the effect of H2O2 on PMA-stimulated superoxide production (Fig. 4B). Since imatinib is not totally specific for c-Abl tyrosine kinase, we also used stable K562 cell lines over-expressing either the GFP-tagged wild-type c-Abl (GFP-c-Abl), or the GFP-tagged kinase-dead c-Abl (GFP-KD-c-Abl), and transiently transfected with the NOX2 system components (gp91phox/p67phox/p47phox). Over-expression of the enzymatically active GFP-c-Abl significantly enhanced both the basal and H2O2-induced activity of NOX2, whereas over-expression of the dominant-negative GFP-KD-c-Abl markedly reduced both basal superoxide production and the response to H2O2 (Fig. 4C). Similar effects were observed for the effect of H2O2 on PMA-stimulated NOX2 activity (Fig. 4D). These results show that c-Abl is a key intermediate in the NOX2-activating effects of H2O2.
Fig. 4. Role of c-Abl tyrosine kinase in H2O2-NOX2 regulation.
Superoxide production was determined with (hatched bars) or without (solid bars) 100 µM H2O2 for 10 min (A, C), or after further stimulation with PMA (1 µg/ml) for an additional 10 min (B, D) using K562/NOX2 cells pre-treated or not (–) with imatinib mesylate (10 µM, overnight) (A, B) or using K562 cells over-expressing GFP-KD-c-Abl or GFP-WT-c-Abl and the NOX2 system (C, D). Superoxide production was expressed as in Fig. 1C as a percent of control cells without H2O2 (A, B) or versus K562/NOX2 cells without H2O2 (C, D). The data shown are the means +/− SEM of 3–4 independent experiments. Asterisks indicate statistical significance versus nontreated cells without H2O2 (A, B) or versus K562/NOX2 cells without H2O2 (C, D). *P < 0.05; ***P < 0.001.
Role of PKCδ in NOX2 activation by H2O2
PKC is an important mediator of neutrophil NOX2 activation. Since both Ca2+ influx and c-Abl can induce PKC activation, we analyzed the effect of PKC inhibitors, namely staurosporine, a broad inhibitor of all PKC subtypes, and rottlerin, which is specific for PKCδ. A member of the novel (n)PKC subfamily of isoforms requiring diacyglycerol (DAG), but not Ca2+, PKCδ participates in NOX2 activation in response to fMLF or zymosan by phosphorylating p47phox [15, 16]. PKCδ and c-Abl also cross-phosphorylate and activate each other [17]. We observed in K562/NOX2 cells that both H2O2 induction of NOX2 activity and its effect on PMA activation were abrogated by staurosporine (data not shown). When the cells were pre-treated with rottlerin, a significant reduction in stimulation of NOX2 activity by H2O2 was observed (Fig. 5A). In addition, rottlerin interfered with both the effect of H2O2 on PMA stimulation of NOX2 and the activation of NOX2 by PMA alone (Fig. 5B). Western blot analysis of PKCδ demonstrated that H2O2 induced phosphorylation of tyrosine311, a reaction that was blocked by rottlerin, BAPTA, or imatinib (Fig. 5C). PMA, used here as a positive control, predictably induced phosphorylation of PKCδ, but no additional phosphorylation was observed upon subsequent exposure to H2O2 (Fig. 5C). This observation correlates with marked superoxide production stimulated by PMA, but no further increment upon subsequent addition of H2O2. These results altogether suggest that PKCδ activation plays a major role in the regulation of NOX2 by H2O2 and that it lies downstream of Ca2+ entry and c-Abl activation.
Fig. 5. Role of PKCδ in H2O2-NOX2 regulation downstream of Ca2+ and c-Abl.
(A, B) K562/NOX2 cells were pre-treated with rottlerin 20 µM for 3h, then tested for superoxide production with (hatched bars) or without (solid bars) 100 µM H2O2 for 10 min (A) or after further stimulation with PMA (1 µg/ml) for an additional 10 min (B). Superoxide production was expressed as in Fig. 1C as a percent of control cells in the absence of H2O2. The data shown are the means +/− SEM of 3–9 independent experiments. Asterisks indicate statistical significance versus non-treated cells without H2O2. *P < 0.05, ***P < 0.001. (C) A representative immunoblot experiment using antibodies against the phosphorylated tyrosine311-PKCδ and total PKCδ and cell lysates prepared from K562/NOX2 cells pre-treated with the indicated inhibitors [BAPTA (50 µM, 5 min), rottlerin (20 µM, 3 h), imatinib (10 µM, overnight)], or PMA (1 µg/ml, 5 min) and then stimulated (+) or not (−) with 100 µM H2O2 for 10 min.
Role of Rac in NOX2 activation by H2O2
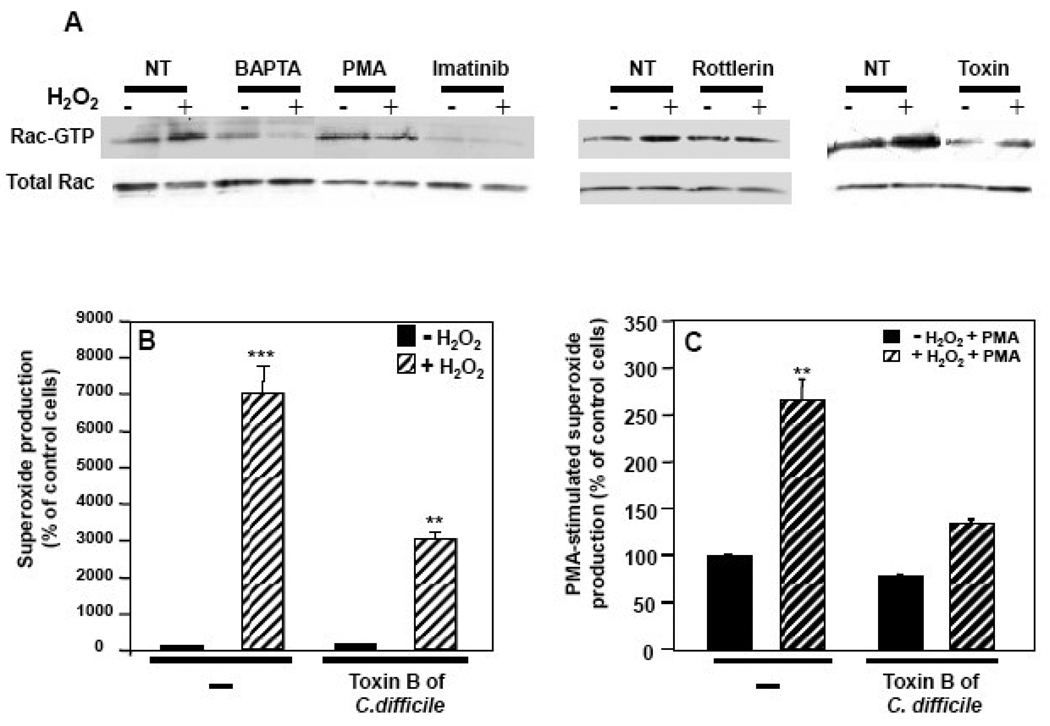
Since Rac is required for agonist-stimulated NOX2 activation and a connection between c-Abl and Rac activation has been reported [18], we analyzed the potential role of Rac activation in NOX2 regulation by H2O2. In K562/NOX2 cells, H2O2 exposure resulted in activation of Rac, as assessed by the GST-PAK pull-down assay (Fig. 6A). Pre-treatment of the cells with the Rac inhibitor toxin B of C. difficile, BAPTA, imatinib, or rottlerin abrogated or reduced the effect of H2O2 on Rac activation. PMA-induced Rac activation was not affected by the subsequent addition of H2O2 (Fig. 6A). Pre-treatment with toxin B significantly reduced the production of superoxide stimulated by H2O2 (Fig. 6B). In cells pre-treated with the toxin, the effect of H2O2 on PMA-stimulated superoxide production was significantly reduced, and the stimulation by PMA itself was reduced by approximately 20% (Fig. 6C). These results suggest a common pathway for PMA and H2O2 in the activation of Rac through at least PKCδ and demonstrate that the regulation of NOX2 by H2O2 requires activation of Rac downstream of Ca2+ influx and c-Abl activation.
Fig. 6. Role of Rac activation in H2O2-NOX2 regulation.
(A) Total cell lysates (100 µg of protein) were prepared from K562/NOX2 cells that had been pre-treated with the indicated inhibitors [BAPTA (50 µM, 5 min), imatinib (10 µM, overnight), rottlerin (20 µM, 3 h), C. difficile toxin B (1 nM, overnight)], or PMA (1 µg/ml, 5 min) and then stimulated (+) or not (−) with 100 µM H2O2 for 10 min. The levels of active Rac (Rac-GTP) were determined using the GST-PAK-CRIB pull-down assay. (B, C) K562/NOX2 cells were pre-treated with toxin B of C. difficile, 1 nM overnight, then tested for superoxide production with (hatched bars) or without (solid bars) 100 µM H2O2 for 10 min (B) or after further stimulation with PMA (1 µg/ml) for an additional 10 min (C). Superoxide production was expressed as in Fig. 1C as a percent of control cells without H2O2. Data shown are the mean +/− SEM of three independent experiments. Asterisks indicate statistical significance versus non-treated cells without H2O2. ** P <0.01, ***P < 0.001.
Similar H2O2-NOX2 regulation pathways in neutrophils and K562/NOX2 cells
As for K562/NOX2 cells, H2O2-induced superoxide production by neutrophils was significantly reduced by BAPTA, mibefradil, or staurosporine, but not by thapsigargin (Fig. 7A). The synergistic effect of H2O2 pre-treatment for superoxide stimulation by PMA was also observed in these cells (see Fig. 1E).
Fig. 7. Assessment of H2O2-NOX2 signaling pathways in neutrophils.
(A) Neutrophils were pre-treated with the indicated inhibitors: BAPTA (50 µM, 5 min), thapsigargin (TG; 100 nM, 30 min), mibefradil (15 µM, 1 h), staurosporine (1 µM, 30 min). The cells were then tested for superoxide production with (hatched bars) or without (solid bars) 100 µM H2O2 for 10 min. Superoxide production was expressed as in Fig. 1C as a percent of control cells without H2O2. Data shown are the mean +/− SEM of 3-7 independent experiments. Asterisks indicate statistical significance versus control cells without H2O2. * P <0.05, ** P <0.01, ***P < 0.001. (B) NOX2 assembly was assessed by immunoblot analysis of membrane preparations from neutrophils stimulated (+) or not (−) with 100 µM H2O2 using antibodies directed against NOX2, p47phox, or p67phox. (C) Total cell lysates (100 µg of protein) were prepared from neutrophils that had been stimulated (+) or not (−) with 100 µM H2O2. The levels of total Rac and active Rac were determined as in Fig 6A. (D, E) Activated (phosphorylated) c-Abl and PKCδ were assessed by immunoblot analysis with general and phosphospecific antibodies to c-Abl-Tyr245 (D) and PKCδTyr311 (E), respectively, on whole cell lysates from neutrophils stimulated (+) or not (−) with 100 µM H2O2. (F) Reconstitution of NADPH oxidase activity in the broken cell system was performed as described in experimental procedures using membrane and cytosol fractions from neutrophils pre-treated (+) or not (−) with 100 µM H2O2 for 10 min. Superoxide production was measured as SOD-inhibitable cytochrome c reduction and expressed in nmol/min/mg of protein. Data shown are the mean +/− SEM of 3 independent experiments. Asterisks indicate statistical significance versus superoxide produced by the combination of membranes and cytosol isolated from untreated neutrophils. * P <0.05.
Western blot analysis performed on neutrophil crude membranes showed that H2O2 induced the translocation to the membrane of the cytosolic factors p67phox and p47phox (Fig. 7B), a key feature of classical agonist-stimulated NOX2 activation. H2O2 also induced Rac activation in neutrophils (Fig. 7C). Furthermore, induction of superoxide production by H2O2 correlated with an increase in phosphorylation of both c-Abl (Fig. 7D) and PKCδ (Fig. 7E). These observations suggest that the signaling pathways involved in H2O2-NOX2 regulation in neutrophils are very similar to those demonstrated in K562/NOX2 cells.
Broken cell system analysis of effects of H2O2 pre-treatment
Reconstitution of NOX2 activity in the broken cell system was examined using different combinations of membranes and cytosol isolated from neutrophils pre-treated or not with 100 µM H2O2 (Fig. 7F). The rate of superoxide generation in the broken cell assay performed with membranes plus cytosol isolated from H2O2-treated cells, was much higher than in assays performed with membranes plus cytosol isolated from untreated control cells (p<0.05). Moreover, the level of superoxide produced by H2O2-treated cytosol plus untreated membranes tended to be increased relative to untreated cytosol plus untreated membranes and similar to the amount produced by untreated cytosol plus H2O2-treated membranes, although neither of these combinations generated as much activity as when both cytosol and membranes were derived from H2O2-treated cells (Fig. 7F). These observations may reflect both the presence of activated co-factors in H2O2-treated cytosol and translocated cytosolic factors in the plasma membranes isolated from H2O2-treated cells. The fact that superoxide generation was highest with the combination of treated membranes plus treated cytosol suggests that gp91phox might itself be a final target of H2O2, particularly since the increased superoxide production was not attributable to an increase in NOX2 content of the membranes from H2O2-treated cells.
Lack of a priming effect of H2O2 on fMLF-stimulated superoxide production
Since H2O2 increases superoxide production induced by PMA, we investigated whether H2O2 could act as a priming factor for receptor-mediated superoxide production. We found that pre-incubation for 10 min with 10 nM to 100 µM H2O2 did not prime the cells for activation by 100 nM fMLF (Fig. 8A). On the contrary, a decrease in superoxide production was observed, suggesting that common signaling intermediates may be involved in H2O2 and fMLF-mediated superoxide production. Since signaling initiated by fMLF binding to its receptor proceeds through PTX-sensitive G proteins, which can be activated by H2O2 via cysteine oxidation [19], we investigated whether these G proteins might be involved in the effect of H2O2 on superoxide production. We observed a significant decrease in H2O2-induced superoxide production by PMNs pre-treated with PTX, an inhibitor of Gi/o proteins (Fig. 8B). A similar effect of PTX was observed in K562/NOX2 cells (data not shown). These observations point to at least one common proximal target of H2O2 and fMLF, namely a PTX-sensitive G protein.
Fig. 8. Effect of H2O2 on fMLF-stimulated superoxide production.
(A) Neutrophils were pre-treated with various amounts of H2O2 and then stimulated with 100 nM fMLF. (B) Neutrophils were pre-treated with 10 µg/ml PTX for 1 h and then tested for superoxide production with (hatched bars) or without (solid bars) 100 µM H2O2 for 10 min. In A and B superoxide production was measured and expressed as in Fig. 1C as a percent of control cells without H2O2. Data shown are the mean +/− SEM of three independent experiments. Asterisks indicate statistical significance versus non-treated cells without H2O2. * P <0.05, ** P <0.01, ***P < 0.001. (C) The schema represents a model for H2O2-NOX2 regulatory pathways.
Discussion
The work reported herein investigated whether NOX2 is regulated by H2O2 and examined the signaling pathways involved in this regulation (see Fig. 8C for a schema of proposed pathways). We found that NOX2 is indeed positively regulated by H2O2 in both primary cells (i.e. human blood neutrophils) and K562 human leukemia cells over-expressing NOX2 and its cofactors. These results are in accord with our studies on NOX5 [5], as well as reports showing that H2O2 induces superoxide production by one or more non-phagocytic NADPH oxidases in vascular tissues [20]. We observed that Ca2+ influx is an essential proximal event required for the induction of NOX2-dependent superoxide production by H2O2. In accord with our findings, it has been shown recently that NOX2 regulates neutrophil membrane potential and Ca2+ influx not only via its electrogenic activity (i.e. catalytic transmembrane electron transfer), but also as a downstream consequence of the generation of ROS [21]. It is well known that Ca2+ mobilization plays an important role in classical receptor-mediated activation of superoxide production by phagocytic cells [22–24]. For example, similar to our observations in H2O2-treated cells, Ca2+ chelating agents block agonist/receptor-stimulated superoxide generation [22, 25, 26].
The receptor-mediated Ca2+ influx required for activation of NOX2-dependent superoxide production in phagocytic cells is predominantly a result of the activation of so-called capacitative Ca2+ entry via store-operated Ca2+ (SOC) channel(s), which are activated by the emptying of intracellular Ca2+ stores [27]. Although store-operated Ca2+ entry (SOCE) provides a mechanistic link between cytosolic [Ca2+] elevation and superoxide anion production, it cannot by itself account for the Ca2+ entry involved in H2O2-NOX2 regulation. For example, we observed that thapsigargin, an inhibitor of SERCA-mediated Ca2+ re-uptake that permits slow endoplasmic reticulum emptying followed by SOCE, did not significantly reduce superoxide production induced by H2O2 in either K562-NOX2 cells or neutrophils. The previous observation that Ca2+ ionophore and thapsigargin were unable to activate plasma membrane-associated NADPH oxidase in neutrophils [28], along with our finding that NOX2 activation by H2O2 is Ca2+-dependent but thapsigargin-resistant, supports the involvement of Ca2+ entry mechanisms other than SOC channels. Indeed, inhibitor experiments suggested that T-type voltage-gated channels were involved in H2O2-NOX2-regulation. However, given the long latency for NOX2 activation in neutrophils, it may be that after initial SOC channel-independent Ca2+ entry, SOCE is activated in a second phase. Such Ca2+ reinforcement/propagation has been observed in other cells [29].
Positive feedback regulation of NOX2 by H2O2 was blocked by imatinib mesylate, an inhibitor of Bcr-Abl as well as c-Abl [30], both of which are expressed in K562 leukemia cells. The essential role of native c-Abl in H2O2-NOX2 activation is suggested by the fact that H2O2 induced NOX2-dependent superoxide production in normal blood neutrophils, which do not express the Bcr-Abl fusion protein. Furthermore, in K562 cells stably over-expressing wild-type GFP-c-Abl, baseline NOX2 activity was enhanced and induction of activity by H2O2 was preserved, whereas over-expression of dominant-negative c-Abl abrogated the stimulatory effect of H2O2 on NOX2 activity. Overall, these results are in keeping with studies showing not only that c-Abl is activated by H2O2 [31], but that it induces an increase of ROS production when over-expressed in hematopoietic cells [32].
In our previous experiments, we showed that while Ca2+ was essential for the translocation of c-Abl to the membrane, it was not required for its activation by phosphorylation [5]. These data suggest that while Ca2+ is a key determinant of H2O2-NOX2 regulation, a Ca2+-independent pathway is also activated by H2O2. Several lines of evidence implicate Src as an upstream activator of c-Abl [33], although in H2O2-treated K562/NOX5 cells [5] and K562/NOX2 cells (data not shown), we were unable to demonstrate a role of c-Src upstream of c-Abl. Thus, the signaling intermediates directly responsible for c-Abl phosphorylation remain to be determined.
Activation of NOX2 is absolutely dependent on the presence of cytosolic co-factor proteins p67phox, p47phox, and Rac. PKC is a major signaling protein kinase required for assembly and activation of the NOX2 complex, acting at least in part through the phosphorylation of multiple serines on p47phox. The PKC family, comprising eleven members, is categorized into three classes on the basis of structure and activation requirements. The classical PKC isoforms (α, β1, β2 and γ) are regulated by both Ca2+ and DAG, the novel PKC isoforms (δ, ε, θ and η) are regulated by DAG, but not Ca2+, and the atypical PKC isoforms (ζ, τ and λ) require neither Ca2+ nor DAG for their activation. Several studies have shown that PKCδ is involved in NOX2 activation [15, 16, 34]. We found that H2O2 induced PKCδ tyrosine phosphorylation, an effect that was inhibited by BAPTA, imatinib, or rottlerin. Also, while PKCδ is known as a Ca2+-independent PKC isoform, our results show that H2O2 induced Ca2+/c-Abl-dependent regulation of PKCδ. In accord with these findings, Ca2+ ionophore [35] and c-Abl [17] were shown to induce PKCδ tyrosine phosphorylation. In addition, we found that inhibition of PKCδ by rottlerin reduced the effect of H2O2 on NOX2 activation. However, these results only partially correlate with the total abrogation of PKCδ tyrosine311 phosphorylation by rottlerin, suggesting that the activation of NOX2, while mediated in large part through PKCδ, may also involve a Ca2+-dependent PKC [36]. Supporting this observation is the fact that staurosporine, a broad inhibitor of PKC, or Go6976, an inhibitor of classical PKC, either abrogated or reduced, respectively, superoxide production induced by H2O2 (data not shown). The potent effect of BAPTA on H2O2-NOX2 regulation is probably related to the fact that classical PKC directly and PKCδ indirectly are regulated by Ca2+. Furthermore, Ca2+ is implicated in the NOX2 assembly process through the regulation of cytoskeletal elements [37].
Since the activation of NOX2 is dependent upon activation of Rac through GTP/GDP exchange and c-Abl can activate small GTPases, including Rac1 [18, 38, 39], we investigated the role of Rac1/2 in H2O2-NOX2 regulation. Furthermore, c-Abl tyrosine phosphorylates Sos-1, a guanine nucleotide-exchange factor (GEF) that can elicit Rac-GEF activity in vitro [40]. In accord with those observations, we found that the pre-treatment of K562/NOX2 cells with toxin B of C. difficile (Fig. 6) decreased significantly the effect of H2O2 on superoxide production. Furthermore, BAPTA, imatinib, or rottlerin counteracted the activation of Rac by H2O2. These observations suggest that Rac is regulated by H2O2 downstream of the Ca2+/c-Abl/PKCδ pathway. In accord with our results, both Ca2+-dependent and Ca2+-independent PKC have been implicated in the activation and translocation of Rac through the phosphorylation of Rho family GTPases [36, 41, 42].
Although a number of mechanisms for the priming of NOX2 activation have been proposed, current evidence appears to favor partial phosphorylation of p47phox [43–45] and/or mobilization of the flavocytochrome from intracellular stores to the plasma membrane [44]. In these scenarios, Ca2+ signaling and tyrosine kinase pathways exhibit complementary roles [46–48]. In our study, while a synergistic effect of H2O2 was consistently observed with PMA (Fig. 1G, Fig. 2E), no priming was observed with 100 nM fMLF in PMNs (Fig. 8A). The absence of priming suggests that common signaling pathways are engaged by H2O2 and fMLF. Of note, fMLF involves a PTX-sensitive Gi protein and interestingly, PTX-sensitive Gi/Go proteins can be activated in a receptor-independent manner by H2O2 [19]. In PMNs, as well as K562/NOX2 cells, we found that pre-treatment with PTX significantly decreased the level of superoxide production induced by H2O2. Also, it is possible that H2O2 inhibits fMLF signaling by competing for Gi proteins. The participation of Gi proteins is furthermore supported by the observation that they regulate Ca2+ channels [49, 50]. Consistent with these mechanisms, pre-treatment with PMA induced extensive superoxide production that was not further enhanced by the subsequent addition of H2O2, suggesting that maximal recruitment and NOX2 assembly by PMA sequestered or masked one or more targets of H2O2.
Quite apart from the activation and translocation of the cytosolic cofactors (p67phox, p47phox, Rac) by H2O2, one cannot exclude that the catalytic core of the enzyme itself is modified by the treatment. If we assume that only the cytosolic factors are affected by H2O2, then reconstitution using any combination of membranes and/or cytosol isolated from treated cells should give the same result. The fact that superoxide produced by NOX2 reconstitution in the broken cell system using membranes plus cytosol obtained from treated cells is significantly higher than any other combination raises the possibility that the activity of the enzyme is increased. Since this increase was not correlated with any increase in NOX protein levels, it suggests that post-translational modification of NOX2 itself could contribute to superoxide production induced by H2O2. In accord with this hypothesis, it was recently reported that direct phosphorylation on serine/threonine in the C-terminal domain of NOX2 increased the diaphorase activity of a NOX2 fusion protein in the presence of p47phox, p67phox, and Rac2 [51]. Whether phosphorylation of NOX2 is involved in H2O2-NOX2 regulation remains to be determined, since our observations could also be explained by H2O2-induced activation events such that the membrane fractions harbor assembled complexes of flavocytochrome plus translocated cytosolic subunits.
Observations in many cell types suggest that Ca2+ and ROS signaling are interdependent [52–55]. Furthermore, sustained increases in either Ca2+ or ROS have been consistently associated with pathological processes. However, the mechanisms by which they mediate tissue injury remain poorly understood. Positive feedback redox regulation of NOX2, as well as other NOX family members [5], might represent a common mechanism for amplification of ROS generation. The potentially broad relevance of this mechanism is underscored by several facts: 1) NOX isoforms are expressed in all major organs and tissues [2]; 2) c-Abl is an ubiquitously expressed protein; and 3) H2O2 is a diffusible molecule that could act as either an autocrine or paracrine signaling factor. Furthermore, given the importance of neutrophils in tissue injury associated with immune reactions [56] and the enhancement of ROS production in many human diseases, such as acute respiratory distress syndrome [57], rheumatoid arthritis [58], atherosclerosis, ischemia/reperfusion-induced tissue injury, hypertension, and kidney disease [59–62], it appears worthwhile to consider NOX regulation by Ca2+ influx and c-Abl activation as both mechanisms and therapeutic targets in diseases associated with oxidative stress.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01-AI020866 and T32-HL04776) and the Department of Veterans Affairs. The confocal microscopy studies were performed in the University of Texas Health Science Center Institutional Optical Imaging Facility, which is supported by NIH grants P30-CA054174 (Cancer Therapy and Research Center), P30-AG013319 (Nathan Shock Center), and P01-AG019316 (Aging, Oxidative Stress and Cell Death). The authors would like to thank Alain Virion and Yves Gorin for helpful discussion and critical review of the manuscript, Victoria Frohlich for expert guidance on the confocal microscope image acquisition and analysis, John Cornell for insightful advice on statistical analysis, and Maria Gamez and Doran Pearson for expert technical assistance.
Abbreviations
- BAPTA
1,2-bis (2-aminophenoxy) ethane-N,N,N,N-tetraacetic acid
- DAG
diacylglycerol
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- fMLF
formyl-methionyl-leucyl-phenylalanine
- GEF
guanine nucleotide-exchange factor
- GFP
green fluorescent protein
- GSH
gluthatione
- GST
glutathione S-transferase
- DHE
dihydroethidium
- H2O2
hydrogen peroxide
- NOX
NADPH oxidase
- PBS
phosphate-buffered saline
- PBS-G
PBS containing 10 mM glucose
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PTX
pertussis toxin
- ROS
reactive oxygen species
- SERCA
sarco-endoplasmic reticulum Ca2+-ATPase
- SOC channels
store-operated Ca2+ channels
- SOCE
store-operated Ca2+ entry
- TG
thapsigargin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosure Statement
Dr. Clark is a scientific co-founder, member of the Scientific Advisory Board, and equity holder in GenKyoTex SA, a Swiss biotechnology start-up company with a primary goal of developing clinically useful inhibitors of the NOX family of NADPH oxidases. No other competing financial interests exist.
References
- 1.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radical Biology and Medicine. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedard K, Krause K-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 3.Han CH, Freeman JL, Lee T, Motalebi SA, Lambeth JD. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox) J. Biol. Chem. 1998;273:16663–16668. doi: 10.1074/jbc.273.27.16663. [DOI] [PubMed] [Google Scholar]
- 4.Bokoch GM, Ulla GK. NADPH oxidases: not just for leukocytes anymore! Trends Biochem. Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 5.El Jamali A, Valente AJ, Lechleiter JD, Gamez M, W PD, Nauseef MW, Clark R. A. Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic. Biol. Med. 2008;44:868–881. doi: 10.1016/j.freeradbiomed.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause K-H, Cox JA. Mechanism of Ca2+ Activation of the NADPH Oxidase 5 (NOX5) J. Biol. Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 7.Brechard S, Tschirhart EJ. Regulation of superoxide production in neutrophils: role of calcium influx. Journal of leukocyte biology. 2008;84:1223–1237. doi: 10.1189/jlb.0807553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishibashi K, Okazaki S, Hiramatsu M. Simultaneous measurement of superoxide generation and intracellular Ca2+ concentration reveals the effect of extracellular Ca2+ on rapid and transient contents of superoxide generation in differentiated THP-1 cells. Biochemical and Biophysical Research Communications. 2006;344:571–580. doi: 10.1016/j.bbrc.2006.02.173. [DOI] [PubMed] [Google Scholar]
- 9.Borregaard N, Heiple JM, Simons ER, Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J. Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark RA, Valente AJ. Nuclear factor kappa B activation by NADPH oxidases. Mech. Ageing Dev. 2004;125:799–810. doi: 10.1016/j.mad.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Schaffner W, Weissman C. A rapid, sensitive and specific method for the determination of protein in dilute solution. Anal. Biochem. 1973;56:502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- 12.Massey V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochem.Biophys.Acta. 1959;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- 13.Benard V, Bohl BP, Bokoch GM. Characterization of Rac and Cdc42 Activation in Chemoattractant-stimulated Human Neutrophils Using a Novel Assay for Active GTPases. J. Biol. Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- 14.Burritt JB, Quinn MT, Jutila MA, Bond CW, Jesaitis AJ. Topological Mapping of Neutrophil Cytochrome b Epitopes with Phage-display Libraries. J. Biol. Chem. 1995;270:16974–16980. doi: 10.1074/jbc.270.28.16974. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Xu B, Bhattacharjee A, Oldfield CM, Wientjes FB. Protein kinase Cdelta regulates p67phox phosphorylation in human monocytes. J. Leukoc. Biol. 2005;77:414–420. doi: 10.1189/jlb.0504284. [DOI] [PubMed] [Google Scholar]
- 16.Bey EA, Xu B, Bhattacharjee A, Oldfiled CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM, Wientjes FB, Cathcart MK. Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J. Immunol. 2004;173:5730–5738. doi: 10.4049/jimmunol.173.9.5730. [DOI] [PubMed] [Google Scholar]
- 17.Sun X, Wu F, Datta R, Kharbanda S, Kufe D. Interaction between Protein Kinase C delta and the c-Abl Tyrosine Kinase in the Cellular Response to Oxidative Stress. J. Biol. Chem. 2000;275:7470–7473. doi: 10.1074/jbc.275.11.7470. [DOI] [PubMed] [Google Scholar]
- 18.Boureux A, Furstoss O, Simon V, Roche S. Abl tyrosine kinase regulates a Rac/JNK and a Rac/Nox pathway for DNA synthesis and Myc expression induced by growth factors. J Cell Sci. 2005;118:3717–3726. doi: 10.1242/jcs.02491. [DOI] [PubMed] [Google Scholar]
- 19.Nishida MMY, Tanaka R, Kontani K, Nagao T, Kurose H. G alpha(i) and G alpha(o) are target proteins of reactive oxygen species. Nature. 2000;408:492–495. doi: 10.1038/35044120. [DOI] [PubMed] [Google Scholar]
- 20.Li W-G, Miller FJ, Jr, Zhang HJ, Spitz DR, Oberley LW, Weintraub NL. H2O2-induced O-.2 Production by a Non-phagocytic NAD(P)H Oxidase Causes Oxidant Injury. J. Biol. Chem. 2001;276:29251–29256. doi: 10.1074/jbc.M102124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tintinger GR, Annette JT, Moliehi P, Ronald A. Reactive oxidants regulate membrane repolarization and store-operated uptake of calcium by formyl peptide-activated human neutrophils. Free Rad. Biol. Med. 2007;42:1851–1857. doi: 10.1016/j.freeradbiomed.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 22.Foyouzi-Youssefi R, Petersson F, Lew DP, Krause KH, Nusse O. Chemoattractant-induced respiratory burst: increases in cytosolic Ca2+ concentrations are essential and synergize with a kinetically distinct second signal. Biochem. J. 1997;322:709–718. doi: 10.1042/bj3220709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valentin F, Bueb JL, Capdeville-Atkinson C, Tschirhart E. Rac-1-mediated superoxide secretion requires Ca2+influx in neutrophil-like HL-60 cells. Cell Calcium. 2001;29:409–415. doi: 10.1054/ceca.2001.0203. [DOI] [PubMed] [Google Scholar]
- 24.Granfeldt D, Samuelsson M, Karlsson A. Capacitative Ca2+ influx and activation of the neutrophil respiratory burst. Different regulation of plasma membrane- and granule-localized NADPH-oxidase. J Leukoc Biol. 2002;71:611–617. [PubMed] [Google Scholar]
- 25.Pozzan T, Lew DP, Wollheim CB, Tsien RY. Is cytosolic ionized calcium regulating neutrophil activation? Science (New York, N.Y.) 1983;221:1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- 26.Gallois A, Bueb JL, Tschirhart E. Effect of SK&F 96365 on extracellular Ca2+-dependent O2- production in neutrophil-like HL-60 cells. Eur. J. Pharmacol. 1998;361:293–298. doi: 10.1016/s0014-2999(98)00728-6. [DOI] [PubMed] [Google Scholar]
- 27.Putney JW. Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drøbak BK, Bjerrum PJ, Christensen SB, Hanley M. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents and actions. 1989;27:17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- 29.Boulware MJ, Marchant JS. Timing in cellular Ca2+ signaling. Curr. Biol. 2008;18:R769–R776. doi: 10.1016/j.cub.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Druker BJTS, Buchdunger E, Ohno S, Segal GM, Fanning S, Zimmermann J, Lydon NB. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat. Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 31.Kumar S, Mishra N, Raina D, Saxena S, Kufe D. Abrogation of the Cell Death Response to Oxidative Stress by the c-Abl Tyrosine Kinase Inhibitor STI571. Mol Pharmacol. 2003;63:276–282. doi: 10.1124/mol.63.2.276. [DOI] [PubMed] [Google Scholar]
- 32.Sattler M, Verma S, Shrikhande G, Byrne CH, Pride YB, Winkler T, Greenfield EA, Salgia R, Griffin JD. The BCR/ABL Tyrosine Kinase Induces Production of Reactive Oxygen Species in Hematopoietic Cells. J. Biol. Chem. 2000;275:24273–24278. doi: 10.1074/jbc.M002094200. [DOI] [PubMed] [Google Scholar]
- 33.Furstoss O, Dorey K, Simon V, Barila D, Superti-Furga G, Roche S. c-Abl is an effector of Src for growth factor-induced c-myc expression and DNA synthesis. EMBO J. 2002;21:514–524. doi: 10.1093/emboj/21.4.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 35.Woolfolk EA, Eguchi S, Ohtsu H, Nakashima H, Ueno H, Gerthoffer WT, Motley ED. Angiotensin II-induced activation of p21-activated kinase 1 requires Ca2+ and protein kinase C{delta} in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2005;289:C1286–C1294. doi: 10.1152/ajpcell.00448.2004. [DOI] [PubMed] [Google Scholar]
- 36.Majumdar S, Kane LH, Rossi MW, Volpp BD, Nauseef WM, Korchak HM. Protein kinase C isotypes and signal-transduction in human neutrophils: selective substrate specificity of calcium-dependent beta-PKC and novel calcium-independent nPKC. Biochim. Biophys. Acta. 1993;1176:276–286. doi: 10.1016/0167-4889(93)90056-u. [DOI] [PubMed] [Google Scholar]
- 37.Howard TH, Wand D. Calcium ionophore, phorbol ester, and chemotactic peptide-induced cytoskeleton reorganization in human neutrophils. J. Clin. Invest. 1987;79:1359–1364. doi: 10.1172/JCI112962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skorski T, Wlodarski P, Daheron L, Salomoni P, Nieborowska-Skorska M, Majewski M, Wasik M, Calabretta B. BCR/ABL-mediated leukemogenesis requires the activity of the small GTP-binding protein Rac. PNAS. 1998;95:11858–11862. doi: 10.1073/pnas.95.20.11858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizuchi D, Kurosu T, Kida A, Jin Z-H, Jin A, Arai A, Miura O. BCR/ABL activates Rap1 and B-Raf to stimulate the MEK/Erk signaling pathway in hematopoietic cells. Biochem. Biophys. Res. Commun. 2005;326:645–651. doi: 10.1016/j.bbrc.2004.11.086. [DOI] [PubMed] [Google Scholar]
- 40.Sini P, Cannas A, Koleske AJ, Paolo Di Fiore P, Scita G. Abl-dependent tyrosine phosphorylation of Sos-1 mediates growth-factor-induced Rac activation. Nat. Cell Biol. 2004;6:268–274. doi: 10.1038/ncb1096. [DOI] [PubMed] [Google Scholar]
- 41.Price LS, Langeslag M, Klooster JPt, Hordijk PL, Jalink K, Collard JG. Calcium Signaling Regulates Translocation and Activation of Rac. J. Biol. Chem. 2003;278:39413–39421. doi: 10.1074/jbc.M302083200. [DOI] [PubMed] [Google Scholar]
- 42.Buchanan FG, Elliot CM, Gibbs M, Exton JH. Translocation of the Rac1 Guanine Nucleotide Exchange Factor Tiam1 Induced by Platelet-derived Growth Factor and Lysophosphatidic Acid. J. Biol. Chem. 2000;275:9742–9748. doi: 10.1074/jbc.275.13.9742. [DOI] [PubMed] [Google Scholar]
- 43.Dang PM, Stensballe A, Boussetta T, Raad H, Dewas C, Kroviarski Y, Hayem G, Jensen ON, Gougerot-Pocidalo MA, El-Benna J. A specific p47phox -serine phosphorylated by convergent MAPKs mediates neutrophil NADPH oxidase priming at inflammatory sites. J. Clin. Invest. 2006;116:2033–2043. doi: 10.1172/JCI27544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeLeo FR, Renee J, McCormick S, Nakamura M, Apicella M, Weiss JP, Nauseef WM. Neutrophils exposed to bacterial lipopolysaccharide upregulate NADPH oxidase assembly. J. Clin. Invest. 1998;101:455–463. doi: 10.1172/JCI949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown GE, Stewart MQ, Bissonnette SA, Elia AEH, Wilker E, Yaffe MB. Distinct Ligand-dependent Roles for p38 MAPK in Priming and Activation of the Neutrophil NADPH Oxidase. J. Biol. Chem. 2004;279:27059–27068. doi: 10.1074/jbc.M314258200. [DOI] [PubMed] [Google Scholar]
- 46.Walker BA, Ward PA. Priming and signal transduction in neutrophils. Biological signals. 1992;1:237–249. doi: 10.1159/000109329. [DOI] [PubMed] [Google Scholar]
- 47.Finkel TH, Pabst MJ, Suzuki H, Guthrie LA, Forehand JR, Phillips WA, Johnston RB., Jr Priming of neutrophils and macrophages for enhanced release of superoxide anion by the calcium ionophore ionomycin. Implications for regulation of the respiratory burst. J. Biol. Chem. 1987;262:12589–12596. [PubMed] [Google Scholar]
- 48.McPhail LC, Clayton CC, Snyderman R. The NADPH oxidase of human polymorphonuclear leukocytes. Evidence for regulation by multiple signals. J. Biol. Chem. 1984;259:5768–5775. [PubMed] [Google Scholar]
- 49.Hu C, Hu C, Depuy SD, Yao J, McIntire WE, Barrett PQ. Protein kinase A activity controls the regulation of T-type CaV3.2 channels by Gbetagamma dimers. The Journal of biological chemistry. 2009;284:7465–7473. doi: 10.1074/jbc.M808049200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DePuy SD, Yao J, Hu C, McIntire W, Bidaud I, Lory P, Rastinejad F, Gonzalez C, Garrison JC, Barrett PQ. The molecular basis for T-type Ca2+ channel inhibition by G protein beta2gamma2 subunits. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:14590–14595. doi: 10.1073/pnas.0603945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raad H, Paclet M-H, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo M-A, Dang PM-C, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. FASEB J. 2008 doi: 10.1096/fj.08-114553. fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lounsbury KM, Hu Q, Ziegelstein RC. Calcium signaling and oxidant stress in the vasculature. Free Radic. Biol. Med. 2000;28:1362–1369. doi: 10.1016/s0891-5849(00)00222-7. [DOI] [PubMed] [Google Scholar]
- 53.Tabet F, Savoia C, Schriffrin EL, Touyz RM. Differential Calcium Regulation by Hydrogen Peroxide and Superoxide in Vascular Smooth Muscle Cells from Spontaneously Hypertensive Rats. J. Cardio. Pharmacol. 2004;44:200–208. doi: 10.1097/00005344-200408000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Hidalgo C, Donoso P. Crosstalk between calcium and redox signaling: from molecular mechanisms to health implications. Antiox. Redox Signal. 2008;10:1275–1312. doi: 10.1089/ars.2007.1886. [DOI] [PubMed] [Google Scholar]
- 55.Hu Q, Zheng G, Zweier JL, Deshpande S, Irani K, Ziegelstein RC. NADPH Oxidase Activation Increases the Sensitivity of Intracellular Ca2+ Stores to Inositol 1,4,5-Trisphosphate in Human Endothelial Cells. J. Biol. Chem. 2000;275:15749–15757. doi: 10.1074/jbc.M000381200. [DOI] [PubMed] [Google Scholar]
- 56.Weiss SJ. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 57.Chollet-Martin S, Montravers P, Gibert C, Elbim C, Desmonts JM, Fagon JY, Gougerot-Pocidalo MA. Subpopulation of hyperresponsive polymorphonuclear neutrophils in patients with adult respiratory distress syndrome. Role of cytokine production. Am. Rev. Respir. Dis. 1992;146:990–996. doi: 10.1164/ajrccm/146.4.990. [DOI] [PubMed] [Google Scholar]
- 58.Nurcombe HL, Bucknall RC, Edwards SW. Neutrophils isolated from the synovial fluid of patients with rheumatoid arthritis: priming and activation in vivo. Ann. Rheum. Dis. 1991;50:147–153. doi: 10.1136/ard.50.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cai H. NAD(P)H Oxidase-Dependent Self-Propagation of Hydrogen Peroxide and Vascular Disease. Circ Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 60.Fan J, Li Y, Levy RM, Fan JJ, Hackam DJ, Vodovotz Y, Yang H, Tracey KJ, Billiar TR, Wilson MA. Hemorrhagic Shock Induces NAD(P)H Oxidase Activation in Neutrophils: Role of HMGB1-TLR4 Signaling. J Immunol. 2007;178:6573–6580. doi: 10.4049/jimmunol.178.10.6573. [DOI] [PubMed] [Google Scholar]
- 61.Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor WR, Griendling KK. Role of NADH/NADPH Oxidase–Derived H2O2 in Angiotensin II-Induced Vascular Hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]
- 62.Sela S, Shurtz-Swirski R, Cohen-Mazor M, Mazor R, Chezar J, Shapiro G, Hassan K, Shkolnik G, Geron R, Kristal B. Primed Peripheral Polymorphonuclear Leukocyte: A Culprit Underlying Chronic Low-Grade Inflammation and Systemic Oxidative Stress in Chronic Kidney Disease. J Am Soc Nephrol. 2005;16:2431–2438. doi: 10.1681/ASN.2004110929. [DOI] [PubMed] [Google Scholar]