Abstract
Skin plays an essential role in adaptation to environmental stimuli that is mediated in part by its remarkable vascular plasticity. Certain vertebrates, such as amphibians, respond to hypoxia in part through the skin; but it is unknown whether this tissue can influence mammalian systemic adaptation to low oxygen levels. We have found that epidermal deletion of the hypoxia responsive transcription factor HIF-1α inhibits renal erythropoietin (EPO) synthesis in response to hypoxia. Conversely, mice with an epidermal deletion of the von Hippel Lindau (VHL) factor, a negative regulator of HIF, have increased EPO synthesis and polycythemia. We show that nitric oxide release induced by the HIF pathway acts on cutaneous vascular flow to increase systemic erythropoietin expression. These results demonstrate that in mice the skin is a critical mediator of systemic responses to environmental oxygen.
Introduction
Mammalian skin acts as an essential buffer against the environment (Tobin, 2006). In this role, the skin can act to protect internal tissues as a barrier, e.g., by conserving water and guarding against pathogens. It can also respond to environmental stresses. These latter changes are accomplished in part by regulated alterations in blood flow through the cutaneous circulation.
Cutaneous vascular flow in mammals controls a wide range of physiological parameters through an intricate system of vascular plexi (Tobin, 2006). Body heat is tightly linked to both the external environment and internal metabolic processes and the relationship of skin and surface area to metabolism is one of the oldest concerns of biology. For example, some of the first formulas relating oxygen use, skin/surface area, metabolism and heat were proposed in the mid-1800’s, in the pioneering work of Bergmann and Rubner (Bergmann, 1847; Rubner, 1883).
Amongst vertebrates, systemic metabolism is closely tied to dermal physiology, particularly in amphibians where the skin has a clear and important respiratory function. In mammals this relationship has been relatively unexplored. However, one recent study has argued that human epidermis does not obtain its oxygen from the dermal circulation, but rather utilizes oxygen directly from the atmosphere (Stucker et al., 2002). Thus, skin may be unique as a tissue in not being directly reliant on cardiopulmonary delivery of oxygen for its survival. Interestingly, when the air overlying the epidermis becomes hypoxic, keratinocytes are able to induce vasodilation in the underlying dermal vasculature, in a nitric oxide-dependent fashion (Minson, 2003). This vasodilation is independent of changes in respiration or temperature, and may allow oxygen delivery to the keratinocytes under circumstances where insufficient oxygen is present in the atmosphere.
A primary mammalian response to hypoxia is the increased synthesis of the hormone erythropoietin (EPO): an erythropoietic agent that is chiefly produced by the kidney and liver (Fandrey, 2004). Several non-erythropoietic roles for EPO have been demonstrated (Lewis, 2004), and it has also been shown that a number of tissues and cells outside of the hematopoietic system express the EPO receptor (EPO-R)(Lewis, 2004). These include neurons as well as vascular endothelial cells and cardiac myocytes, all of which are susceptible to damage during hypoxic insult (Gassmann et al., 2003).
Recent work has shown that EPO and its receptors are key factors in ventilatory adaptations to hypoxia (Soliz et al., 2007). Thus the induction of EPO synthesis may be one of the most primary responses of the body to lowered oxygen levels, influencing or even coordinating a cascade of systemic responses to hypoxia that extend beyond erythropoiesis.
Differential vascular flow in the kidney can influence EPO expression, which is coupled to changes in renal blood gas levels acting on oxygen sensing cells near the proximal tubules of the renal nephron (Fandrey, 2004). The molecular mechanisms for this involve hypoxia inducible transcription factors (HIFs), which are primary modulators of the transcriptional hypoxic response (Semenza, 2004) and are negatively regulated by the von Hippel Lindau (VHL) factor (Ivan et al., 2001; Maxwell et al., 2001). The VHL gene acts to ubiquitinate the HIF-α transcription factors (HIF-1α and HIF-2α), and induce their turnover in normoxia due to recognition of a prolyl hydroxylation motif, wherein oxygen-dependent hydroxylases modify the HIF-α proteins.
Loss of VHL prevents the ubiquitination of hydroxylated HIF-α proteins, and causes increases in HIF-mediated transcription due to accumulation of the transcription factors (Ivan et al., 2001; Maxwell et al., 2001; Maxwell et al., 1999). Increased HIF-α levels in the skin can contribute to vascular expansion, and thus alteration of cutaneous vascular flow (Elson et al., 2001; Kim et al., 2006b). We wished to determine whether this altered HIF-α function can influence physiological homeostasis, and, more generally, whether the skin plays a role in hypoxic adaptive responses in mammals.
We provide evidence that the skin is a primary coordinator of the systemic hypoxic response, and acts to modulate cutaneous blood flow to potentiate renal and hepatic EPO synthesis. This occurs in a clearly HIF-dependent manner. These findings indicate a previously unappreciated and fundamental role for mammalian skin in responding to environmental oxygenation.
Results
HIF-1α is extensively expressed in the normal epidermis
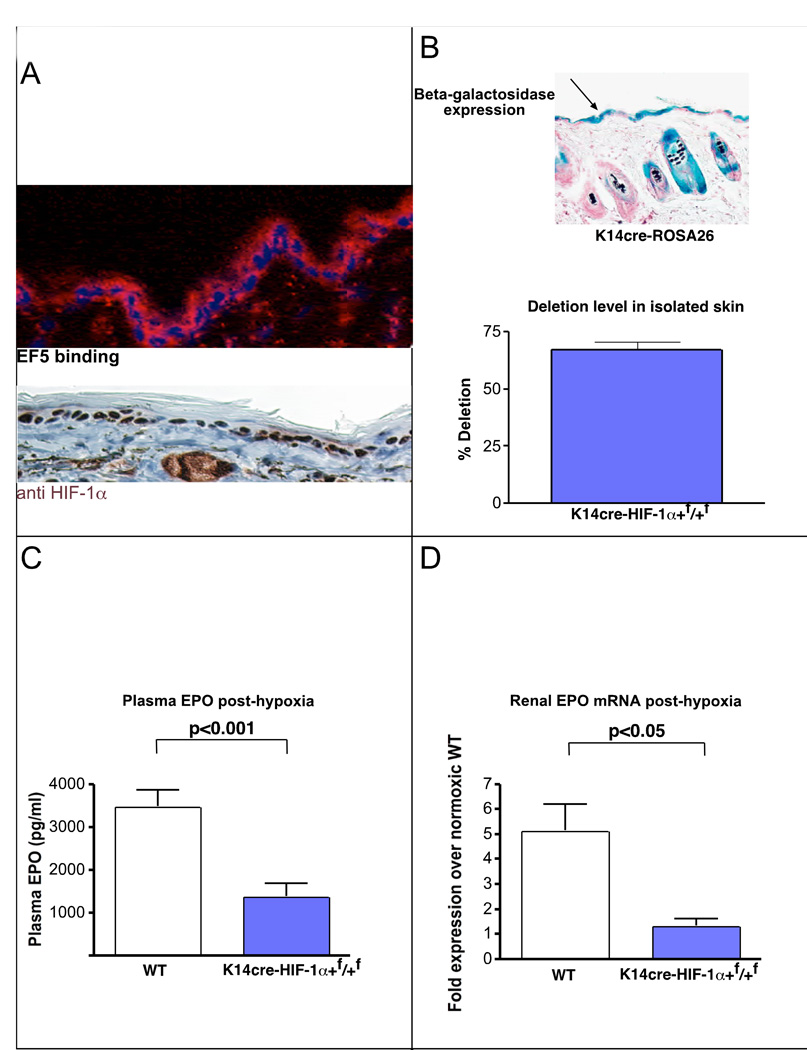
It has been previously shown that normal rodent and human skin has many characteristics of a constitutively hypoxic tissue, including the binding of hypoxia-detection agents, e.g., the nitroimidazole EF5 (Evans et al., 2006). Mouse epidermis demonstrates extensive binding of this hypoxia-sensitive compound, particularly in basal keratinocytes (Figure 1A). Consistent with a constitutive low level of tissue oxygenation are high levels of expression of the hypoxia-inducible transcription factor HIF-1α in the nuclei of keratinocytes (Figure 1A, Supplemental Figure 3).
Figure 1. Loss of HIF-1α in the epidermis diminishes renal EPO production during hypoxia.
A, The normal mouse epidermis is hypoxic, based on EF5 binding (red), and expresses HIF-1α protein (red). B, K14 cre recombinase transgene expression was verified by β-galactosidase expression in epidermis in the ROSA25 reporter strain; deletion efficiency of the loxP-flanked allele in the epidermis and hair follicle was 70%. C, EPO protein in the plasma following hypoxic exposure for 14 hours at 9% oxygen is significantly reduced in mice lacking HIF-1α in the epidermis (wt n = 25, K14cre-HIF-1α+f/+f n = 11). D, Renal EPO mRNA expression is reduced to normoxic levels in hypoxic K14cre-HIF-1α+f/+f mice (wt n = 6, K14cre-HIF-1α+f/+f n = 3). All graphs represent mean ± SEM.
Loss of HIF-1α in the epidermis
To determine the role of HIF-1α expression in the epidermis it was deleted tissue-specifically by crossing the HIF-1α+f/+f allele with a strain expressing cre recombinase driven by the keratin-14 (K14) promoter (Jonkers et al., 2001). This transgene limits expression to basal keratinocytes and a small number of other epithelial lineages (Jonkers et al., 2001). We verified tissue-specificity of cre recombinase expression by a cross of this transgene into the ROSA26 cre reporter strain (Soriano, 1999), which gave rise to cre-induced beta-galactosidase expression in epidermis (Figure 1B). No beta-galactosidase expression was seen in brain, liver, kidney, lung, or other visceral organs (data not shown), indicating the K14cre transgene is not active in those tissues. Deletion analysis of genomic DNA by real time-PCR also indicated extensive deletion in the epidermis of K14cre-HIF-1α+f/+f mice, and no deletion was detectable in liver or kidney (Figure 1B and data not shown).
Loss of HIF-1α in keratinocytes prevents a systemic hypoxic response
K14cre-HIF-1α+f/+f mutant animals develop normally (data not shown). Given the central role for EPO in the physiological response to hypoxia, including its modulation of adaptive mechanisms such as erythropoiesis and ventilation (Soliz et al., 2007), we focused on it as a key readout of systemic responses to hypoxia.
Basal expression of EPO was unchanged in K14cre-HIF-1α+f/+f mutant animals, and their hematocrits were also normal (data not shown). To test hypoxic response, we placed K14cre-HIF-1α+f/+f mice in chambers in which they were subjected to normobaric hypoxia (9% O2) for 14 hours. This level of hypoxia induces an approximately 30-fold increase in plasma levels of EPO in wild type mice, an increase from basal levels of approximately 100pg/ml plasma to a mean of approximately 3500 pg/ml (Figure 1C). We found that blood EPO concentration in the mutants following this hypoxic challenge were only 30% of those found in wild-type animals (Figure 1C). This was correlated with a loss of hypoxically-induced EPO expression in the kidney: renal EPO mRNA expression was not significantly elevated in mutants (Figure 1D).
Analysis of vascular flow in the mutants indicated a non-significant trend towards increased flow towards the kidney and liver, and away from the skin in these mutants (data not shown).
This demonstrates that a HIF-1α-dependent response in the skin is an important element for triggering renal synthesis of EPO. We carried out similar experiments on K14cre-HIF-2αf/+f mutants; however, we saw no difference in hypoxia-induced plasma EPO levels in these animals compared to wild type littermates (data not shown). This indicates that epidermal induction of EPO is a HIF-1α-mediated response, differentiating it from the role played by HIF-2α in the direct regulation of EPO in the kidney (Gruber et al., 2007).
Deletion of VHL in the epidermis
To further study the mechanisms underlying HIF response in the epidermis, a model for constitutively increased HIF expression was employed. This utilized a tissue-specific deletion of a negative regulator of HIF-α function, the von Hippel-Lindau gene (VHL) (Maxwell et al., 1999). This deletion results in up-regulation of both HIF-1α and HIF-2α in keratinocytes (Haase, 2005; Kim et al., 2006a).
Loss of VHL in the epidermis causes an approximate 20% increase in vascularization of the skin (Kim et al., 2006b). As shown in Figure 2A, it also causes increases in HIF target gene expression.
Figure 2. Deletion of VHL in the epidermis induces EPO production during normoxia.
A, Upon deletion of VHL in the epidermis, HIF-1α protein (red) is stabilized and HIF target gene expression is increased in the skin (for each graph wt n = 3, K14cre-VHL+f/+f n = 3, for RNA isolation). B, Constitutive or Tamoxifen-induced epidermal deletion of VHL results in highly elevated plasma EPO (wt n = 36, K14cre-VHL+f/+f n = 23, K14TAMcre-VHL+f/+f n = 5). C, In the K14cre-VHL+f/+f mouse, EPO mRNA expression is suppressed in the kidney but increased in the liver. In the tamoxifen inducible K14TAMcre-VHL+f/+f, where deletion occurs in the adult, EPO expression is increased in the kidney, and unaffected in the liver (wt n = 9, K14cre-VHL+f/+f n = 6, K14TAMcre-VHL+f/+f n = 5). D, As blood hematocrit levels increase in the constitutively deleted K14cre-VHL+f/+f mice (wt n = 43, K14cre-VHL+f/+f 1.5 wks n = 3, K14cre-VHL+f/+f 10 wks n = 32), renal EPO mRNA expression is suppressed and hepatic EPO increases, indicating that hematocrit can selectively affect renal EPO expression (wt n = 9, K14cre-VHL+f/+f 1.5 wks n = 3, K14cre-VHL+f/+f 10 wks n = 6). All graphs represent mean ± SEM.
Constitutive or induced loss of VHL in the epidermis dramatically increases blood EPO levels
Adult K14cre-VHL+f/+f mutant animals have high levels of plasma EPO (Figure 2B). This is accompanied by an increase in reticulocytes (Supplemental Figure 1A) and a significant increase in hematocrit, to an approximate level of 0.70 (Figure 2D). Skin barrier function is normal, and there is no evidence of dehydration (data not shown), indicating that the high hematocrit is due to a high level of erythropoiesis. Blood volume is significantly higher than that found in wild type animals, and is consistent with a non-leaky vasculature and an expansion of blood volume due to an increase in erythrocyte mass (supplemental Figure 1A).
Induced deletion of VHL also induces EPO expression
Since the K14cre transgene is expressed as early as embryonic day 14.5 (Vasioukhin et al., 1999), it was important to ascertain whether this dramatic increase in EPO production was related to the role for HIF described above, and thus represented a physiological stimulation of the EPO pathway via the skin, or whether it is due to some developmental alteration in EPO regulation.
To separate physiological from developmental effects, we crossed the VHL+f/+f allele into a conditional K14cre-transgenic background (Vasioukhin et al., 1999). This conditional transgenic strain allows tamoxifen treatment to be used to induce keratinocyte-specific deletion of a loxP-flanked allele in adults (Vasioukhin et al., 1999). As seen in Figure 2B, 6 weeks after tamoxifen-induced cre recombinase activation in 4 week old mice, plasma EPO levels have risen significantly compared to wild type control littermates treated with tamoxifen. This demonstrates that loss of VHL in the skin induces systemic EPO elevation even when the epidermis has developed normally.
Extensive analysis of protein and mRNA found no evidence that either skin or isolated keratinocytes are capable of expressing EPO under normoxia or hypoxia, or when VHL deletion occurs (data not shown). All EPO expression is thus generated in these mutant mice through physiological signaling.
Hepatic EPO expression in constitutive VHL mutants is correlated with high hematocrit
We found that in the constitutively deleted, K14cre-VHL+f/+f mutant animals, the liver has the highest levels of increased EPO expression (Figure 2C). Generally, the kidney, rather than the liver, is the predominant site of basal and induced EPO synthesis. To determine whether this shift in site of EPO expression was specific to epidermal signaling, tissue EPO expression levels in K14TAMcre-VHL+f/+f mice were analyzed 6 weeks after induced deletion. As can be seen in Figure 2C, mean expression levels are increased in the kidney, and not the liver, of these mice.
Next we analyzed EPO levels, synthesis, and hematocrit in wild type and K14cre-VHL+f/+f 10 day old pups and in mature animals at 10 weeks (Figure 2D). In the pups, hematocrits are not different, although K14cre-VHL+f/+f mutant plasma EPO levels are significantly elevated. Both hepatic and renal EPO mRNA synthesis are slightly elevated in 10 day old pups. However, at 10 weeks of age, renal EPO mRNA expression has been suppressed compared to wild type; and only hepatic EPO mRNA levels are still high. This is correlated with a high hematocrit in the adult K14cre-VHL+f/+f mutant. Suppression of renal EPO mRNA synthesis by a high hematocrit has been documented by others in studies of hypobaric hypoxia (Bozzini et al., 2005; Lezon et al., 1995); since young K14cre-VHL+f/+f mice and induced deletion K14TAMcre-VHL+f/+f mice both have elevated renal EPO mRNA expression, the epidermal VHL mutation can affect both the kidney and the liver physiologically.
Double and triple deletions of HIF-α’s and VHL in skin demonstrate a predominant role for HIF-2α
To address the mechanisms responsible for EPO response induced by VHL deletion in the skin, a genetic analysis was carried out to determine whether the effects from VHL deletion are directly HIF-related, since the VHL gene product has been proposed to regulate non-HIF targets (Russell and Ohh, 2007). Double and triple deletions were carried out, by crossing HIF-1α and HIF-2α conditional alleles alone, and in combination, into the background of the VHL conditional and K14cre alleles (Figures 3A). Deletion of HIF-1α lowers mean serum EPO levels, but deletion of HIF-2α causes a dramatic reduction, restoring them to wild type levels. Interestingly, deletion of both HIF-1α and HIF-2α causes a decrease in EPO to levels significantly below those seen in wild type mice (Figure 3A).
Figure 3. K14cre-VHL+f/+f demonstrates altered blood flow, increased internal hypoxia, and increased EPO expression. Restoration to wild type levels by co-deletion of HIF-2α.
A, Deletion of HIF-2α but not HIF-1α in the K14cre-VHL+f/+f background restores plasma EPO (wt n = 36, K14cre-VHL+f/+f n = 23, K14cre-VHL+f/+fHIF-1α+f/+f n = 15, K14cre-VHL+f/+f HIF-2α+f/+f n = 3, K14cre-VHL+f/+f HIF-1α+f/+f f HIF-2α+f/+f n = 3) and blood hematocrit (B) to wild type levels (wt n = 43, K14cre-VHL+f/+f n = 32, K14cre-VHL+f/+f HIF-1α+f/+f n = 17, K14cre-VHL+f/+f HIF-2α+f/+f n = 3, K14cre-VHL+f/+f HIF-1α+f/+f f HIF-2α+f/+f n = 3). Deletion of both HIF-1α and HIF-2α is similar in effect to deletion of HIF-2α alone. C, Blood oxygen saturation is normal in K14cre-VHL+f/+f animals (wt n = 7, K14cre-VHL+f/+f n = 6), but animals are hypotensive (wt n = 17, K14cre-VHL+f/+f n = 10). D, Blood flow in the K14cre-VHL+f/+f is shifted away from the liver and kidney, and toward the skin, as measured by microsphere distribution (wt n = 11, K14cre-VHL+f/+f n = 4). E, The shift in blood flow corresponds to increased EF5 binding/hypoxia in the kidney and liver of K14cre-VHL+f/+f (wt n = 8, K14cre-VHL+f/+f n = 4). F, Nitric oxide metabolites are increased in K14cre-VHL+f/+f plasma, demonstrating increased NO production (wt n = 33, K14cre-VHL+f/+f n = 8). All graphs represent mean ± SEM.
In Figure 3B it can be seen that loss of HIF-2α, but not HIF-1α, restores hematocrit in K14cre-VHL+f/+f mice to wild type levels. This indicates that the drop in EPO seen in Figure 3A, following loss of HIF-1α, was not sufficient to affect erythropoiesis. These data together indicate that VHL deletion is acting to effect changes in EPO expression through HIF-2α. As discussed above, the epidermal deletion of HIF-1α (and not HIF-2α) alone affects systemic hypoxic response; but as shown here VHL deletion causes HIF-2α to act as the primary transcription factor in the same response. Thus this is evidence that gene regulation via the HIF pathway can differentially employ HIF-1α or HIF-2α, dependent on VHL status. This coincides with experimental observations in other settings of VHL loss of function that indicate that loss of VHL preferentially increases HIF-2α activity (Carroll and Ashcroft, 2006; Kim et al., 2006b; Kondo et al., 2002; Rankin et al., 2007; Raval et al., 2005; Scortegagna et al., 2005).
NO levels and blood flow shifts correlate with increasing EPO levels
To address physiological mechanisms underlying this HIF-mediated effect on systemic erythropoiesis, we assayed blood oxygen and blood pressure levels in K14cre-VHL+f/+f mutants (Figure 3C). Here we saw no significant effects on overall blood oxygenation, but a highly significant decrease in blood pressure in K14cre-VHL+f/+f mutants (Figure 3C). Since blood flow through the renal and hepatic circulatory beds is a key determinant of organ oxygenation and hypoxic response, we wished to determine whether differential changes in blood flow could be occurring in K14cre-VHL+f/+f mutant mice. To assay this, we injected fluorescent microspheres into the left atria of experimental mice; these microspheres lodge in capillaries and their distribution relative to tissue mass within an animal gives a ratio of differential blood flow to differing tissues. As can be seen in Figure 3D, there is a significant shift in flow towards the skin, and away from the liver and kidney, in K14cre-VHL+f/+f mutant mice.
To assay whether this shift in blood flow was accompanied by a change in tissue oxygenation, we injected animals with the nitroimidazole EF5 to determine whether there was an increase in the binding of this hypoxia marker in K14cre-VHL+f/+f mutant kidney and liver, and thus a change in tissue oxygenation (Figure 3E). Interestingly, while there was a trend to increased hypoxia in the kidney, it was only significantly increased in the liver of K14cre-VHL+f/+f mutant mice (Figure 3E). This agrees with data (Figure 2C) demonstrating that the liver has a 25-fold increased EPO expression in the K14cre-VHL+f/+f mutant animals.
Role of NO in mediating cutaneous induction of EPO synthesis
Nitric oxide (NO) is a critical mediator of cutaneous vasodilation in response to local heat, injury, and hypoxia (Harbrecht, 2006; Houghton et al., 2006), and can induce vascular hypotension. One key target gene of the HIF transcriptional response in many tissues is the inducible nitric oxide synthase gene (iNOS) (Figure 2A). As can be seen in figure 3f, K14cre-VHL+f/+f mice have a highly significant increase in plasma NO, indicating a large increase in NO synthesis has occurred in mutants relative to wild type animals.
We next wished to determine the role played by increased NO levels in the increased synthesis of EPO, and in particular establish whether an NO-induced shift in dermal circulation could act to reduce blood flow to tissues such as the liver and kidney. We first established whether alterations in systemic, as opposed to tissue-specific NO, would increase EPO synthesis. This is important in part because significant evidence indicates that NO is capable of inducing HIF activation/stabilization (Hagen et al., 2003; Mateo et al., 2003; Metzen et al., 2003). It has been shown in rats that broad pharmacological inhibition of NO synthesis causes systemic increases in EPO production (Todorov et al., 2000). We found the same to be true of mice (Figure 4A): after 4 days of treatment with the NO synthesis inhibitor L-NAME, plasma EPO levels rise approximately 20% in wild type mice. Thus systemic inhibition of NO synthesis acts to raise EPO production. A simple explanation for this observation would be a role for NO-mediated vasorelaxation in directly increasing blood flow to the kidney and liver; absence of NO thus decreasing flow and increasing EPO response.
Figure 4. Nitric Oxide production in the skin mediates shift in blood flow and renal EPO expression in K14cre-VHL+f/+f mice.
A, Systemic inhibition of NO synthesis by L-NAME increases plasma EPO in wild type mice (wt n = 36, wt + L-NAME n = 12). Global iNOS knockout mice show significantly elevated EPO plasma levels following hypoxia relative to wild type mice (wt n = 25, iNOS−/− n = 7). B, NO synthesis inhibition by treatment with L-NAME restores plasma EPO levels to levels similar to L-NAME-treated wild type mice, when administered to K14cre-VHL+f/+f mice (K14cre-VHL+f/+f n = 23, K14cre-VHL+f/+f + L-NAME 3 days n = 3, K14cre-VHL+f/+f + L-NAME 4 days n = 4). C, NO donor (nitroglycerine) applied to the skin of wild type mice increases plasma EPO levels; similar systemic doses of NO donor do not significantly affect plasma EPO levels (control n = 20, Systemic nitroglycerine n = 3, Epidermal nitroglycerine n = 9). D, Epidermal NO donor administration induces renal EPO mRNA expression (control n = 9, Epidermal nitroglycerine n = 3). E, Epidermal nitroglycerin shifts blood flow (control n = 11, Epidermal nitroglycerine n = 6) towards the skin and increases renal and hepatic hypoxia (F), in a manner similar to that seen in K14cre-VHL+f/+f mice (control n = 8, Epidermal nitroglycerine n = 5). All graphs represent mean ± SEM.
Inhibition of NO synthesis specifically blocks dermal induction of EPO synthesis
To determine more specifically the role of the HIF target gene iNOS in EPO regulation, we assayed the hypoxic response of mice with a global deletion of the NOS2 gene encoding iNOS (Laubach et al., 1995). These mice showed no differences in basal levels of plasma EPO or in hematocrit relative to wild type mice (data not shown); but they did show a highly significant increase in EPO response following 14 hours of hypoxia (Figure 4A). This demonstrates again that reduced systemic NO can increase EPO signaling. The global nature of the iNOS knockout does prevent drawing conclusions about tissue-specific effects of NO production, however.
To assay for the role of NO production via epidermal deletion of VHL, we treated the K14cre-VHL+f/+f mice with the NO synthase inhibitor L-NAME, and found that 4 days of treatment significantly lowered EPO levels in mutant mice, reducing them to levels seen in wild type mice treated with this compound (Figure 4B). This is the opposite of the effect of L-NAME seen in either the wild type mice described above, or the iNOS −/− mice; and indicates that NO signaling is essential in the special case of HIF-mediated up-regulation of EPO in the skin. This in turn argues for a direct link between VHL deletion in the skin, NO synthesis, and changes in physiology leading to EPO expression.
Dermal, but not systemic, administration of NO donor increases EPO levels
The observation above suggested an intriguing new method to induce EPO expression. We treated C57Bl6 mice with either a systemic nitric oxide donor (nitroglycerine) via oral gavage of a slow release formulation; or used a similar dosing of nitroglycerine through a patch on the skin. As is shown in figure 4C, systemic administration of the NO donor does not result in an increase in EPO after 7 hours of treatment; however, epidermal administration causes an almost 7-fold rise in plasma EPO levels. Plasma NO metabolite levels at 7 hours are similar following both treatments (Supplemental Figure 2A), however, only epidermal administration causes a significant rise in renal EPO synthesis (Figure 4D). It was subsequently found that other stimuli of epidermal NO release, such as mustard oil, also induce significant renal EPO synthesis when administered cutaneously, and that concurrent L-NAME treatment partially blocks this induction (Supplemental Figure 1B).
As can be seen in Figures 4E and 4F, epidermal administration of nitroglycerin shifts blood flow away from the splanchnic sites of EPO production, and significantly induces hypoxia as visualized by increased EF5 binding in those organs after treatment.
Short-term hypoxic response reveals a differential role for the skin in EPO response to hypoxia
The data above demonstrate an important role for HIF response in the skin in the regulation of EPO synthesis. However, a great deal of adaptation to hypoxia occurs immediately upon exposure to low oxygen, through changes in heart and respiration rates and through pulmonary vasoconstriction (Powell et al., 1998). As part of this physiological response, EPO synthesis begins almost immediately (Abbrecht and Littell, 1972).
In acute hypoxia, blood is distributed toward the brain and liver, and is shunted away from the skin (Kuwahira et al., 1993). Acute hypoxia also induces an immediate, ion-channel mediated vasoconstriction in the lung (Moudgil et al., 2005, 2006); interestingly, this same phenomenon has been shown to occur within seconds in the skin of amphibians (Malvin and Walker, 2001).
We wished to determine whether acute responses to hypoxia are influenced by changes in skin oxygenation. To test this, we placed C57Bl6 wild type mice in chambers that enclosed their heads in one compartment, and their bodies in a separately ventilated compartment (Figure 5A, Supplemental Figure 1C). We then determined the level of EPO response following respiration and skin exposure to different levels of normobaric oxygen for 5 hours. As can be seen in Figure 5B, although there is no effect from changing skin exposure when mice are breathing normally oxygenated air, as expected, a large increase in EPO levels is seen when mice respire in a hypoxic environment.
Figure 5. Skin hypoxia directly affects overall systemic hypoxic response in wild type mice exposed to acute hypoxia.
A, Special chambers were constructed to isolate inhaled oxygen concentration from the oxygen concentration exposed to the skin. B, In mice breathing 21% O2, skin hypoxia was not enough to induce increased EPO synthesis. However, in mice breathing 10% O2, plasma EPO levels were significantly elevated when skin was normoxic (Respired 21% O2 Skin 21% O2 n = 2, Respired 21% O2 Skin 10% O2 n = 3, Respired 10% O2 Skin 10% O2 n = 8, Respired 10% O2 Skin 21% O2 n = 9); mice exposed to differential gases for 5 hours. C, Renal EPO expression was higher in mice breathing 10% O2 while exposed to 21% O2 (Respired 10% O2 Skin 10% O2 n = 6, Respired 10% O2 Skin 21% O2 n = 7); mice exposed to differential gases for 5 hours. D, Blood flow is shifted from the skin to the kidney and liver when the skin is hypoxic for 1 hour. This shift is absent when the skin is exposed to normoxia (Respired 10% O2 Skin 10% O2 n = 7, Respired 10% O2 Skin 21% O2 n = 8). All graphs represent mean ± SEM.
Surprisingly, when mice have their bodies exposed to normoxia while they breath hypoxic air, the hypoxic EPO response is more than doubled relative to animals that are both breathing and have body exposure to hypoxia. It is clear that this exposure affects renal EPO synthesis, since there is a correlated doubling of EPO mRNA in the kidney in these mice (Figure 5C).
We wished to determine whether these changes were also correlated with a shift in vascular flow from the skin towards splanchnic organs. Repeating the experiment above, with a shorter (1 hour) time of exposure, it was found that in this acute response, normoxia surrounding the body while breathing hypoxic air, caused a shift in blood flow towards the skin, and significantly reduced relative flow to the liver (Figure 5D). The mean shift was an almost 10-fold change relative to mice both breathing and surrounded by a hypoxic environment. This finding demonstrates that there is an acute hypoxic response in the skin that modulates vascular flow, and regulates systemic hypoxic response. Interestingly, there is evidence that this acute response may be mediated by the same oxygen-sensitive potassium channels that control pulmonary vasoconstriction. We found that Kv1.5 potassium channels, which are essential for hypoxia-induced pulmonary vasoconstriction (Moudgil et al., 2006), are also present in cutaneous blood vessels in the skin (Supplemental Figure 1D).
To determine the relationship of these findings to HIF-1α function, we carried out the experiments described above on K14cre-HIF-1α+f/+f mutant animals. This revealed that HIF-1α also plays a key role in the regulation of the immediate adaptation to hypoxia. As shown in Supplemental Figure 2C, K14cre-HIF-1α+f/+f mutant animals breathing hypoxia have a higher level of plasma EPO than wild type littermates at this early time point. Plasma EPO levels in the mutants do not change significantly with skin exposure to normoxia (Supplemental Figure 2C). Thus the adaptation to skin oxygenation seen in wild type animals in Figure 5B is eliminated when HIF-1α is absent from the epidermis. This indicates that HIF response in the skin in hypoxic adaptation is at least bipartite, with an early role in modulating EPO response (as shown in Supplemental Figure 2C), and a later role in its maintenance (as shown in Figure 1C). This is a further demonstration of a central role for the epidermal HIF response in regulating adaptation to environmental oxygen levels.
Discussion
Although the skin is essential for adaptation to environmental oxygenation in some other vertebrates, notably amphibians, no such role has been proposed before for mammals. Clearly, the physiologic function of mammalian skin differs in some respects from that of other vertebrates. It is also certain that an organisms’ surface to volume ratio, its dermal vasculature, as well as its metabolic rate, will influence how vascular conductance affects the hypoxic response that we demonstrate here. All of these will need to be taken into account in considering the relevance of these observations to other mammals. In addition, it should be noted that the differential oxygenation experiments were necessarily carried out under anesthesia, and thus some level of anesthesia-related change in vascular responsiveness could play a role in what we observed; further experimentation along these lines will be needed to establish how anesthetic agents might affect these results.
We show here that acute hypoxia reduces blood flow to the skin and that this in turn correlates with decreased renal EPO production; a longer term hypoxic challenge requires a HIF response in the skin to potentiate EPO production. These apparently disparate results both demonstrate an essential role for the skin in hypoxic EPO synthesis. Although this leads to the possibility of numerous complex models of response, a simple model to explain this disparity would be a dual role for the dermal hypoxic response in modulating circulation and EPO production (shown in Figure 6). Initially, hypoxic vasoconstriction acts in a rapid manner in the skin to increase flow to the kidney, and modulate effects of decreases in blood pO2. Regulation of potassium channel function and other HIF-dependent mechanisms of vasoconstriction (such as regulation of the HIF target gene endothelin-1) act to control vascular flow at this stage (Whitman et al., 2007). Transcriptional response through HIF in a more chronic hypoxic state would subsequently act to this acute dermal vasoconstriction, and over a period of hours gradually lessen blood flow to the kidney, acting to increase EPO production. This model argues for a dynamic role for both HIF and for skin response to hypoxia in regulating internal blood flow during hypoxic response.
Figure 6. Acute and chronic adaptation to hypoxia is influenced by dermal response.
Hypoxia may act through mechanisms similar to those found in the lung to induce pulmonary vasoconstrictions under acute hypoxic stress; a more chronic stress allows a HIF-induced modulation of response that also affects blood flow and EPO expression.
Hypoxia-induced pulmonary vasoconstriction (HPV) is essential to maintain blood flow to aerated sections of the lung following injury or blockage of other regions. However, in generalized hypoxia, and in states of chronic and pervasive lung damage, it can lead to pulmonary edema and death (Weir et al., 2005). Although a number of mechanisms to explain HPV have been proposed, there is still considerable controversy about the nature of the oxygen sensing mechanisms involved, as well as the various components required for the response (Weir et al., 2005). We have demonstrated the presence in dermal vasculature of one key modulator of HPV, the potassium channel Kv1.5. Interestingly, Kv1.5 is also a HIF transcriptional target (Moudgil et al., 2006), as is a key modulator of voltage-gated potassium channels, the peptide endothelin-1 (Whitman et al., 2007). Further work on smooth muscle-specific knockouts of HIF factors may prove useful in delineating how the hypoxic response in the vasculature of the skin impacts the physiological response we have described.
Durand and Martineaud first showed in 1969 that humans demonstrate an immediate and persistent cutaneous vasoconstriction when exposed to high altitudes (Durand et al., 1969). Weil and colleagues subsequently found that although high altitudes resulted in cutaneous vasoconstriction in human subjects, similar levels of hypoxia inhaled at normal altitudes through breathing tubes did not alter dermal vascular tension (Weil et al., 1969). These data correspond to our findings in mice following acute exposure, and indicate that similar mechanisms for hypoxia-induced skin vasoconstriction exist in humans. Further study will need to be done to show whether EPO levels are similarly regulated by dermal vascular flow in human subjects.
We demonstrate here for the first time a novel pharmacological mechanism for induction of EPO expression, application of a nitric oxide donor to the skin. We have also found that a number of factors that increase skin blood flow increase EPO expression in mice; one such factor is the compound allyl isothiocyanate (mustard oil) (Supplemental Figure 1B). It is interesting to note that mustard oil massage of newborns is a wide-spread folk tradition amongst millions of people in Asia (Fikree et al., 2005; Mullany et al., 2005). It is intriguing to speculate that this massage could in part also influence neonatal erythropoietin synthesis.
In summary, mice regulate EPO response, and by extension systemic response to hypoxia, through a mechanism that is dependent on the skin and is correlated with changes in cutaneous blood flow. Thus, an intriguing new role for the skin as an independent oxygen sensor and as a regulator of systemic response to environmental hypoxia is evident.
Materials and Methods
Mouse Breeding
The K14 Cre mouse line (Haase et al., 2001; Jonkers et al., 2001) was obtained from A. Berns (Netherlands Cancer Institute). Intercrosses were generated by standard genetic techniques. K14 Tamoxifen-inducible Cre mice (Vasioukhin et al., 1999) were a kind gift of E. Fuchs (Rockefeller). Control mice, when used for comparison to mutants, were always littermates of the mutant mice containing the same floxed alleles, but lacking the K14cre transgene they are referred to as “wt” for brevity.
Nitroimidazole staining and detection
The nitroimidazole EF5 and the anti-EF5 antibodies were provided by C. Koch (University of Pennsylvania). EF5 fluorescence for each section was calculated by subtracting the competed antibody intensity from primary antibody intensity.
Immunohistochemistry
Rabbit polyclonal HIF-1α antibody, a gift of R. Abraham (Burnham Institute), was used at 1:100 overnight. Anti-potassium channel Kv1.5 antibody (Chemicon) was used at 1:100.
Gene Deletion Efficiency
Deletion efficiency was determined by quantitative real time PCR on genomic DNA isolated from the adult mouse epidermis.
EPO ELISA
EPO protein levels in blood plasma were determined with the Quantikine Mouse EPO ELISA kit (R&D Systems).
mRNA Expression
RNA was harvested from flash frozen tissues using the Trizol reagent and protocol from Invitrogen. Primers were taken from (Tam et al., 2006).
Blood Hematocrit and Volume
Hematocrit was measured by retro-orbital bleed into heparinized hematocrit tubes. Plasma volume was calculated by injecting 50 µl of 10 mg/ml Evans Blue dye i.v. into the tail vein and measurement of dilution.
Blood Oxygen Saturation
Measurements were made by pulse oximetery, using the MouseOx by Starr Life Sciences Corp.
Blood Pressure
Tail cuff measurements were made on the Kent Scientific XBP1000.
Blood flow analysis
Red fluorescent 15 µm FluoSpheres (Molecular Probes) were used. 50 µl of the microsphere mixture (50,000 spheres) was injected through the body wall into the left atrium of live mice immediately after experimental treatment; ratios of tissue microsphere fluorescence within mice were used to control for variations in microsphere injection efficiency. For skin harvest, the back of each mouse was shaved, and a 2 cm × 2 cm section of dorsal skin was taken.
Griess Assay
The measurement of plasma nitric oxide species was carried out according to (Miranda et al., 2001).
L-NAME
NG-nitro-L-arginine methyl ester (L-NAME; Sigma) was administered in drinking water at 0.5 g / L for 3 or 4 days.
Nitroglycerin experiments
3.5 cm2 Nitroglycerin patches (Hercon Laboratories) releasing 0.1 mg/hr for 7 hours were used for skin administration. To test the same dose orally we administered 0.7 mg of slow release Nitroglycerin (Ethex Corp.) by gavage; doses were calculated to approximate similar release rates. Blood was taken at the end of 7 hours by retro-orbital bleed or cardiac puncture.
Mustard Oil
Mustard oil (allyl isothiocyanate; Sigma) was diluted 1:10 in mineral oil. The dorsal skin of nude mice or shaved C57BL mice was painted with 10% mustard oil (MO) or mineral oil. Mice were painted 5 times over 7 hours.
Inhaled vs. Skin Hypoxia Chambers
Individual mouse chambers were constructed from 50 ml Falcon tubes and plastic jars. Latex gaskets separated the body chamber from the head chamber. Mice were anesthetized with Ketamine/Xylazine during the experiment. Head and body chambers were perfused alternatively with 10% oxygen or 21% oxygen with significant positive pressure. Exhaled air was captured by a nose cone for metabolic measurements.
Statistics
All statistical analysis performed using Prism (GraphPad Software). All error bars = SEM. All t-tests are two-tailed unpaired t-tests.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbrecht PH, Littell JK. Plasma erythropoietin in men and mice during acclimatization to different altitudes. J Appl Physiol. 1972;32:54–58. doi: 10.1152/jappl.1972.32.1.54. [DOI] [PubMed] [Google Scholar]
- Bergmann C. Ueber die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Grösse. Göttinger Studien. 1847:595–708. [Google Scholar]
- Bozzini CE, Barcelo AC, Conti MI, Martinez MP, Alippi RM. Enhanced erythropoietin production during hypobaric hypoxia in mice under treatments to keep the erythrocyte mass from rising: implications for the adaptive role of polycythemia. High Alt Med Biol. 2005;6:238–246. doi: 10.1089/ham.2005.6.238. [DOI] [PubMed] [Google Scholar]
- Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66:6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- Durand J, Verpillat JM, Pradel M, Martineaud JP. Influence of altitude on the cutaneous circulation of residents and newcomers. Fed Proc. 1969;28:1124–1128. [PubMed] [Google Scholar]
- Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS, Arbeit JM. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1alpha. Genes Dev. 2001;15:2520–2532. doi: 10.1101/gad.914801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Schrlau AE, Chalian AA, Zhang P, Koch CJ. Oxygen Levels in Normal and Previously Irradiated Human Skin as Assessed by EF5 Binding. J Invest Dermatol. 2006 doi: 10.1038/sj.jid.5700451. [DOI] [PubMed] [Google Scholar]
- Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression. Am J Physiol Regul Integr Comp Physiol. 2004;286:R977–R988. doi: 10.1152/ajpregu.00577.2003. [DOI] [PubMed] [Google Scholar]
- Fikree FF, Ali TS, Durocher JM, Rahbar MH. Newborn care practices in low socioeconomic settlements of Karachi, Pakistan. Soc Sci Med. 2005;60:911–921. doi: 10.1016/j.socscimed.2004.06.034. [DOI] [PubMed] [Google Scholar]
- Gassmann M, Heinicke K, Soliz J, Ogunshola OO. Non-erythroid functions of erythropoietin. Adv Exp Med Biol. 2003;543:323–330. doi: 10.1007/978-1-4419-8997-0_22. [DOI] [PubMed] [Google Scholar]
- Gruber M, Hu CJ, Johnson RS, Brown EJ, Keith B, Simon MC. Acute postnatal ablation of Hif-2{alpha} results in anemia. Proc Natl Acad Sci U S A. 2007;104:2301–2306. doi: 10.1073/pnas.0608382104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH. The VHL tumor suppressor in development and disease: functional studies in mice by conditional gene targeting. Semin Cell Dev Biol. 2005;16:564–574. doi: 10.1016/j.semcdb.2005.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A. 2001;98:1583–1588. doi: 10.1073/pnas.98.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen T, Taylor CT, Lam F, Moncada S. Redistribution of intracellular oxygen in hypoxia by nitric oxide: effect on HIF1alpha. Science. 2003;302:1975–1978. doi: 10.1126/science.1088805. [DOI] [PubMed] [Google Scholar]
- Harbrecht BG. Therapeutic use of nitric oxide scavengers in shock and sepsis. Curr Pharm Des. 2006;12:3543–3549. doi: 10.2174/138161206778343000. [DOI] [PubMed] [Google Scholar]
- Houghton BL, Meendering JR, Wong BJ, Minson CT. Nitric oxide and noradrenaline contribute to the temperature threshold of the axon reflex response to gradual local heating in human skin. J Physiol. 2006;572:811–820. doi: 10.1113/jphysiol.2005.104067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006a;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kim WY, Safran M, Buckley MR, Ebert BL, Glickman J, Bosenberg M, Regan M, Kaelin WG., Jr Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. Embo J. 2006b;25:4650–4662. doi: 10.1038/sj.emboj.7601300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–246. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- Kuwahira I, Gonzalez NC, Heisler N, Piiper J. Changes in regional blood flow distribution and oxygen supply during hypoxia in conscious rats. J Appl Physiol. 1993;74:211–214. doi: 10.1152/jappl.1993.74.1.211. [DOI] [PubMed] [Google Scholar]
- Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci U S A. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis LD. Preclinical and clinical studies: a preview of potential future applications of erythropoietic agents. Semin Hematol. 2004;41:17–25. doi: 10.1053/j.seminhematol.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Lezon C, Alippi RM, Barcelo AC, Martinez MP, Conti MI, Bozzini CE. Depression of stimulated erythropoietin production in mice with enhanced erythropoiesis. Haematologica. 1995;80:491–494. [PubMed] [Google Scholar]
- Malvin GM, Walker BR. Sites and ionic mechanisms of hypoxic vasoconstriction in frog skin. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1308–R1314. doi: 10.1152/ajpregu.2001.280.5.R1308. [DOI] [PubMed] [Google Scholar]
- Mateo J, Garcia-Lecea M, Cadenas S, Hernandez C, Moncada S. Regulation of hypoxia-inducible factor-1alpha by nitric oxide through mitochondria-dependent and -independent pathways. Biochem J. 2003;376:537–544. doi: 10.1042/BJ20031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Pugh CW, Ratcliffe PJ. The pVHL-hIF-1 system. A key mediator of oxygen homeostasis. Adv Exp Med Biol. 2001;502:365–376. [PubMed] [Google Scholar]
- Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470–3481. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minson CT. Hypoxic regulation of blood flow in humans. Skin blood flow and temperature regulation. Adv Exp Med Biol. 2003;543:249–262. doi: 10.1007/978-1-4419-8997-0_18. [DOI] [PubMed] [Google Scholar]
- Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- Moudgil R, Michelakis ED, Archer SL. The role of k+ channels in determining pulmonary vascular tone, oxygen sensing, cell proliferation, and apoptosis: implications in hypoxic pulmonary vasoconstriction and pulmonary arterial hypertension. Microcirculation. 2006;13:615–632. doi: 10.1080/10739680600930222. [DOI] [PubMed] [Google Scholar]
- Mullany LC, Darmstadt GL, Khatry SK, Tielsch JM. Traditional massage of newborns in Nepal: implications for trials of improved practice. J Trop Pediatr. 2005;51:82–86. doi: 10.1093/tropej/fmh083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol. 1998;112:123–134. doi: 10.1016/s0034-5687(98)00026-7. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117:1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, Pugh CW, Maxwell PH, Harris AL, Ratcliffe PJ. Contrasting Properties of Hypoxia-Inducible Factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-Associated Renal Cell Carcinoma. Mol Cell Biol. 2005;25:5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubner M. Ueber den Einfluss der Körpergrösse auf Stoff- und Kraftwechsel. Zeitschrift für Biologie. 1883;19:535–562. [Google Scholar]
- Russell RC, Ohh M. The role of VHL in the regulation of E-cadherin: a new connection in an old pathway. Cell Cycle. 2007;6:56–59. doi: 10.4161/cc.6.1.3668. [DOI] [PubMed] [Google Scholar]
- Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, Shelton JM, Richardson JA, Moe O, Garcia JA. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood. 2005;105:3133–3140. doi: 10.1182/blood-2004-05-1695. [DOI] [PubMed] [Google Scholar]
- Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol. 2004;96:1173–1177. doi: 10.1152/japplphysiol.00770.2003. discussion 1170–1172. [DOI] [PubMed] [Google Scholar]
- Soliz J, Gassmann M, Joseph V. Soluble erythropoietin receptor is present in the mouse brain and is required for the ventilatory acclimatization to hypoxia. J Physiol. 2007;583:329–336. doi: 10.1113/jphysiol.2007.133454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Stucker M, Struk A, Altmeyer P, Herde M, Baumgartl H, Lubbers DW. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J Physiol. 2002;538:985–994. doi: 10.1113/jphysiol.2001.013067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam BY, Wei K, Rudge JS, Hoffman J, Holash J, Park SK, Yuan J, Hefner C, Chartier C, Lee JS, et al. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006;12:793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- Tobin DJ. Biochemistry of human skin--our brain on the outside. Chem Soc Rev. 2006;35:52–67. doi: 10.1039/b505793k. [DOI] [PubMed] [Google Scholar]
- Todorov V, Gess B, Godecke A, Wagner C, Schrader J, Kurtz A. Endogenous nitric oxide attenuates erythropoietin gene expression in vivo. Pflugers Arch. 2000;439:445–448. doi: 10.1007/s004249900192. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil JV, Battock DJ, Grover RF, Chidsey CA. Venoconstriction in man upon ascent to high altitude: studies on potential mechanisms. Fed Proc. 1969;28:1160–1164. [PubMed] [Google Scholar]
- Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman EM, Pisarcik S, Luke T, Fallon M, Wang J, Sylvester JT, Semenza GL, Shimoda LA. Endothelin-1 Mediates Hypoxia-Induced Inhibition of Voltage-Gated K+ Channel Expression in Pulmonary Arterial Myocytes. Am J Physiol Lung Cell Mol Physiol. 2007 doi: 10.1152/ajplung.00091.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.