Abstract
The hippocampus has been hypothesized to function as a “spatial” or “cognitive” map, however, the functional cellular organization of the spatial map remains a mystery. The majority of electrophysiological studies, thus far, have supported the view of a random-type organization in the hippocampus. However, using immediate early genes (IEGs) as an indicator of neuronal activity, we recently observed a cluster-type organization of hippocampal principal cells, whereby a small number (~4) of nearby cells were activated in animals exposed to a restricted part of an environment. To determine the fine structure of these clusters and to provide a 3D image of active hippocampal cells that encode for different parts of an environment, we established a functional mapping of immediate early genes (IEGs) zif268 and Homer1a, using in situ hybridization and 3D-reconstruction imaging methods. We found that, in animals exposed to the same location twice, there were significantly more double IEG-expressing cells, and the clusters of nearby cells were more “tightly” formed, in comparison to animals exposed to two different locations. We propose that spatial encoding recruits specific cell ensembles in the hippocampus and that with repeated exposure to the same place the ensembles become better organized to more accurately represent the “spatial map”.
Keywords: place cells, functional organization, cell assemblies, nearest neighbor distance, zif268, Homer 1a
INTRODUCTION
Based on the discovery of “place cells” (O’Keefe and Dostrovsky, 1971), one of the functions of the hippocampus is proposed to be that of a “spatial” or “cognitive” map (O’Keefe and Nadel, 1978). The functional cellular organization of place cells, within the hippocampus, however, remains a mystery. Part of the problem in discerning a functional organization could be that single unit recordings are limited to a few neurons at a time which could account for the lack of a correlation between topographical and spatial organization. Perhaps due to this limitation, the majority of studies have reported little or no correlation between neighboring neurons and adjacent place fields. If anything, adjacent place cells could have very disparate place fields, and further, there is very little predictability between place fields in different environments (Muller and Kubie, 1987, 1989; Thompson and Best, 1989; Wilson and McNaughton, 1993; Tanila et al., 1997; Knierim et al., 1998). Thus, the consensus view, to date, is that the “spatial map” is randomly arranged, i.e., there is no topographic organization of place cells.
A handful of electrophysiological studies, however, have hinted at a cluster-type functional organization in the hippocampus. For example, Eichenbaum et al. (1989) reported that place cells in the CA1 field formed clusters, whereby, neighboring cells had adjacent and overlapping place fields. Furthermore, by recording from multiple cells with an array of electrodes in the CA1 and CA3, Hampson, Deadwyler and associates found that place cells encoding for space were concentrated in clusters, spaced approximately 200-400μm apart (Deadwyler et al., 1996; Hampson et al., 1996). Interestingly, a discrete cluster-type organization was also observed in animals performing a spatial task (Hampson et al., 1999, 2002). All of the above mentioned studies, however, are limited in the ability to determine possible cluster-type organization in the hippocampus due to the small number of simultaneously recorded cells. A more representative organization, therefore, could only be achieved through the use of different methods which allow the sampling of the entire neuronal population.
Immediate-early genes (IEGs) have been identified as critical indicators of neuronal activity (Worley et al., 1993; Clayton, 2000). In hippocampal pyramidal neurons, spatial environmental stimuli rapidly and transiently induce expression of IEGs, such as zif268 (also known as Egr1 and NGFI-A; Cole et al., 1989; for reviews, see Davis et al., 2003; Knapska and Kaczmarek, 2004), Arc (Link et al., 1995; Lyford et al., 1995; for a review, see Guzowski, 2002), and Homer1a (Brakeman et al., 1997; Kato et al., 1998; for a review, see de Bartolomeis and Iasevoli, 2003). In situ hybridization (ISH) and immunohistochemical techniques have detected context-specific cell ensemble activity in the hippocampus and neocortex at the single cell level (Chaudhuri et al., 1997; Guzowski et al., 1999; Vanzdarjanova et al., 2002). We have recently found that in animals exposed to a restricted part of an environment, zif268-immunoreactive cells formed clusters of a few (3-5) active cells adjacent to clusters of non-active cells in the CA1 and CA3 (Pavlides C, Donishi T, Ribeiro S, Mello C, Ogawa S, unpublished observation). A critical question that arises then is how this “spatial map” is formed in the hippocampus - does a cluster of neighboring neurons encode for each spatial component, or does the same cluster of cells become engaged in many different components of an environment, or a combination of both? The present study was aimed at investigating the precise configuration of cells within the IEG clusters, and determining how different clusters may participate in different parts of an environment. We have investigated patterns of active nearby CA1 neurons in animals exploring spatial environments, using ISH with zif268 and Homer1a mRNAs and three dimensional (3D)-reconstruction imaging methods.
EXPERIMENTAL PROCEDURES
Subjects and Behavioral Manipulations
All procedures performed on animals were in accordance with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised 1996) and were approved by The Rockefeller University Animal Care and Use Committee. Adult male Sprague-Dawley rats (275-300g on arrival; Charles River laboratories, Wilmington, MA) were housed with food and water available ad libitum, under a 12/12h light/dark cycle in a temperature-controlled (22°C) facility. Each animal was handled for 10 min daily for 3-4 weeks before the experiment. A radial eight-arm maze with only two arms attached was placed in a black painted room approximately 2.5 m2 in diameter (Fig.1A). Visual cues were placed distally to each of the arms. Proximal to one arm there was a single cue while proximal to the other arm there were several cues. The size of the animal’s location on both arms was set to 10 × 30 cm2 because place fields have been estimated to be on the average 20-25 cm region of space (Maurer et al., 2006; estimated from Wilson and McNaughton, 1993). Since place fields form rather quickly but are stabilized with repeated exposure (Kubie and Muller, 1991; Best and White, 1999), on two consecutive days before the experiments, each animal was exposed to both of the arms for 15 min each, and then returned to their home cage, which was located in a dark isolation chamber within the test room.
Fig. 1. Behavioral setup and experimental paradigm.
A: Environment. Behavioral testing was performed in a black painted room approximately 2.5m2 in diameter. An elevated 8-arm maze, with only two arms attached, was placed in the center of the room. The arms, which were 60-cm long, were sectioned off with a barrier in the middle of the arm and the animals were placed on Locations A or B (size: 10 × 30 cm2 each) on the end of the arms. Distal to each arm were a number of cues. The room was always illuminated with red light. During place exposure of an animal, a white light was turned on. Included in the room was the isolation chamber in which the animals were kept. B: Qualitative time course of peak expression of Homer1a and zif268 mRNAs. The differential time course of these two genes allows for identification of neuronal activity of the two exposures. C: Experimental Paradigm. Three experimental groups of animals were used – non-exposed controls (CON), exposed to two different locations once (DL), or exposed to the same location twice (SL). All animals were habituated to the arms for 15 min on two consecutive days. They were then returned to their home cage and placed in an isolation chamber for 48 hr. On the day of the experiment, they were exposed to the arms as indicated and 30 min after the initial exposure were anesthetized and sacrificed.
Peak expression of zif268 and Homer1a mRNAs (Fig.1B) has been reported to be at approximately 5min and 30min, respectively (Guzowski et al., 1999; Bottai et al., 2002; Vanzdarjanova et al., 2002). Thus, for labeling cells in two environments using the two genes, we divided animals into the following three experimental groups (Fig.1C): (i) two different locations exposure (DL, n=6), in which each animal was exposed on one arm for 5 min, returned to its home cage in the dark for 20 min and exposed on the other arm for 5 min; (ii) the same location exposure twice (SL, n=7), in which each animal was exposed on one arm for 5 min, returned to its home cage in the dark for 20 min and exposed on the same arm for 5 min; and (iii) the home-cage control group (CON, n=5), in which each animal was kept in its home cage in the dark. Immediately following the second exposure, animals were anesthetized with Ketamine (120 mg/kg)/ Xylazine (12 mg/kg) under red light, and then decapitated. In addition, to conform the time course of peak expression of zif268 and Homer1a, we performed exploratory studies with different groups of animals: (i) exposed for 5min (5min-group, n=2); (ii) exposed for 5 min and kept in dark for 15min (20min-group, n=2); and (iii) exposed for 5min and kept in dark for 25min (30min-group, n=2). At the end of each time period, the animals were sacrificed, the brains were rapidly removed and frozen in isopentane at −30°C and then in powdered dry ice, and cut at 12-μm-thick coronal sections on a cryostat (Jung Frigocut 2800N, Leica Microsystems, Bannockburn, IL) at −15°C. Sections were individually collected on a gelatin-coated slide and stored at −80°C before in situ hybridization. All efforts were made to minimize the number of animals used and their suffering.
Template and riboprobe preparation
Riboprobes were generated by RT-PCR. For the zif268 DNA template, PCR primers (Integrated DNA Technologies, Coralville, IA) were designed to amplify a fragment from bases 627-1266 of the rat zif268 cDNA. For rat Homer1a DNA template, PCR primers were designed from the 1.3kb sequence in the 3’-UTR region in the rat Homer1a sequence, modeled as previously described (Brakeman et al., 1997; Vazdarjanova et al., 2002, Vazdarjanva and Guzowski, 2004). Non-isotopic riboprobes were synthesized with digoxigenin-labeled UTP (Roche Diagnostics, Indianapolis, IN), and with a fluorescein-labeled UTP (Roche Diagnositcs) mixture (Ishii et al., 2004). Isotopic riboprobes were synthesized with [35S]-labeled UTP (PerkinElmer, Boston, MA) mixture (Ambion, Austin, TX) (Nakamura and McEwen, 2005).
In situ hybridization double-labeling with DAB and silver-grains
ISH double-labeling with DAB and silver-grains was performed as described previously (Stone et al., 1999; Yang et al., 1999; Nakamura and McEwen, 2005) with modifications. Fresh-frozen sections were processed at the same time with two riboprobes using identical conditions. In brief, sections were fixed with 4% formaldehyde in sterile 1X PBS. Sections were rinsed in PBS, incubated with proteinase K in digestion buffer (50mM Tris-HCl pH 7.4, 5mM EDTA), and acetylated with 0.25% acetic anhydride in 0.1M triethanolamine-HCl. Sections were rinsed in sterile 2X standard saline citrate (SSC), dehydrated with increasing ethanol concentrations, delipidated with chloroform, and allowed to air dry.
Hybridization solution (50% formamide, 600mM NaCl, 10mM Tris-HCl pH 7.4, 1X Denhardt’s solution, 1mM EDTA, 10mM DTT, 10% dextran sulfate, 100μg/ml denatured salmon sperm DNA, and 0.1ng/μl of digoxigenin-labeled Homer1a probes and 1.2 × 107 c.p.m. of [35S]-labeled zif268 probes), was applied to each slide (150μl), and the sections were coverslipped and incubated in a humidified environment at 68°C for 17h.
Following the hybridization, the coverslips were removed and the sections were rinsed in 50% formamide and 2X SSC at 64°C and in 2X SSC alone. Sections were incubated with 20 μg/ml RNase A in digestion buffer (500mM NaCl, 10mM Tris-HCl Ph 7.4, 1mM EDTA) at 37°C, and rinsed in 0.5X SSC, 0.25X SSC, and TN buffer (0.1 M Tris-HCl pH 7.4, 0.15 M NaCl) at room temperature. Sections were incubated with 3% H2O2 in TN buffer and incubated with 3% bovine serum albumin (BSA) in TNT buffer (0.1 M Tris-HCl pH 7.4, 0.15 M NaCl, 0.05% Tween 20) at room temperature, and then incubated with anti-digxigenin-POD antisera (1/500 dilution, Roche Diagnostics) in BSA/TNT at 4°C for 16h. Sections were rinsed in TNT, and incubated using the Tyramide Signal Amplification (TSA) biotin system (PerkinElmer) at room temperature for 10 min. Sections were incubated with streptavidin-horseradish peroxidase (PerkinElmer) for 1h, reacted with 0.02% 3,3’-diaminobenzidine (DAB, Sigma, St. Louis, MO) and 0.006% H2O2 in 0.05M Tris-HCl for 15 min, dehydrated in increasing ethanol, and allowed to air dry. Dried sections were dipped in Kodak NTB emulsion (Kodak, Rochester, NY) and stored at 4°C. Emulsion slides were developed with Kodak D-19 developer (Kodak) at 15°C, air-dried, counterstained with 0.2% Cresyl Violet, dehydrated using graded concentrations of ethanol and xylene, and coverslipped.
In situ hybridization double-labeling with two-color fluorescence
ISH double-labeling with two-color fluorescence was performed with fresh-frozen sections as described previously (Ishii et al., 2004) with modifications. In brief, hybridization solution, consisting of 50% formamide, 600mM NaCl, 10mM Tris-HCl pH 7.4, 1X Denhardt’s solution, 1mM EDTA, 10% dextran sulfate, 100μg/ml denatured salmon sperm DNA, 0.1ng/μl of digoxigenin-labeled Homer1a probes and 0.1ng/μl of fluorescein-labeled zif268 probes, was applied, and sections were coverslipped and incubated in a humidified environment at 68°C for 17 hr. After stringent hybridization washes, sections were incubated in BSA/TNT at room temperature, and then incubated with both anti-digoxigenin-AP antisera (1/500 dilution, Roche Diagnostics) and anti-fluorescein-POD antisera (1/100 dilution, Roche Diagnostics) in BSA/TNT at 4°C for 16 hr. Sections were rinsed in TNT, incubated using TSA biotin system (PerkinElmer), and then incubated with streptavidin Alexa 488 (1/800 dilution, Molecular Probe, Eugene, OR) for 1 hr in the dark. Sections were also incubated with HNPP and Fast Red (Roche Diagnostics) in detection buffer (0.1 M Tris-HCl pH 8.0, 0.15 M NaCl, 10mM MgCl2) at room temperature for 30 min, counterstained with TOPRO-3 (1/10,000 dilution, Molecular Probe), and coverslipped. Sections were captured using laser scanning confocal microscope (LSM510, Carl Zeiss, Thornwood, NY).
Signal detection of IEG’s
The spatial distribution of Homer1a and zif268 expressing cells was analyzed in the CA1 field of the right dorsal hippocampus (3.4-4.0 mm posterior from bregma), according to the rat atlas (Paxinos and Watson, 1998). The pyramidal cells were visualized on a computer screen using a 40x objective under bright-field microscopy (Olympus BX41, Japan) and a CCD camera (Olympus Q-Color3, Japan). Double IEG-labeled cells were analyzed by identifying DAB-labeled dots and silver grains for Homer1a and zif268, respectively. The cells were also stained with Cresyl Violet for positive identification of nuclei. DAB-labeled dots in the nuclei were identified as brown dots, while silver grains in nuclei were identified by small black dots over the nuclei. Since the grains are localized on the emulsion, they are at a higher focal point than the DAB stain which is localized on the tissue. To distinguish grains from DAB-labeled dots, we performed image processing as follows: (i) the same frame images (each image: 351 × 263 μm2) were captured at two different focal planes. One focal plane was “on-emulsion” level for grains (small black; Fig.2NO), and the other was “on-tissue” level for DAB (brown) and Cresyl Violet staining (Fig.2PQ); (ii) When on-emulsion grains were focused, on-tissue DAB-labeled signals caused a diffused reflection (Fig.2NO, TU) because of the focal range; (iii) Once the two images with on-emulsion level and on-tissue level were subtracted (Image J 1.41, NIH, Bethesda, ML), the diffused reflection signals appeared as white signals (Fig.2VW) and these represented the DAB-labeled signals; (iv) This subtracted image was labeled red and then overlaid on the on-tissue image (Fig.2XY) and used as “a signal-detection image” for further analysis.
Fig. 2. Specificity, time-course, and dual-labeling in situ hybridization for Homer1a and zif268 mRNAs.

A-D: Autoradiographs showing expression of Homer1a (B) and zif268 (D) mRNAs with [35S]-labeled antisense and sense riboprobes for Homer1a (A) and zif268 (C). Contrary to the sense signals, strong antisense signals for Homer1a and zif268 were found in the cortex and the principal cell layers of the hippocampus. E-M: Expression of DAB-labeled Homer1a (E-G), and DAB- (H-J) and grain-labeled (K-M) zif268 in the CA1 region. There were three experimental groups: control (E,H,K), and groups exposed to one arm for 5min (5min-group, F,I,L) and exposed for 5min and kept in dark for 25min (30min-group, G,J,M). N-Q: Double-labeling ISH showing expression of DAB-labeled Homer1a (arrow) and grain-labeled zif268 (arrowheads) in CA1 pyramidal cell nuclei (violet) “on-emulsion” (N,O), and “on-tissue” (P,Q) focal planes. R-S: Expression of Homer1a (red) and zif268 (green) mRNAs in nuclei (blue) by two color-fluorescent ISH showing a similar distribution as with double DAB/grain-labeling ISH. T-U: Image of "on-emulsion" focal plane for DAB (brown) and Cresyl Violet staining. V-W: Brown DAB-labeled signals appear as white signals when the “on-emulsion” and “on-tissue” images in the same frame were subtracted (see Methods). X-Y: Signal-detection image overlaid with the on-tissue image and the subtracted image, in which the signals were colored in red. The higher magnification of the insets shows the nucleus with the staining in O, Q, S, U, W, and Y. Scale bars, A-D: 2mm; E-J: 100μm; K-M: 25μm; N,P: 20μm; R,T,V,X: 50μm.
For the IEG-labeling analysis, adjacent signal-detection images were captured along a 1200-μm length of the CA1 pyramidal cell layer (1.0-2.5 mm lateral from midline suture) in each section. According to grain and DAB-labeled signal detection, the position of a positive cell was marked on the signal-detection image by a person blind to the experimental groups.
Reference images and 3D-reconstructed images
For 3D image reconstructions, alternate 12μm-thick sections were used in order to avoid double counting of cells whose nucleus may appear in two neighboring sections since cell nuclear diameter is at most 12 μm. A hippocampal image, containing the CA1, CA3 and dentate gyrus regions, was captured as follows: (i) each region of interest was visualized with a 20X objective using a bright-field microscope (Olympus BX61, Japan), and was captured with a CCD camera (Olympus Q-Image Retiga 1300, Japan); (ii) The selected regions of interest consisted of 90 adjacent frames on each section; (iii) These were automatically captured and stitched together to form a single image as “a reference image”, using the software Slidebook 4.1 (SciTech Pty Ltd., Preston South VIC, Australia); (iv) On the stitched reference image, the position of a positive cell was marked, according to the signal-detection imaging described above; (v) Then, 8 serial reference images per animal (each image: 4646 × 2484 μm2, pixel size: 1 × 1μm2) were sequentially aligned slice-by-slice, based on contrast/brightness of cellular distribution containing cell-layer curves, using the image registration software FLIRT 5.3 (University of Oxford, Oxford, UK) (Jenkinson and Smith, 2001); (vi) The aligned reference images and marked dots were then 3D reconstructed, volume rendered and visualized using the software 3D Slicer (http://www.slicer.org/). An interval between the reference images was 24 μm for 3D image reconstruction. We measured areal cell density ρ (1/μm3), in the CA1 field of each animal, using the following formula:
where λ is the evenly scattered, nearest-neighbor distance (μm)
Distances based on nearest neighboring cells
Based on methods used to identify spatial patterns in 2D and 3D (Ripley, 1977, 1979), we characterized clusters of the nearby cells in the CA1 field. Using software IDL 6.2 (RSI Inc., Boulder, CO), we quantified a distance from a cell to the nearest neighboring cell using the following formula:
where N1 (μm) is the nearest-neighbor distance, and nj is the maximum cell number per animal (Fig.5A). Individual values of the N1 (N1,i,j) were quantified from each position of positive cells in each 3D-reconstructed image, and then in the DL and SL groups. We successively quantified the following values:
where N2 is the distance from a cell to the second nearest neighboring cell (second nearest-neighbor distance); N3 is the distance from a cell to the third cell …; and, Nnj is the distance from a cell to the furthest cell.
Fig. 5. Nearest-neighbor distances and cell density of the double IEG-labeled cells.
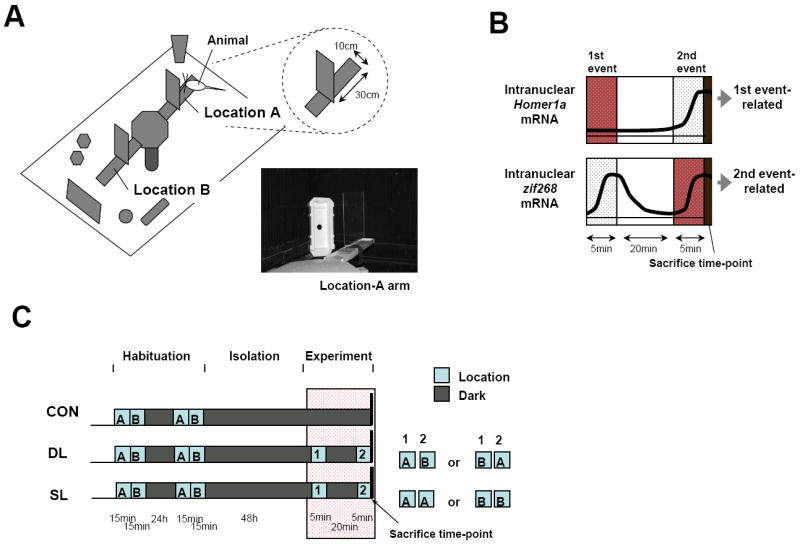
A: Schematic drawing showing the nearest neighbor distance between the cells (N1,i,j), the second nearest neighbor distance (N2,i,j), and the third nearest neighbor distance (N3,i,j). Cell i,j(1) is shown as the nearest neighboring cell from cell i in animal j (Cell i,j(0)) and Cell i,j(2) as the second neighboring cell, and Cell i,j(3) as the third nearest neighboring cell. B: Histograms showing the distribution of the nearest-neighbor distance (N1) cell frequency in the DL (left) and SL (right) groups. C: Bar-plots showing cell density ρ in CA1 pyramidal cell layers in the DL and SL groups. D: Schematic drawings showing sizes of evenly scattered, nearest-neighbor distances λ (λj = ρ j-1/3 (μm); animal: 1 ≦ j; see methods) in the DL and SL groups. Error bars indicate S.E.M. in each group. †p< 0.05, significant difference between the DL and SL groups.
Furthermore, for the further second-order process, we calculated with the following formulas:
where D1-2 (μm) is the difference between the first and second nearest-neighbor distances in each animal, D2-3 is the differences between the second and third nearest-neighbor distances, D3-4 is the differences between the third and forth nearest-neighbor distances, …, and Dnj-1− nj is the difference between subsequent-order nearest-neighbor distances.
Distributions of the N1 and D1-2, D2-3, D3-4, …. D9-10, were simulated for the DL and SL groups using Monte Carlo simulations on software IDL 6.2. Monte Carlo simulations were performed in the CA1 pyramidal layer of each animal. Based on the “signal-detection images”, positions of all cells were identified and extracted on a 1200 μm length in the CA1 for each “reference image” and these “reference images” were reconstructed in 3D montages. In total, 9,946 cells in DL (n=6 animals) and 11,566 cells in SL (n=7 animals) were used. Monte Carlo simulation samples of positive cells were positioned randomly in a set of all cells identical in space in CA1 pyramidal cell layers in each animal. The simulation was performed 100 times in each animal by repeated sampling in identical cell positions. Then, we measured the distance between the simulated samples.
To directly compare the values between the DL and SL groups, we used the following values:
where N1’ is the normalized nearest-neighbor distance, and D1-2’, D2-3’, D3-4’, …., and D9-10’are the normalized differences between subsequent-order nearest-neighbor distances.
Nearest neighbor topographical arrangement
In many instances the clusters appeared to consist of a small number (2-4) of double labeled cells. To more precisely analyze this arrangement we grouped these cells into what we labeled a “triad” which consisted of three closely located cells. We determined the nearest neighbor of each of the cells and calculated the distances between the cells as the “triad size”. Differences in triad size were then compared between the DL and SL groups.
Statistical analysis
For double IEG-labeled cells, one-way analysis of variance (ANOVA) was carried out to evaluate the significance of the main effect on the three experimental groups. Further, post hoc comparisons by Tukey-Kramer test were performed between relevant pairs. Student’s two-tailed t-test was also used to compare between the DL and SL groups.
The values of the nearest-neighbor distances and the differences between subsequent-order nearest-neighbor distances were not normally distributed: hence, the data were analyzed using the nonparametric two-sample Kolmogorov-Smirnov test (two-tailed), to compare between relevant pairs. Moreover, skewness (SkQ) and kurtosis (KuDQ) of the distribution (Q1: lower quartile, Q2: median, Q3: upper quartile, D1: first decile, D9: ninth decile) were defined as follows:
All statistical analyses were carried out using the software StatView (SAS, Cary, NC) and R 2.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Specificity of expression for Homer1a or zif268 mRNAs
To investigate IEG expression in CA1 neurons encoding spatial environments, we employed ISH labeling of zif268 and Homer1a mRNAs. It has previously been reported that peak expression of zif268 mRNAs can be detected within 2-5 min at the genomic sites of transcription as intranuclear foci after an animal has been exposed to a “place or event” (Guzowski et al., 1999), and this intranuclear active transcription disappears within 15-20 min. Peak Homer1a mRNAs appear within 25-40 min as intranuclear foci, which can be detected after exposure of an animal to a “place or event” (Bottai et al., 2002; Vanzdarjanova et al., 2002). Our experimental protocol, therefore, involved exposing animals for 5 min to one location (e.g. Location A, shown in Fig.1A), placing them in isolation for 20 min and then exposing them either to the same (i.e., Location A) or to a different location (i.e., Location B, shown in Fig.1A). Thus, intra-nuclear Homer1a expression should correspond to the first “place or event” while intra-nuclear zif268 expression should correspond to the second “place or event” (Fig.1B). We hypothesized that in animals exposed to the same location twice, there should be a larger number of double zif268- and Homer1a-expressing cells, in comparison to exposing animals to two different locations once (Guzowski et al., 1999; Vanzdarjanova et al., 2002).
The patterns of zif268- and Homer1a-expressing cells were detected by single isotopic ISH autoradiography (Fig. 2A-D). We also examined the time course of expression of intra-nuclear mRNAs using single ISH for DAB-labeled Homer1a (Fig. 2E-G), DAB-labeled zif268 (Fig. 2H-J), and grain-labeled zif268 (Fig. 2K-M). Expression of DAB-labeled Homer1a mRNAs in nuclei increased in the 30min-group (Fig. 2G) as compared to the 5min-group (Fig. 2F), 20min-group, and non-exposed controls (Fig. 2E). Meanwhile, similar to expression of DAB-labeled zif268 (Fig. 2H-J), expression of grain-labeled zif268 mRNAs increased in the 5min-group (Fig. 2L), as compared to the 20min-group, 30min-group (Fig. 2M), and non-exposed control group (Fig. 2K). These time peaks of Homer1a and zif268 mRNA expression are comparable to what has been previously reported (Guzowski et al., 1999; Bottai et al., 2002; Vanzdarjanova et al., 2002).
Double-labeling with Homer1a and zif268 mRNAs
We employed ISH double-labeling for DAB-labeled Homer1a and grain-labeled zif268 (Fig.2N-Q), in three experimental groups of animals: (i) non-exposed controls (CON); (ii) exposed to two different locations once (DL); and (iii) exposed to the same location twice (SL) (Fig. 1C). We then analyzed the population of single (Table 1) and double (Table 2) IEG-labeled cells for Homer1a and zif268. Zif268-expressing cells were analyzed by counting the cumulative number of intra-nuclear grains. IEG expression levels in non-exposed animals were taken as background. For a cell to qualify as double IEG-labeled, cells with less than 5 zif268 grains were eliminated due to the fact that the background ratio became minimum from 5 grains (Table 1, 2, Fig. 3A). The cells, which contained DAB-labeled Homer1a, a Cresyl Violet-counterstained nucleus, and cumulative values from 5 zif268 grains, were then quantified for the three experimental groups. ANOVA showed that the DL and SL groups had a higher population of double IEG-labeled cells than the CON group (F(2, 15) = 17.69, p< 0.0001; Table 2 and Fig. 3B). Furthermore, post hoc comparisons by Tukey-Kramer test showed that the SL group had a significantly higher double IEG-labeled cell population than the DL group (Table 2). In terms of single IEG-labeled cell population, there were no differences in either the number of Homer1a or zif268 alone expressing cells between the SL and DL groups (Table 1 and 2).
Fig. 3. Quantification of zif268- and Homer1a-labeled CA1 neurons.

A: Line-plots showing the distribution of the number of zif268 grains in CA1 neurons that also contain DAB-labeled Homer1a and Cresyl Violet stained nuclei in non-exposed home-cage animals (CON), exposed to two different locations once (DL), or exposed to the same location twice (SL). Arrow shows the threshold of the number of zif268 grains in the nuclei used for the double IEG-labeling analysis. B: Bar-plots showing the cell population with cumulative values of more than 5 zif268 grains in the CON, DL, and SL groups. C: Bar-plots showing the average numbers of zif268 grains in the double IEG-labeled cells between the DL and SL groups. Error bars indicate S.E.M. in each group. *p< 0.01, significant difference between control and experimental groups. †p< 0.05, significant difference between the DL and SL groups.
To determine whether zif268 expression was at a similar level following either the same place exposure twice or two different place exposures, we quantified the average number of zif268-labeled grains in the double IEG-labeled cells and compared them between the DL and SL groups. Student’s t-test did not reveal a difference in the average number of zif268 grains in the double IEG-labeled cells between the DL and SL groups (Fig. 3C). Thus, zif268 expression was similar in the two experimental groups.
Nearest-neighbor distance
For the 3D analysis, the scanned hippocampal images with double IEG-labeled cells were first sequentially aligned (Fig. 4A,B). We then used previously described methods for spatial mapping patterns in 2D and 3D (Ripley, 1977, 1979) that involved calculating the nearest neighbor distance N1 (μm; Fig.5A), and the further second-order process. One of the peaks at 24μm (arrow) in the DL group corresponded to a peak (arrow) in the SL group. This peak appears to contain aligned values of N1 when each 24-μm-thick slice was sequentially aligned for 3D reconstruction. This peak was not caused by double counting the same positive cell, since the error of an overlapped positive cell between alternate 12-μm-thick slices was less than 5 % (data not shown). Distribution histograms of the N1 did not reveal dispersed peaks in the DL and SL groups (Fig.5B). Rather, the distribution of the SL group (SkQ = 0.331, KuDQ = 0.862) was noticeably skewed to the left and compressed to that of the DL group (SkQ = 0.202, KuDQ = 0.736).
Fig. 4. Representative high magnification 3D-reconstructed images of zif268 and Homer1a double-labeled cell populations (red dots) in the dorsal CA1 field in DL (A) and SL (B) exposed animals.
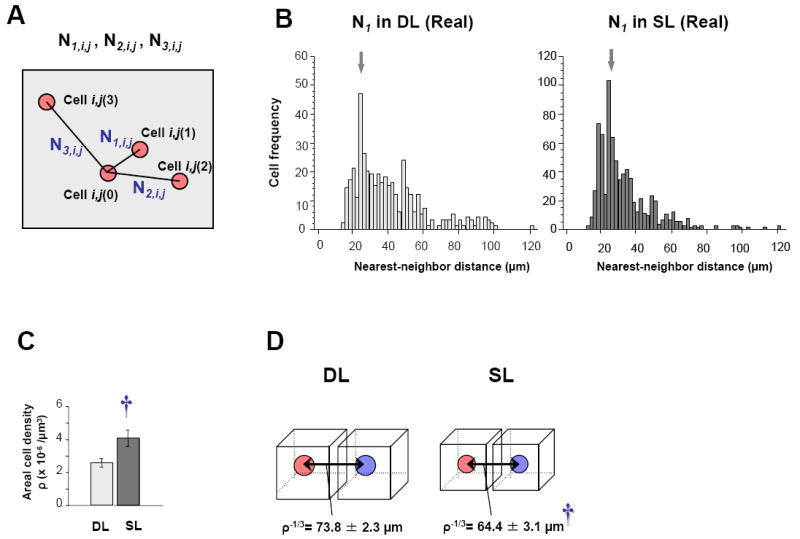
The upper and lower levels showing two representative 3D-reconstructed images in each group. The inset in the corner shows a lower magnification of the image. The total number of double IEG-labeled cells was 87 (A upper), 91 (A lower), 110 (B upper), and 133 (B lower) per 2.4 - 2.9 × 107 μm3 (192 μm thickness in the anterio-posterior axis by 8 sections and 1200-μm-long CA1 pyramidal cell layer in coronal section). There appears to be clusters of nearby cells in both of the DL and SL groups.
We took the cell density ρ and further calculated the evenly scattered nearest-neighbor distance λ (see Methods) to investigate the 3D spatial patterns of double IEG-labeled cells in CA1. Student’s t-test showed that the SL group had a higher cell density ρ than the DL group (p< 0.05, +57.1%, Fig.5C). On the other hand, the SL group had an evenly scattered nearest-neighbor distance λ lower than the DL group (p< 0.05, -12.8%, Fig.5D). This shows that the SL group had a denser active cell mass than the DL group.
Since the cell density ρ was different between the DL and SL groups, we performed a Monte Carlo simulation to compare the N1 distribution between real and simulated samples in both DL and SL groups. The DL group had a lower N1 distribution than simulated samples (Two-sample Kolmogorov-Smirnov test, p< 0.005; Mann-Whitney U test, p<0.05; Fig.6A). Also, the SL group had a lower distribution than simulated samples (Two-sample Kolmogorov-Smirnov test, p< 1 × 10-13; Mann-Whitney U test, p< 5 × 10-8; Fig.6B). These results indicate that the cell distribution in the DL and SL groups are more clustered than would be expected by chance.
Fig. 6. Nearest-neighbor distances of the double IEG-labeled cells.

A-B: Cumulative line-plots showing the N1 distribution between real and simulated samples in the DL (A) and SL (B) groups. Histograms also show the subtracted values of the N1 between real and simulated samples (dark-gray area). C: Cumulative line-plots showing the normalized N1 (N1’) distribution between the DL and SL groups. Histograms also show the subtracted values of the N1’ between the DL and SL groups (dark-gray area). †p< 0.05, ††p< 0.005, †††p< 1 × 10-13, significant difference between pairs by Two-sample Kolmogorov-Smirnov test.
Since cell density ρ was larger in the SL than in the DL, the distribution of N1 should be lower in the SL. Thus, we re-analyzed the normalized values of the N1 (N1’), in which each CA1 space size was normalized to the cell density (see Methods) to directly compare the N1 between the DL and SL groups of real samples. The SL group had a lower N1’ distribution than the DL group (Two-sample Kolmogorov-Smirnov test, p< 0.05, Fig.6C). This indicates that the clustering of double IEG-expressing cells is tighter in the SL group, even though the total number of cells is larger.
Differences between subsequent-order nearest-neighbor distances
To further characterize a 3D spatial mapping of double-IEG labeled cells, we estimated the second-order process, which indicates differences between subsequent-order nearest-neighbor distances (Ds; e.g., the difference between the first and second nearest-neighbor distances, see Methods). If a D shows a higher distribution, it can be interpreted that the gap between the active neighboring cells is due to formations of clusters of active cells in the hippocampus (Fig. 7). The DL group had a higher distribution of D1-2, D2-3, and D3-4 than simulated samples (Two-sample Kolmogorov-Smirnov test, D1-2: p<0.05, D2-3: p<0.0005, D3-4: p<0.05; Mann-Whitney U test, D1-2: p<0.005, D2-3: p< 5 × 10-7, D3-4: p<0.01; Fig.8A). There was no difference in the distributions of D4-5, D5-6, D6-7, D7-8, D8-9, and D9-10 between real and simulated samples in the DL group. The SL group had a higher distribution of D1-2, D2-3, and D3-4 than simulated samples (Two-sample Kolmogorov-Smirnov test, D1-2: p<0.00005; D2-3: p<0.005; D3-4: p<0.05; Mann-Whitney U test, D1-2: p< 5 × 10-6; D2-3: p<0.001; D3-4: p<0.05; Fig. 8B). There was no difference in the distributions of D4-5, D5-6, D6-7, D7-8, D8-9 and D9-10 between real and simulated samples in the SL group. These results show that the SL and DL groups had clusters of nearby double IEG-expressing cells.
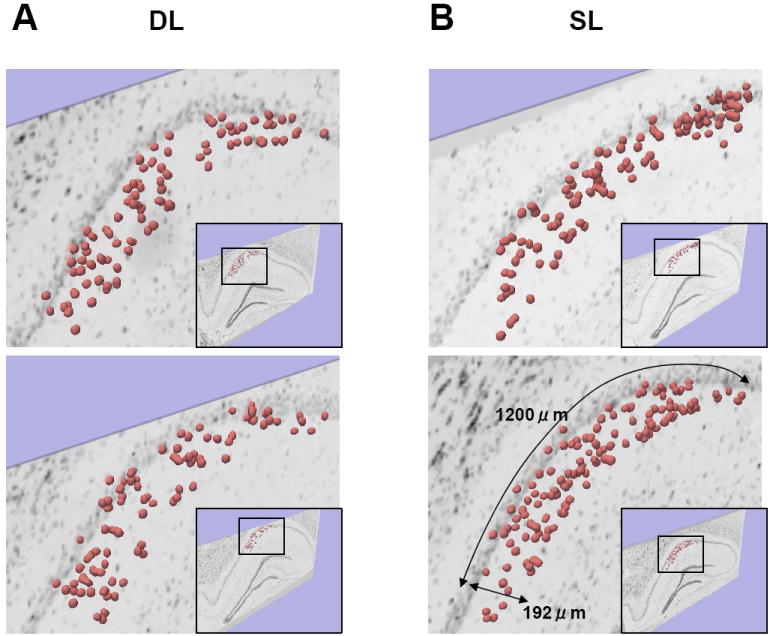
Fig. 7. Schematic drawing comparing cluster formation in two different animals (animals j vs. l).
A: Representative comparisons of D1-2 (D1-2,i,j < D1-2,k,l). In animal j, cell i,j(1) is shown as the nearest neighboring cell, and Cell i,j(2) is shown as the second nearest neighboring cell from cell i, which is cell i,j(0) as a starting point. As compared to animal j, animal l has a larger gap between Cell k,l(2) and Cell k,l(0). This can be interpreted as either forming a cluster of the two nearby cells in animal l, or forming a cluster of the three nearby cells in animal j. B: Another example of comparisons of D2-3 (D2-3,i,j< D2-3,k,l). As compared to animal j, animal l has a larger gap between Cell k,l(3) and Cell k,l(0). This can be interpreted as either forming a cluster of the three nearby cells in animal l, or forming a cluster of the four nearby cells in animal j.
Fig. 8. Differences between subsequent-order nearest-neighbor distances.
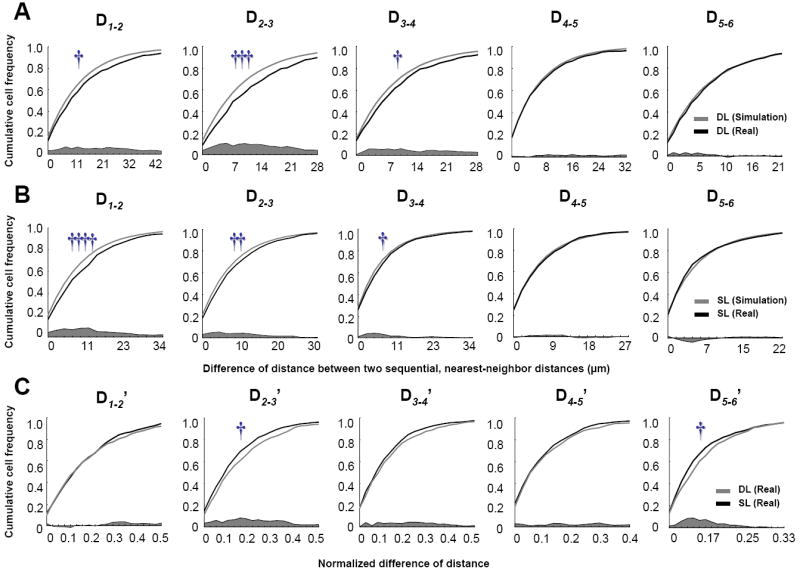
A-B: Cumulative line-plots showing differences between subsequent-order nearest-neighbor distances (D1-2, D2-3, …, and D5-6) for real and simulated samples in the DL (A) and SL (B) groups, and normalized differences of distance between two sequential, nearest-neighbors (D1-2’, D2-3’, …, and D5-6’) of real samples between the DL and SL groups (C). Histograms also show the subtracted values of these differences between real and simulated samples (dark-gray area). †p< 0.05, ††p< 0.005, †††p< 0.0005, ††††p< 0.00005, significant difference between relevant pairs by Two-sample Kolmogorov-Smirnov test.
As for the normalized Ds (Ds’), the DL group had a higher distribution of D2-3’ and D5-6’ than the SL group (Two-sample Kolmogorov-Smirnov test, D2-3’: p<0.05; and D5-6’: p< 0.05; Mann-Whitney U test, D2-3’: p<0.01; and D5-6’: p< 0.05; Fig.8C). Therefore, the SL and DL groups of animals showed a differential distribution of subsets of active nearby CA1 pyramidal cell clusters.
Nearest neighbor topographical arrangement
We further estimated a basic cluster arrangement of nearby cells which consisted of a triad (three cells) that is closely arranged neighboring cells. The triad size analysis showed that the SL group had a lower distribution of triads with closer located cells than the DL group (Two-sample Kolmogorov-Smirnov test, p< 0.05; Mann-Whitney U test, p<0.05; Fig.9). Thus, these results showed that the SL group had subsets of tighter clusters of active nearby cells than the DL group.
Fig. 9. Nearest neighbor topographical arrangement.
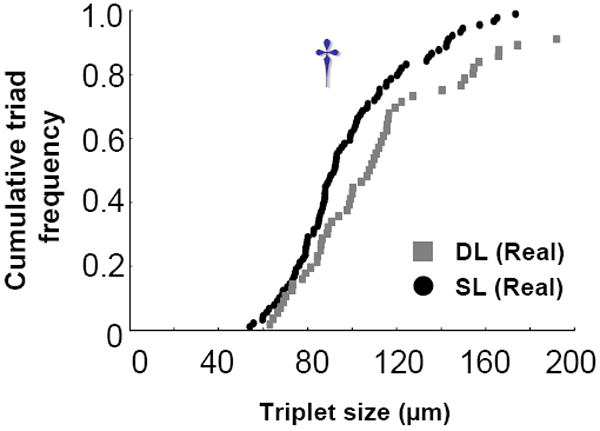
A: Cumulative dot-plots showing triad sizes of between the DL and SL groups. †p< 0.05, significant difference between relevant pairs by Two-sample Kolmogorov-Smirnov test.
DISCUSSION
The main finding from the present study was the observation that in animals exposed to a restricted part of an environment (30-cm long by 10-cm wide), IEG expressing cells in the dorsal hippocampus formed clusters of a few active cells, adjacent to non-IEG expressing cells. Further, exposing animals to the same environment twice produced a larger number of double IEG labeled (zif268 and Homer1a) cells than exposing them to two different environments. The ratio of double IEG-expressing cells in the same environment exposure, in comparison to different environment exposure, was 2:1. Perhaps of greater significance was our finding that in the animals that were exposed to the same place twice, there was a “tighter” cohesion of the clusters of active nearby cells, as revealed by the shorter distance to the nearest neighbor analysis. These results suggest a topographic organization of hippocampal pyramidal neurons to encode for space.
Topographic organization in the hippocampus
Our findings suggest that small clusters of active nearby hippocampal cells (~4) encode a specific place of an environment. Unlike the results with the functional IEG mapping method, recordings of hippocampal neurons have failed to provide conclusive evidence with regards to any type of topographical organization. It has been estimated that the majority (perhaps > 80%) of pyramidal cells within the CA1 and CA3 hippocampal fields respond to an animal’s position in space (for a review, see O’Keefe, 1999). However, as stated above, how these cells may be organized to represent a “spatial map” remains a mystery. A common observation from a number of multi-unit recoding studies is that neighboring cells typically do not have adjacent place fields. Perhaps more intriguing is the fact that knowing the place fields in one environment, does not allow one to predict the relationship of place fields to cells in a different environment. Nonetheless, there is some evidence suggesting that hippocampal units are functionally organized (O’Keefe and Speakman, 1987; Eichenbaum et al., 1989; Deadwyler et al., 1996; Hampson et al., 1996; Hampson et al., 1999; Hampson et al., 2002). Anatomical evidence also supports cluster-type organization in the hippocampus. For example, CA3 neurons, which are upstream to CA1 neurons, have robust associational connections to neighboring CA3 neurons (Ishizuka et al., 1990; Li et al., 1994). Neighboring CA3 neurons were reported to project inputs to topographically organized nearby CA1 neurons at the septo-temporal axis (Brivanlou et al., 2004). This would indicate that neighbor-to-neighbor relations of pyramidal neurons are preserved in the CA3 and CA1 fields. Therefore, it is reasonable to suggest that in the CA1 field there is a high probability that neurons can be active in clusters following CA3 cell activation.
Hippocampal place cell characteristics
In animals restricted to a 10 × 30 cm2 region of space, we observed approximately 2% of double IEG-expressing cells in the CA1, in response to the same place exposure twice. These IEG results are supported by single unit recording studies showing that 2-5% of all dorsal CA1 neurons, that are place cells, are active within a 20-25 cm region of space (Maurer et al., 2006; estimated from Wilson and McNaughton, 1993). Many of the firing characteristics of place cells have been described (Kubie and Muller, 1991; Best and White, 1999) and some of these characteristics pertinent to this discussion are as follows: 1) place fields form rather quickly but are stabilized with repeated exposure; 2) once stabilized, exposure to the same environment activates the same place cells; 3) place cells depend mainly on distal cues, although in the absence of prominent distal cues they can be affected by proximal cues; 4) place cells can have place fields in multiple environments. Thus, space is represented by the activity of a network of cells, although, empirically, the likelihood of a cell having place fields in two sampled environments is low (9-16%) (Muller and Kubie, 1987). It is also relevant to mention that the remapping of place fields, which occurs when an animal is placed in different environments or the environments are intentionally changed, usually requires that the two environments are significantly different (Kubie and Muller, 1991; Wills et al., 2005; Leutgeb et al., 2005). Given these observations, it would be safe to speculate that exposing an animal to a restricted environment with limited cues would activate a subset of place cells. Also, exposing an animal to the same space twice would activate the same place cells twice. In the present study, the two locations were relatively different, but the general environment (e.g., room) was the same. Thus, clusters of active cells were also observed in the different place exposure but the cluster size was smaller and the nearest neighbor distances were farther than those observed in the same place exposure. While the majority of active cells may have been encoding the overall environment or other undetermined stimuli, it is reasonable to assume that a small percentage of the cells were activated specifically due of the animal’s location. Therefore, the small percentage and tighter clusters of active cells we observed from repeated exposure to the same place could result from the place cell activity having more stable place fields.
Functional IEG mapping methods
A number of previous studies by Guzowski and associates have used the IEG detection method to map neuronal activity in the hippocampus, in animals exploring space (Guzowski et al., 1999; Vazdarjanva et al., 2002; Vazdarjanva and Guzowski, 2004; Chawla et al., 2005). Similar to the present study, the behavioral procedures used in these studies has been to let animals explore an environment for 5 min, return them to their home cage for 20 min, expose them to either the same or a different environment for 5 min and sacrifice them immediately. A number of differences, however, should be noted between their paradigm and ours: 1) A major difference was that they deliberately exposed animals to all parts of the environment (picking them up by hand and placing them to a different location every 15 sec, see Vazdarjanva et al., 2002). In contrast, our objective was to minimize the extent of the environment that the animal was exposed to, in an attempt to reduce the space to be encoded and thus the number of active place cells; 2) Related to this, the size of the space and complexity of the sensory stimuli were different between the two studies: (10 × 30 cm2 space with simple distal cues in the present study, and 61 × 61 cm2 and complex proximal and distal cues in their studies); 3) Furthermore, while in their studies the two environments were completely different, in the present study animals were exposed to two different arms of an elevated maze which was located in the same room, with only the visual cues facing each of the arms being different; 4) A more subtle, but potentially significant, difference between the paradigms was the fact that in their studies the animals were returned to the vivarium following exposure to the first environment, while the animals in the present study were returned to an isolation chamber in complete darkness; 5) in the present study animals were exposed to the environment previously - repeated exposure to an environment has been shown to stabilize place fields (Kubie and Muller, 1991; Kentros et al., 1998; Best and White, 1999); 6) Finally, in their studies IEG expression was determined using fluorescent ISH, with either Arc or Arc/Homer1a mRNAs, while in the present study we used grain- and DAB- labeled ISH with zif268 and Homer1a mRNAs, respectively.
While the results from Guzowski and associates and the present study agree in certain aspects, they differ in others. The previous studies reported that following exploration in a novel environment, 40-45% of CA1 neurons were either Arc (intranuclear or cytoplasmic) or Homer1a positive (Guzowski et al., 1999; Vazdarjanva et al., 2002; Vazdarjanva and Guzowski, 2004). In contrast, we observed that following exposure to an environment, 10 % of CA1 cells were zif268 positive. Vazdarjanva and Guzowski (2004) also reported that exposure to two different environments, produced a significantly higher number of double IEG labeled cells (15% increase from control), in comparison to exposure to the same environment which was twice as high (30% increase from control). Our findings are in agreement with this result, in that approximately twice as many double IEG-labeled cells were observed in the same place exposure group (4.06% increase from control), in comparison to the different place exposure group (2.22% increase from control), albeit at much lower numbers. Furthermore, our results show tighter clusters of active nearby cells in the same place exposure group than in the different place exposure group.
Within a small environment only a subset of place cells would be expected to be active, as confirmed by electrophysiological recordings (Wilson and McNaughton, 1993; Maurer et al., 2006). Thus, in an animal placed in a large part of the environment, a greater number of active cells should be engaged (Pavlides C, Donishi T, Ribeiro S, Mello C, Ogawa S, unpublished observation). Sensory stimulation would cause cells to be more active. In an animal exposed to an environment with richer cues, a greater expression of zif268 and Arc mRNAs was induced in the hippocampus (Pinaud et al., 2002). The active handling of animals would provide sufficient sensory stimulation, which along with more active exploration of a larger space could have contributed to the higher number of IEG-expressing cells. Furthermore, active handling of the animals during exposure to the environment could have produced stress, which increases levels of zif268 mRNAs and proteins in the hippocampus (Revest et al., 2005).
In contrast to our observation of clusters of active nearby cells, Redish et al. (2001) reported that in animals exposed to two novel environments (60 × 60 cm2 boxes) there was no evidence of clustering as indicated either by Arc mRNA expression or by unit recordings. What are possible reasons for the discrepancies? The most plausible explanation is that in the previous study, free exploration of the environments by the animals activated 40-45% of the CA1 pyramidal cells, thus obscuring the observation of any cluster-type functional organization. Restricting animals to a small part of the environment allowed for a small number of cells to be activated and revealed the formation of small clusters. The spacing between cells within a cluster may still be sufficiently far apart for cells to be recorded simultaneously from a single electrode. Another problem is that tetrodes produce relatively large damage and deformation of the brain, especially when multiple tetrodes are used. Thus, whether cells within a cluster are being sampled is not certain. Assuming for the moment that the functional organization of the hippocampus to encode for space is that of clusters, a question that still remains is the arrangement of the “spatial map”. The present findings reveal a functional unit of cell ensembles in the hippocampus, however, it remains unclear whether each cluster of cells encodes for each spatial component or multiple spatial components. Knowing the anatomical configuration of the clusters, is only the first step in deciphering the “spatial map” which could only be achieved by determining the interactions of unit discharges within the clusters. More refined unit recordings from the clusters would be necessary to decode the foundation of the “cognitive map”.
Acknowledgments
We like to thank Ms. Jilda A. Caccavo and Ms. Christine R. McPherson (Rockefeller University) for technical support, and Drs. Yuji Ikegaya (University of Tokyo), Stephan Haupt, and Robert Sinclair (Okinawa Institute of Science and Technology) for theoretical comments. This work was supported by National Institutes of Mental Health grant MH067283 to CP.
Comprehensive list of abbreviations
- IEGs
Immediate-early genes
- ISH
in situ hybridization
- 3D
three dimensional
- DL
two different locations exposure
- SL
the same location exposure twice
- CON
home-cage control
- N1
the nearest-neighbor distance
- N2
the second nearest-neighbor distance
- N3
the third nearest-neighbor distance
- D1-2
the difference between the first and second nearest-neighbor distances
- D2-3
the difference between the second and third nearest-neighbor distances
- D3-4
the difference between the third and fourth nearest-neighbor distances
- N1’
the normalized nearest-neighbor distance
- D1-2’
the normalized difference between the first and second nearest-neighbor distances
- SkQ
skewness
- KuDQ
kurtosis
- ρ
the average cell density
- λ
the evenly scattered nearest-neighbor distance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Best PJ, White AM. Placing hippocampal single-unit studies in a historical context. Hippocampus. 1999;9:346–351. doi: 10.1002/(SICI)1098-1063(1999)9:4<346::AID-HIPO2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Bottai D, Guzowski JF, Schwarz MK, Kang SH, Xiao B, Lanahan A, Worley PF, Seeburg PH. Synaptic activity-induced conversion of intronic to exonic sequence in Homer 1 immediate early gene expression. J Neurosci. 2002;22:167–175. doi: 10.1523/JNEUROSCI.22-01-00167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Brivanlou IH, Dantzker JL, Stevens CF, Callaway EM. Topographic specificity of functional connections from hippocampal CA3 to CA1. Proc Natl Acad Sci USA. 2004;101:2560–2565. doi: 10.1073/pnas.0308577100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A, Nissanov J, Larocque S, Rioux L. Dual activity maps in primate visual cortex produced by different temporal patterns of zif268 mRNA and protein expression. Proc Natl Acad Sci USA. 1997;94:2671–2675. doi: 10.1073/pnas.94.6.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Clayton DF. The genomic action potential. Neurobiol Learn Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Davis S, Bozon B, Laroche S. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav Brain Res. 2003;142:17–30. doi: 10.1016/s0166-4328(02)00421-7. [DOI] [PubMed] [Google Scholar]
- Deadwyler SA, Bunn T, Hampson RE. Hippocampal ensemble activity during spatial delayed-nonmatch-to-sample performance in rats. J Neurosci. 1996;16:354–372. doi: 10.1523/JNEUROSCI.16-01-00354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeis A, Iasevoli F. The Homer family and the signal transduction system at glutamatergic postsynaptic density: potential role in behavior and pharmacotherapy. Psychopharmacol Bull. 2003;37:51–83. [PubMed] [Google Scholar]
- Eichenbaum H, Wiener SI, Shapiro ML, Cohen NJ. The organization of spatial coding in the hippocampus: a study of neural ensemble activity. J Neurosci. 1989;9:2764–2775. doi: 10.1523/JNEUROSCI.09-08-02764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12:86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Simeral JD, Deadwyler SA. “Keeping on track”: firing ofhippocampal neurons during delayed-nonmatch-to-sample performance. J Neurosci. 2002;22:RC198. doi: 10.1523/JNEUROSCI.22-02-j0002.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Simeral JD, Deadwyler SA. Distribution of spatial and nonspatial information in dorsal hippocampus. Nature. 1999;402:610–614. doi: 10.1038/45154. [DOI] [PubMed] [Google Scholar]
- Hampson RE, Byrd DR, Konstantopoulos JK, Bunn T, Deadwyler SA. Hippocampal place fields: relationship between degree of field overlap and cross-correlations within ensembles of hippocampal neurons. Hippocampus. 1996;6:281–293. doi: 10.1002/(SICI)1098-1063(1996)6:3<281::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Ishii T, Omura M, Mombaerts P. Protocols for two- and three-color fluorescent RNA in situ hybridization of the main and accessory olfactory epithelia in mouse. J Neurocytol. 2004;33:657–669. doi: 10.1007/s11068-005-3334-y. [DOI] [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimization method for robust affine registration of brain images. Med Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998;273:23969–23975. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, Muller RV. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science. 1998;280:2121–2126. doi: 10.1126/science.280.5372.2121. [DOI] [PubMed] [Google Scholar]
- Knapska E, Kaczmarek L. A gene for neuronal plasticity in the mammalian brain: Zif268/Egr-1/NGFI-A/Krox-24/TIS8/ZENK? Prog Neurobiol. 2004;74:183–211. doi: 10.1016/j.pneurobio.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL. Interactions between idiothetic cues and external landmarks in the control of place cells and head direction cells. J Neurophysiol. 1998;80:425–446. doi: 10.1152/jn.1998.80.1.425. [DOI] [PubMed] [Google Scholar]
- Kubie JL, Muller RU. Multiple representations in the hippocampus. Hippocampus. 1991;1:240–242. doi: 10.1002/hipo.450010305. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Treves A, Meyer R, Barnes CA, McNaughton BL, Moser MB, Moser EI. Progressive transformation of hippocampal neuronal representations in “morphed” environments. Neuron. 2005;48:345–358. doi: 10.1016/j.neuron.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Li XG, Somogyi P, Ylinen A, Buzsáki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994;339:181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Maurer AP, Cowen SL, Burke SN, Barnes CA, McNaughton BL. Organization of hippocampal cell assemblies based on theta phase precession. Hippocampus. 2006;16:785–794. doi: 10.1002/hipo.20202. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The firing of hippocampal place cells predicts the future position of freely moving rats. J Neurosci. 1989;9:4101–4110. doi: 10.1523/JNEUROSCI.09-12-04101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7:1951–1968. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura NH, McEwen BS. Changes in interneuronal phenotypes regulated by estradiol in the adult rat hippocampus: a potential role for neuropeptide Y. Neuroscience. 2005;136:357–369. doi: 10.1016/j.neuroscience.2005.07.056. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. Do hippocampal pyramidal cells signal non-spatial as well as spatial information? Hippocampus. 1999;9:352–64. doi: 10.1002/(SICI)1098-1063(1999)9:4<352::AID-HIPO3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res. 1987;68:1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon Press; 1978. [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. fourth edition. San Diego: Academic Press; 1998. [Google Scholar]
- Petersen CC. The functional organization of the barrel cortex. Neuron. 2007;56:339–355. doi: 10.1016/j.neuron.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Pinaud R, Tremere LA, Penner MR, Hess FF, Robertson HA, Currie RW. Complexity of sensory environment drives the expression of candidate-plasticity gene, nerve growth factor induced-A. Neuroscience. 2002;112:573–582. doi: 10.1016/s0306-4522(02)00094-5. [DOI] [PubMed] [Google Scholar]
- Redish AD, Battaglia FP, Chawla MK, Ekstrom AD, Gerrard JL, Lipa P, Rosenzweig ES, Worley PF, Guzowski JF, McNaughton BL, Barnes CA. Independence of firing correlates of anatomically proximate hippocampal pyramidal cells. J Neurosci. 2001;21:RC134. doi: 10.1523/JNEUROSCI.21-05-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest JM, Di Blasi F, Kitchener P, Rougé-Pont F, Desmedt A, Turiault M, Tronche F, Piazza PV. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nat Neurosci. 2005;8:664–672. doi: 10.1038/nn1441. [DOI] [PubMed] [Google Scholar]
- Ripley BD. Tests of randomness for spatial point patterns. J R Statistical Soc B (Methodological) 1979;41:368–374. [Google Scholar]
- Ripley BD. Modelling spatial patterns. J R Statistical Soc B (Methodological) 1977;39:172–212. [Google Scholar]
- Stone DJ, Walsh J, Benes FM. Localization of cells preferentially expressing GAD(67) with negligible GAD(65) transcripts in the rat hippocampus. A double in situ hybridization study. Mol Brain Res. 1999;71:201–209. doi: 10.1016/s0169-328x(99)00185-0. [DOI] [PubMed] [Google Scholar]
- Tanila H, Shapiro ML, Eichenbaum H. Discordance of spatial representation in ensembles of hippocampal place cells. Hippocampus. 1997;7:613–623. doi: 10.1002/(SICI)1098-1063(1997)7:6<613::AID-HIPO4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Best PJ. Place cells and silent cells in the hippocampus of freely-behaving rats. J Neurosci. 1989;9:2382–2390. doi: 10.1523/JNEUROSCI.09-07-02382.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24:6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills TJ, Lever C, Cacucci F, Burgess N, O’Keefe J. Attractor dynamics in the hippocampal representation of the local environment. Science. 2005;308:873–876. doi: 10.1126/science.1108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Worley PF, Bhat RV, Baraban JM, Erickson CA, McNaughton BL, Barnes CA. Thresholds for synaptic activation of transcription factors in hippocampus: correlation with long-term enhancement. J Neurosci. 1993;13:4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Wanner IB, Roper SD, Chaudhari N. An optimized method for in situ hybridization with signal amplification that allows the detection of rare mRNAs. Histochem Cytochem. 1999;47:431–446. doi: 10.1177/002215549904700402. [DOI] [PubMed] [Google Scholar]


