Summary
In vertebrate olfactory receptor neurons (ORNs), the odorant-triggered receptor current flows through two distinct ion channels on the sensory cilia: Ca2+ influx through a cyclic nucleotide-gated (CNG) channel followed by Cl− efflux through a Ca2+-activated anion channel. The excitatory Cl− current amplifies the small CNG current and crucially depends on a high intracellular Cl− concentration. We show here that a Na+-K+-2Cl− cotransporter, NKCC1, is required for this Cl− current, in that ORNs deficient in Nkcc1 or incubated with an NKCC blocker (bumetanide) lack the Cl− current. Surprisingly, immunocytochemistry indicates that NKCC1 is located on the somata and dendrites of ORNs rather than the cilia, where transduction occurs. This topography is remarkably similar to the situation in secretory epithelial cells, where basolateral Cl− uptake and apical Cl− efflux facilitate transepithelial fluid movement. Thus, a single functional architecture serves two entirely different purposes, probably underscoring the epithelial origin of the ORNs.
Introduction
Olfactory receptor neurons (ORNs) are bipolar cells in the olfactory epithelium of the nasal cavity. Each ORN sends a single dendrite to the epithelial border where it terminates in a swelling called the dendritic knob. Approximately 20 cilia, the site of olfactory transduction, protrude from the knob into the nasal mucus. Odorants dissolved in the mucus bind to receptor proteins in the ciliary membrane and activate a G protein that in turn stimulates an adenylyl cyclase (for review, see Gold, 1999; Schild and Restrepo, 1998). The ensuing increase in cAMP concentration opens a cyclic nucleotide-gated (CNG) channel that conducts mainly Ca2+ and little Na+ (Dzeja et al., 1999). The rise in ciliary Ca2+ concentration due to Ca2+ influx through the CNG channel (Kurahashi and Shibuya, 1990; Leinders-Zufall et al., 1997) activates a Ca2+-gated Cl− channel, also on the cilia (Kleene, 1993; Kleene and Gesteland, 1991; Reisert et al., 2003). Quite exceptionally, the Cl− current at the cilia of ORNs is inward, hence excitatory, carrying up to 80% of the odorant-induced receptor current in rodents (Lowe and Gold, 1993) and 36%–65% in amphibians (Kurahashi and Yau, 1993; Zhainazarov and Ache, 1995). This secondary anionic current acts as a nonlinear low-noise amplifier of the primary cationic CNG current (Kleene, 1993, 1997; Lowe and Gold, 1993), reduces the cell's dependence on extracellular Na+ for the depolarizing response (Kurahashi and Yau, 1994), and contributes to action-potential frequency and oscillatory response behavior (Reisert and Matthews, 2001b). To provide an excitatory role for Cl−, the Cl− concentration gradient across the ciliary membrane must be such that the Cl− equilibrium potential is positive with respect to the firing threshold of the cell. Indeed, with a variety of experimental approaches, the Cl− concentration in the mucus has been found to be 55 mM to 93 mM (Chiu et al., 1988; Reuter et al., 1998), similar to the intracellular concentration estimated to range from 40 to 100 mM (Kaneko et al., 2001; Nakamura et al., 1997; Reuter et al., 1998; Zhainazarov and Ache, 1995). The mechanism by which internal Cl− is maintained at such an elevated level, however, has not been investigated.
A family of cation-Cl− cotransporters are known to transport Cl− across the cell membrane by coupling it to cotransport (symport) of cations. The electroneutral Na+-K+-2Cl− cotransporter (NKCC) has two isoforms, both serving to elevate intracellular Cl− concentration above its electrochemical equilibrium. Nkcc2 is exclusively expressed in the kidney, whereas Nkcc1 is widely expressed in tissues, including neurons and epithelial cells (for review, see Haas and Forbush, 2000; Russell, 2000). In some neuronal cell types, the presence of NKCC1 renders GABA, normally an inhibitory neurotransmitter in the central nervous system, excitatory (Ben-Ari, 2002). Accordingly, we have sought to find out whether NKCC underlies the depolarizing Cl− current in ORNs. Indeed, we have identified the presence of the NKCC1 isoform in these cells and found that it is crucial for the existence of the inward chloride current in the olfactory response. Surprisingly, however, NKCC1 is present only on the dendrite and soma of ORNs, but not the cilia.
Results
Nkcc1 Expression
We first checked the expression patterns of Nkcc1 and Nkcc2 in the olfactory epithelium (OE) by RT-PCR (Figure 1A). We found expression of Nkcc1, but not Nkcc2, in this tissue. As controls, we found that the kidney expressed both Nkcc1 and Nkcc2, and the brain, only Nkcc1 (Delpire, 2000). As another control, we used primers directed against the CNG B-subunit gene, Cngb1 (Bönigk et al., 1999). As expected, this subunit of the olfactory CNG channel was expressed only in the OE.
Figure 1. Nkcc1 Is Expressed in ORNs of Mouse Olfactory Epithelium.

(A) RT-PCR. Primers specific for mouse Nkcc1, Nkcc2, or the olfactory CNG channel subunit Cngb1 were used to amplify oligo-dT primed cDNA made from RNA of various adult tissues. The figure is an image of an ethidium bromide-stained agarose gel.
(B) X-gal staining. (top) Medial view, with septum removed, of whole-mount olfactory epithelium and olfactory bulb from an Nkcc1+/− mouse, with one copy of Nkcc1 and one copy of lacZ. OB, olfactory bulb; OE, olfactory epithelium; RE, respiratory epithelium. (bottom) 18 μm coronal section of the olfactory epithelium. SC, sustentacular cell layer; ORN, olfactory receptor neuron layer.
Cell Type-Specific Expression and Localization of NKCC1 in Olfactory Epithelium
To examine the cell type-specific expression of Nkcc1 in the OE, we dissected the olfactory turbinates from mice engineered to be deficient in Nkcc1 by knockin of the reporter gene lac-Z to exon 9 of Nkcc1 (Delpire et al., 1999). Using X-gal staining, we found β-galactosidase (β–GAL) activity in the sensory epithelium across the entire medial face of the endoturbinates (Figure 1B, top). The respiratory epithelium appeared to be devoid of Nkcc1 promoter activity. 18 μm cryostat sections of the whole-mount staining pattern confirmed Nkcc1 promoter activity in ORNs, but not in the sustentacular cells of the sensory epithelium (Figure 1B, bottom). In coronal sections, we did not see any gross differences in the X-gal staining pattern between medial and lateral olfactory tissue (data not shown).
To examine the subcellular localization of NKCC1 in the ORNs, we stained OE sections from Nkcc1+/− and Nkcc1−/− animals with a rabbit antibody against NKCC1 (Kaplan et al., 1996). As control, a mouse monoclonal antibody against the olfactory CNG channel subunit CNGA2 was used, and, in agreement with previous work (Meyer et al., 2000), CNGA2 was localized to the cilia-containing layer of the olfactory epithelium in both Nkcc1+/− and Nkcc1−/− animals (Figures 2A and 2D). In contrast, immunolabeling of NKCC1 was found not in the cilia of Nkcc1+/− mice, but more proximally (Figure 2B). In the merged image of the two staining patterns (which includes nuclear DAPI staining), NKCC1 appears localized to the proximal dendrite and soma, but not the dendritic knob or cilia that are in contact with the nasal mucus (Figure 2C). In addition, there is a strong signal for NKCC1 in a subset of cells that may be a subclass of sustentacular cells, microvillar cells, or possibly duct cells (Zhuo et al., 2001). The lack of signal for Nkcc1 expression in tissue from Nkcc1−/− mice (Figure 2E) demonstrates the specificity of the antibody. The normal appearance of CNGA2 immunostaining (Figure 2D) and the olfactory isoform of adenylyl cyclase (data not shown) in Nkcc1−/− tissue indicated that no gross perturbations of the OE had resulted from the lack of Nkcc1 expression.
Figure 2. Immunolocalization of NKCC1 and Olfactory CNG Channel Subunit CNGA2.
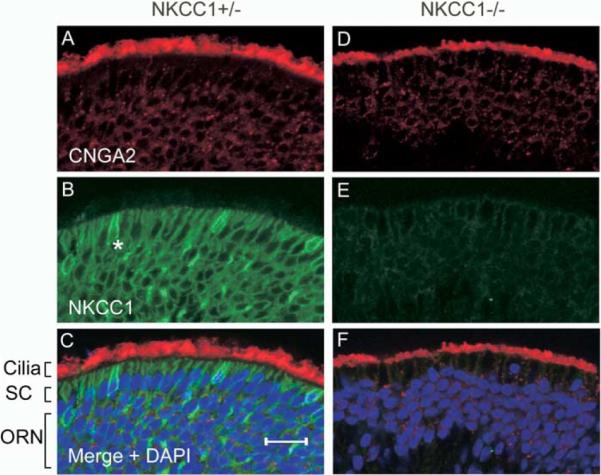
Specific primary antibodies were applied to 18 μm coronal sections of the olfactory epithelium from Nkcc1+/− (A–C) or Nkcc1−/− animals (D–F). CNGA2 ([A] and [D]) was visualized with a Cy3-conjugated secondary antibody, and NKCC1 signal ([B] and [E]), with an Alexa 488-conjugated secondary anti-body. Merged images ([C] and [F]) are of (A) + (B) and (D) + (E), respectively, together with DAPI (blue) staining for cell nuclei. SC, sustentacular cell layer and apical olfactory dendrites; ORN, olfactory receptor neuron layer; *, microvillar-like cell. Scale bar, 20μm.
To further confirm the localization pattern of NKCC1, we used another antibody (5030, see Experimental Procedures) raised against the C-terminal 21 amino acid residues of mouse NKCC1. The staining patterns of Nkcc1+/− and Nkcc1−/− olfactory tissues with this antibody were identical to those in Figures 2B and 2E (data not shown).
NKCC1 Is Required for the Generation of the Inward Cl− Current
The magnitude of the inward Cl− current depends on the intracellular Cl− concentration. To test the importance of NKCC1 in maintaining high intracellular Cl− concentration in ORNs, we recorded the odorant-induced receptor current from dissociated ORNs of Nkcc1+/+ and Nkcc1−/− mice, using the suction-pipette technique, a noninvasive method that leaves the intracellular milieu and ion concentrations unperturbed. All suction-pipette experiments were performed at 37°C. To facilitate odorant stimulation, ORNs used also ubiquitously expressed a heptanal odorant receptor (I7, see Experimental Procedures). In response to a 1 s pulse of the odorant heptanal (100 μM), wild-type (Nkcc1+/+) ORNs generated a large receptor current (Figure 3A, black trace). Addition of the Cl− channel blocker niflumic acid during the odorant pulse reduced the receptor current substantially (red trace). Niflumic acid alone did not elicit a response (green trace). The cyan trace, which was recorded at the end of the experiment, demonstrates the reversibility of the blocker and the stability of the cells chosen for analysis. Any neuron responsive to heptanal was included for analysis, and all showed a reduction in current when niflumic acid was applied (irrespective of its peak current amplitude, ranging from −10 pA to −135 pA).
Figure 3. Contribution of the Cl− Current to the Odorant-Induced Response by ORNs with or without Functioning NKCC1.
The response of ORNs to a 1 s exposure to 100 μM heptanal at 37°C was recorded with the suction-pipette technique under three different conditions: (A) wild-type, (B) wild-type + 50 μM bumetanide, and (C) Nkcc1−/− neurons. Black and cyan traces are exposures to heptanal before and after exposure to heptanal + 300 μM niflumic acid (red, NFA) and to niflumic acid alone (green). (D) Mean suction current responses. The slightly larger current of wild-type ORNs in niflumic acid compared to knockout ORNs possibly reflected an incomplete block of the Cl− channel. Only in wild-type ORNs was the odorant-induced current significantly reduced by niflumic acid (Student's t test, 5% level). (E) For each individual ORN, the heptanal response in niflumic acid was normalized with respect to its response in heptanal alone. This ratio represents the fraction of current carried by the CNG channel. Numbers in parentheses indicate the number of neurons recorded for each experiment. Error bars indicate SEM.
To assess the role of NKCC1 in maintaining high intracellular Cl− in Nkcc1+/+ cells, we used bumetanide, an NKCC blocker (Russell, 2000). Incubation of Nkcc1+/+ ORNs with 50 μM bumetanide for 30 min reduced the receptor current substantially, after which the current was no longer affected by niflumic acid (Figure 3B). Similarly, with Nkcc1−/− ORNs, the receptor current was initially small and remained unaffected by niflumic acid (Figure 3C). From collected results, the average current decreased approximately 7-fold when NKCC1 function was disrupted (Nkcc1+/+ in bumetanide or untreated Nkcc1−/−), and niflumic acid had only a small effect, indicating that most of the Cl− current was absent (Figure 3D). On average, 80% of the response in wild-type cells was carried by the Cl− channel, calculated from 1 − (response to heptanal + niflumic acid/response to heptanal alone) (Figure 3E, left bar). The remaining 20% of the response came from the CNG channel. For Nkcc1+/+ cells treated with bumetanide, ~30% of the average odorant-induced current was carried by Cl− (Figure 3E, middle bar); this residual percentage presumably reflected an incomplete abolition of the Cl− accumulation by bumetanide, perhaps due to insufficient exposure time. For Nkcc1−/− cells, there was essentially no receptor current carried by Cl− (Figure 3E, right bar). Interestingly, an increase in response was occasionally observed when niflumic acid was applied to individual Nkcc1−/− ORNs; this effect accounted for the relatively large SEM in the measurements (see Discussion). An increase in response with niflumic acid was never recorded for Nkcc1+/+ neurons.
The large reduction in receptor current carried by Cl− in Nkcc1+/+ cells treated with bumetanide was not due to pharmacological block of the Cl− channel or to non-specific effects on olfactory transduction by the chemical. ORNs gave similar responses whether stimulated by heptanal alone or by heptanal + bumetanide (i.e., with bumetanide present only during the 1 s odorant stimulation instead of its continuous presence as in the above experiments) (n = 6, data not shown). Thus, bumetanide did not seem to affect the odorant-induced response in ways other than blocking NKCC1, at least when applied for 1 s during odorant stimulation. It also did not stimulate ORNs if heptanal was omitted (n = 6, data not shown).
Density of Ciliary Cl− Channels Unchanged in Nkcc1−/− ORNs
To test whether the loss of Cl− current in Nkcc1−/− ORNs was possibly due to the disappearance of Cl− channels or to a decrease in their sensitivity to Ca2+, we recorded from inside-out membrane patches excised from dendritic knobs of Nkcc1+/+ and Nkcc1−/− animals (Reisert et al., 2003). To compare the magnitudes of the Cl− and CNG currents (both being present in a patch) in ORNs from both mouse lines, we exposed patches cytosolically to concentrations of Ca2+ (67 μM) and cAMP (100 μM) that would maximally activate the Cl− and CNG currents (Figures 4A and 4B). As observed previously in rat, the Cl− current ran down over time, but not the CNG current (Reisert et al., 2003). The average Cl− current from a patch recorded immediately after excision was similar for both Nkcc1+/+ and Nkcc1−/− ORNs (102 ± 15 pA and 97 ± 25 pA, respectively), indicating similar densities of Cl− channels. Interestingly, on average, patches from Nkcc1−/− ORNs showed a larger CNG current than those from Nkcc1+/+ ORNs (134 ± 33 pA [n = 7] versus 64 ± 19 pA [n = 8], respectively), reflecting a higher CNG channel density on patches of Nkcc1−/− ORNs (Figure 4C). The reason for this difference is unclear.
Figure 4. Ca2+-Activated Cl− Channels in Nkcc1−/− ORNs Are Normal in Density and Function.
Patches excised from dendritic knobs of (A) Nkcc1+/+ and (B) Nkcc1−/− ORNs were exposed to 67 μM Ca2+ and 100 μM cAMP. The holding potential was −40 mV. The Cl− current exhibited “rundown” over time, and the time after patch excision is noted next to the recordings. (C) Mean Cl− and CNG currents in patches from Nkcc1+/+ (eight patches) and Nkcc1−/− (six patches) neurons. (D and E) Dose-response relationships for a 10 s exposure to Ca2+ in patches from Nkcc1+/+ and Nkcc1−/− neurons, respectively. The Ca2+ concentrations were: 67 μM, black and magenta (first and last recorded trace); 11 μM, cyan; 2.4 μM, dark blue; 0.75 μM, green; 0.25 μM, red; 0, yellow. (F) The peak Cl− currents were plotted against the Ca2+ concentration for patches from Nkcc1+/+ (black ▽, average of eleven patches) and Nkcc1−/− neurons (red Δ, five patches). Data sets were fitted with the Hill equation such that K1/2 = 3.5 μM and 2.5 μM and the Hill coefficient n = 1.4 and n = 1.7 for Nkcc1+/+ and Nkcc1−/− neurons, respectively. Error bars indicate SEM.
To investigate whether the Ca2+ sensitivity of the Cl− channels in Nkcc1−/− cells had changed, we obtained dose-response relationships for the activation of the channel on excised patches (performed after the above-mentioned rundown had reached steady state). As found previously for the rat Cl− channel (Reisert et al., 2003), a moderate, reversible channel inactivation was observed during the 10 s Ca2+ exposure (Figures 4D and 4E). The dependence of the peak Cl− current on Ca2+ concentration was similar for patches from Nkcc1+/+ ORNs (K1/2 = 3.5 ± 0.2 μM) and Nkcc1−/− ORNs (K1/2 = 2.5 ± 0.2 μM) (Figure 4F). The current-voltage relationships were also similar, being inwardly rectifying in both cases (data not shown). Taken together, these results indicate that loss of NKCC1 does not affect the density or the biophysical properties of the Cl− channel. Thus, we can discount the notion that a defect in the Cl− channel, or a defect in its expression, underlies the reduced Cl− component of the receptor current in Nkcc1−/− cells.
Experiments in 0-Ca2+
Ringer The experiments described so far relied on niflumic acid to reveal the CNG current component in the overall response, but under physiological ionic conditions, i.e., in the presence of external Ca2+, the CNG component is small. Another way to remove the Cl− current is to remove extracellular Ca2+, in which case the lack of Ca2+ influx through CNG channels during odorant stimulation should lead to no Cl− current. In 0-Ca2+ Ringer, the receptor current tripled in size compared to control (Figure 5A, compare red to black and cyan traces), which was attributed to the removal of an extracellular block of the CNG channel by Ca2+ (Dzeja et al., 1999; Zufall and Firestein, 1993) and to the removal of Ca2+- mediated feedback inhibition on the CNG channel (Bradley et al., 2004; Chen and Yau, 1994). As expected, the current in 0-Ca2+ Ringer was hardly affected by niflumic acid (Figure 5A, green trace), indicating that the receptor current was composed exclusively of CNG current. The abrupt drop in current at the end of the 1 s stimulation reflected the resumption of Ca2+ block and Ca2+ feedback inhibition of the CNG channel upon return to normal Ringer.
Figure 5. ORNs Devoid of NKCC1 Function Still Can Generate Large CNG Currents.
Comparison of odorant-induced responses to 100 μM heptanal in normal Ringer and in 0-Ca2+ Ringer.
(A) Nkcc1+/+, (B) Nkcc1+/+ in the steady presence of 50 μM bumetanide, and (C) Nkcc1−/− neurons. Black and cyan traces are responses in normal Ringer before and after odorant stimulation in 0-Ca2+ Ringer in the absence (red) and presence of 300 μM niflumic acid (green). (D) Mean suction current responses. (E) Fractional current carried by the CNG channel. The heptanal response in 0-Ca2+ Ringer + niflumic acid was normalized to the response in 0-Ca2+ Ringer alone. (F) Ratio of the response in Ringer to the response in 0-Ca2+ Ringer. A much smaller current is generated in Ringer when NKCC1 is nonfunctional. Numbers in parentheses indicate number of recorded neurons. In 0-Ca2+ Ringer, most ORNs developed a slow inward current that only reached a few pA by the time that the odorant response in 0-Ca2+ Ringer reached its peak. This current was subtracted from the peak current determination. Error bars indicate SEM.
With bumetanide present (Figure 5B), or with Nkcc1−/−ORNs (Figure 5C), very small currents were observed in Ringer (black and cyan), but removal of external Ca2+ produced much larger CNG currents (red traces), and niflumic acid (green) had little or no effect. Collected results indicated that, in 0-Ca2+ Ringer, the receptor current of Nkcc1+/+ ORNs treated with bumetanide or that of untreated Nkcc1−/− ORNs, was on average only 55% of wild-type (Figure 5D, left). This reduction, also found in normal Ringer with niflumic acid (Figure 3D, right set of bars), may reflect some changes in intraciliary Na+ and K+ concentrations secondary to the shutdown of Na+-K+-2Cl− cotransport. The average 20% reduction in receptor current in 0-Ca2+ Ringer due to niflumic acid (Figure 5E) possibly originated from niflumic acid acting as a competitive inhibitor of the heptanal odorant receptor (see Experimental Procedures). On average, the response of Nkcc1−/− cells or bumetanide-treated Nkcc1+/+ cells increased 10-fold in 0-Ca2+ Ringer versus only 3-fold for untreated Nkcc1+/+cells (Figure 5F). This difference results from the small receptor current of bumetanide-treated Nkcc1+/+ ORNs and of Nkcc1−/− ORNs in Ringer being purely a CNG current. Taken together, these data indicate that the ol-factory transduction cascade upstream of the Cl− channel, in Nkcc1+/+ ORNs treated with bumetanide or in Nkcc1−/− ORNs, functions normally.
Experiments in Low-Cl− Ringer
If the lack of Cl− current in Nkcc1−/− ORNs is due simply to a low internal Cl− concentration, a shift in the Cl− equilibrium potential to more positive values, such as by an acute reduction of external Cl− concentration, should restore an inward Cl− current. To test this idea, low-Cl− Ringer was applied to the cilia during the 1 s odorant application. This protocol in itself should not affect NKCC1 function (and therefore intracellular Cl−), because NKCC1 is situated on the dendrite and soma (Figure 2), which, under our recording conditions, resided largely inside the suction pipette and were constantly exposed to 140 mM Cl−. As expected, an increased receptor current due to low-Cl− Ringer was observed even in wild-type ORNs (Figures 6A and 6C). With Nkcc1−/− ORNs, the small response in Ringer increased substantially in low-Cl− solution (Figures 6B and 6C). Practically all additional current induced by low-Cl− Ringer was blocked by niflumic acid (green trace), confirming it as a Cl− current. These results indicate that the Cl− channel on Nkcc1−/− ORNs did open during the odorant-induced response, but no Cl− current was generated simply because of a low intracellular Cl− concentration. Only when an excitatory Cl− gradient was established could a Cl− efflux exist. Compared to the response in normal Ringer, the response in low-Cl− solution increased an average of 1.6-fold (SEM ± 0.2) in wild-type neurons and 5-fold (5.2 ± 0.9) in Nkcc1−/− neurons (Figures 6C and 6D).
Figure 6. Cl− Current in Low-Cl− Ringer.
An odorant-induced response (black and cyan traces, 100 μM heptanal) could be increased by lowering the external Cl− concentration (red); this increase was reduced by niflumic acid (green). (A) Nkcc1+/+ and (B) Nkcc1−/− ORNs. (C) Mean suction current responses. (D) Response in low-Cl− solution compared to response in Ringer. Error bars indicate SEM.
Discussion
When a Ca2+-activated Cl− channel on olfactory cilia was first reported (Kleene and Gesteland, 1991), it was not clear whether the Cl− current flowing through this channel was inhibitory or excitatory. Later, this current was shown to be inward and therefore excitatory (Kurahashi and Yau, 1993; Lowe and Gold, 1993; Reisert and Matthews, 1998; Zhainazarov and Ache, 1995). An inward Cl− current requires that the intraciliary Cl− concentration be high. We have now found that this Cl− accumulation is achieved by the Na+-K+-2Cl− cotrans-porter NKCC1. Interestingly, our immunocytochemical data suggest that NKCC1 is situated on the ORN dendrite and soma rather than the cilia, where olfactory transduction occurs. While this location may seem surprising initially, it is actually in line with what is known about secretory epithelial cells, where Cl− uptake via a cotransporter occurs at the basolateral membrane, and Cl− secretion via one or more Cl channel types occurs at the ciliated apical membrane (for review, see Haas and Forbush, 2000; Russell, 2000). This direction of Cl− flux, with accompanying fluid secretion, takes advantage of a high interstitial Cl− concentration on the basolateral side and a lower Cl− concentration on the mucosal side. A low Cl− concentration has also been demonstrated in the nasal mucus (Chiu et al., 1988; Reuter et al., 1998). With respect to olfactory transduction, the advantage of a dendrosomatic location of NKCC1 is that the cell body provides a much larger reservoir for Cl− homeostasis than do the cilia (Lindemann, 2001). This location also ensures that the uptake of Cl− into the ORN does not depend on mucosal Na+ and K+ concentrations, which may fluctuate, especially for aquatic or amphibian species, because the nasal cavity is open to the external environment. The striking similarity in Cl− movement between ORNs and secretory epithelial cells reflects a kinship between these two types of cells. In the case of ORNs, however, the Cl− flux has taken on a function entirely different from fluid secretion—namely, sensory signaling. Our study also demonstrates that a protein crucial for olfactory transduction need not be situated on the cilia.
Our electrophysiological experiments with respect to NKCC1 employed two complementary approaches: recording from ORNs of Nkcc1−/− mice and blocking NKCC1 function in wild-type ORNs pharmacologically with the loop diuretic bumetanide. The important difference between these two approaches is that, while Nkcc1−/− ORNs could have altered properties with unknown functional consequences as a result of this transporter's absence during development, these changes would not occur in wild-type ORNs treated acutely with bumetanide. Conversely, bumetanide could have pharmacological side effects (possibly occurring slowly over the 30 min bumetanide incubation time), but these would not be of concern when recording from Nkcc1−/− ORNs. In both cases, the odorant-induced receptor current decreased dramatically and lost most, or all, of its Cl− component. Moreover, we found that this loss of the Cl− component was due neither to a loss of Cl− channels in Nkcc1−/− animals nor to nonspecific effects of bumetanide on the olfactory transduction cascade. In both experimental approaches, a large CNG current was observed in 0-Ca2+ Ringer, demonstrating that the signal transduction cascade upstream of the Cl− channel was still functional. The lack of Cl− current in ORNs with defunct NKCC1 suggests that the intracellular Cl− concentration is simply too low to support an excitatory Cl− current. This speculation was confirmed when we recorded odorant-induced currents from Nkcc1−/− ORNs in an external solution with acutely reduced Cl− concentration and found a large niflumic acid-sensitive component. Thus, despite the 45% reduction of CNG current in bumetanide-treated wild-type ORNs or Nkcc1−/− ORNs (presumably due to secondary changes in intraciliary concentrations of Na+ and K+; see Results), ciliary Ca2+ can still reach levels high enough to activate the Cl− channel. So, what is the Cl− concentration in Nkcc1−/− ORNs? The observation that the small response in individual Nkcc1−/− ORNs increased or decreased when niflumic acid was applied (Figure 3E) suggests that the internal Cl− concentration distributes more or less passively so that the Cl− equilibrium potential is near the resting membrane potential.
Mice with a component of the olfactory transduction cascade ablated, such as CNGA2−/−, AC-III (adenylyl cyclase type III)−/−; and Golf−/−, all show severe impairment in their olfactory sense (Belluscio et al., 1998; Brunet et al., 1996; Wong et al., 2000). Survival of neonates from these mouse lines is also compromised, presumably because of the olfactory deficit interfering with nursing and food intake. The Nkcc−/− animals have the same problems (Delpire et al., 1999), and, owing to the cotransporter's ubiquitous expression in tissues, additional dysfunctions including deafness, imbalance (Delpire et al., 1999), altered pain perception (Laird et al., 2004), reduced salivation (Evans et al., 2000), and male sterility (Pace et al., 2000). To assess olfactory function behaviorally in such a severely affected animal may prove challenging. One solution would be to ablate Nkcc1 selectively in ORNs by using a conditional knockout strategy.
Experimental Procedures
Animal Breeding
Nkcc1+/− animals, which express the reporter gene lac-Z at exon 9 of the Nkcc1 locus (Delpire et al., 1999), were crossed into a line (UbI7)(Zhao and Reed, 2001) engineered to express a mouse olfactory receptor for heptanal (Zhao et al., 1998). UbI7 specifically and ubiquitously expresses I7 in all ORNs under the control of the olfactory marker protein (OMP) promoter, as has been shown by in situ hybridization and Northern blot analysis (Zhao and Reed, 2001).
PCR
RT-PCR was performed essentially as described (Bradley et al., 1997). To confirm the authenticity of the Nkcc amplicons, a second round of PCR was done with a nested pair of primers, using a 100-fold dilution of the primary reaction as template. The final product was then restriction mapped. For Nkcc1 (GenBank U13174), primer pairs were 614F, 5′ CCAACGTGAGCTTCCAGAAC; 1417R, 5′ CAA TCTGAGCCTTTGCTTCC; 712F, 5′ GACACCCACACCAACACCTA; and 1166R, 5′ TACGCTCCTCCTCCTCTCAC. For Nkcc2 (GenBank U61381), primer pairs were 1357F, 5′ GCTGCTACTGGGATTCTTGC; 2322R, 5′ GCAGAGGCCACTATTCTTCG; 971F, 5′ GCTTGATCTTTGC TTTTGCC; and 1079R, 5′ GATTCAATGATGGTGGATCC. For Cngb1 (GenBank AF068572), primer pairs were 735F, 5′ AGGAGCTCCA TCCGTCGCCTG; and 1031R, 5′ GTCCTCGCCATCAGCCTCCTG.
Immunofluorescence and X-Gal Staining
Three- to five-month-old homozygous and control mice were anesthetized with avertin (0.2 ml/g) and perfused with ice-cold PBS followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Olfactory turbinates were dissected and cyroprotected in 30% sucrose, then embedded in tissue freezing medium (Triangle Biomedical Sciences), and sectioned by cryostat. 18 μm thick sections were thaw-mounted onto glass slides (Superfrost/Plus, Fischer) and air-dried overnight, then stored at −80°C before use. Antibody staining was as described by Sung et al. (2000). Briefly, sections were hydrated in PBS, treated with 1% SDS/8% 2-mercaptoethanol for 5 min, and blocked in 5% normal goat serum (Sigma G-6767)/1% BSA/0.4% Triton X-100 for 30 min, followed by incubation overnight at 4°C in primary antibody in blocking solution. After two 30 min and one 15 min wash in 2% BSA/0.2% Triton X-100, sections were incubated at room temperature for 60 min with fluorophore-conjugated secondary antibodies in washing solution, then washed and mounted with Vectashield with DAPI (Vector Laboratories). Sections were analyzed with a Zeiss confocal microscope (LSM-510). To facilitate a comparative analysis of homozygous knockout and wild-type stained sections, data for a given antibody combination were collected with the same laser excitation and photomultiplier detection intensities. Primary antibodies were: rabbit anti-NKCC1, diluted 1:200 (Kaplan et al., 1996); rabbit anti-NKCC1 (5030), diluted 1:25; mouse monoclonal anti-CNGA2, diluted 1:200 (Meyer et al., 2000); and rabbit anti-AC III, diluted 1:2000 (Santa Cruz Biotechnology). Secondary antibodies—Cy3-conjugated goat anti-mouse IgG and Alexa 488-conjugated goat anti-rabbit—were both diluted 1:500 (Jackson).
X-gal staining of sections and whole mounts was as described previously (Mombaerts et al., 1996). The 5030 Nkcc1 antibody was generated against the C-terminal 21 amino acid residues (KADL PPVLLVRGNHQSVLTFYS) of mouse NKCC1 (GenBank U13174). The peptide was conjugated via its N-terminal lysine residue to thyroglobulin and used for immunization of rabbits (Covance, Denver, PA). Rabbit antisera were affinity-purified on a peptide-BSA column. Specificity of the NKCC1 antibodies was confirmed by comparison of signals from staining tissue sections of Nkcc1+/− and Nkcc1−/− littermates.
Electrophysiology
Tissue Preparation
Following euthanasia of the animal by methods approved by Johns Hopkins University Institutional Animal Care and Use Committee, olfactory turbinates were removed from the nasal cavity and stored in Ringer solution at 4°C until use. For suction-pipette recordings, a small piece of epithelium was placed in an Eppendorf tube containing 200 μl Ringer solution without enzymes and briefly vortexed. Cells were allowed to settle for 30 min in a recording chamber mounted on an inverted microscope and were identified by their typical morphology. For excised-patch experiments, the olfactory epithelium was peeled off its supporting cartilage, incubated in 0.2 mg/ml trypsin (Sigma-Aldrich) in divalent-free solution for 30 min at 37°C, and then transferred to a solution containing 0.4 mg/ml DNase 1 (Roche Diagnostics), 140 mM NaCl, and 2 mM MgCl2. After gentle trituration, 0.5 ml of cell suspension was transferred to the recording chamber and allowed to settle.
Solutions and Solution Application
Mammalian Ringer solution contained
140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 0.01 mM EDTA, 10 mM HEPES, and 10 mM glucose. For low-Cl− solution, 120 mM NaCl was replaced with 120 mM Na-methanesulfonate, producing a +50 mV shift in Cl− equilibrium potential. The 0-Ca2+ Ringer contained 1 mM EGTA, 140 mM NaCl, 5 mM KCl, 3.2 mM MgCl2 (3 mM free Mg2+), and 10 mM HEPES. In all solutions, the pH was adjusted to 7.5 with NaOH. Solutions containing heptanal were made daily from a 20 mM heptanal/DMSO stock solution. Bumetanide was dissolved in DMSO at a stock concentration of 100 mM and diluted with Ringer solution to achieve a final bumetanide concentration of 50 μM. In the bumetanide experiments, dissociated cells settled in the recording chamber in bumetanide-containing Ringer for at least 30 min before recording began. All solutions, including the pipette solution, contained bumetanide in these experiments, with the exception of odorant solutions. Excised-patch experiments were performed in 140 mM symmetrical LiCl solution, which included 10 mM HEPES and 10 mM HEDTA, with pH adjusted to 7.2 with NMDG. Ca2+-containing solutions were made as described in Reisert et al. (2003).
Solution exchange was achieved by transferring the tip of the recording pipette across the interface of neighboring streams of solution using the Perfusion Fast-Step SF-77B solution changer (Warner Instrument Corp.). Solution exchange was complete within 15 ms. All experiments were performed at 37°C, and solutions were heated with a solution heater (based on Matthews, 1990) just prior to entering the solution changer.
Recording
The suction-pipette technique was used to record from isolated mouse ORNs (Baylor et al., 1979; Lowe and Gold, 1991; Reisert and Matthews, 2001a). The cell body of a single ORN was sucked into the tip of the recording pipette, leaving the cilia exposed to the bath solution. The odorant-induced suction-pipette current was recorded with an Axopatch-1D patch-clamp amplifier, Digidata 1322A interface, and pClamp software (Axon Instruments). Irrespective of bumetanide treatment or Nkcc1 expression, approximately 20% of ORNs responded to 100 μM heptanal (17% for Nkcc1+/+, 24% for Nkcc1 +/+ treated with bumetanide, and 19% for Nkcc1−/−), indicating that no ORNs escaped from analysis due to lack of Cl− current. All responders were used in the analysis. Solution changes from normal Ringer to low Cl− solution generated large currents due to the junction potential between the two solutions. To isolate the odorant-induced current elicited in low-Cl− solution, a current trace corresponding to a repeat of the solution change to low-Cl− solution but without odorant was subtracted.
Patch pipettes were made from borosilicate glass and fire-polished to yield an open pipette resistance of 6–8 MΩ. Inside-out patches were excised from the dendritic knob of ORNs, and recordings were sampled at 500 Hz and low-pass filtered at 100 Hz.
Acknowledgments
We thank Philippe Ascher, Vikas Bhandawhat, Chih-Ying Su, Michael Do, and Samar Hattar for comments; Eric Delpire for his kind gift of Nkcc1+/− mice and the NKCC1 antibody; Haiqing Zhao and Randy Reed for the kind gift of the UbI7 mice; and Frank Müller and U.B. Kaupp for the kind gift of the CNGA2 monoclonal antibody. This work was supported by the Howard Hughes Medical Institute and NIH (DC 06904).
Footnotes
Note Added in Proof A recently published paper (Kaneko et al., 2004) uses Cl− imaging and pharmacological tools to suggest that Nkcc1 participates in Cl− accumulation in olfactory sensory neurons. Those authors concluded that Cl− uptake in ORNs occurs apically from the mucus and not basolaterally, which is in contrast to our findings reported here.
References
- Baylor DA, Lamb TD, Yau K-Y. The membrane current of single rod outer segments. J. Physiol. 1979;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in Golf are anosmic. Neuron. 1998;20:69–81. doi: 10.1016/s0896-6273(00)80435-3. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat. Rev. Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bönigk W, Bradley J, Müller F, Sesti F, Boekhoff I, Ronnett GV, Kaupp UB, Frings S. The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. J. Neurosci. 1999;19:5332–5347. doi: 10.1523/JNEUROSCI.19-13-05332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Zhang YN, Bakin R, Lester HA, Ronnett GV, Zinn K. Functional expression of the heteromeric “olfactory” cyclic nucleotide-gated channel in the hippocampus: a potential effector of synaptic plasticity in brain neurons. J. Neurosci. 1997;17:1993–2005. doi: 10.1523/JNEUROSCI.17-06-01993.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Bönigk W, Yau KW, Frings S. Calmodulin permanently associates with rat olfactory CNG channels under native conditions. Nat. Neurosci. 2004;7:705–710. doi: 10.1038/nn1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet LJ, Gold GH, Ngai J. General anosmia caused by a targeted disruption of the mouse olfactory cyclic nucleotide-gated cation channel. Neuron. 1996;17:681–693. doi: 10.1016/s0896-6273(00)80200-7. [DOI] [PubMed] [Google Scholar]
- Chen T-Y, Yau K-W. Direct modulation by Ca2+-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Chiu D, Nakamura T, Gold GH. Ionic composition of toad olfactory mucus measured with ion-selective microelectrodes. Chem. Senses. 1988;13:677–678. [Google Scholar]
- Delpire E. Cation-chloride cotransporters in neuronal communication. News Physiol. Sci. 2000;15:309–312. doi: 10.1152/physiologyonline.2000.15.6.309. [DOI] [PubMed] [Google Scholar]
- Delpire E, Lu J, England R, Dull C, Thorne T. Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat. Genet. 1999;22:192–195. doi: 10.1038/9713. [DOI] [PubMed] [Google Scholar]
- Dzeja C, Hagen V, Kaupp UB, Frings S. Ca2+ permeation in cyclic nucleotide-gated channels. EMBO J. 1999;18:131–144. doi: 10.1093/emboj/18.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RL, Park K, Turner RJ, Watson GE, Nguyen HV, Dennett MR, Hand AR, Flagella M, Shull GE, Melvin JE. Severe impairment of salivation in Na+/K+/2Cl− cotransporter (Nkcc1)-deficient mice. J. Biol. Chem. 2000;275:26720–26726. doi: 10.1074/jbc.M003753200. [DOI] [PubMed] [Google Scholar]
- Gold GH. Controversial issues in vertebrate olfactory transduction. Annu. Rev. Physiol. 1999;61:857–871. doi: 10.1146/annurev.physiol.61.1.857. [DOI] [PubMed] [Google Scholar]
- Haas M, Forbush B., 3rd. The Na-K-Cl cotransporter of secretory epithelia. Annu. Rev. Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Nakamura T, Lindemann B. Noninvasive measurement of chloride concentration in rat olfactory receptor cells with use of a fluorescent dye. Am. J. Physiol. 2001;280:C1387–C1393. doi: 10.1152/ajpcell.2001.280.6.C1387. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Putzier I, Frings S, Kaupp UB, Gensch T. Chloride accumulation in mammalian olfactory sensory neurons. J. Neurosci. 2004;24:7931–7938. doi: 10.1523/JNEUROSCI.2115-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MR, Plotkin MD, Brown D, Hebert SC, Delpire E. Expression of the mouse Na-K-2Cl cotransporter, mBSC2, in the terminal inner medullary collecting duct, the glomerular and extraglomerular mesangium, and the glomerular afferent arteriole. J. Clin. Invest. 1996;98:723–730. doi: 10.1172/JCI118844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene SJ. Origin of the chloride current in olfactory transduction. Neuron. 1993;11:123–132. doi: 10.1016/0896-6273(93)90276-w. [DOI] [PubMed] [Google Scholar]
- Kleene SJ. High-gain, low-noise amplification in olfactory transduction. Biophys. J. 1997;73:1110–1117. doi: 10.1016/S0006-3495(97)78143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene SJ, Gesteland RC. Calcium-activated chloride conductance in frog olfactory cilia. J. Neurosci. 1991;11:3624–3629. doi: 10.1523/JNEUROSCI.11-11-03624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T, Shibuya T. Ca2+-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Res. 1990;515:261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Yau K-W. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993;363:71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Yau K-W. Tale of an unusual chloride current. Curr. Biol. 1994;4:256–258. doi: 10.1016/s0960-9822(00)00058-0. [DOI] [PubMed] [Google Scholar]
- Laird JM, Garcia-Nicas E, Delpire EJ, Cervero F. Presynaptic inhibition and spinal pain processing in mice: a possible role of the Nkcc1 cation-chloride co-transporter in hyperalgesia. Neurosci. Lett. 2004;361:200–203. doi: 10.1016/j.neulet.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Rand MN, Shepherd GM, Greer CA, Zufall F. Calcium entry through cyclic nucleotide-gated channels in individual cilia of olfactory receptor cells: spatiotemporal dynamics. J. Neurosci. 1997;17:4136–4148. doi: 10.1523/JNEUROSCI.17-11-04136.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B. Predicted profiles of ion concentrations in olfactory cilia in the steady state. Biophys. J. 2001;80:1712–1721. doi: 10.1016/S0006-3495(01)76142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G, Gold GH. The spatial distributions of odorant sensitivity and odorant-induced currents in salamander olfactory receptor cells. J. Physiol. 1991;442:147–168. doi: 10.1113/jphysiol.1991.sp018787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366:283–286. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- Matthews HR. A flow heater for fast solution and temperature changes. J. Physiol. 1990;425:20. [Google Scholar]
- Meyer MR, Angele A, Kremmer E, Kaupp UB, Müller F. A cGMP-signaling pathway in a subset of olfactory sensory neurons. Proc. Natl. Acad. Sci. USA. 2000;97:10595–10600. doi: 10.1073/pnas.97.19.10595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Kaneko H, Nishida N. Direct measurement of the chloride concentration in newt olfactory receptors with the fluorescent probe. Neurosci. Lett. 1997;237:5–8. doi: 10.1016/s0304-3940(97)00794-5. [DOI] [PubMed] [Google Scholar]
- Pace AJ, Lee E, Athirakul K, Coffman TM, O'Brien DA, Koller BH. Failure of spermatogenesis in mouse lines deficient in the Na+-K+-2Cl− cotransporter. J. Clin. Invest. 2000;105:441–450. doi: 10.1172/JCI8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. J. Gen. Physiol. 1998;112:529–535. doi: 10.1085/jgp.112.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Response properties of isolated mouse olfactory receptor cells. J. Physiol. 2001a;530:113–122. doi: 10.1111/j.1469-7793.2001.0113m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Responses to prolonged odour stimulation in frog olfactory receptor cells. J. Physiol. 2001b;534:179–191. doi: 10.1111/j.1469-7793.2001.t01-1-00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Bauer PJ, Yau KW, Frings S. The Ca-activated Cl channel and its control in rat olfactory receptor neurons. J. Gen. Physiol. 2003;122:349–364. doi: 10.1085/jgp.200308888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter D, Zierold K, Schröder WH, Frings S. A depolarizing chloride current contributes to chemoelectrical transduction in olfactory sensory neurons in situ. J. Neurosci. 1998;18:6623–6630. doi: 10.1523/JNEUROSCI.18-17-06623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JM. Sodium-potassium-chloride cotransport. Physiol. Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol. Rev. 1998;78:429–466. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- Sung KW, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA receptor-mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J. Neurosci. 2000;20:7531–7538. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron. 2000;27:487–497. doi: 10.1016/s0896-6273(00)00060-x. [DOI] [PubMed] [Google Scholar]
- Zhainazarov AB, Ache BW. Odor-induced currents in Xenopus olfactory receptor cells measured with perforated-patch recording. J. Neurophysiol. 1995;74:479–483. doi: 10.1152/jn.1995.74.1.479. [DOI] [PubMed] [Google Scholar]
- Zhao HQ, Reed RR. OMP-directed ubiquitous expression of an odorant receptor in mouse olfacotry receptor neurons. Chem. Senses. 2001;26:1110–1111. [Google Scholar]
- Zhao HQ, Ivic L, Otaki JM, Hashimoto M, Mikoshiba K, Firestein S. Functional expression of a mammalian odorant receptor. Science. 1998;279:237–242. doi: 10.1126/science.279.5348.237. [DOI] [PubMed] [Google Scholar]
- Zhuo X, Schwob JE, Swiatek PJ, Ding X. Mouse cyp2g1 gene: promoter structure and tissue-specific expression of a cyp2g1-lacz fusion gene in transgenic mice. Arch. Biochem. Biophys. 2001;391:127–136. doi: 10.1006/abbi.2001.2410. [DOI] [PubMed] [Google Scholar]
- Zufall F, Firestein S. Divalent cations block the cyclic nucleotide-gated channel of olfactory receptor neurons. J. Neurophysiol. 1993;69:1758–1768. doi: 10.1152/jn.1993.69.5.1758. [DOI] [PubMed] [Google Scholar]






