Abstract
Mercury is an immunotoxic substance that has been shown to induce autoimmune disease in rodent models, characterized by lymphoproliferation, overproduction of immunoglobulin (IgG and IgE), and high circulating levels of autoantibodies directed at antigens located in the nucleus (anti-nuclear autoantibodies, or ANA) or the nucleolus (anti-nucleolar autoantibodies, or ANoA). We have reported elevated levels of ANA and ANoA in human populations exposed to mercury in artisanal gold mining, though other confounding variables that may also modulate ANA/ANoA levels were not well-controlled. The goal of this study is to specifically test whether occupational and environmental conditions (other than mercury exposure) that are associated with artisanal gold mining affect the prevalence of markers of autoimmune dysfunction. We measured ANA, ANoA, and cytokine concentrations in serum and compared results from mercury-exposed artisanal gold miners to those from diamond and emerald miners working under similar conditions and with similar socioeconomic status and risks of infectious disease. Mercury-exposed gold miners had higher prevalence of detectable ANA and ANoA and higher titers of ANA and ANoA as compared to diamond and emerald miners with no occupational mercury exposure. Also, mercury-exposed gold-miners with detectable ANA or ANoA in serum had significantly higher concentrations of pro-inflammatory cytokines IL-1β, TNF-α, and IFN-γ in serum as compared to the diamond and emerald miners. This study provides further evidence that mercury exposure may lead to autoimmune dysfunction and systemic inflammation in affected populations.
Keywords: Mercury, autoimmunity, inflammation, antinuclear/antinucleolar autoantibodies, cytokines
Introduction
Mercury is a ubiquitous global environmental pollutant, with human exposure to organic, inorganic, and elemental species of mercury occurring in many diverse settings. Mercury has primarily been recognized for its effects on the developing nervous system, first noted in tragic poisonings occurring in Japan as a result of contaminated fish consumption and Iraq as a result of contaminated grain consumption (Bakir et al., 1973; Harada, 1995). Lower levels of methylmercury exposures in children resulting from a diet high in fish and other seafood have also been associated with persistent adverse neuro-developmental effects on motor, verbal, and attention abilities (Debes et al., 2006).
Through the use of rodent models, awareness of the direct effects of mercury on the immune system has increased. In these models, genetically susceptible strains of mice exposed to relatively high levels of inorganic mercury develop a lupus-like autoimmune syndrome, characterized by lymphoproliferation; high levels of circulating immunoglobulin, including auto-antibodies to nuclear and nucleolar targets (anti-nuclear autoantibodies, ANA, and anti-nucleolar antibodies, ANoA, respectively); and glomerulonephritis as a result of immune-complex deposition (Abedi-Valugerdi and Moller, 2000; Pollard et al., 2005). Female mice tend to be more susceptible to mercury-induced autoimmunity, consistent with sex differences observed in autoimmune diseases in humans (Fairweather et al., 2008; Nielsen and Hultman, 2002). In susceptible strains of mice, treatment with organic species of mercury also elicits an autoimmune response, though there are differences in toxicokinetic and the toxicodynamic responses based on the species of mercury used. Eventually, thimerosal is equipotent to inorganic mercury in eliciting a lupus-like immune response in susceptible animals, but mice treated with methylmercury do not develop renal or systemic immune complex deposition (Haggqvist et al., 2005; Havarinasab et al., 2007; Havarinasab et al., 2005; Havarinasab and Hultman, 2005). In mouse strains prone to lupus-like disease, exposure to either organic or inorganic mercury exacerbates and accelerates autoimmune disease (Havarinasab and Hultman, 2006; Hultman et al., 2006; Pollard et al., 2001).
Relatively few studies exist in the literature on the relationship of mercury exposure and biomarkers of autommunity or autoimmune disease in human populations. Cases of mercury-induced autoimmune kidney disease mediated by immune complex deposition have been noted historically in highly exposed populations, though there is some debate as to the role of autoimmunity in these cases given that mercury also causes direct damage to the kidneys (Barr et al., 1973; Bigazzi, 1999; Cardenas et al., 1993; Tubbs et al., 1982). Several studies, using levels of circulating anti-laminin antibodies and immune complex as a marker for disease, failed to find a correlation between occupational mercury exposure and markers of immune dysfunction (Barregard et al., 1997; Bernard et al., 1987; Ellingsen et al., 2000; Langworth et al., 1992). One case-control study reported a correlation between self-reported occupational exposure to mercury and increased risk of lupus (Cooper et al., 2004). A case-control study of scleroderma patients found an association between urinary mercury levels and severity of the disease, though the mercury levels were low and the sample size was small so the authors could not rule out confounding effects of kidney function in the patients (Arnett et al., 1996).
ANA and ANoA presence in serum are used in the clinical diagnosis of lupus and scleroderma (Ho and Reveille, 2003; Kurien and Scofield, 2006), and some similarities have been noted between ANA/ANoA profiles in mercury-induced autoimmunity models and in some patients with scleroderma (Takeuchi et al., 1995). In studies of communities in Brazil, we have reported that exposure to either methyl or inorganic mercury is associated with elevated titers of detectable ANA and ANoA (Alves et al., 2006; Silva et al., 2004). These findings were replicated by Alves et al. in a study of fish-consuming populations exposed to methylmercury also in Brazil (Alves et al., 2006). Exposure to mercury in these populations is related to the use of mercury in riverine small-scale artisanal gold mining operations, in which miners are directly exposed to inorganic and elemental mercury, and downstream communities can be exposed by consumption of fish contaminated by methylmercury in impacted watersheds (de Andrade Lima et al., 2008; Dominique et al., 2007; Silbergeld et al., 2005).
Our previous study of persons at the gold mining camp located at Rio-Rato in the state of Pará, Brazil reported an association between length of time in gold mining and elevated serum titers of both ANA and ANoA as compared to persons exposed to methylmercury in fish and a community with no direct or indirect contact with gold-mining or consumption of methylmercury-contaminated fish. However, the referent community also differed from the gold-mining community in many important respects, such as occupation, infectious disease status, and socioeconomic status. For that reason we undertook this study to specifically test whether occupational and environmental conditions (other than mercury exposure) that are associated with small-scale mining could be associated with the prevalence of markers of autoimmune dysfunction, ANA and ANoA. We hypothesize that it is specifically the mercury exposure related to gold-mining that is associated with increases in ANA and ANoA prevalence and titers. In order to test this hypothesis, we compared ANA/ANoA prevalence and titers in serum samples from residents of the gold-mining camp at Rio-Rato to serum samples from residents of four different mining communities, two diamond-mining camps and two emerald-mining camps in a watershed unaffected by gold-mining or mercury contamination. We chose these sites because of similarities in environmental and social factors among these different types of mining as compared to gold mining. Diamond mining, like small-scale gold mining in Amazonia, also involves hydraulic excavation of a riverbed, whereas emerald mining in this region involves subterranean excavation. Neither involves the use of mercury. Levels of physical exertion are similar in all three occupations. Socio-economic status and educational levels tend to be low in all these communities, as diamond, emerald, and gold miners are all seeking the poverty-alleviating benefits of their work.
In addition to comparing ANA and ANoA prevalence and titers, we further characterized biomarkers of immune response to mercury in mercury-exposed miners as compared to non-exposed miners by measuring serum cytokine levels. For this study, we selected cytokines that have been shown to respond to mercury exposure in animal and in vitro models (Haggqvist and Hultman, 2003; Haggqvist and Hultman, 2005; Hemdan et al., 2007; Hu et al., 1999; Kono et al., 1998; Silva et al., 2005). In order to test the hypothesis that mercury exposure will modulate serum cytokine levels, we measured the signature TH1/TH2 cytokines Interferon-γ (IFN-γ) and Interleukin-4 (IL-4), the pro-inflammatory cytokines Tumor Necrosis Factor-α (TNF-α) and IL-1β, and the anti-inflammatory cytokines IL-1 Receptor antagonist (IL-1Ra) and IL-10. In addition, we measured IL-17, which is produced by a recently characterized subset of T helper cells, TH17 cells, and may play a role in the etiology of autoimmune diseases (Langrish et al., 2005; Wilson et al., 2007).
Materials and Methods
Human subjects
We studied five separate populations in this study. All studies were conducted in collaboration with the Fundaçao Nacional de Saúde (FUNASA) of the Brazilian Ministério da Saúde in the states of Pará and Goias. The locations of study sites within Brazil are shown on a map in Figure 1. At the gold mining camp, Rio-Rato in the state of Pará, the population was directly involved in gold extraction and crude refining, resulting in relatively high but episodic exposures to elemental mercury, as has been described in detail elsewhere (Silbergeld et al., 2005). Mercury exposures are relatively high, based upon both air sampling and biomarker measurement as reported previously (Aks et al., 1995; de Jesus et al., 2001). Davinopolis, Sao Antonio Rio Verde, Campo Verde—Itaobi and Campo Verde—Vereador are located in the state of Goías, and were examined as part of a specific request of the Secretariat of Health of the state of Goías. Davinopolis and Sao Antonio Rio Verde are small diamond mining sites situated on small rivers, and Itaobi and Vereador (both in Campo Verde) are emerald mines organized around a central subterranean emerald deposit.
Figure 1. Map of study sites within Brazil.
Gold miners were recruited at Rio-Rato, a gold mining camp located along Rio Tapajós in the state of Pará. Emerald and diamond miners were recruited from small mining camps located in the state of Goiás.
At each location, enrollment and sampling locations were set up in central public locations. At the two emerald mines in Campo Verde, enrollment was conducted directly at the mine for one day each and covered two shifts of workers at each site. Study enrollment also took place over the course of one day each at Sao Antonio Rio Verde and Davinopolis. During an initial visit by study personnel, information was disseminated to the mine owners and workers, and the day for the study was announced through flyers and public meetings. All miners at each site were invited to take part in the study, and we estimate that we enrolled 100% of the population present at each site. At Rio-Rato, all residents of the camp were also invited to take part in the study, but it is difficult to judge the participation rate because of the dispersion of the gold-miners at distant locations from the camp. For that reason, at Rio-Rato we conducted enrollment for five days to ensure participation of as many persons as possible. At all sites, 100% of those asked to participate agreed to be a part of the study. The only exclusion criterion was self-disclosed information that indicated that the subject was not involved in mining.
Information was collected by interview, administered in Portuguese by trained personnel, to provide information on demographics (age, gender, educational attainment), birthplace, current/previous occupation (including past uses of mercury), income, health status, reproductive history (women), drug and alcohol use, past and current malaria infections, occupational history, prior work in mining, length of time residing at the site, and self-reported medical history. A short clinical examination was conducted and samples of hair, urine, blood, and/or stool were collected for laboratory analysis. Malaria was assessed by a questionnaire used in infectious disease surveillance by FUNASA in order to determine past self-reported history of malaria. Prevalent malaria was determined by thick smears taken at the time of interview; slides were read by trained technicians in the clinical malaria service at IEC in Belem, Pará.
This study was approved and supervised by the institutional review board of FUNASA, as well as the Committee on Human Research at the Johns Hopkins Bloomberg School of Public Health (JHSPH).
Mercury exposure
Mercury exposure was determined by occupational and past exposure information gathered with the questionnaire and also by biomarker sampling. Mercury exposure at Rio-Rato was determined by urine analysis. For the diamond and emerald miners, mercury exposure was determined by hair analysis. Standard cold vapor atomic absorption spectrophotometry methods were utilized to measure mercury in both hair and urine. Mercury measurements were conducted by a laboratory of FUNASA that participates in an international QA/QC program with the Universite de Quebec a Montreal (Dolbec et al., 2001; Lebel et al., 1997; Roulet et al., 1998). Because of the remote locations of sample collection, it was not possible to carry out measurements of urine creatinine. The limit of detection (LOD) for the urine samples was 0.50 µg Hg/L urine. All values that were LOD were assigned the value of LOD/√2 for plotting and analysis. No hair mercury levels were below the LOD.
Blood and serum sample collection
Blood was collected by venipuncture by a trained phlebotomist after completion of each interview. Samples were allowed to clot, centrifuged on site, aliquoted, and immediately frozen on liquid nitrogen. Samples were first transported by air to the IEC in Belém, Pará. Aliquots were then transported to Baltimore by air on dry ice. Serum samples were stored at −80°C until analysis.
Detection of ANA/ANoA
After transfer to JHSPH, the coded serum samples were thawed on ice. Serum samples were serially diluted two-fold from 1:10 to 1:320 and analyzed by indirect immunofluorescence (IIF) microscopy using commercially available HEp-2 slides (INOVA Diagnostics), according to the manufacturer’s instructions. Briefly, samples (or manufacturer supplied positive and negative controls) were applied to slide wells coated with HEp-2 cells and incubated for 30 minutes in a humidified chamber. Slides were washed and incubated with FITC-conjugated goat anti-human IgG detection antibodies. Slides were washed again and stored at 4°C until analysis. Slides were analyzed with at least 100X magnification by three blinded readers (RMG, IAS, and JFN). ANA/ANoA data are expressed as the inverse of the highest titer, or the dilution factor, at which fluorescence could still be detected.
Cytokine measurement
Sample selection
For these analyses, a subset of the population was utilized. Sample identity was unmasked after ANA and ANoA analysis. Participants were divided into four groups, either high or low Hg with +/− ANA and/or ANoA (AN(o)A): 1) High Hg/AN(o)A+, 2) High Hg/AN(o)A−, 3) Low Hg/AN(o)A+, and 4) Low Hg/AN(o)A−.
The High Hg groups were selected from the Rio-Rato gold miners based on elevated urinary Hg concentrations. We chose to use a cut-off of 3.0 µg Hg/L urine, which represents the 95th percentile for adults within the US imputed from the geometric mean and standard deviation reported by Dye et al. (0.72 µg Hg/L + 2*(1.14 µg Hg/L) = 3.0 µg Hg/L) (Dye et al., 2005). This resulted in range of 3.12 – 81.37 µg Hg/L urine within the High Hg groups. The Low Hg groups were selected from the diamond and emerald miners based on their lower hair mercury levels. Participants were included in the Low Hg groups if their hair mercury level was less than 1.73 µg Hg/g hair, based on the 95th percentile reported by McDowell et al. for hair mercury levels within US adults, for a range of 0.16 µg Hg/g – 1.63 µg Hg/g in the Low Hg groups (McDowell et al., 2004).
The High Hg and Low Hg groups were further divided based on their detectable ANA and ANoA titers. Participants with any detectable ANA or ANoA in their serum were labeled as AN(o)A+. Participants with no detectable ANA or ANoA in their serum were labeled as AN(o)A−.
Cytokine Analysis
An aliquot of each selected serum sample was analyzed for cytokine content using the Bio-Plex multiplex bead-based cytokine assay (Bio-Rad) according to the manufacturer’s instructions. The following seven cytokines were measured in serum from a subset of participants: IL-1β, IL-1Ra, IL-4, IL-10, IL-17, IFN-γ, and TNF-α. Briefly, color-coded fluorescent beads conjugated with antibodies specific for the cytokine of interest were incubated at room temperature with the serum sample. After washing, the beads were incubated with a biotinylated detection antibody. The beads were washed and treated with streptavidin-PE to detect the bound antibodies. The beads were then analyzed using the Bio-Plex Suspension Array system, a flow-based instrument with the ability to detect the presence of streptavidin-PE bound antibodies and the fluorescence range of each bead. Using information from a standard curve for each cytokine measured, it was possible to infer the concentration of each cytokine present in the sample. Cytokine measurements that were below the LOD (as determined by the standard curve for each cytokine individually) were assigned a value of the LOD/√2 for statistical analysis and plotting. These samples were included in both the plots presented and the statistical analysis conducted, except in the case of IL-4, IL-17, and IL-10 where a majority of the samples had a value below the LOD.
Statistical Analysis
Given the skewed distribution of both observed mercury biomarker measurements and serum cytokine concentrations, concentration measurements of mercury biomarker levels and cytokine levels were log-transformed for plotting and analysis, though median and interquartile ranges are given for each measurement on their natural scale. Log-transformed cytokine values were compared using one-way analysis of variance (ANOVA) followed by pairwise post-tests using the Bonferroni correction.
We used simple logistic regression in order to model the log-odds of ANA or ANoA positivity (at any titer) among individual (i) diamond (DM) or gold miners (GM) compared to emerald miners. Here Yi represents the ANA or ANoA status (positive or negative) of the ith individual:
Model 1
Emerald miners were chosen as a referent group, though both diamond miners and emerald miners could be considered appropriate referent groups, given the similarities in occupational conditions. Because sex and malarial status could potentially influence the odds of ANA or ANoA positivity, these were also tested in a simple logistic regression model:
Model 2
Because we used repeated measures of individual serum samples at increasing dilution factors, we also modeled the cumulative distribution of ANA or ANoA positivity as a function of occupational group (diamond or gold miner, with emerald miners again as the referent population) and dilution factor (DF = 10, 20, 40, 80, 160, or 320):
Model 3
In this model, Yij represents the ANA or ANoA status (positive or negative) of the ith individual at the jth dilution factor (DF).
Sex and malarial status were also tested in models of the cumulative distribution ANA or ANoA positivity:
Model 4
The generalized estimating equation (GEE) with robust estimation of standard errors (Zeger and Liang, 1986) was used to generate marginal, population-averaged estimates of the log-odds of ANA/ANoA positivity for models 3 and 4. This method of estimation accounts for the correlation structure of the dataset so that valid standard errors of parameter estimates can be drawn. The correlation structure that minimized the “quasi-likelihood under the independence model criterion” (QIC) was chosen as the correlation structure for the final model (Pan, 2001a; Pan, 2001b).
All analyses were conducted in STATA (Stata Corp, version 10IC). STATA, R (R project, version 2.8.0), and ArcGIS (ESRI, version 9) were used to create data plots, graphs and maps.
Results
Mercury exposure and population characteristics
Population characteristics for each of the mining populations are summarized in Table 1. Most of the participants were male in each population.
Table 1.
Population characteristics by recruitment site.
| Type of mining |
Population | N | Hg Biomarker (Median, Interquartile range) |
Sex | Prevalent Malaria |
|---|---|---|---|---|---|
| Gold | Rio-Rato | n = 98 | 3.67 µg/L urine (0.87 – 8.93) |
M = 64 (65%) F = 34 (35%) |
Y = 92 (94%) N = 6 (6%) |
| Emerald | Campo Verde, Vereador |
n = 22 | 0.268 µg/g hair (0.217 – 0.398) |
M = 22 (100%) F = 0 (0%) |
Y = 1 (5%) N = 21 (95%) |
| Campo Verde, Itaobi |
n = 69 | 0.290 µg/g hair (0.220 – 0.470) |
M = 35 (51%) F = 34 (49%) |
Y = 1 (1%) N = 68 (99%) |
|
| Diamond | Davinopolis | n = 24 | 0.48 µg/g hair (0.29 – 0.63) |
M = 20 (83%) F = 4 (17%) |
Y = 8 (33%) N = 16 (67%) |
| Sao Antonio Rio Verde |
n = 33 | 0.96 µg/g hair (0.6 – 1.29) |
M = 31 (94%) F = 2 (6%) |
Y = 14 (42%) N = 19 (58%) |
Rio-Rato is a gold mining site, and participants were directly involved in some aspect of gold extraction or refining, as described previously (Silbergeld et al., 2002; Silva et al., 2004). Urine mercury concentrations ranged from below the LOD (0.50 µg Hg/L urine) to 81.4 µg Hg/L urine, with a median value of 3.67 (Figure 2). There was a high proportion of both past and prevalent malaria among Rio-Rato participants, with 93% having prevalent malaria at the time of the study.
Figure 2. Biomarkers of mercury exposure in five different mining communities.
A. In Rio-Rato, urine mercury levels (µg Hg/L) were measured for each participant. Data are plotted on the log scale, with each circle representing the urine mercury level for a single participant. The inter-quartile range and median of the data are also represented. B. In the emerald mining communities of Campo Verde, Itaobi (CVI) and Campo Verde, Vereador (CVV) and in the diamond mining communities of Davinopolis (DAV) and Sao Antonio Rio Verde (SARV), hair mercury levels (µg Hg/g hair) were measured for each participant. Data are represented on the log scale as described in A.
Participants from the four diamond and emerald mining communities had relatively low hair Hg concentrations (overall median = 0.37 µg Hg/g hair; range 0.129 – 3.00 µg Hg/g hair; see Table 1 and Figure 2 for summaries by site), especially when compared with other Brazilian populations as reported by us and others (Crompton et al., 2002; Santos et al., 2000). The levels are elevated as compared to the median for adults in the US population, and the distribution is considerably wider (McDowell et al., 2004). Also as expected, participants from the two diamond-mining communities had a higher rate of prevalent malaria (33% with prevalent malaria in Davinopolis, and 42% in Sao Antonio Rio Verde), as compared to residents of emerald-mining communities, where prevalent malaria was found in only one participant at each site. This is likely due to the ecology of the diamond mining operations, in rivers, as compared to diamond mines.
Gold miners are more likely to have detectable ANA/ANoA in serum as compared to diamond or emerald miners
ANA and ANoA were analyzed in serum samples from all participants. For analysis, we grouped the data by type of mining, as shown in Table 2.
Table 2.
Odds of detectable ANA/ANoA in mining populations. Emerald miners were used as the referent population.
| ANA | ANoA | |||||
|---|---|---|---|---|---|---|
| Population | OR | p-value | 95% CI | OR | p-value | 95% CI |
| Model 1a | ||||||
| Diamond miners | 0.7 | 0.529 | 0.2 – 2.1 | 2.5 | 0.330 | 0.4– 15.2 |
| Gold miners | 8.6 | <0.001 | 4.1 – 18.0 | 30.6 | <0.001 | 7.1– 131.9 |
| Model 2b | ||||||
| Diamond miners | 0.7 | 0.613 | 0.2 – 2.4 | 2.9 | 0.256 | 0.45 – 19.2 |
| Gold miners | 6.9 | 0.003 | 1.9 – 24.9 | 35.6 | <0.001 | 5.3– 235.6 |
| Model 3c | ||||||
| Diamond miners | 0.6 | 0.400 | 0.2 – 1.9 | 2.1 | 0.384 | 0.4– 12.3 |
| Gold miners | 17.2 | <0.001 | 8.2 – 36.5 | 46.7 | <0.001 | 11.1– 197.6 |
| Model 4d | ||||||
| Diamond miners | 0.6 | 0.494 | 0.2 – 2.2 | 2.6 | 0.407 | 0.6 – 22.9 |
| Gold miners | 16.1 | <0.001 | 4.0 – 65.2 | 51.9 | <0.001 | 8.7– 309.8 |
Simple logistic regression.
Simple logistic regression adjusted for sex and malarial status.
GEE model of cumulative distribution.
GEE model adjusted for sex and malarial status.
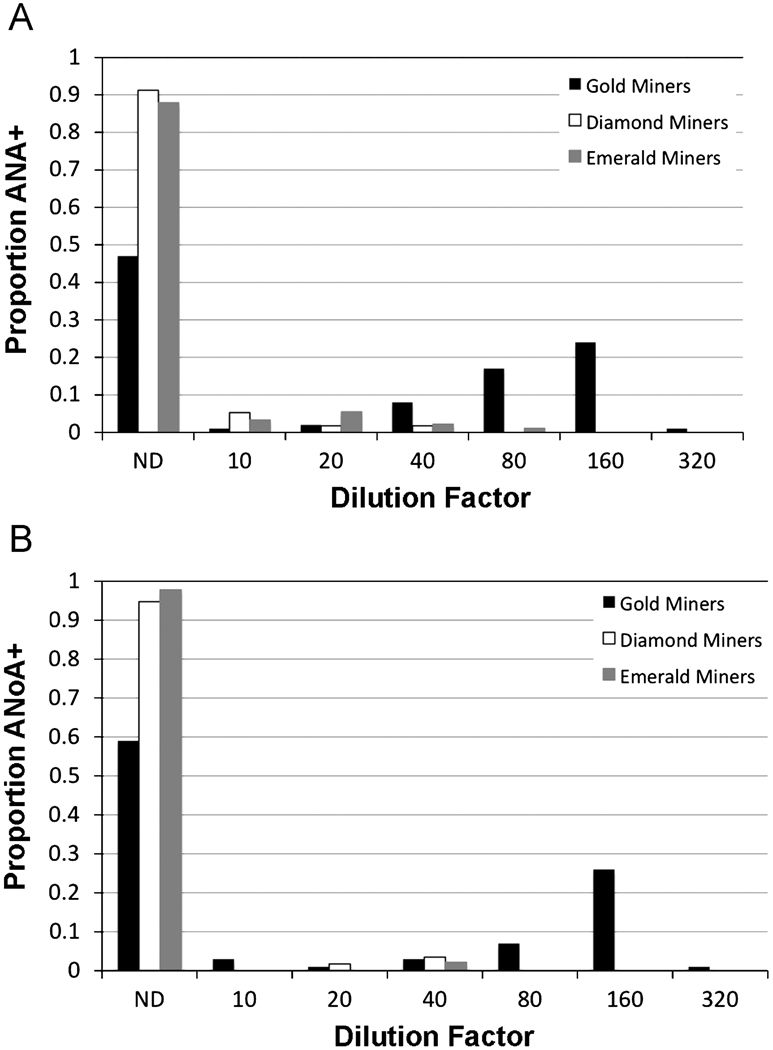
A higher proportion of gold miners had detectable ANA in their serum compared to either diamond or emerald miners, and titers at which ANA could be detected were higher in this group (Figure 3A). 47% of the gold-mining population at Rio-Rato had no ANA detectable in their serum, and 51% of the population had ANA at dilution factors ≥ 40. 91% of diamond miners and 88% of emerald miners had no detectable ANA, and only 2% of emerald miners had ANA detectable at dilution factors ≥ 40. Among the diamond miners, no individual had ANA detectable at a dilution factor greater than 40. In a simple logistic regression of ANA positivity (Model 1), gold miners had an odds ratio (OR) of 8.6 (p<0.001) compared to emerald miners, while diamond miners were not significantly different from emerald miners (Table 2). Sex (p = 0.162) and malarial status (p = 0.638) were not significant in the model (Model 2). Sex- and malaria-adjusted OR estimates for diamond and gold miners are also shown in Table 2.
Figure 3. Detectable ANA and ANoA are more prevalent in a mercury exposed, gold mining community compared to emerald and diamond mining communities.
Serum samples from gold miners (n=98, from Rio-Rato), diamond miners (n=57, from Sao Antonio Rio Verde and Davinopolis), and emerald miners (n=91, from Campo Verde, Vereador and Campo Verde, Itaobi) were serially diluted and assayed for ANA and ANoA. Results show the proportion of the population that has a detectable amount of ANA (A) or ANoA (B) at the inverse titer shown, with ND indicating that no ANA or ANoA was detectable at any dilution.
A similar trend is observed for ANoA (Figure 3B). 59% of the gold-mining population had no detectable ANoA, and 37% of the population had ANoA detectable at dilution factors ≥ 40. 95% of the diamond miners and 98% of the emerald miners had no detectable ANoA. 4% of diamond miners had ANoA detectable at a dilution factor of 40, and 2% of emerald miners had ANoA at this level. No diamond or emerald miner had ANoA detectable at dilution factors greater than 40. In a simple logistic regression of ANoA positivity (Model 1), gold miners had an OR of 30.6 (p<0.001) compared to emerald miners, while diamond miners were not significantly different from emerald miners (Table 2). Sex (p = 0.217) and malarial status (p = 0.856) were not significant in the model (Model 2); adjusted OR estimates for diamond and gold miners are shown in Table 2.
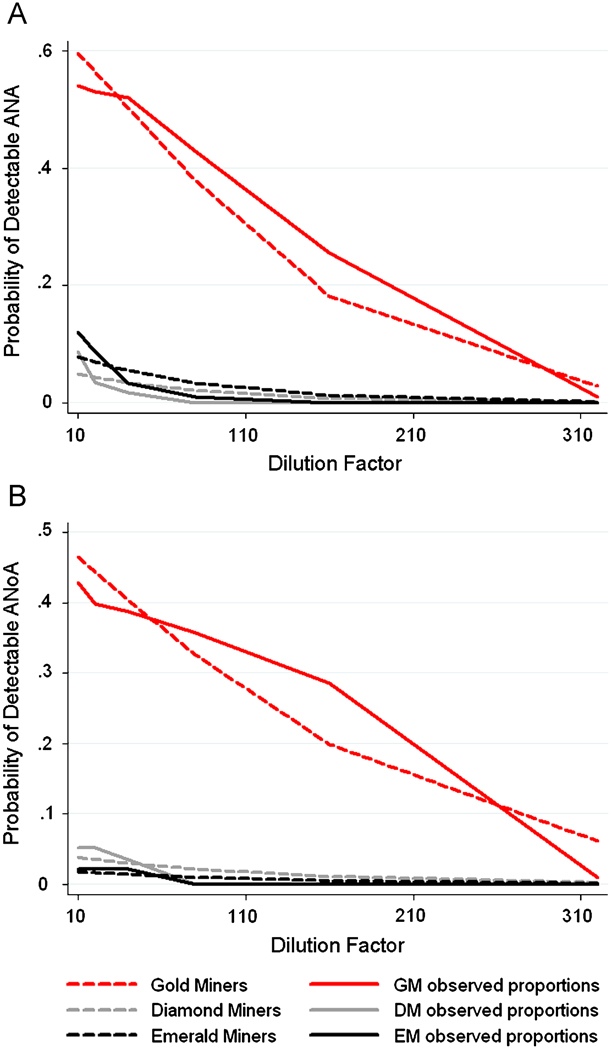
To formally test the difference in the cumulative distribution of titers of ANA and ANoA in the three mining populations, we fit a logistic regression model with an independent correlation structure using GEE which predicts the log-odds of having detectable ANA or ANoA over the range of dilution factors (inverse titers) used (Model 3). The predicted probability of detectable ANA and ANoA for Model 3 is shown in Figure 4, along with the observed proportion of detectable ANA and ANoA. Gold miners are more likely than emerald miners to have ANA detected at higher titers (OR = 17.1, p < 0.001), and were also significantly more likely to have ANoA detected at higher titers (OR = 46.7, p < 0.001) compared to emerald miners. Diamond miners were not significantly different from emerald miners for both ANA and ANoA. Sex (ANA p = 0.312; ANoA p = 0.331) and malaria (ANA p = 0.880; ANoA p = 0.885) were not significant in models of the cumulative distribution of ANA and ANoA positivity (Model 4); sex- and malaria-adjusted ORs are shown in Table 2.
Figure 4. Predicted probabilities and observed proportions of ANA and ANoA in mining populations.
The predicted probabilities obtained from fitting Model 3 to the data are plotted in dashed lines, and compared to the observed proportion of positive responses for ANA (A) and ANoA (B) for each population of miners.
Interestingly, we found upon decoding the identities of the participants that among the diamond or emerald miners there were 21 subjects with detectable ANA or ANoA from diamond or emerald miners. Of these, six were persons who reported past occupational exposure to mercury through past work in small-scale gold mining. In a simple logistic regression model of the ANA or ANoA status accounting for past history of mercury exposure (adjusted for sex and malarial status) and using data from diamond and emerald miners only, past mercury exposure yields an odds ratio of 1.98 (95% CI: 0.65–6.01). This result was suggestive but not statistically significant given the very small numbers of people within each group.
AN(o)A+ gold-miners have higher concentrations of pro-inflammatory cytokines in serum compared to diamond and emerald miners
For these analyses, we divided the serum samples into four groups based on ANA or ANoA status and mercury exposure, as described in Materials and Methods. Group characteristics and cytokine measurements by group are reported in Table 3. Both High Hg groups (High Hg/AN(o)A+ and High Hg/AN(o)A−) had similar ranges of mercury levels in urine. Similarly, both Low Hg groups (Low Hg/AN(o)A+ and Low Hg/AN(o)A−) had similar ranges of mercury levels in hair. Given the different prevalence and distribution of titers between the gold miners of Rio-Rato and the diamond and emerald miners (see Figure 3) for both ANA and ANoA, participants selected for the High Hg/AN(o)A+ group had higher titers on average compared to the Low Hg/AN(o)A+ group. 88% of the High Hg/AN(o)A+ group had an ANA or ANoA titer greater than 1:40, while 36% of the Low Hg/AN(o)A+ group had a titer greater than 1:40.
Table 3.
Serum cytokine measurements according to ANA or ANoA status and mercury exposure.
| Group | N | Hg biomarker: Median (Range) |
Sex | IL-1β | IL-1ra | TNF-α | IFN-γ |
|---|---|---|---|---|---|---|---|
| High Hg/ AN(o)A+ |
25 | 9.74 µg/L urine (4.92 – 74.28) |
M = 16 F = 9 |
7.33a (1.85 – 22.5) |
19.2 (2.27 – 30. 7) |
4.59 (0.71 – 11.8) |
10.1 (2.09 – 22.9) |
| High Hg/ AN(o)A− |
10 | 9.06 µg/L urine (3.12 – 81.37) |
M = 7 F = 3 |
1.47 (1.00 – 12.9) |
11.2 (2.27 – 51.2) |
0.98 (0.212 – 15.9) |
2.28 (0.233 – 48.9) |
| Low Hg/ AN(o)A+ |
14 | 0.342 µg/g hair (0.20 – 1.63) |
M = 6 F = 8 |
0.45 (0.12 – 1.74) |
12.4 (2.27 – 22.8) |
0.212 (0.212 – 1.79) |
0.233 (0.233 – 48.9) |
| Low Hg/ AN(o)A− |
28 | 0.450 µg/g hair (0.16 – 1.22) |
M = 24 F = 4 |
1.11 (0.46 – 2.55) |
18.6 (12.7 – 40.6) |
0.624 (0.212 – 2.41) |
0.504 (0.233 – 2.09) |
All cytokine levels are in pg/ml, with the median (interquartile range) represented.
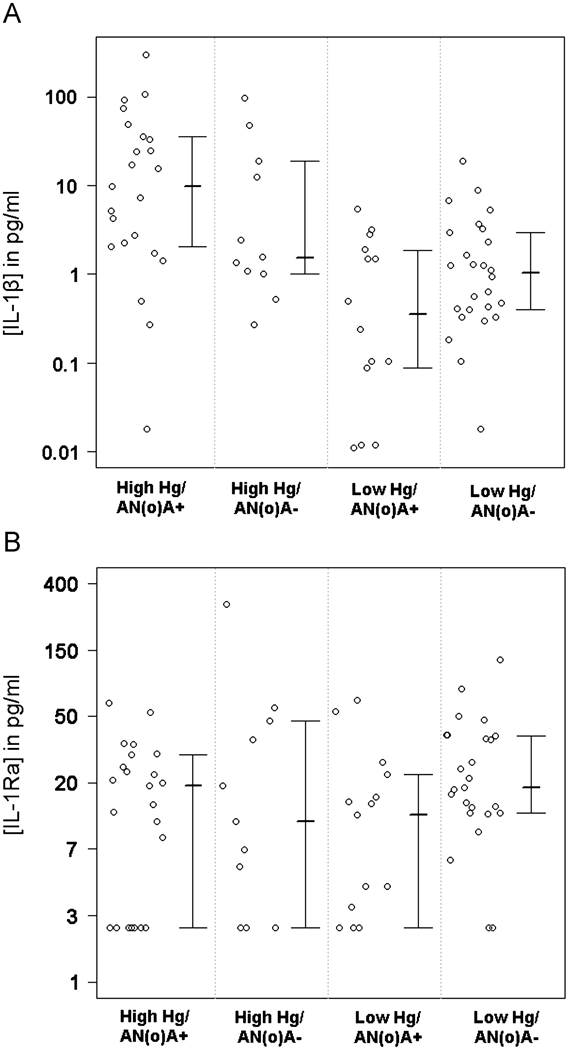
In the High Hg/AN(o)A+ group, significantly higher levels of serum IL-1β were found, as compared to individuals in the low mercury exposure groups, regardless of the AN(o)A status (Figure 5A; p < 0.001 compared to the Low Hg/AN(o)A+ group; p = 0.003 compared to the Low Hg/AN(o)A− group). In contrast, the concentrations of the IL-1β antagonist, IL-1Ra, were not significantly different between the four groups (ANOVA p = 0.120, Figure 5B). 15 samples were below the LOD for the IL-1Ra assay (8.9 pg/ml).
Figure 5. Serum concentrations of the pro-inflammatory cytokine IL-1β are higher in occupationally Hg-exposed, ANA+ or ANoA+ individuals compared to non-Hg-exposed individuals, though serum concentrations of the anti-inflammatory cytokine IL-1Ra do not differ between groups.
A. Log-transformed data are shown for the populations described in Table 2, with each circle representing the IL-1β level for a single participant. The inter-quartile range and median of the data are also represented. The mean of High Hg/AN(o)A+ group is significantly different from both the Low Hg/AN(o)A+ (p < 0.001) and Low Hg/AN(o)A− (p=0.003) groups. B. Data are shown for IL-1Ra as in A. The means of the four groups did not differ significantly (p=0.120).
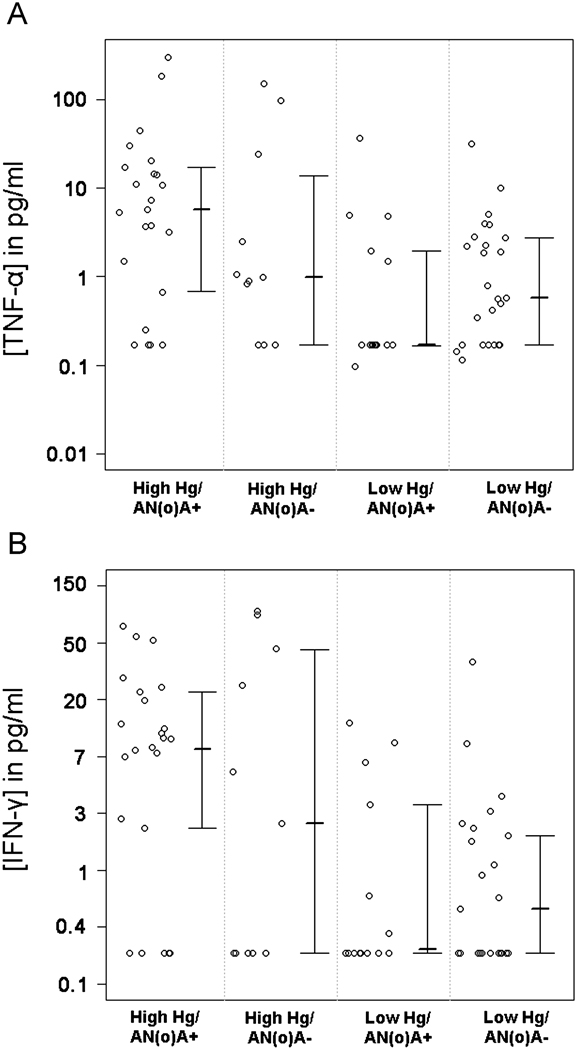
TNF-α, another pro-inflammatory cytokine, was also elevated in the High Hg/AN(o)A+ group as compared to the Low Hg/AN(o)A+ group (p < 0.007) and the Low Hg/AN(o)A− group (p=0.027, Figure 6A). 20 samples were below the LOD for TNF-α of the assay (0.3 pg/ml).
Figure 6. Serum concentrations of the pro-inflammatory cytokines TNF-α and IFN-γ are higher in occupationally Hg-exposed, ANA+ or ANoA+ individuals compared to non-Hg-exposed individuals.
A. Log-transformed data are shown for the populations described in Table 2, with each circle representing the TNF-α level for a single participant. The inter-quartile range and median of the data are also represented. The mean of the observed log-transformed TNF-α concentrations for the High Hg/AN(o)A+ group is significantly different from both the Low Hg/AN(o)A+ (p < 0.007) and Low Hg/AN(o)A−(p=0.027) groups. B. Data are shown for IFN-γ as in A. The mean of the observed log-transformed IFN-γ concentrations for the High Hg/AN(o)A+ group is significantly different from both the Low Hg/AN(o)A+ (p = 0.006) and Low Hg/AN(o)A− (p=0.010) groups.
IFN-γ, a cytokine generally associated with inflammatory responses of TH1 cells, was elevated in the High Hg/AN(o)A+ group compared to the Low Hg/AN(o)A+ group (p = 0.006) and the Low Hg/AN(o)A− group (p = 0.010, Figure 6B). However, it should be noted that 42% of the serum samples (n = 32) were below the LOD for IFN-γ (0.33 pg/ml), with a higher proportion of non-detect values occurring in the Low Hg groups.
IL-4, IL-10, and IL-17 were also measured in the serum samples, but 69%, 52%, and 81% respectively, of the total cytokine measurements were lower than the LOD, preventing analysis and comparison of the groups. Samples below the LOD were present in all groups.
Discussion
In this study, we found that the associations between detectable and elevated ANA or ANoA are associated specifically with exposure to mercury in the context of small scale gold mining, as compared to other miners in similar situations where mercury is not utilized. Gold mining involves high levels of physical exertion, and the population is at high risk of exposure to infectious diseases, such as malaria, due to the extraction of gold from riverine environments. In addition, these miners are exposed to elemental and inorganic mercury occupationally and in many cases also to methylmercury through consumption of fish from the contaminated river. The levels of urinary mercury in the gold miners are lower than previously described in other gold-mining populations, likely due to the episodic nature of exposure and timing of the data collection during the dry season when gold mining activities are lessened. However, the levels are elevated compared to urine mercury levels found within a non-occupationally exposed US population of women by the NHANES study (both unadjusted for creatinine) (Dye et al., 2005).
Diamond and emerald miners experience many similar physical and environmental stresses compared to the gold miners, with the exception of occupational exposure to mercury. While hair mercury levels in this population are elevated compared to US populations captured by the NHANES study, the hair levels are much lower than those reported in other Amazonian communities (Crompton et al., 2002; McDowell et al., 2004; Passos et al., 2008; Passos et al., 2007; Silva et al., 2004). It is also important to consider that hair mercury levels for men were only recently added to the NHANES study, and only urine and hair mercury levels in women and children have yet been reported (Nesheim et al., 2007). Men and women may have important difference in their exposure to mercury as well as in the toxicokinetic and toxicodynamic handling of those metals (Vahter et al., 2007). Given that the majority of our participants were male, comparisons to other populations should be cautious.
The statistical modeling approach used in this study allowed us to directly contrast the distribution of ANA and ANoA in the different mining populations, and formally test the statistical significance of the difference in titer distribution. Gold miners were much more likely to have ANA or ANoA present in their serum, and were also much more likely to have elevated levels of these biomarkers compared to either emerald or diamond miners. The prevalence and distribution of titers of serum ANA and ANoA in the non-mercury exposed diamond and emerald miners were comparable to other non-mercury exposed populations that have been evaluated both within Brazil and the United States, including the referent population of Tabatinga in the state of Pará, Brazil originally measured by us (Fernandez et al., 2003; Silva et al., 2004; Tan et al., 1997). However, the prevalence and distribution of titers within the mercury-exposed, gold-mining population are clearly different from both the referent groups used in this study and healthy populations documented in the literature. Among the diamond and emerald miners with detectable ANA or ANoA, past mercury exposure as a gold-miner was commonly reported. While a formal test of this relationship did not indicate a statistical significance, this result is quite suggestive that past mercury exposure may lead to a persistent effect on the human immune system. This question requires further study in a larger population of people previously, but not currently, exposed to mercury.
In addition to these findings, we report the first evidence that mercury may modulate cytokine signaling in exposed human populations. We found that levels of the pro-inflammatory cytokines IL-1β, TNF-α, and IFN-γ are significantly higher in mercury-exposed, AN(o)A+ individuals compared to individuals with lower mercury exposures. Excessive and unchecked production of pro-inflammatory cytokines plays a critical role in the etiology and progression of autoimmune diseases, in addition to many other diseases characterized by excessive inflammation (Apostolakis et al., 2008; Arend, 2002; Dinarello, 1996; Eklund et al., 2007; Prudhomme et al., 1995). Concentrations of the direct antagonist of IL-1β, IL-1Ra, were not found to be significantly different in the groups characterized by AN(o)A status and mercury exposure. IL-10, another anti-inflammatory cytokine, was not highly expressed, and levels were below the LOD for most participants regardless of mercury exposure or AN(o)A status. These results indicate that mercury exposure may be elevating systemic levels of pro-inflammatory cytokines without a concomitant increase in anti-inflammatory cytokine levels, especially within those individuals who are producing auto-antibodies.
A recent study by Hemdan et al. reported that TNF-α expression is suppressed in human peripheral blood mononuclear cells that are stimulated by monoclonal antibodies to the T-cell receptor or with heat-killed gram(−) bacteria. IFN-γ expression was modulated by mercury, dependent on the pathway of immune stimulation. Considerable variation in cytokine production was observed between participants and over the concentrations of mercury used (Hemdan et al., 2007). We report that expression of TNF-α and IFN-γ are upregulated in a mercury-exposed, AN(o)A+ population as compared to non-exposed populations. These differences may be related to both the activation state of the affected immune cells and genetic differences between the populations in susceptibility to mercury-induced immune dysfunction.
We measured IFN-γ, IL-4, and IL-17 as markers of T cell subsets that have been shown to be important in the etiology of progression of autoimmune disease. IFN-γ levels were significantly higher in mercury-exposed, AN(o)A+ individuals compared to non-exposed individuals. IFN-γ has been demonstrated to be required for the induction of murine mercury-induced autoimmunity (Hu et al., 1999; Kono et al., 1998). Increases in IFN-γ have also been documented in other murine models of lupus and in human populations affected by autoimmune disease (Berner et al., 2000; Prudhomme et al., 1995). Most of our participants, regardless of mining occupation and AN(o)A status, did not have detectable levels of IL-4 and IL-17. Given the well-documented mutual antagonism of T helper subsets (Palmer and Restifo, 2009), generally low levels of circulating IL-4 and IL-17 are not surprising.
This study was somewhat limited by the difference in biomarkers used in the different mining populations, which did not allow for a direct comparison of mercury levels between the gold-mining community and the diamond and emerald mining communities and prevented integration of an individual-level mercury biomarker into our statistical model to evaluate the risk of developing ANA and ANoA. Urine mercury concentrations are the most appropriate biomarker to use when monitoring inorganic and elemental mercury exposures, especially in the case of occupational exposures. Hair mercury levels are the most appropriate to explore exposure to organic mercury species, given that hair incorporates organic mercury into its matrix and integrates exposure levels over a time period of years. Given the potential exposures of the diamond and emerald miners, hair mercury was a logical choice for a biomarker of mercury exposure. Nevertheless, even with different biomarkers, it is clear that the Rio-Rato miners were exposed to mercury and the diamond and emerald miners were not exposed to mercury, as compared to persons with occupational exposures or high levels of fish consumption.
This study has relevance for human populations all over the world, given the ubiquitous nature of mercury exposure from multiple sources (National Research Council (U.S.) Board on Environmental Studies and Toxicology., 2000). Most directly, this study suggests that exposure to elemental and inorganic mercury, such as that associated with artisanal gold mining, has significant impacts on the human immune system. The modulation of cytokine and antibody responses by mercury may affect the susceptibility of exposed persons to diseases of immune activation, such as autoimmunity and allergy, but could also significantly alter host-pathogen interactions and have consequences for susceptibility to infectious disease. This is of concern given the prevalence of this practice worldwide (Appleton et al., 2006; Donkor et al., 2006; Gammons et al., 2006; Ikingura et al., 2006; Limbong et al., 2003; Spiegel et al., 2006; Taylor et al., 2005; Wu et al., 2006). Our previous work showed that exposure to organic mercury species may have a similar, though less severe, impact on the human immune system in terms of ANA and ANoA responses in exposed populations (Silva et al., 2004). Work in animal models has similarly shown that organic mercury species induce autoimmunity in susceptible strains of mice, similar to the effects of inorganic mercury (Havarinasab and Hultman, 2005; Silva et al., 2004). The primary source of mercury exposure for much of the world’s population is due to consumption of methylmercury-contaminated fish, which leads to chronic, low-dose mercury exposures (National Research Council (U.S.) Board on Environmental Studies and Toxicology., 2000). This study reflects the importance of the consideration of the effects of mercury exposure on immune response in humans when evaluating the public health impact of mercury, and the further need to evaluate the impact of low-dose and chronic exposures to mercury on the human immune system.
Acknowledgements
The authors would like to thank Beth Feingold for her help in constructing the map used in Figure 1.
Funding sources:
This research was supported by grants from Cure Autism Now and the United States National Institute of Environmental Health Sciences (Grant numbers 1R21ES014857-01, ES07141, K99ES015426).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Human subjects research approval:
This study was approved and supervised by the institutional review board of Fundaçao Nacional de Saúde (FUNASA), as well as the Committee on Human Research at the Johns Hopkins Bloomberg School of Public Health (JHSPH).
References
- Abedi-Valugerdi M, Moller G. Contribution of H-2 and non-H-2 genes in the control of mercury-induced autoimmunity. Int Immunol. 2000;12:1425–1430. doi: 10.1093/intimm/12.10.1425. [DOI] [PubMed] [Google Scholar]
- Aks SE, et al. Fractional mercury levels in Brazilian gold refiners and miners. J Toxicol Clin Toxicol. 1995;33:1–10. doi: 10.3109/15563659509020208. [DOI] [PubMed] [Google Scholar]
- Alves MF, et al. Fish consumption, mercury exposure and serum antinuclear antibody in Amazonians. Int J Environ Health Res. 2006;16:255–262. doi: 10.1080/09603120600734147. [DOI] [PubMed] [Google Scholar]
- Apostolakis S, et al. IL-1 cytokines in cardiovascular disease: diagnostic, prognostic and therapeutic implications. Cardiovasc Hematol Agents Med Chem. 2008;6:150–158. doi: 10.2174/187152508783955006. [DOI] [PubMed] [Google Scholar]
- Appleton JD, et al. Impacts of mercury contaminated mining waste on soil quality, crops, bivalves, and fish in the Naboc River area, Mindanao, Philippines. Sci Total Environ. 2006;354:198–211. doi: 10.1016/j.scitotenv.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- Arnett FC, et al. Autoantibodies to fibrillarin in systemic sclerosis (scleroderma). An immunogenetic, serologic, and clinical analysis. Arthritis Rheum. 1996;39:1151–1160. doi: 10.1002/art.1780390712. [DOI] [PubMed] [Google Scholar]
- Bakir F, et al. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Barr RD, et al. Levels of mercury in urine correlated with the use of skin lightening creams. Am J Clin Pathol. 1973;59:36–40. doi: 10.1093/ajcp/59.1.36. [DOI] [PubMed] [Google Scholar]
- Barregard L, et al. A study of autoantibodies and circulating immune complexes in mercury-exposed chloralkali workers. Int Arch Occup Environ Health. 1997;70:101–106. doi: 10.1007/s004200050193. [DOI] [PubMed] [Google Scholar]
- Bernard AM, et al. Search for anti-laminin antibodies in the serum of workers exposed to cadmium, mercury vapour or lead. Int Arch Occup Environ Health. 1987;59:303–309. doi: 10.1007/BF00377742. [DOI] [PubMed] [Google Scholar]
- Berner B, et al. Analysis of Th1 and Th2 cytokines expressing CD4+ and CD8+ T cells in rheumatoid arthritis by flow cytometry. J Rheumatol. 2000;27:1128–1135. [PubMed] [Google Scholar]
- Bigazzi PE. Metals and kidney autoimmunity. Environ Health Perspect. 1999;107 Suppl 5:753–765. doi: 10.1289/ehp.99107s5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, et al. Markers of early renal changes induced by industrial pollutants. I. Application to workers exposed to mercury vapour. Br J Ind Med. 1993;50:17–27. doi: 10.1136/oem.50.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GS, et al. Occupational risk factors for the development of systemic lupus erythematosus. J Rheumatol. 2004;31:1928–1933. [PubMed] [Google Scholar]
- Crompton P, et al. Assessment of mercury exposure and malaria in a Brazilian Amazon riverine community. Environ Res. 2002;90:69–75. doi: 10.1006/enrs.2002.4358. [DOI] [PubMed] [Google Scholar]
- de Andrade Lima LR, et al. Characterization and treatment of artisanal gold mine tailings. J Hazard Mater. 2008;150:747–753. doi: 10.1016/j.jhazmat.2007.05.028. [DOI] [PubMed] [Google Scholar]
- de Jesus IM, et al. Exposure to elemental mercury in urban workers and gold miners from the Tapajos Region, Para, Brazil. Bull Environ Contam Toxicol. 2001;67:317–323. doi: 10.1007/s001280127. [DOI] [PubMed] [Google Scholar]
- Debes F, et al. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28:536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- Dolbec J, et al. Sequential analysis of hair mercury levels in relation to fish diet of an Amazonian population, Brazil. Sci Total Environ. 2001;271:87–97. doi: 10.1016/s0048-9697(00)00835-4. [DOI] [PubMed] [Google Scholar]
- Dominique Y, et al. Simulation of the chemical fate and bioavailability of liquid elemental mercury drops from gold mining in Amazonian freshwater systems. Environ Sci Technol. 2007;41:7322–7329. doi: 10.1021/es070268r. [DOI] [PubMed] [Google Scholar]
- Donkor AK, et al. Mercury in different environmental compartments of the Pra River Basin, Ghana. Sci Total Environ. 2006;368:164–176. doi: 10.1016/j.scitotenv.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Dye BA, et al. Urinary mercury concentrations associated with dental restorations in adult women aged 16–49 years: United States, 1999–2000. Occup Environ Med. 2005;62:368–375. doi: 10.1136/oem.2004.016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund KK, et al. Serum IL-1beta levels are associated with the presence of erosions in recent onset rheumatoid arthritis. Clin Exp Rheumatol. 2007;25:684–689. [PubMed] [Google Scholar]
- Ellingsen DG, et al. Renal and immunologic markers for chloralkali workers with low exposure to mercury vapor. Scand J Work Environ Health. 2000;26:427–435. doi: 10.5271/sjweh.564. [DOI] [PubMed] [Google Scholar]
- Fairweather D, et al. Sex differences in autoimmune disease from a pathological perspective. Am J Pathol. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SA, et al. Prevalence of antinuclear autoantibodies in the serum of normal blood dornors. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:315–319. doi: 10.1590/s0041-87812003000600005. [DOI] [PubMed] [Google Scholar]
- Gammons CH, et al. Mercury concentrations of fish, river water, and sediment in the Rio Ramis-Lake Titicaca watershed, Peru. Sci Total Environ. 2006;368:637–648. doi: 10.1016/j.scitotenv.2005.09.076. [DOI] [PubMed] [Google Scholar]
- Haggqvist B, et al. The immunosuppressive effect of methylmercury does not preclude development of autoimmunity in genetically susceptible mice. Toxicology. 2005;208:149–164. doi: 10.1016/j.tox.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Haggqvist B, Hultman P. Effects of deviating the Th2-response in murine mercury-induced autoimmunity towards a Th1-response. Clin Exp Immunol. 2003;134:202–209. doi: 10.1046/j.1365-2249.2003.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggqvist B, Hultman P. Interleukin-10 in murine metal-induced systemic autoimmunity. Clin Exp Immunol. 2005;141:422–431. doi: 10.1111/j.1365-2249.2005.02878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- Havarinasab S, et al. Mercury species in lymphoid and non-lymphoid tissues after exposure to methyl mercury: correlation with autoimmune parameters during and after treatment in susceptible mice. Toxicol Appl Pharmacol. 2007;221:21–28. doi: 10.1016/j.taap.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Havarinasab S, et al. Immunosuppressive and autoimmune effects of thimerosal in mice. Toxicol Appl Pharmacol. 2005;204:109–121. doi: 10.1016/j.taap.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Havarinasab S, Hultman P. Organic mercury compounds and autoimmunity. Autoimmun Rev. 2005;4:270–275. doi: 10.1016/j.autrev.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Havarinasab S, Hultman P. Alteration of the spontaneous systemic autoimmune disease in (NZB x NZW)F1 mice by treatment with thimerosal (ethyl mercury) Toxicol Appl Pharmacol. 2006;214:43–54. doi: 10.1016/j.taap.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Hemdan NY, et al. Immunomodulation by mercuric chloride in vitro: application of different cell activation pathways. Clin Exp Immunol. 2007;148:325–337. doi: 10.1111/j.1365-2249.2007.03338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho KT, Reveille JD. The clinical relevance of autoantibodies in scleroderma. Arthritis Res Ther. 2003;5:80–93. doi: 10.1186/ar628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, et al. Mechanism of mercury-induced autoimmunity: both T helper 1- and T helper 2-type responses are involved. Immunology. 1999;96:348–357. doi: 10.1046/j.1365-2567.1999.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman P, et al. The effect of xenobiotic exposure on spontaneous autoimmunity in (SWR x SJL)F1 hybrid mice. J Toxicol Environ Health A. 2006;69:505–523. doi: 10.1080/15287390500354904. [DOI] [PubMed] [Google Scholar]
- Ikingura JR, et al. Environmental assessment of mercury dispersion, transformation and bioavailability in the Lake Victoria Goldfields, Tanzania. J Environ Manage. 2006;81:167–173. doi: 10.1016/j.jenvman.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Kono DH, et al. The prototypic Th2 autoimmunity induced by mercury is dependent on IFN-gamma and not Th1/Th2 imbalance. J Immunol. 1998;161:234–240. [PubMed] [Google Scholar]
- Kurien BT, Scofield RH. Autoantibody determination in the diagnosis of systemic lupus erythematosus. Scand J Immunol. 2006;64:227–235. doi: 10.1111/j.1365-3083.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- Langrish CL, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langworth S, et al. Renal and immunological effects of occupational exposure to inorganic mercury. Br J Ind Med. 1992;49:394–401. doi: 10.1136/oem.49.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel J, et al. Fish diet and mercury exposure in a riparian Amazonian population. Water Air and Soil Pollution. 1997;97:31–44. [Google Scholar]
- Limbong D, et al. Emissions and environmental implications of mercury from artisanal gold mining in North Sulawesi, Indonesia. Sci Total Environ. 2003;302:227–236. doi: 10.1016/s0048-9697(02)00397-2. [DOI] [PubMed] [Google Scholar]
- McDowell MA, et al. Hair mercury levels in U.S. children and women of childbearing age: reference range data from NHANES 1999–2000. Environ Health Perspect. 2004;112:1165–1171. doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (U.S.) Board on Environmental Studies and Toxicology. Toxicological effects of methylmercury. Washington, DC: National Academy Press; 2000. [Google Scholar]
- Nesheim MC, et al. Seafood choices : balancing benefits and risks. Washington, D.C: National Academies Press; 2007. [Google Scholar]
- Nielsen JB, Hultman P. Mercury-induced autoimmunity in mice. Environ Health Perspect. 2002;110 Suppl 5:877–881. doi: 10.1289/ehp.02110s5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DC, Restifo NP. Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 2009;30:592–602. doi: 10.1016/j.it.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W. Akaike's information criterion in generalized estimating equations. Biometrics. 2001a;57:120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Pan W. Model selection in estimating equations. Biometrics. 2001b;57:529–534. doi: 10.1111/j.0006-341x.2001.00529.x. [DOI] [PubMed] [Google Scholar]
- Passos CJ, et al. Daily mercury intake in fish-eating populations in the Brazilian Amazon. J Expo Sci Environ Epidemiol. 2008;18:76–87. doi: 10.1038/sj.jes.7500599. [DOI] [PubMed] [Google Scholar]
- Passos CJ, et al. Fish consumption and bioindicators of inorganic mercury exposure. Sci Total Environ. 2007;373:68–76. doi: 10.1016/j.scitotenv.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Pollard KM, et al. Immunology and genetics of induced systemic autoimmunity. Autoimmun Rev. 2005;4:282–288. doi: 10.1016/j.autrev.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Pollard KM, et al. Xenobiotic acceleration of idiopathic systemic autoimmunity in lupus-prone bxsb mice. Environ Health Perspect. 2001;109:27–33. doi: 10.1289/ehp.0110927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudhomme GJ, et al. Quantitative Polymerase Chain-Reaction Analysis Reveals Marked Overexpression of Interleukin-1-Beta, Interleukin-10 and Interferon-Gamma Messenger-Rna in the Lymph-Nodes of Lupus-Prone Mice. Molecular Immunology. 1995;32:495–503. doi: 10.1016/0161-5890(95)00024-9. [DOI] [PubMed] [Google Scholar]
- Roulet M, et al. The geochemistry of mercury in central Amazonian soils developed on the Alter-do-Chao formation of the lower Tapajos River Valley, Para state, Brazil. Sci Total Environ. 1998;223:1–24. doi: 10.1016/s0048-9697(98)00265-4. [DOI] [PubMed] [Google Scholar]
- Santos EC, et al. Mercury exposures in riverside Amazon communities in Para, Brazil. Environ Res. 2000;84:100–107. doi: 10.1006/enrs.2000.4088. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, et al. Mercury exposure and malaria prevalence among gold miners in Para, Brazil. Rev Soc Bras Med Trop. 2002;35:421–429. doi: 10.1590/s0037-86822002000500001. [DOI] [PubMed] [Google Scholar]
- Silbergeld EK, et al. Mercury and autoimmunity: implications for occupational and environmental health. Toxicol Appl Pharmacol. 2005;207:282–292. doi: 10.1016/j.taap.2004.11.035. [DOI] [PubMed] [Google Scholar]
- Silva IA, et al. In vitro HgCl2 exposure of immune cells at different stages of maturation: effects on phenotype and function. Environ Res. 2005;98:341–348. doi: 10.1016/j.envres.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Silva IA, et al. Mercury exposure, malaria, and serum antinuclear/antinucleolar antibodies in Amazon populations in Brazil: a cross-sectional study. Environ Health. 2004;3:11. doi: 10.1186/1476-069X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel SJ, et al. Mercury reduction in Munhena, Mozambique: homemade solutions and the social context for change. Int J Occup Environ Health. 2006;12:215–221. doi: 10.1179/oeh.2006.12.3.215. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, et al. Analysis of the autoantibody response to fibrillarin in human disease and murine models of autoimmunity. J Immunol. 1995;154:961–971. [PubMed] [Google Scholar]
- Tan EM, et al. Range of antinuclear antibodies in "healthy" individuals. Arthritis Rheum. 1997;40:1601–1611. doi: 10.1002/art.1780400909. [DOI] [PubMed] [Google Scholar]
- Taylor H, et al. Environmental assessment of mercury contamination from the Rwamagasa artisanal gold mining centre, Geita District, Tanzania. Sci Total Environ. 2005;343:111–133. doi: 10.1016/j.scitotenv.2004.09.042. [DOI] [PubMed] [Google Scholar]
- Tubbs RR, et al. Membranous glomerulonephritis associated with industrial mercury exposure. Study of pathogenetic mechanisms. Am J Clin Pathol. 1982;77:409–413. doi: 10.1093/ajcp/77.4.409. [DOI] [PubMed] [Google Scholar]
- Vahter M, et al. Gender differences in the disposition and toxicity of metals. Environ Res. 2007;104:85–95. doi: 10.1016/j.envres.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Wilson NJ, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Wu Y, et al. Trends in anthropogenic mercury emissions in China from 1995 to 2003. Environ Sci Technol. 2006;40:5312–5318. doi: 10.1021/es060406x. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]