Abstract
The proproliferative transcription factor KLF5 plays an important role in promoting cell proliferation and tumorigenesis. KLF5 is a short-lived protein that can be rapidly degraded through the ubiquitin-proteasome pathway in cancer cells. However, the mechanisms regulating protein stability remain poorly understood. In this study, the tumor suppressor Fbw7, a component of the SCF complex (SCFFbw7) E3 ubiquitin ligase, specifically promoted the degradation and ubiquitination of KLF5 but had little effect on the stability of KLF4. Fbw7 interacted with KLF5 in a CDC4 phosphodegron (CPD)-dependent manner. Three CPDs were found in the KLF5 protein. Simultaneous mutation of these CPDs significantly abolished Fbw7-mediated ubiquitination and degradation. Furthermore, Fbw7 deficiency dramatically delayed KLF5 turnover and led to the accumulation of KLF5 protein in cancer cells. Glycogen synthase kinase-3β could phosphorylate and promote Fbw7-mediated KLF5 degradation. More importantly, Fbw7 negatively regulated the biological activity of KLF5 in gene regulation and cell proliferation. Taken together, these data indicate that Fbw7 is a key negative regulator controlling KLF5-mediated cell proliferation and suggest an additional mechanism linking the loss of Fbw7 function to tumorigenesis.
Keywords: E3 Ubiquitin Ligase, Protein-Protein Interactions, Transcription Factors, Ubiquitin, Zinc Finger, Fbw7, KLF5
Introduction
Sp/Krüppel-like factor (KLF)2 transcription factors are involved in various biological processes and human diseases (1, 2). KLF5 (also known as IKLF and BTEB2) is a basic KLF transcription factor that regulates cell proliferation and plays an important role in diverse physiological and pathological processes, including stemness, inflammation, and atherogenesis (3, 4). As a proproliferative factor, KLF5 also has essential functions in tumorigenesis (3). Increasing evidence indicates that KLF5 can function as an oncogenic protein by promoting cell proliferation in many cancers (5–10). For example, a high expression level of KLF5 correlates with a shorter survival time in breast cancer patients (11). Inhibition of KLF5 expression by pharmacological or genetic methods significantly reduces colorectal cancer cell proliferation (6, 12). However, under certain conditions, KLF5 can also act as a tumor suppressor in some cancers (13, 14). The exact mechanisms underlying these apparently contradictory functions are not completely understood.
The function of KLF5 is regulated at multiple levels. KLF5 transcription is regulated by several signaling molecules such as Wnt and lysophosphatidic acid (15, 16). At the post-translational level, KLF5 function is modulated by phosphorylation, sumoylation, and acetylation (3). Phosphorylation of KLF5 by protein kinase C enhances its transactivation activity and its interaction with CBP (cAMP-responsive element-binding protein-binding protein) (17), whereas sumoylation regulates KLF5-mediated lipid metabolism and its subcellular localization (18, 19).
KLF5 is an unstable protein with a short half-life in cells. Its protein levels are regulated negatively by the ubiquitin-proteasome pathway (20). The E3 ubiquitin ligase WWP1 targets KLF5 for ubiquitination and degradation in cancer and epithelial cells (21). However, the WWP1 gene is commonly overexpressed in numerous cancers and functions as a potential oncoprotein promoting cell proliferation and tumorigenesis (7, 22, 23). Given that KLF5 promotes cell proliferation in cancer cells as well, the possibility that other factors might regulate KLF5 stability during tumorigenesis remains to be investigated.
The tumor suppressor Fbw7 (also called CDC4), an F-box protein component of the SCF (SKP1/CUL1/F-box protein) E3 ubiquitin ligase, is a critical regulator of several oncoproteins (e.g. c-Myc, cyclin E, c-Jun, Notch, mTOR (mammalian target of rapamycin), and c-Myb) by targeting them for degradation and ubiquitination (24–31). Fbw7 contains an F-box domain of ∼40 amino acids (which interacts directly with SKP1 to recruit ubiquitin-conjugating enzymes) and eight WD40 repeats (which are required for its association with substrates) (32, 33). Fbw7 binds to its targets through a consensus phospho-binding motif called the CDC4 phosphodegron (CPD) in its substrates (24, 29, 34). Numerous cancer-associated mutations of Fbw7 have been found in various cancers (35), and loss of Fbw7 function results in tumorigenesis (36, 37). However, the mechanisms that link Fbw7 deficiency to tumorigenesis are not completely understood.
In this study, we identify Fbw7 as a novel regulator of KLF5. The data show that Fbw7 binds to and facilitates the ubiquitination and degradation of KLF5 through multiple phosphodegrons. This process is promoted by glycogen synthase kinase-3β (GSK3β)-mediated phosphorylation of KLF5 and is ultimately reflected by the down-regulation of KLF5 biological activity.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
Human embryonic kidney 293T cells and human epithelial carcinoma HeLa cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% heat-inactivated fetal bovine serum at 37 °C in 5% CO2. HCT116, HCT116 Fbw7−/−, DLD1, and DLD1 Fbw7−/− cells (kindly provided by Dr. Jonathan Cherry, The Johns Hopkins University) were cultured in McCoy's 5A medium (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum. Transfections were performed using calcium phosphate-DNA coprecipitation (for 293T cells), SunbioTrans-EZ (for HeLa cells; Shanghai Sunbio Medical Biotechnology Co., Ltd.), and Lipofectamine 2000 (for HCT116 cells; Invitrogen) according to the manufacturers' instructions.
Plasmids
Expression plasmids of FLAG-Fbw2, FLAG-Fbw5, FLAG-Fbw7, FLAG-Fbw8, and β-TrCP1 were kindly provided by Dr. Michele Pagano (New York University). pcDNA3.1-FLAG-Fbw7α, pcDNA3.1-FLAG-Fbw7β, and pcDNA3.1-FLAG-Fbw7γ were subcloned from glutathione S-transferase (GST)-tagged human CDC4α, CDC4β, and CDC4γ (kindly provided by Dr. Steven I. Reed, The Scripps Research Institute). KLF4 and KLF5 expression constructs were provided by Dr. Hitoshi Niwa (RIKEN Center for Developmental Biology, Kobe, Japan) and Dr. Mukesh K. Jain (Case Cardiovascular Research Institute, Cleveland, OH). Hemagglutinin (HA)-ubiquitin plasmids were described previously (38). Deletion mutants of Fbw7, human KLF5, and mouse KLF5 and KLF4 and their mutants were subcloned into the pcDNA3.1 vector with a FLAG, HA, or green fluorescent protein (GFP) tag at the N terminus by standard cloning methods. GST-KLF5 CPD and GST-Fbw7 WD40 were cloned into pGEX-4T-1. GSK3β plasmids were provided by Dr. Kunliang Guan. WWP1 was amplified from 293T cDNA and cloned into a pcDNA3.1-FLAG vector within the EcoRI and NotI restriction sites. All vectors were confirmed by DNA sequencing.
Immunoprecipitation and Immunoblotting
Transfected cells were lysed in lysis buffer (50 mm Tris-HCl (pH 7.4), 150 mm NaCl, 10% glycerol, 1 mm EDTA, 0.5% Nonidet P-40, and a mixture of protease inhibitors) and cleared by centrifugation. Cleared cell lysates were incubated with 1 μg of anti-HA or anti-FLAG antibody (Sigma) and 16 μl of protein A/G beads (Santa Cruz Biotechnology, Santa Cruz, CA) for 3 h.
To detect endogenous protein interactions, HeLa cells were treated with the proteasome inhibitor MG132 (20 μm; benzyloxycarbonyl-l-leucyl-l-leucyl-l-leucinal, Sigma) for 6 h before harvesting. Cells were lysed in ice-cold lysis buffer. Cleared cell lysates were incubated with 4 μg of anti-KLF5 antibody (AF3758, R&D Systems) or 5 μg of anti-Fbw7 antibody (Ab12292, Abcam, Hong Kong) and 16 μl of protein A/G beads for 3 h at 4 °C. After extensive washing, beads were boiled at 100 °C for 5 min. Proteins were resolved by SDS-PAGE and transferred onto nitrocellulose membranes (Millipore), followed by immunoblotting using anti-HA (1:1000), anti-FLAG (1:1000), or anti-GFP (1:1000; Santa Cruz Biotechnology) antibody. Endogenous KLF5 or Fbw7 was detected using anti-KLF5 antibody (1:1000; sc-22797, Santa Cruz Biotechnology) or anti-Fbw7 antibody (1:500; Ab12292, Abcam), respectively. Immunoblots were analyzed using the Odyssey system (LI-COR Biosciences).
Flow Cytometry-based Protein Degradation Assay
KLF5 was subcloned into a DsRed-IRES-EGFP vector (kindly provided by Dr. Stephen J. Elledge, Harvard Medical School). Two μg of DsRed-IRES-EGFP vector or DsRed-IRES-EGFP-KLF5 were transfected into 293T cells in 6-well plates. Transfected cells were collected and suspended in phosphate-buffered saline (PBS) and analyzed using a FACSCalibur flow cytometer (BD Biosciences).
Immunofluorescence
293T cells cultured on coverslips were transfected with 1 μg of GFP-KLF5 and 1 μg of FLAG-Fbw7 in 24-well plates. The cells were treated with 10 μm MG132 for 4 h before fixation with 4% paraformaldehyde. Fbw7 was stained with mouse anti-FLAG antibody (1:100).
For degradation assays, DLD1 cells, Fbw7-deleted DLD1 cells, or GFP-Fbw7-expressing HeLa cells were fixed with 4% paraformaldehyde, permeabilized using 0.1% Triton X-100, blocked with 1% bovine serum albumin in PBS for 1 h, and stained with rabbit anti-KLF5 antibody (1:100; Santa Cruz Biotechnology), followed by Texas Red-labeled anti-rabbit secondary antibody (1:100; Invitrogen). Fixed permeabilized HeLa cells were also labeled with rabbit anti-KLF5 antibody (1:100) and mouse anti-Fbw7 antibody (1:100; Ab74054, Abcam). The slides were then stained with Texas Red-labeled anti-rabbit secondary antibody and fluorescein isothiocyanate-labeled anti-mouse secondary antibody (1:100; Invitrogen) to detect co-localization of endogenous KLF5 and Fbw7. The nuclei were stained with 0.5 μg/ml 4′,6-diamidino-2-phenylindole (Sigma). Images were analyzed using a Leica SP5 confocal microscope.
Reverse Transcription (RT)-PCR Assay
Total RNA was isolated from cells using TRIzol reagent (Invitrogen) according to the manufacturer's directions, treated with RNase-free DNase, and subjected to reverse transcription with random hexanucleotide primers. The following primer sequences were used: KLF5, 5′-CACTACTGCGATTACCCTG-3′ (sense) and 5′-GGTCTGTCATTTGAGGGAG-3′ (antisense); Fbw7, 5′-GACGCCGAATTACATCTGTC-3′ (sense) and 5′-GTAGCAGGTCTTTGGGTTC-3′ (antisense); KLF4, 5′-CCATTACCAAGAGCTCATGC-3′ (sense) and 5′-TAGTGCCTGGTCAGTTCATC-3′ (antisense); survivin, 5′-GACTTGGCCCAGTGTTTCTTC-3′ (sense) and 5′-CACTTTCTCCGCAGTTTCCTC-3′ (antisense); and GSK3β, 5′-CTCAAATTAAGGCACATCC-3′ (sense) and 5′-CGGTCTCCAGTATTAGCAT-3′ (antisense).
In Vitro Phosphorylation Assay
Wild-type or CPD-deficient (3A) GST-KLF5 CPD protein (amino acids 221–356) was purified from Escherichia coli by standard protocols. FLAG-KLF5 was expressed in 293T cells and purified using 1 μg of anti-FLAG antibody for immunoprecipitation. After washing twice with kinase buffer (25 mm Tris-HCl (pH 7.5), 5 mm β-glycerophosphate, 10 mm MgCl2, 2 mm dithiothreitol, and 0.1 mm Na3VO4), purified FLAG-KLF5 or 2 μg of purified GST-KLF5 CPD protein were incubated with 200 ng of recombinant GSK3β kinase (R&D Systems) in 25 μl of kinase buffer containing 200 μm ATP and 5 μCi of [γ-32P]ATP at 30 °C for 1 h. The reaction was terminated by the addition of 8 μl of 4× Laemmli SDS sample buffer. The reaction mixtures were boiled and separated by SDS-PAGE and subjected to autoradiography.
GST Pulldown Assay
FLAG-KLF5 was expressed in 293T cells. The transfected cells were lysed and cleared by centrifugation. GST-Fbw7 WD40 was purified from E. coli. Ten μg of purified GST or GST-Fbw7 WD40 protein were incubated with KLF5 cell lysates for 1 h, and GST proteins were purified using glutathione-Sepharose 4B (Amersham Biosciences). Bound KLF5 was detected by immunoblotting.
Small Interfering RNA (siRNA) Knockdown and Cycloheximide Chase Assay
HeLa cells were transfected with siRNA oligonucleotides using Lipofectamine. Two different oligonucleotides against Fbw7 were used: siRNA1 (5′-ACCUUCUCUGGAGAGAGAAAUGCTT-3′) and siRNA2 (5′-GUGUGGAAUGCAGAGACUGGAGATT-3′) (27). Forty-eight h after transfection, cells were treated with 50 μg/ml cycloheximide for 0–4 h. The transfected cells were lysed and analyzed by immunoblotting using anti-KLF5 or anti-tubulin (Sigma) antibody.
Pulse-Chase Assay
HeLa cells were plated in 60-mm dishes at 5 × 105 cells and transfected using Lipofectamine with control siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′) or siRNA against Fbw7 (see above) or GSK3β (5′-GUAUUGCAGGACAAGAGAUTT-3′). Forty-eight h after transfection, cells were washed twice with PBS and cultured in Met/Cys-free Dulbecco's modified Eagle's medium (21013-024, Invitrogen) supplemented with 2 mm l-glutamine (G8540, Sigma) and 5% dialyzed fetal calf serum (30067-334, Invitrogen) for 30 min at 37 °C. The cells were labeled with 50 μl of EXPRE35S35S protein labeling mixture (100 μCi/dish; NEG072007MC, PerkinElmer Life Sciences) for 40 min at 37 °C. After extensive washing, cells were lysed in radioimmune precipitation assay buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 10 mm N-ethylmaleimide, 1 mm phenylmethylsulfonyl fluoride, 1 mm NaV3O4, and 1 mm NaF). Endogenous KLF5 was immunoprecipitated with goat anti-KLF5 antibody (2 μg/ml; R&D Systems). Proteins were separated by SDS-PAGE. The gel was treated with Amplify solution (EnlightningTM, PerkinElmer Life Sciences) to enhance the 35S signal, dried, and autoradiographed.
Ubiquitination Assay
In vivo ubiquitination assays were performed as described previously (38). Briefly, FLAG-KLF5 was transiently transfected into 293T cells with or without HA-ubiquitin and GFP-Fbw7α. Transfected cells were treated with 10 μm MG132 for 6 h before harvesting. Cells were lysed in 500 μl of radioimmune precipitation assay buffer with protease inhibitors, and KLF5 was immunoprecipitated using 1 μg of anti-FLAG antibody for 4 h at 4 °C. Polyubiquitinated KLF5 was detected using anti-HA antibody.
To detect the ubiquitination of endogenous KLF5 under denaturing conditions, HeLa cells were transfected using Lipofectamine with control or Fbw7 siRNA oligonucleotides. Twenty-four h later, cells were transfected with HA-ubiquitin. Transfected cells were treated with 20 μm MG132 for 6 h and lysed in 60 μl of modified radioimmune precipitation assay buffer (1× PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1% SDS, 10 mm N-ethylmaleimide, 1 mm phenylmethylsulfonyl fluoride, 1 mm NaV3O4, and 1 mm NaF). Cell lysates were boiled for 10 min, diluted in 10 volumes of lysis buffer without SDS, and subjected to immunoprecipitation using 4 μg of anti-KLF5 antibody (R&D Systems). Ubiquitination of KLF5 was detected by immunoblotting using anti-HA antibody (1:1000). Endogenous KLF5 was detected using anti-KLF5 antibody (1:1000; Santa Cruz Biotechnology).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide Assay
HCT116 cells were seeded in 96-well plates and cotransfected with control siRNA or KLF5 siRNA (5′-ACCUUCUCUGGAGAGAGAAAUGCTT-3′) and mouse KLF5 plasmids (HA-KLF5 and HA-KLF5-3A). Forty-eight h after transfection, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (T0793, Sangon Biotech Co., Ltd., Shanghai, China) was added to each well for 4 h. Me2SO (150 μl) was then added, and the absorbance was measured at 490 nm using a microplate spectrophotometer (SpectraMax 190, Molecular Devices, Inc.).
Colony Formation Assay
HCT116 cells (2.5 × 103) transfected with wild-type KLF5 or its CPD-deficient mutant (KLF5-3A) in the presence or absence of Fbw7 plasmids were mixed with 0.8% agarose in warm 2× Dulbecco's modified Eagle's medium containing 20% fetal bovine serum and plated in each well of a 6-well plate on top of a prepared 1.2% agar base. Colony formation in the soft agar was performed as described previously (18). The number of colonies was counted under a microscope 2 weeks later.
Luciferase Assay
The survivin gene promoter was amplified from human genomic DNA by PCR using primer sequences 5′-CTCGAGCTCAAAGTGTTGGGATTACAGG-3′ and 5′-AAGCTTTGGTCCTTGAGAAAGGGCTGC-3′. Two restriction endonuclease recognition sites, XhoI and HindIII, were attached to the primers for subsequent cloning. The PCR products were purified from agarose gels, digested, and subcloned into a pGL3-Basic plasmid (Promega). The construct was verified by restriction digestion and DNA sequencing. Luciferase activity was measured using the Dual-Luciferase assay system (Promega) according to the manufacturer's protocol.
RESULTS
KLF5 Is Degraded by Tumor Suppressor Fbw7
SCFFbw7 ubiquitin ligase can efficiently ubiquitinate and degrade substrates through its association with CPD motifs (24). The protein sequence of KLF5 contains three potential conserved CPDs identified in numerous Fbw7 substrates (Fig. 1A). To determine whether Fbw7 promotes the degradation of KLF5, HA-tagged KLF5 was expressed either alone or in the presence of various FLAG-tagged F-box ubiquitin ligases in 293T cells. Coexpression of Fbw7 significantly decreased KLF5 protein levels (Fig. 1B). In contrast, other F-box proteins (β-TrCP1, Fbw2, Fbw5, and Fbw8) had little effect, suggesting that Fbw7 is a specific E3 ubiquitin ligase of KLF5.
FIGURE 1.
Degradation of KLF5 by Fbw7. A, KLF5 contains three putative CPD sequences. X indicates any residue other than Lys or Arg. h, human; m, mouse; r, rat. B, shown is the degradation of KLF5 by Fbw7α. HA-KLF5 was cotransfected with empty vector (control (Ctl)), β-TrCP1, Fbw2, Fbw5, Fbw8, or Fbw7α into 293T cells. All transfections contained equivalent amounts of GFP to monitor transfection efficiency. Transfected cells were lysed, and the levels of KLF5 were detected by immunoblotting (IB) using anti-HA antibody. Expression of Fbw proteins and GFP was detected with anti-FLAG and anti-GFP antibodies, respectively. C, HA-KLF5 was coexpressed with empty vector or different Fbw7 isoforms. Transfected cells were treated with or without 10 μm MG132 for 6 h before harvesting. The levels of KLF5, Fbw7, and GFP were detected by immunoblotting. D, HA-KLF5 was transfected into 293T cells in the presence of FLAG-tagged wild-type Fbw7α (WT), F-box-deleted mutant Fbw7α (ΔF), or the WD40 domain from Fbw7α. The levels of KLF5 were detected using anti-HA antibody. E, 293T cells were transfected with FLAG-Fbw7α and HA-KLF5 or HA-KLF4. The degradation of KLFs was monitored by immunoblotting.
Thus far, three Fbw7 isoforms (Fbw7α, Fbw7β, and Fbw7γ) with different subcellular localizations have been reported (39). Fbw7α is localized mainly in the nucleoplasm, whereas Fbw7β is found in the cytoplasm and Fbw7γ in the nucleolus (40). 293T cells were transfected with KLF5 in the absence or presence of each individual Fbw7 isoform. The nuclear α- and γ-isoforms significantly promoted the degradation of KLF5, but Fbw7β did not (Fig. 1C). Furthermore, the degradation of KLF5 mediated by the α- and γ-isoforms was significantly blocked by the proteasome inhibitor MG132, suggesting that this process is dependent on the ubiquitin-proteasome pathway (Fig. 1C).
The domains of Fbw7α required for KLF5 degradation were also explored (Fig. 1D). Relative to the wild type, expression of the F-box domain deletion mutant (Fbw7αΔF) or the WD40 domain alone could not degrade KLF5, suggesting that the F-box domain is critical for Fbw7-mediated KLF5 degradation.
To illustrate the specificity of Fbw7-mediated KLF5 degradation, we examined whether the protein levels of KLF4 could be affected by Fbw7. KLF4 is a close family member of KLF5 but without any potential CPDs (as analyzed using Scansite of the Massachusetts Institute of Technology). As expected, increasing the amount of Fbw7α greatly promoted KLF5 degradation but had little effect on KLF4 protein levels (Fig. 1E).
To study the degradation of KLF5 by Fbw7 in cells more precisely, a recently reported fluorescence-activated cell sorter (FACS) analysis-based protein degradation system was used (41). KLF5 was fused to GFP in an expression vector, and DsRed was included in the same transcript as an internal control (Fig. 2A). When expressed alone in 293T cells, the relative expression levels of GFP-KLF5 were much lower than those of GFP (Fig. 2B). Treatment with MG132 significantly increased GFP-KLF5 but had little effect on GFP protein levels (Fig. 2C), suggesting that GFP-KLF5 is constitutively degraded by the ubiquitin-proteasome pathway in cells. Coexpression of Fbw7α dramatically decreased KLF5 protein levels, whereas deletion of the F-box produced the opposite effect (Fig. 2, D and E). These results are consistent with the notion that Fbw7αΔF functions as a dominant-negative mutant to block the binding of substrate to endogenous Fbw7. Collectively, these data demonstrate that Fbw7α specifically degrades KLF5 by the ubiquitin-proteasome pathway.
FIGURE 2.
FACS-based analysis of KLF5 stability. A, shown is diagram of the DsRed-IRES-EGFP vector and the DsRed-IRES-EGFP-KLF5 construct used for FACS analysis. B, empty vector or GFP-KLF5 was transfected into 293T cells and analyzed by FACS analysis. Relative GFP levels were normalized to the expression level of DsRed. C, shown are the results from FACS analysis of the relative protein levels from cells transfected with empty vector or GFP-KLF5 and treated with or without MG132 for 6 h. D, shown are the results from FACS analysis of the relative levels of GFP or GFP-KLF5 from 293T cells cotransfected with empty vector or GFP-KLF5 and Fbw7α or the Fbw7αΔF mutant. E, shown are the results from graphic analysis of flow cytometry data normalized to the expression of DsRed. Data are presented as means ± S.D. (Student's t test). *, p < 0.05; **, p < 0.01 (n = 3). Ctrl, control.
Fbw7α Interacts with KLF5
We next used a co-immunoprecipitation assay to examine how KLF5 interacts with Fbw7α. GFP-KLF5 was cotransfected into 293T cells with or without FLAG-Fbw7α in the presence of MG132 to prevent degradation of KLF5 by Fbw7α. FLAG-Fbw7α was precipitated using anti-FLAG antibody, and the immunoprecipitates were analyzed by immunoblotting with anti-GFP or anti-FLAG antibody. KLF5 copurified efficiently with Fbw7. In contrast, KLF4 was not detected in Fbw7α immunoprecipitates (Fig. 3A).
FIGURE 3.
Interaction of KLF5 with Fbw7α. A, GFP-KLF4 or GFP-KLF5 was transfected into 293T cells with empty vector or FLAG-Fbw7α. Transfected cells were lysed after treatment with 10 μm MG132 for 6 h and immunoprecipitated with anti-FLAG antibody, and the immunoprecipitates (IP) and the original whole cell extracts (WCE) were analyzed by immunoblotting (IB) using anti-GFP or anti-FLAG antibody. B, 293T cells were cotransfected with GFP-KLF5 and wild-type Fbw7α or its Fbw7αΔF or WD40 mutant. After treatment with MG132, cells were lysed and immunoprecipitated with anti-FLAG antibody, and the immunoprecipitates and cell lysates were analyzed by immunoblotting using anti-GFP or anti-FLAG antibody. *, IgG band. C, GST and GST-WD40 proteins were purified from E. coli and incubated with the cell lysates expressing HA-KLF5. The products of GST pulldown assay were analyzed by immunoblotting using anti-HA or anti-GST antibody. D and E, endogenous Fbw7 and KLF5, respectively, were immunoprecipitated from HeLa cells by an antibody specific for that protein. The immunoprecipitates and the original cell lysates were subjected to immunoblotting using anti-KLF5 or anti-Fbw7 antibody. F, shown is the co-localization of KLF5 and Fbw7 in the nucleus. GFP-KLF5 and FLAG-Fbw7α were coexpressed in 293T cells. Transfected cells were treated MG132 and fixed, and KLF5 was stained with anti-FLAG antibody. Images were analyzed by confocal microscopy. G, Fbw7 and KLF5 were localized by specific antibodies in fixed HeLa cells and analyzed by confocal microscopy.
To identify the domains of Fbw7 involved in its interaction with KLF5, full-length Fbw7α, Fbw7αΔF, and the WD40 domain were FLAG-tagged and coexpressed with GFP-KLF5. Fbw7α or its mutants were precipitated using anti-FLAG antibody, and copurified KLF5 was detected using anti-GFP antibody (Fig. 3B). Both Fbw7αΔF and the WD40 domain alone interacted efficiently with KLF5, indicating that Fbw7 binds to KLF5 through its WD40 domain. This was confirmed using a GST pulldown assay (Fig. 3C). Relative to GST, GST-Fbw7 WD40 efficiently bound KLF5. Taken together, these data show that Fbw7 binds to KLF5 through its WD40 domain.
Co-immunoprecipitation studies showed that endogenous Fbw7 copurified with endogenous KLF5 (Fig. 3, D and E). The co-localization of Fbw7 and KLF5 in HeLa cells was confirmed by immunofluorescence. Fbw7 co-localized with KLF5 in the nucleus of cotransfected cells (Fig. 3F) and untransfected HeLa cells (Fig. 3G), consistent with the results from the interaction assays.
Fbw7α Degrades and Interacts with KLF5 via Three CPDs
Three putative CPDs have been identified in KLF5 (Fig. 1A). To determine whether these CPDs are involved in Fbw7-mediated KLF5 degradation, key amino acid residues (Thr227, Ser292, and Thr312 in mouse KLF5, which are also conserved in human and rat) were mutated to Ala. These mutants were then subjected to degradation analysis. Surprisingly, mutating these sites individually had only minor effects on Fbw7-mediated degradation (Fig. 4, A and B). However, some Fbw7 substrates, such as PGC-1α and cyclin E, contain more than one degron (34, 42). Therefore, Fbw7 might target KLF5 for degradation via multiple degrons as well. The double mutants T227A/S292A, T227A/T312A, and S292A/T312A and the triple mutant T227A/S292A/T312A (KLF5-3A) were thus created and subjected to the degradation assay (Fig. 4C). The double mutant T227A/S292A was more resistant to Fbw7α (∼35% degradation) compared with T227A/T312A (67% degradation) and S292A/T312A (57% degradation), suggesting that CPD1 and CPD2 might play more important roles than CPD3 in Fbw7-mediated degradation. In contrast, the triple CPD mutant (KLF5-3A) was strongly resistant to Fbw7α degradation (Fig. 4, C and D). Taken together, these data indicate that each individual CPD of KLF5 is a functional Fbw7 degradation signal.
FIGURE 4.
Identification of CPDs in KLF5. HA-tagged wild-type KLF5 (WT) and its mutants with single-point mutations (A and B) or double- or triple-point mutations (C and D) were cotransfected with empty vector or FLAG-Fbw7α into 293T cells. 3A is the triple mutation of T227A/S292A/T312A of mouse KLF5. All transfections contained equivalent amounts of GFP to monitor transfection efficiency. The levels of KLF5 and its mutants were detected by immunoblotting (IB) using anti-HA antibody. In B and D, quantitative expression of data from A and C was analyzed using Odyssey V3.0 software and normalized to the GFP level. The relative degradation of KLF5 by Fbw7α was calculated by the protein levels of cell lysates with or without Fbw7 (n = 3). E, simultaneous mutation of all three CPDs completely abolishes the interaction of KLF5 with Fbw7α. GFP-KLF5 or GFP-KLF5-3A was coexpressed in 293T cells in the presence or absence of FLAG-Fbw7α. The cells were treated with MG132, lysed, and immunoprecipitated (IP) with anti-FLAG antibody. The resulting immunoprecipitates were analyzed by anti-GFP or anti-FLAG antibody. WCE, whole cell lysate. F, HA-KLF5 and its 3A mutant were coexpressed with FLAG-Fbw7α or FLAG-WWP1. The cells were lysed, and the levels of KLF5 were analyzed using anti-HA antibody. The expression of Fbw7α and WWP1 was detected using anti-FLAG antibody.
We next examined whether these CPDs are required for the interaction of KLF5 with Fbw7. Compared with wild-type KLF5, the triple KLF5 mutant failed to interact with Fbw7 (Fig. 4E), thereby proving that KLF5 interacts with Fbw7 via these phosphodegrons.
As noted above, WWP1 can promote the degradation of KLF5 (21). We therefore determined if the triple mutation also affects WWP1-mediated degradation. However, KLF5-3A was still efficiently degraded by WWP1 (Fig. 4F), suggesting that Fbw7 and WWP1 use different mechanisms to degrade KLF5.
Fbw7 Targets KLF5 for Ubiquitination
Fbw7 is a component of E3 ubiquitin ligase that promotes the degradation of target proteins through ubiquitination (24). We thus used an in vivo ubiquitination assay to test whether Fbw7 promotes KLF5 ubiquitination. 293T cells transfected with FLAG-KLF5 and HA-ubiquitin in the absence or presence of GFP-Fbw7α were treated with MG132 for 6 h to stabilize the ubiquitinated proteins before lysis. In the absence of Fbw7α, KLF5 was weakly ubiquitinated (Fig. 5A). The addition of Fbw7α greatly enhanced KLF5 ubiquitination, whereas mutation of all three CPDs completely abolished Fbw7α-induced KLF5 ubiquitination (Fig. 5B).
FIGURE 5.
Promotion of KLF5 ubiquitination by Fbw7α. A, 293T cells were transfected with the expression constructs of FLAG-KLF5 with or without GFP-Fbw7α and with or without HA-ubiquitin (Ubi). Transfected cells were treated with MG132, lysed, and immunoprecipitated (IP) with anti-FLAG antibody. The resulting precipitates and their corresponding whole cell lysates (WCL) were analyzed using anti-FLAG, anti-HA, or anti-GFP antibody. B, FLAG-KLF5 or KLF5-3A was coexpressed in 293T cells with HA-tagged ubiquitin and with or without GFP-Fbw7α. Ubiquitination was analyzed by immunoblotting (IB). WT, wild-type KLF5. C, HeLa cells expressing HA-ubiquitin were lysed under denaturing conditions. Endogenous KLF5 was immunoprecipitated from control or Fbw7 knockdown cells, and ubiquitination was examined by immunoblotting using anti-HA antibody. siCon, control siRNA; siFbw7, Fbw7 siRNA.
To test whether endogenous Fbw7 can ubiquitinate endogenous KLF5, HeLa cells were transfected with control or Fbw7 siRNA. HA-ubiquitin was then expressed in the transfected cells. After treatment with MG132, endogenous KLF5 was purified under denaturing conditions, and ubiquitination was detected by immunoblotting (Fig. 5C). Knockdown of Fbw7 significantly decreased the ubiquitination of endogenous KLF5. Taken together, these results support the hypothesis that Fbw7 negatively regulates the stability of KLF5 through ubiquitination in a CPD-dependent manner.
Endogenous KLF5 Is Degraded by Endogenous Fbw7 in Cancer Cell Lines
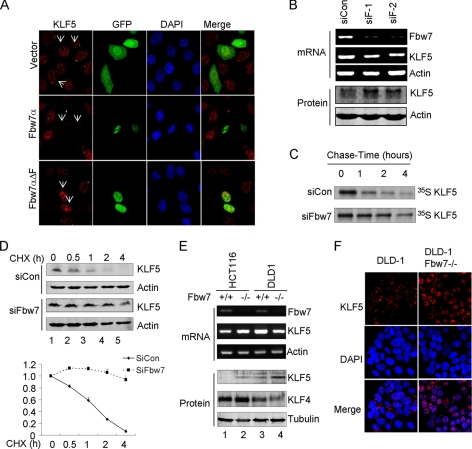
To examine whether endogenous KLF5 is regulated by Fbw7, GFP-Fbw7α was transfected into HeLa cells expressing high levels of endogenous KLF5 protein. Immunofluorescence staining showed that expression of wild-type Fbw7α dramatically decreased endogenous KLF5 levels relative to the GFP control, whereas Fbw7αΔF significantly increased KLF5 levels (Fig. 6A), suggesting that the mutant might function as a dominant-negative form to block the Fbw7-mediated degradation of endogenous KLF5.
FIGURE 6.
Degradation of endogenous KLF5 by endogenous Fbw7. A, HeLa cells were transfected with GFP, GFP-Fbw7α, or GFP-Fbw7αΔF. Fixed cells were stained with anti-KLF5 antibody, followed by secondary antibody. The images were analyzed by confocal microscopy. Blue, DNA; red, KLF5; green, GFP. B, knockdown of Fbw7 elevated endogenous KLF5 protein levels. HeLa cells were transfected with control siRNA (siCon) or siRNA against Fbw7 (siF-1 and siF-2). The mRNA levels of Fbw7 and KLF5 were analyzed by RT-PCR, and the protein levels of KLF5 and actin were analyzed by immunoblotting. C, knockdown of Fbw7 delayed the turnover of endogenous KLF5. HeLa cells were transfected with control siRNA or Fbw7 siRNA (siFbw7). The half-life of endogenous KLF5 was monitored by pulse-chase assay. D, the half-life of endogenous KLF5 in control or Fbw7 knockdown cells was detected by cycloheximide (CHX) chase assay. Quantitative data from three independent experiments were analyzed using Odyssey V3.0 software. E, the abundance of Fbw7 and KLF5 mRNAs in Fbw7-deficient HCT116 or DLD1 cells and their parent cells was analyzed by RT-PCR. The protein levels of KLF5, KLF4, and tubulin were analyzed by immunoblotting. F, the protein levels of KLF5 in Fbw7-deficient and parental DLD1 cells were analyzed by immunofluorescence. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
To determine whether endogenous KLF5 could be targeted for degradation by endogenous Fbw7, KLF5 protein levels were measured when endogenous Fbw7 was knocked down. Transfection of siRNA against Fbw7 into HeLa cells significantly inhibited its expression (as measured by RT-PCR). Knockdown of Fbw7 in turn efficiently elevated the amount of KLF5 protein but had little effect on its mRNA levels (Fig. 6B). In addition, the half-life of KLF5 protein increased significantly in Fbw7 knockdown cells compared with controls as assessed by both pulse-chase and cycloheximide chase assays (Fig. 6, C and D), suggesting that the elevated KLF5 protein levels in Fbw7-deficient cells are indeed the result of reduced protein degradation.
To confirm this, the Fbw7-deficient colon carcinoma cells HCT116 and DLD1 (43) were used to examine the expression of Fbw7 and KLF5. Deletion of Fbw7 weakly increased KLF5 mRNA levels in HCT116 cells but produced a modest reduction in DLD1 cells (Fig. 6E). In contrast, the protein levels of KLF5, but not KLF4, significantly increased in both Fbw7-deficient cell lines compared with their parental cells. These data were confirmed by immunofluorescence assays: deletion of Fbw7 in DLD1 cells significantly increased nuclear KLF5 protein levels (Fig. 6F). Taken together, our data indicate that endogenous KLF5 protein levels are regulated by endogenous Fbw7 ubiquitin ligase activity in cancer cell lines.
GSK3β Is Required for Fbw7-mediated Degradation of KLF5
Many known Fbw7 substrates (e.g. c-Myc, c-Myb, SREBP-1, PGC-1α, etc.) are phosphorylated by GSK3β. Phosphorylation by GSK3β promotes the binding of substrate to Fbw7 and enhances substrate degradation (25, 27, 34, 44). Interestingly, Scansite software analysis revealed that KLF5 CPDs are also potential GSK3 phosphorylation motifs (Fig. 1A). To investigate whether KLF5 is a substrate of GSK3β, we performed in vitro phosphorylation assays. HA-KLF5 was expressed in 293T cells and purified with anti-HA antibody. KLF5 was indeed significantly phosphorylated by recombinant GSK3β (Fig. 7A). We next tested whether the CPDs of KLF5 are phosphorylated directly by GSK3. The KLF5 fragment containing wild-type or mutant CPDs was fused with GST, purified from E. coli, and subjected to in vitro phosphorylation assays. As expected, phosphorylation of wild-type KLF5 by GSK3β was much stronger than that of the CPD-deficient fragment (Fig. 7B). These data indicate that KLF5 is a substrate of GSK3 and that its CPDs are potential GSK3 phosphorylation sites.
FIGURE 7.
Phosphorylation and degradation of KLF5 by GSK3β. A, phosphorylation of full-length KLF5 by GSK3β. FLAG-KLF5 was expressed, immunoprecipitated with anti-FLAG antibody, phosphorylated in vitro, and analyzed by autoradiography. B, direct phosphorylation of KLF5 CPDs by GSK3β. Wild-type (WT) and CPD-deficient GST-KLF5 (GST-KLF5-3A) fragments were purified from E. coli, phosphorylated in vitro, and analyzed by autoradiography. C, interaction of GSK3β with KLF5. Myc-GSK3β was transfected into 293T cells with or without FLAG-KLF5. The cells were lysed and immunoprecipitated (IP) using anti-FLAG antibody, and the resulting precipitates and whole cell extracts (WCE) were analyzed by immunoblotting. D, GSK3β promotes Fbw7α-mediated degradation. Wild-type or CPD-deficient KLF5 (KLF5-3A) was coexpressed with Fbw7α and/or GSK3β-S9A. Expression of KLF5 and GSK3β was analyzed by immunoblotting. E, HeLa cells were transfected with control or Fbw7 (siFbw7) siRNA for 48 h, and then GSK3β-S9A was expressed for another 24 h. Expression of KLF5, GSK3β, and actin was analyzed by immunoblotting. F, the expression construct DsRed-IRES-EGFP-KLF5 was transfected into 293T cells. Twenty-four h after transfection, cells were treated with 40 mm LiCl for 2 h, followed by treatment with 10 μm MG132 for 6 h. The level of KLF5 was analyzed by flow cytometry. **, p < 0.01, relative to treatment with Me2SO (Student's t test). G, knockdown of GSK3β extends the half-life of endogenous KLF5. HeLa cells were transfected with control (siCon) or GSK3β (siGSK3β) siRNA. The half-life of endogenous KLF5 was monitored by pulse-chase assay. The mRNA levels of GSK3β were monitored by RT-PCR. H, HeLa cells expressing HA-ubiquitin (HA-Ubi) were treated with LiCl and MG132 as indicated. The ubiquitination of endogenous KLF5 under denaturing conditions was examined by immunoblotting. NSB, nonspecific band. I, HeLa cells were treated with MG132 and LiCl as indicated. Cells were lysed, endogenous KLF5 was immunoprecipitated, and the immunoprecipitates were analyzed by immunoblotting using anti-KLF5 or anti-Fbw7 antibody.
We next examined the interaction between GSK3 and KLF5 by co-immunoprecipitation. FLAG-KLF5 was cotransfected with or without Myc-GSK3 into 293T cells, and KLF5 was immunoprecipitated using anti-FLAG antibody. GSK3β copurified with KLF5 only when they were cotransfected (Fig. 7C), indicating that GSK3β interacts specifically with KLF5.
We further explored whether GSK3β promotes KLF5 degradation. KLF5 was coexpressed with Fbw7α in the presence or absence of constitutively active GSK3β (GSK3β-S9A) in 293T cells, and KLF5 protein levels were monitored by immunoblotting. KLF5 protein levels decreased in the presence of Fbw7α and were further reduced when GSK3β-S9A was coexpressed (Fig. 7D). In contrast, both Fbw7α and GSK3β-S9A had little effect on protein levels in the KLF5-3A mutant, suggesting that the GSK3β promotion of Fbw7α-mediated KLF5 degradation depends on KLF5 CPDs.
The role of endogenous Fbw7 in GSK3-promoted KLF5 degradation was also examined by siRNA knockdown. Expression of GSK3β-S9A significantly reduced endogenous KLF5 protein levels, but the reduction was inhibited by Fbw7 knockdown (Fig. 7E), suggesting that Fbw7 is required for GSK3β-promoted KLF5 degradation.
To confirm that GSK3β activity is required for KLF5 degradation, we took advantage of the FACS-based degradation system (Fig. 7F). Treatment with the GSK3 inhibitor LiCl significantly elevated KLF5 protein levels, and this was increased further by the addition of MG132. Furthermore, GSK3β knockdown using specific siRNAs clearly extended the half-life of endogenous KLF5 as measured by pulse-chase assay (Fig. 7G). Similar results were obtained when the cells were treated with LiCl (data not shown). Treatment with LiCl also significantly inhibited the ubiquitination of endogenous KLF5 (Fig. 7H), indicating that endogenous GSK3 activity is required for KLF5 ubiquitination and degradation.
To further understand the mechanism by which GSK3 affects KLF5 stability, we examined the effect of GSK3 activity on the interaction between Fbw7 and KLF5. Treatment with LiCl clearly decreased the interaction between endogenous Fbw7 and KLF5 (Fig. 7I), suggesting that GSK3 regulates KLF5 stability by promoting the binding of KLF5 to Fbw7.
Fbw7 Negatively Regulates KLF5 Biological Activity in Colorectal Cancer Cells
It has been reported that expression of KLF5 promotes the growth and proliferation of colorectal cancer cells (18). We thus tested whether Fbw7-mediated degradation inhibits KLF5-dependent cell proliferation using a soft agar formation assay. Compared with empty vector, more colonies were formed when wild-type KLF5 was overexpressed, whereas the Fbw7-resistant mutant KLF5-3A was able to stimulate more HCT116 cells to form colonies compared with the wild type (Fig. 8A). In addition, coexpression of Fbw7α significantly inhibited KLF5-mediated cell proliferation but had little effect on KLF5-3A-mediated proliferation (Fig. 8B). Furthermore, knocking down endogenous KLF5 using human KLF5-specific siRNA (which has little effect on mouse KLF5) significantly inhibited the proliferation of HCT116 cells (Fig. 8C). Re-introducing mouse KLF5 rescued the activity induced by KLF5 knockdown, suggesting that KLF5 is critical for HCT116 cell proliferation. As expected, expression of mouse KLF5-3A had a stronger effect on cell proliferation compared with the wild type. Thus, these data support an inhibitory role for Fbw7α-mediated degradation in the KLF5-stimulated anchorage-independent growth of cancer cells.
FIGURE 8.
Inhibition of KLF5-mediated survivin expression and cell proliferation by Fbw7-mediated degradation. A and B, Fbw7 inhibits KLF5-mediated cell proliferation. Empty vector, HA-KLF5, and HA-KLF5-3A were transfected into HCT116 cells with or without FLAG-Fbw7α. The number of colonies was counted 2 weeks later. C, HCT116 cells were transfected as indicated. Cell proliferation was examined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The mRNA level of KLF5 was detected by RT-PCR using primers suitable for both human and mouse KLF5 (mKLF5). Data are presented as means ± S.D. (Student's t test). *, p < 0.05; **, p < 0.01 (n = 3). siKLF, KLF5 siRNA; siCon, control siRNA. D, the abundance of survivin and platelet-derived growth factor-A (PDGFA) mRNA in Fbw7-deficient and parental cells was analyzed by RT-PCR. E, Fbw7α inhibits KLF5-mediated survivin promoter activity. The survivin promoter reporter gene was transfected with KLF5 and Fbw7α into HCT116 cells as indicated. Promoter activity was analyzed by Dual-Luciferase assay. RLU, relative luciferase units.
On the basis of our results thus far, we speculated that Fbw7 might inhibit the expression of KLF5-transactivated genes. We therefore measured by RT-PCR the expression levels of the known KLF5-targeted genes platelet-derived growth factor-A (45), survivin (46), and cyclin D1 (47) in wild-type or Fbw7-deficient HCT116 and DLD1 cancer cells. Survivin mRNA levels in particular were elevated significantly in both types of Fbw7-deficient cells (Fig. 8D). Subsequent luciferase reporter assays showed that the expression of KLF5 significantly enhanced the activity of the survivin essential promoter in HCT116 cells, whereas this effect was inhibited efficiently by coexpression of Fbw7α (Fig. 8E). Thus, Fbw7 appears to down-regulate the transcriptional activity of KLF5.
DISCUSSION
As an essential proproliferative factor, KLF5 can serve as an oncogene and promote the proliferation and survival of many cancer cells (3). KLF5 is an unstable protein that is negatively regulated by the ubiquitin-proteasome pathway in epithelial and cancer cells (20). A previous study showed that the WWP1 E3 ubiquitin ligase can bind to the KLF5 PY motif to ubiquitinate and degrade the factor (21). However, although WWP1 might have context-dependent tumor suppressor activity, other evidence is accumulating that the WWP1 gene is overexpressed in a wide variety of cancers and promotes cancer cell proliferation and survival (22, 23). These apparently contradictory data suggest that other effectors might be involved in the regulation of KLF5 ubiquitination and degradation. In this study, we have identified the tumor suppressor Fbw7 as a novel regulator of KLF5.
Fbw7 is dysregulated in many human malignancies (24). Deletion or mutations of Fbw7 are frequently found in a numerous cancers (e.g. breast, colon, and prostate) (24, 35). Although numerous Fbw7 substrates have been identified, the mechanism by which Fbw7 mediates tumor suppression remains unknown. Our data show that expression of Fbw7 significantly inhibits KLF5-mediated cell proliferation. Therefore, it is reasonable to speculate that Fbw7 deficiency might result in increased KLF5 protein levels and that up-regulated KLF5, together with other Fbw7 substrates such as mTOR (28), cyclin E (29), and c-Myc (26), promotes cancer cell proliferation and tumorigenesis. Indeed, the regulation of KLF5 by Fbw7 suggests a novel mechanism for oncogenesis resulting from the deletion or mutation of Fbw7.
Ubiquitin-proteasome-dependent protein degradation usually requires direct interaction between the substrate and E3 ubiquitin ligase (24). Fbw7 contains several conserved protein-protein interaction domains. Among them, a stretch of eight WD40 repeats makes multiple contacts with the CPDs in the Fbw7 substrate (24). Our data demonstrate that Fbw7 indeed binds to KLF5 through the interaction between the CPDs of KLF5 and the WD40 domain of Fbw7. Interestingly, any of the three CPDs found in KLF5 can function as a degron to target KLF5 for degradation, although their relative efficacies differ. Under physiological conditions, it is possible that all three CPDs are necessary for the regulation of KLF5 by Fbw7.
In this study, KLF4 and KLF5 were regulated differentially by Fbw7α in cells. Both KLF4 and KLF5 belong to the KLF transcription factor family with a close zinc finger domain and share similar functions under certain conditions, such as self-renewal of embryonic stem cells (48). However, numerous distinctive features have been identified in other cells (4). KLF4 is enriched in terminally differentiated epithelial cells toward the luminal surface, whereas KLF5 is present in highly proliferating epithelial cells lining the bases of the crypts in the intestinal tract (49). KLF4 usually inhibits cell proliferation, but KLF5 plays a role in promoting cell cycle regulation in colon cells (4). Although both factors are unstable proteins regulated by the ubiquitin-proteasome pathway (20, 50), Fbw7α appears to regulate their stability differently. Selective regulation of protein levels might be one mechanism by which the distinct functions of KLF4 and KLF5 are controlled in cells.
In this study, the protein kinase GSK3β phosphorylated and promoted the degradation of KLF5. Phosphorylation by GSK3 usually requires its substrates to be primed by protein kinases at position +4 (51). Priming by p38 promotes the GSK3-mediated phosphorylation of PGC-1α and consequently induces its degradation by Fbw7 (34). Several protein kinases, such as CK1 and Plk, can function as priming kinases for various GSK3 substrates (52, 53). Here, unprimed KLF5 is revealed as a novel substrate for GSK3β in vitro. Further studies must be performed to determine the ability of GSK3 to phosphorylate primed and unprimed KLF5 in vivo.
The exact mechanism by which GSK3 phosphorylates KLF5 also remains to be determined. Interestingly, among the three KLF5 CPDs, the +4 residue of CPD1 is Asp, which usually mimics phosphorylated Ser or Thr. This might explain the ability of GSK3β to phosphorylate purified KLF5 without being primed by other kinases. We also cannot exclude the possibility that other protein kinases phosphorylate and promote KLF5 degradation. Specifically, future work will be required to identify the priming kinases of CPD2 and CPD3.
In summary, we have identified the tumor suppressor Fbw7 as a novel E3 ubiquitin ligase of KLF5. Fbw7 binds and promotes the degradation and ubiquitination of KLF5 via its three CPDs, which are required for phosphorylation by GSK3β. Our data further demonstrate that Fbw7 is a negative regulator of KLF5-mediated transactivation and cell proliferation.
Acknowledgments
We thank Michele Pagano, Steven I. Reed, Kunliang Guan, Mukesh K. Jain, and Hitoshi Niwa for kindly providing plasmids used in this study and Dr. Jonathan Cherry for providing HCT116, HCT116 Fbw7−/−, DLD1, and DLD1 Fbw7−/− cells. We are grateful to Dr. Yuan Wang, Dianqing Wu, Xin Ge, and Ceshi Chen for critical comments on manuscript. We thank Xianling Zhu and other members of the Wang laboratory for assistance.
This work was supported in part by Grant 06DZ22923 from the Research Platform of Cell Signaling Networks of the Science and Technology Commission of Shanghai Municipality, Grants 30800587 and 30971521 from the National Natural Science Foundation of China, and 973 Program Grant 2010CB529704 from the National Basic Research Program of China.
- KLF
- Krüppel-like factor
- CPD
- CDC4 phosphodegron
- GSK3β
- glycogen synthase kinase-3β
- GST
- glutathione S-transferase
- HA
- hemagglutinin
- GFP
- green fluorescent protein
- IRES
- internal ribosome entry site
- EGFP
- enhanced green fluorescent protein
- PBS
- phosphate-buffered saline
- RT
- reverse transcription
- siRNA
- small interfering RNA
- FACS
- fluorescence-activated cell sorter.
REFERENCES
- 1.Bieker J. J. (2001) J. Biol. Chem. 276, 34355–34358 [DOI] [PubMed] [Google Scholar]
- 2.Black A. R., Black J. D., Azizkhan-Clifford J. (2001) J. Cell. Physiol. 188, 143–160 [DOI] [PubMed] [Google Scholar]
- 3.Dong J. T., Chen C. (2009) Cell. Mol. Life Sci. 66, 2691–2706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nandan M. O., Yang V. W. (2009) Histol. Histopathol. 24, 1343–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng H. Q., Zhou Z., Huang J., Chaudhury L., Dong J. T., Chen C. (2009) Oncogene 28, 3702–3713 [DOI] [PubMed] [Google Scholar]
- 6.McConnell B. B., Bialkowska A. B., Nandan M. O., Ghaleb A. M., Gordon F. J., Yang V. W. (2009) Cancer Res. 69, 4125–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C., Zhou Z., Sheehan C. E., Slodkowska E., Sheehan C. B., Boguniewicz A., Ross J. S. (2009) Int. J. Cancer 124, 2829–2836 [DOI] [PubMed] [Google Scholar]
- 8.Chen C., Benjamin M. S., Sun X., Otto K. B., Guo P., Dong X. Y., Bao Y., Zhou Z., Cheng X., Simons J. W., Dong J. T. (2006) Int. J. Cancer 118, 1346–1355 [DOI] [PubMed] [Google Scholar]
- 9.McConnell B. B., Klapproth J. M., Sasaki M., Nandan M. O., Yang V. W. (2008) Gastroenterology 134, 1007–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun R., Chen X., Yang V. W. (2001) J. Biol. Chem. 276, 6897–6900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong D., Czerwenka K., Heinze G., Ryffel M., Schuster E., Witt A., Leodolter S., Zeillinger R. (2006) Clin. Cancer Res. 12, 2442–2448 [DOI] [PubMed] [Google Scholar]
- 12.Bialkowska A. B., Du Y., Fu H., Yang V. W. (2009) Mol. Cancer Ther. 8, 563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen C., Bhalala H. V., Vessella R. L., Dong J. T. (2003) Prostate 55, 81–88 [DOI] [PubMed] [Google Scholar]
- 14.Bateman N. W., Tan D., Pestell R. G., Black J. D., Black A. R. (2004) J. Biol. Chem. 279, 12093–12101 [DOI] [PubMed] [Google Scholar]
- 15.Ziemer L. T., Pennica D., Levine A. J. (2001) Mol. Cell. Biol. 21, 562–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H., Bialkowska A., Rusovici R., Chanchevalap S., Shim H., Katz J. P., Yang V. W., Yun C. C. (2007) J. Biol. Chem. 282, 15541–15549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z., Teng C. T. (2003) Nucleic Acids Res. 31, 2196–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du J. X., Bialkowska A. B., McConnell B. B., Yang V. W. (2008) J. Biol. Chem. 283, 31991–32002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oishi Y., Manabe I., Tobe K., Ohsugi M., Kubota T., Fujiu K., Maemura K., Kubota N., Kadowaki T., Nagai R. (2008) Nat. Med. 14, 656–666 [DOI] [PubMed] [Google Scholar]
- 20.Chen C., Sun X., Ran Q., Wilkinson K. D., Murphy T. J., Simons J. W., Dong J. T. (2005) Oncogene 24, 3319–3327 [DOI] [PubMed] [Google Scholar]
- 21.Chen C., Sun X., Guo P., Dong X. Y., Sethi P., Cheng X., Zhou J., Ling J., Simons J. W., Lingrel J. B., Dong J. T. (2005) J. Biol. Chem. 280, 41553–41561 [DOI] [PubMed] [Google Scholar]
- 22.Chen C., Sun X., Guo P., Dong X. Y., Sethi P., Zhou W., Zhou Z., Petros J., Frierson H. F., Jr., Vessella R. L., Atfi A., Dong J. T. (2007) Oncogene 26, 2386–2394 [DOI] [PubMed] [Google Scholar]
- 23.Chen C., Zhou Z., Ross J. S., Zhou W., Dong J. T. (2007) Int. J. Cancer 121, 80–87 [DOI] [PubMed] [Google Scholar]
- 24.Welcker M., Clurman B. E. (2008) Nat. Rev. Cancer 8, 83–93 [DOI] [PubMed] [Google Scholar]
- 25.Kitagawa K., Hiramatsu Y., Uchida C., Isobe T., Hattori T., Oda T., Shibata K., Nakamura S., Kikuchi A., Kitagawa M. (2009) Oncogene 28, 2393–2405 [DOI] [PubMed] [Google Scholar]
- 26.Yada M., Hatakeyama S., Kamura T., Nishiyama M., Tsunematsu R., Imaki H., Ishida N., Okumura F., Nakayama K., Nakayama K. I. (2004) EMBO J. 23, 2116–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welcker M., Orian A., Jin J., Grim J. E., Grim J. A., Harper J. W., Eisenman R. N., Clurman B. E. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 9085–9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao J. H., Kim I. J., Wu D., Climent J., Kang H. C., DelRosario R., Balmain A. (2008) Science 321, 1499–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koepp D. M., Schaefer L. K., Ye X., Keyomarsi K., Chu C., Harper J. W., Elledge S. J. (2001) Science 294, 173–177 [DOI] [PubMed] [Google Scholar]
- 30.Nateri A. S., Riera-Sans L., Da Costa C., Behrens A. (2004) Science 303, 1374–1378 [DOI] [PubMed] [Google Scholar]
- 31.O'Neil J., Grim J., Strack P., Rao S., Tibbitts D., Winter C., Hardwick J., Welcker M., Meijerink J. P., Pieters R., Draetta G., Sears R., Clurman B. E., Look A. T. (2007) J. Exp. Med. 204, 1813–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao B., Oehlmann S., Sowa M. E., Harper J. W., Pavletich N. P. (2007) Mol. Cell 26, 131–143 [DOI] [PubMed] [Google Scholar]
- 33.Orlicky S., Tang X., Willems A., Tyers M., Sicheri F. (2003) Cell 112, 243–256 [DOI] [PubMed] [Google Scholar]
- 34.Olson B. L., Hock M. B., Ekholm-Reed S., Wohlschlegel J. A., Dev K. K., Kralli A., Reed S. I. (2008) Genes Dev. 22, 252–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akhoondi S., Sun D., von der Lehr N., Apostolidou S., Klotz K., Maljukova A., Cepeda D., Fiegl H., Dafou D., Marth C., Mueller-Holzner E., Corcoran M., Dagnell M., Nejad S. Z., Nayer B. N., Zali M. R., Hansson J., Egyhazi S., Petersson F., Sangfelt P., Nordgren H., Grander D., Reed S. I., Widschwendter M., Sangfelt O., Spruck C. (2007) Cancer Res. 67, 9006–9012 [DOI] [PubMed] [Google Scholar]
- 36.Mao J. H., Perez-Losada J., Wu D., Delrosario R., Tsunematsu R., Nakayama K. I., Brown K., Bryson S., Balmain A. (2004) Nature 432, 775–779 [DOI] [PubMed] [Google Scholar]
- 37.Rajagopalan H., Lengauer C. (2004) Cell Cycle 3, 693–694 [DOI] [PubMed] [Google Scholar]
- 38.Wang P., Gao H., Ni Y., Wang B., Wu Y., Ji L., Qin L., Ma L., Pei G. (2003) J. Biol. Chem. 278, 6363–6370 [DOI] [PubMed] [Google Scholar]
- 39.Grim J. E., Gustafson M. P., Hirata R. K., Hagar A. C., Swanger J., Welcker M., Hwang H. C., Ericsson J., Russell D. W., Clurman B. E. (2008) J. Cell Biol. 181, 913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welcker M., Orian A., Grim J. E., Grim J. A., Eisenman R. N., Clurman B. E. (2004) Curr. Biol. 14, 1852–1857 [DOI] [PubMed] [Google Scholar]
- 41.Yen H. C., Elledge S. J. (2008) Science 322, 923–929 [DOI] [PubMed] [Google Scholar]
- 42.Welcker M., Clurman B. E. (2007) Cell Div. 2, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajagopalan H., Jallepalli P. V., Rago C., Velculescu V. E., Kinzler K. W., Vogelstein B., Lengauer C. (2004) Nature 428, 77–81 [DOI] [PubMed] [Google Scholar]
- 44.Sundqvist A., Bengoechea-Alonso M. T., Ye X., Lukiyanchuk V., Jin J., Harper J. W., Ericsson J. (2005) Cell Metab. 1, 379–391 [DOI] [PubMed] [Google Scholar]
- 45.Usui S., Sugimoto N., Takuwa N., Sakagami S., Takata S., Kaneko S., Takuwa Y. (2004) J. Biol. Chem. 279, 12300–12311 [DOI] [PubMed] [Google Scholar]
- 46.Zhu N., Gu L., Findley H. W., Chen C., Dong J. T., Yang L., Zhou M. (2006) J. Biol. Chem. 281, 14711–14718 [DOI] [PubMed] [Google Scholar]
- 47.Suzuki T., Sawaki D., Aizawa K., Munemasa Y., Matsumura T., Ishida J., Nagai R. (2009) J. Biol. Chem. 284, 9549–9557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang J., Chan Y. S., Loh Y. H., Cai J., Tong G. Q., Lim C. A., Robson P., Zhong S., Ng H. H. (2008) Nat. Cell Biol. 10, 353–360 [DOI] [PubMed] [Google Scholar]
- 49.McConnell B. B., Ghaleb A. M., Nandan M. O., Yang V. W. (2007) BioEssays 29, 549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z. Y., Wang X., Zhou Y., Offner G., Tseng C. C. (2005) Cancer Res. 65, 10394–10400 [DOI] [PubMed] [Google Scholar]
- 51.Wu D., Pan W. (2010) Trends Biochem. Sci. 35, 161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X., Liu J., Gao T. (2009) Mol. Cell. Biol. 29, 6192–6205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang T., Wei Y., Honaker Y., Yamaguchi H., Appella E., Hung M. C., Piwnica-Worms H. (2008) Cancer Cell 13, 36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]