Abstract
HK97 is an exceptionally amenable system for characterizing major conformational changes associated with capsid maturation in dsDNA bacteriophage. HK97 undergoes a capsid expansion of ~20%, accompanied by major subunit rearrangements during genome packaging. A previous 3.44 Å resolution crystal structure of the mature capsid, Head II, and Cryo-EM studies of other intermediate expansion forms of HK97 suggested that primarily rigid body movements facilitated the maturation process. We recently reported a 3.65 Å resolution structure of the pre-expanded particle form, Prohead II, and found that the capsid subunits undergo significant refolding and twisting of the tertiary structure to accommodate expansion. The Prohead II study focused on the major twisting motions in the P-domain, and refolding of the spine helix during the transition. Here we extend the crystallographic comparison between Prohead II and Head II, characterizing the refolding events occurring in each of the four major domains of the capsid subunit and their effect on quaternary structure stabilization. In addition, H/2H exchange coupled to mass spectrometry, was used to characterize the structural dynamics of three distinct capsid intermediates, Prohead II, EI, and the nearly mature Head I. Differences in solvent accessibilities of the 7 quasi-equivalent capsid subunits were observed in P-II, attributed to differences in secondary and quaternary structure. Nearly all differences in solvent accessibility among subunits disappear after the first transition to Expansion Intermediate (EI). We show that most of the refolding is coupled to this transformation, an event associated with the transition from asymmetric to symmetric hexamers.
Keywords: Virus assembly, X-ray crystallography, Hydrogen/Deuterium Exchange, Mass spectrometry, Protein folding
Introduction
Most complex viruses undergo a maturational transition between the initially assembled state, referred to as a procapsid, and the mature, infectious form, termed the capsid. Such systems include the bacteriophage λ, P22, and HK97, and animal viruses such as Herpes and Adenovirus, all of which package their genomes into a pre-formed procapsid1–5. The capsid acts as a molecular container that houses the viral genome, and is comprised of a complex arrangement of hundreds of protein subunits. The enormity of the complex requires a fragile precursor assembly state from which further conformational rearrangements and reorganization enhance virus stability and infectivity6. Studies on bacteriophage HK97 have shown that extensive increases in buried surface area occur during the maturation process7. The heavily intercalated quaternary interactions that comprise the mature capsid lattice are crucial both to the virus’s stability in the extra-cellular environment and, in the case of dsDNA bacteriophage, to accommodate the high pressure generated by the packaged viral genome.
HK97 is an ideal system to study virus maturation. Isometric particles can be made in E.coli, void of the portal and terminase proteins, required for genome packaging, yet still able to undergo expansion and molecular rearrangement in vitro. The expression system contains gene products for just the capsid protein (gp5) and the internally packaged protease (gp4). The gp5 subunits first assemble into capsomers consisting of either hexamers (arrangement of 6-gp5 subunits) or pentamers (5-gp5 subunits)8. The capsomers then assemble into the initial procapsid state, Prohead I (P-I), which contains ~60 copies of gp49. Proteolytic cleavage of 102 N-terminal residues of each gp5 subunit following assembly results in the pre-expanded particle called Prohead II (P-II), composed entirely of 31kDa gp5* subunits. The expressed P-II capsid is unable to package DNA, but expands upon treatment with low pH buffers, isobutanol and many other conditions10, ultimately resulting in a particle that has a similar morphology and topology of the HK97 virion11,12. During expansion, the diameter of the particle increases from 540Å in Prohead II to 660Å in Head II (H-II). In vitro expansion proceeds through distinct intermediate states, the first one termed Expansion Intermediate (EI), followed by Balloon, and finally Head II (Figure 1A). The transition to EI from Prohead II was previously characterized as a stochastic, highly cooperative two-step process13 with an ensemble half-life of ~3 minutes at pH 4, but that occurs on a time scale of less than 1 second per particle (data unpublished). Nearly 60% of the particle expansion occurs during this transition. Cryo-EM and crystallographic studies revealed that the hexons of P-II are skewed, with their subunits oriented in a roughly radial direction, resulting in a thicker capsid shell7,14. Cryo-EM analysis of EI showed that symmetrization and reorganization of the hexamers occurred during this transition15. Subsequent to the morphological change to EI, the E-loop domain of the gp5 subunit is repositioned near the P-domain of a subunit from a neighboring capsomer. The repositioning allows a self-catalyzed isopeptide bond to form between K169 of the E-loop and N356 of the P-domain11,12. These cross-links occur about quasi and icosahedral 3-fold symmetry axes of the capsid. Increasing numbers of cross-links accumulate, resulting in an additional expansion event to the Balloon intermediate, followed by a final expansion to H-II, the mature particle form with a molecular topology similar to chainmail. A crosslink defective mutant, K169Y, is able to expand from P-II to the nearly mature conformation, Head I (H-I), which is indistinguishable from the Balloon state except for the lack of crosslinks. Balloon/H-I differs from the mature H-II particle primarily in the radial extension of pentamers, with the latter characterized by an additional ~14 Å protrusion of the pentamers that only occurs in crosslink competent particles27. Calorimetric studies have shown that crosslink formation leads to an enhanced thermal stability for the fully mature Head II structure when compared to the pre-expanded P-II particle and the crosslink defective H-I16.
Figure 1. HK97 maturation.
(a) The schematic depicts the assembly and expansion of HK97 in an E.coli expression system, lacking the portal protein and other machinery required for genome packaging. The expansion pathways of both WT and crosslink defective mutant, K169Y, are shown in response to chemical perturbation in vitro. (b) Structural model of P-II, subunit A, color-coded by the four major domains labeled in bold. (c) P-II and H-II particle forms are shown rendered as a surface representation of the corresponding crystal structures.
Raman spectroscopy studies of T4 and P22 indicated significant subunit refolding accompanying capsid maturation17,18, but structural studies of HK97 with Cryo-EM suggested rigid body rotations as the predominant dynamic enabling capsid subunit rearangement7. Recently we solved the crystal structure of the metastable, pre-expanded P-II form of HK97 at 3.65Å resolution14, allowing direct comparison, at near atomic resolution, with the previously solved 3.44Å19,12 resolution structure of the mature, H-II, revealing the structural changes associated with viral capsid maturation. The comparison of the two states show that a tertiary twist of each subunit accompanied by the bending and refolding of a 40Å helix occurs and that the transition is certainly not the movement of rigid bodies. The motions of the subunits occur with respect to a set of fixed interactions between subunits at all quasi and icosahedral 3-fold sites that are inter-capsomer “staples.” Previously we proposed a model in which quaternary associations in P-II stabilized a high-energy intermediate state generated by the distorted tertiary structures and this was primed to undergo conformational changes upon triggering maturation. Here we extend the scope of the study of structural transitions to characterize the roles of each of the subunit domains in the P-II state, and during maturation. By comparing the pre-expanded and expanded states, we identified the tertiary and quaternary contacts that stabilize the intermediate folded form in P-II, the contacts that change during the maturation, and the interactions that guide the subunit trajectories.
Hydrogen/Deuterium exchange (H/2H) coupled to MALDI-mass spectrometry was used to follow the changes in solvent accessibility during capsid maturation and quantitatively measured the changes in solvent protection of the various domains due to refolding and or changes in binding at subunit interfaces. H/2H exchange coupled to MALDI-mass spectrometry has been a widely used technique probing the structural environment of proteins based on the rate of backbone amide proton exchange with deuterium20,21. H/2H exchange has been applied to the study of a variety of protein complexes, including viral capsids, to identify regions of strict protein interactions as well as those of flexibility and disorder22,23,24. The method uses pepsin digestion to cleave previously deuterated protein and quantifies the solvent accessibilities of those fragments that appear in subsequent mass spectrometry analysis. H/2H data was taken for three distinct intermediate states, Prohead II (P-II), Expansion Intermediate (EI), and Head I (H-I).
The changes in solvent accessibility and dynamics were measured for multiple expansion states to characterize the sequence and extent of the structural rearrangements during expansion. As opposed to the previous study which only presented limited H/2H exchange data for the spine helix and the P-loop, the current study extends the coverage to fragments in each major domain of the subunits. We have also increased the residue coverage in the spine helix, which has allowed us to now resolve multiple modes of exchange due to a breakdown in quasi-symmetry. H/2H data affirmed that refolding occurred within the A-domain, spine helix, and N-arm as also identified by crystallography, and pinpointed that most of the changes in solvent accessibility occurred during the first particle transition to EI. H/2H data also showed that exchange in the P-II state occurs in a non-uniform manner in certain domains, due to differences in environments and structure for the 7 quasi-equivalent subunits. The multiple modes of exchange disappeared after the transition to EI, a state that shows overall more solvent protection than P-II and very similar protection values as the nearly mature H-I state. The data indicate that the quasi-equivalent differences seen in P-II are gone upon reaching EI and that the subunits reach their nearly mature tertiary structures during this transition.
Results
Analysis of Buried Surface Area (B.S.A)
The 3.65 Å resolution structure of P-II provides the first glimpse into the binding interfaces maintaining a metastable intermediate capsid assembly of a dsDNA bacteriophage. Three-fold interactions are the staples maintaining inter-capsomer linkages and individual subunits undergo bending and refolding motions within these constraints during capsid expansion. A combination of salt bridges and a putative metal binding site were identified as the source for the stabilization. The boundary for this fixed region is colored in blue in Figure 2a. Outside of this invariant region most of the subunit undergoes structural changes, including alterations in quaternary contacts that bolster particle stability during maturation. We evaluated the quaternary rearrangement associated with maturation by comparing the crystal structures of P-II and H-II and calculating the changes in Buried Surface Area (B.S.A.)25,26 (Figure 2b). The hexamers of P-II appear as 2-fold related, skewed trapezoids with each subunit having a different tertiary structure. In contrast H-II hexamers are formed by subunits with virtually identical tertiary structures and have near-perfect 6-fold symmetry12. Our data indicates that subunits that are related by 2-fold symmetry in the P-II hexamer have similar structures and environments, with similar B.S.A. values. The three 2-fold pairs are subunits A and D, B and E and C and F. B.S.A. measurements are shown for only one subunit of the pair because they are closely similar. B.S.A measurements are also shown for only one of the five pentamer subunits (G) as they are identical by icosahedral symmetry. Regions highlighted in Figure 2b, other than the P-loop, have significant change in the buried surface. The major B.S.A changes include significant burial of the N-arm, E-loop, and A-loop (in hexamer subunits only), as well as the A-domain helix of subunits B and E. On the other hand, the spine helix undergoes a decrease in quaternary contacts. Table 1 shows the cumulative B.S.A. calculations for inter-capsomer quaternary contacts, and intra-capsomer contacts, revealing a significant increase in overall buried surface area occurring during the transition from P-II to H-II.
Figure 2. Buried surface area calculations.
(a) The structure of a section of the P-II capsid including several hexon capsomers (containing subunits A–F), and one penton capsomers (containing subunit G). The view is from the exterior of the capsid, looking down the 5-fold axis. The sections colored represent regions that have significant changes in their quaternary interfaces during expansion (excluding the P-loop, colored blue, which maintains nearly identical 3-fold contacts throughout expansion) as identified by the B.S.A. calculations shown in panel b. Note, the color scheme in b are unrelated to the color scheme in panel a. The N-arm (colored green) is unlabeled as it is hidden in the interior of the capsid and is poorly visible, though it undergoes a major structural change during expansion. (b) Graph shows the changes in buried surface area (B.S.A) of quaternary interactions for subunits A (orange trace), B (purple trace), F (sky-blue trace), and G (yellow trace) during expansion from P-II to H-II states, using calculations derived from their respective crystal structures. Regions of interest are circled and labeled. Note, the P-loop which was described above as invariant, has minor B.S.A fluctuation primarily due to interactions with the E-loop domain upon the final maturation step to H-II27.
Table 1. Buried surface area comparisons between 3.65 Å Prohead II crystal structure and the 3.44 Å Head II crystal structure.
Values are in Å2. Measurements are based on buried surface area within each hexon and penton (intra-capsomer), or between neighboring capsomers (inter-capsomer).
| Subunit contacts |
P-II Hexamer |
H-II Hexamer |
P-II Pentamer |
H-II Pentamer |
|---|---|---|---|---|
| Intra-capsomer | 19,529 | 23.227 | 19,212 | 21,720 |
| Intra-capsomer | 17,258 | 24,950 | 15,523 | 21,030 |
Using an “orthogonal” approach, H/2H exchange data coupled to mass spectrometry was used to characterize the solvent accessibility of these regions in solution. The K169Y mutant was used for the study, as covalent crosslinks prevent efficient pepsin digestion and consequent analysis by mass spectrometry. Particles were exposed to deuterated buffer, pH 7.5, for 0–15 minutes. The peptide fragments analyzed for the three distinct capsid states; P-II, EI, and H-I, are shown in Figure 3. To clearly see the overall changes in solvent accessibility between P-II and H-I, we color coded their corresponding subunits in Figure 4 by the percentage that each analyzed fragment exchanges with deuterium after a 10 minute incubation. The data illustrate that the regions that are shown to refold by crystallography, including the N-arm, A-loop, and spine helix, undergo major increases in solvent protection. The H/2H exchange results for the individual domains of the different capsid states are presented in following sections. H/2H exchange data for regions not explicitly discussed are shown in Supplementary Figure 1.
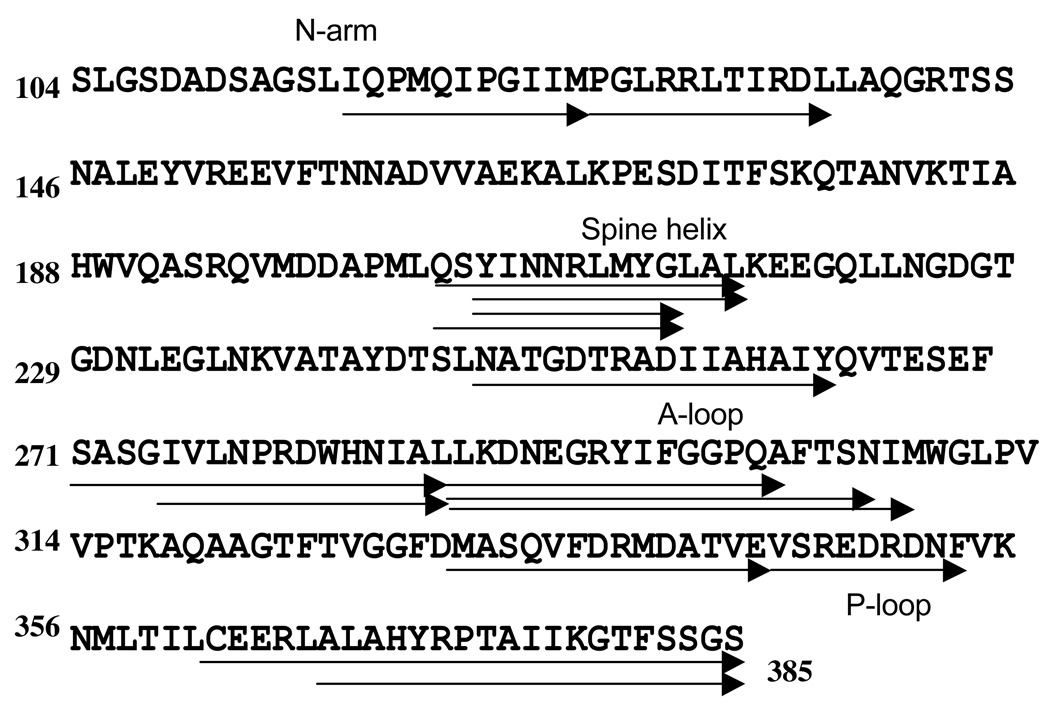
Figure 3. Fragments used in the H/2H exchange study for the P-II, EI, and H-I intermediate states.
Arrows represent the sequence of a single fragment generated by proteolytic digestion and analyzed by mass spectroscopy. Arrows stacked upon each other represent separate fragments that have overlapping sequences. All fragments were generated by pepsin digestion after native capsid incubation with the deuterium solution. Identical fragments spanning 138/282 (49%) possible residues have been characterized for the three expansion states.
Figure 4. H/2H exchange of P-II and H-I.
(a) Subunit E of P-II is color coded (spectrum below) according to the percentage of amide protons that exchange with deuterium after 10-min for each peptide fragment. Residues corresponding to overlaps in peptide fragment coverage are also color coded according to their exchange percentage. (b) SubunitE of H-II is color-coded based on the percentage of amide protons exchanging in H-I state after 10-min incubations. The H-II model was used since it was previously determined that H-II subunit E is nearly structurally identical to that of H-I.
A-domain Helix
All of the aforementioned regions except for the A-domain helix (colored yellow in Figure 2a) undergo either a hinging and or refolding transition that results in B.S.A. changes. In the pentamer (G) subunits, the A-domain helix maintains the same interactions in P-II as H-II, despite the overall flattening of the pentamer. This is also true for the A-domain helices in subunits C and F, since their interactions with neighboring subunits, B and E respectively, don’t change. In contrast, subunits A and D transition from a recessed position in P-II to one that is equivalent with the other subunits in H-II (Suppl. movie 1). This causes an increase in B.S.A. of the A-domain helices of subunits B and E, the two subunits that neighbor A and D, respectively. The differences between B.S.A. values in quasi-equivalent subunits demonstrates the breakdown of the quasi-symmetry in the procapsid structure where the conformations of the different subunits vary significantly. The conformational changes during expansion result in a nearly perfect 6-fold symmetry in the hexon subunits.
The N-arm
The N-arm, residues 104–130 undergoes a major increase in B.S.A. during expansion. The N-arm is mostly disordered in the P-II crystal structure with continuous density beginning at residue 119 in subunit A, 120 for subunit E, and ranging from residues 121 to 128 for the other 5 subunits. Though disordered, significant density can be observed past a hinging region of the N-arm at residue 130, observed as a 60 degree hinging motion (average of the 7-subunits) between the P-II and H-II conformations. The N-arm refolds and adds a strand to a β-sheet formed with a neighboring subunit in the H-II state.
H/2H exchange data of two fragments, one spanning residues before the hinge, 117–126, and the other 127–136, which consists of mostly residues after the hinge, show changes in protection only upon reaching the H-I conformation (Figure 5). Residues 117–126 are completely solvent accessible in both the P-II and EI states, but are significantly protected in H-I. The protection corresponds to the beta strand formation with the neighboring subunit as seen in a previous crystal structure of H-I27. The fragment spanning residues following the hinge (127–136) shows a decrease in protection, likely attributed to the opening of the arm during the hinging. The data confirm the dynamics of the N-arm in the P-II state as implied in the crystal structure, but also extends our understanding of N-arm dynamics throughout maturation, showing ordering of this region does not occur in the EI state, but only forms a beta strand upon reaching H-I. These results also confirm the previous NMR study of the maturation where it was clear that the N-arm mobility disappeared in H-I28.
Figure 5. H/2H exchange of N-arm.
(a) Mass envelopes of a fragment spanning residues 117–126 in the N-arm, shown at 1-min and 10-min time-points for P-II, and 1-min time-points for EI and H-I. (b-c) H/2H exchange curves comparing P-II (■), EI (○), and H-I (Δ) solvent accessibility for N-arm fragments 117–126, and 127–138 respectively. Error bars represent standard deviations from a triplicate set of experiments with 2–3 measurements taken in each experiment.
The A-loop
The A-loops form intra-capsomer interactions at the center of capsomers and refold during the transitions to the mature capsid. Figure 6 illustrates the contacts that contribute to the A-loop conformation, with side chain interactions and distances highlighted for the subunits with different binding interfaces. Unlike the symmetric arrangement of hexamer subunits in H-II where each A-loop makes extensive interactions with its neighboring subunit’s A-loop, there are 3 different environments for A-loops in the P-II hexamer subunits. The A-loops for the pseudo-2-fold partners A and D are closer to the center of the hexamer, resulting in just these 2-fold interactions. Subunits B and E are furthest from the center of the hexamer and have the least amount of buried surface area. A-loops of subunits F and C are intermediate between these two extremes. All hexon subunits in P-II have more tenuous A-loop interactions than in H-II (Figure 6). In the case of the penton subunit (G), which exhibits 5-fold symmetry in the P-II state, the B.S.A of the A-loops stay essentially constant during expansion (Figure 2B), as do their conformations (Figure 7).
Figure 6. Quaternary interactions of A-loops and spine helix.
(a) Stereo-view of the Prohead II hexon with subunits labeled A-F. Quaternary Interactions between subunits are shown for the A-loop (center circle) of the A-domain (panel b shows zoomed in view), and the interface between the spine helix of subunit F with the neighboring subunit A (outer circle, with zoomed-in view in panel c). (d) Stereo-view of Head II hexon. (E) Quaternary interactions between subunits A and F, a zoomed-in view of center circle from panel d. (f) Interface between spine helix in H-II subunit F with subunit A, a zoomed-in view of the outer circle from panel d. The various side chain interactions described in the text are shown in their respective panels, along with distance values for the interactions.
Figure 7. Refolding of A-loop.
(a–d) Each subunit from P-II was individually aligned by least squares fitting with its corresponding subunit in H-II. Panels a–d show A-loop differences for subunits A,B,F, and G respectively. This pertains to the regions circled in the center of the capsomers as shown in figure 6. (e–g) H/2H exchange curves of three overlapping peptide fragments in the A-loop, shown for P-II (■), EI (○), and H-I (Δ) capsid states. (H) Mass envelopes after 10 minute deuterium incubations pertaining to the fragment spanning residues 288–306, shown for P-II, EI, and H-I.
The H/2H data show that the A-loops are more protected in EI than in P-II, and that there is little change in this protection throughout further maturation to the H-I state (Figure 7). The A-loop is much more accessible to deuterium exchange in the P-II state as seen in the curve for a peptide fragment spanning residues 288–306, revealing that nearly 15/17 exchangeable amide protons exchange deuterium within 10 minutes. This is in contrast to approximately 10 exchangeable amides in both the EI and H-I states. Comparison with an overlapping fragment, spanning residues 288–302, reveals that residues 303–306 are fully exchanged at the 1-minute time-point (a measure of fast exchange amides) for all expansion states. These residues lie towards the C-terminal region of the refolding segment of the A-loop. Residues 303–306 therefore remain highly solvent accessible throughout the refolding process, indicating that solvent protection is occurring between residues 288–302. Another overlapping fragment spanning residues 288–308 showed nearly identical exchange kinetics as residues 288–306 for all expansion states, demonstrating that residues 307 and 308 are well protected from exchange. This region directly follows the residues that refold, indicating that the high solvent accessibility is limited to the refolding region. The nearly identical exchange values between EI and H-I for the A-loop reveals that the refolding and quaternary reorganization in this region is occurring during the first expansion step, even though the particle is only about 60% through its expansion process. A previous EM map of EI showed the hexamers display 6-fold symmetry unlike their P-II counterparts15. The symmetrization at the A-loop interface during the first expansion stage therefore likely results in the formation of quaternary associations that remain preserved up to H-II, though the subunits undergo additional rotational motions during the late stages of expansion. The increased deuterium exchange accessibility in the P-II state as compared to the H-I state agree with the crystallographic data that showed a much more extensive interface between neighboring A-loops in the 6-fold symmetric mature hexamer when compared to the skewed P-II hexamer.
Spine Helix
The spine helix was previously characterized as bent and partially unfolded in the P-II state. The bending and quasi-equivalent differences in helix conformations for the different subunits can be seen in Figure 8. Unlike most of the changes in the B.S.A calculations shown in Figure 3, where the majority of the domains become more buried during expansion, residues 201–213 of the spine helix become less buried by 190–238 Å2 for the different subunits. The helix of each hexon subunit in P-II lies directly below the E-loop and part of the P-domain of its neighboring subunit (Figure 6C). There are multiple interactions including stacking of aromatic rings, a salt bridge, hydrogen bonds, and Van der Waals contacts that stabilize the bent conformation (Figure 6c and Suppl. movie 2). During expansion, the majority of quaternary interactions between the helix and the neighboring subunit are abrogated, except for a salt bridge between R210 of the helix and E153 of the E-loop (Figures 6C and 6F), that we conclude serves to guide the base of the E-loop into an orientation suitable for the crosslink to form during expansion in WT particles. Comparisons of the E-loop in the P-II and H-II conformations show a nearly 30 degree rotation due to the ordering of the E-loop.
Figure 8. Bending and dynamics of the spine helix.
(a–d) Each subunit from P-II has been individually aligned by least squares fitting with its corresponding subunit in H-II. Panels a–d show differences in spine helix conformation for subunits A,B,F, and G respectively. (E–G) H/2H exchange curves of three overlapping peptide fragments in the spine helix, shown for P-II (■), EI (○), and H-I (Δ) capsid states. (H) The table compares the number of amides that exchange at both fast and intermediate/slow rates for the overlapping helix peptides in the P-II state. The actual rate of fast exchange was approximated as 10 deuterons/minute, while the intermediate/slow rates were measured experimentally and are listed as well.
The ordering and conformational changes in the spine helix are particularly important in understanding the thermodynamic basis of expansion. The spine helix is more solvent exposed in P-II compared to the mature subunit conformation14, indicating that the helix is partially unfolded in P-II. The data presented here illustrates the dynamics of the helix, with overlapping peptide fragments resolving differences in exchange between different parts of the helix (Figure 8e–h). Comparisons between a fragment spanning 204–214 and another fragment spanning 206–214 allows direct measurement of the solvent accessibility of the overlapped regions, residues 205–206. These residues show little to no solvent accessibility at early time-points that measure fast exchange, with ~0 deuterons exchanged. At later time-points, that measure intermediate to slow exchange (~5–15 minutes), an additional deprotection of ~0.5 amides is seen for the two residues. A similar comparison can be made between the fragments spanning 206–214 and 206–216 to specifically resolve the protection of residues 215–216. There is an exchange of ~1 amide proton at early time-points (corresponding to the fast rate of exchange), indicating high solvent accessibility for these residues. These two comparisons reveal that the majority of rapidly exchanging amide protons in the spine helix of P-II (~ 2–3 amides), are localized in the region spanning residues 207 and 214, with an additional ~4 residues exchanging at the intermediate/slow rate. As identified in the crystal structure, these residues are situated in the region of the helix with the most pronounced bend, a region in which a break in secondary structure can be seen for some subunits as shown in Figure 8d. The H/2H data shows that the backbone hydrogen bonding is, in fact, compromised in the bent regions of the helix, unlike the mature subunit conformation which shows significantly increased solvent protection. Exchange curves for the helix in the EI and H-I states are nearly identical to each other, and show nearly two-thirds less solvent accessibility than P-II, indicating strong differences in backbone hydrogen bonding. This indicates that virtually all of the ordering in secondary structure for this region occurs during the initial expansion event from P-II to EI, a result similar to that seen for the A-loop.
Several of the peptides in the spine helix in P-II show either a wide or bimodal mass envelope, indicative of quasi-equivalent behavior with multiple extents of exchange. The peptide spanning residues 204–216 best illustrates this behavior. The bimodal mass envelope can be resolved into multiple Gaussians at early time-points. Figure 9 shows the two-gaussian fit of the mass envelope of the helix fragment following a 1-minute deuterium incubation. Both the centroid and percentage of overall envelope area were calculated for each of the gaussians as shown in the Table in Figure 9. After back exchange and side chain corrections, the more protected envelope showed that 0 amides exchanged for these subunits, while the larger, more exposed population exchanged nearly 7 deuterons. The fraction of the total area attributed to the first Gaussian was multiplied by the number of quasi-equivalent subunits, and is equal to 2.13 subunits. The fraction of the total area attributed to the second gaussian corresponds to 4.83 subunits. The result is nearly identical to the crystallographic conclusions, which indicate that 5 of the 7 subunits have bent helices while subunits A and D are nearly linear as in H-II. The bimodal envelope disappears after the first transition to EI, indicating that the spine helices in different subunits are nearly equivalent at the earliest stage of expansion.
Figure 9. Bimodal distribution of helix residues 204–216 indicates major differences in conformations between quasi-equivalent subunits.
(a) Mass envelopes of helix fragment in P-II state after 0, 1, 5, and 10 minutes of exchange. (b) Mass envelopes of the helix fragment after 1-minute of exchange for P-II, EI, and H-I expansion states. (c) Bimodal envelope from P-II 1min spectra is fitted to 2 gaussian distributions. (d) Table showing calculations from the bimodal gaussian fit in panel c. The centroids of the resolved gaussians are shown as well as the number of amide protons corresponding to each envelope (after 0 min subtraction and back exchange correction). Finally, the number of subunits attributed to each gaussian is calculated by dividing the area of each gaussian by the total area and multiplying the value by 7, which corresponds to the number of subunits in the viral asymmetric unit.
Disscussion
Bimodal distributions of solvent exchange seen in Figure 9 can be attributed to two possible mechanisms. The first, which is more commonly seen in protein unfolding studies and occasionally protein dynamics studies, involves the EX1 mechanism of exchange29,30,31. This exchange mechanism is characterized by the kinetic equation, kclose ≪ kint. The mechanism is predicated on the closing rate of a protein (kclose ) to be much slower than the intrinsic chemical exchange rate of amide protons (kint)32. This may be seen in an unfolded state of a protein, or may be caused by a local conformational opening, in which all of the amide protons of the peptide fragment have completely exchanged its protons before the closing event occurs. The result is a fully deuterated peptide fragment with no intermediate exchange profile during incremental exposures to deuterium. In many systems, the conformational opening event occurs for only a subpopulation of the protein by the time early measurements (seconds) are taken. The result is a bimodal distribution of a fully deuterated subpopulation that has undergone the opening event, and a solvent protected population that has yet to open. During longer time-points, the fully deuterated envelope grows larger with respect to the protected envelope, with no gaussian distribution indicative of an intermediately exchanged subpopulation. Macromolecular complexes that have been shown to exhibit such a transition include the scaffold for phage phi2933.
In the EX2 mechanism, characterized by the kinetic equation, kclose ≫ kint, the peptide region of interest is not fully deuterated after a single opening event, resulting in a mass envelope that progressively shifts towards higher masses during longer exposures to deuterium. By this mechanism, if all of the protein in the sample behaves uniformly, the envelope will generally have a unimodal appearance. In P-II it was noticed both in the crystal structure (Figure 8) and by H/2H exchange that the 7 quasi-equivalent subunits have different tertiary structures and quaternary structure environments. Head II, in contrast has nearly identical subunits that are virtually superimposable. The helices of the subunits that undergo the least amount of tertiary and secondary structure changes, A and D, have a straighter conformation, one that is similar to their H-II form. The other 5 subunits, however, show a large bend and twist in the helices that resolve to canonical helices during expansion. The bimodal distribution is therefore a result of variation between subunits. The definitive proof that the spine helix exchanges amide protons by an EX2 mechanism is seen by the way mass envelopes gradually shift to higher masses during incrementally longer exposure times (Figure 9a). Both the more solvent exposed and solvent protected parts of the mass envelope shift to the right, a scenario not possible if the behavior was EXI, characterized by an all or nothing exchange.
The data presented here demonstrate complicated changes in H/2H exchange dynamics that characterize P-II, an intermediately folded procapsid state of a dsDNA bacteriophage. The large differences in quasi-equivalent subunits are imposed during assembly, likely directed by the scaffolding region of the capsid subunit, termed the Δ-domain. Once the Δ-domain is cleaved off by protease in the initially assembled P–I state, a metastable particle form, P-II, is primed to undergo a large rearrangement of protein interactions, guided by tertiary and secondary structure changes. Data presented here revealed a partially unfolded helix maintained in 5 of the 7 subunits by extensive tertiary interactions between the helical residues and neighboring side chains. The B.S.A measurements from the crystal structures revealed that the helices in H-II have minimal interactions with neighboring residues, allowing the formation of equivalent, canonical helices. The loss of energy in disrupting the hydrogen bonds of the spine helix is counteracted by the increased quaternary interface present in P-II. We conclude that the quaternary interactions described for P-II state counterbalance the local high energy conformations of individual subunits, thereby stabilizing the pre-expanded state. Such a model explains the energy release associated with capsid maturation measured in phage such as P22 (90kJ/mol)34, and the sensitivity of P-II to undergo expansion, triggered by a variety of chemicals and pH changes in vitro.
The large differences in quasi-equivalent subunits in the immature state as well as significant differences in orientation and binding interfaces of the subunits revealed a less discriminate arrangement of proteins, compared to the near perfect symmetry seen in mature capsid forms of HK97 as well as all other mature icosahedral bacteriophage. The capsid of HK97 requires extensive flexibility in regions such as the A-loops, to maintain the quaternary arrangement initially present in the P-II, providing an inherent molecular switch capable of guiding the molecular rearrangements. Regions of the A-loops that show increased dynamics in P-II are in fact the regions that also undergo refolding, facilitating a more extensive interface within capsomers that adds to the overall increased stability of the mature capsid states. The expansion mechanism is not coupled to any chemical energy sources such as NTP’s. It requires only the packaging of a fraction of the DNA to trigger the event35,36,37, hypothesized to be a result of electrostatic repulsion with the negatively charged DNA7. Such a trigger likely provides enough energy to force the particle out of a local energy minimum, transitioning downhill to the more favorable, symmetric capsid forms. Interestingly, the study has shown that the major refolding and structural rearrangements occur during the first transition to the EI state, implicating the symmetrization of the hexamers as the basis for the increased and more uniform solvent protection values. Symmetry in protein complexes has long been postulated as a basis for increased protein stability38. The breakdown in symmetry and extensive differences between subunits in P-II as presented here, explains the meta-stability of the procapid state and its trajectory towards symmetrization in order to attain increased stability. Once the EI state is attained, it is the crosslinking and/or E-loop conformational changes that further alter the energy landscape, leading to additional expansion through a Brownian ratchet mechanism15. The N-arm was shown to be highly dynamic until reaching the late expansion states, indicating its secondary structure formation is not crucial to the major subunit conformational changes, but acts as final quaternary clamp, adding to the overall highly intercalated topology of the capsid lattice.
Bacteriophage with related folds such as P22, T4, Phi29, T7, and Lambda2,39,40,41,42, probably share extensive differences in structure among quasi-equivalent subunits in the procapsid state, as documented for HK97. H/2H exchange studies of P22 revealed increased solvent dynamics in the procapsid particle compared to the mature capsid form, but bimodal data illustrating major differences between subunits was not reported24. Since a high-resolution crystal structure of P22 and other dsDNA phage have yet to be determined, attributing such data to specific domains is difficult. All of the dsDNA phage studied have a fold homologous to HK97, and therefore also have a spine helix in their structure. We believe that it is likely that they also exhibit a partially unfolded helix in their procapsid forms, a possible mechanism for energy storage and exothermic expansion in these systems.
Methods and Materials
Protein Preparation and Crystallization
Crystals were grown from a W336F/Δ-E-loop truncation mutant of P-II. Site directed mutagenesis was used to introduce the W336F mutation, a construct that is slower to expand than WT and therefore provides a more homogeneous population of P-II particles, necessary for crystallization. A 5.2 Å resolution structure was previously solved using the W336F construct, which identified the E-loop as a dynamic region that may prevent efficient crystal packing. Unlike its role in the H-II state, the E-loop makes no quaternary contacts with neighboring subunits, and therefore made this region a good candidate for truncation. Splicing by overlap extension was performed on the W336F construct, replacing residues 159–171 with residues APGD, a sequence known to promote formation of a reverse turn 43. The result was a truncated loop that was fully visible in the electron density of the crystal structure. Expression of gp5 capsid protein and gp4 protease was completed in an E.coli T7 expression system and purification of HK97 Prohead II was performed as previously described10. The mutant assembles into an intact capsid with similar efficiency as wild type and is able to expand, though at a slower rate than WT.
The mother liquor for crystallization contained 0.1 M CHES buffer, pH 9.0, 200 mM manganese chloride and 2.3–3.0% Peg 4000. Upon mixing the manganese chloride with CHES buffer and Peg, much of the manganese chloride precipitated. The precipitant was allowed to settle overnight, and only the supernatant was used for crystallization. To 4ul of mother liquor, 1 ul of 2M NDSB-211 (additive from Hampton Research) was added. 1ul of P-II protein (25mg/ml) was added to 1ul of this mixture and allowed to crystallize by hanging drop and vapor diffusion.
Crystallographic processing
An atomic model for the P-II structure was initially derived by rigid-body fitting of the refined 3.44 Å structure of the mature H-II coordinates (Protein Data Bank 1OHG) into the prohead II electron density. The initial phases for molecular replacement were derived from a previously solved 12 Å cryo-electron microscopy structure of P-II7. Crystallographic data was collected at the Advanced Photon Source synchrotron at Argonne National Laboratories, beamlines 23-IDD and 14-BMC. The Room-temperature diffraction data from twenty nine crystals was indexed, integrated, and scaled using HKL2000 suite44. The crystals belong to the space group, I222. Details of Indexing, scaling, structure determination, and refinement were performed as previously described14.
Buried surface area calculations were performed on the Viper database, using crystallographic coordinates of P-II and H-II 25,26. Bending angles calculated between the hinging N-arm and E-loop domains were calculated by deconvoluting matrices needed to align coordinates representing the refined P-II structure with that of H-II structure fit into the P-II map. The two particle states were initially aligned by least squares fitting of residues 230–383. These residues represent the subunit core which mainly remains identical in conformation, except for local refolding in the A-loop. R.M.S. deviations of the alignment ranged from 1.1Å to 1.3Å.
H/2H exchange
K169Y P-II and H-I particles were expressed and purified as described previously. Expansion states were determined prior to H/2H exchange using agarose gel electrophoresis and intrinsic fluorescence measurements. Evaluation of HK97 particle states by these methods have been previously described10,46. EI particles were formed after treating P-II particles with 10% isobutanol for 15 minutes. Isobtuanol is one of many conditions that triggers in vitro particle expansion, but was used for this study due to its compatibility with MALDI mass spectrometry. Concentrated K169Y HK97 protein (20–40mg/ml) was diluted 7-fold in an 85% final D2O concentration, buffered with 20mM tris pH 7.5, and containing either 200mM sodium chloride or 200mM potassium chloride. Samples were incubated in D20 at pH 7.5 for various time periods followed by addition of pH 2.5 non-deuterated quench solution (final D2O concentration of 9.0%). Following quench, all samples were handled on ice in a 4°C. Protein was digested with 50ul pepsin coated beads for 5min. The beads were removed by centrifugation, and the supernatant was flash frozen in liquid N2. Samples were thawed individually, mixed 1:1 with alpha C matrix solution and vacuum crystallized on a Maldi plate, followed by analysis on Maldi-TOF Mass spectrometer45. Back exchange was calculated as 42% using the N-terminal peptide (residues 117–126), which exchanged amide protons for deuterium completely within 20 seconds. The sodium adduct of this peptide was present in much greater abundance in the spectra. All future H/2H measurements of this fragment were therefore performed on the adducted species. The total number of deuterons exchanged was calculated by subtracting the centroid of the mass envelope from the non-deuterated control from the centroid of each deuterated mass envelope. The error (standard deviation) was estimated from the average of 3 independent experiments with 2–3 measurements recorded for each experiment (Total of 6–9 measurements for each timepoint). A more detailed description of H/2H exchange protocol, data analysis and resulting kinetic plots were followed as previously described21,45.
H/2H exchange reactions were performed for up to 15 minutes, enabling measurement of amide protons exchanging at both a fast (> 1 min−1) and intermediate rate (0.01 to 1 min−1)24. Longer incubations measuring slow exchange rates (< 0.01 min−1) were not done in this study. Amide protons exchanging at intermediate to slow rates are generally a result of solvent protection due to either secondary structure or protein-protein interactions. The measured data for all fragments was best fit to either a single or two-exponential model accounting for deuterons exchanging at only a fast rate, or both a fast and intermediate rate respectively. The following equation represents the two-exponential fit:
where D is the total number of deuterons exchanged at time t, Nfast is the number of deuterons exchanging at a fast rate, kfast, and Ninter is the number of deuterons exchanging at an intermediate rate, kinter. The fast exchanging amide protons had nearly all exchanged by the first timepoint, so kfast was estimated as described previously21. The mass envelope of helix fragment spanning residues 204–216 could be resolved as a combination of two gaussians at early timepoints. The fitting to multiple guassians as seen in figure 7 was performed in the software package, Origin. All fragments were identified with tandem mass spectrometry (MS/MS) using a Q-STAR mass spectrometer or a MALDI TOF-TOF (ABI 4800).
Supplementary Material
H/2H exchange curves of P-domain and A-domain peptide fragments. Curves are shown for various peptide fragments in the aforementioned domains that are also color coded on the subunits in figure 4. Data is shown for P-II (■), EI (○) , and H-I (Δ) capsid states.
Quasi-equivalence in P-II hexon. The movie depicts the organization of subunits within a P-II hexon, as well as their change in conformation as compared to the H-II state. The transition of the hexon between the two expansion states was done as a morph in the program Chimera, using the coordinates for the P-II and H-II crystal structures. The movie focuses on the differences in orientation for two subunits, specifically D (salmon color), and E (white). Subunit D assumes a more flattened orientation and is recessed closer to the center of the hexon than E. In order to attain the near equivalent subunit conformations as seen in H-II, subunit E undergoes a much greater rotational motion as compared to subunit D, and the interface between the two subunits also undergoes a significant change.
Quaternary interface of the spine helix. The movie consists of a morph between P-II and H-II coordinates, also created in Chimera. The movie depicts the major quaternary interactions that stabilize the bent helix structure in P-II for subunits F (blue), and A (yellow). These include hydrogen bonds, aromatic ring stacking interactions, a salt bridge, and Van der Waals contacts. The morph to H-II shows that nearly all of these quaternary contacts are abrogated during maturation, except for a single salt bridge between R210 and E153, a contact likely responsible for the quaternary positioning of the E-loop during maturation. The extensive quaternary interface at the spine helix in P-II illustrates how the metastable particle is stabilized in its bent conformation.
Acknowledgements
We thank Brian Firek and Crystal Moyer for mutagenesis of the HK97 constructs used in the study. We thank Chi-Yu Fu with help in processing H/2H exchange data. We thank Lu Gan and Jeff Speir for assistance with crystallographic studies. We also thank Blair Szymczyma for material used in the study. We thank Roger Hendrix, Bob Duda, Rick Huang, and Kelly Lee for helpful discussions. We thank the staffs at beamlines 14-BMC and 23-ID-D of the Advanced Photon Source for assistance in data collection. This work was supported by NIH Grants RO1 AI40101 and NIH Training Grant GM08326.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dokland T, Murialdo H. Structural transitions during maturation of bacteriophage lambda capsids. J Mol Biol. 1993;233:682–694. doi: 10.1006/jmbi.1993.1545. [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Li Z, Zhang Z, Baker ML, Prevelige PE, Chiu W. Protein Fold and Maturation of Bacteriophage P22 seen at Subnanometer Resolutions. Nature Structural Biology. 2003;10:131–135. doi: 10.1038/nsb891. [DOI] [PubMed] [Google Scholar]
- 3.Steven AC, Spear PG. The herpes simplex virus procapsid; structure, conformational changes upon maturation, and roles of the triplex proteins VP19c and VP23 in assembly. Journal of molecular biology. 1996;263:447–462. doi: 10.1016/s0022-2836(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 4.Heymann JB, Cheng N, Newcomb WW, Trus BL, Brown JC, Steven AC. Dynamics of Herpes Simplex Virus Maturation Visualized by Time-lapse Cryo Electron Microscopy. Nature Structural Biology. 2003;10:334–341. doi: 10.1038/nsb922. [DOI] [PubMed] [Google Scholar]
- 5.D’Halluin JCM. Adenovirus type 2 assembly analysed by reversible cross-linking labile intermediates. Journal of Virology. 1978;26:357–363. doi: 10.1128/jvi.26.2.357-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steven AC, Heymann JB, Cheng N, Trus BL, Conway JF. Virus maturation: dynamics and mechanism of a stabilizing structural transition that leads to infectivity. Curr Opin Struct Biol. 2005;15:227–236. doi: 10.1016/j.sbi.2005.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conway JF, Wikoff WR, Cheng N, Duda RL, Hendrix RW, Johnson JE, Steven AC. Virus maturation involving large subunit rotations and local refolding. Science. 2001;292:744–748. doi: 10.1126/science.1058069. [DOI] [PubMed] [Google Scholar]
- 8.Xie Z, Hendrix RW. Assembly in vitro of bacteriophage HK97 proheads. J Mol Biol. 1995;253:74–85. doi: 10.1006/jmbi.1995.0537. [DOI] [PubMed] [Google Scholar]
- 9.Duda RL, Martincic K, Hendrix RW. Genetic basis of bacteriophage HK97 prohead assembly. J Mol Biol. 1995;247:636–647. doi: 10.1006/jmbi.1994.0169. [DOI] [PubMed] [Google Scholar]
- 10.Duda RL, Hempel J, Michel H, Shabanowitz J, Hunt D, Hendrix RW. Structural transitions during bacteriophage HK97 head assembly. J Mol Biol. 1995;247:618–635. doi: 10.1006/jmbi.1995.0168. [DOI] [PubMed] [Google Scholar]
- 11.Duda RL. Protein chainmail: catenated protein in viral capsids. Cell. 1998;94:55–60. doi: 10.1016/s0092-8674(00)81221-0. [DOI] [PubMed] [Google Scholar]
- 12.Wikoff WR, Liljas L, Duda RL, Tsuruta H, Hendrix RW, Johnson JE. Topologically linked protein rings in the bacteriophage HK97 capsid. Science. 2000;289:2129–2133. doi: 10.1126/science.289.5487.2129. [DOI] [PubMed] [Google Scholar]
- 13.Lee KK, Tsuruta H, Hendrix RW, Duda RL, Johnson JE. Cooperative reorganization of a 420 subunit virus capsid. J Mol Biol. 2005;352:723–735. doi: 10.1016/j.jmb.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 14.Gertsman I, Gan L, Guttman M, Lee K, Speir JA, Duda RL, Hendrix RW, Komives EA, Johnson JE. An unexpected twist in viral capsid maturation. Nature. 2009;458:646–650. doi: 10.1038/nature07686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KK, Gan L, Tsuruta H, Moyer C, Conway JF, Duda RL, Hendrix RW, Steven AC, Johnson JE. Virus capsid expansion driven by the capture of mobile surface loops. Structure. 2008;16:1491–1502. doi: 10.1016/j.str.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ross PD, Cheng N, Conway JF, Firek BA, Hendrix RW, Duda RL, Steven AC. Crosslinking renders bacteriophage HK97 capsid maturation irreversible and effects an essential stabilization. Embo J. 2005;24:1352–1363. doi: 10.1038/sj.emboj.7600613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steven AC, Greenstone H, Bauer AC, Williams RW. The maturation-dependent conformational change of the major capsid protein of bacteriophage T4 involves a substantial change in secondary structure. Biochemistry. 1990;29:5556–5561. doi: 10.1021/bi00475a020. [DOI] [PubMed] [Google Scholar]
- 18.Tuma R, Prevelige PE, Jr, Thomas GJ., Jr Mechanism of capsid maturation in a double-stranded DNA virus. Proc Natl Acad Sci U S A. 1998;95:9885–9890. doi: 10.1073/pnas.95.17.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helgstrand C, Wikoff WR, Duda RL, Hendrix RW, Johnson JE, Liljas L. The refined structure of a protein catenane: the HK97 bacteriophage capsid at 3.44 A resolution. J Mol Biol. 2003;334:885–899. doi: 10.1016/j.jmb.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 20.Croy CH, Bergqvist S, Huxford T, Ghosh G, Komives EA. Biophysical characterization of the free IkappaBalpha ankyrin repeat domain in solution. Protein Sci. 2004;13:1767–1777. doi: 10.1110/ps.04731004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandell JG, Baerga-Ortiz A, Akashi S, Takio K, Komives EA. Solvent accessibility of the thrombin-thrombomodulin interface. J Mol Biol. 2001;306:575–589. doi: 10.1006/jmbi.2000.4416. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Smith DL. Capsid structure and dynamics of a human rhinovirus probed by hydrogen exchange mass spectrometry. Protein Sci. 2005;14:1661–1672. doi: 10.1110/ps.051390405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanman J, Lam TT, Emmett MR, Marshall AG, Sakalian M, Prevelige PE., Jr Key interactions in HIV-1 maturation identified by hydrogen-deuterium exchange. Nat Struct Mol Biol. 2004;11:676–677. doi: 10.1038/nsmb790. [DOI] [PubMed] [Google Scholar]
- 24.Kang SaP, P E., Jr Domain Study of Bacteriophage P22 Coat Protein and characterization of the capsid lattice transformation by Hydrogen/Deuterium Exchange. Journal of molecular biology. 2005;347:935–948. doi: 10.1016/j.jmb.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd CM, Borelli IA, Lander G, Natarajan P, Siddavanahalli V, Bajaj C, Johnson JE, Brooks CL, Reddy VS., 3rd VIPERdb: a relational database for structural virology. Nucleic Acids Res. 2006;34:D386–D389. doi: 10.1093/nar/gkj032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Godzik A, Kolinski A, Skolnick J. Topology fingerprint approach to the inverse protein folding problem. J Mol Biol. 1992;227:227–238. doi: 10.1016/0022-2836(92)90693-e. [DOI] [PubMed] [Google Scholar]
- 27.Gan L, Speir JA, Conway JF, Lander G, Cheng N, Firek BA, Hendrix RW, Duda RL, Liljas L, Johnson JE. Capsid conformational sampling in HK97 maturation visualized by X-ray crystallography and cryo-EM. Structure. 2006;14:1655–1665. doi: 10.1016/j.str.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Szymczyna BR, Gan L, Johnson JE, Williamson JR. Solution NMR studies of the maturation intermediates of a 13 MDa viral capsid. J Am Chem Soc. 2007;129:7867–7876. doi: 10.1021/ja071118j. [DOI] [PubMed] [Google Scholar]
- 29.Deng Y, Smith DL. Rate and equilibrium constants for protein unfolding and refolding determined by hydrogen exchange-mass spectrometry. Anal Biochem. 1999;276:150–160. doi: 10.1006/abio.1999.4347. [DOI] [PubMed] [Google Scholar]
- 30.Konermann L, Simmons DA. Protein-folding kinetics and mechanisms studied by pulse-labeling and mass spectrometry. Mass Spectrom Rev. 2003;22:1–26. doi: 10.1002/mas.10044. [DOI] [PubMed] [Google Scholar]
- 31.Weis DD, Wales TE, Engen JR, Hotchko M, Ten Eyck LF. Identification and characterization of EX1 kinetics in H/D exchange mass spectrometry by peak width analysis. J Am Soc Mass Spectrom. 2006;17:1498–1509. doi: 10.1016/j.jasms.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 32.Englander SW, Sosnick TR, Englander JJ, Mayne L. Mechanisms and uses of hydrogen exchange. Curr Opin Struct Biol. 1996;6:18–23. doi: 10.1016/s0959-440x(96)80090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fu CY, Prevelige PE., Jr Dynamic motions of free and bound O29 scaffolding protein identified by hydrogen deuterium exchange mass spectrometry. Protein Sci. 2006;15:731–743. doi: 10.1110/ps.051921606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galisteo ML, King J. Conformational transformations in the protein lattice of phage P22 procapsids. Biophys J. 1993;65:227–235. doi: 10.1016/S0006-3495(93)81073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjornsti MA, Reilly BE, Anderson DL. Morphogenesis of bacteriophage phi 29 of Bacillus subtilis: oriented and quantized in vitro packaging of DNA protein gp3. J Virol. 1983;45:383–396. doi: 10.1128/jvi.45.1.383-396.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jardine PJ, McCormick MC, Lutze-Wallace C, Coombs DH. The bacteriophage T4 DNA packaging apparatus targets the unexpanded prohead. J Mol Biol. 1998;284:647–659. doi: 10.1006/jmbi.1998.2178. [DOI] [PubMed] [Google Scholar]
- 37.Hohn B. DNA sequences necessary for packaging of bacteriophage lambda DNA. Proc Natl Acad Sci U S A. 1983;80:7456–7460. doi: 10.1073/pnas.80.24.7456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blundell TL, Srinivasan N. Symmetry, stability, and dynamics of multidomain and multicomponent protein systems. Proc Natl Acad Sci U S A. 1996;93:14243–14248. doi: 10.1073/pnas.93.25.14243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fokine A, Leiman PG, Shneider MM, Ahvazi B, Boeshans KM, Steven AC, Black LW, Mesyanzhinov VV, Rossmann MG. Structural and functional similarities between the capsid proteins of bacteriophages T4 and HK97 point to a common ancestry. Proc Natl Acad Sci U S A. 2005;102:7163–7168. doi: 10.1073/pnas.0502164102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morais MC, Choi KH, Koti JS, Chipman PR, Anderson DL, Rossmann MG. Conservation of the capsid structure in tailed dsDNA bacteriophages: the pseudoatomic structure of phi29. Mol Cell. 2005;18:149–159. doi: 10.1016/j.molcel.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 41.Agirrezabala X, Velazquez-Muriel JA, Gomez-Puertas P, Scheres SH, Carazo JM, Carrascosa JL. Quasi-atomic model of bacteriophage t7 procapsid shell: insights into the structure and evolution of a basic fold. Structure. 2007;15:461–472. doi: 10.1016/j.str.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Lander GC, Evilevitch A, Jeembaeva M, Potter CS, Carragher B, Johnson JE. Bacteriophage lambda stabilization by auxiliary protein gpD: timing, location, and mechanism of attachment determined by cryo-EM. Structure. 2008;16:1399–1406. doi: 10.1016/j.str.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dyson HJ, Bolinger L, Feher VA, Osterhout JJ, Jr, Yao J, Wright PE. Sequence requirements for stabilization of a peptide reverse turn in water solution--proline is not essential for stability. Eur J Biochem. 1998;255:462–471. doi: 10.1046/j.1432-1327.1998.2550462.x. [DOI] [PubMed] [Google Scholar]
- 44.Otwiniowski Z, Minor W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods in Enzymology. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 45.Mandell JG, Falick AM, Komives EA. Measurement of amide hydrogen exchange by MALDI-TOF mass spectrometry. Anal Chem. 1998;70:3987–3995. doi: 10.1021/ac980553g. [DOI] [PubMed] [Google Scholar]
- 46.Lee KK, Gan L, Tsuruta H, Hendrix RW, Duda RL, Johnson JE. Evidence that a local refolding event triggers maturation of HK97 bacteriophage capsid. J Mol Biol. 2004;340:419–433. doi: 10.1016/j.jmb.2004.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
H/2H exchange curves of P-domain and A-domain peptide fragments. Curves are shown for various peptide fragments in the aforementioned domains that are also color coded on the subunits in figure 4. Data is shown for P-II (■), EI (○) , and H-I (Δ) capsid states.
Quasi-equivalence in P-II hexon. The movie depicts the organization of subunits within a P-II hexon, as well as their change in conformation as compared to the H-II state. The transition of the hexon between the two expansion states was done as a morph in the program Chimera, using the coordinates for the P-II and H-II crystal structures. The movie focuses on the differences in orientation for two subunits, specifically D (salmon color), and E (white). Subunit D assumes a more flattened orientation and is recessed closer to the center of the hexon than E. In order to attain the near equivalent subunit conformations as seen in H-II, subunit E undergoes a much greater rotational motion as compared to subunit D, and the interface between the two subunits also undergoes a significant change.
Quaternary interface of the spine helix. The movie consists of a morph between P-II and H-II coordinates, also created in Chimera. The movie depicts the major quaternary interactions that stabilize the bent helix structure in P-II for subunits F (blue), and A (yellow). These include hydrogen bonds, aromatic ring stacking interactions, a salt bridge, and Van der Waals contacts. The morph to H-II shows that nearly all of these quaternary contacts are abrogated during maturation, except for a single salt bridge between R210 and E153, a contact likely responsible for the quaternary positioning of the E-loop during maturation. The extensive quaternary interface at the spine helix in P-II illustrates how the metastable particle is stabilized in its bent conformation.