Abstract
CD80 expressed on the surface of antigen presenting cells provides a positive costimulatory signal to naïve CD4+ T cells via CD28 during activation. However, CD80 is also expressed on the surface of activated CD4+ T cells, and cross-linking CD80 on the surface of CD4+ T cells activated in the presence of Th1-promoting cytokines induces a direct upregulation of T-bet, IFN-γ, and Bcl(XL) expression in primary CD4+ T cells. The present data show that naïve CD4+ T cells activated in Th1-promoting conditions in the presence of anti-CD80 mAb increase the level of IFN-γ produced by increasing the rate of IFN-γ mRNA transcription, which is supported by an increase in the level of T-bet phosphorylation and T-bet binding to the 3rd intronic enhancer in the IFN-γ locus. Furthermore, anti-CD80 mAb-induced increase in IFN-γ expression and T-bet phosphorylation is dependent upon the activation of a Ca2+-dependent pathway as shown by anti-CD80 mAb-induced intracellular Ca2+ flux following CD80 cross-linking. These findings indicate a novel regulatory role for CD80-mediated intracellular signals in CD4+ T cells and have important implications for disease therapies employing anti-costimulatory monoclonal Abs (mAbs) as use of an intact CD80 mAb may lead to CD80 cross-linking on activated T cells and enhanced proinflammatory cytokine production.
Keywords: T cell, Costimulation, CD80, Cell Signaling, Tolerance/Suppression/Anergy
Introduction
According to the two-signal hypothesis for the activation of naïve CD4+ T cells, the first signal necessary for naive CD4+ T cell activation is antigen-specific T cell receptor (TCR)2 binding of antigenic peptide-MHC II complex on an antigen presenting cell (APC). The second signal, a costimulatory signal, is largely provided via CD28 expressed on the CD4+ T cell surface binding one of the B7 family members (CD80 or CD86) expressed on the surface of activated APCs (1-4). While this model of CD4+ T cell activation and differentiation is over simplistic, the two-signal hypothesis has been the basis of multiple therapeutic strategies for treatment of autoimmune disease. For example, it has been hypothesized that blockade of these costimulatory signals may serve as an effective immunotherapy in relapsing-remitting experimental autoimmune encephalomyelitis (R-EAE) (5, 6), diabetes (7), and transplantation (8, 9). As such, multiple studies have detailed the effects of anti-CD80 monoclonal antibody (mAb) treatment during the course of R-EAE. Depending on the time of treatment, varying effects on disease outcome have been reported (10). These findings suggest that the blockade of CD80 interacting with its ligand is able to modulate T cell, B cell, and dendritic cell function both in a positive or negative manner (11-13). Although the signaling events following CD28-B7 interaction have been studied for the CD28 signaling cascade in CD4+ T cells, the ability of CD80 cross-linking to induce an alteration in the function of “classic” CD80-expressing cell types, such as B cells and dendritic cells (1, 2), as well as primary CD4+ T cells (11) has not been thoroughly elucidated.
Besides APCs, activated CD4+ T cells also express CD80 and CD86 (11, 14, 15), and these costimulatory molecules potentially signal to a CD4+ T cell directly following cross-linking with mAb (11). Since CD4+ T cells express CD80 following activation, and CD80 cross-linking induces a putative intracellular signal, the possibility exists that CD80 stimulation of CD4+ T cells may affect T cell effector function. Previous work from our laboratory has shown that cross-linking of CD80 on CD4+ T cells induces an increase in IFN-γ and T-bet transcripts and protein expression (11). The transcriptional regulation of IFN-γ has been widely studied. DNase I hypersensitivity mapping of the IFN-γ locus regulatory regions in Th1 cells have identified three main regulatory regions for the IFN-γ locus. Of these three regions, the distal enhancer sequence within the 3rd intron appears to contain the highest level of cis-regulatory activity in transfection assays (16). Initiation and maintenance of transcriptional control at the IFN-γ locus is primarily via T-bet (17).
Our previous results demonstrated that anti-CD80 mAb treatment induces an increase in IFN-γ and T-bet, without altering the number of IFN-γ secreting cells (11). Considering the aforementioned finding that the 3rd intronic enhancer regulates transcriptional activity at the IFN-γ locus, we hypothesized that cross-linking CD80 on the surface of a CD4+ T cell activated in Th1-promoting conditions induces an increase in T-bet phosphorylation and binding to the 3rd intronic enhancer. Here we show that CD80 cross-linking increases the level of IFN-γ by increasing the rate of IFN-γ transcription, and binding of T-bet to the 3rd intronic enhancer. Further, we show that cross-linking CD80 on CD4+ T cells induces the activation of a Ca2+-dependent signaling pathway. The present data help to illustrate the complexity of using intact monoclonal antibodies for therapeutic use in vivo, given that bivalent monoclonal antibodies may bind and cross-link the same surface molecules on multiple cell types leading to diverse functional outcomes.
Materials and Methods
Mice and CD4+ Th cell-promoting culture conditions
Female SJL (Harlan Labs; Indianapolis, IN), C57Bl/6 (Jackson Laboratories; Bar harbor, ME), and PD-L1 knockout (kindly provided by L. Chen at Johns Hopkins University) were housed under SPF conditions in the Northwestern Univ. Animal Facility. Naïve CD4+ T cells were purified using AutoMacs Magnetic Bead cell separation technology (Miltenyi Biotech; Auburn, CA) from total lymph node cells isolated from unprimed mice. The purity of the isolated naïve CD4+ T cells (L-selectinhi CD4+ cells) was routinely found to be 98-99.9%. For in vitro activation, 3-5×105 naïve CD4+ T cells were activated in the presence of 5-25×105 latex beads coated with 1 μg of anti-CD3 and/or 1 μg anti-CD28 in neutral (IL-2 at 200 U/ml), Th1-driving (IL-2 at 200 U/ml; IL-12 at 40 U/ml; anti-IL-4 at 10 μg/ml), or Th2-driving (IL-2 at 200 U/ml; IL-4 at 500 U/ml; anti-IFN-γ at 10 μg/ml) conditions in the presence or absence of either control antibody [Armenian Hamster IgG (eBioscience; San Diego, CA)] or intact anti-CD80 antibody (????).
ELISA and Ca2+ flux
Naïve CD4+ T cells were isolated and activated as described above. After 24 hours in culture the cells were collected and labeled with 1 μM Indo-1 (Invitrogen; Carlsbad, CA) for 15 min at 37°C followed by repeated washes and continuedincubation at 37°C for an additional 2 h. In some experiments cells were treated with cell signaling inhibitors [Wortmannin (50nM), GF109203X (20nM), Go6967 (2.3-20nM), p38 MAPK inhibitor (35nM), SB202190 (30nM), or U73122 (1.5μM); (Biosource; Camarillo, CA)] during the final 30 min before analysis prior to the addition of either Control Ig or anti-CD80 mAb. Cell samples were analyzed on a LSRII for 30 s before the addition of an increasing concentration of either isotype control, anti-CD3, antiCD80 mAb, and/or anti-CD80 Fab. Sample analysis was immediately continued following Ab addition for an additional 3 min. Data are presented as the ratio of 398 nm (Indo-1 bound to Ca2+)/482 nm (unbound Ca2+) in CD4+ T cells over a period of 3.5 min.
Nuclear run-on
The rate of IFN-γ transcription was determined by nuclear run-on, as described in detail elsewhere (18, 19). Briefly, 20×106 naïve CD4+ cells were activated as described above, collected on day 3 following activation. Nuclear run-on and RNA isolation were preformed in the presence of biotin-16-UTP (Roche; Indianapolis, IN). To control for the possibility of non-biotin-labeled RNA contamination, replicate sets of nuclei were used in the nuclear run-on that did not contain biotin-16-UTP. Dynabeads M-280 (Dynal; Carlsbad, CA) were used to capture the biotin-labeled molecules from the purified nuclear RNA. RT-PCR was preformed from serially diluted cDNA samples for the level of actin and IFN-γ transcripts. PCR reactions were run on a 1.5% agarose gel with ethidium bromide and densitometry was preformed using NIH Image 1.61 software (National Institutes of Health, USA). Actin served as an internal control to ensure the efficiency of the reverse transcription and the amount of RNA utilized in each reaction. The optical density (O.D.) values obtained for actin were used to normalize the IFN-γ optical density values using ImageJ 1.39 (NIH). All samples that did not contain biotin-16-UTP were found to be negative for actin and IFN-γ transcripts.
IFN-γ Transcript Stability and Real-time PCR
On day 3 following the initial activation of naïve CD4+ T cells in Th1-promoting conditions, 20 μg/ml of Actinomycin D (Sigma) was added to each culture to stop the further production of mRNA transcripts. T cells were collected from the cultures over a 16 h time course following the addition of Actinomycin D, cell viability was analyzed by trypan blue exclusion, and total mRNA isolated. Total mRNA was isolated with TRIZOL Reagent (Invitrogen) and was reversed transcribed into cDNA using random hexamer primers. Briefly, a common master mix [LightCycler-FastStartDNA SYBR Green I (Roche), 2 mM MgCl2, 0.5 μM gene-specific primer], and 1.5 μl of cDNA for a final reaction volume of 15 μl was used. After each real-time reaction, a melting curve was generated and samples were run on a 1.2% agarose gel to ensure that only one gene-specific PCR product was generated. Real-time PCR was preformed using the Roto-gene 2000 Real-time Cycler (Phoenix Research Products; Hayward, CA). The following primers were used. β-actin 5'- TACAGCTTCACCACCACAGC-3' and 5'- AAGGAAGGCTGGAAAAGAGC-3' (annealing temp 60 °C, 206-bp product); IFN-γ 5'-CACGGCACAGTCATTGAAAG-3' and 5'-GCTGATGGCCTGATTGTCTT-3' (annealing temp 60°C, 198-bp product); T-bet 5'-CGGAGAATGGACTCCAGAGA-3' and 5'-CTGTTTGGCTGGCTGTTGTA-3' (annealing temp 60°C, 201-bp products).
Western blot
5×106 naïve CD4+ T cells were activated as described above. For total cellular protein, cells were collected, washed three times with PBS, lysed with 1% Triton X-100 lysis buffer, and frozen at –80°C until analysis. Protein samples (5-10 μg) were run on a denaturing 7.5% polyacrylamide gel and transferred to Immobilon-P PVDF membranes (Millipore, Bedford, MA). As described previously (19), membranes were blocked with TBST+ 5% dried milk, probed with primary antibodies diluted in TBST + 5% dried milk, and washed . Membranes were then probed with horse radish peroxidase (HRP)-labeled secondary antibodies diluted in TBST + 5% dried milk, washed, and HRP-labeled antibodies were detected using the LumiGlo Detection Kit (Cell Signaling, Inc., Beverly, MA) and specific bands were visualized on Kodak Biomax MS film using an intensifying screen enabled film cassette. Antibodies used were anti-T-bet (C-15) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-actin (C-11), and anti-phosphorylated tyrosine (clone PY20) (Upstate).
Chromatin immunoprecipitation (ChIP)
ChIP analysis was carried out essentially as described previously (20). Naïve T cells (10×106 cells) activated as described above were collected on day 3, fixed with 1.1% formaldehyde solution, and cross-linking was stopped by the addition of glycine at a final concentration of 0.125 M for 5 min. Cells were rinsed with cold PBS and resuspended in 0.25% Triton X-100 lysis buffer. Nuclei were pelleted, resuspended, and sonicated ten times for 30 s, with a 1 min cooling period on ice in-between. Debris was removed and samples were precleared with protein A/protein G agarose beads that had been blocked with sonicated salmon sperm DNA and BSA. The beads were removed and chromatin samples were incubated at 4 °C with various antibodies overnight. Immune complexes were precipitated for 3 h by the addition of blocked protein A/protein G agarose beads. The precipitates were washed and resuspended in 100 μl of TE buffer. The samples were adjusted to 0.5% SDS, 100 μg/ml RNase A, and 200 μg/ml of proteinase K and incubated at 55 °C for 3 h, followed by an overnight incubation at 65 °C to reverse the formaldehyde cross-links. The DNA was purified by phenol-chloroform extraction, precipitated in the presence of 20 μg of glycogen and resuspended in 100 μl of TE buffer. PCR was done with 2 μl of the immunoprecipitated DNA for 30 cycles (45 s at 95 °C, 45 s at 56 °C and 2 min at 72 °C, completed by 10 min in 72 °C) with various primers. As a control, the PCR was done directly on input DNA purified from chromatin before immunoprecipitation. PCR products were resolved on 1.5% agarose gels and visualized with ethidium bromide. The antibodies used were anti-T-bet and anti-p50 as a control Ab (Santa Cruz Biotechnology). The following primers were used: IFN-γ promoter 5'-CATACCCTTTCCTTGCTTTTC-3' and 5'-TTGTGGGATTCTCTTGAAAGCA-3'; and 3rd Intron enhancer, 5'-GTGTGTTAGTGGAAAGAGCAG-3' and 5'-GATGTCTACTATTGCTTCGCC-3'.
Statistical analyses
Data were analyzed by a one-way ANOVA to determine whether an overall statistically significant change existed before using the two-tailed unpaired Student's t test. Statistically significant differences were reported when the p value was < 0.05.
Results
Cross-Linking CD80 induces an increase in IFN-γ produced
Our previous findings indicated that addition of an intact anti-CD80 mAb may send a signal to the CD4+ T cell resulting in increased IFN-γ production (11). Alternatively, the addition of anti-CD80 mAb may block a negative costimulatory molecule that interacts with CD80, such as CTLA-4 (2) or PD-L1 (21). PD-L1 is hypothesized to act as a negative regulatory molecule for effector CD4+ T cell function. Therefore, we wished to determine if the addition of anti-CD80 mAb was indeed sending a direct signal as opposed to blocking a negative regulatory signal. Since the addition of the non-cross-linking anti-CD80 Fab does not induce an increase in the level of IFN-γ produced, the later possibility appeared to be unlikely (11). However, we wished to test further which of the two aforementioned possibilities may play a role in the anti-CD80 mAb-induced increase in IFN-γ production. Naïve CD4+ T cells were activated in the presence of Th1 cell-promoting conditions and the level of CD80 and CTLA-4 was determined over a 48 hour time course. As shown in Figure 1A, CD80 is expressed by the 38.7% of the CD4+ T cells by 12 h, and increases to 63.3% by 24 h. CD80 expression appears to peak at 48 h, and a small percentage of CD80/CTLA-4 double positive cells (10.5%) are observed by 48 h. We also found that both CD28 (22, 23) and PD-L1 (21) were constitutively expressed by the cultured CD4+ T cells (data not shown).
Figure 1. Anti-CD80 mAb induces an increase in IFN-γ in the absence of PD-L1.
CD4+ T cells were isolated from naïve wild-type SJL mice and cultured (106 cells) with anti-CD3 + anti-CD28 coated beads + IL-2 and IL-12 (4 ng/ml) + anti-IL-4 (10 μg/ml) (Th1-promoting conditions). Cells were collected over a 48 h time course and the percent of CD4+/CD28+ T cells expressing (A) CD80 versus CTLA-4 was determined by flow cytometry. (B) Naïve CD4+ T cells were activated in the presence of Th1-promoting conditions in the presence of Control Ig, intact anti-CD80 mAb or CTLA-4 Ig (1μg/ml) plus increasing concentrations of anti-CD80 Fab (0-100 μg/ml) and culture supernatants were collected on day 3 to assay the amount of IFN-γ produced via ELISA. (C) Likewise, naïve CD4+ T cells from wildtype and PD-L1−/− C57BL/6 mice were activated in the presence of Th1-promoting conditions plus Control Ig, anti-CD80 mAb, anti-CD80 Fab, CTLA-4 Ig, anti-CTLA-4, or soluble anti-CD28 in the absence or presence of anti-CD80 mAb (1μg/ml). Data is presented as the mean units of IFN-γ/ml produced from three replicate wells. Asterisk (*) indicates a statistically significant increase in the level of total IFN-γ produced in comparison to control Ig treated cells, p < 0.05. One representative of two independent experiments is shown.
We next determined if the addition of a natural ligand, CTLA-4, for CD80 was able to induce an increase in the level of IFN-γ produced. As shown in Figure 1B, the addition of CTLA-4 Ig induced an increase in the level of IFN-γ produced by naïve CD4+ T cells activated in Th1-promoting conditions. Furthermore, both the anti-CD80 mAb- and CTLA-4 Ig-induced increases in IFN-γ production were blocked by the addition of anti-CD80 Fab. To determine if the addition of anti-CD80 mAb potentially blocks a negative regulatory signal resulting from CD80 interacting with PD-L1 during T cell-T cell interactions, naïve CD4+ T cells from wildtype and PD-L1−/− mice were activated in Th1-promoting conditions. As shown in Figure 1C, the addition of anti-CD80 mAb, CTLA-4 Ig, or anti-CD28 induces an increase in the level of IFN-γ produced in both the presence or absence of PD-L1 expression. Furthermore, the anti-CD80 mAb-induced increase in IFN-γ is blocked by the addition of anti-CD80 Fab. Collectively, these findings suggest that the anti-CD80 mAb-induced increase in the level of IFN-γ is due to cross-linking CD80 on the surface of CD4+ T cells, not due to blockade of a negative regulatory signal.
Cross-linking CD80 induces T-bet phoshorylation
Naïve CD4+ T cells activated in Th1-promoting conditions demonstrate a time-dependent increase in the levels of CD80 expression (Figure 1A) (11, 15, 24), and cross-linking CD80 by the addition of an anti-CD80 mAb increases the level of IFN-γ message and protein, and T-bet message expressed (11, 25). To determine if the CD80-induced increase in the level of T-bet message is translated into the functional protein, naïve CD4+ T cells were activated in neutral (anti-CD3/28 plus IL-2), Th1-promoting (added IL-12 and anti-IL-4 mAb), or Th2-promoting (added IL-4, anti-IL-12 mAb, and anti-IFN-γ mAb) conditions in the presence of either a species and isotype matched control Ig or anti-CD80 mAb. As shown in Figure 2A the level of T-bet protein present within CD4+ T cells activated in Th1-promoting conditions in the presence of anti-CD80 mAb was increased as determined by western blot. However, when naïve CD4+ T cells were activated in Th2-promoting conditions, T-bet expression was not induced in either the presence or absence of anti-CD80 mAb.
Figure 2. anti-CD80 mAb treatment-induced increase in T-bet expression and tyrosine phosphorylation.
Naïve CD4+ T cells were activated in culture as described in Figure 1, plus the addition of cells cultured in the presence of anti-CD3 + anti-CD28 (1 μg/ml) coated beads + IL-2 (200 U/ml) (Th0-promoting conditions); or anti-CD3 + anti-CD28 coated beads + IL-2 + IL-4 (11.1 ng/ml) + anti-IL-12 and anti-IFN-γ (10 μg/ml) (Th2-promoting conditions). After 24 h in culture, either a species and isotype matched Control Ig, or anti-CD80 mAb (1 μg/ml) was added to the cultures and total cellular protein isolated 3 hours later. (A) The level of T-bet expression was determined by western blot, and the level of tyrosine phosphorylated T-bet was determined by first immunoprecipitating total T-bet, and probing the blots with either anti-T-bet or anti-phosphotyrosine antibodies. (B) The fold increase in the level of total T-bet expression and the level of tyrosine phosphorylated T-bet following the addition of anti-CD80 mAb as determined by densitometry compared to cells that received Control Ig treatment from three individual experiments is shown. Asterisk (*) indicates a statistically significant increase in the level of total T-bet and phosphorylated T-bet in comparison to control Ig treated cells, p < 0.05.
Tyrosine phosphorylation of T-bet has been shown to regulate both the transcription of the IFN-γ gene and to repress the Th2 genetic program through the physical interaction with GATA-3 (26). To further determine if the role of the anti-CD80 mAb-induced increase in T-bet protein is functionally relevant, we evaluated the level of T-bet phosphorylation. T-bet was immunoprecipitated and membranes probed with either an anti-T-bet mAb to determine the level of T-bet or an anti-phosphorylated tyrosine antibody to determine the level of tyrosine phosphorylated T-bet. The results show that the level of phosphorylated T-bet is significantly increased following CD80 cross-linking (Figure 2B). In summary, these data show that the level of T-bet protein expression and phosphorylation is increased following cross-linking of CD80 on CD4+ T cells activated in Th1-promoting conditions.
CD80 cross-linking increases the rate of IFN-γ transcription
The finding of increased expression of T-bet protein and phosphorylation upon CD80 cross-linking correlates with our previous findings that cross-linking CD80 on CD4+ T cells activated in Th1-promoting conditions increases the level of IFN-γ transcript and protein. The level of transcript present within a cell is regulated by both the rate of specific gene transcription and the stability of that transcript within the cell (27). To determine the mechanism by which cross-linking CD80 induces increased levels of IFN-γ expression, the effect of anti-CD80 mAb treatment on both the rate of IFN-γ transcription and the stability of the IFN-γ transcript produced was determined. First, naïve CD4+ T cells were activated in Th1-promoting conditions in the presence of a species and isotype matched control Ig or various concentrations of anti-CD80 mAb. As shown in Figure 3A, as the concentration of anti-CD80 mAb added to the cultures was increased, the rate of IFN-γ transcription increased as determined by nuclear run-on. Furthermore, the anti-CD80 mAb-induced increase in the rate IFN-γ transcription was blocked by the addition of a non-cross-linking anti-CD80 Fab fragment.
Figure 3. Cross-linking CD80 increases the rate of IFN-γ transcription.
Naïve CD4+ T cells were activated in culture as described in Figure 1. (A) After 3 days in culture, nuclei were isolated from 20×106 cells and nuclear run-on was performed in the presence of biotin-16-UTP. Biotin-labeled RNA was incubated with streptavidin-labeled magnetic beads, and isolated by magnetic separation. The level of IFN-γ transcript was analyzed by RT-PCR. The level of IFN-γ transcript produced was determined for cultures that received Control Ig (■), anti-CD80 mAb (1 μg/ml) (▲), anti-CD80 mAb (1 μg/ml) + anti-CD80 Fab (1 μg/ml) (•), and anti-CD80 mAb (10 μg/ml) (◆). Data are presented as the normalized optical density of IFN-γ transcript from one representative of two independent experiments. Asterisk (*) indicates a statistically significant increase in the level of total IFN-γ in comparison to control Ig treated cells, p < 0.05. (B) To determine the stability of the IFN-γ transcript, 106 cells were collected (t=0), and then Actinomycin D (20 μg/ml) was added to the remaining cells in culture. Cells with actinomycin D were collected over a 4 hour time course. Cell viability was analyzed by trypan blue exclusion and total cellular RNA was collected for quantitation of IFN-γ transcript by real-time PCR. Linear regression was done to determine the rate of IFN-γ degradation. One representative experiment of three independent experiments is shown. (C) After three days, 10×106 cells were fixed, nuclei isolated, lysed, and DNA was sheared into 200-500 base pair fragments by sonication. T-bet bound to the DNA was then immunoprecipitated by the use of an anti-T-bet antibody. The amount of T-bet bound to the IFN-γ promoter and 3rd intronic enhancer was analyzed by RT-PCR. The amount of T-bet-specific PCR product (T) for each sample was determined by subtracting out the non-specific PCR product from samples that received a species and isotype matched control Ab (C) during the immunoprecipitation and normalized to the amount of in-put DNA (I). One representative experiment of two is shown.
To determine if the stability of IFN-γ transcript was affected by cross-linking CD80, actinomycin D was added to the in vitro cultures on day 3 after initial activation. Cells were collected before, and at various times after, the addition of actinomycin D. Cell viability was assessed at each of these time points by trypan blue exclusion, and the overall viability of the cells did not change significantly until 4-16 hours after the addition of actinomycin D. Since a two-fold decrease in cell viability was seen at the 6 hour time point, these cells were eliminated from analysis. The level of IFN-γ transcript present at the remaining time points was quantified by real-time PCR. Linear regression was done for each of the treatment groups, so that the stability of the mature transcript could be determined following cross-linking CD80 in our in vitro model system. As shown in Figure 3B, stimulation of CD80 does not affect the stability of IFN-γ transcript as compared to the species- and isotype-matched control Ab group. These results indicate that cross-linking CD80 on a CD4+ T cell activated in Th1-promoting conditions increases the rate of IFN-γ transcription, while not affecting the stability of the transcript produced.
We next determined if anti-CD80 mAb-induced increase in T-bet expression and phosphorylation is relevant to the increase in the rate of IFN-γ transcription by assessing the level of T-bet bound to the IFN-γ regulatory regions, i.e., the IFN-γ promoter and the 3rd intronic enhancer, using chromatin immunoprecipitation (ChIP). PCR primer sets were designed to amplify regions of DNA that are known to contain the IFN-γ promoter and the 3rd intronic enhancer, and of the regions containing T-bet binding sequences (16, 28). The data show that the addition of anti-CD80 mAb induces an increase in the level of T-bet bound to the 3rd intronic enhancer (Figure 3C). In contrast, the level of T-bet bound to the IFN-γ promoter was not altered by the addition of anti-CD80 mAb. Collectively, cross-linking CD80 on the surface of CD4+ T cells activated in Th1-promoting conditions increases the expression, phosphorylation, and binding of T-bet to the IFN-γ regulatory regions.
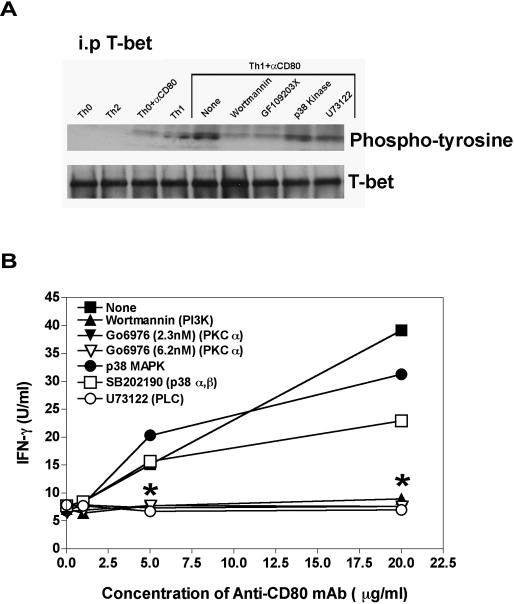
Inhibition of Anti-CD80 mAb-induced T-bet Phosphorylation and IFN-γ
Since cross-linking CD80 on CD4+ T cells activated in Th1-promoting conditions increases the level of T-bet phosphorylation, a putative starting point for the identification of the CD80 signaling pathway has been identified. Published data show that T-bet is phosphorylated on tyrosine residues through the activation of Ca2+-dependent pathways (26, 29, 30). Therefore, we determined if the inhibition of various signaling pathways that involve tyrosine phosphorylation was able to inhibit the anti-CD80 mAb-induced increase in T-bet phosphorylation. Following initial activation for 24 hours in Th1-promoting conditions, cells were treated with inhibitors of various signaling pathways or vehicle for 30 minutes, at which time anti-CD80 mAb was added and total cellular protein isolated after an additional 3 hours. As shown in Figure 4A, blockade of the Ca2+-dependent pathways was able to inhibit the phosphorylation of T-bet. To determine if the inhibition of T-bet was functionally relevant, the level of IFN-γ protein secreted was determined via ELISA. The data show that blockade of the Ca2+-dependent pathways also inhibits the anti-CD80 mAb-induced increase in the level of secreted IFN-γ (Figure 4B). These findings indicate that cross-linking CD80 on CD4+ T cells activated in Th1-promoting conditions activates a putative Ca2+-dependent pathway.
Figure 4. Inhibition of Anti-CD80 mAb-induced T-bet Phosphorylation and IFN-γ.
Naïve CD4+ T cells were activated in culture as described in Figure 1. After 24 h, either a vehicle control or specific inhibitors [Wortmannin (50 nM), Go6967 (2.3-20 nM), p38 MAPK inhibitor (35 nM), GF109203X (20nM), SB202190 (30 nM), or U73122 (1.5 μM)] were added, followed thirty minutes later by the addition of either a species and isotype matched Control Ig or anti-CD80 mAb (1 μg/ml). (A) After 3 h in culture, total cellular protein was isolated and the level of tyrosine phosphorylated T-bet was determined by first immunoprecipitating T-bet and probing the blots with either anti-T-bet or anti-phosphotyrosine antibodies. (B) After 48 h, culture supernatants were collected and the level of IFN-γ produced was determined by ELISA. One representative experiment of three is shown. Asterisk (*) indicates a statistically significant decrease in the level of total IFN-γ in comparison to anti-CD80 mAb treated cells in the vehicle, p < 0.05.
Cross-lining CD80 induces an intracellular Ca2+ Flux
Since the above findings suggest that cross-linking CD80 on the surface of CD4+ T cells activated in the Th1-promoting conditions activates a Ca2+-dependent signaling pathway, we sought to determine if cross-linking CD80 on naïve CD4+ T cells activated in Th1-promoting conditions induces a Ca2+-flux within CD4+ T cells. Naïve CD4+ T cells activated in Th1-promoting conditions for 24 hours induces the surface expression of CD80 (11). CD4+ T cells were then treated with a vehicle control (DMSO), specific signaling pathway inhibitors, or anti-CD80 Fab for 30 minutes prior to stimulation with anti-CD80 mAb or anti-CD3 mAb as a control for the induction of a Ca2+-flux. As shown in Figure 5, the addition of anti-CD3 mAb or anti-CD80 mAb to CD4+ T cells activated in the Th1 cell-promoting conditions and DMSO induces a Ca2+-flux. Pretreatment of naïve CD4+ T cell activated in Th1-promoting conditions cells with anti-CD80 Fab was able to inhibit the anti-CD80 mAb-induced Ca2+-flux demonstrating the specificity of the induction. In addition, pretreatment with the inhibitors, wortmannin and U73122, blocked the induction of the anti-CD80 mAb-induced Ca2+-flux. In contrast, when cells pretreated with the PKCα inhibitor Go6976, which is down stream of the Ca2+ flux, had no effect on the anti-CD80 mAb-induced Ca2+-flux. Collectively, the present findings suggest that cross-linking CD80 on CD4+ T cells activated in Th1-promoting conditions induces a Ca2+ flux.
5. Cross-linking CD80 induces a Ca2+-flux.
Naïve CD4+ T cells were activated in culture as described in Figure 1. After 24 h, either a vehicle control or specific inhibitors [Wortmannin (50nM), Go6967 (2.3nM), or U73122 (1.5 μM)] or anti-CD80 Fab were added. The cells were then labeled with Indo-1 and the ability of anti-CD3 mAb (1 μg/ml) or anti-CD80 mAb (1 μg/ml) to induce a Ca2+-flux was analyzed via FACS analysis. One representative experiment of three is presented.
Discussion
Although limited data are available to support a functional role of CD80 expression by CD4+ T cells during an immune response (31, 32), the current results support the hypothesis that stimulation via CD80 on CD4+ T cells positively regulates effector Th1 function. Cross-linking CD80 on T cells was previous shown to induce an increase in the tyrosine phosphorylation of multiple intracellular proteins (33), and increases the level of IFN-γ and T-bet transcription (11). To our knowledge, this is the first report that begins to identify a putative signaling pathway induced following cross-linking of CD80 on CD4+ T cells or any other cell type. Our findings indicate that cross-linking CD80 on CD4+ T cells activated in Th1-promoting conditions increases the level of IFN-γ produced by increasing the rate of IFN-γ transcription via the activation of a Ca2+-dependent signaling pathway.
Over the past several years there has been an increase in the number of findings that suggest the ability of two of the B7 family members, i.e., CD80 and CD86, to induce intracellular signals directly to the cell that the molecule is expressed on. For example, cross-linking CD80 on the surface of B cells activated in the presence of IFN-γ increases the level of IgG2a produced, as well as increases pro-apoptotic genes (12). Likewise, dendritic cells activated in the presence of soluble CD80/86 increases the expression of express IL-6 and IFN-γ in a CD80-, CD86-, and p38 MAP kinase-dependent manner (13). Considerable progress has been made towards determining the signaling pathway activated following cross-linking CD86. Cross-linking CD86 on the surface of B cells activated in the presence of IL-4 increases the rate of mature IgG1 transcript produced via PI3K-dependent activation of the NF-κB subunits p65 and p50. Likewise, activation of these same NF-κB subunits is induced in macrophage cell lines following the cross-linking of CD80 and CD86 (34). The present findings suggest that the CD80-induced increase in IFN-γ is similarly PI3K-dependent. Taken together, it is tempting to conclude that the same intracellular signaling pathway is activated following both CD80 and CD86 cross-linking. However, cross-linking CD80 on B cells induces pro-apoptotic gene expression, while cross-linking CD86 induces anti-apoptotic gene expression (12). Therefore, while there may be common intracellular signaling intermediates activated by both CD80 and CD86, receptor-specific intracellular signaling intermediates must also be activated.
The high affinity interaction between CD4+ T cell-expressed CD80 and CD4+ T cell-expressed CTLA-4 is hypothesized to play a regulatory role during T cell-T cell interactions. It has been observed that Th2 cells express higher levels of CTLA-4 than Th1 cells. This finding was correlated with data showing that Th2 cells, but not Th1 cells, show variations in the organization of the immunological synapse dependent on B7 expression by the APC (35). The current data show that CD80 and CTLA-4 are co-expressed by a small percentage of the CD4+ T cells by 48 h after activation allowing for CD80-CTLA-4 interaction. Cross-linking CTLA-4 inhibits CD4+ T cell activity by decreasing tyrosine phosphorylation of Fyn and ZAP70 and Ca2+ mobilization following TCR stimulation (36-39). Our results show that cross-linking CD80 induces a Ca2+-flux. Since studies in human T cells line suggest that an increase in intracellular Ca2+ would increase the level of CTLA-4 expressed (40, 41), we are currently investigating if cross-linking CD80 also alters the level of CTLA-4 expressed. These data may point to a putative T cell-T cell regulatory mechanism by which CD80 interaction with its ligand CTLA-4 would induce a temporal increase in cytokine production and survival signals for CD4+ T cells expressing CD80 during the early stages of an immune response. This hypothesis is supported by the finding that transfer of CD80/CD86−/− T cells resulted in a significantly increased severity of GVHD as compared to transferred wildtype T cells, while transfer of T cells over-expressing CD86 decreased GVHD as compared to wildtype T cells (15). This finding suggests that CD80/CD86 expression on T cells down-regulated allogeneic responses through CTLA-4 ligation. In contrast, CD4+ T cell-expressed CD80 has been shown to play a critical role in CD4+ T cell-mediated anti-tumor activity suggesting that inflammatory CD4+ T cell responses may be increased by CD80 expressed on tumor-infiltrating CD4+ T cells (14, 42). However, since cross-linking CD80 may then also increase the level of CTLA-4 expressed by the activated CD4+ T cells, this would eventually result in down-regulation of the response.
The importance of CD80-mediated costimulation in autoimmune disease progression, such as in R-EAE, is supported by studies analyzing the temporal expression of B7 costimulatory molecules. Active immunization of SJL mice with PLP139-151 in CFA, resulted in a temporal upregulation of surface CD80 expression, relative to CD86, on B cells, T cells, and macrophages in the spleen (43). Furthermore, a CD80-dominant expression pattern is seen on all peripheral inflammatory cell types in the CNS-infiltrating population (44). The relevance of CD80 expression by CD4+ T cells is not limited to the mouse system. CD80 is also expressed by human peripheral blood CD4+ T cells, and the ability of CD80 to initiate an intracellular signal has also been shown to exist in human immune cells (12, 33). Analysis of lesions from MS patients has identified an increased level of CD80 expressed at the lesion site (45). Genetic analyses of MS patients support the hypothesis that CD80 expressed by autoreactive CD4+ T cells may contribute to the disease exacerbation. There is a positive correlation between the expression of allelic variants of CTLA-4 that have an increased affinity for binding to CD80 in MS patients (46). Beside the anti-CD80 mAb-induced increase in the level of IFN-γ, ongoing studies in our laboratory have begun to focus on the effect anti-CD80 mAb treatment has on the level of IL-17 produced by encephalitogenic CD4+ T cells in ex vivo recall responses, and naïve CD4+ T cell differentiation into Th17 cells. In this scenario, the expression of CTLA-4 might have the opposite effect of the “classic” negative regulatory role CTLA-4 has on CD4+ T cell activity (47-49). Instead, CTLA-4, with its increased affinity for CD80, might act as a cross-linker for CD80 sending a positive signal to the autoreactive T cell during T cell-T cell interactions.
Considering the current in vitro data along with the aforementioned in vivo data, a putative mechanism is suggested to explain why the timing of anti-CD80 mAb treatment has differing effects on disease outcome in the R-EAE model where it has been reported that mice treated with anti-CD80 mAb during the onset of disease exhibited decreased disease severity (10) while mice treated during remission with an intact anti-CD80 mAb exhibited increased disease severity (44). In contrast, treatment with an anti-CD80 Fab fragment led to significantly decreased disease severity and relapse frequency when administered during disease remission. Both the in vitro and in vivo data support the hypothesis that the initial steps of activation of naïve CD4+ T cells are inhibited by anti-CD80 due to a lack of costimulation through CD28 interacting with CD80 expressed on APCs. For example, the addition of either an intact anti-CD80 mAb, anti-CD80 Fab, or CTLA4-Ig at the time of naïve CD4+ T cell activation in the presence of antigenic peptide and APCs blocks T cell activation (50, 51). These culture conditions represent the treatment regimen in which anti-CD80 mAb is administered to the mice at the time of priming with encephalitogenic CD4+ T cell epitopes. In contrast, the culture conditions employed in this report more closely mimic the in vivo treatment regimen wherein anti-CD80 treatment is initiated during ongoing disease. Therefore, these results support the conclusion that cross-linking CD80 on effector CD4+ T cells induces increased production of IFN-γ (and possibly IL-17) leading to enhanced tissue destruction and increased epitope spreading by increasing CD4+ T cell effector function. The present findings also strongly suggest that extreme caution must be taken when designing treatment regimens for autoimmune diseases such as MS based on antibody-mediated blockade of costimulatory molecules. Any treatment that has the potential to cross-link CD80 must be analyzed with the utmost care in that not only can blocking and/or cross-linking of CD80 on an APC affect cellular activity (12), but these same regimens may also directly upregulate autoreactive CD4+ T cell effector functions, thereby exacerbating disease.
Abbreviation used in this paper
- APC
antigen-presenting cell
- ChIP
chromatin immunoprecipitation
- mAb
monoclonal antibody
- R-EAE
relapsing experimental autoimmune encephalomyelitis
- TCR
T cell receptor
Footnotes
This work was supported in part by U.S. Public Health Service, National Institutes of Health Grants NS-034819 and NS-026543, and by support from the Myelin Repair Foundation. J.R.P. is supported by National Multiple Sclerosis Society Postdoctoral Fellowship Grant FG-1667A1/2.
References
- 1.Hathcock KS, Laszlo G, Dickler HB, Bradshaw J, Linsley P, Hodes RJ. Identification of an alternative CTLA-4 ligand costimulatory for T cell activation. Science. 1993;262:905–907. doi: 10.1126/science.7694361. [DOI] [PubMed] [Google Scholar]
- 2.Lenschow DJ, Su GH, Zuckerman LA, Nabavi N, Jellis CL, Gray GS, Miller J, Bluestone JA. Expression and functional significance of an additional ligand for CTLA-4. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11054–11058. doi: 10.1073/pnas.90.23.11054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damle NK, Klussman K, Linsley PS, Aruffo A. Differential costimulatory effects of adhesion molecules B7, ICAM-1, LFA-3, and VCAM-1 on resting and antigen-primed CD4 + T lymphocytes. J. Immunol. 1992;148:1985–1992. [PubMed] [Google Scholar]
- 4.Norton SD, Zuckerman L, Urdahl KB, Shefner R, Miller J, Jenkins MK. The CD28 ligand, B7, enhances IL-2 production by providing a costimulatory signal to T cells. J. Immunol. 1992;149:1556–1561. [PubMed] [Google Scholar]
- 5.Karandikar NJ, Vanderlugt CL, Walunas TL, Miller SD, Bluestone JA. CTLA-4: A negative regulator of autoimmune disease. J. Exp. Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrin PJ, Scott D, Davis TA, Gray GS, Doggett MJ, Abe R, June CH, Racke MK. Opposing effects of CTLA4-Ig and anti-CD80 (B7-1) plus anti-CD86 (B7-2) on experimental allergic encephalomyelitis. J. Neuroimmunol. 1996;65:31–39. doi: 10.1016/0165-5728(95)00172-7. [DOI] [PubMed] [Google Scholar]
- 7.Lenschow DJ, Herold KC, Rhee L, Patel B, Koons A, Qin HY, Fuchs E, Singh B, Thompson CB, Bluestone JA. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 8.Lenschow DJ, Zeng Y, Hathcock KS, Zuckerman LA, Freeman G, Thistlethwaite JR, Gray GS, Hodes RJ, Bluestone JA. Inhibition of transplant rejection following treatment with anti-B7-2 and anti-B7-1 antibody. Transplantation. 1995;60:1171–1178. doi: 10.1097/00007890-199511270-00019. [DOI] [PubMed] [Google Scholar]
- 9.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, Linsley PS, Bluestone JA. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–792. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 10.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH. B7-1 and B7-2 costimulatory molecules differentially activate the Th1/Th2 developmental pathways: Application to autoimmune disease therapy. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 11.Podojil JR, Kohm AP, Miller SD. CD4+ T cell expressed CD80 regulates central nervous system effector function and survival during experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:2948–2958. doi: 10.4049/jimmunol.177.5.2948. [DOI] [PubMed] [Google Scholar]
- 12.Suvas S, Singh V, Sahdev S, Vohra H, Agrewala JN. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J. Biol. Chem. 2002;277:7766–7775. doi: 10.1074/jbc.M105902200. [DOI] [PubMed] [Google Scholar]
- 13.Orabona C, Grohmann U, Belladonna ML, Fallarino F, Vacca C, Bianchi R, Bozza S, Volpi C, Salomon BT, Fioretti MC, Romani L, Puccetti P. CD28 induces immunostimulatory signals in dendritic cells via CD80 and CD86. Nat. Immunol. 2004;5:1134–1142. doi: 10.1038/ni1124. [DOI] [PubMed] [Google Scholar]
- 14.Nickoloff BJ, Nestle FO, Zheng XG, Turka LA. T lymphocytes in skin lesions of psoriasis and mycosis fungoides express B7-1: a ligand for CD28. Blood. 1994;83:2580–2586. [PubMed] [Google Scholar]
- 15.Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions. J. Immunol. 2004;172:34–39. doi: 10.4049/jimmunol.172.1.34. [DOI] [PubMed] [Google Scholar]
- 16.Shnyreva M, Weaver WM, Blanchette M, Taylor SL, Tompa M, Fitzpatrick DR, Wilson CB. Evolutionarily conserved sequence elements that positively regulate IFN-gamma expression in T cells. Proc. Natl. Acad. Sci. U. S. A. 2004;101:12622–12627. doi: 10.1073/pnas.0400849101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in T(H)1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 18.Patrone G, Puppo F, Cusano R, Scaranari M, Ceccherini I, Puliti A, Ravazzolo R. Nuclear run-on assay using biotin labeling, magnetic bead capture and analysis by fluorescence-based RT-PCR. BioTechniques. 2000;29:1012–+. doi: 10.2144/00295st02. [DOI] [PubMed] [Google Scholar]
- 19.Podojil JR, Kin NW, Sanders VM. CD86 and beta2-adrenergic receptor signaling pathways, respectively, increase Oct-2 and OCA-B expression and binding to the 3'-IgH enhancer in B cells. J. Biol. Chem. 2004;279:23394–23404. doi: 10.1074/jbc.M313096200. [DOI] [PubMed] [Google Scholar]
- 20.Avni O, Lee D, Macian F, Szabo SJ, Glimcher LH, Rao A. T(H) cell differentiation is accompanied by dynamic changes in histone acetylation of cytokine genes. Nat Immunol. 2002;3:643–651. doi: 10.1038/ni808. [DOI] [PubMed] [Google Scholar]
- 21.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J. Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 23.Linsley PS, Bradshaw J, Urnes M, Grosmaire L, Ledbetter JA. CD28 engagement by B7/BB-1 induces transient down-regulation of CD28 synthesis and prolonged unresponsiveness to CD28 signaling. J. Immunol. 1993;150:3161–3169. [PubMed] [Google Scholar]
- 24.Wyss-Coray T, Mauri-Hellwg D, Baumann K, Bettens F, Grunow R, Pichler WJ. The B-7 adhesion molecule is expressed on activated human T cells: functional involvement in T-T interactions. In Eur. J. Immunol. 1993:2175–2180. doi: 10.1002/eji.1830230919. [DOI] [PubMed] [Google Scholar]
- 25.Kohm AP, Podojil JR, Williams JS, McMahon JS, Miller SD. CD28 regulates glucocorticoid-induced TNF receptor family-related gene (GITR) expression on CD4+ T cells via IL-2 dependent mechanisms. Cell. Immunol. 2005;235:56–64. doi: 10.1016/j.cellimm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 27.Chan SH, Kobayashi M, Santoli D, Perussia B, Trinchieri G. Mechanisms of IFN-gamma induction by natural killer cell stimulatory factor (NKSF/IL-12). Role of transcription and mRNA stability in the synergistic interaction between NKSF and IL-2. J. Immunol. 1992;148:92–98. [PubMed] [Google Scholar]
- 28.Cho JY, Grigura V, Murphy TL, Murphy K. Identification of cooperative monomeric Brachyury sites conferring T-bet responsiveness to the proximal IFN-gamma promoter. Int. Immunol. 2003;15:1149–1160. doi: 10.1093/intimm/dxg113. [DOI] [PubMed] [Google Scholar]
- 29.Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 30.Liu KQ, Bunnell SC, Gurniak CB, Berg LJ. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J. Exp. Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 32.Doty RT, Clark EA. Subcellular localization of CD80 receptors is dependent on an intact cytoplasmic tail and is required for CD28-dependent T cell costimulation. J. Immunol. 1996;157:3270–3279. [PubMed] [Google Scholar]
- 33.Hirokawa M, Kuroki J, Kitabayashi A, Miura AB. Transmembrane signaling through CD80 (B7-1) induces growth arrest and cell spreading of human B lymphocytes accompanied by protein tyrosine phosphorylation. Immunol. Lett. 1996;50:95–98. doi: 10.1016/0165-2478(96)02526-6. [DOI] [PubMed] [Google Scholar]
- 34.Khan N, Ghousunnissa S, Jegadeeswaran SM, Thiagarajan D, Hasnain SE, Mukhopadhyay S. Anti-B7-1/B7-2 antibody elicits innate-effector responses in macrophages through NF-kappaB-dependent pathway. Int. Immunol. 2007;19:477–486. doi: 10.1093/intimm/dxm012. [DOI] [PubMed] [Google Scholar]
- 35.Jackman RP, Balamuth F, Bottomly K. CTLA-4 differentially regulates the immunological synapse in CD4 T cell subsets. J. Immunol. 2007;178:5543–5551. doi: 10.4049/jimmunol.178.9.5543. [DOI] [PubMed] [Google Scholar]
- 36.Balamuth F, Brogdon JL, Bottomly K. CD4 raft association and signaling regulate molecular clustering at the immunological synapse site. J. Immunol. 2004;172:5887–5892. doi: 10.4049/jimmunol.172.10.5887. [DOI] [PubMed] [Google Scholar]
- 37.Sloan-Lancaster J, Evavold BD, Allen PM. Induction of T-cell anergy by altered T-cell-receptor ligand on live antigen-presenting cells. Nature. 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 38.Gajewski TF, Schell SR, Fitch FW. Evidence implicating utilization of different T cell receptor-associated signaling pathways by TH1 and TH2 clones. J. Immunol. 1990;144:4110–4120. [PubMed] [Google Scholar]
- 39.Fanger CM, Neben AL, Cahalan MD. Differential Ca2+ influx, KCa channel activity, and Ca2+ clearance distinguish Th1 and Th2 lymphocytes. J. Immunol. 2000;164:1153–1160. doi: 10.4049/jimmunol.164.3.1153. [DOI] [PubMed] [Google Scholar]
- 40.Linsley PS, Bradshaw J, Green J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996;4:535–543. doi: 10.1016/s1074-7613(00)80480-x. [DOI] [PubMed] [Google Scholar]
- 41.Vendetti S, Riccomi A, Sacchi A, Gatta L, Pioli C, De Magistris MT. Cyclic adenosine 5'-monophosphate and calcium induce CD152 (CTLA-4) up-regulation in resting CD4+ T lymphocytes. J. Immunol. 2002;169:6231–6235. doi: 10.4049/jimmunol.169.11.6231. [DOI] [PubMed] [Google Scholar]
- 42.Stephan MT, Ponomarev V, Brentjens RJ, Chang AH, Dobrenkov KV, Heller G, Sadelain M. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat. Med. 2007;13:1440–1449. doi: 10.1038/nm1676. [DOI] [PubMed] [Google Scholar]
- 43.Karandikar NJ, Vanderlugt CL, Eagar TN, Tan L, Bluestone JA, Miller SD. Tissue-specific up-regulation of B7-1 expression and function during the course of murine relapsing experimental autoimmune encephalomyelitis. J. Immunol. 1998;161:192–199. [PubMed] [Google Scholar]
- 44.Miller SD, Vanderlugt CL, Lenschow DJ, Pope JG, Karandikar NJ, Dal Canto MC, Bluestone JA. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 45.Windhagen A, Newcombe J, Dangond F, Strand C, Woodroofe MN, Cuzner ML, Hafler DA. Expression of costimulatory molecules B7-1 (CD80), B7-2 (CD80), and interleukin 12 cytokine in multiple sclerosis lesions. J. Exp. Med. 1995;182:1985–1996. doi: 10.1084/jem.182.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kantarci OH, Hebrink DD, Achenbach SJ, Atkinson EJ, Waliszewska A, Buckle G, McMurray CT, de Andrade M, Hafler DA, Weinshenker BG. CTLA4 is associated with susceptibility to multiple sclerosis. J. Neuroimmunol. 2003;134:133–141. doi: 10.1016/s0165-5728(02)00395-8. [DOI] [PubMed] [Google Scholar]
- 47.Gribben JG, Freeman GJ, Boussiotis VA, Rennert P, Jellis CL, Greenfield E, Barber M, Resvito VA, Ke X, Gray GS, Nadler LM. CTLA4 mediates antigen-specific apoptosis of human T cells. Proc. Natl. Acad. Sci. U.S.A. 1995;92:811–815. doi: 10.1073/pnas.92.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boise LH, Noel PJ, Thompson CB. CD28 and apoptosis. Curr. Opin. Immunol. 1995;7:620–625. doi: 10.1016/0952-7915(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 49.Fallarino F, Fields PE, Gajewski TF. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J. Exp. Med. 1998;188:205–210. doi: 10.1084/jem.188.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Azuma H, Chandraker A, Nadeau K, Hancock WW, Carpenter CB, Tilney NL, Sayegh MH. Blockade of T-cell costimulation prevents development of experimental chronic renal allograft rejection. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12439–12444. doi: 10.1073/pnas.93.22.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McKnight AJ, Perez VL, Shea CM, Gray GS, Abbas AK. Costimulator dependence of lymphokine secretion by naive and activated CD4+ T lymphocytes from TCR transgenic mice. J. Immunol. 1996;152:5220–5225. [PubMed] [Google Scholar]