Abstract
Background and purpose
Anticipatory postural adjustments (APAs), prior to step initiation, are bradykinetic in advanced Parkinson's disease (PD) and may be one of the factors associated with ‘start hesitation’. However, little is known about APAs in the early stage of PD. In this study, we determined whether body-worn accelerometers could be used to characterize step initiation deficits in subjects with early-to-moderate, untreated PD.
Methods
Eleven PD and 12 healthy control subjects were asked to take two steps. Postural adjustments were compared from center of pressure (COP) and from acceleration of the trunk at the center of mass level (L5).
Results
Our findings show that APAs measured from the peak COP displacement towards the swing leg and the peak trunk acceleration towards the stance leg were smaller in untreated PD compared to control subjects. The magnitude of APAs measured from peak COP displacements and accelerations were correlated.
Conclusion
These results suggest that quantitative analysis of step initiation from one accelerometer on the trunk could provide useful information for the characterization of patients in early stages of PD, when clinical evidence of start hesitation may not be detectable. Ambulatory monitoring of step initiation is also promising for monitoring patient progression in the home environment, and eventually providing feedback for preventing freezing of gait episodes.
Keywords: Parkinson's Disease, step initiation, freezing, posture, accelerometers
INTRODUCTION
When attempting to voluntarily initiate the first step to begin walking, many patients with PD exhibit start hesitation and freezing, especially in advanced stages of the disease [1;2]. Patients with advanced PD generally show bradykinetic step initiation, measured as increased movement preparation time, reduced lateral shift of the body mass over the stance limb and decreased propulsive forces [3-5]. These abnormalities of step initiation are sensitive to levodopa replacement [5;6], external cues [5], initial stance width [6], and to bilateral deep brain stimulation of the Subthalamic nucleus [7-9].
However, it has been debated whether step initiation is impaired in early stages of PD, before the start of dopaminergic medication [10]. The only study of step initiation in early to moderate PD reported smaller than normal initial backward displacement of the center of pressure under the feet compared to control subjects, but many of these subjects were taking medications that may affect step initiation [11]. Since patients in early stages of PD do not show clinical signs of balance or gait problems, quantitative detection of deficits of step initiation could provide early markers for later developing problems. For these reasons, the evaluation of step initiation in untreated PD represents a novel, valid, and objective measure to test the effects of potential neuroprotective drugs.
Step initiation requires a tight proprioceptive coordination between motor commands for postural adjustments and for stepping [12-14]. Immediately prior to step initiation, anticipatory postural adjustments (APAs) act to accelerate the center of body mass forward and laterally over the stance foot by moving the center of pressure (COP) posteriorly and toward the stepping leg [15]. APAs are thought to be initiated via motor circuits including the supplementary motor area (SMA), that are independent from the more volitional lifting of the foot during step initiation [14]. APAs prior to step initiation are usually described using force plates and EMG activation patterns [16-18]. The backward COP displacement results from a deactivation of bilateral gastrocnemius and soleus muscles, and activation of tibialis anterior; the lateral COP displacement is a consequence of preloading of the stepping foot by the hip abductors [15;19].
However, the cost and complexity of measuring APAs using traditional motion analysis, force plate, and EMG systems limit their application to clinical practice. Recently, small, inexpensive, wearable inertial sensors such as accelerometers have been used to quantify gait and postural sway [20-22]. Our group has recently demonstrated how to detect APAs, represented by anticipatory trunk accelerations, prior to step initiation in young healthy subjects [23].
The purpose of this study was to determine if APAs prior to step initiation were bradykinetic in early, untreated PD and if trunk acceleration measures could be used to differentiate the magnitude of APAs in untreated PD from age-matched controls.
METHODS
Participants
Eleven patients with idiopathic PD and 12 age-matched healthy control subjects participated in this study. The diagnosis of idiopathic PD was made by a movement disorders expert by clinical exam, history and any pertinent laboratory results. Only patients who were early-to-middle stage in disease course and had never been treated with dopaminergic or anti-parkinsonian medication were invited to participate. Subjects were excluded if they presented any neurological disorders other than PD, orthopedic disorders or other impairments that could potentially interfere with gait, or if they used orthotic devices or had artificial joints.
Patients were clinically rated by a trained examiner on the Motor Section (III) of the Unified Parkinson's Disease Rating Scale and the Hoehn and Yahr Scale immediately before the experimental sessions. Table 1 summarizes subject characteristics, ordered by severity of PD. All participants provided informed consent according to the Oregon Health & Sciences University Institutional Review Board.
Table 1.
Characteristics (individual means and group means ±S.E.M) of subjects with Parkinson's disease, sorted according to severity of UPDRS Motor Score. Abbreviations: H&Y=Hoehn and Yahr Scale, PIGD=Postural Instability and Gait Disorder subscore for the UPDRS III, Peak ML-COP= peak lateral center of pressure excursion toward the swing foot during the APA.
| Subj | AGE (years) |
Disease onset (months) |
H&Y | UPDRS III Total Motor Score |
Bradykinesia (Items 23:27) subscore |
PIGD (Items 27:30) subscore |
Peak ML- COP (mm) |
|---|---|---|---|---|---|---|---|
| P1 | 63 | 26 | 3 | 46 | 21 | 5 | 19.03 |
| P2 | 77 | 13 | 2.5 | 45 | 19 | 2 | 25.17 |
| P3 | 70 | 15 | 2.5 | 35 | 15 | 4 | 39.9 |
| P4 | 50 | 10 | 2 | 35 | 19 | 1 | 25.28 |
| P5 | 53 | 26 | 2 | 33 | 17 | 0 | 36.6 |
| P6 | 64 | 11 | 2 | 32 | 13 | 1 | 8.65 |
| P7 | 61 | 13 | 2 | 27 | 17 | 0 | 21.38 |
| P8 | 61 | 8 | 2 | 21 | 9 | 1 | 24.25 |
| P9 | 58 | 48 | 1.5 | 21 | 10 | 0 | 28.83 |
| P10 | 58 | 22 | 1 | 17 | 10 | 0 | 17.1 |
| P11 | 49 | 5 | 1 | 7 | 2 | 0 | 28.12 |
|
| |||||||
| Mean | 60.3 | 17.9 | 1.9 | 29 | 13.82 | 1.27 | 24.94 |
| SEM | 0.76 | 1.11 | 0.05 | 1.08 | 0.51 | 0.16 | 0.79 |
Experimental Setup
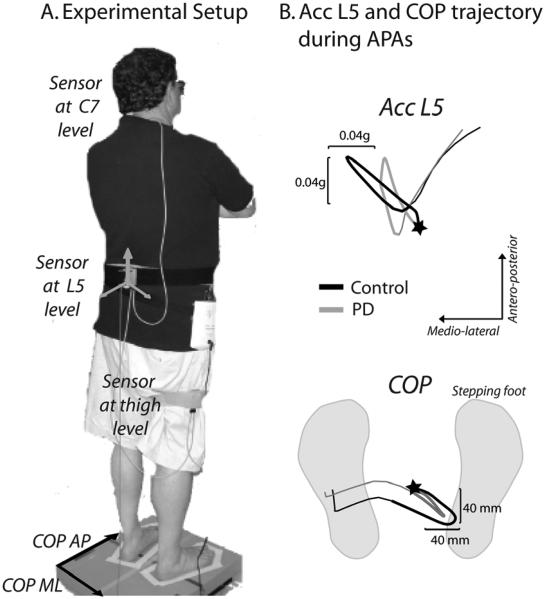
At the beginning of each trial, the subjects stood on a force plate (AMTI OR6-6, Watertown, MA) with feet externally rotated at their comfortable stance but with heel-to-heel distance fixed at 10 cm for all subjects. Initial stance position was consistent from trial-to-trial by tracing foot outlines on the force plate and by coaching subjects to maintain their initial COP position prior to each trial based on oscilloscope COP traces. Subjects wore 3 MTX Xsens sensors (49A33G15, XSens, Enschede, NL) with 3-D accelerometers (±1.7g range), and 3-D gyroscopes, (±300°/s range) mounted on: i) the posterior trunk at the level of L5, near the body center of mass, ii) lateral aspect of the right thigh, limb that took the first step, and iii) the spinous process of C7. Accelerations sensed at C7 level were not reliable measures of APAs because of inadvertent head and trunk motion, and will not be considered further. The sensing axes were oriented along the body antero-posterior, medio-lateral, and vertical directions. Figure 1A shows the experimental setup. Subjects were instructed to self-initiate two steps, starting with the right foot, at their normal, comfortable pace as if they were going to start walking. Three trials of step initiation were acquired.
Figure 1.
Experimental set-up and representative trunk acceleration and center of pressure (COP) trajectory during APA.
A. Experimental setup: sensors placement on a subject and tape around feet for consistent foot placement on the force plate.
B. The trajectories of trunk acceleration sensed at L5 level and of COP from the force plate during APAs in two representative subjects: control subject (black) and PD subject (grey). The stars represent the start of APAs. The total APA phase is represented by the bold COP and acceleration trajectories.
APAs were evaluated from COP displacements recorded from the force plate with a 100-Hz sampling frequency after applying a 10-Hz cut-off, zero phase, low-pass Butterworth filter to the ground reaction forces. APAs were also evaluated from L5 acceleration data collected with a 50-Hz sampling frequency, transformed to horizontal-vertical coordinate system [24] and filtered with a 3.5 Hz cut-off, zero-phase, low-pass Butterworth filter.
Data analysis and extracted features
A specific set of step initiation features was automatically extracted by ad hoc algorithms and then visually inspected to check for possible errors in the event classification. Figure 2 shows examples of COP (A and C) and acceleration (B and D) signals collected from a representative control and PD subject both in medio-lateral (ML) and antero-posterior (AP) directions, together with the extracted features.
Figure 2.
COP and trunk acceleration at L5 during APAs in two representative subjects: control subject (black) and PD subject (grey). Left panel: medio-lateral direction, A. COP trajectories and B. trunk acceleration trajectories. Right panel: antero-posterior direction, C. COP trajectories and D. trunk acceleration trajectories. Parameters computed from the trajectories (peak, time-to-peak, and duration of APAs) are shown. The onset and end of the APAs are represented by the dashed lines.
The following APA characteristics were compared from the COP displacements and trunk accelerations: 1) APA duration (from the onset to the end of APAs), 2) APA ML amplitude, that is (i) Peak ML-COP, peak lateral COP excursion toward the swing foot from baseline and (ii) Peak ML-Acc, peak acceleration toward the stance foot of the lateral trunk acceleration; and 3) APA AP amplitude, that is (i) Peak AP-COP, peak of backward COP excursion and (ii) Peak AP-Acc, forward trunk acceleration from the baseline. Relative time-to-peak, from the onset of APAs to the instant of each peak APA, was also measured.
The onset of APAs (first measurable change in COP from baseline) was detected by an automated threshold-based algorithm, with threshold set as twice the standard deviation of signal during the initial, pre-step initiation, period of each trial. The APAs were considered completed (end of APAs) when both the AP and ML COP went back to their baseline values.
To determine the relationship between the APAs and the velocity and length of the first step, the gyroscope sensing AP thigh angular velocity was used to determine: i) time-to-peak angular velocity (from the onset of APAs to the instant of peak angular velocity), and ii) range of motion of the thigh (calculated from the integrated sagittal angular velocity), respectively, approximate indicator of the velocity and length of the first step.
Statistical analyses
The relation between extracted APA and first step properties was investigated by linear regression analysis, as well as the relation between the UPDRS Motor scores and APA features. For each feature, a separate one-way ANOVA was used to detect differences between the control versus the PD group. For the entire set of statistical analyses the level of significance was set at p<0.05. All the analyses were performed with NCSS Software, Kaysville, Utah.
RESULTS
Force plate measures of APAs
The COP trajectory typical for gait initiation (Figure 1B) is maintained in untreated PD subjects, although quantitative changes, especially in lateral COP excursion, were detected.
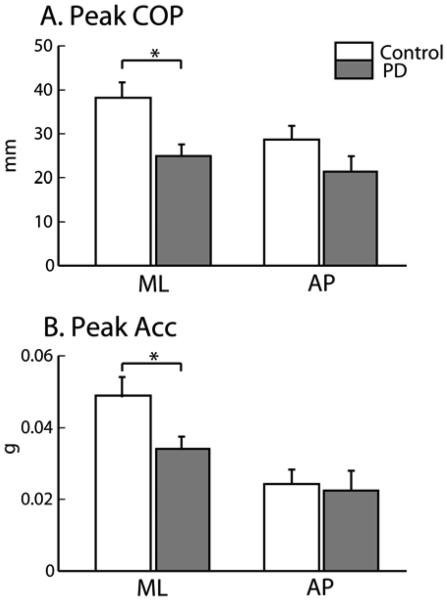
Peak ML-COP in the PD group is clearly reduced compared to control subjects, while Peak AP-COP is not, as shown in Figure 2-A and 2-C by examples in representative control and PD subjects. Figure 3-A shows the same result for the groups: the mean value of Peak ML-COP in the PD group was significantly smaller than in the control group, p=0.007, (mean ± SEM; 24.94 ± 0.79 mm in PD versus 38.21 ± 1.02 mm in control), while Peak AP-COP was not significantly smaller in the PD than control group (mean ± SEM; 21.34 ± 1.1 mm in PD versus 28.7 ± 0.9 mm in control).
Figure 3.
Comparison of group mean APAs in PD and control subjects. The mean values (±S.E.M) of Peak COP (A) and Peak Acc (B) are presented. Significant differences are showed with * p<0.05.
Inertial sensor measures of APAs and step kinematics
As expected, the trunk accelerometer detected a pattern for the center of body mass motion reciprocally linked with the COP displacement pattern during the APA; the trunk acceleration showed a medial-forward excursion in correspondence to the lateral-backward displacement of the COP. Similar to the Peak ML-COP, Peak ML-Acc was significantly smaller in PD compared to control subjects, p=0.01, (mean ± SEM; 0.034 ± 0.001 g in PD versus 0.049 ± 0.0015 g in control), while Peak AP-Acc was not (mean ± SEM; 0.022 ± 0.001 g in PD versus 0.024 ± 0.001 g in control). See Figure 2B and 2D for representative subject APA trajectories and Figure 3B for group comparisons.
Unlike APA peak magnitudes, the durations of the APAs were similar between the PD and control groups (mean ± SEM; 0.55 ± 0.01 s in PD, and 0.54 ± 0.01 s in control subjects). Also, time-to-peak APAs, detected from both COP and accelerations in both the ML and AP directions, were similar across the two groups (mean ± SEM from COP; ML direction: 0.40 ± 0.08 s in PD, and 0.39 ± 0.01 s in control subjects; AP direction: 0.37 ± 0.008 s in PD, and 0.38 ± 0.01 s in control subjects).
Despite the very small peak APA magnitude in subjects with PD, the velocity and length of the first step were not significantly compromised in untreated PD subjects. The time-to-peak forward angular velocity, indicator of step velocity (mean ± SEM; 0.99 ± 0.38 s versus 0.77 ± 0.17 s) and range of thigh motion, indicator of step length, were similar across groups (mean ± SEM; 22.62 ± 0.36° versus 22.66 ± 0.22°).
Correlation between force plate-based and acceleration-based features of APAs
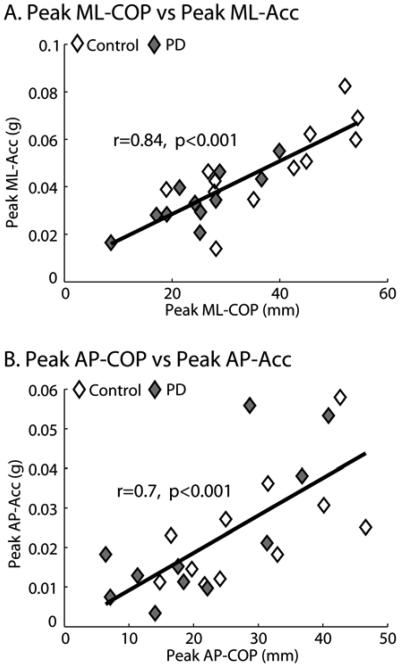
A significant linear correlation was found between COP and acceleration measures of the APA peaks in the ML direction ( r=0.84, p=0.00001; Figure 4.A), and in the AP direction (r=0.7, p=0.0004; Figure 4.B).
Figure 4.
Linear correlation between Peak COP and Peak Acc presented for the medio-lateral (A) and antero-posterior (B) directions. Data are combined for PD (grey rhombus) and control subjects (white rhombus).
The time-to-peak forward thigh angular velocity correlated significantly with the magnitude of the APAs: Peak ML-COP (r=−0.52, p=0.01); Peak ML-Acc (r=−0.49, p=0.04); and Peak AP-COP (r=−0.61 p=0.004); Peak AP-Acc (r=−0.58, p=0.006).
However, the Peak ML-COP, Peak AP-COP and Peak ML-Acc, Peak AP-Acc variables were not correlated with the length of the first step. All correlations between Peak ML, AP-COP, or Peak ML, AP-Acc, and UPDRS Motor Section scores were not significant.
DISCUSSION
The results of this study show, for the first time, that lateral APAs are impaired in early, untreated PD, and how wearable inertial sensors can detect this impairment.
APAs are hypometric in the ML direction
Reduced APAs are a specific, primary symptom of PD, responsible for severe balance and mobility problems [13;25;26]. In the current study, we found small peak APAs in subjects with mild PD, even though the velocity and length of their first steps were not slower or smaller than steps of control subjects. These results are consistent with separate, interacting motor programs for neural control of APAs and the step itself [14;25;27-29]. Early PD may affect the supplementary motor cortex, responsible for APAs before it affects the primary motor cortex and other areas responsible for generating force for stepping [4;14;16;18].
We found a significantly smaller lateral, but not backward, COP displacement during the APAs in subjects with untreated PD, compared to control subjects. This result is in contrast with Carpinella et al. [11] who found a significant reduction of backward displacement of the COP in early PD, but did not report subjects' lateral COP displacements. This discrepancy may be explained by differences in the required task, since our subjects were instructed to take only two steps, whereas their subjects initiated gait, or to differences in the starting foot placement position. We required all subjects to stand with a standard stance width. If the Carpinella's study allowed narrow or self-selected, variable stance width, it would be difficult to detect differences in lateral APAs between groups [6]. In addition, inclusion criteria for PD subjects were different, since all of our subjects were untreated, whereas several subjects in the Carpinella's study were tested on dopaminergic medication. Similar to our results, Carpinella et al. [11] showed similar APA durations for PD and control subjects.
The smaller lateral, but not backward, APA magnitudes suggests that the pathology of PD may have a specific effect on loading/unloading of the legs early in the disease, consistent with abnormal ML, but not AP, sway during quiet stance in PD [30]. Later in the disease, the magnitude of both lateral and backward APAs becomes bradykinetic in PD [18]. Unlike subjects with early PD, healthy elderly subjects show reduced backward, but not lateral, APA magnitudes compared to young subjects, consistent with separate neural control of these two directions of APAs, which have separate functions [11]. Force for lateral APAs come primarily from hip abductors and ankle extensors, so weakness of these muscles could contribute to small lateral APAs in PD. In contrast, force for anterior-posterior APAs come from tibialis anterior muscles, so weakness of ankle dorsiflexors may contribute to small APAs in the elderly. PD subjects also show particularly impaired control of ML sway in quiet stance, consistent with deficits in neural control or proprioception affecting loading and unloading mechanisms that may be important for freezing [31;32]. Since the application of smaller forces to initiate movement results in smaller self-induced postural perturbations, it is also possible that small lateral APAs represent a strategy to minimize postural instability [11;19;33]. We consider lateral postural instability an unlikely explanation for small lateral APAs because as the disease progresses, PD subjects gradually narrow their stance width to compensate for their inability to scale up the size of their bradykinetic APAs [6].
Although step length and step velocity were not different between groups, first step velocity was significantly correlated with backward and lateral APA magnitude for both groups, consistent with previous studies in healthy subjects [23;34]. Breniere et al., 1987 [35] showed that steady state gait velocity is correlated with the size and duration of backward APAs, but also to differences in the initial posture and proprioceptive availablity on the sole of the foot.
It is unclear whether the magnitude of lateral APAs before step initiation is a good biomarker for PD progression. Although APA magnitude was significantly smaller in early PD, than control, subjects, it was not correlated with their UPDRS Motor Scores (ranging from 7 to 46) or PIDG (Items 27, 28, 29, and 30) or bradykinesia (Items 23, 24, 25, and 26) subscores. This could be due to the fact that APA magnitude may not be a measure of PD severity or bradykinesia, but rather a precursor to freezing. In addition to the small amplitude of lateral APAs observed in our mild subjects, subjects with more advanced PD also show prolonged APA durations, small backward APA amplitude, and significantly slower execution of the first step [4-6;18]. Longitudinal studies are needed to determine which characteristics of postural preparation for step initiation are related to disease progression.
Acceleration sensed at the trunk can detect impaired APAs in untreated PD
Accelerations of the trunk prior to step initiation reliably characterize APAs, similar to a mirror image of COP displacements, as have been measured traditionally. This reflection of trunk acceleration from COP displacements likely reflects center of mass accelerations during the APA phase [14;34]. In addition, our results showed that acceleration-based extracted features in the APA phase can detect the same impairments in untreated PD as the force plate-based extracted features, specifically, reduced lateral displacement to unload the initial stepping leg. As a compliment to measuring APAs, Breniere et al. [36], demonstrated how to use accelerometers to analyze motions of the hips, shoulders, and estimated body center of gravity before and at heel-off, prior to a step.
Clinicians cannot readily observe small postural preparation or reduced velocity of the first step related to start hesitation. APA detection via accelerometers provides a new, promising application for clinical practice and clinical trials. The acceleration signal via a sensor on a belt is easy to acquire and wireless, portable versions of inertial sensors are becoming available. In this way, step initiation can be measured in a clinical or home environment to monitor progression of start hesitation and sensitivity to interventions, and eventually, to provide biofeedback for preventing freezing of gait.
In conclusion, quantitative analysis of step initiation in untreated PD, with one accelerometer on the trunk, provides useful information for the characterization of patients in early stages of PD, when clinical evidence of start hesitation may not be detectable. Future studies are needed to determine whether patients with the smallest APAs are more likely to later develop freezing with start hesitation.
Acknowledgements
The authors thank Triana Nagel-Nelson for assistance in data collection and Arash Salarian and Edward King for technical assistance. Supported by the Kinetics Foundation and by the National Institutes on Aging (NIA 006457).
Reference List
- 1.Giladi N, McMahon D, Przedborski S, Flaster E, Guillory S, Kostic V, Fahn S. Motor blocks in Parkinson's disease. Neurology. 1992;42:333–339. doi: 10.1212/wnl.42.2.333. [DOI] [PubMed] [Google Scholar]
- 2.Nieuwboer A, Dom R, De WW, Desloovere K, Janssens L, Stijn V. Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson's disease. Brain. 2004;127:1650–1660. doi: 10.1093/brain/awh189. [DOI] [PubMed] [Google Scholar]
- 3.Gantchev N, Viallet F, Aurenty R, Massion J. Impairment of posturo-kinetic co-ordination during initiation of forward oriented stepping movements in parkinsonian patients. Electroencephalogr Clin Neurophysiol. 1996;101:110–120. doi: 10.1016/0924-980x(95)00253-h. [DOI] [PubMed] [Google Scholar]
- 4.Rosin R, Topka H, Dichgans J. Gait initiation in Parkinson's disease. Mov Disord. 1997;12:682–690. doi: 10.1002/mds.870120509. [DOI] [PubMed] [Google Scholar]
- 5.Burleigh-Jacobs A, Horak FB, Nutt JG, Obeso JA. Step initiation in Parkinson's disease: influence of levodopa and external sensory triggers. Mov Disord. 1997;12:206–215. doi: 10.1002/mds.870120211. [DOI] [PubMed] [Google Scholar]
- 6.Rocchi L, Chiari L, Mancini M, Carlson-Kuhta P, Gross A, Horak FB. Step initiation in Parkinson's disease: influence of initial stance conditions. Neurosci Lett. 2006;406:128–132. doi: 10.1016/j.neulet.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Burleigh AL, Horak FB, Burchiel KJ, Nutt JG. Effects of thalamic stimulation on tremor, balance, and step initiation: a single subject study. Mov Disord. 1993;8:519–524. doi: 10.1002/mds.870080419. [DOI] [PubMed] [Google Scholar]
- 8.Crenna P, Carpinella I, Rabuffetti M, Rizzone M, Lopiano L, Lanotte M, Ferrarin M. Impact of subthalamic nucleus stimulation on the initiation of gait in Parkinson's disease. Exp Brain Res. 2006;172:519–532. doi: 10.1007/s00221-006-0360-7. [DOI] [PubMed] [Google Scholar]
- 9.Rocchi L, Mancini M, Ferraresi G, Sensi M, Manca M, Chiari L. Influence of deep brain stimulation surgery on the initiation of gait in Parkinson's disease. Gait Posture. 2008;28(Suppl 1):S25. [Google Scholar]
- 10.Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- 11.Carpinella I, Crenna P, Calabrese E, Rabuffetti M, Mazzoleni P, Nemni R, Ferrarin M. Locomotor function in the early stage of Parkinson's disease. IEEE Trans Neural Syst Rehabil Eng. 2007;15:543–551. doi: 10.1109/TNSRE.2007.908933. [DOI] [PubMed] [Google Scholar]
- 12.Dietz V. Neurophysiology of gait disorders: present and future applications. Electroencephalogr Clin Neurophysiol. 1997;103:333–355. doi: 10.1016/s0013-4694(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 13.Frank JS, Horak FB, Nutt J. Centrally initiated postural adjustments in parkinsonian patients on and off levodopa. J Neurophysiol. 2000;84:2440–2448. doi: 10.1152/jn.2000.84.5.2440. [DOI] [PubMed] [Google Scholar]
- 14.Massion J. Movement, posture and equilibrium: interaction and coordination. Prog Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- 15.Winter DA. A.B.C of Balance during Standing and Walking. Waterloo Biomechanics; Waterloo: 1995. [Google Scholar]
- 16.Crenna P, Frigo C. A motor programme for the initiation of forward-oriented movements in humans. J Physiol. 1991;437:635–653. doi: 10.1113/jphysiol.1991.sp018616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elble RJ, Moody C, Leffler K, Sinha R. The initiation of normal walking. Mov Disord. 1994;9:139–146. doi: 10.1002/mds.870090203. [DOI] [PubMed] [Google Scholar]
- 18.Halliday SE, Winter DA, Frank JS, Patla AE, Prince F. The initiation of gait in young, elderly, and Parkinson's disease subjects. Gait Posture. 1998;8:8–14. doi: 10.1016/s0966-6362(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 19.Vaugoyeau M, Viallet F, Mesure S, Massion J. Coordination of axial rotation and step execution: deficits in Parkinson's disease. Gait Posture. 2003;18:150–157. doi: 10.1016/s0966-6362(03)00034-1. [DOI] [PubMed] [Google Scholar]
- 20.Bonato P. Advances in wearable technology and applications in physical medicine and rehabilitation. J Neuroeng Rehabil. 2005;2:2. doi: 10.1186/1743-0003-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiari L, Dozza M, Cappello A, Horak FB, Macellari V, Giansanti D. Audio-biofeedback for balance improvement: an accelerometry-based system. IEEE Trans Biomed Eng. 2005;52:2108–2111. doi: 10.1109/TBME.2005.857673. [DOI] [PubMed] [Google Scholar]
- 22.Salarian A, Russmann H, Vingerhoets FJ, Dehollain C, Blanc Y, Burkhard PR, Aminian K. Gait assessment in Parkinson's disease: toward an ambulatory system for long-term monitoring. IEEE Trans Biomed Eng. 2004;51:1434–1443. doi: 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- 23.Rocchi L, Mancini M, Chiari L, Cappello A. Dependence of anticipatory postural adjustments for step initiation on task movement features: a study based on dynamometric and accelerometric data. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1489–1492. doi: 10.1109/IEMBS.2006.260731. [DOI] [PubMed] [Google Scholar]
- 24.Moe-Nilssen R. A new method for evaluating motor control in gait under real-life environmental conditions. Part 1: The instrument. Clin Biomech (Bristol , Avon ) 1998;13:320–327. doi: 10.1016/s0268-0033(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 25.MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, Zhang Y, Mille ML, Rogers MW. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol. 2007;97:4368–4379. doi: 10.1152/jn.01136.2006. [DOI] [PubMed] [Google Scholar]
- 26.Mille ML, Johnson HM, Martinez KM, Simuni T, Rogers MW. Acute effects of a lateral postural assist on voluntary step initiation in patients with Parkinson's disease. Mov Disord. 2007;22:20–27. doi: 10.1002/mds.21139. [DOI] [PubMed] [Google Scholar]
- 27.Fahn S, Elton RL, The UPDRS Development Committee . Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne D, Goldstein M, editors. Recent Developments in Parkinson's Disease. Macmillan Healthcare Information; Florham Park, New Jersey: 1987. pp. 153–163. [Google Scholar]
- 28.Jacobs JV, Horak FB. External postural perturbations induce multiple anticipatory postural adjustments when subjects cannot pre-select their stepping foot. Exp Brain Res. 2007;179:29–42. doi: 10.1007/s00221-006-0763-5. [DOI] [PubMed] [Google Scholar]
- 29.Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215:334–341. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell SL, Collins JJ, De Luca CJ, Burrows A, Lipsitz LA. Open-loop and closed-loop postural control mechanisms in Parkinson's disease: increased mediolateral activity during quiet standing. Neurosci Lett. 1995;197:133–136. doi: 10.1016/0304-3940(95)11924-l. [DOI] [PubMed] [Google Scholar]
- 31.Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture. 2000;11:102–110. doi: 10.1016/s0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 32.Vaugoyeau M, Viel S, Assaiante C, Amblard B, Azulay JP. Impaired vertical postural control and proprioceptive integration deficits in Parkinson's disease. Neuroscience. 2007;146:852–863. doi: 10.1016/j.neuroscience.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 33.Schieppati M, Nardone A. Free and supported stance in Parkinson's disease. The effect of posture and ‘postural set’ on leg muscle responses to perturbation, and its relation to the severity of the disease. Brain. 1991;114(Pt 3):1227–1244. doi: 10.1093/brain/114.3.1227. [DOI] [PubMed] [Google Scholar]
- 34.Breniere Y, Do MC. Control of gait initiation. J Mot Behav. 1991;23:235–240. doi: 10.1080/00222895.1991.9942034. [DOI] [PubMed] [Google Scholar]
- 35.Brenière Y, Do MC, Bouisset S. Are dynamic phenomena prior to stepping essential to walking? J Mot Behav. 1987;19:62–76. doi: 10.1080/00222895.1987.10735400. [DOI] [PubMed] [Google Scholar]
- 36.Breniere Y, Dietrich G. Heel-off perturbation during gait initiation: biomechanical analysis using triaxial accelerometry and a force plate. J Biomech. 1992;25:121–127. doi: 10.1016/0021-9290(92)90269-7. [DOI] [PubMed] [Google Scholar]