Abstract
Purpose
To analyze the frequency and associations with prognostic markers and outcome of mutations in IDH genes encoding isocitrate dehydrogenases in adult de novo cytogenetically normal acute myeloid leukemia (CN-AML).
Patients and Methods
Diagnostic bone marrow or blood samples from 358 patients were analyzed for IDH1 and IDH2 mutations by DNA polymerase chain reaction amplification/sequencing. FLT3, NPM1, CEBPA, WT1, and MLL mutational analyses and gene- and microRNA-expression profiling were performed centrally.
Results
IDH mutations were found in 33% of the patients. IDH1 mutations were detected in 49 patients (14%; 47 with R132). IDH2 mutations, previously unreported in AML, were detected in 69 patients (19%; 13 with R172 and 56 with R140). R172 IDH2 mutations were mutually exclusive with all other prognostic mutations analyzed. Younger age (< 60 years), molecular low-risk (NPM1-mutated/FLT3-internal tandem duplication–negative) IDH1-mutated patients had shorter disease-free survival than molecular low-risk IDH1/IDH2-wild-type (wt) patients (P = .046). R172 IDH2-mutated patients had lower complete remission rates than IDH1/IDH2wt patients (P = .007). Distinctive microarray gene- and microRNA-expression profiles accurately predicted R172 IDH2 mutations. The highest expressed gene and microRNAs in R172 IDH2-mutated patients compared with the IDH1/IDH2wt patients were APP (previously associated with complex karyotype AML) and miR-1 and miR-133 (involved in embryonal stem-cell differentiation), respectively.
Conclusion
IDH1 and IDH2 mutations are recurrent in CN-AML and have an unfavorable impact on outcome. The R172 IDH2 mutations, previously unreported in AML, characterize a novel subset of CN-AML patients lacking other prognostic mutations and associate with unique gene- and microRNA-expression profiles that may lead to the discovery of novel, therapeutically targetable leukemogenic mechanisms.
INTRODUCTION
Despite progress in understanding mechanisms of leukemogenesis and improvement in treatment, only approximately 40% of younger (age < 60 years) and 10% of older (age ≥ 60 years) adults with acute myeloid leukemia (AML) achieve long-term survival.1–4 These results underscore the need for novel therapeutic strategies that would improve outcome. To this end, identification of subsets of patients with distinct clinical and biologic features that would help to stratify them to specific risk-adapted and/or molecularly targeted therapies is imperative.5,6
Cytogenetically normal AML (CN-AML) is the largest group among both younger and older AML patients and the best characterized molecularly.5–7 During the last 15 years, recurring mutations with prognostic significance in genes such as FLT3,8,9 NPM1,10,11 CEBPA,12,13 WT1,14,15 and MLL16,17 have been identified in de novo CN-AML. These markers are not mutually exclusive, and combinations of them may further refine prediction of the risk of adverse events. Thus, patients who carry an NPM1 mutation but not an FLT3 internal tandem duplication (ITD) are in the molecular low-risk group because they have a better outcome than patients who lack NPM1 mutations and/or carry an FLT3-ITD and therefore are in the molecular high-risk group.5,6 Among the latter, however, those who also harbor CEBPA mutations have outcomes similar to that of patients with mutated NPM1 and no FLT3-ITD.13 While the majority of CN-AML patients harbor one or more of the aforementioned mutations, in approximately 15% of the patients, no mutations have been detected,18 suggesting the existence of hitherto undiscovered genetic alterations contributing to leukemogenesis and defining molecular risk for these patients.
Recent studies revealed that mutations in IDH1 and IDH2, the genes encoding isoforms of the nicotinamide adenine dinucleotide phosphate–dependent isocitrate dehydrogenases, are recurrent in brain tumors, including WHO grade 4 gliomas and WHO grade 2 and 3 astrocytomas and oligodendrogliomas, and are associated with favorable outcome.19,20 Importantly, an IDH1 mutation was also discovered through massively parallel DNA sequencing analysis of the genome of a patient with CN-AML.21 In the same study,21 15 IDH1 mutations, but no IDH2 mutations, were also found in a validation set of 187 AML patients. An analysis of overall survival (OS) of this patient population (n = 188), which was heterogeneous with regard to AML type (de novo v secondary), age, and cytogenetics, showed no independent prognostic significance of IDH1 mutations. However, a subgroup analysis showed that IDH1 mutations were associated with CN-AML, being detected in 13 (16%) of 80 such patients, and that they conferred adverse prognosis in the absence of NPM1 mutations.21
To corroborate these preliminary data, we analyzed IDH1 and IDH2 mutations in a homogeneous cohort of 358 adults with de novo CN-AML treated with age-adapted intensive chemotherapy regimens on Cancer and Leukemia Group B (CALGB) first-line protocols and comprehensively characterized other gene mutations associated with outcome.
PATIENTS AND METHODS
Patients, Cytogenetic Analysis, and Treatment
We studied pretreatment bone marrow and blood samples with ≥ 20% blasts from 358 patients age 19 to 83 years with de novo CN-AML. Cytogenetic analyses at diagnosis were confirmed by central karyotype review.22 To establish CN-AML, ≥ 20 metaphase cells from diagnostic bone marrow had to be analyzed and the karyotype had to be normal.23 Institutional review board–approved informed consent for participation in the studies was obtained from all patients. Younger patients (age < 60 years; n = 159) were treated on CALGB 962124 and 1980825 protocols and older patients (age ≥ 60 years; n = 199) were enrolled on protocols 8525,26 8923,27 9420,28 9720,29 or 1020130 (for treatment details, see Appendix, online only). No patient included in our analysis received allogeneic transplantation in first complete remission (CR). The median follow-up for younger and older patients alive and included in this analysis was 7.0 and 3.8 years, respectively.
Molecular Analyses
For IDH1 and IDH2 mutational analyses, DNA fragments spanning exons 4 of IDH1 and IDH2, previously identified as “hot spots” for mutations in these genes,20 were amplified by polymerase chain reaction and directly sequenced as detailed in the Appendix. Other molecular markers—FLT3-ITD,9 FLT3 tyrosine kinase domain (FLT3-TKD) mutations,31 MLL partial tandem duplication (MLL-PTD),17,32 and mutations in the NPM1,33 CEBPA,13 and WT114 genes—were assessed centrally as previously reported.
Genome-Wide Expression Analyses
Gene- and microRNA-expression profiling were conducted using the Affymetrix U133 Plus 2.0 array (Affymetrix, Santa Clara, CA) and the Ohio State University custom microRNA array (OSU_CCC version 4.0), respectively, as reported previously,34,35 and described in the Appendix.
Statistical Analysis
Definitions of clinical end points—CR, disease-free survival (DFS), and OS—are provided in the Appendix. The differences among patients in baseline clinical and molecular features according to their IDH1 and IDH2 mutational status were tested using the Fisher's exact and Wilcoxon rank sum tests for categoric and continuous variables, respectively. Estimated probabilities of DFS and OS were calculated using the Kaplan-Meier method, and the log-rank test evaluated differences between survival distributions.
For expression profiling, summary measures of gene- and microRNA-expression levels were computed, normalized, and filtered (see Appendix). Expression signatures were derived by comparing gene- and microRNA-expression levels among patients with distinct types of IDH mutations and patients with wild-type IDH1 and IDH2 (IDH1/IDH2wt). Univariable significance levels of 0.001 for gene- and 0.005 for microRNA-expression profiling were used to determine the gene probe sets and microRNA probes comprising the signatures, respectively. Prediction of IDH mutation status using gene- and microRNA-expression profiles is described in the Appendix. All analyses were performed by the CALGB Statistical Center.
RESULTS
Frequency of IDH2 and IDH2 Mutations
Of the 358 AML patients analyzed, 118 (33%) harbored missense mutations in IDH genes. Forty-nine patients (14%) had IDH1 mutations, including R132 mutations detected in 46 patients, V71 in two patients, and concurrent R132 and V71 mutations in one patient (Table 1). Sixty-nine patients (19%) had IDH2 mutations: 56 were R140 and 13 were R172 mutations (Table 1). No patient had both IDH1 and IDH2 mutations.
Table 1.
Types of IDH1 and IDH2 Mutations in CN-AML
|
IDH1 Mutations |
IDH2 Mutations |
||||
|---|---|---|---|---|---|
| Nucleotide Change | Amino Acid Change | No. of Patients | Nucleotide Change | Amino Acid Change | No. of Patients |
| c.395G>A | R132H | 23 | c.419G>A | R140Q | 53 |
| c.394C>T | R132C | 15 | c.418C>T | R140W | 1 |
| c.394C>A | R132S | 5 | |||
| c.394C>G | 3 | c.418G>T | R140L | 2 | |
| c.211G>A | V71I | 2 | c.515G>A | R172K | 13 |
| c.395G>A and c.211G>A | R132H & V71I | 1 | — | — | |
NOTE. All mutations are missense mutations. The letters in the Amino Acid Change columns denote which amino acid from the wild-type sequence (first letter) is substituted by another amino acid in the mutated sequence (second letter). The number between the letters denotes the codon position. Amino acid abbreviations: C, cysteine; G, glycine; H, histidine; I, isoleucine; K, lysine; L, leucine; Q, glutamine; R, arginine; S, serine; V, valine; W, tryptophan.
Abbreviations: CN, cytogenetically normal; AML, acute myeloid leukemia.
Associations of IDH Mutations With Pretreatment Characteristics
Comparisons of pretreatment clinical characteristics of IDH1- or IDH2-mutated patients with those of IDH1/IDH2wt patients are reported in Table 2. All types of IDH mutations were significantly associated with higher platelet counts, IDH2 mutations were associated with older age, and R172 IDH2 mutations were associated with low WBC and a low percentage of circulating blasts.
Table 2.
Clinical Characteristics According to IDH Mutational Status in De Novo Cytogenetically Normal Acute Myeloid Leukemia
| Clinical Characteristic |
IDH1-Mutated (n = 49) |
R140 IDH2-Mutated (n = 56) |
R172 IDH2-Mutated (n = 13) |
IDH1/IDH2wt (n = 240) |
P (IDH1-Mutated v IDH1/IDH2wt) | P (R140 IDH2-Mutatedv IDH1/IDH2wt) | P (R172 IDH2-Mutated v IDH1/IDH2wt) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||||
| Age, years | .49 | .006 | .02 | ||||||||
| Median | 62 | 64 | 70 | 60 | |||||||
| Range | 21-82 | 29-83 | 38-83 | 19-81 | |||||||
| Male sex | 23 | 47 | 32 | 57 | 7 | 54 | 118 | 49 | .88 | .30 | .78 |
| Race | .10 | .78 | .30 | ||||||||
| White | 41 | 84 | 52 | 95 | 11 | 85 | 218 | 92 | |||
| Nonwhite | 8 | 16 | 3 | 5 | 2 | 15 | 19 | 8 | |||
| Hemoglobin, g/dL | .73 | .08 | .77 | ||||||||
| Median | 9.3 | 9.8 | 9.4 | 9.3 | |||||||
| Range | 7.1-12.9 | 4.8-13.6 | 7.7-12.3 | 4.6-15.0 | |||||||
| Platelet count, ×109/L | < .001 | .008 | < .001 | ||||||||
| Median | 98 | 73 | 131 | 53 | |||||||
| Range | 11-850 | 11-270 | 64-309 | 7-510 | |||||||
| WBC count, ×109/L | .18 | .30 | < .001 | ||||||||
| Median | 24.6 | 22.5 | 1.5 | 28.4 | |||||||
| Range | 0.9-152.1 | 1.1-434.1 | 0.9-10.5 | 0.9-450.0 | |||||||
| Percentage of PB blasts | .26 | .85 | .002 | ||||||||
| Median | 59 | 45 | 7 | 50 | |||||||
| Range | 0-99 | 0-97 | 0-79 | 0-97 | |||||||
| Percentage of BM blasts | .06 | .27 | .58 | ||||||||
| Median | 73 | 74 | 66 | 63 | |||||||
| Range | 33-99 | 25-96 | 30-86 | 7-99 | |||||||
| Extramedullary involvement | 11 | 24 | 12 | 22 | 1 | 8 | 75 | 32 | .38 | .19 | .12 |
Abbreviations: wt, wild-type; PB, peripheral blood; BM, bone marrow.
At diagnosis, patients with IDH1 mutations were less frequently FLT3-ITD–positive (P = .02), were more often categorized in the molecular low-risk group (NPM1-mutated/FLT3-ITD–negative; P = .003), and had a trend for lower frequencies of WT1 (P = .06) and CEBPA mutations (P = .08) compared with IDH1/IDH2wt patients (Table 3).
Table 3.
Molecular Characteristics According to IDH Mutational Status in De Novo Cytogenetically Normal Acute Myeloid Leukemia
| Molecular Characteristic |
IDH1-Mutated (n = 49) |
R140 IDH2-Mutated (n = 56) |
R172 IDH2- Mutated (n = 13) |
IDH1/IDH2wt (n = 240) |
P (IDH1-Mutated v IDH1/IDH2wt) | P (R140 IDH2-Mutated v IDH1/IDH2wt) | P (R172 IDH2-Mutated v IDH1/IDH2wt) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||||
| NPM1 | .19 | .76 | < .001 | ||||||||
| Mutated | 34 | 71 | 31 | 57 | 0 | 0 | 143 | 60 | |||
| Wild-type | 14 | 29 | 23 | 43 | 13 | 100 | 96 | 40 | |||
| FLT3-ITD | .02 | .12 | .005 | ||||||||
| Present | 10 | 20 | 15 | 27 | 0 | 0 | 91 | 38 | |||
| Absent | 39 | 80 | 41 | 73 | 13 | 100 | 149 | 62 | |||
| Molecular risk group* | .003 | .63 | .01 | ||||||||
| Low | 27 | 55 | 19 | 35 | 0 | 0 | 75 | 31 | |||
| High | 22 | 45 | 35 | 65 | 13 | 100 | 164 | 69 | |||
| FLT3-TKD | .32 | .47 | .37 | ||||||||
| Present | 3 | 6 | 4 | 7 | 0 | 0 | 29 | 12 | |||
| Absent | 45 | 94 | 50 | 93 | 13 | 100 | 210 | 88 | |||
| WT1 | .06 | .007 | .37 | ||||||||
| Mutated | 1 | 2 | 0 | 0 | 0 | 0 | 27 | 11 | |||
| Wild-type | 47 | 98 | 54 | 100 | 13 | 100 | 211 | 89 | |||
| CEBPA | .08 | .13 | .13 | ||||||||
| Mutated | 2 | 6 | 4 | 9 | 0 | 0 | 38 | 18 | |||
| Wild-type | 34 | 94 | 42 | 91 | 13 | 100 | 169 | 82 | |||
| MLL-PTD | .32 | 1.00 | 1.00 | ||||||||
| Present | 1 | 2 | 3 | 6 | 0 | 0 | 16 | 7 | |||
| Absent | 44 | 98 | 49 | 94 | 12 | 100 | 215 | 93 | |||
Abbreviations: wt, wild-type; ITD, internal tandem duplication; TKD, tyrosine kinase domain; PTD, partial tandem duplication.
Molecular low risk: NPM1 mutated and FLT3-ITD-negative; high risk: NPM1 wild-type and/or FLT3-ITD–positive.
Patients with R140 IDH2 mutations had a lower frequency of WT1 mutations (P = .007) than IDH1/IDH2wt patients. Strikingly, patients with R172 IDH2 mutations did not carry any other prognostic mutations, including FLT3-ITD, FLT3-TKD, MLL-PTD, or mutations in the NPM1, WT1, or CEBPA genes (Table 3).
Associations of IDH Mutations With Clinical Outcome
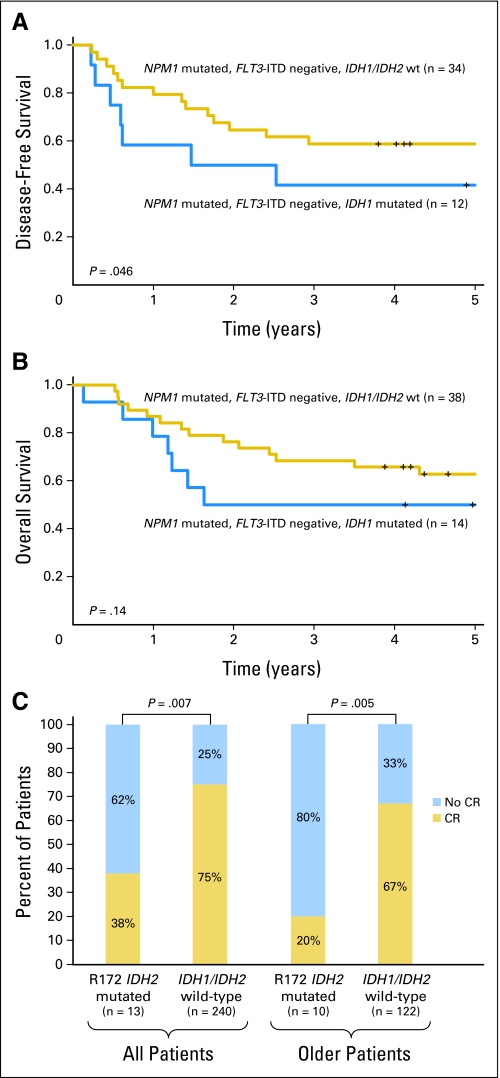
Considering all patients with IDH1 mutations, there was no difference in outcome compared with IDH1/IDH2wt patients (Appendix Table A1, online only). However, in an age group–stratified analysis, we observed a prognostic impact of IDH1 mutations on the subset of younger (age < 60 years) patients in the molecular low-risk group (NPM1-mutated/FLT3-ITD–negative). IDH1-mutated patients (all with R132 IDH1 mutation; see Appendix Table A2, online only for other clinical and molecular characteristics) had a significantly worse DFS (P = .046; 5-year DFS rates, 42% v 59%) and a trend for worse OS (P = .14; 5-year OS rates, 50% v 63%) compared with IDH1/IDH2wt patients (Figs 1A and 1B).
Fig 1.
Impact of distinct IDH mutation types on clinical outcome of patients with cytogenetically normal acute myeloid leukemia. (A) Disease-free survival and (B) overall survival of younger molecular low-risk patients according to IDH1 mutation status. (C) Complete remission rates according to R172 IDH2 mutation status. ITD, internal tandem duplication; wt, wild-type; CR, complete remission.
With regard to IDH2 mutations, the outcome of patients with R140 IDH2 mutations did not differ significantly from the outcome of IDH1/IDH2wt patients (Appendix Table A1). However, R172 IDH2-mutated patients had a significantly lower CR rate than those with IDH1/IDH2wt (38% v 75%; P = .007; Fig 1C). When analysis was limited to older patients, who represented most patients with this mutation (77%), those with R172 IDH2 mutations had a lower CR rate (20% v 67%; P = .005) than IDH1/IDH2wt patients (Fig 1C). The estimated 3-year OS rates were 0% versus 17% for older patients with R172 IDH2 mutations compared with IDH1/IDH2wt patients, but the difference in OS duration for the two groups was not significant.
Since R172 IDH2 mutations were mutually exclusive with NPM1 mutations, to account for the potentially favorable clinical impact of NPM1 mutations on achievement of CR,33 we compared the outcome of patients with R172 IDH2 mutations with that of IDH1/IDH2wt patients without NPM1 mutations. We focused only on older patients because they represented the vast majority of patients with R172 IDH2 mutation. In this prognostically unfavorable subset, patients with R172 IDH2 mutations showed a trend for a lower CR rate (20% v 56%; P = .08).
Biologic Insights Concerning R172 IDH2 Mutations
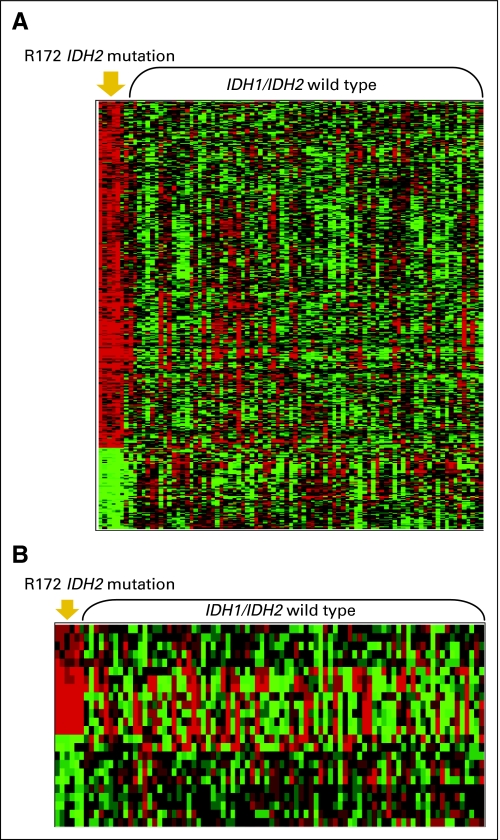
To gain biologic insights into the potentially unfavorable prognostic significance of R172 IDH2 mutations, which are mutually exclusive with any other prognostically relevant mutations and therefore likely to identify a novel subset of CN-AML, we derived a gene-expression signature by comparing R172 IDH2-mutated patients with IDH1/IDH2wt patients. Because 77% of patients with R172 IDH2 mutations were older, to eliminate age-dependent bias, we analyzed only patients age ≥ 60 years. Of the 451 differentially expressed probe sets (P < .001), 365 were upregulated and 86 were downregulated in patients with R172 IDH2 mutations (Fig 2A; Appendix Table A3, online only).
Fig 2.
Genome-wide gene- and microRNA-expression profiles associated with R172 IDH2 mutations. (A) Gene-expression and (B) microRNA-expression signatures, derived from comparing older patients with R172 IDH2 mutations and those with the wild-type IDH1/IDH2 genes. Rows represent gene probe sets (A) or microRNA probes (B), and columns represent patients. Patients are grouped by IDH2 mutational status (R172 or wild-type). Expression values of the probe sets and microRNA probes are represented by color: green indicates expression less than the median value and red indicates expression greater than the median value for the given gene probe set or microRNA probe.
To assess the accuracy of this gene-expression signature to correctly identify R172 IDH2-mutated patients versus those with IDH1/IDH2wt, we conducted a leave-one-out cross-validated prediction analysis. The mutation status of 95.5% of patients (including five of six R172 IDH2-mutated patients) was correctly predicted (Table 4).
Table 4.
Accuracy of Prediction of the R172 IDH2 Mutational Status in Older CN-AML Using Leave-One-Out Cross-Validated Prediction Analysis From Gene- and MicroRNA-Expression Profiles
| Classification | Overall Accuracy (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Gene-expression signature | |||
| R172 (n = 6) v wild-type (n = 83) | 95.5 | 83.3 | 96.4 |
| MicroRNA-expression signature | |||
| R172 (n = 6) v wild-type (n = 82) | 93.2 | 83.3 | 93.9 |
Abbreviations: CN, cytogenetically normal; AML, acute myeloid leukemia.
Among the most upregulated probe sets in R172 IDH2-mutated patients were those representing APP (nine-fold), CXCL12 (eight-fold), PAWR (eight-fold), CDC42BPA (eight-fold), and SPARC (seven-fold; Appendix Table A3). APP was previously reported to be upregulated in AML patients with complex karyotype.36 Polymorphism in CXCL12 (also known as SDF1) was associated with increased circulating blasts and extramedullary disease in AML.37 PAWR was found to regulate WT1 activity and to be overexpressed in myelodysplastic syndromes progressing to AML.38 In addition, CDC42BPA, although not directly associated with AML, seemingly participates in tumor cell invasion.39 In contrast, SPARC, encoding a matricellular glycoprotein with growth-inhibitory and antiangiogenic functions, was found to have lower expression in MLL-associated AML and tumor suppressor activity.40 Other genes of interest upregulated in the R172 IDH2-mutated patients were ID1 (four-fold), whose expression was recently correlated with poor outcome in AML41; ABCB1 (MDR1; five-fold) mediating chemoresistance42; and KRAS2 (2.6-fold), which is constitutively activated in several human cancers including AML.43
The downregulated genes we found include KYNU, which encodes a protein participating in the biosynthesis of NAD cofactors from tryptophan44; SUCLG2, involved in the Krebs cycle and mutated in Leigh-like syndrome45; CD93, involved in regulating phagocytosis of apoptotic cells and angiogenesis46; LY86 and LIST1, associated with immune response pathways47,48; and PTHR2, a receptor for the parathyroid hormone.49 To the best of our knowledge, none of these genes have previously been associated with AML.
Genome-wide profiling identifies aberrantly expressed microRNAs associated with distinct molecular subsets of CN-AML patients.50 Therefore, we derived a microRNA-expression signature associated with older R172 IDH2-mutated CN-AML. The signature comprised 24 differentially expressed (P < .005) probes, 13 of which were upregulated and 11 of which were downregulated in R172 IDH2-mutated patients (Fig 2B; Appendix Table A4, online only). In leave-one-out cross-validated prediction analysis, the mutation status of 93.2% of patients (including five of six R172 IDH2-mutated patients) was correctly predicted (Table 4).
Among the microRNAs most upregulated (> four-fold) in R172 IDH2-mutated patients were members of the miR-125 family (miR-125a-5p and miR-125b), miR-1, and miR-133. miR-125b has been shown to target the tumor suppressor gene TP53 and inhibit myeloid differentiation,51 whereas miR-1 and miR-133 have not been previously associated with human cancer, but they participate in cell fate decision mechanisms of pluripotent embryonic stem cells.52 Among the most downregulated probes were those representing mir-194-1, miR-526, miR-520a-3p, and mir-548b, none of which have previously been associated with normal hematopoiesis or AML.
DISCUSSION
Mutations in the IDH1 and IDH2 genes have been found in patients with glioma and predict favorable outcome.19 Using whole-genome sequencing and validation analyses, Mardis et al21 recently reported that IDH1 mutations can also be found in AML and are associated with normal karyotypes. Therefore, we analyzed a larger, more homogeneous cohort of de novo CN-AML (n = 358), comprising both younger and older patients treated with age-adapted chemotherapy regimens on first-line CALGB clinical trials.
The first salient finding of our study was that we not only confirmed the presence of IDH1 mutations but also found previously unreported IDH2 mutations in CN-AML.5 IDH1 mutations were found in 14% of our patients, which is similar to the findings of Mardis et al.21 In addition to the previously reported R132 IDH1 mutations, we identified three patients with a V71I IDH1 allele. Although this allele has been recently reported as a single nucleotide polymorphism (SNP), Bleeker et al53 did not find it in any of the 672 tumor samples and 84 cell lines they sequenced. This suggests that if V71I IDH1 is an SNP, it is rare and, therefore, the possibility that V71I IDH1 represents a novel IDH1 somatic or germline mutation associated with AML cannot be excluded.
Moreover, unlike Mardis et al,21 we also detected two different types of mutations in the IDH2 gene (ie, R140 and R172), which occurred with even greater frequency (19%) and, to the best of our knowledge, have not been previously reported in AML. Interestingly, while the R172 IDH2 mutation was previously found in gliomas, to the best of our knowledge, the R140 IDH2 mutation has not been previously reported in human cancer or normal tissue. Since changes in codon 140 detected in our patients led to the substitution of the arginine with three different amino acids (Table 1), it is likely that R140 IDH2 represents a somatic mutation associated with AML rather than a newly discovered SNP. Studies of normal tissues from R140 IDH2 AML patients are underway to confirm (or refute) the somatic nature of R140 IDH2. Both IDH2 mutations were associated with older age but, remarkably, only R172 IDH2 mutations were found in the absence of other recurrent mutations thereby identifying a novel subset of patients among those 15% of CN-AML patients for whom no prognostic gene mutation has been hitherto reported. When considered together, the frequency of mutations in genes encoding the isocitrate dehydrogenases is relatively high in CN-AML (33%), placing them among the most frequent mutations in CN-AML.
The second important finding relates to the prognostic significance of IDH mutations in specific age and molecular subsets of CN-AML. We showed that although IDH1 mutations did not affect outcome in the whole cohort of CN-AML patients, they conferred worse prognosis in younger patients with molecular low-risk CN-AML. These results differ from two previous studies reporting that IDH1 mutations conferred adverse outcome in NPM1wt patients with CN-AML21 or various karyotypes.54 Differences in sizes of patient cohorts analyzed, varying inclusion criteria (eg, we studied only de novo AML patients whereas Schnittger et al54 also analyzed secondary AML), age, and treatment administered might contribute to these discrepancies among studies, which require further investigation for resolution.
Most patients with R172 IDH2 mutations failed to achieve a CR following intensive cytarabine/anthracycline-based induction chemotherapy. Because NPM1 mutations are a strong, favorable prognosticator in older CN-AML patients,33 we also separately analyzed older patients without NPM1 mutations; even then, the CR rate of patients with R172 IDH2 mutations tended to be lower than that of IDH1/IDH2wt patients. These results suggest that it is the presence of the R172 IDH2 mutation itself rather than the absence of NPM1 mutations that decreases the odds of achieving CR. However, given the relatively small number of R172 IDH2-mutated patients in our cohort, larger studies should corroborate our results. Notably, in contrast with our data in CN-AML, R172 IDH2 mutations were reported to predict a favorable outcome in patients with gliomas,20 thereby supporting the notion that the prognostic significance of molecular markers may vary according to distinct biologic and/or therapeutic contexts in which they are evaluated. Furthermore, in contrast with R172 IDH2 mutations, the outcome of patients with R140 IDH2 mutations was not different from the outcome of patients with wt IDH1 and IDH2 genes, thereby suggesting different contributions to leukemogenesis from these two mutation types.
Finally, we showed that R172 IDH2 mutations in CN-AML are associated with unique gene- and microRNA-expression signatures. Although the signature did not include previously reported unfavorable prognosticators in CN-AML (ie, BAALC, ERG, and MN1),55–61 it comprised other upregulated genes associated with adverse karyotypes (APP),36 unfavorable outcome (ID1),41 increased rate of extramedullary disease (CXCL12),37 or increased chemoresistance (ABCB1) in AML,42 supporting the negative prognostic significance of this mutation type. Furthermore, among microRNAs differentially expressed in R172 IDH2-mutated patients, we noted upregulation of miR-125b, previously found to block myeloid differentiation,51 and miR-1 and miR-133, not reported previously in AML but involved in embryonal stem-cell differentiation.52 Importantly, both gene- and microRNA-expression signatures appeared to predict the R172 IDH2 mutational status with high accuracy, thus supporting the view that patients with R172 IDH2 mutations profoundly differ biologically and clinically from patients with wt IDH1 and IDH2 alleles.
The mechanisms through which IDH1 and IDH2 mutations contribute to malignant transformation are under investigation. Thompson62 postulated that IDH1 and IDH2 mutations result in gain rather than loss of function, given the high frequency of somatic mutations affecting a single codon and the absence of other mutations causing gene inactivation. Indeed, Dang et al63 showed that the R132 IDH1 mutation causes the encoded enzyme to acquire the ability to convert α-ketoglutarate to 2-hydroxy-glutarate, which accumulates in the affected cells. This likely contributes to malignant transformation since inborn errors of 2-hydroxy-glutarate metabolism have been associated with an increased risk of brain tumors.63 While similar mechanisms might be operative in patients harboring IDH2 mutations, to the best of our knowledge, no functional study of the mutant proteins has been reported.
In summary, we report here that IDH1 mutations predict shorter DFS in younger molecular low-risk CN-AML patients, R172 IDH2 mutations are mutually exclusive with other known prognostic mutations and denote a novel subset of older CN-AML patients characterized by resistance to induction chemotherapy, and R140 IDH2 mutations do not appear to confer prognostic significance. By deriving gene- and microRNA-expression signatures, we uncovered intriguing features in R172 IDH2-mutated patients that may lead to better understanding of the biologic role of this mutation and to the design of novel therapies targeting aberrant isocitrate dehydrogenase–driven activation of metabolic pathways.
Acknowledgment
We thank Professor Albert de la Chapelle for the helpful discussion.
Appendix
The following Cancer and Leukemia Group B institutions, principal investigators, and cytogeneticists participated in this study: Wake Forest University School of Medicine, Winston-Salem, NC: David D. Hurd, P. Nagesh Rao, Wendy L. Flejter, and Mark J. Pettenati (Grant No. CA03927); The Ohio State University Medical Center, Columbus, OH: Clara D. Bloomfield, Karl S. Theil, Diane Minka, and Nyla A. Heerema (Grant No. CA77658); North Shore–Long Island Jewish Health System, Manhasset, NY: Daniel R. Budman and Prasad R.K. Koduru (Grant No. CA35279); University of Iowa Hospitals, Iowa City, IA: Daniel A. Vaena and Shivanand R. Patil (Grant No. CA47642); Roswell Park Cancer Institute, Buffalo, NY: Ellis G. Levine and AnneMarie W. Block (Grant No. CA02599); Duke University Medical Center, Durham, NC: Jeffrey Crawford, Mazin B. Qumsiyeh, John Eyre, and Barbara K. Goodman (Grant No. CA47577); University of Chicago Medical Center, Chicago, IL: Hedy L. Kindler, Diane Roulston, Katrin M. Carlson, Yanming Zhang, and Michelle M. Le Beau (Grant No. CA41287); Washington University School of Medicine, St. Louis, MO: Nancy L. Bartlett, Michael S. Watson, Eric C. Crawford, Peining Li, and Jaime Garcia-Heras (Grant No. CA77440); University of North Carolina, Chapel Hill, NC: Thomas C. Shea and Kathleen W. Rao (Grant No. CA47559); University of Massachusetts Medical Center, Worcester, MA: William V. Walsh, Vikram Jaswaney, Michael J. Mitchell, and Patricia Miron (Grant No. CA37135); Dartmouth Medical School, Lebanon, NH: Konstantin Dragnev, Doris H. Wurster-Hill, and Thuluvancheri K. Mohandas (Grant No. CA04326); Dana-Farber Cancer Institute, Boston, MA: Harold J. Burstein, Ramana Tantravahi, Leonard L. Atkins, Paola Dal Cin, and Cynthia C. Morton (Grant No. CA32291); Vermont Cancer Center, Burlington, VT: Steven M. Grunberg, Elizabeth F. Allen, and Mary Tang (Grant No. CA77406); Ft. Wayne Medical Oncology/Hematology, Ft. Wayne, IN: Sreenivasa Nattam and Patricia I. Bader; Eastern Maine Medical Center, Bangor, ME: Harvey M. Segal and Laurent J. Beauregard (Grant No. CA35406); Weill Medical College of Cornell University, New York, NY: John Leonard, Ram S. Verma, Prasad R.K. Koduru, Andrew J. Carroll, and Susan Mathew (Grant No. CA07968); Mount Sinai School of Medicine, New York, NY: Lewis R. Silverman and Vesna Najfeld (Grant No. CA04457); University of Puerto Rico School of Medicine, San Juan, PR: Eileen I. Pacheco, Paola Dal Cin, Leonard L. Atkins, and Cynthia C. Morton; Rhode Island Hospital, Providence, RI: William Sikov, Teresita Padre-Mendoza, Hon Fong L. Mark, Shelly L. Kerman, and Aurelia Meloni-Ehrig (Grant No. CA08025); State University of New York Upstate Medical University, Syracuse, NY: Stephen L. Graziano and Constance K. Stein (Grant No. CA21060); Minneapolis Veterans Administration Medical Center, Minneapolis, MN: Vicki A. Morrison and Sugandhi A. Tharapel (Grant No. CA47555); University of California at San Diego, San Diego, CA: Barbara A. Parker, Renée Bernstein, and Marie L. Dell'Aquila (Grant No. CA11789); Christiana Care Health Services, Newark, DE: Stephen S. Grubbs, Jeanne M. Meck, and Digamber S. Borgaonkar (Grant No. CA45418); Long Island Jewish Medical Center Community Clinical Oncology Program, Lake Success, NY: Kanti R. Rai and Prasad R.K. Koduru (Grant No. CA11028); University of Illinois at Chicago, Chicago, IL: David J. Peace, Maureen M. McCorquodale, and Kathleen E. Richkind (Grant No. CA74811); Western Pennsylvania Hospital, Pittsburgh, PA: John Lister and Gerard R. Diggans; University of Minnesota, Minneapolis, MN: Bruce A. Peterson, Diane C. Arthur, and Betsy A. Hirsch (Grant No. CA16450); University of Missouri/Ellis Fischel Cancer Center, Columbia, MO: Michael C. Perry and Tim H. Huang (Grant No. CA12046); University of Maryland Cancer Center, Baltimore, MD: Martin J. Edelman, Joseph R. Testa, Maimon M. Cohen, and Yi Ning (Grant No. CA31983); Walter Reed Army Medical Center, Washington, DC: Brendan M. Weiss, Rawatmal B. Surana, and Digamber S. Borgaonkar (Grant No. CA26806); Georgetown University Medical Center, Washington, DC: Minnetta C. Liu and Jeanne M. Meck (Grant No. CA77597); McGill Department of Oncology, Montreal, Quebec, Canada: J.L. Hutchison and Jacqueline Emond (Grant No. CA31809); Medical University of South Carolina, Charleston, SC: Mark R. Green, G. Shashidhar Pai, and Daynna J. Wolff (Grant No. CA03927); University of Nebraska Medical Center, Omaha, NE: Anne Kessinger and Warren G. Sanger (Grant No. CA77298); University of Alabama at Birmingham, Birmingham, AL: Robert Diasio and Andrew J. Carroll (Grant No. CA47545); University of Cincinnati Medical Center, Cincinnati, OH: Orlando J. Martelo and Ashok K. Srivastava (Grant No. CA47515); Columbia-Presbyterian Medical Center, New York, NY: Rose R. Ellison and Dorothy Warburton (Grant No. CA12011); Massachusetts General Hospital, Boston, MA: Jeffrey W. Clark, Paola Dal Cin, and Cynthia C. Morton (Grant No. CA 12449); Virginia Commonwealth University Minority-Based Community Clinical Oncology Program, Richmond, VA: John D. Roberts and Colleen Jackson-Cook (Grant No. CA52784); State University of New York Maimonides Medical Center, Brooklyn, NY: Sameer Rafla and Ram S. Verma (Grant No. CA25119); Southern Nevada Cancer Research Foundation Community Clinical Oncology Program, Las Vegas, NV: John A. Ellerton and Marie L. Dell'Aquila (Grant No. CA35421); University of California at San Francisco, San Francisco, CA: Charles J. Ryan and Kathleen E. Richkind (Grant No. CA60138).
Patients and Methods
Treatment.
Patients enrolled on Cancer and Leukemia Group B (CALGB) 19808 were randomly assigned to receive induction chemotherapy with cytarabine, daunorubicin, and etoposide with or without PSC 833 (valspodar), a multidrug resistance protein inhibitor.24 On achievement of complete response (CR), patients were assigned to intensification with high-dose cytarabine and etoposide for stem-cell mobilization followed by myeloablative treatment with busulfan and etoposide supported by autologous peripheral blood stem-cell transplantation. Patients enrolled on CALGB 9621 were treated similarly to those on CALGB 19808, as previously reported.25
Older patients were all treated with cytarabine/daunorubicin-based induction therapy followed by cytarabine-based consolidation therapy. Patients on CALGB 8525 were treated with induction chemotherapy consisting of cytarabine in combination with daunorubicin and were randomly assigned to consolidation with different doses of cytarabine followed by maintenance treatment.26 Patients on CALGB 8923 were treated with induction chemotherapy consisting of cytarabine in combination with daunorubicin and were randomly assigned to receive postremission therapy with cytarabine alone or in combination with mitoxantrone.27 Patients on CALGB 9420 and 9720 received induction chemotherapy consisting of cytarabine in combination with daunorubicin and etoposide, with (CALGB 9420) or with/without (CALGB 9720) the multidrug resistance protein modulator valspodar.28,29 Patients on CALGB 9420 received postremission therapy with cytarabine (2 g/m2/d) alone, and patients on CALGB 9720 received a single cytarabine/daunorubicin consolidation course identical to the induction regimen and were then randomly assigned to low-dose recombinant interleukin-2 maintenance therapy or none.28 Patients on CALGB 10201 received induction chemotherapy consisting of cytarabine and daunorubicin, with or without the BCL2 antisense oblimersen sodium. The consolidation regimen included two cycles of cytarabine (2 g/m2/d) with or without oblimersen (Marcucci G: J Clin Oncol 25:360s, 2007 [suppl; abstr 7012]).
Sample preparation.
Patients enrolled on the treatment protocols were also enrolled on the companion protocols CALGB 9665 (leukemia tissue bank), CALGB 8461 (cytogenetic studies), and CALGB 20202 (molecular studies in AML) and consented to pretreatment bone marrow (BM) and peripheral blood collection. Samples were subjected to Ficoll-Hypaque gradient separation, and mononuclear cells were cryopreserved until use. Genomic DNA extraction and quality control of the extracted nucleic acids were performed as reported elsewhere.14
The primers used for polymerase chain reaction (PCR) amplification were IDH1F: AGCTCTATATGCCATCACTGC, IDH1R: AACATGCAAAATCACATTATTGCC, IDH2F: AATTTTAGGACCCCCGTCTG, and IDH2R: CTGCAGAGACAAGAGGATGG.
PCR conditions for IDH1 and IDH2 amplifications were identical, using 20 to 50 ng genomic DNA and HotStar Taq DNA polymerase kit (Qiagen, Valencia, CA) in a 50-μL reaction with the following amplification program: denaturing at 95°C for 1 minute, annealing at 57°C for 1 minute, and extension at 72°C for 1 minute, for 35 cycles. The reactions were run in a DNA Engine Dyad Peltier Thermal Cycler (Bio-Rad, Hercules, CA). The PCR products were then sequenced as previously reported.20
Definition of clinical end points.
CR required absolute neutrophil counts ≥ 1,500/μL, platelet counts ≥ 100,000/μL, no leukemic blasts in the blood, BM cellularity greater than 20% with maturation of all cell lines, no Auer rods, less than 5% BM blast cells, and no evidence of extramedullary leukemia, all of which had persisted for at least 1 month. Relapse was defined by ≥ 5% BM blasts, circulating leukemic blasts, or the development of extramedullary leukemia. Overall survival was measured from the date the patient was enrolled onto the study until the date of death, and patients alive at last follow-up were censored. Disease-free survival was measured from the date of CR until the date of relapse or death; patients alive and relapse-free at last follow-up were censored (Cheson BD: J Clin Oncol 8:813-819, 1990).
Genome-wide gene- and microRNA-expression analyses.
For gene-expression microarrays, summary measures of gene expression were computed for each probe set using the robust multichip average method, which incorporates quantile normalization of arrays. Expression values were logged (base 2) before analysis. A filtering step was performed to remove probe sets that did not display significant variation in expression across arrays. In this procedure, a χ2 test was used to test whether the observed variance in expression of a probe set was significantly larger than the median observed variance in expression for all probe sets using α = .01 as the significance level. A total of 24,437 probe sets passed the filtering criterion. Comparisons of gene expression were made between R172 IDH2-mutated and IDH1/IDH2-wild-type (wt) patients (R172 IDH2-mutated, n = 6; IDH1/IDH2wt, n = 83) using a univariable significance level of 0.001.
For microRNA microarrays, signal intensities were calculated for each spot making an adjustment for local background. Intensities were log-transformed and log-intensities from replicate spots were averaged. Quantile normalization was performed on arrays using all human and mouse microRNA probes represented on the array. For each microRNA probe, an adjustment was made for batch effects (ie, differences in expression related to the batch in which arrays were hybridized). Further analysis was limited to the 895 unique human probes represented on the array. Comparisons of microRNA expression were made between R172 IDH2-mutated and IDH1/IDH2wt patients (R172 IDH2-mutated, n = 6; IDH1/IDH2wt, n = 82) using a univariable significance level of 0.005. Analyses were performed using BRB-ArrayTools Version 3.8.0 Beta_2 Release developed by Richard Simon, DSc, and Amy Peng Lam.
Prediction of IDH2 mutation status from expression profiles.
We implemented compound covariate prediction using leave-one-out cross-validation to predict R172 IDH2-mutated versus IDH1/IDH2wt status of patients from gene- and microRNA-expression profiles (Radmacher MD: J Comput Biol 9:505-511, 2002).56 For gene-expression arrays, each patient, one at a time, was removed from analysis and the expression profiles of the remaining R172 IDH2-mutated versus IDH1/IDH2wt patients were compared to derive a gene- or microRNA-expression signature. A compound covariate was then computed for each patient on the basis of this signature: the value of the compound covariate for patient i was ci = Σ wj xij, where xij is the log-transformed expression value for probe set j in patient i and wj is the weight assigned to probe set j (in this case, wj was set equal to the two-sample t statistic for the comparison of the R172 IDH2-mutated and IDH1/IDH2wt groups for probe set j). The sum is over all j probe sets included in the signature. A classification threshold was computed to be the midpoint of the means of the compound covariate values for the R172 IDH2-mutated and IDH1/IDH2wt groups. The compound covariate was then calculated for the left-out patient, and its IDH2 status was predicted by comparing its value to the classification threshold. This entire process was repeated until every patient had been left out one time and mutation status had been predicted. The overall accuracy of the prediction is indicated, as are the sensitivity and specificity for prediction of R172 IDH2 mutations.
Table A1.
Clinical Outcome of Patients With IDH1 or Patients With R140 IDH2 Mutation
| Outcome Endpoint |
IDH1-Mutated (n = 49) |
R140 IDH2-Mutated (n = 56) |
IDH1/IDH2wt (n = 240) |
P (IDH1-Mutated v IDH1/IDH2-wt) | P (R140 IDH2 Mutated v IDH1/IDH2wt) | |||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | |||
| Complete remission | 73 | 70 | 75 | .86 | .40 | |||
| Overall survival | .33 | .58 | ||||||
| Median, years | 1.3 | 1.4 | 1.4 | |||||
| Alive at 3 years | 29 | 17 to 41 | 39 | 26 to 52 | 33 | 27 to 39 | ||
| Disease-free survival | .30 | .82 | ||||||
| Median, years | 1.1 | 1.3 | 1.1 | |||||
| Disease-free at 3 years | 28 | 14 to 43 | 28 | 15 to 43 | 32 | 25 to 39 | ||
Abbreviation: wt, wild-type.
Table A2.
Clinical and Molecular Characteristics According to IDH1 Mutational Status
| Characteristic |
IDH1-Mutated* (n = 14) |
IDH1/IDH2wt (n = 38) |
P | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .48 | ||||
| Median | 42 | 49 | |||
| Range | 21-57 | 19-59 | |||
| Male sex | 4 | 29 | 21 | 55 | .12 |
| Race | .65 | ||||
| White | 12 | 86 | 34 | 89 | |
| Nonwhite | 2 | 14 | 4 | 11 | |
| Hemoglobin, g/dL | .45 | ||||
| Median | 9.6 | 9.2 | |||
| Range | 7.1-12.4 | 6.4-12.3 | |||
| Platelet count, ×109/L | .07 | ||||
| Median | 147 | 61 | |||
| Range | 11-380 | 12-445 | |||
| WBC count, ×109/L | .40 | ||||
| Median | 31.4 | 22.1 | |||
| Range | 1.7-127.7 | 1.6-146.0 | |||
| Percentage of PB blasts | .003 | ||||
| Median | 82 | 40 | |||
| Range | 10-89 | 0-90 | |||
| Percentage of BM blasts | .04 | ||||
| Median | 77 | 64 | |||
| Range | 42-92 | 10-91 | |||
| Extramedullary involvement | 4 | 33 | 19 | 50 | .34 |
| FLT3-TKD | .41 | ||||
| Present | 1 | 7 | 8 | 21 | |
| Absent | 13 | 93 | 30 | 79 | |
| WT1 | 1.0 | ||||
| Mutated | 1 | 7 | 3 | 8 | |
| Wild-type | 13 | 93 | 35 | 92 | |
| CEBPA | 1.0 | ||||
| Mutated | 0 | 0 | 1 | 3 | |
| Wild-type | 14 | 100 | 37 | 97 | |
| MLL-PTD | 1.0 | ||||
| Present | 0 | 0 | 2 | 5 | |
| Absent | 14 | 100 | 36 | 95 | |
NOTE. Patients were younger than age 60 years and were molecular low-risk (ie, NMP1-mutated and FLT3-ITD–negative) with de novo cytogenetically normal acute myeloid leukemia.
Abbreviations: wt, wild-type; PB, peripheral blood; BM, bone marrow; ITD, internal tandem duplication; TKD, tyrosine kinase domain; PTD, partial tandem duplication.
All patients harbored the R132IDH1 mutation.
Table A3.
Differentially Expressed (P < .001) Probe Sets Between R172 IDH2-Mutated (n = 6) and IDH1/IDH2wt (n = 83) Older Patients
| Probe Set | Gene Symbol | Description | Fold-Change: R172/wt |
|---|---|---|---|
| Upregulated gene probes | |||
| 200602_at | APP | Amyloid beta (A4) precursor protein | 9.50 |
| 209687_at | CXCL12 | Chemokine (C-X-C motif) ligand 12 (stromal cell-derived factor 1) | 8.01 |
| 204004_at | PAWR | PRKC, apoptosis, WT1, regulator | 7.91 |
| 214464_at | CDC42BPA | CDC42 binding protein kinase alpha (DMPK-like) | 7.73 |
| 203666_at | CXCL12 | Chemokine (C-X-C motif) ligand 12 (stromal cell-derived factor 1) | 7.48 |
| 226192_at | 7.39 | ||
| 200665_s_at | SPARC | Secreted protein, acidic, cysteine-rich (osteonectin) | 7.30 |
| 218901_at | PLSCR4 | Phospholipid scramblase 4 | 7.28 |
| 214953_s_at | APP | Amyloid beta (A4) precursor protein | 7.10 |
| 204005_s_at | PAWR | PRKC, apoptosis, WT1, regulator | 6.79 |
| 215116_s_at | DNM1 | Dynamin 1 | 6.77 |
| 230896_at | BEND4 | BEN domain containing 4 | 6.66 |
| 221530_s_at | BHLHE41 | Basic helix-loop-helix family, member e41 | 6.60 |
| 213506_at | F2RL1 | Coagulation factor II (thrombin) receptor-like 1 | 6.50 |
| 236635_at | ZNF667 | Zinc finger protein 667 | 6.23 |
| 1554182_at | TRIM74 | Tripartite motif-containing 74 | 6.18 |
| 218086_at | NPDC1 | Neural proliferation, differentiation and control, 1 | 5.98 |
| 236793_at | 5.90 | ||
| 235759_at | 5.85 | ||
| 205839_s_at | BZRAP1 | Benzodiazapine receptor (peripheral) associated protein 1 | 5.77 |
| 201069_at | MMP2 | Matrix metallopeptidase 2 (gelatinase A, 72 kda gelatinase, 72 kda type IV collagenase) | 5.73 |
| 222862_s_at | AK5 | Adenylate kinase 5 | 5.51 |
| 226223_at | 5.35 | ||
| 223885_at | CALN1 | Calneuron 1 | 5.09 |
| 239082_at | 5.07 | ||
| 225342_at | AK3L1 | Adenylate kinase 3-like 1 | 5.06 |
| 209993_at | ABCB1 | ATP-binding cassette, subfamily B (MDR/TAP), member 1 | 4.96 |
| 223125_s_at | C1orf21 | Chromosome 1 open reading frame 21 | 4.92 |
| 1570412_at | 4.90 | ||
| 202018_s_at | LTF | Lactotransferrin | 4.87 |
| 230266_at | RAB7B | RAB7B, member RAS oncogene family | 4.82 |
| 204348_s_at | AK3L1 | Adenylate kinase 3-like 1 | 4.60 |
| 37005_at | NBL1 | Neuroblastoma, suppression of tumorigenicity 1 | 4.44 |
| 208937_s_at | ID1 | Inhibitor of DNA binding 1, dominant negative helix-loop-helix protein | 4.23 |
| 240671_at | 4.18 | ||
| 204352_at | TRAF5 | TNF receptor-associated factor 5 | 4.16 |
| 219569_s_at | TMEM22 | Transmembrane protein 22 | 4.14 |
| 244741_s_at | MGC9913 | Hypothetical protein MGC9913 | 4.14 |
| 222281_s_at | 4.04 | ||
| 226587_at | SNRPN | Small nuclear ribonucleoprotein polypeptide N | 4.02 |
| 230698_at | CALN1 | Calneuron 1 | 3.97 |
| 237591_at | FLJ42957 | FLJ42957 protein | 3.89 |
| 242457_at | 3.85 | ||
| 227415_at | DGKH | Diacylglycerol kinase, eta | 3.81 |
| 229715_at | 3.74 | ||
| 209487_at | RBPMS | RNA binding protein with multiple splicing | 3.73 |
| 202073_at | OPTN | Optineurin | 3.72 |
| 226125_at | 3.67 | ||
| 209994_s_at | ABCB1 | ATP-binding cassette, subfamily B (MDR/TAP), member 1 | 3.67 |
| 230224_at | ZCCHC18 | Zinc finger, CCHC domain containing 18 | 3.56 |
| 227108_at | STARD9 | Star-related lipid transfer (START) domain containing 9 | 3.56 |
| 223126_s_at | C1orf21 | Chromosome 1 open reading frame 21 | 3.54 |
| 226591_at | SNRPN | Small nuclear ribonucleoprotein polypeptide N | 3.53 |
| 209982_s_at | NRXN2 | Neurexin 2 | 3.53 |
| 1555912_at | ST7OT1 | ST7 overlapping transcript 1 (non-protein coding) | 3.51 |
| 218966_at | MYO5C | Myosin VC | 3.48 |
| 215811_at | 3.43 | ||
| 1558103_a_at | 3.40 | ||
| 230175_s_at | 3.40 | ||
| 221272_s_at | C1orf21 | Chromosome 1 open reading frame 21 | 3.36 |
| 201621_at | NBL1 | Neuroblastoma, suppression of tumorigenicity 1 | 3.34 |
| 238127_at | FLJ41484 | Hypothetical LOC650669 | 3.33 |
| 207120_at | ZNF667 | Zinc finger protein 667 | 3.26 |
| 211110_s_at | AR | Androgen receptor | 3.23 |
| 241916_at | 3.23 | ||
| 207550_at | MPL | Myeloproliferative leukemia virus oncogene | 3.23 |
| 212667_at | SPARC | Secreted protein, acidic, cysteine-rich (osteonectin) | 3.22 |
| 204347_at | AK3L1 | Adenylate kinase 3-like 1 | 3.20 |
| 214373_at | 3.19 | ||
| 221974_at | IPW | Imprinted in Prader-Willi syndrome (non-protein coding) | 3.18 |
| 213484_at | 3.18 | ||
| 212607_at | AKT3 | V-akt murine thymoma viral oncogene homolog 3 (protein kinase B, gamma) | 3.14 |
| 1557961_s_at | LOC100127983 | Hypothetical protein LOC100127983 | 3.04 |
| 1558102_at | 3.02 | ||
| 243364_at | AUTS2 | Autism susceptibility candidate 2 | 2.97 |
| 232511_at | 2.95 | ||
| 232653_at | 2.93 | ||
| 209291_at | ID4 | Inhibitor of DNA binding 4, dominant negative helix-loop-helix protein | 2.89 |
| 212463_at | CD59 | CD59 molecule, complement regulatory protein | 2.84 |
| 219228_at | ZNF331 | Zinc finger protein 331 | 2.83 |
| 235831_at | 2.83 | ||
| 212842_x_at | RGPD5 | RANBP2-like and GRIP domain containing 5 | 2.82 |
| 219308_s_at | AK5 | Adenylate kinase 5 | 2.81 |
| 238861_at | 2.80 | ||
| 237571_at | 2.80 | ||
| 243880_at | GOSR2 | Golgi SNAP receptor complex member 2 | 2.77 |
| 1554183_s_at | TRIM74 | Tripartite motif-containing 74 | 2.76 |
| 235421_at | MAP3K8 | Mitogen-activated protein kinase kinase kinase 8 | 2.76 |
| 238097_at | LOC100128430 | Hypothetical protein LOC100128430 | 2.73 |
| 242406_at | 2.71 | ||
| 240182_at | 2.70 | ||
| 1559494_at | 2.68 | ||
| 1555168_a_at | CALN1 | Calneuron 1 | 2.67 |
| 244726_at | 2.66 | ||
| 233379_at | FLJ14213 | Protor-2 | 2.65 |
| 226922_at | RANBP2 | RAN binding protein 2 | 2.63 |
| 1563369_at | FLJ42957 | FLJ42957 protein | 2.63 |
| 1553204_at | C20orf200 | Chromosome 20 open reading frame 200 | 2.61 |
| 218094_s_at | DBNDD2 | Dysbindin (dystrobrevin binding protein 1) domain containing 2 | 2.61 |
| 227845_s_at | SHD | Src homology 2 domain containing transforming protein D | 2.60 |
| 218898_at | FAM57A | Family with sequence similarity 57, member A | 2.60 |
| 235094_at | 2.58 | ||
| 235408_x_at | ZNF117 | Zinc finger protein 117 | 2.58 |
| 224998_at | CMTM4 | CKLF-like MARVEL transmembrane domain containing 4 | 2.58 |
| 1559203_s_at | KRAS | V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog | 2.58 |
| 240090_at | 2.57 | ||
| 235099_at | CMTM8 | CKLF-like MARVEL transmembrane domain containing 8 | 2.57 |
| 229876_at | PHKA1 | Phosphorylase kinase, alpha 1 (muscle) | 2.57 |
| 222786_at | CHST12 | Carbohydrate (chondroitin 4) sulfotransferase 12 | 2.55 |
| 243952_at | psiTPTE22 | TPTE pseudogene | 2.55 |
| 233029_at | OBSCN | Obscurin, cytoskeletal calmodulin and titin-interacting rhogef | 2.55 |
| 1555898_at | LOC400986 | Protein immunoreactive with anti-PTH polyclonal antibodies | 2.54 |
| 228580_at | HTRA3 | Htra serine peptidase 3 | 2.53 |
| 238643_at | 2.52 | ||
| 236610_at | 2.51 | ||
| 220450_at | 2.50 | ||
| 1556474_a_at | FLJ38379 | Hypothetical FLJ38379 | 2.48 |
| 240903_at | 2.46 | ||
| 1562245_a_at | ZNF578 | Zinc finger protein 578 | 2.45 |
| 235697_at | 2.44 | ||
| 225305_at | SLC25A29 | Solute carrier family 25, member 29 | 2.44 |
| 225306_s_at | SLC25A29 | Solute carrier family 25, member 29 | 2.43 |
| 233055_at | 2.42 | ||
| 225354_s_at | SH3BGRL2 | SH3 domain binding glutamic acid-rich protein like 2 | 2.40 |
| 244740_at | MGC9913 | Hypothetical protein MGC9913 | 2.39 |
| 219355_at | CXorf57 | Chromosome X open reading frame 57 | 2.37 |
| 221207_s_at | NBEA | Neurobeachin | 2.36 |
| 1553982_a_at | RAB7B | RAB7B, member RAS oncogene family | 2.34 |
| 1555960_at | HINT1 | Histidine triad nucleotide binding protein 1 | 2.34 |
| 208498_s_at | AMY1A | Amylase, alpha 1A (salivary) | 2.33 |
| 223377_x_at | CISH | Cytokine inducible SH2-containing protein | 2.33 |
| 1556008_a_at | 2.33 | ||
| 1555833_a_at | IRGQ | Immunity-related gtpase family, Q | 2.32 |
| 241897_at | 2.32 | ||
| 226197_at | 2.31 | ||
| 230861_at | DKFZP434L187 | Hypothetical LOC26082 | 2.31 |
| 206693_at | IL7 | Interleukin 7 | 2.30 |
| 240539_at | 2.29 | ||
| 209068_at | HNRPDL | Heterogeneous nuclear ribonucleoprotein D-like | 2.26 |
| 227524_at | 2.26 | ||
| 221877_at | IRGQ | Immunity-related gtpase family, Q | 2.25 |
| 232114_at | MED12L | Mediator complex subunit 12-like | 2.25 |
| 1557557_at | LOC100129196 | Similar to hcg2033298 | 2.24 |
| 218735_s_at | ZNF544 | Zinc finger protein 544 | 2.24 |
| 226503_at | RIF1 | RAP1 interacting factor homolog (yeast) | 2.23 |
| 231947_at | MYCT1 | Myc target 1 | 2.23 |
| 241840_at | 2.23 | ||
| 221832_s_at | LUZP1 | Leucine zipper protein 1 | 2.23 |
| 203410_at | AP3M2 | Adaptor-related protein complex 3, mu 2 subunit | 2.23 |
| 229005_at | 2.22 | ||
| 221223_x_at | CISH | Cytokine inducible SH2-containing protein | 2.22 |
| 216247_at | RPS20 | Ribosomal protein S20 | 2.22 |
| 230630_at | AK3L1 | Adenylate kinase 3-like 1 | 2.21 |
| 239734_at | 2.21 | ||
| 222857_s_at | KCNMB4 | Potassium large conductance calcium-activated channel, subfamily M, beta member 4 | 2.20 |
| 239567_at | 2.20 | ||
| 223593_at | AADAT | Aminoadipate aminotransferase | 2.18 |
| 228569_at | PAPOLA | Poly(A) polymerase alpha | 2.18 |
| 64488_at | IRGQ | Immunity-related gtpase family, Q | 2.17 |
| 244753_at | 2.17 | ||
| 209525_at | HDGFRP3 | Hepatoma-derived growth factor, related protein 3 | 2.16 |
| 239252_at | 2.16 | ||
| 203794_at | CDC42BPA | CDC42 binding protein kinase alpha (DMPK-like) | 2.15 |
| 212851_at | DCUN1D4 | DCN1, defective in cullin neddylation 1, domain containing 4 (S. cerevisiae) | 2.15 |
| 236474_at | 2.15 | ||
| 226541_at | FBXO30 | F-box protein 30 | 2.15 |
| 205450_at | PHKA1 | Phosphorylase kinase, alpha 1 (muscle) | 2.15 |
| 230002_at | CLCC1 | Chloride channel CLIC-like 1 | 2.15 |
| 239067_s_at | PANX2 | Pannexin 2 | 2.14 |
| 204269_at | PIM2 | Pim-2 oncogene | 2.14 |
| 232280_at | SLC25A29 | Solute carrier family 25, member 29 | 2.14 |
| 205240_at | GPSM2 | G-protein signaling modulator 2 (AGS3-like, C. elegans) | 2.12 |
| 212179_at | SFRS18 | Splicing factor, arginine/serine-rich 18 | 2.12 |
| 1556735_at | 2.12 | ||
| 242494_at | 2.12 | ||
| 229631_at | DNHD1 | Dynein heavy chain domain 1 | 2.11 |
| 218224_at | PNMA1 | Paraneoplastic antigen MA1 | 2.11 |
| 47560_at | LPHN1 | Latrophilin 1 | 2.11 |
| 211163_s_at | TNFRSF10C | Tumor necrosis factor receptor superfamily, member 10c, decoy without an intracellular domain | 2.11 |
| 211621_at | AR | Androgen receptor | 2.11 |
| 238038_at | 2.10 | ||
| 212609_s_at | AKT3 | V-akt murine thymoma viral oncogene homolog 3 (protein kinase B, gamma) | 2.09 |
| 204453_at | ZNF84 | Zinc finger protein 84 | 2.08 |
| 233479_at | 2.08 | ||
| 230680_at | 2.07 | ||
| 223249_at | CLDN12 | Claudin 12 | 2.06 |
| 236202_at | 2.05 | ||
| 201999_s_at | DYNLT1 | Dynein, light chain, Tctex-type 1 | 2.05 |
| 227412_at | PPP1R3E | Protein phosphatase 1, regulatory (inhibitor) subunit 3E | 2.04 |
| 206222_at | TNFRSF10C | Tumor necrosis factor receptor superfamily, member 10c, decoy without an intracellular domain | 2.04 |
| 207623_at | ABCF2 | ATP-binding cassette, subfamily F (GCN20), member 2 | 2.04 |
| 219623_at | ACTR5 | ARP5 actin-related protein 5 homolog (yeast) | 2.03 |
| 227093_at | USP36 | Ubiquitin specific peptidase 36 | 2.02 |
| 219236_at | PAQR6 | Progestin and adipoq receptor family member VI | 2.02 |
| 241788_x_at | 2.02 | ||
| 235680_at | 2.01 | ||
| 235779_at | LOC284408 | Hypothetical protein LOC284408 | 2.01 |
| 221480_at | HNRNPD | Heterogeneous nuclear ribonucleoprotein D (AU-rich element RNA binding protein 1, 37 kda) | 2.01 |
| 218162_at | OLFML3 | Olfactomedin-like 3 | 2.01 |
| 236916_at | 2.01 | ||
| 243365_s_at | AUTS2 | Autism susceptibility candidate 2 | 2.00 |
| 227963_at | 2.00 | ||
| 213124_at | ZNF473 | Zinc finger protein 473 | 2.00 |
| 214461_at | LBP | Lipopolysaccharide binding protein | 1.99 |
| 244647_at | 1.99 | ||
| 227234_at | LOC100132815 | Hypothetical protein LOC100132815 | 1.98 |
| 235533_at | COX19 | COX19 cytochrome c oxidase assembly homolog (S. cerevisiae) | 1.98 |
| 214996_at | 1.97 | ||
| 212427_at | KIAA0368 | Kiaa0368 | 1.97 |
| 227573_s_at | OBSL1 | Obscurin-like 1 | 1.96 |
| 223600_s_at | KIAA1683 | Kiaa1683 | 1.96 |
| 1556121_at | 1.96 | ||
| 226586_at | ANKS6 | Ankyrin repeat and sterile alpha motif domain containing 6 | 1.96 |
| 228259_s_at | EPB41L4A | Erythrocyte membrane protein band 4.1 like 4A | 1.96 |
| 220117_at | ZNF385D | Zinc finger protein 385D | 1.95 |
| 1556694_a_at | 1.94 | ||
| 232614_at | 1.94 | ||
| 224719_s_at | C12orf57 | Chromosome 12 open reading frame 57 | 1.93 |
| 244534_at | 1.93 | ||
| 37408_at | MRC2 | Mannose receptor, C type 2 | 1.93 |
| 1562244_at | ZNF578 | Zinc finger protein 578 | 1.93 |
| 1558604_a_at | 1.93 | ||
| 228002_at | IDI2 | Isopentenyl-diphosphate delta isomerase 2 | 1.92 |
| 1560562_a_at | ZNF677 | Zinc finger protein 677 | 1.92 |
| 228192_at | C6orf125 | Chromosome 6 open reading frame 125 | 1.92 |
| 1552381_at | SRrp35 | Serine-arginine repressor protein (35 kda) | 1.91 |
| 34726_at | CACNB3 | Calcium channel, voltage-dependent, beta 3 subunit | 1.91 |
| 227206_at | 1.91 | ||
| 241912_at | ZNF814 | Zinc finger protein 814 | 1.91 |
| 243480_at | 1.91 | ||
| 219448_at | TMEM70 | Transmembrane protein 70 | 1.90 |
| 1552423_at | ETV3 | Ets variant 3 | 1.90 |
| 203488_at | LPHN1 | Latrophilin 1 | 1.90 |
| 236815_at | 1.90 | ||
| 1552347_at | CRYZL1 | Crystallin, zeta (quinone reductase)-like 1 | 1.90 |
| 227333_at | 1.89 | ||
| 243951_at | ABCB1 | ATP-binding cassette, subfamily B (MDR/TAP), member 1 | 1.88 |
| 236543_at | 1.88 | ||
| 230757_at | 1.87 | ||
| 212923_s_at | C6orf145 | Chromosome 6 open reading frame 145 | 1.87 |
| 222147_s_at | ACTR5 | ARP5 actin-related protein 5 homolog (yeast) | 1.87 |
| 1553247_a_at | ZNF709 | Zinc finger protein 709 | 1.87 |
| 238191_at | 1.86 | ||
| 238376_at | 1.86 | ||
| 231116_at | 1.86 | ||
| 225629_s_at | ZBTB4 | Zinc finger and BTB domain containing 4 | 1.86 |
| 229328_at | ZNF540 | Zinc finger protein 540 | 1.85 |
| 227992_s_at | NCRNA00085 | Non-protein coding RNA 85 | 1.85 |
| 228826_at | 1.85 | ||
| 211929_at | HNRNPA3 | Heterogeneous nuclear ribonucleoprotein A3 | 1.84 |
| 220459_at | MCM3APAS | MCM3AP antisense RNA (non-protein coding) | 1.84 |
| 239329_at | 1.83 | ||
| 239295_at | SRrp35 | Serine-arginine repressor protein (35 kda) | 1.83 |
| 226567_at | USP14 | Ubiquitin specific peptidase 14 (trna-guanine transglycosylase) | 1.83 |
| 212772_s_at | ABCA2 | ATP-binding cassette, subfamily A (ABC1), member 2 | 1.83 |
| 212855_at | DCUN1D4 | DCN1, defective in cullin neddylation 1, domain containing 4 (S. cerevisiae) | 1.82 |
| 239829_at | 1.82 | ||
| 225888_at | C12orf30 | Chromosome 12 open reading frame 30 | 1.82 |
| 229654_at | ZNF44 | Zinc finger protein 44 | 1.82 |
| 238484_s_at | 1.82 | ||
| 229234_at | ZC3H12B | Zinc finger CCCH-type containing 12B | 1.82 |
| 201067_at | PSMC2 | Proteasome (prosome, macropain) 26S subunit, atpase, 2 | 1.81 |
| 202743_at | PIK3R3 | Phosphoinositide-3-kinase, regulatory subunit 3 (gamma) | 1.80 |
| 221735_at | WDR48 | WD repeat domain 48 | 1.80 |
| 202259_s_at | N4BP2L2 | NEDD4 binding protein 2-like 2 | 1.80 |
| 205145_s_at | MYL5 | Myosin, light chain 5, regulatory | 1.80 |
| 244665_at | 1.80 | ||
| 215137_at | 1.79 | ||
| 231978_at | TPCN2 | Two pore segment channel 2 | 1.79 |
| 223185_s_at | BHLHE41 | Basic helix-loop-helix family, member e41 | 1.79 |
| 227308_x_at | LTBP3 | Latent transforming growth factor beta binding protein 3 | 1.79 |
| 209020_at | C20orf111 | Chromosome 20 open reading frame 111 | 1.79 |
| 236106_at | 1.78 | ||
| 230616_at | LAMB2L | Laminin, beta 2-like | 1.78 |
| 225710_at | GNB4 | Guanine nucleotide binding protein (G protein), beta polypeptide 4 | 1.78 |
| 221976_s_at | HDGFRP3 | Hepatoma-derived growth factor, related protein 3 | 1.78 |
| 243178_at | 1.78 | ||
| 227272_at | C15orf52 | Chromosome 15 open reading frame 52 | 1.77 |
| 227314_at | ITGA2 | Integrin, alpha 2 (CD49B, alpha 2 subunit of VLA-2 receptor) | 1.77 |
| 238454_at | ZNF540 | Zinc finger protein 540 | 1.77 |
| 229854_at | OBSCN | Obscurin, cytoskeletal calmodulin and titin-interacting rhogef | 1.76 |
| 204376_at | VPRBP | Vpr (HIV-1) binding protein | 1.76 |
| 212206_s_at | H2AFV | H2A histone family, member V | 1.76 |
| 242109_at | SYTL3 | Synaptotagmin-like 3 | 1.75 |
| 238700_at | 1.75 | ||
| 229720_at | BAG1 | BCL2-associated athanogene | 1.75 |
| 243924_at | LOC100127980 | Hypothetical protein LOC100127980 | 1.74 |
| 227865_at | C9orf103 | Chromosome 9 open reading frame 103 | 1.74 |
| 209983_s_at | NRXN2 | Neurexin 2 | 1.74 |
| 200690_at | HSPA9 | Heat shock 70 kda protein 9 (mortalin) | 1.74 |
| 223321_s_at | FGFRL1 | Fibroblast growth factor receptor-like 1 | 1.74 |
| 232866_at | ZNF135 | Zinc finger protein 135 | 1.74 |
| 231902_at | ZNF827 | Zinc finger protein 827 | 1.73 |
| 210484_s_at | MGC31957 | Hypothetical protein MGC31957 | 1.73 |
| 219266_at | ZNF350 | Zinc finger protein 350 | 1.73 |
| 225120_at | PURB | Purine-rich element binding protein B | 1.73 |
| 244597_at | LOC26010 | Viral DNA polymerase-transactivated protein 6 | 1.73 |
| 221831_at | LUZP1 | Leucine zipper protein 1 | 1.72 |
| 1569713_at | 1.72 | ||
| 235564_at | ZNF117 | Zinc finger protein 117 | 1.72 |
| 220328_at | PHC3 | Polyhomeotic homolog 3 (Drosophila) | 1.72 |
| 220032_at | C7orf58 | Chromosome 7 open reading frame 58 | 1.72 |
| 235406_x_at | 1.72 | ||
| 1554076_s_at | TMEM136 | Transmembrane protein 136 | 1.71 |
| 237856_at | RAP1GDS1 | RAP1, GTP-GDP dissociation stimulator 1 | 1.71 |
| 44696_at | TBC1D13 | TBC1 domain family, member 13 | 1.70 |
| 237389_at | 1.70 | ||
| 234998_at | 1.70 | ||
| 226944_at | HTRA3 | Htra serine peptidase 3 | 1.70 |
| 223849_s_at | MOV10 | Mov10, Moloney leukemia virus 10, homolog (mouse) | 1.70 |
| 209838_at | COPS2 | COP9 constitutive photomorphogenic homolog subunit 2 (Arabidopsis) | 1.70 |
| 228709_at | TPR | Translocated promoter region (to activated MET oncogene) | 1.69 |
| 238483_at | 1.69 | ||
| 212293_at | HIPK1 | Homeodomain interacting protein kinase 1 | 1.68 |
| 236127_at | ZBTB17 | Zinc finger and BTB domain containing 17 | 1.68 |
| 211277_x_at | APP | Amyloid beta (A4) precursor protein | 1.68 |
| 1558459_s_at | LOC401320 | Hypothetical LOC401320 | 1.68 |
| 225487_at | TMEM18 | Transmembrane protein 18 | 1.67 |
| 207693_at | CACNB4 | Calcium channel, voltage-dependent, beta 4 subunit | 1.67 |
| 205079_s_at | MPDZ | Multiple PDZ domain protein | 1.67 |
| 232553_at | PCYT1B | Phosphate cytidylyltransferase 1, choline, beta | 1.67 |
| 214961_at | KIAA0774 | Kiaa0774 | 1.67 |
| 207561_s_at | ACCN3 | Amiloride-sensitive cation channel 3 | 1.67 |
| 1557248_at | ZNF814 | Zinc finger protein 814 | 1.67 |
| 219944_at | CLIP4 | CAP-GLY domain containing linker protein family, member 4 | 1.66 |
| 209733_at | 1.65 | ||
| 219145_at | LPHN1 | Latrophilin 1 | 1.65 |
| 238131_at | PHC2 | Polyhomeotic homolog 2 (Drosophila) | 1.65 |
| 1570329_at | 1.65 | ||
| 209530_at | CACNB3 | Calcium channel, voltage-dependent, beta 3 subunit | 1.64 |
| 227505_at | 1.64 | ||
| 232716_at | 1.64 | ||
| 218596_at | TBC1D13 | TBC1 domain family, member 13 | 1.64 |
| 206142_at | ZNF135 | Zinc finger protein 135 | 1.63 |
| 231941_s_at | MUC20 | Mucin 20, cell surface associated | 1.63 |
| 65591_at | WDR48 | WD repeat domain 48 | 1.62 |
| 235530_at | 1.62 | ||
| 1552986_at | LOC142937 | Hypothetical protein BC008131 | 1.61 |
| 232758_s_at | 1.60 | ||
| 219287_at | KCNMB4 | Potassium large conductance calcium-activated channel, subfamily M, beta member 4 | 1.60 |
| 228012_at | MATR3 | Matrin 3 | 1.60 |
| 243398_at | 1.60 | ||
| 214289_at | PSMB1 | Proteasome (prosome, macropain) subunit, beta type, 1 | 1.59 |
| 237561_x_at | 1.58 | ||
| 1558164_s_at | PEX13 | Peroxisomal biogenesis factor 13 | 1.58 |
| 242307_at | ZNF789 | Zinc finger protein 789 | 1.58 |
| 235814_at | 1.57 | ||
| 213367_at | LOC791120 | Hypothetical LOC791120 | 1.57 |
| 241318_at | 1.56 | ||
| 1552643_at | ZNF626 | Zinc finger protein 626 | 1.55 |
| 213444_at | ZNF862 | Zinc finger protein 862 | 1.55 |
| 205964_at | ZNF426 | Zinc finger protein 426 | 1.54 |
| 213306_at | MPDZ | Multiple PDZ domain protein | 1.53 |
| 215370_at | 1.52 | ||
| 232919_at | AFG3L2 | AFG3 atpase family gene 3-like 2 (yeast) | 1.52 |
| 1558969_a_at | RPL32P3 | Ribosomal protein L32 pseudogene 3 | 1.50 |
| 234034_at | 1.49 | ||
| 227504_s_at | 1.49 | ||
| 215296_at | CDC42BPA | CDC42 binding protein kinase alpha (DMPK-like) | 1.48 |
| 231260_at | BC036928 | Hypothetical protein BC036928 | 1.48 |
| 211947_s_at | BAT2D1 | BAT2 domain containing 1 | 1.48 |
| 218655_s_at | CCDC49 | Coiled-coil domain containing 49 | 1.47 |
| 232199_at | 1.45 | ||
| 218002_s_at | CXCL14 | Chemokine (C-X-C motif) ligand 14 | 1.44 |
| 1558819_at | LOC100131819 | Similar to hcg1778814 | 1.35 |
| Downregulated gene probes | |||
| 217840_at | DDX41 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 41 | 0.66 |
| 200618_at | LASP1 | LIM and SH3 protein 1 | 0.66 |
| 219074_at | TMEM184C | Transmembrane protein 184C | 0.65 |
| 230363_s_at | INPP5F | Inositol polyphosphate-5-phosphatase F | 0.64 |
| 209188_x_at | DR1 | Down-regulator of transcription 1, TBP-binding (negative cofactor 2) | 0.63 |
| 223329_x_at | SUGT1 | SGT1, suppressor of G2 allele of SKP1 (S. cerevisiae) | 0.63 |
| 215774_s_at | SUCLG2 | Succinate-coa ligase, GDP-forming, beta subunit | 0.63 |
| 228578_at | RBM45 | RNA binding motif protein 45 | 0.63 |
| 218327_s_at | SNAP29 | Synaptosomal-associated protein, 29 kda | 0.62 |
| 223299_at | SEC11C | SEC11 homolog C (S. cerevisiae) | 0.62 |
| 207654_x_at | DR1 | Down-regulator of transcription 1, TBP-binding (negative cofactor 2) | 0.61 |
| 225389_at | BTBD6 | BTB (POZ) domain containing 6 | 0.61 |
| 224309_s_at | SUGT1 | SGT1, suppressor of G2 allele of SKP1 (S. cerevisiae) | 0.61 |
| 203732_at | TRIP4 | Thyroid hormone receptor interactor 4 | 0.61 |
| 221688_s_at | IMP3 | IMP3, U3 small nucleolar ribonucleoprotein, homolog (yeast) | 0.60 |
| 222503_s_at | WDR41 | WD repeat domain 41 | 0.60 |
| 215075_s_at | GRB2 | Growth factor receptor-bound protein 2 | 0.60 |
| 225890_at | C20orf72 | Chromosome 20 open reading frame 72 | 0.59 |
| 222537_s_at | CDC42SE1 | CDC42 small effector 1 | 0.59 |
| 224874_at | POLR1D | Polymerase (RNA) I polypeptide D, 16 kda | 0.58 |
| 202579_x_at | HMGN4 | High mobility group nucleosomal binding domain 4 | 0.58 |
| 209787_s_at | HMGN4 | High mobility group nucleosomal binding domain 4 | 0.58 |
| 216652_s_at | DR1 | Down-regulator of transcription 1, TBP-binding (negative cofactor 2) | 0.57 |
| 221492_s_at | ATG3 | ATG3 autophagy related 3 homolog (S. cerevisiae) | 0.57 |
| 230592_at | NSL1 | NSL1, MIND kinetochore complex component, homolog (S. cCerevisiae) | 0.57 |
| 224511_s_at | TXNDC17 | Thioredoxin domain containing 17 | 0.57 |
| 217909_s_at | MLX | MAX-like protein X | 0.56 |
| 209786_at | HMGN4 | High mobility group nucleosomal binding domain 4 | 0.56 |
| 211725_s_at | BID | BH3 interacting domain death agonist | 0.56 |
| 229742_at | C15orf61 | Chromosome 15 open reading frame 61 | 0.55 |
| 225917_at | 0.55 | ||
| 211936_at | HSPA5 | Heat shock 70 kda protein 5 (glucose-regulated protein, 78 kda) | 0.55 |
| 224643_at | PRRC1 | Proline-rich coiled-coil 1 | 0.55 |
| 203846_at | TRIM32 | Tripartite motif-containing 32 | 0.55 |
| 205055_at | ITGAE | Integrin, alpha E (antigen CD103, human mucosal lymphocyte antigen 1; alpha polypeptide) | 0.55 |
| 225850_at | SFT2D1 | SFT2 domain containing 1 | 0.54 |
| 226242_at | C1orf131 | Chromosome 1 open reading frame 131 | 0.54 |
| 209748_at | SPAST | Spastin | 0.54 |
| 220140_s_at | SNX11 | Sorting nexin 11 | 0.54 |
| 236846_at | LOC284757 | Hypothetical protein LOC284757 | 0.54 |
| 239342_at | DGKZ | Diacylglycerol kinase, zeta 104 kda | 0.53 |
| 227413_at | UBLCP1 | Ubiquitin-like domain containing CTD phosphatase 1 | 0.53 |
| 202101_s_at | RALB | V-ral simian leukemia viral oncogene homolog B (ras related; GTP binding protein) | 0.53 |
| 218987_at | ATF7IP | Activating transcription factor 7 interacting protein | 0.52 |
| 1562648_at | CCDC88A | Coiled-coil domain containing 88A | 0.51 |
| 229120_s_at | C1orf56 | Chromosome 1 open reading frame 56 | 0.50 |
| 1568742_at | 0.49 | ||
| 218157_x_at | CDC42SE1 | CDC42 small effector 1 | 0.49 |
| 226276_at | TMEM167A | Transmembrane protein 167A | 0.48 |
| 204862_s_at | NME3 | Nonmetastatic cells 3, protein expressed in | 0.48 |
| 227624_at | TET2 | Tet oncogene family member 2 | 0.48 |
| 207724_s_at | SPAST | Spastin | 0.46 |
| 204608_at | ASL | Argininosuccinate lyase | 0.46 |
| 204810_s_at | CKM | Creatine kinase, muscle | 0.46 |
| 1554077_a_at | TMEM53 | Transmembrane protein 53 | 0.45 |
| 206907_at | TNFSF9 | Tumor necrosis factor (ligand) superfamily, member 9 | 0.44 |
| 238063_at | TMEM154 | Transmembrane protein 154 | 0.44 |
| 204880_at | MGMT | O-6-methylguanine-DNA methyltransferase | 0.43 |
| 204385_at | KYNU | Kynureninase (L-kynurenine hydrolase) | 0.41 |
| 207131_x_at | GGT1 | Gamma-glutamyltransferase 1 | 0.37 |
| 222752_s_at | TMEM206 | Transmembrane protein 206 | 0.37 |
| 204994_at | MX2 | Myxovirus (influenza virus) resistance 2 (mouse) | 0.36 |
| 1558770_a_at | PIK3R6 | Phosphoinositide-3-kinase, regulatory subunit 6 | 0.36 |
| 1554628_at | ZNF57 | Zinc finger protein 57 | 0.34 |
| 210629_x_at | LST1 | Leukocyte specific transcript 1 | 0.34 |
| 205147_x_at | NCF4 | Neutrophil cytosolic factor 4, 40 kda | 0.34 |
| 207677_s_at | NCF4 | Neutrophil cytosolic factor 4, 40 kda | 0.34 |
| 223059_s_at | FAM107B | Family with sequence similarity 107, member B | 0.33 |
| 219033_at | PARP8 | Poly (ADP-ribose) polymerase family, member 8 | 0.32 |
| 214574_x_at | LST1 | Leukocyte specific transcript 1 | 0.31 |
| 241464_s_at | 0.31 | ||
| 223058_at | FAM107B | Family with sequence similarity 107, member B | 0.31 |
| 211581_x_at | LST1 | Leukocyte specific transcript 1 | 0.31 |
| 219506_at | C1orf54 | Chromosome 1 open reading frame 54 | 0.31 |
| 210663_s_at | KYNU | Kynureninase (L-kynurenine hydrolase) | 0.30 |
| 214181_x_at | LST1 | Leukocyte specific transcript 1 | 0.29 |
| 211582_x_at | LST1 | Leukocyte specific transcript 1 | 0.29 |
| 1553311_at | C20orf197 | Chromosome 20 open reading frame 197 | 0.24 |
| 212459_x_at | SUCLG2 | Succinate-coa ligase, GDP-forming, beta subunit | 0.24 |
| 215772_x_at | SUCLG2 | Succinate-coa ligase, GDP-forming, beta subunit | 0.22 |
| 214835_s_at | SUCLG2 | Succinate-coa ligase, GDP-forming, beta subunit | 0.20 |
| 206772_at | PTH2R | Parathyroid hormone 2 receptor | 0.19 |
| 202878_s_at | CD93 | CD93 molecule | 0.15 |
| 200923_at | LGALS3BP | Lectin, galactoside-binding, soluble, 3 binding protein | 0.14 |
| 205859_at | LY86 | Lymphocyte antigen 86 | 0.14 |
| 217388_s_at | KYNU | Kynureninase (L-kynurenine hydrolase) | 0.09 |
NOTE. False discovery rate = 0.054; global test P = .007). Ordered by fold changes.
Abbreviation: wt, wild-type.
Table A4.
Differentially Expressed (P < .005) Probes Between R172 IDH2-Mutated (n = 6) and IDH1/IDH2wt (n = 82) Patients
| Target MicroRNA | Sequence (unique ID) | Fold-Change: R172/wt |
|---|---|---|
| Upregulated microRNA probes | ||
| hsa-miR-1 | AATGCTATGGAATGTAAAGAAGTATGTATTTTTGGTAGGC | 10.35 |
| hsa-miR-1 | AATGCTATGGAATGTAAAGAAGTATGTATTTTTGGTAGGC | 7.11 |
| hsa-miR-133a | TTGGTCCCCTTCAACCAGCTGTAGCTGTGCATTGATGGCG | 7.00 |
| hsa-miR-125b | TCCCTGAGACCCTAACTTGTGATGTTTACCGTTTAAATCC | 4.99 |
| hsa-miR-125a-5p | TCTAGGTCCCTGAGACCCTTTAACCTGTGAGGACATCCAG | 4.96 |
| hsa-miR-133a | CCTCTTCAATGGATTTGGTCCCCTTCAACCAGCTGTAGCT | 4.24 |
| hsa-miR-1 | TGGACCTGCTAAGCTATGGAATGTAAAGAAGTATGTATCT | 4.08 |
| hsa-miR-125b | ACCAGACTTTTCCTAGTCCCTGAGACCCTAACTTGTGAGG | 3.91 |
| hsa-miR-421 | AATGAATCATCAACAGACATTAATTGGGCGCCTGCTCTGT | 1.94 |
| hsa-miR-374a | ACATCGGCCATTATAATACAACCTGATAAGTGTTATAGCA | 1.84 |
| hsa-miR-361-5p | GGA TTT GGG AGC TTA TCA GAA TCT CCA GGG GTA CTT TAT A | 1.65 |
| hsa-miR-26a | GTGGCCTCGTTCAAGTAATCCAGGATAGGCTGTGCAGGTC | 1.61 |
| hsa-miR-30d | TTGTAAACATCCCCGACTGGAAGCTGTAAGACACAGCTAA | 1.61 |
| Downregulated microRNA probes | ||
| hsa-miR-7 | GGACCGGCTGGCCCCATCTGGAAGACTAGTGATTTTGTTG | 0.76 |
| hsa-mir-345 | CTGAACGAGGGGTCTGGAGGCCTGGGTTTGAATATCGACA | 0.72 |
| hsa-miR-129-5p | TGGATCTTTTTGCGGTCTGGGCTTGCTGTTCCTCTCAACA | 0.69 |
| hsa-mir-632 | TCCTACCGCAGTGCTTGACGGGAGGCGGAGCGGGGAACGA | 0.69 |
| hsa-miR-615-5p | CTCGGGAGGGGCGGGAGGGGGGTCCCCGGTGCTCGGATCT | 0.65 |
| hsa-miR-1301 | CAGGGGCTGGGCCTGCAGCTGCCTGGGCAGAGCGGCTCCT | 0.60 |
| hsa-mir-639 | ACGGGGCGCGCGCGGCCTGGAGGGGCGGGGCGGACGCAGA | 0.57 |
| hsa-mir-548b | CAGACTATATATTTAGGTTGGCGCAAAAGTAATTGTGGTT | 0.46 |
| hsa-miR-520a-3p | GAG AGA AAA GAA AGT GCT TCC CTT TGG ACT GTT TCG GTT T | 0.41 |
| hsa-miR-526a | CTC AGG CTG TGA CCC TCT AGA GGG AAG CAC TTT CTG TTG C | 0.33 |
| hsa-mir-194-1 | CCAATTTCCAGTGGAGATGCTGTTACTTTTGATGGTTACC | 0.32 |
NOTE. False discovery rate = 0.166; global P = .03. Ordered by fold-change.
Abbreviations: wt, wild-type; ID, identification.
Footnotes
See accompanying article on page 2356
Supported in part by National Cancer Institute Grants No. CA101140, CA114725, CA31946, CA33601, CA16058, CA77658, CA129657, and CA140158, by The Coleman Leukemia Research Foundation, and by the Deutsche Krebshilfe Dr Mildred Scheel Cancer Foundation (H.B.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Guido Marcucci, Clara D. Bloomfield
Financial support: Guido Marcucci, Clara D. Bloomfield
Administrative support: Michael A. Caligiuri, Clara D. Bloomfield
Provision of study materials or patients: Guido Marcucci, Bayard L. Powell, Thomas H. Carter, Jonathan E. Kolitz, Meir Wetzler, Andrew J. Carroll, Maria R. Baer, Michael A. Caligiuri, Richard A. Larson, Clara D. Bloomfield
Collection and assembly of data: Guido Marcucci, Kati Maharry, Yue-Zhong Wu, Michael D. Radmacher, Susan P. Whitman, Heiko Becker, Sebastian Schwind, Klaus H. Metzeler, Andrew J. Carroll, Clara D. Bloomfield
Data analysis and interpretation: Guido Marcucci, Kati Maharry, Yue-Zhong Wu, Michael D. Radmacher, Krzysztof Mrózek, Dean Margeson, Kelsi B. Holland, Clara D. Bloomfield
Manuscript writing: Guido Marcucci, Kati Maharry, Michael D. Radmacher, Krzysztof Mrózek, Susan P. Whitman, Heiko Becker, Sebastian Schwind, Klaus H. Metzeler, Clara D. Bloomfield
Final approval of manuscript: Guido Marcucci, Kati Maharry, Yue-Zhong Wu, Michael D. Radmacher, Krzysztof Mrózek, Dean Margeson, Kelsi B. Holland, Susan P. Whitman, Heiko Becker, Sebastian Schwind, Klaus H. Metzeler, Bayard L. Powell, Thomas H. Carter, Jonathan E. Kolitz, Meir Wetzler, Andrew J. Carroll, Maria R. Baer, Michael A. Caligiuri, Richard A. Larson, Clara D. Bloomfield
REFERENCES
- 1.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Farag SS, Ruppert AS, Mrózek K, et al. Outcome of induction and postremission therapy in younger adults with acute myeloid leukemia with normal karyotype: A Cancer and Leukemia Group B study. J Clin Oncol. 2005;23:482–493. doi: 10.1200/JCO.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 3.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Estey E. Acute myeloid leukemia and myelodysplastic syndromes in older patients. J Clin Oncol. 2007;25:1908–1915. doi: 10.1200/JCO.2006.10.2731. [DOI] [PubMed] [Google Scholar]
- 5.Mrózek K, Marcucci G, Paschka P, et al. Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: Are we ready for a prognostically prioritized molecular classification? Blood. 2007;109:431–448. doi: 10.1182/blood-2006-06-001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaidzik V, Döhner K. Prognostic implications of gene mutations in acute myeloid leukemia with normal cytogenetics. Semin Oncol. 2008;35:346–355. doi: 10.1053/j.seminoncol.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Mrózek K, Heerema NA, Bloomfield CD. Cytogenetics in acute leukemia. Blood Rev. 2004;18:115–136. doi: 10.1016/S0268-960X(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 8.Whitman SP, Archer KJ, Feng L, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: A Cancer and Leukemia Group B study. Cancer Res. 2001;61:7233–7239. [PubMed] [Google Scholar]
- 9.Thiede C, Steudel C, Mohr B, et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 10.Falini B, Mecucci C, Tiacci E, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 11.Döhner K, Schlenk RF, Habdank M, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: Interaction with other gene mutations. Blood. 2005;106:3740–3746. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 12.Fröhling S, Schlenk RF, Stolze I, et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: Prognostic relevance and analysis of cooperating mutations. J Clin Oncol. 2004;22:624–633. doi: 10.1200/JCO.2004.06.060. [DOI] [PubMed] [Google Scholar]
- 13.Marcucci G, Maharry K, Radmacher MD, et al. Prognostic significance of, and gene and microRNA expression signatures associated with, CEBPA mutations in cytogenetically normal acute myeloid leukemia with high-risk molecular features: A Cancer and Leukemia Group B study. J Clin Oncol. 2008;26:5078–5087. doi: 10.1200/JCO.2008.17.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paschka P, Marcucci G, Ruppert AS, et al. Wilms' tumor 1 gene mutations independently predict poor outcome in adults with cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2008;26:4595–4602. doi: 10.1200/JCO.2007.15.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Virappane P, Gale R, Hills R, et al. Mutation of the Wilms' tumor 1 gene is a poor prognostic factor associated with chemotherapy resistance in normal karyotype acute myeloid leukemia: The United Kingdom Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2008;26:5429–5435. doi: 10.1200/JCO.2008.16.0333. [DOI] [PubMed] [Google Scholar]
- 16.Caligiuri MA, Strout MP, Lawrence D, et al. Rearrangement of ALL1 (MLL) in acute myeloid leukemia with normal cytogenetics. Cancer Res. 1998;58:55–59. [PubMed] [Google Scholar]
- 17.Whitman SP, Ruppert AS, Marcucci G, et al. Long-term disease-free survivors with cytogenetically normal acute myeloid leukemia and MLL partial tandem duplication: A Cancer and Leukemia Group B study. Blood. 2007;109:5164–5167. doi: 10.1182/blood-2007-01-069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 19.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mardis ER, Ding L, Dooling DJ, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–1066. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrd JC, Mrózek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: Results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100:4325–4336. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- 23.Mrózek K, Carroll AJ, Maharry K, et al. Central review of cytogenetics is necessary for cooperative group correlative and clinical studies of adult acute leukemia: The Cancer and Leukemia Group B experience. Int J Oncol. 2008;33:239–244. [PMC free article] [PubMed] [Google Scholar]
- 24.Kolitz JE, George SL, Dodge RK, et al. Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: Final induction results of Cancer and Leukemia Group B study 9621. J Clin Oncol. 2004;22:4290–4301. doi: 10.1200/JCO.2004.11.106. [DOI] [PubMed] [Google Scholar]
- 25.Kolitz JE, George SL, Marcucci G, et al. A randomized comparison of induction therapy for untreated acute myeloid leukemia (AML) in patients < 60 years using P-glycoprotein (Pgp) modulation with Valspodar (PSC833): Preliminary results of Cancer and Leukemia Group B study 19808. Blood. 2005;106:122a–123a. abstr 407. [Google Scholar]
- 26.Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331:896–903. doi: 10.1056/NEJM199410063311402. [DOI] [PubMed] [Google Scholar]
- 27.Stone RM, Berg DT, George SL, et al. Granulocyte-macrophage colony-stimulating factor after initial chemotherapy for elderly patients with primary acute myelogenous leukemia. N Engl J Med. 1995;332:1671–1677. doi: 10.1056/NEJM199506223322503. [DOI] [PubMed] [Google Scholar]
- 28.Lee EJ, George SL, Caligiuri M, et al. Parallel phase I studies of daunorubicin given with cytarabine and etoposide with or without the multidrug resistance modulator PSC-833 in previously untreated patients 60 years of age or older with acute myeloid leukemia: Results of Cancer and Leukemia Group B study 9420. J Clin Oncol. 1999;17:2831–2839. doi: 10.1200/JCO.1999.17.9.2831. [DOI] [PubMed] [Google Scholar]
- 29.Baer MR, George SL, Caligiuri MA, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B study 9720. J Clin Oncol. 2008;26:4934–4939. doi: 10.1200/JCO.2008.17.0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcucci G, Moser B, Blum W, et al. A phase III randomized trial of intensive induction and consolidation chemotherapy ± oblimersen, a pro-apoptotic Bcl-2 antisense oligonucleotide in untreated acute myeloid leukemia patients > 60 years old. J Clin Oncol. 2007;25(suppl):360s. abstr 7012. [Google Scholar]
- 31.Whitman SP, Ruppert AS, Radmacher MD, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008;111:1552–1559. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caligiuri MA, Strout MP, Schichman SA, et al. Partial tandem duplication of ALL1 as a recurrent molecular defect in acute myeloid leukemia with trisomy 11. Cancer Res. 1996;56:1418–1425. [PubMed] [Google Scholar]
- 33.Becker H, Marcucci G, Maharry K, et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: A Cancer and Leukemia Group B study. J Clin Oncol. 2010;28:596–604. doi: 10.1200/JCO.2009.25.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radmacher MD, Marcucci G, Ruppert AS, et al. Independent confirmation of a prognostic gene-expression signature in adult acute myeloid leukemia with a normal karyotype: A Cancer and Leukemia Group B study. Blood. 2006;108:1677–1683. doi: 10.1182/blood-2006-02-005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calin GA, Liu CG, Sevignani C, et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proc Natl Acad Sci U S A. 2004;101:11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldus CD, Liyanarachchi S, Mrózek K, et al. Acute myeloid leukemia with complex karyotypes and abnormal chromosome 21: Amplification discloses overexpression of APP, ETS2, and ERG genes. Proc Natl Acad Sci U S A. 2004;101:3915–3920. doi: 10.1073/pnas.0400272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng Z, Shi YX, Samudio IJ, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113:6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mahadevan D, DiMento J, Croce KD, et al. Transcriptosome and serum cytokine profiling of an atypical case of myelodysplastic syndrome with progression to acute myelogenous leukemia. Am J Hematol. 2006;81:779–786. doi: 10.1002/ajh.20690. [DOI] [PubMed] [Google Scholar]
- 39.Wilkinson S, Paterson HF, Marshall CJ. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 40.DiMartino JF, Lacayo NJ, Varadi M, et al. Low or absent SPARC expression in acute myeloid leukemia with MLL rearrangements is associated with sensitivity to growth inhibition by exogenous SPARC protein. Leukemia. 2006;20:426–432. doi: 10.1038/sj.leu.2404102. [DOI] [PubMed] [Google Scholar]
- 41.Tang R, Hirsch P, Fava F, et al. High Id1 expression is associated with poor prognosis in 237 patients with acute myeloid leukemia. Blood. 2009;114:2993–3000. doi: 10.1182/blood-2009-05-223115. [DOI] [PubMed] [Google Scholar]
- 42.Mahadevan D, List AF. Targeting the multidrug resistance-1 transporter in AML: Molecular regulation and therapeutic strategies. Blood. 2004;104:1940–1951. doi: 10.1182/blood-2003-07-2490. [DOI] [PubMed] [Google Scholar]
- 43.Scholl C, Gilliland DG, Fröhling S. Deregulation of signaling pathways in acute myeloid leukemia. Semin Oncol. 2008;35:336–345. doi: 10.1053/j.seminoncol.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Mizutani K, Sugimoto K, Okuda T, et al. Kynureninase is a novel candidate gene for hypertension in spontaneously hypertensive rats. Hypertens Res. 2002;25:135–140. doi: 10.1291/hypres.25.135. [DOI] [PubMed] [Google Scholar]
- 45.Ostergaard E. Disorders caused by deficiency of succinate-CoA ligase. J Inherit Metab Dis. 2008;31:226–229. doi: 10.1007/s10545-008-0828-7. [DOI] [PubMed] [Google Scholar]
- 46.Greenlee MC, Sullivan SA, Bohlson SS. Detection and characterization of soluble CD93 released during inflammation. Inflamm Res. 2009;58:909–919. doi: 10.1007/s00011-009-0064-0. [DOI] [PubMed] [Google Scholar]
- 47.Gorczynski RM, Kai Y, Miyake K. MD1 expression regulates development of regulatory T cells. J Immunol. 2006;177:1078–1084. doi: 10.4049/jimmunol.177.2.1078. [DOI] [PubMed] [Google Scholar]
- 48.Wang C, Hayashi H, Harrison R, et al. Modulation of Mac-1 (CD11b/CD18)-mediated adhesion by the leukocyte-specific protein 1 is key to its role in neutrophil polarization and chemotaxis. J Immunol. 2002;169:415–423. doi: 10.4049/jimmunol.169.1.415. [DOI] [PubMed] [Google Scholar]
- 49.Usdin TB, Modi W, Bonner TI. Assignment of the human PTH2 receptor gene (PTHR2) to chromosome 2q33 by fluorescence in situ hybridization. Genomics. 1996;37:140–141. doi: 10.1006/geno.1996.0532. [DOI] [PubMed] [Google Scholar]
- 50.Marcucci G, Mrózek K, Radmacher MD, et al. MicroRNA expression profiling in acute myeloid and chronic lymphocytic leukaemias. Best Pract Res Clin Haematol. 2009;22:239–248. doi: 10.1016/j.beha.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 51.Bousquet M, Quelen C, Rosati R, et al. Myeloid cell differentiation arrest by miR-125b-1 in myelodysplastic syndrome and acute myeloid leukemia with the t(2;11)(p21;q23) translocation. J Exp Med. 2008;205:2499–2506. doi: 10.1084/jem.20080285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takaya T, Ono K, Kawamura T, et al. MicroRNA-1 and microRNA-133 in spontaneous myocardial differentiation of mouse embryonic stem cells. Circ J. 2009;73:1492–1497. doi: 10.1253/circj.cj-08-1032. [DOI] [PubMed] [Google Scholar]
- 53.Bleeker FE, Lamba S, Leenstra S, et al. IDH1 mutations at residue p.R132 (IDH1R132) occur frequently in high-grade gliomas but not in other solid tumors. Hum Mutat. 2009;30:7–11. doi: 10.1002/humu.20937. [DOI] [PubMed] [Google Scholar]
- 54.Schnittger S, Haferlach C, Ulke M, et al. IDH1 mutations are detected in 9.3% of all AML and are strongly associated with intermediate risk karyotype and unfavourable prognosis: A study of 999 patients. Blood. 2009;114 doi: 10.1182/blood-2010-02-267955. abstr LBA-3. [DOI] [PubMed] [Google Scholar]
- 55.Baldus CD, Tanner SM, Ruppert AS, et al. BAALC expression predicts clinical outcome of de novo acute myeloid leukemia patients with normal cytogenetics: A Cancer and Leukemia Group B study. Blood. 2003;102:1613–1618. doi: 10.1182/blood-2003-02-0359. [DOI] [PubMed] [Google Scholar]
- 56.Langer C, Radmacher MD, Ruppert AS, et al. High BAALC expression associates with other molecular prognostic markers, poor outcome and a distinct gene-expression signature in cytogenetically normal patients younger than 60 years with acute myeloid leukemia: A Cancer and Leukemia Group B (CALGB) study. Blood. 2008;111:5371–5379. doi: 10.1182/blood-2007-11-124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marcucci G, Baldus CD, Ruppert AS, et al. Overexpression of the ETS-related gene, ERG, predicts a worse outcome in acute myeloid leukemia with normal karyotype: A Cancer and Leukemia Group B study. J Clin Oncol. 2005;23:9234–9242. doi: 10.1200/JCO.2005.03.6137. [DOI] [PubMed] [Google Scholar]
- 58.Marcucci G, Maharry K, Whitman SP, et al. High expression levels of the ETS-related gene, ERG, predict adverse outcome and improve molecular risk-based classification of cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2007;25:3337–3343. doi: 10.1200/JCO.2007.10.8720. [DOI] [PubMed] [Google Scholar]
- 59.Heuser M, Beutel G, Krauter J, et al. High meningioma 1 (MN1) expression as a predictor for poor outcome in acute myeloid leukemia with normal cytogenetics. Blood. 2006;108:3898–3905. doi: 10.1182/blood-2006-04-014845. [DOI] [PubMed] [Google Scholar]
- 60.Langer C, Marcucci G, Holland KB, et al. Prognostic importance of MN1 transcript levels, and biologic insights from MN1-associated gene and microRNA expression signatures in cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J Clin Oncol. 2009;27:3198–3204. doi: 10.1200/JCO.2008.20.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Metzeler KH, Dufour A, Benthaus T, et al. ERG expression is an independent prognostic factor and allows refined risk stratification in cytogenetically normal acute myeloid leukemia: A comprehensive analysis of ERG, MN1, and BAALC transcript levels using oligonucleotide microarrays. J Clin Oncol. 2009;27:5031–5038. doi: 10.1200/JCO.2008.20.5328. [DOI] [PubMed] [Google Scholar]
- 62.Thompson CB. Metabolic enzymes as oncogenes or tumor suppressors. N Engl J Med. 2009;360:813–815. doi: 10.1056/NEJMe0810213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dang L, White DW, Gross S, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature. 2009;462:739–744. doi: 10.1038/nature08617. [DOI] [PMC free article] [PubMed] [Google Scholar]