Abstract
Context: Mothers who exclusively breastfeed lose up to 10% of their bone mass. This is primarily mediated by PTHrP, in combination with low estrogen levels. The mechanisms underlying this marked bone loss are unknown. Uncoupling of bone turnover, which is seen in other prototypical states of bone loss, would seem the likely explanation. However, the most current markers of bone turnover have not been studied in human lactation.
Objectives: The purpose of this study was to assess bone formation in lactating humans using the most current bone turnover markers.
Design and Participants: We conducted a prospective cohort study with repeated measures of bone metabolism in a volunteer sample of 49 women, recruited into three study groups: lactating, bottle feeding, and healthy controls. The postpartum women were studied at 6–8 and 12–14 wk postpartum, whereas the controls were studied at the follicular phase of their menstrual cycles.
Outcome Measures: Biochemical markers of bone turnover were assessed.
Results: Mean serum C-telopeptide of type I collagen, a sensitive marker of bone resorption, was approximately 2-fold higher in lactating women as compared with bottle-feeding and healthy controls (P = 0.037 and P < 0.001, respectively). Surprisingly, amino-terminal telopeptides of procollagen 1, the most current marker of bone formation, bone-specific alkaline phosphatase, and osteocalcin were all significantly higher in the lactating group as compared with controls (P < 0.001, P = 0.002, and P < 0.001, respectively).
Conclusions: In contrast to prototypical states of rapid bone loss (myeloma, cancer, and immobilization) in which markers of bone turnover display marked uncoupling, lactational bone loss, as assessed in this small exploratory study, is distinct, showing comparably rapid bone loss in the face of apparent osteoclast-osteoblast coupling.
In contrast to prototypical states of rapid bone loss when bone turnover displays marked uncoupling, lactational bone loss is characterized by apparent osteoclast-osteoblast coupling.
Lactation is a state of physiologically altered calcium metabolism that has a significant impact on bone mass (1). During lactation in humans, it is estimated that 600–1000 ml milk are produced per day, which contains 200–400 mg calcium (1). The majority of this calcium comes from osteoclastic bone resorption of the maternal skeleton (2,3). Mothers who exclusively breastfeed for 6 months have been shown to lose up to 10% of their bone mass (1,4). In rodents, skeletal loss during 3 wk lactation has been shown to approach 30% of total mineral content (5). Interestingly, there is rapid recovery of bone density with weaning and resumption of menses (1).
Mammary gland-derived PTHrP has been shown to be the physiological mediator of bone loss during lactation, in combination with the suppressed estrogen levels characteristic of lactation (2,3,6). PTHrP concentrations are significantly higher in lactating women than in nonlactating controls. The source of PTHrP is predominantly the mammary gland, because PTHrP levels are elevated 10,000-fold in milk compared with plasma (7), and circulating maternal PTHrP levels are increased further with suckling (8). Most importantly, mammary-specific ablation of the PTHrP gene during lactation in the mouse results in loss of circulating PTHrP, reduced bone resorption rates, and marked attenuation of lactational bone loss (9).
Although it is well established that PTHrP plays a central role in mobilization of calcium from the bone during lactation, the specific pathways at the level of the skeleton underlying this rapid bone loss may differ from other states of rapid bone loss. In humoral hypercalcemia of malignancy (HHM), when circulating PTHrP is pathologically elevated, trabecular bone volume is reduced by approximately 4% (10). In this setting, there is a marked increase in bone resorption as assessed using quantitative histomorphometry and bone biopsy specimens (7,10). This is accompanied by completely suppressed bone formation (10). This striking uncoupling accounts for the marked bone loss and hypercalcemia seen in this condition. The uncoupling of bone formation and resorption in HHM also has been documented by measuring markers of bone turnover (11). Two other prototypical states of rapid bone loss include multiple myeloma (12) and immobilization after acute spinal cord injury. During immobilization, there is acute loss in bone mineral density of approximately 30% over 16 months, or approximately 10% in 6 months (13). The mechanism underlying the rapid bone loss in these two states is again complete uncoupling of bone formation and resorption (14). As another example of PTHrP-mediated complete uncoupling of bone resorption from formation, young healthy volunteers continuously infused with PTHrP for 48–96 h develop markedly increased bone resorption, as measured by serum cross-linked N-telopeptide of procollagen I (NTX) and cross-linked C-telopeptide of type I collagen (CTX) (15). In contrast, bone formation, as measured by amino-terminal telopeptides of procollagen 1 (P1NP), is dramatically suppressed by continuous PTHrP infusion (15). Thus, in humans, continuous exposure to elevated PTHrP concentrations, either in HHM or in response to PTHrP infusion, leads to complete uncoupling of bone resorption and formation.
Interestingly, although bone loss during lactation appears to occur at a rate comparable to that in HHM, multiple myeloma, and immobilization, the mechanisms that account for this are less well studied. As might be anticipated, it is accompanied by a marked increase in osteoclastic activity, as assessed both by bone resorption markers as well as by quantitative bone histomorphometry in rodents and nonhuman primates (2,5,16,17,18). In contrast, however, to the consistent increase in bone resorption, the status of bone formation during lactation is less clear. Bone formation in animals has been reported to be increased, decreased, or unchanged using quantitative bone histomorphometry and/or bone turnover markers (16,17,18). The single primate histomorphometric study available did not quantify osteoblast numbers or osteoblast activity (19). Studies in human lactation have shown a consistent increase in bone resorption as assessed using bone turnover markers. In contrast, formation markers have been reported to be increased or unchanged (17,18). In a longitudinal study of bone turnover during lactation, bone resorption, as measured by urine NTX, was shown to be increased in women who exclusively breastfed for 6 months compared with women who breastfed for less than 1 month. Bone formation, as measured by osteocalcin and bone-specific alkaline phosphatase (BSAP), was also noted to be increased in mothers who lactated for 6 months (18). Similarly, a study of bone metabolism in human lactation confirmed elevated bone resorption, as measured by carboxyl-terminal telopeptide of type 1 collagen (ICTP), in women who exclusively lactate for 6 months compared with healthy controls. In addition, bone formation, as measured by osteocalcin, BSAP, and carboxyl-terminal propeptide of type 1 collagen (P1CP), was also increased (8). A third study showed an increase in bone resorption, as measured by urine deoxypyrinoline, in mothers who lactated for 13 wk compared with women who had not been pregnant. However, in contrast to the first two studies, bone formation, as measured by osteocalcin and BSAP, did not significantly rise (17). To date, no study in humans has used the most current formation marker, P1NP (20). Finally, quantitative bone histomorphometry data evaluating the status of bone formation are not available in lactating humans, nor are they ever likely to be, because of the risks and inconvenience of bone biopsy and attendant tetracycline labeling.
The purpose of this study, therefore, was to assess bone formation in lactating humans using the most current state-of-the-art bone formation marker, P1NP (20). We anticipated that P1NP would reveal a clear-cut uncoupling of bone formation from resorption, as is seen in other prototypical disorders of bone loss, HHM, multiple myeloma, and immobilization, as well as continual PTHrP infusion. Instead, we report, to our surprise, that P1NP is markedly increased and apparently coupled to bone resorption in human lactation, unlike the other disorders of rapid bone loss. This is corroborated by measurement of BSAP and osteocalcin. Possible mechanisms are discussed.
Subjects and Methods
Study subjects
Forty-nine female Caucasian or Asian subjects between the ages of 24 and 41 yr were enrolled. Subjects were recruited into three study groups: lactating (n = 20), bottle feeding (n = 9), and healthy controls (n = 20). The lactating group included 20 postpartum (singleton pregnancy) women who were breastfeeding exclusively. This was defined as using one or fewer bottles of supplemental formula per day. The bottle-feeding group included postpartum (singleton pregnancy) women who had weaned their infant from breastfeeding at least 4 wk before the study. This group was originally designed to include 20 subjects, but only nine subjects could be recruited. The healthy-control group included 20 healthy, nonpregnant women who were race and age matched to women in the lactating group and who had not been lactating or pregnant within the past year. Exclusion criteria included disorders associated with skeletal metabolism (e.g. renal, endocrine, or gastrointestinal disorders), significant alcohol or tobacco use, or use of any chronic medication (with the exception of stable doses of thyroid hormone, vitamin supplementation, and oral contraceptives). One woman from the bottle-feeding group and one from the control group were excluded from analysis because of hyperthyroidism identified during the initial visit. All women provided informed written consent. The study protocol was approved by the University of Pittsburgh Institutional Review Board.
Study design
This was a prospective cohort study with repeated measures of bone metabolism in lactating women compared with nonlactating postpartum women and healthy controls. The study involved two outpatient visits to the Clinical and Translational Research Center at the University of Pittsburgh. The first visit for the lactation and bottle-feeding groups occurred 6–8 wk after delivery. The second visit occurred 12–14 wk postpartum. Healthy controls were studied twice during the follicular phase of their menstrual cycle, at intervals similar to their matched lactating women. A medical and obstetric history, dietary calcium intake questionnaire, vital signs, and body mass index were obtained at both visits. Fasting blood and urine samples were also collected at each visit for measurement of serum total and ionized calcium, phosphorus, creatinine, PTH (1-84), 1,25-dihydroxyvitamin D [1,25(OH)2D], and urinary calcium, phosphorus, and creatinine as well as markers of bone turnover. Estradiol, TSH, and 25-hydroxyvitamin D (25-OHD) were also measured at the first visit.
Analyses
Serum total calcium, ionized calcium, phosphorus, creatinine, TSH, and urine calcium, creatinine, and phosphorus were measured using standard automated chemistries in the University of Pittsburgh Medical Center Clinical Chemistry Laboratory on the day of collection. The fractional excretion of calcium and tubular maximum for phosphorus were calculated as described previously (21,22). Plasma 1,25(OH)2D and 25-OHD were measured using a previously described assay (23). PTH (1-84) was measured by immunochemiluminometric assay (Quest Laboratories, San Juan Capistrano, CA). Plasma osteocalcin was measured by RIA [intraassay coefficient of variation (CV) = 7.3%] as described previously (24). P1NP was measured using kits from Orion Diagnostics (Espoo, Finland) RIA (CV = 2.6%). NTX was measured using kits from Osteomark (Ostex International, Seattle, WA) ELISA (CV = 7.9%). CTX was measured using kits from Crosslaps (Nordic Biosciance Diagnostics, Herlev, Denmark) ELISA (CV = 7.3%). BSAP was measured using commercial kits from Ostase (Hybritech Inc., Fullerton, CA) enzyme immunoassay (CV = 1.8%). Markers of bone turnover, 25-OHD, 1,25(OH)2D, and estradiol concentrations were assayed in single batches at the conclusion of the study.
Statistics
The projected sample size for this descriptive, exploratory study was determined with the intent of parameter estimation of group means and sd with margin of error (in terms of the half-width of the two-sided confidence interval) of 0.468s and 0.350s, respectively, with a confidence coefficient of 0.95, where s is the group sd. Based on the reduced group sample size observed for the bottle-feeding group (n = 9), the margin of error when estimating means and sd would be 0.769s and 0.620s, respectively. When conducting two-sided hypothesis testing at a significance level of 0.05 comparing means across the three groups using ANOVA with the F test, the small detectable effect size in terms of f (the signal-to-noise ratio defined as the ratio of the sd for group means to the within-group sd) with 80% power is f = 0.46. Further details of the statistical methods are provided in the Supplemental Material (published on The Endocrine Society’s Journals Online web site at http://jcem.endojournals.org).
Results
Baseline demographics and estradiol concentrations
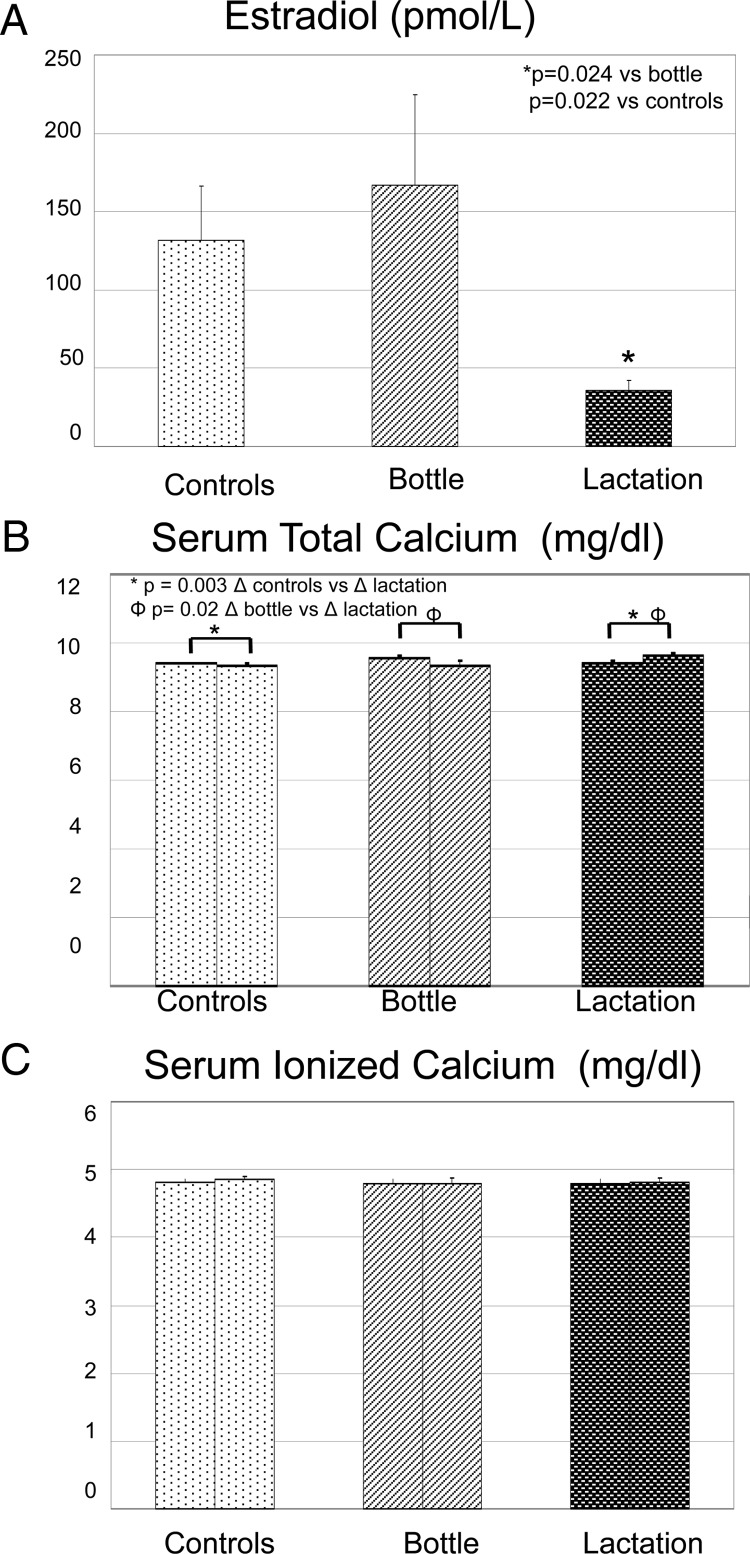
The baseline demographics of the three study groups are shown in Table 1. Subjects were well matched with regard to age and race and similar with respect to height and parity. The women who were bottle-feeding had significantly higher weight (P = 0.035) and body mass index (P = 0.033) compared with healthy controls but were not different from women in the lactation group. The women in the lactation group had significantly higher total daily calcium intake compared with the control group (P = 0.007) but were not different from bottle-feeding women. As anticipated, estradiol concentrations were significantly lower in the lactation group compared with bottle-feeding and control groups (P = 0.024 and P = 0.022, respectively; Fig. 1A).
Table 1.
Baseline demographics
| Control (n = 19) | Bottle (n = 8) | Lactation (n = 20) | |
|---|---|---|---|
| Age (yr) | |||
| Mean ± se | 31.5 ± 1.5 | 30.9 ± 0.7 | 32.1 ± 1.1 |
| Median (range) | 28.0 (24–44) | 31.0 (28–33) | 31.5 (26–42) |
| Race | |||
| Caucasian (%) | 95 | 100 | 95 |
| Asian (%) | 5 | 0 | 5 |
| Height (m) | |||
| Mean ± se | 1.62 ± 0.01 | 1.64 ± 0.02 | 1.65 ± 0.01 |
| Median (range) | 1.62 (1.51–1.73) | 1.64 (1.54–1.72) | 1.66 (1.53–1.72) |
| Weight (kg) | |||
| Mean ± se | 64.3 ± 2.9 | 78.7 ± 7.9 | 70.6 ± 1.9 |
| Median (range) | 61.0 (46.9–88.4) | 76.7 (52.7–113.2) | 71.7 (58.8–85.0) |
| BMI (kg/m2) | |||
| Mean ± se | 24.3 ± 0.9 | 29.2 ± 2.7 | 26.0 ± 0.7 |
| Median (range) | 24.6 (19.0–29.9) | 28.2 (20.0–43.1) | 25.9 (21.6–33.1) |
| Parity | |||
| Mean ± se | 1.1 ± 0.4 | 2.1 ± 0.4 | 2 ± 0.3 |
| Median (range) | 0.0 (0–4) | 2.0 (1–4) | 2.0 (1–6) |
| Total daily calcium intake (mg/d) | |||
| Mean ± se | 799.4 ± 83.7 | 914.9 ± 230.7 | 1425.1 ± 167.8 |
| Median (range) | 717.0 (350–1728) | 750.0 (249–2091) | 1343.0 (476–2805) |
Results are shown as mean ± se or median (range). BMI, Body mass index.
P = 0.035 bottle vs. control.
P = 0.033 bottle vs. control.
P = 0.007 lactation vs. control.
Figure 1.
Estradiol and total and ionized calcium concentrations in lactating, bottle-feeding, and control women at two different time points. The first bar represents the mean and se for each group during the first study visit (6–8 wk postpartum for the lactation and bottle-feeding groups and follicular phase of the menstrual cycle for controls), and the second bar represents the second visit (12–14 wk postpartum or 6–8 wk after visit 1). A, Estradiol levels were significantly lower in the lactation group compared with the other two groups at both time points. B, There were no statistical differences among groups at visit 1 with regard to serum total calcium. When comparing the change in serum calcium from visit 1 to visit 2, there was a minimal but significant increase in serum calcium in the lactation group compared with bottle-feeding and controls. C, There were no statistical differences among groups or time points with regard to ionized calcium levels.
Calcium metabolism
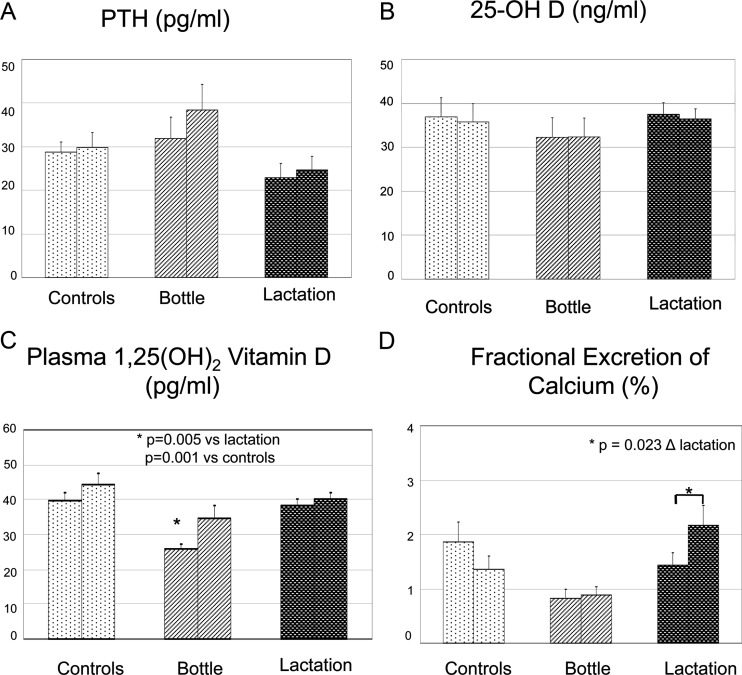
Total and ionized serum calcium were within normal range in all three groups of women and not different from each other at the initial visit (P = 0.746 and 0.836, respectively; Fig. 1, B and C). When comparing the change in serum calcium from visit 1 to visit 2 among the three groups, there was a minimal but significant increase in total serum calcium in the lactation group compared with bottle-feeding (P = 0.02) and control (P = 0.03) groups, as shown in Fig. 1B. There were no changes in ionized serum calcium (Fig. 1C). Intact PTH [PTH (1-84)] concentrations appeared lower in lactating women compared with both bottle-feeding and control women at both time points, but this finding was not statistically significant (P = 0.178 and 0.547, respectively; Fig. 2A). At the initial visit, mean levels of 25-OHD were 37.5, 32.3, and 36.9 ng/ml for the lactation, bottle-feeding, and control groups, respectively (Fig. 2B). This difference was not statistically significant (P = 0.886). All subjects in the lactation group, one in the bottle-feeding group, and half the patients in the control group were taking 200–400 IU supplemental vitamin D. Lactating women had higher plasma 1,25(OH)2D concentrations compared with bottle-feeding women (P = 0.005) at visit 1 but were not significantly different from controls (P = 0.69; Fig. 2C). Controls also had significantly higher levels of 1,25(OH)2D compared with bottle-feeding women (P = 0.001). The change in plasma 1,25(OH)2D between the two study visits for all three groups was not statistically significant (P = 0.3). Initial fractional excretion of calcium (FECa) was similar among controls and lactating women (P = 0.13; Fig. 2D). In the time period between the two study visits, FECa significantly increased in the lactation group (P = 0.023) but did not change in controls or bottle-feeding women.
Figure 2.
PTH, 25-OHD, 1,25(OH)2D, and FECa in the three groups. The two bars for each group represent study visits 1 and 2 as previously described. Means and sebars are shown. A and B, There were no statistical differences among groups or time points in regard to PTH or plasma 25-OHD; C, plasma 1,25(OH)2D was significantly lower in the bottle-feeding group compared with both lactation and control groups at baseline; D, There were no statistical differences among groups in regard to FECa at baseline, but in the time period between the two study visits, FECa significantly increased in the lactation group (P = 0.023). FECa remained stable in the control and bottle-feeding groups.
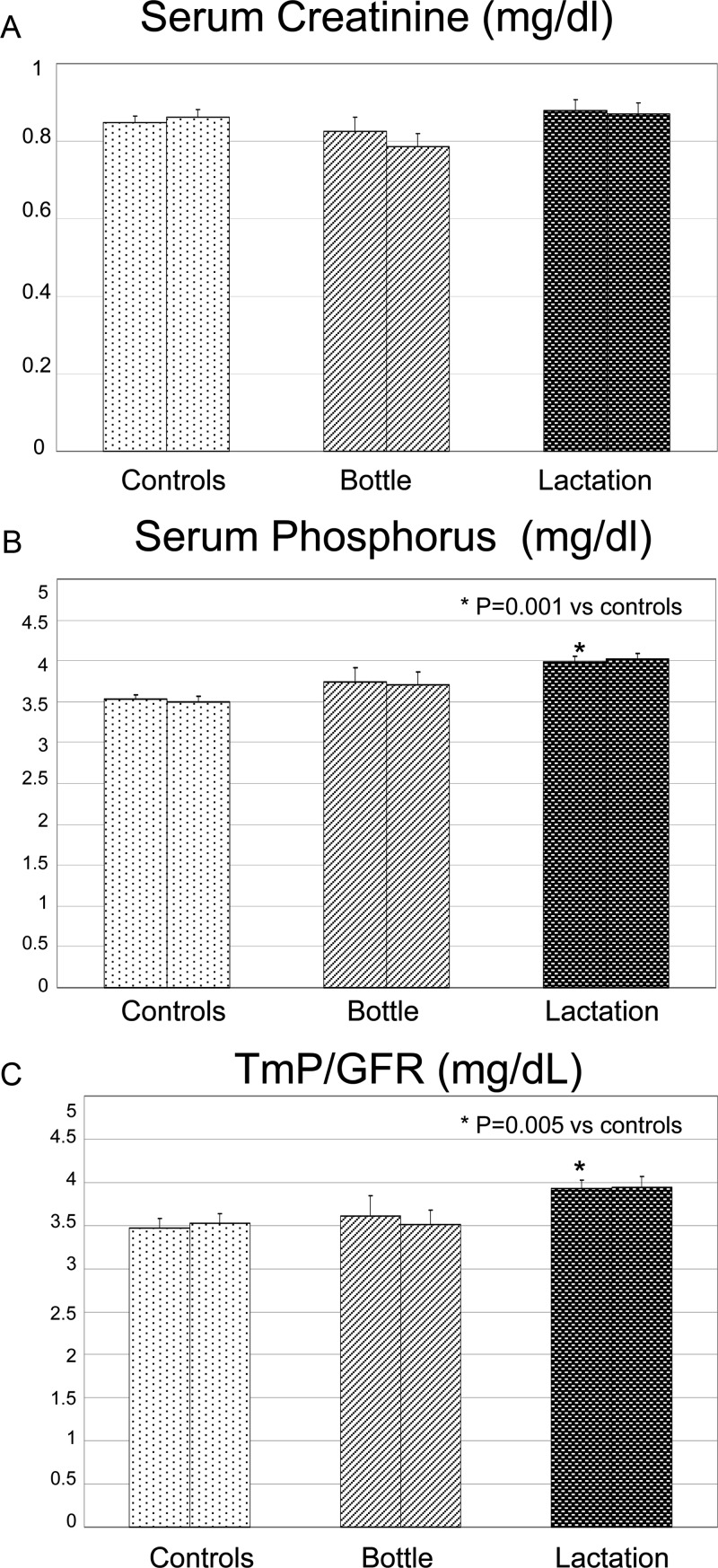
Serum creatinine was within normal range in all three groups (Fig. 3A). Mean serum phosphorus concentrations were significantly higher in lactating women compared with controls on the initial visit (P = 0.001), although they were not significantly different from bottle-feeding women (P = 0.2), as shown in Fig. 3B. Similarly, the tubular maximum of phosphorus (TmP) corrected for glomerular filtration rate (GFR) was significantly higher in lactating women compared with controls (P = 0.005) at visit 1 (Fig. 3C).
Figure 3.
Serum creatinine, serum phosphorus, and the TmP/GFR for each group at two different time points. The two bars for each group represent study visits 1 and 2 as previously described. Means and sebars are shown. A, There were no statistical differences between groups or time points for serum creatinine; B, serum phosphorus was significantly higher in the lactating group at visit 1 compared with controls; C, TmP/GFR was statistically higher in the lactating group at visit 1 compared with controls.
Bone turnover markers
Circulating concentrations of plasma CTX and NTX, markers of bone resorption, are shown at the two different time points in Fig. 4. Mean serum NTX appeared to be higher in visit 1 in both lactating and bottle-feeding women compared with controls, but this was not statistically significant (P = 0.75 and P = 0.2, respectively; Fig. 4A). Mean serum CTX was approximately 2-fold higher in lactating women compared with both bottle-feeding women (P = 0.037) and controls (P < 0.001) at visit 1. Mean serum CTX seemed higher in bottle-feeding women compared with controls at visit 1, but although close, it did not reach statistical significance (P = 0.057; Fig. 4B). There was no significant change in NTX or CTX between groups from visit 1 to visit 2 (P = 0.3 and 0.6, respectively).
Figure 4.
Markers of bone resorption in the three study groups at two different time points. The two bars for each group represent study visits 1 and 2 as previously described. Means and sebars are shown. A, Bone resorption appeared increased in both the lactating and bottle-feeding groups as assessed by NTX, but this did not achieve statistical significance; B, CTX was significantly higher in the lactating group as compared with the other two groups.
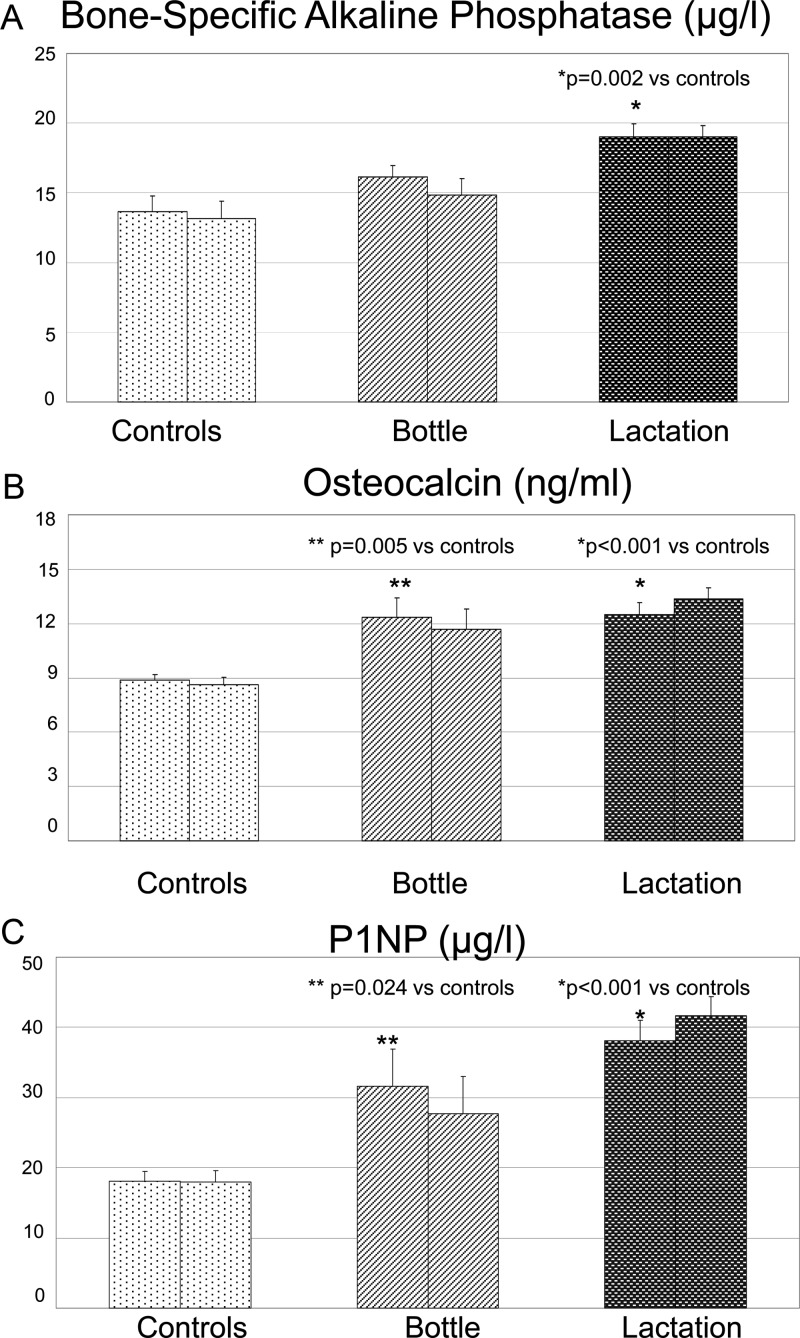
Circulating concentrations of BSAP, osteocalcin, and P1NP, markers of bone formation, are shown in Fig. 5. Surprisingly, and in contrast to our expectations, all three markers of bone formation were significantly higher in the lactating women compared with controls (P = 0.002 for BSAP, P < 0.001 for osteocalcin, and P < 0.001 for P1NP) at visit 1 and also at visit 2. Levels of osteocalcin and P1NP were also higher in bottle-feeding women compared with controls (P = 0.005 and P = 0.024, respectively, for visit 1). There was no significant difference in bone formation markers between lactating and bottle-feeding women (P = 0.2 for BSAP, P = 0.9 for osteocalcin, and P = 0.2 for P1NP) at visit 1.
Figure 5.
Markers of bone formation in the three study groups at two different time points. The two bars for each group represent study visits 1 and 2 as previously described. Means and sebars are shown. A, BSAP was significantly higher in the lactation group compared with controls at both visits (P = 0.002 for visit 1, and P < 0.001 for visit 2). B, Osteocalcin was significantly increased in the lactation and bottle-feeding groups compared with controls at both visits (P < 0.001 for visits 1 and 2). In the time period between the two study visits, osteocalcin levels significantly decreased in the bottle-feeding women and increased in the lactating women (P = 0.038). C, P1NP, the most current marker of bone formation, was significantly increased in the lactation and bottle-feeding groups compared with controls at both visits (P < 0.001 for both visits 1 and 2). In the time period between the two study visits, P1NP levels significantly decreased in the bottle-feeding women and increased in the lactating women (P = 0.029).
Collectively, these results reveal an unanticipated apparent tight coupling of bone formation and bone resorption at two different time points in human lactation. More specifically, in quantitative terms, bone resorption, assessed by CTX, was almost 2-fold higher in the lactation group as compared with the control group (mean CTX lactation and control groups were 0.9 and 0.5 μg/liter, respectively). Concurrently, bone formation, as assessed by P1NP, was more than 2-fold higher in the lactation group than in the control group (mean P1NP in lactation and control groups were 76.1 and 36.2 μg/liter, respectively).
Discussion
This is a unique study because it is the first prospective cohort study of lactation to measure the most current markers of bone turnover, namely serum CTX and P1NP (20,25). This study demonstrates a significant 2-fold increase in bone resorption as assessed by CTX during the first 2–3 months lactation, as expected. Surprisingly, however, we also observed a similar increase in bone formation, as assessed by P1NP, when comparing the lactation group to controls. Our results show that bone turnover appears to remain coupled in lactation with significant increases in both resorption and formation. These results appear to indicate that the mechanism underlying the rapid and substantial bone loss in lactation is not due to uncoupling of bone turnover, i.e. an increase in bone resorption and suppression in bone formation, which is so characteristic of other prototypical examples of rapid bone loss: myeloma, HHM, and immobilization as well as continuous PTHrP infusions. These unanticipated observations raise several interesting questions.
How might one explain the rapid and profound bone loss that is well described in lactation (10% in six months) (3,4), when the results show that rates of formation and resorption appear to be coupled, or quantitatively equivalent? The trivial explanation would be that bone turnover markers are not quantitatively reflective of actual rates of bone formation and resorption and therefore that marked uncoupling could be occurring in the face of apparently coupled increase in markers of formation and resorption. This explanation seems unlikely, because in the other states of comparably rapid skeletal loss and resorption-formation uncoupling, marked uncoupling of resorption and formation markers occurs (10,11,12,14,15) and accurately predicts histomorphometric measures of bone resorption and formation. Thus, other explanations are required.
In the absence of human histomorphometric data, one can only speculate as to what may be happening at the level of the bone remodeling unit. On the resorption side of the equation, it seems reasonable to accept the current dogma that elevated PTHrP concentrations associated with lactation, accompanied by suppressed estrogen concentrations, causes an increase in osteoclast-mediated bone resorption (2,6,7). On the formation side of the equation, the increased markers of bone formation would appear to suggest that the complete bone formation program proceeds at a rapid and coupled rate. However, if that were true, bone mass should not decline. We speculate, therefore, that the increase in markers of bone formation may indicate that the bone formation program during lactation is occurring but is incomplete. More specifically, we speculate that lactation-associated continuous elevation in PTHrP recruits osteoblast precursors and initiates the osteoblast differentiation program but that complete osteoblast differentiation and/or osteoid mineralization does not occur. The evidence for this is strongly supported by the in vitro and rodent studies (26,27,28) that demonstrate that continuous exposure to PTH or PTHrP recruits and initiates osteoblast differentiation but also causes an arrest of osteoblast differentiation at the pre-osteoblast to osteoblast transition. This would also account for the accumulation of peritrabecular fibroblasts (or pre-osteoblasts) during continuous PTH infusion described by Dobnig and Turner (27). The accumulation of pre-osteoblasts might lead to increases in formation markers, particularly P1NP, which is a marker of type 1 collagen synthesis. In this scenario, accelerated bone resorption would occur, and would appear to be unopposed by bone formation, as a result of the absence or paucity of fully differentiated osteoblasts and lack of mineralization, reflected in a fall in bone mineral density. Although in vitro studies have described osteocalcin as a marker of matrix mineralization and thus osteoblast maturation (29), osteocalcin also may be released during bone resorption, so the serum concentration may reflect components of both formation and resorption (30).
Interestingly, if this exploratory hypothesis is correct, it would also provide an explanation for the rapid reaccumulation of bone mineral after cessation of lactation. An abundance of pre-osteoblasts, recruited during lactation, would be allowed to complete their differentiation program with weaning, and the withdrawal of mammary-derived PTHrP secretion, a phenomenon that is well documented in vitro (26,27,28). In fact, rodent studies of weaning have shown a dramatic rise in bone formation as measured by osteocalcin and/or histomorphometry during weaning compared with lactation (31,32,33). Of course, this remains a hypothesis, and documentation of these changes in humans would require serial bone biopsies during lactation as well as weaning, a study that is not likely to occur for logistical reasons.
Despite the well documented skeletal calcium losses observed in lactation, serum total and ionized calcium remained within normal limits. This is likely attributable to transport of large quantities of mobilized calcium (200–400 mg/d) from the maternal circulation into milk (34). Serum phosphorus was minimally but significantly higher in lactating women compared with controls. These findings have been well documented in previous epidemiological studies (2). The elevated phosphorus concentrations have been attributed to increased skeletal resorption combined with reduced phosphorus excretion (2). Renal excretion of phosphorus was indeed statistically lower in the lactation group compared with controls in our study. The renal excretion of calcium in lactating women has been reported to be low due to a reduction in GFR resulting from dehydration (2). In most studies of lactating women, 24-h urine collections demonstrate hypocalciuria relative to controls (35). In our study, we measured morning fractional calcium excretion, but postprandial urinary calcium measures were not obtained. FECa values were low and comparable in the lactation and control groups, indicating that the bone metabolism changes observed during lactation may be independent of the usual regulators of calcium metabolism, PTH and 1,25(OH)2D. Large, epidemiological studies have shown that PTH is reduced 50% or more during lactation compared with the prepregnant state (2). In our study, there appeared to be a trend toward lower PTH concentrations in lactating women, although it was not statistically different compared with the other groups. Had our sample size been larger, perhaps this trend would have become significant. The concentrations of 1,25(OH)2D in lactating women remained within normal limits, which has also been shown in previous epidemiological studies (2,36). The normal 1,25(OH)2D concentrations in the lactation group are in contrast to the suppressed concentrations of 1,25(OH)2D found in HHM, two syndromes mediated by PTHrP. This may reflect differences in PTHrP secretion pattern (pulsatile in lactation due to suckling vs. continuous in HHM) and binding to the PTH1 receptor (37). We hypothesize that pulsatile PTHrP acts as a weak agonist of 1,25(OH)2D (22,37). In contrast, in HHM, hypercalcemia occurs, PTH is suppressed, and PTHrP is continuously elevated, resulting in lower concentrations of 1,25(OH)2D.
Interestingly, bone formation was also significantly increased in the bottle-feeding group compared with controls. We suspect that the underlying pathophysiology is different from that observed in the lactation group. In bottle-feeders, estrogen production resumes (Fig. 1A) and may activate bone formation as previously described (38).
This study has limitations. First, the sample size was relatively small for each group, especially the bottle-feeding group. The World Health Organization and American Academy of Pediatrics recommend 6 months exclusive infant breastfeeding for achievement of optimal growth, development, and health (39). Due to widespread implementation of this recommendation, recruitment of bottle-feeding mothers was challenging. Although we had no difficulty in recruiting lactating women and controls in 1 yr active recruitment at several sites, we simply were unable to recruit more than the nine subjects in the bottle-feeding group. Having said this, we did observe statistical differences between groups and therefore anticipate that with larger sample sizes, the significance would only be more pronounced. Second, we did not directly confirm bone loss in our lactating patients, but marked bone loss has been consistently shown by others to occur during lactation (1,2,3,4). Third, circulating concentrations of PTHrP were not measured in this study because, to the best of our knowledge, there are no sufficiently sensitive and specific PTHrP assays currently available to distinguish normal from lactation values. Lastly, we examined only two time points in what is certainly a dynamic state of bone metabolism. Future larger longitudinal studies of late pregnancy, lactation, and postlactation may help further elucidate the mechanisms of rapid bone loss during lactation and subsequent reaccumulation during weaning.
In summary, this study demonstrates that bone turnover in human lactation is likely unique and may differ from pathological states of comparably rapid bone loss as well as from human clinical models of continuous PTHrP infusion. The precise histomorphometric changes that explain these discrepancies in humans are unknown and will only be definitively defined by bone biopsy during lactation.
Acknowledgments
We thank the staff of the Clinical Translational Research Center for helping to support this study. We also thank the many study participants who volunteered their time and commitment to this study.
Footnotes
This study was supported by National Institutes of Health Grants R-01 DK51081 and R-01 DK07303 as well as the University of Pittsburgh Clinical Translational Sciences Award, NIH/NCRR/CTSA UL1 RR024153 and MO-1 RR000056.
Disclosure Summary: R.M.C., L.P., M.B.T., S.M.S., M.H., C.M.G., A.F.S., and M.J.H. have nothing to declare. B.W.H. consults for Diasorin Corp.
First Published Online February 11, 2010
Abbreviations: BSAP, Bone-specific alkaline phosphatase; CTX, cross-linked C-telopeptide of type I collagen; CV, coefficient of variation; FECa, fractional excretion of calcium; GFR, glomerular filtration rate; HHM, humoral hypercalcemia of malignancy; NTX, cross-linked N-telopeptide of procollagen I; 25-OHD, 25-hydroxyvitamin D; 1,25(OH)2, 1,25-dihydroxyvitamin D; P1NP, amino-terminal telopeptides of procollagen 1; TmP, tubular maximum of phosphorus.
References
- Sowers M 1996 Pregnancy and lactation as risk factors for subsequent bone loss and osteoporosis. J Bone Miner Res 11:1052–1060 [DOI] [PubMed] [Google Scholar]
- Kovacs CS 2005 Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia 10:105–118 [DOI] [PubMed] [Google Scholar]
- Sowers MF, Hollis BW, Shapiro B, Randolph J, Janney CA, Zhang D, Schork A, Crutchfield M, Stanczyk F, Russell-Aulet M 1996 Elevated parathyroid hormone-related peptide associated with lactation and bone density loss. JAMA 276:549–554 [PubMed] [Google Scholar]
- Kalkwarf HJ, Specker BL, Bianchi DC, Ranz J, Ho M 1997 The effect of calcium supplementation on bone density during lactation and after weaning. N Engl J Med 337:523–528 [DOI] [PubMed] [Google Scholar]
- Kovacs CS, Kronenberg HM 1997 Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 18:832–872 [DOI] [PubMed] [Google Scholar]
- VanHouten JN, Wysolmerski JJ 2003 Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology 144:5521–5529 [DOI] [PubMed] [Google Scholar]
- Burtis WJ, Brady TG, Orloff JJ, Ersbak JB, Warrell Jr RP, Olson BR, Wu TL, Mitnick ME, Broadus AE, Stewart AF 1990 Immunochemical characterization of circulating parathyroid hormone-related protein in patients with humoral hypercalcemia of cancer. N Engl J Med 322:1106–1112 [DOI] [PubMed] [Google Scholar]
- Dobnig H, Kainer F, Stepan V, Winter R, Lipp R, Schaffer M, Kahr A, Nocnik S, Patterer G, Leb G 1995 Elevated parathyroid hormone-related peptide levels after human gestation: relationship to changes in bone and mineral metabolism. J Clin Endocrinol Metab 80:3699–3707 [DOI] [PubMed] [Google Scholar]
- VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, Wysolmerski JJ 2003 Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest 112:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AF, Vignery A, Silverglate A, Ravin ND, LiVolsi V, Broadus AE, Baron R 1982 Quantitative bone histomorphometry in humoral hypercalcemia of malignancy: uncoupling of bone cell activity. J Clin Endocrinol Metab 55:219–227 [DOI] [PubMed] [Google Scholar]
- Nakayama K, Fukumoto S, Takeda S, Takeuchi Y, Ishikawa T, Miura M, Hata K, Hane M, Tamura Y, Tanaka Y, Kitaoka M, Obara T, Ogata E, Matsumoto T 1996 Differences in bone and vitamin D metabolism between primary hyperparathyroidism and malignancy-associated hypercalcemia. J Clin Endocrinol Metab 81:607–611 [DOI] [PubMed] [Google Scholar]
- Jakob C, Zavrski I, Heider U, Brux B, Eucker J, Langelotz C, Sinha P, Possinger K, Sezer O 2002 Bone resorption parameters [carboxy-terminal telopeptide of type-I collagen (ICTP), amino-terminal collagen type-I telopeptide (NTx), and deoxypyridinoline (Dpd)] in MGUS and multiple myeloma. Eur J Haematol 69:37–42 [DOI] [PubMed] [Google Scholar]
- Garland DE, Stewart CA, Adkins RH, Hu SS, Rosen C, Liotta FJ, Weinstein DA 1992 Osteoporosis after spinal cord injury. J Orthop Res 10:371–378 [DOI] [PubMed] [Google Scholar]
- Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, McWhinney B, Hickman PE 1998 Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab 83:415–422 [DOI] [PubMed] [Google Scholar]
- Horwitz MJ, Tedesco MB, Sereika SM, Syed MA, Garcia-Ocaña A, Bisello A, Hollis BW, Rosen CJ, Wysolmerski JJ, Dann P, Gundberg C, Stewart AF 2005 Continuous PTH and PTHrP infusion causes suppression of bone formation and discordant effects on 1,25(OH)2 vitamin D. J Bone Miner Res 20:1792–1803 [DOI] [PubMed] [Google Scholar]
- Lees CJ, Jerome CP, Register TC, Carlson CS 1998 Changes in bone mass and bone biomarkers of cynomolgus monkeys during pregnancy and lactation. J Clin Endocrinol Metab 83:4298–4302 [DOI] [PubMed] [Google Scholar]
- Prentice A, Jarjou LM, Stirling DM, Buffenstein R, Fairweather-Tait S 1998 Biochemical markers of calcium and bone metabolism during 18 months of lactation in Gambian women accustomed to a low calcium intake and in those consuming a calcium supplement. J Clin Endocrinol Metab 83:1059–1066 [DOI] [PubMed] [Google Scholar]
- Sowers M, Eyre D, Hollis BW, Randolph JF, Shapiro B, Jannausch ML, Crutchfield M 1995 Biochemical markers of bone turnover in lactating and nonlactating postpartum women. J Clin Endocrinol Metab 80:2210–2216 [DOI] [PubMed] [Google Scholar]
- Lees CJ, Jerome CP 1998 Effects of pregnancy and lactation on bone in cynomolgus macaques: histomorphometric analysis of iliac biopsies. Bone 22:545–549 [DOI] [PubMed] [Google Scholar]
- Dominguez Cabrera C, Sosa Henríquez M, Traba ML, Alvarez Villafañe E, de la Piedra C 1998 Biochemical markers of bone formation in the study of postmenopausal osteoporosis. Osteoporos Int 8:147–151 [DOI] [PubMed] [Google Scholar]
- Syed MA, Horwitz MJ, Tedesco MB, Garcia-Ocaña A, Wisniewski SR, Stewart AF 2001 Parathyroid hormone-related protein-(1-36) stimulates renal tubular calcium reabsorption in normal human volunteers: implications for the pathogenesis of humoral hypercalcemia of malignancy. J Clin Endocrinol Metab 86:1525–1531 [DOI] [PubMed] [Google Scholar]
- Horwitz MJ, Tedesco MB, Sereika SM, Hollis BW, Garcia-Ocaña A, Stewart AF 2003 Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) [hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J Clin Endocrinol Metab 88:1603–1609 [DOI] [PubMed] [Google Scholar]
- Hollis BW, Kamerud JQ, Kurkowski A, Beaulieu J, Napoli JL 1996 Quantification of circulating 1,25-dihydroxyvitamin D by radioimmunoassay with 125I-labeled tracer. Clin Chem 42:586–592 [PubMed] [Google Scholar]
- Gundberg CM, Nieman SD, Abrams S, Rosen H 1998 Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab 83:3258–3266 [DOI] [PubMed] [Google Scholar]
- Rosen HN, Moses AC, Garber J, Iloputaife ID, Ross DS, Lee SL, Greenspan SL 2000 Serum CTX: a new marker of bone resorption that shows treatment effect more often than other markers because of low coefficient of variability and large changes with bisphosphonate therapy. Calcif Tissue Int 66:100–103 [DOI] [PubMed] [Google Scholar]
- Wang YH, Liu Y, Buhl K, Rowe DW 2005 Comparison of the action of transient and continuous PTH on primary osteoblast cultures expressing differentiation stage-specific GFP. J Bone Miner Res 20:5–14 [DOI] [PubMed] [Google Scholar]
- Dobnig H, Turner RT 1997 The effects of programmed administration of human parathyroid hormone fragment (1–34) on bone histomorphometry and serum chemistry in rats. Endocrinology 138:4607–4612 [DOI] [PubMed] [Google Scholar]
- van der Horst G, Farih-Sips H, Löwik CW, Karperien M 2005 Multiple mechanisms are involved in inhibition of osteoblast differentiation by PTHrP and PTH in KS483 Cells. J Bone Miner Res 20:2233–2244 [DOI] [PubMed] [Google Scholar]
- Stein GS, Lian JB, Owen TA 1990 Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J 4:3111–3123 [DOI] [PubMed] [Google Scholar]
- Camacho P, Kleerekoper M 2006 Biochemical markers of bone turnover. In: Favus MJ, ed. Primer on the metabolic bone diseases and disorders of mineral metabolism. 6th ed. Washington, DC: American Society for Bone and Mineral Research; 127–133 [Google Scholar]
- Ardeshirpour L, Dann P, Adams DJ, Nelson T, VanHouten J, Horowitz MC, Wysolmerski JJ 2007 Weaning triggers a decrease in receptor activator of nuclear factor-κB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology 148:3875–3886 [DOI] [PubMed] [Google Scholar]
- Bowman BM, Siska CC, Miller SC 2002 Greatly increased cancellous bone formation with rapid improvements in bone structure in the rat maternal skeleton after lactation. J Bone Miner Res 17:1954–1960 [DOI] [PubMed] [Google Scholar]
- Miller SC, Bowman BM 2004 Rapid improvements in cortical bone dynamics and structure after lactation in established breeder rats. Anat Rec A Discov Mol Cell Evol Biol 276:143–149 [DOI] [PubMed] [Google Scholar]
- Budayr AA, Halloran BP, King JC, Diep D, Nissenson RA, Strewler GJ 1989 High levels of a parathyroid hormone-like protein in milk. Proc Natl Acad Sci USA 86:7183–7185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A 2000 Maternal calcium metabolism and bone mineral status. Am J Clin Nutr 71:1312S–1316S [DOI] [PubMed] [Google Scholar]
- Wysolmerski JJ 2002 The evolutionary origins of maternal calcium and bone metabolism during lactation. J Mammary Gland Biol Neoplasia 7:267–276 [DOI] [PubMed] [Google Scholar]
- Dean T, Vilardaga JP, Potts Jr JT, Gardella TJ 2008 Altered selectivity of parathyroid hormone (PTH) and PTH-related protein (PTHrP) for distinct conformations of the PTH/PTHrP receptor. Mol Endocrinol 22:156–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC, Jilka RL, Girasole G, Passeri G, Bellido T 1993 Estrogen, cytokines, and the control of osteoclast formation and bone resorption in vitro and in vivo. Osteoporos Int 3(Suppl 1):114–116 [DOI] [PubMed] [Google Scholar]
- Hoddinott P, Tappin D, Wright C 2008 Breast feeding. BMJ 336:881–887 [DOI] [PMC free article] [PubMed] [Google Scholar]