Abstract
The authors investigated the association between age at menarche and risk of type 2 diabetes mellitus (T2DM) among 101,415 women from the Nurses’ Health Study (NHS) aged 34–59 years (1980–2006) and 100,547 women from Nurses’ Health Study II (NHS II) aged 26–46 years (1991–2005). During 2,430,274 and 1,373,875 person-years of follow-up, respectively, 7,963 and 2,739 incident cases of T2DM were documented. Young age at menarche was associated with increased risk of T2DM after adjustment for potential confounders, including body figure at age 10 years and body mass index (BMI; weight (kg)/height (m)2) at age 18 years. Relative risks of T2DM across age-at-menarche categories (≤11, 12, 13, 14, and ≥15 years) were 1.18 (95% confidence interval (CI): 1.10, 1.27), 1.09 (95% CI: 1.02, 1.17), 1.00 (referent), 0.92 (95% CI: 0.83, 1.01), and 0.95 (95% CI: 0.84, 1.06), respectively, in the NHS (P for trend < 0.0001) and 1.40 (95% CI: 1.24, 1.57), 1.13 (95% CI: 1.00, 1.27), 1.00 (referent), 0.98 (95% CI: 0.82, 1.18), and 0.96 (95% CI: 0.78, 1.19), respectively, in NHS II (P for trend < 0.0001). Associations were substantially attenuated after additional control for updated time-varying BMI. These data suggest that early menarche is associated with increased risk of T2DM in adulthood. The association may be largely mediated through excessive adult adiposity. The association was stronger among younger women, supporting a role for sex hormones in younger onset of T2DM, in addition to BMI.
Keywords: adiposity; body mass index; diabetes mellitus, type 2; menarche; risk factors; weight gain
The prevalence of type 2 diabetes mellitus in the United States has increased rapidly during the last several decades, in parallel with the obesity epidemic (1–3). By the time type 2 diabetes is diagnosed, some individuals have already developed serious complications (2). Therefore, it has become increasingly important to identify persons at risk in early life so they may benefit from early interventions. Early menarche has been associated with risk factors for diabetes, such as excessive adiposity in childhood (4–6) and adulthood (7–10), as well as elevated blood glucose levels (11–14) and insulin resistance (15–17), independently of adiposity. Moreover, coincidental with the increasing prevalence of obesity and type 2 diabetes, age at menarche in US girls has been declining over the past 30 years (18, 19).
Few studies have been conducted to examine the association between age at menarche and risk of type 2 diabetes. Findings from existing studies have been inconclusive because of small samples and lack of information on childhood or adulthood characteristics that could confound the association (14, 20, 21). Notably, one important characteristic of the escalating type 2 diabetes epidemic is a shift toward a younger age of onset. The association of age at menarche with type 2 diabetes among younger, middle-aged women was not addressed in previous studies, in which study populations were predominantly postmenopausal women (ages 40–92 years; average age, >57 years) (14, 20, 21). Therefore, we conducted prospective analyses in 2 large, independent cohorts including both old and young women, to investigate the association between age at menarche and risk of type 2 diabetes.
MATERIALS AND METHODS
Study population
The Nurses’ Health Study (NHS) was established in 1976, when 121,700 female US registered nurses aged 30–55 years completed a mailed questionnaire about their medical history and lifestyle. Nurses’ Health Study II (NHS II) was established in 1989, when 116,609 female US registered nurses aged 24–44 years completed an initial questionnaire. Both cohorts have been and continue to be followed with biennial mailed questionnaires for the collection of updated information on health-related behaviors and characteristics and for determination of incident disease outcomes. The follow-up rate exceeds 90% for every 2-year period.
For the present analyses, we excluded participants if they had a history of diabetes, cancer, or cardiovascular disease at baseline or had reported any of these conditions on a previous questionnaire. After exclusions, 101,415 NHS participants (followed between 1980 and 2006) and 100,547 NHS II participants (followed between 1991 and 2005) remained.
Assessment of age at menarche
Age at menarche was defined as age at the first menstrual period (in years). This information was ascertained by recall on the 1976 (NHS) or 1989 (NHS II) questionnaire. In the NHS, the question was open-ended: “At what age did your menstrual periods begin? ___ years of age.” In NHS II, the question asked, “At what age did your menstrual periods begin?” Response categories were “9 or younger; 10; 11; 12; 13; 14; 15; 16; 17 or older.” We excluded NHS women who reported ages at menarche greater than 18 years (n = 66), since such delays (outside the spectrum of normal variation) are likely to have a pathologic cause.
Ascertainment of type 2 diabetes
On the baseline and biennial questionnaires, we inquired about whether diabetes had been newly diagnosed. Women who reported diabetes were sent a supplementary questionnaire for confirmation of the report and ascertainment of the date of diagnosis and the details of the diagnostic tests, presenting symptoms, and medications prescribed. We excluded women classified as having only gestational diabetes, as well as those diagnosed with type 1 diabetes during follow-up. Consistent with the criteria of the National Diabetes Data Group, the diagnosis of type 2 diabetes was established if 1 or more of the following criteria were met: 1) an elevated glucose concentration (fasting plasma glucose level ≥7.8 mmol/L (140 mg/dL), random plasma glucose level ≥11.1 mmol/L (200 mg/dL), or plasma glucose level ≥11.1 mmol/L (200 mg/dL) after an oral glucose load) and at least 1 symptom related to diabetes (excessive thirst, polyuria, weight loss, or hunger); 2) no symptoms but elevated glucose concentrations on 2 occasions; and 3) treatment with insulin or oral hypoglycemic medication. For cases identified after 1997, the cutoff for fasting plasma glucose concentration was lowered to 7.0 mmol/L (126 mg/dL) according to the American Diabetes Association criteria (22). The validity of this diagnostic procedure has been verified in a subsample of this study population (23). Of a random sample of 62 nurses reporting type 2 diabetes, 61 cases (98%) were confirmed after medical record review by an endocrinologist blinded to the supplementary questionnaire information. In addition, another substudy assessing the prevalence of undiagnosed diabetes suggested a very low rate of false-negative findings (24).
Assessment of covariates
A semiquantitative food frequency questionnaire (first sent to NHS women in 1980 and to NHS II women in 1991) has been mailed to study participants every 4 years for assessment and updating of dietary information. Data on the reproducibility and validity of the food frequency questionnaires have been reported elsewhere (25). Glycemic load was calculated by multiplying the grams of carbohydrate in each serving by its glycemic index value. The dietary score was calculated as the sum of the quintile values of cereal fiber and polyunsaturated fat:saturated fat ratio in ascending order and trans-fat and glycemic load in descending order. A higher quintile of dietary score reflected a higher ratio of polyunsaturated fat to saturated fat, a higher intake of cereal fiber, a low intake of trans-fat, and a low glycemic load (26).
Information on age, weight, smoking status, menopause status, use of postmenopausal hormone therapy, use of oral contraceptives (for NHS II), parity (for NHS II), personal history of diabetes, cardiovascular disease, and cancer was collected on the baseline questionnaire by self-report and was updated every 2 years during follow-up. Questions on oral contraceptive use and parity for NHS women were asked at baseline and in 1982; family history of diabetes and height were assessed only at baseline. We calculated body mass index (BMI) as weight in kilograms divided by height in meters squared. The validity of self-reported body weight in the NHS was reported previously (for correlation between self-reported weight and measured weight, Pearson's r = 0.96) (27). Recalled weight at age 18 years was also highly correlated with measured weight from the physical examination records (r = 0.87) in NHS II (28).
Participants recalled their body figures at age 10 years using a 9-level figure drawing (29). Women's recall of their body figure in childhood has been validated against weight and height measurements taken in childhood (r = 0.70) (30). Information on participants’ birth weights and whether they had been breastfed during infancy was collected in 1992 (NHS) or 1991 (NHS II) and was validated as described elsewhere (31). Women reported their waist circumference in 1986 for the NHS and in 1993 for NHS II. Physical activity data were assessed in 1980, 1982, 1986, 1988, 1992, 1996, 1998, and 2000 for the NHS and in 1991 and 1997 for NHS II. A validation study indicated relatively good validity and reproducibility for the physical activity measurements in NHS II (32). Childhood socioeconomic status was assessed as father's occupation on the 1976 (NHS) or 2005 (NHS II) questionnaire. Adulthood socioeconomic status was assessed as spouse's educational level on the 1992 (NHS) or 1999 (NHS II) questionnaire. Race/ethnicity (Caucasian, Hispanic, Asian, or African-American) was reported in 1992 (NHS) or 1989 (NHS II).
Statistical analysis
Person-years of follow-up were calculated from the date of return of the baseline questionnaire (1980 for NHS, 1991 for NHS II) to the date of type 2 diabetes diagnosis, the date of death, or the end of follow-up (June 2006 for NHS and June 2005 for NHS II), whichever came first. Age at menarche was modeled as either a categorical variable (≤11, 12, 13, 14, or ≥15 years) or a continuous variable (in years). We conducted Cox proportional hazards analysis stratified by 5-year age category and 2-year follow-up interval to estimate the relative risk of type 2 diabetes according to age at menarche. For example, in the NHS, the starting point of one stratum was 1980, and the end point of that stratum was 1982 (the next questionnaire cycle). The time-varying covariates included in the multivariate analysis were updated during follow-up, using the most recent data for each 2-year follow-up interval. We first tested a multivariate Cox model, adjusting for potential confounders, including age, family history of diabetes, race/ethnicity, and childhood and adolescent variables such as birth weight, whether the participant had been breastfed, childhood socioeconomic status, and perceived body figure at age 10 years. We further adjusted for adult lifestyle or risk factors for type 2 diabetes, including smoking, alcohol use, physical activity, adult socioeconomic status, diet score, hypertension and hypercholesterolemia at baseline, menopausal status/postmenopausal hormone use, parity, oral contraceptive use, and menstrual cycle regularity.
Because we only measured body figure at age 10 years instead of premenarcheal BMI, we included BMI at age 18 years in the main model, since it was strongly correlated with premenarcheal BMI (33). Because adult BMI might represent intermediate factors rather than confounders for the association between age at menarche and type 2 diabetes, we adjusted for adult BMI (time-varying BMIs during follow-up) in separate models. To test for a linear trend across menarcheal age categories, we modeled menarcheal age as a continuous variable using the median value for each category. We evaluated whether the association between age at menarche and risk of diabetes was modified by family history of diabetes, smoking status, or physical activity using analyses stratified by these variables and by modeling interaction terms. To compare similar age groups across the NHS and NHS II and to test whether the association was modified by age, we used the mean age at baseline in the NHS (45 years) as the cutoff point to conduct stratified analysis in the NHS only, since most women in NHS II (99.6%) were younger than age 45 years at baseline. We also tested whether the association was modified by menopausal status in the NHS only, because most women in NHS II (96.1%) were premenopausal at baseline.
In addition, we examined the relation between age at menarche and adult BMI at baseline (1980 for NHS, 1991 for NHS II) and weight gain from age 18 years through baseline. We performed logistic regression analyses to estimate the relative risks of adult obesity (defined by baseline BMI ≥ 30) and excessive weight gain (weight gain over 10 kg since age 18 years) by menarcheal age category and performed linear regression to estimate the mean baseline BMI or weight-gain difference corresponding to each 1-year increase in age at menarche, adjusting for age, socioeconomic status in childhood and adulthood, smoking, physical activity, dietary score, menopausal status/use of hormone replacement therapy, alcohol intake, parity, body figure at age 10 years, and BMI at age 18 years.
All tests of statistical significance were 2-sided, and statistical significance was defined at the α = 0.05 level. All analyses were performed using SAS statistical software, version 9.1 (SAS Institute Inc., Cary, North Carolina).
RESULTS
In general, women in NHS II were younger than women in the NHS and tended to have earlier menarche, which reflects the well-recognized birth cohort effect (Table 1) (34). In each cohort, women with a younger age at menarche had a lower birth weight, a larger body figure at age 10 years, and a greater BMI at age 18 years and at baseline (adulthood) and had gained more weight since age 18 years. In addition, women with a younger age at menarche were more likely to have a family history of diabetes, a history of hypertension or hypercholesterolemia at baseline, and regular menstrual cycles between ages 18 and 22 years.
Table 1.
Age-Standardized Characteristics of the Study Population by Age at Menarche, Nurses’ Health Study (1980–2006) and Nurses’ Health Study II (1991–2005)a
| Age at Menarche, yearsb |
||||||||||
| ≤11 |
12 |
13 |
14 |
≥15 |
||||||
| % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | % | Mean (SD) | |
| Nurses’ Health Study | ||||||||||
| 22.2 | 26.6 | 31.1 | 12.3 | 7.9 | ||||||
| Childhood and adolescent characteristics | ||||||||||
| Birth weight, poundsc | ||||||||||
| <5.5 | 11.6 | 10.6 | 10.2 | 9.5 | 10.9 | |||||
| 5.5–7.0 | 30.8 | 32.1 | 30.5 | 30.2 | 31.8 | |||||
| 7.–8.5 | 44.6 | 45.0 | 45.8 | 45.7 | 42.7 | |||||
| ≥8.5 | 13.0 | 12.4 | 13.5 | 14.6 | 14.7 | |||||
| Having been breastfed | 61.9 | 63.2 | 64.1 | 65.8 | 65.8 | |||||
| Body figured at age 10 years | ||||||||||
| 1 | 23.2 | 28.1 | 33.5 | 39.9 | 44.6 | |||||
| 2 | 23.4 | 27.4 | 27.4 | 28.3 | 25.6 | |||||
| 3 | 20.3 | 19.1 | 17.3 | 14.3 | 13.5 | |||||
| 4 | 16.9 | 13.8 | 11.4 | 9.3 | 8.3 | |||||
| ≥5 | 16.3 | 11.7 | 10.4 | 8.2 | 8.0 | |||||
| BMIe at age 18 years | 22.0 (3.1) | 21.4 (2.8) | 21.1 (2.8) | 20.7 (2.6) | 20.6 (2.9) | |||||
| Regular menstrual cycles at ages 18–22 years | 67.1 | 68.0 | 65.9 | 61.5 | 50.2 | |||||
| Baseline (adult) characteristics in 1980 | ||||||||||
| Age, years | 45.1 (7.1) | 45.6 (7.3) | 45.9 (7.1) | 46.8 (7.1) | 47.6 (7.0) | |||||
| BMI | 25.1 (4.8) | 24.3 (4.2) | 23.8 (4.0) | 23.4 (3.8) | 23.3 (3.9) | |||||
| Weight gain since age 18 years, kg | 6.9 (12.3) | 6.4 (11.6) | 6.3 (11.1) | 6.2 (10.9) | 6.0 (11.2) | |||||
| Family history of diabetes | 22.1 | 21.5 | 20.7 | 20.4 | 20.8 | |||||
| Current smoker | 28.3 | 27.6 | 27.5 | 28 | 29.1 | |||||
| Alcohol consumption, g/day | 6.1 (10.2) | 6.4 (10.4) | 6.7 (10.7) | 6.9 (10.9) | 6.6 (10.5) | |||||
| Physical activity, hours/week | 4.0 (2.9) | 4.0 (2.9) | 4.0 (2.9) | 4.0 (2.9) | 4.1 (2.9) | |||||
| Hypertension | 16.2 | 13.9 | 13.0 | 11.8 | 12.0 | |||||
| Hypercholesterolemia | 5.1 | 4.4 | 4.0 | 4.2 | 4.4 | |||||
| Glycemic load, g | 85 (26) | 85 (25) | 86 (25) | 86 (25) | 86 (26) | |||||
| Premenopausal | 53.7 | 55.5 | 55.9 | 55.3 | 52.9 | |||||
| Ever use of hormone replacement therapy | 16.7 | 16.0 | 15.6 | 15.6 | 16.9 | |||||
| Ever use of oral contraceptives | 45.1 | 45.8 | 45.8 | 44.9 | 44.0 | |||||
| Parity | ||||||||||
| 0 (nulliparous) | 7.4 | 7.3 | 6.5 | 6.7 | 6.9 | |||||
| 1–2 | 36.7 | 36.8 | 35.1 | 33.3 | 35.6 | |||||
| ≥3 | 55.9 | 55.9 | 58.4 | 60.0 | 57.6 | |||||
| Nurses’ Health Study II | ||||||||||
| 24.3 | 30.2 | 27.6 | 10.4 | 7.5 | ||||||
| Childhood and adolescent characteristics | ||||||||||
| Birth weight, pounds | ||||||||||
| <5.5 | 8.6 | 7.7 | 7.5 | 7.3 | 7.4 | |||||
| 5.5–7.0 | 31.6 | 30.3 | 30.4 | 29.9 | 29.0 | |||||
| 7.0–8.5 | 46.9 | 48.8 | 48.5 | 49.1 | 49.1 | |||||
| ≥8.5 | 12.9 | 13.2 | 13.7 | 13.7 | 14.5 | |||||
| Having been breastfed | 33.3 | 34.0 | 34.2 | 34.0 | 36.1 | |||||
| Body figure at age 10 years | ||||||||||
| 1 | 12.0 | 16.8 | 20.9 | 26.0 | 30.1 | |||||
| 2 | 24.8 | 30.1 | 33.5 | 35.0 | 35.1 | |||||
| 3 | 24.5 | 24.3 | 21.8 | 19.7 | 17.3 | |||||
| 4 | 20.8 | 16.5 | 14.0 | 11.5 | 10.0 | |||||
| ≥5 | 18.0 | 12.3 | 9.8 | 7.8 | 7.6 | |||||
| BMI at age 18 years | 22.0 (3.7) | 21.4 (3.2) | 20.9 (3.0) | 20.5 (3.0) | 20.3 (3.1) | |||||
| Regular menstrual cycles at ages 18–22 years | 80.4 | 78.8 | 76.8 | 69.7 | 59.3 | |||||
| Baseline (adult) characteristics in 1991 | ||||||||||
| Age, years | 36.3 (4.6) | 36.1 (4.6) | 36.2 (4.7) | 35.9 (4.8) | 35.5 (4.8) | |||||
| BMI | 26.1 (6.0) | 24.7 (5.2) | 23.9 (4.8) | 23.4 (4.6) | 23.0 (4.4) | |||||
| Weight gain since age 18 years, kg | 10.7 (12.9) | 9.2 (11.3) | 8.3 (10.5) | 7.8 (10.1) | 7.6 (9.7) | |||||
| Family history of diabetes | 17.9 | 16.3 | 15.4 | 14.2 | 14.2 | |||||
| Current smoker | 13.0 | 11.9 | 12.1 | 11.9 | 13.3 | |||||
| Alcohol consumption, g/day | 2.9 (5.8) | 3.1 (6.2) | 3.3 (6.1) | 3.3 (6.2) | 3.5 (6.6) | |||||
| Physical activity, hours/week | 21.0 (28.1) | 20.7 (26.7) | 21.0 (27.0) | 21.3 (28.1) | 22.9 (30.4) | |||||
| Hypertension | 8.0 | 6.1 | 5.1 | 4.7 | 5.3 | |||||
| Hypercholesterolemia | 16.1 | 14.2 | 13.5 | 12.4 | 13.4 | |||||
| Glycemic load, g | 120 (22) | 121 (21) | 122 (22) | 123 (22) | 123 (22) | |||||
| Premenopausal | 95.3 | 96.3 | 96.4 | 96.4 | 95.8 | |||||
| Ever use of hormone replacement therapy | 7.8 | 6.7 | 6.5 | 6.4 | 7.9 | |||||
| Ever use of oral contraceptives | 85.0 | 84.4 | 84.7 | 83.6 | 84.5 | |||||
| Parity | ||||||||||
| 0 (nulliparous) | 28.7 | 26.5 | 26.3 | 25.8 | 28.1 | |||||
| 1–2 | 51.9 | 52.8 | 53.0 | 52.4 | 51.7 | |||||
| ≥3 | 19.3 | 20.8 | 20.8 | 21.8 | 20.2 | |||||
Abbreviations: BMI, body mass index; NHS, Nurses’ Health Study; SD, standard deviation.
A total of 201,962 women were included (101,415 in NHS and 100,547 in NHS II).
Total numbers of women by age at menarche—NHS: ≤11 years, n = 22,528; 12 years, n = 26,951; 13 years, n = 31,492; 14 years, n = 12,442; 15 years, n = 8,002; NHS II: ≤11 years, n = 24,473; 12 years, n = 30,378; 13 years, n = 27,696; 14 years, n = 10,424; 15 years, n = 7,576.
1 pound = 0.45 kg.
Participants recalled their body figure at age 10 years using a 9-level figure drawing (29).
Weight (kg)/height (m)2.
We documented 7,963 and 2,739 incident cases of type 2 diabetes during 2,430,274 and 1,373,875 person-years of follow-up in the NHS and NHS II, respectively. The age at diagnosis of type 2 diabetes in the NHS was, on average, 16 years older than that in NHS II (63.5 years vs. 47.4 years). Early menarche was significantly associated with increased risk of type 2 diabetes in both the NHS and NHS II, and the association was stronger in younger women (NHS II) than in older women (NHS) (Table 2 and Figure 1). After adjustment for age, parity, race/ethnicity, family history of diabetes, lifestyle and reproductive factors, childhood characteristics (including body fatness at age 10 years, as measured by perceived body figure), and BMI at age 18 years (model 3 in Table 2), corresponding relative risks across menarcheal age categories (≤11, 12, 13, 14, and ≥15 years) were 1.18 (95% confidence interval (CI): 1.10, 1.27), 1.09 (95% CI: 1.02, 1.17), 1.00 (referent), 0.92 (95% CI: 0.83, 1.01), and 0.95 (95% CI: 0.84, 1.06), respectively, in the NHS (P for trend < 0.0001) and 1.40 (95% CI: 1.24, 1.57), 1.13 (95% CI: 1.00, 1.27), 1.00 (referent), 0.98 (95% CI: 0.82, 1.18), and 0.96 (95% CI: 0.78, 1.19), respectively, in NHS II (P for trend < 0.0001). Each 1-year increase in age at menarche was associated with 6% (95% CI: 4, 8) and 10% (95% CI: 7, 12) reductions in type 2 diabetes risk in the NHS and NHS II, respectively. The association was substantially attenuated after further adjustment for adult BMI (model 4 in Table 2). In further analysis carried out among type 2 diabetes cases only, we found that early menarche was associated with a younger age at diagnosis of type 2 diabetes. Among older women in the NHS, each 1-year increase in age at menarche was associated with a 0.27-year (95% CI: 0.14, 0.40) delay in age at type 2 diabetes diagnosis, after adjustment for potential confounders and intermediate variables, including BMI in adulthood (all covariates in model 4 of Table 2).
Table 2.
Relative Risk of Type 2 Diabetes According to Age at Menarche, Nurses’ Health Study (1980–2006) and Nurses’ Health Study II (1991–2005)
| Age at Menarche, years |
P for Trend | RR per Year |
||||||||||
| ≤11 |
12 |
13 | 14 |
≥15 |
||||||||
| RR | 95% CI | RR | 95% CI | (Referent) | RR | 95% CI | RR | 95% CI | RR | 95% CI | ||
| Nurses’ Health Study | ||||||||||||
| No. of cases | 2,128 | 2,188 | 2,297 | 789 | 561 | |||||||
| Person-years of follow-up | 536,956 | 645,206 | 758,274 | 299,179 | 190,659 | |||||||
| Age at diagnosis, yearsa | 62.5 (8.32) | 63.2 (8.22) | 63.9 (7.84) | 64.7 (8.28) | 65.5 (8.24) | |||||||
| Age-adjusted results | 1.34 | 1.26, 1.42 | 1.13 | 1.07, 1.20 | 1.00 | 0.86 | 0.79, 0.93 | 0.94 | 0.86, 1.04 | <0.0001 | 0.91 | 0.89, 0.92 |
| Model 1b | 1.28 | 1.21, 1.37 | 1.12 | 1.05, 1.19 | 1.00 | 0.86 | 0.79, 0.93 | 0.92 | 0.84, 1.02 | <0.0001 | 0.92 | 0.90, 0.93 |
| Model 2c | 1.21 | 1.13, 1.31 | 1.10 | 1.02, 1.18 | 1.00 | 0.91 | 0.82, 1.00 | 0.94 | 0.84, 1.06 | <0.0001 | 0.94 | 0.92, 0.95 |
| Model 3d | 1.18 | 1.10, 1.27 | 1.09 | 1.02, 1.17 | 1.00 | 0.92 | 0.83, 1.01 | 0.95 | 0.84, 1.06 | <0.0001 | 0.94 | 0.92, 0.96 |
| Model 4e | 1.02 | 0.95, 1.10 | 1.02 | 0.95, 1.10 | 1.00 | 0.97 | 0.88, 1.07 | 1.01 | 0.90, 1.14 | 0.42 | 0.99 | 0.97, 1.01 |
| Nurses’ Health Study II | ||||||||||||
| No. of cases | 1,003 | 823 | 578 | 192 | 143 | |||||||
| Person-years of follow-up | 332,344 | 415,145 | 379,623 | 142,929 | 103,834 | |||||||
| Age at diagnosis, yearsa | 46.9 (5.39) | 47.5 (5.23) | 48.0 (5.18) | 47.8 (5.44) | 47.2 (5.46) | |||||||
| Age-adjusted results | 1.98 | 1.79, 2.20 | 1.32 | 1.18, 1.47 | 1.00 | 0.90 | 0.76, 1.06 | 0.95 | 0.79, 1.14 | <0.0001 | 0.80 | 0.78, 0.82 |
| Model 1b | 1.63 | 1.47, 1.80 | 1.22 | 1.10, 1.36 | 1.00 | 0.96 | 0.81, 1.13 | 1.02 | 0.85, 1.23 | <0.0001 | 0.86 | 0.84, 0.89 |
| Model 2c | 1.50 | 1.34, 1.69 | 1.16 | 1.02, 1.31 | 1.00 | 0.99 | 0.82, 1.19 | 0.95 | 0.77, 1.17 | <0.0001 | 0.88 | 0.86, 0.91 |
| Model 3d | 1.40 | 1.24, 1.57 | 1.13 | 1.00, 1.27 | 1.00 | 0.98 | 0.82, 1.18 | 0.96 | 0.78, 1.19 | <0.0001 | 0.90 | 0.88, 0.93 |
| Model 4e | 1.15 | 1.02, 1.29 | 1.03 | 0.91, 1.16 | 1.00 | 1.08 | 0.90, 1.30 | 1.11 | 0.89, 1.37 | 0.19 | 0.97 | 0.94, 1.00 |
Abbreviations: CI, confidence interval; RR, relative risk.
Mean (standard deviation).
In model 1, results were adjusted for age group (≤49, 50–54, 55–59, 60–64, or ≥65 years), birth weight (<5.5, 5.5–7.0, 7.0–8.5, or >8.5 pounds), having been breastfed, childhood socioeconomic status (based on father's occupation), race/ethnicity (Caucasian, Hispanic, Asian, or African-American), family history of diabetes, and perceived body figure at age 10 years (on a 9-level scale; 1, 2, 3, 4, or ≥5).
In model 2, results were adjusted for all of the variables in model 1 plus the baseline factors physical activity (quintiles of hours/week), quintile of dietary score (glycemic load, trans-fat, polyunsaturated:saturated fat ratio, and dietary fiber), alcohol consumption (0, 0.1–4.9, 5.0–14.9, or ≥15 g/day), smoking status (never, past, or current smoker (1–14, 15–24, or ≥25 cigarettes/day)), hypertension, hypercholesterolemia, menopause status (pre- or post-), use of hormone replacement therapy, adult socioeconomic status (based on husband's education), and reproductive factors (parity, oral contraceptive use, and regularity of menstrual cycles at ages 18–22 years).
In model 3, results were adjusted for all of the variables in model 2 plus body mass index (weight (kg)/height (m)2) at age 18 years (<18.5, 18.5–21.9, 22.0–24.9, 25.0–29.9, or ≥30.0).
In model 4, results were adjusted for all of the variables in model 3 plus updated body mass index over the course of follow-up (<21.0, 21.0–22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–32.9, 33.0–34.9, 35.0–39.9, or ≥40.0).
Figure 1.
Relative risk of type 2 diabetes mellitus (T2DM) by age at menarche in A) the Nurses’ Health Study (1980–2006) (P for trend < 0.0001) and B) Nurses’ Health Study II (1991–2005) (P for trend < 0.0001), after adjustment for age, birth weight, having been breastfed, childhood socioeconomic status, race/ethnicity, family history of diabetes, perceived body figure at age 10 years, physical activity, quintile of dietary score, alcohol consumption, smoking status, baseline hypertension, baseline hypercholesterolemia, menopause status, use of hormone replacement therapy, adult socioeconomic status, parity, oral contraceptive use, regularity of menstrual cycles between ages 18 and 22 years, and body mass index at age 18 years (model 3). Bars, 95% confidence interval.
In stratified analyses, the association between age at menarche and type 2 diabetes was stronger in younger women (age <45 years at baseline) than in older women (age ≥45 years at baseline) in the NHS (P for interaction = 0.008) (Table 3). This result was consistent with that from NHS II, in which most women were younger than age 45 years. The inverse association between age at menarche and type 2 diabetes risk was also stronger in premenopausal women than in postmenopausal women, although the test for interaction was marginally significant (P for interaction = 0.056). This association, however, did not differ substantially by family history of diabetes, smoking status, or physical activity in either the NHS or NHS II (Table 3).
Table 3.
Relative Risk of Type 2 Diabetes According to Selected Sociodemographic Factors in the Nurses’ Health Study (1980–2006) and Nurses’ Health Study II (1991–2005)a
| Age at Menarche, years |
P for Trend | |||||||||
| ≤11 |
12 |
13 | 14 |
≥15 |
||||||
| RR | 95% CI | RR | 95% CI | (Referent) | RR | 95% CI | RR | 95% CI | ||
| Nurses’ Health Study | ||||||||||
| Age at baseline (1980), years | ||||||||||
| <45 | 1.31 | 1.16, 1.47 | 1.20 | 1.07, 1.34 | 1.00 | 1.05 | 0.89, 1.24 | 1.00 | 0.80, 1.24 | <0.0001 |
| ≥45 | 1.11 | 1.01, 1.22 | 1.03 | 0.94, 1.13 | 1.00 | 0.85 | 0.75, 0.96 | 0.90 | 0.79, 1.03 | <0.0001 |
| P for interaction | 0.008 | |||||||||
| Menopausal status | ||||||||||
| Premenopausal | 1.39 | 1.06, 1.81 | 0.97 | 0.73, 1.28 | 1.00 | 0.99 | 0.67, 1.46 | 0.86 | 0.52, 1.43 | 0.014 |
| Postmenopausal | 1.17 | 1.08, 1.26 | 1.10 | 1.02, 1.19 | 1.00 | 0.91 | 0.82, 1.01 | 0.94 | 0.84, 1.06 | <0.0001 |
| P for interaction | 0.056 | |||||||||
| Family history of diabetes | ||||||||||
| No | 1.25 | 1.13, 1.38 | 1.13 | 1.02, 1.24 | 1.00 | 0.92 | 0.80, 1.04 | 1.01 | 0.89, 1.17 | <0.0001 |
| Yes | 1.11 | 0.99, 1.24 | 1.06 | 0.95, 1.18 | 1.00 | 0.92 | 0.80, 1.07 | 0.87 | 0.73, 1.04 | 0.0013 |
| P for interaction | 0.20 | |||||||||
| Smoking status | ||||||||||
| Never smoker | 1.18 | 1.06, 1.32 | 1.06 | 0.95, 1.18 | 1.00 | 0.94 | 0.81, 1.08 | 0.91 | 0.77, 1.09 | 0.0001 |
| Ever smoker | 1.18 | 1.07, 1.31 | 1.12 | 1.02, 1.23 | 1.00 | 0.90 | 0.79, 1.03 | 0.97 | 0.83, 1.13 | <0.0001 |
| P for interaction | 0.86 | |||||||||
| Physical activityb | ||||||||||
| Low | 1.14 | 1.04, 1.25 | 1.05 | 0.96, 1.15 | 1.00 | 0.91 | 0.81, 1.03 | 0.91 | 0.79, 1.05 | <0.0001 |
| High | 1.33 | 1.10, 1.61 | 1.19 | 0.99, 1.43 | 1.00 | 1.01 | 0.79, 1.28 | 1.04 | 0.79, 1.37 | 0.0048 |
| P for interaction | 0.74 | |||||||||
| Nurses’ Health Study II | ||||||||||
| Family history of diabetes | ||||||||||
| No | 1.33 | 1.15, 1.53 | 1.08 | 0.93, 1.25 | 1.00 | 1.02 | 0.82, 1.26 | 0.94 | 0.73, 1.22 | <0.0001 |
| Yes | 1.55 | 1.25, 1.93 | 1.24 | 0.99, 1.55 | 1.00 | 0.91 | 0.64, 1.29 | 1.03 | 0.70, 1.53 | <0.0001 |
| P for interaction | 0.59 | |||||||||
| Smoking status | ||||||||||
| Never smoker | 1.37 | 1.18, 1.59 | 1.06 | 0.91, 1.24 | 1.00 | 0.94 | 0.74, 1.19 | 1.00 | 0.76, 1.31 | <0.0001 |
| Ever smoker | 1.46 | 1.20, 1.78 | 1.26 | 1.03, 1.54 | 1.00 | 1.00 | 0.73, 1.35 | 0.93 | 0.66, 1.32 | <0.0001 |
| P for interaction | 0.51 | |||||||||
| Physical activity | ||||||||||
| Low | 1.35 | 1.15, 1.58 | 1.14 | 0.97, 1.34 | 1.00 | 1.05 | 0.83, 1.34 | 0.90 | 0.67, 1.20 | <0.0001 |
| High | 1.44 | 1.20, 1.72 | 1.09 | 0.90, 1.32 | 1.00 | 0.90 | 0.68, 1.21 | 1.06 | 0.78, 1.44 | <0.0001 |
| P for interaction | 0.71 | |||||||||
Abbreviations: CI, confidence interval; RR, relative risk.
Results were adjusted for age, birth weight, having been breastfed, childhood socioeconomic status, race/ethnicity, family history of diabetes, perceived body figure at age 10 years, physical activity, quintile of dietary score, alcohol consumption, smoking status, baseline hypertension, baseline hypercholesterolemia, menopause status (pre- or post-), use of hormone replacement therapy, adult socioeconomic status, reproductive factors (parity, oral contraceptive use, and regularity of menstrual cycles at ages 18–22 years), and body mass index at age 18 years (model 3).
Low physical activity: lowest 2 quintiles of metabolic equivalent task score; high physical activity: highest 3 quintiles of metabolic equivalent task score.
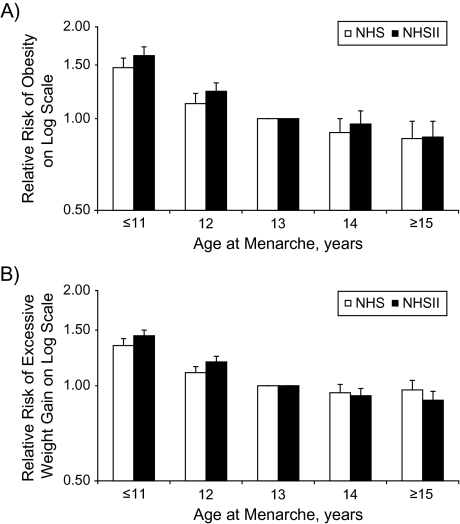
Early menarche was associated with an elevated risk of adult obesity and excessive weight gain after adjustment for age and major potential confounders and mediators (Figure 2). Each 1-year increase in age at menarche was associated with a decrease of 0.20 units in multivariate-adjusted adulthood BMI (95% CI: 0.18, 0.22; P for trend < 0.0001) and a decrease of 0.50 kg in multivariate-adjusted weight gain since age 18 years (95% CI: 0.44, 0.55; P for trend < 0.0001) among women in the NHS. Among women in NHS II, each 1-year increase in age at menarche was associated with a decrease of 0.26 units in adult BMI (95% CI: 0.24, 0.28; P for trend < 0.0001) and a decrease of 0.65 kg in weight gain since age 18 years (95% CI: 0.60, 0.70; P for trend < 0.0001).
Figure 2.
Relative risks of A) adult obesity at baseline and B) excessive weight gain since age 18 years by age at menarche in the Nurses’ Health Study (NHS; 1980–2006) and Nurses’ Health Study II (NHS II; 1991–2005), after adjustment for baseline age, socioeconomic status in childhood and adulthood, smoking status, physical activity, quintile of dietary score, menopause status, use of hormone replacement therapy, alcohol intake, parity, perceived body figure at age 10 years, and body mass index at age 18 years (all P’s for trend < 0.0001). Bars, 95% confidence interval.
DISCUSSION
In this prospective study, carried out among more than 200,000 women with more than 10,000 incident cases of type 2 diabetes, we found that early menarche was significantly associated with increased risk of type 2 diabetes in both older (NHS) and younger (NHS II) women, with the association being stronger among younger women. Established childhood and adulthood risk factors for diabetes did not fully explain the association. After further adjustment for adulthood BMI, the elevated risk associated with younger age at menarche (≤11 years) became attenuated, suggesting that adult adiposity may mediate the observed association between early menarche and type 2 diabetes risk.
The strengths of the current study include the prospective design, the large sample size, the high rates of follow-up, and the availability of data on childhood characteristics and detailed information on adulthood diet and lifestyle factors repeatedly obtained over long-term follow-up. Moreover, 2 independent cohorts comprising women of different age groups were included in the present study, which allowed us not only to assess the consistency of results across birth cohorts but also to evaluate whether the association differed between younger and older women.
Several limitations should also be acknowledged. First, because age at menarche was retrospectively assessed by recall, misclassification was inevitable. However, age at menarche has been associated with breast cancer and other endpoints in both the NHS (35, 36) and NHS II (29, 37), with the magnitude and direction of the association being consistent with the literature, attesting to the validity of the measurement. In addition, because of the prospective study designs, misclassification of age at menarche is likely to have been nondifferential with respect to type 2 diabetes. Second, we did not have direct measurement of premenarcheal body weight. Instead, we adjusted for women's recall of body figure at age 10 years. Although misclassification of this variable is likely, women's recall of their body figure at age 10 years has been shown to be highly correlated with measured BMI in childhood (r = 0.70) (30). In addition, we adjusted for BMI at age 18 years to further control for premenarcheal body weight. Third, diabetes was assessed by self-report and confirmed by means of a supplementary questionnaire. Data from a validation study using medical records indicated that the confirmation procedure was highly accurate for this study population (23). Lastly, because of the observational nature of the current study, we cannot rule out the possibility of residual confounding by additional unmeasured or imperfectly measured confounders.
Studies on the association between age at menarche and risk of type 2 diabetes are sparse (14, 20, 21). Findings from our NHS data are generally in agreement with those from a recent prospective study of the EPIC-Norfolk cohort (women aged 40–75 years), in which earlier menarche was associated with increased risk of type 2 diabetes (per 1-year increase, adjusted relative risk = 0.91, 95% CI: 0.87, 0.96), and this association appeared to be completely mediated by adulthood adiposity (with further adjustment for BMI at baseline, adjusted relative risk = 0.98, 95% CI: 0.93, 1.03) (21). The impact of childhood characteristics on the observed association, however, was not examined. By contrast, in 2 other, smaller studies among women aged 50–92 years and 63–81 years at baseline, respectively, age at menarche was not associated with risk of type 2 diabetes, even before adjustment for BMI (14, 20).
One important characteristic of the escalating type 2 diabetes epidemic is a shift toward a younger age of onset. However, the association of age at menarche with type 2 diabetes among younger, middle-aged women was not addressed in previous studies, in which the study populations were usually older and predominantly postmenopausal (average ages were all above 57 years). In the present study, we were able to examine this association among younger women (the median age at diagnosis of type 2 diabetes was approximately 47 years). Indeed, a stronger association was observed among them than among older women (NHS). To further explore whether the stronger association among younger women was mainly due to the younger age or to differences in other characteristics between the 2 cohorts, we repeated the analyses among NHS women stratified by age at baseline (<45 years vs. ≥45 years). Even within the same cohort, the association between early menarche and risk of type 2 diabetes was stronger among younger women than among older women.
In the present study, the observed association was substantially attenuated after adjustment for adult BMI, suggesting that the relation could be largely mediated by adulthood adiposity. Indeed, earlier menarche was associated with greater adulthood BMI and greater weight gain between age 18 years and baseline in both younger and older women, which is in accordance with findings from several studies reporting significant inverse associations between age at menarche and adult obesity (7–10). However, the underlying mechanisms for such an association remain unclear. It has been hypothesized that earlier menarche may lead to the postmenarcheal accumulation of adipose tissue during pubertal development (33, 38, 39). It is also plausible that the association between early menarche and adult obesity reflects the association between childhood and adult obesity, since girls with early menarche have also been reported to have greater body fatness in childhood (40–42), and BMI tracks between childhood and adulthood (43, 44). However, in our study, the inverse association between age at menarche and adult BMI remained significant after adjustment for body figure at age 10 years and BMI at age 18 years.
In the current study, among younger and middle-aged women (NHS II), the elevated risk of type 2 diabetes associated with early menarche (ages ≤11 years) persisted even after we controlled for adult BMI, suggesting that such an association might be related to pathways beyond excessive adiposity. Early menarche has been associated with higher estrogen levels and decreased serum sex hormone-binding globulin levels that persist in adulthood (45–49). Increasing evidence suggests that endogenous sex hormones play important roles in the pathogenesis of type 2 diabetes (50). Hyperandrogenic conditions, such as polycystic ovarian syndrome, have been strongly associated with glucose intolerance and insulin resistance (11, 51–53). High plasma estradiol and testosterone levels and low sex hormone-binding globulin levels are associated with higher risk of type 2 diabetes in women, independent of adiposity (50, 54–57). It is therefore plausible that age at menarche is related to type 2 diabetes risk through its associated hormonal changes, independent of BMI.
In conclusion, early menarche was associated with increased risk of type 2 diabetes in both young and old women. This association appeared to be mediated through excessive adult adiposity and to be stronger in younger women than in older women. Furthermore, it appeared that the association among younger, middle-aged women could not be fully explained by increased adult BMI, suggesting a risk pathway between age at menarche and type 2 diabetes beyond excessive adiposity. In addition to adiposity in childhood and adolescence, age at menarche might represent another risk factor for the early identification of women who are at increased risk of being overweight or obese and of developing type 2 diabetes in adulthood.
Acknowledgments
Author affiliations: Melvin and Bren Simon Cancer Center and Department of Public Health, School of Medicine, Indiana University, Indianapolis, Indiana (Chunyan He); Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts (David J. Hunter, Susan E. Hankinson, Frank B. Hu); Channing Laboratory, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts (David J. Hunter, Susan E. Hankinson, Frank B. Hu); and Division of Epidemiology, Statistics and Prevention Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, Maryland (Cuilin Zhang, Germaine M. Buck Louis, Mary L. Hediger).
This study was supported by the National Institutes of Health (grants CA87969, CA50385, and DK58845). Drs. Cuilin Zhang, Germaine M. Buck Louis, and Mary L. Hediger were supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Conflict of interest: none declared.
Glossary
Abbreviations
- BMI
body mass index
- CI
confidence interval
- NHS
Nurses’ Health Study
References
- 1.Mokdad AH, Bowman BA, Ford ES, et al. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286(10):1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 2.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 3.Mokdad AH, Ford ES, Bowman BA, et al. Diabetes trends in the U.S.: 1990–1998. Diabetes Care. 2000;23(9):1278–1283. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 4.Garn SM, LaVelle M, Rosenberg KR, et al. Maturational timing as a factor in female fatness and obesity. Am J Clin Nutr. 1986;43(6):879–883. doi: 10.1093/ajcn/43.6.879. [DOI] [PubMed] [Google Scholar]
- 5.Kaplowitz PB, Slora EJ, Wasserman RC, et al. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics. 2001;108(2):347–353. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- 6.Adair LS, Gordon-Larsen P. Maturational timing and overweight prevalence in US adolescent girls. Am J Public Health. 2001;91(4):642–644. doi: 10.2105/ajph.91.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons TJ, Power C, Logan S, et al. Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord. 1999;23(suppl 8):S1–S107. [PubMed] [Google Scholar]
- 8.Laitinen J, Power C, Järvelin MR. Family social class, maternal body mass index, childhood body mass index, and age at menarche as predictors of adult obesity. Am J Clin Nutr. 2001;74(3):287–294. doi: 10.1093/ajcn/74.3.287. [DOI] [PubMed] [Google Scholar]
- 9.Pierce MB, Leon DA. Age at menarche and adult BMI in the Aberdeen children of the 1950s cohort study. Am J Clin Nutr. 2005;82(4):733–739. doi: 10.1093/ajcn/82.4.733. [DOI] [PubMed] [Google Scholar]
- 10.Must A, Naumova EN, Phillips SM, et al. Childhood overweight and maturational timing in the development of adult overweight and fatness: the Newton Girls Study and its follow-up. Pediatrics. 2005;116(3):620–627. doi: 10.1542/peds.2004-1604. [DOI] [PubMed] [Google Scholar]
- 11.Gambineri A, Pelusi C, Manicardi E, et al. Glucose intolerance in a large cohort of Mediterranean women with polycystic ovary syndrome: phenotype and associated factors. Diabetes. 2004;53(9):2353–2358. doi: 10.2337/diabetes.53.9.2353. [DOI] [PubMed] [Google Scholar]
- 12.Remsberg KE, Demerath EW, Schubert CM, et al. Early menarche and the development of cardiovascular disease risk factors in adolescent girls: the Fels Longitudinal Study. J Clin Endocrinol Metab. 2005;90(5):2718–2724. doi: 10.1210/jc.2004-1991. [DOI] [PubMed] [Google Scholar]
- 13.Heys M, Schooling CM, Jiang C, et al. Age of menarche and the metabolic syndrome in China. Epidemiology. 2007;18(6):740–746. doi: 10.1097/EDE.0b013e3181567faf. [DOI] [PubMed] [Google Scholar]
- 14.Saquib N, Kritz-Silverstein D, Barrett-Connor E. Age at menarche, abnormal glucose tolerance and type 2 diabetes mellitus: the Rancho Bernardo Study. Climacteric. 2005;8(1):76–82. doi: 10.1080/13697130500062688. [DOI] [PubMed] [Google Scholar]
- 15.Frontini MG, Srinivasan SR, Berenson GS. Longitudinal changes in risk variables underlying metabolic Syndrome X from childhood to young adulthood in female subjects with a history of early menarche: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2003;27(11):1398–1404. doi: 10.1038/sj.ijo.0802422. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Hong X, Wilker E, et al. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis. 2008;196(2):590–597. doi: 10.1016/j.atherosclerosis.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kivimäki M, Lawlor DA, Smith GD, et al. Association of age at menarche with cardiovascular risk factors, vascular structure, and function in adulthood: the Cardiovascular Risk in Young Finns Study. Am J Clin Nutr. 2008;87(6):1876–1882. doi: 10.1093/ajcn/87.6.1876. [DOI] [PubMed] [Google Scholar]
- 18.Herman-Giddens ME. Recent data on pubertal milestones in United States children: the secular trend toward earlier development. Int J Androl. 2006;29(1):241–246. doi: 10.1111/j.1365-2605.2005.00575.x. [DOI] [PubMed] [Google Scholar]
- 19.Demerath EW, Towne B, Chumlea WC, et al. Recent decline in age at menarche: the Fels Longitudinal Study. Am J Hum Biol. 2004;16(4):453–457. doi: 10.1002/ajhb.20039. [DOI] [PubMed] [Google Scholar]
- 20.Cooper GS, Ephross SA, Sandler DP. Menstrual patterns and risk of adult-onset diabetes mellitus. J Clin Epidemiol. 2000;53(11):1170–1173. doi: 10.1016/s0895-4356(00)00240-7. [DOI] [PubMed] [Google Scholar]
- 21.Lakshman R, Forouhi N, Luben R, et al. Association between age at menarche and risk of diabetes in adults: results from the EPIC-Norfolk cohort study. Diabetologia. 2008;51(5):781–786. doi: 10.1007/s00125-008-0948-5. [DOI] [PubMed] [Google Scholar]
- 22.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 23.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 24.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 25.Willett WC, Sampson L, Browne ML, et al. The use of a self-administered questionnaire to assess diet four years in the past. Am J Epidemiol. 1988;127(1):188–199. doi: 10.1093/oxfordjournals.aje.a114780. [DOI] [PubMed] [Google Scholar]
- 26.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 27.Willett W, Stampfer MJ, Bain C, et al. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117(6):651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 28.Troy LM, Hunter DJ, Manson JE, et al. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19(8):570–572. [PubMed] [Google Scholar]
- 29.Baer HJ, Colditz GA, Rosner B, et al. Body fatness during childhood and adolescence and incidence of breast cancer in premenopausal women: a prospective cohort study. Breast Cancer Res. 2005;7(3):R314–R325. doi: 10.1186/bcr998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Must A, Willett WC, Dietz WH. Remote recall of childhood height, weight, and body build by elderly subjects. Am J Epidemiol. 1993;138(1):56–64. doi: 10.1093/oxfordjournals.aje.a116777. [DOI] [PubMed] [Google Scholar]
- 31.Troy LM, Michels KB, Hunter DJ, et al. Self-reported birthweight and history of having been breastfed among younger women: an assessment of validity. Int J Epidemiol. 1996;25(1):122–127. doi: 10.1093/ije/25.1.122. [DOI] [PubMed] [Google Scholar]
- 32.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23(5):991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 33.Demerath EW, Li J, Sun SS, et al. Fifty-year trends in serial body mass index during adolescence in girls: the Fels Longitudinal Study. Am J Clin Nutr. 2004;80(2):441–446. doi: 10.1093/ajcn/80.2.441. [DOI] [PubMed] [Google Scholar]
- 34.McDowell MA, Brody DJ, Hughes JP. Has age at menarche changed? Results from the National Health and Nutrition Examination Survey (NHANES) 1999–2004. J Adolesc Health. 2007;40(3):227–231. doi: 10.1016/j.jadohealth.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Colditz GA. Epidemiology of breast cancer. Findings from the Nurses’ Health Study. Cancer. 1993;71(4 suppl):1480–1489. doi: 10.1002/cncr.2820710413. [DOI] [PubMed] [Google Scholar]
- 36.Viswanathan AN, Feskanich D, De Vivo I, et al. Smoking and the risk of endometrial cancer: results from the Nurses’ Health Study. Int J Cancer. 2005;114(6):996–1001. doi: 10.1002/ijc.20821. [DOI] [PubMed] [Google Scholar]
- 37.Garland M, Hunter DJ, Colditz GA, et al. Menstrual cycle characteristics and history of ovulatory infertility in relation to breast cancer risk in a large cohort of US women. Am J Epidemiol. 1998;147(7):636–643. doi: 10.1093/oxfordjournals.aje.a009504. [DOI] [PubMed] [Google Scholar]
- 38.de Ridder CM, Thijssen JH, Bruning PF, et al. Body fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. J Clin Endocrinol Metab. 1992;75(2):442–446. doi: 10.1210/jcem.75.2.1639945. [DOI] [PubMed] [Google Scholar]
- 39.van Lenthe FJ, Kemper CG, van Mechelen W. Rapid maturation in adolescence results in greater obesity in adulthood: the Amsterdam Growth and Health Study. Am J Clin Nutr. 1996;64(1):18–24. doi: 10.1093/ajcn/64.1.18. [DOI] [PubMed] [Google Scholar]
- 40.St George IM, Williams S, Silva PA. Body size and the menarche: the Dunedin Study. J Adolesc Health. 1994;15(7):573–576. doi: 10.1016/1054-139x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- 41.Power C, Lake JK, Cole TJ. Body mass index and height from childhood to adulthood in the 1958 British born cohort. Am J Clin Nutr. 1997;66(5):1094–1101. doi: 10.1093/ajcn/66.5.1094. [DOI] [PubMed] [Google Scholar]
- 42.Freedman DS, Khan LK, Serdula MK, et al. Relation of age at menarche to race, time period, and anthropometric dimensions: the Bogalusa Heart Study [electronic article] Pediatrics. 2002;110(4):e43. doi: 10.1542/peds.110.4.e43. [DOI] [PubMed] [Google Scholar]
- 43.Guo SS, Wu W, Chumlea WC, et al. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr. 2002;76(3):653–658. doi: 10.1093/ajcn/76.3.653. [DOI] [PubMed] [Google Scholar]
- 44.Monteiro PO, Victora CG. Rapid growth in infancy and childhood and obesity in later life—a systematic review. Obes Rev. 2005;6(2):143–154. doi: 10.1111/j.1467-789X.2005.00183.x. [DOI] [PubMed] [Google Scholar]
- 45.Apter D, Bolton NJ, Hammond GL, et al. Serum sex hormone-binding globulin during puberty in girls and in different types of adolescent menstrual cycles. Acta Endocrinol (Copenh) 1984;107(3):413–419. doi: 10.1530/acta.0.1070413. [DOI] [PubMed] [Google Scholar]
- 46.Apter D, Vihko R. Early menarche, a risk factor for breast cancer, indicates early onset of ovulatory cycles. J Clin Endocrinol Metab. 1983;57(1):82–86. doi: 10.1210/jcem-57-1-82. [DOI] [PubMed] [Google Scholar]
- 47.Apter D, Reinilä M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer. 1989;44(5):783–787. doi: 10.1002/ijc.2910440506. [DOI] [PubMed] [Google Scholar]
- 48.Vihko RK, Apter DL. The epidemiology and endocrinology of the menarche in relation to breast cancer. Cancer Surv. 1986;5(3):561–571. [PubMed] [Google Scholar]
- 49.Vihko R, Apter D. Endocrine characteristics of adolescent menstrual cycles: impact of early menarche. J Steroid Biochem. 1984;20(1):231–236. doi: 10.1016/0022-4731(84)90209-7. [DOI] [PubMed] [Google Scholar]
- 50.Ding EL, Song Y, Malik VS, et al. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–1299. doi: 10.1001/jama.295.11.1288. [DOI] [PubMed] [Google Scholar]
- 51.Tok EC, Ertunc D, Evruke C, et al. The androgenic profile of women with non-insulin-dependent diabetes mellitus. J Reprod Med. 2004;49(9):746–752. [PubMed] [Google Scholar]
- 52.Toprak S, Yönem A, Cakir B, et al. Insulin resistance in nonobese patients with polycystic ovary syndrome. Horm Res. 2001;55(2):65–70. doi: 10.1159/000049972. [DOI] [PubMed] [Google Scholar]
- 53.Oh JY, Barrett-Connor E, Wedick NM, et al. Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo Study. Diabetes Care. 2002;25(1):55–60. doi: 10.2337/diacare.25.1.55. [DOI] [PubMed] [Google Scholar]
- 54.Golden SH, Dobs AS, Vaidya D, et al. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92(4):1289–1295. doi: 10.1210/jc.2006-1895. [DOI] [PubMed] [Google Scholar]
- 55.Tsai EC, Matsumoto AM, Fujimoto WY, et al. Association of bioavailable, free, and total testosterone with insulin resistance: influence of sex hormone-binding globulin and body fat. Diabetes Care. 2004;27(4):861–868. doi: 10.2337/diacare.27.4.861. [DOI] [PubMed] [Google Scholar]
- 56.Lee CC, Kasa-Vubu JZ, Supiano MA. Androgenicity and obesity are independently associated with insulin sensitivity in postmenopausal women. Metabolism. 2004;53(4):507–512. doi: 10.1016/j.metabol.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 57.Ding EL, Song Y, Manson JE, et al. Plasma sex steroid hormones and risk of developing type 2 diabetes in women: a prospective study. Diabetologia. 2007;50(10):2076–2084. doi: 10.1007/s00125-007-0785-y. [DOI] [PubMed] [Google Scholar]