Abstract
It is assumed that an effective human immunodeficiency virus type 1 (HIV-1) vaccine should be capable of eliciting neutralizing antibodies. However, even the best antibodies known to date lack neutralizing ability against a significant proportion of primary HIV-1 variants and, despite great efforts, still no immunogen is available that can elicit humoral immunity which is protective against infection or disease progression. We tested sera from 35 participants in the Amsterdam Cohort Studies on HIV-1 infection, who were all infected with HIV-1 subtype B and therapy-naïve at the time of sampling, for neutralizing activity against a panel of 23 tier 2–3 HIV-1 variants, with a minimum of five HIV-1 variants per subtype (A, B, C and D). Strong cross-clade neutralizing activity was detected in sera from seven individuals. Strikingly, sera from 22 of 35 individuals (63 %) neutralized three or more of the six tier 2–3 HIV-1 subtype B viruses in the panel. There was a strong correlation between neutralization titre and breadth in serum. Indeed, the IC50 of sera with strong cross-clade neutralizing activity was significantly higher than the IC50 of sera with cross-subtype B activity, which, in turn, had a higher IC50 than sera with the lowest neutralization breadth. These results imply that humoral immunity, at least in HIV-1 subtype B-infected individuals, is often subtype-specific rather than strain-specific and that the breadth of neutralization is correlated with the titre of neutralizing activity in serum. Considering the difficulties in designing a vaccine that is capable of eliciting cross-clade neutralizing activity, subtype-specific vaccines may be explored as an interesting alternative.
INTRODUCTION
Neutralizing antibodies (NAbs) are believed to be crucial for immunity against virus infections and are therefore considered an essential component of a human immunodeficiency virus type 1 (HIV-1) vaccine-elicited immune response (Walker & Burton, 2008). The development of an immunogen that is capable of eliciting NAbs is, however, challenged by the inaccessibility of conserved epitopes and the enormous sequence diversity of the viral envelope (McCutchan, 2000), which is the main target for NAbs. Indeed, the error-prone reverse transcriptase, the lack of proofreading and the extremely rapid virus-turnover rate are responsible for huge sequence variation, which can be as high as 10 % already within the virus quasispecies in a single individual (Gaschen et al., 2002; Malim & Emerman, 2001; Shankarappa et al., 1999). This high diversity has led to a classification of HIV-1 variants into distinct clades or subtypes, which are defined as groups of viruses that resemble each other more closely than viruses from other subtypes. The main (M) group is subdivided into subtypes A–K and different recombinant forms, which have different geographical distributions: subtype B, for instance, predominates in Europe, the Americas and Australia, whereas subtype C predominates in sub-Saharan Africa (Stebbing & Moyle, 2003). The viral envelope currently differs by up to 35 % between subtypes and up to 20 % within subtypes (Gaschen et al., 2002; Hemelaar et al., 2006; Taylor et al., 2008). The enormity of this challenge can be put into perspective by comparison with the influenza vaccine, where a diversity of <2 % in amino acid changes can already cause failure in the cross-reactivity of the polyclonal response elicited by the vaccine (Gaschen et al., 2002). It may therefore be put into question whether a single vaccine capable of eliciting NAbs against all HIV-1 variants is feasible.
In addition to the high sequence diversity, the humoral immune response is thwarted by the inaccessibility of the relevant (conserved) epitopes. The inaccessibility of relevant epitopes on the HIV-1 envelope is due to a high level of glycosylation, occlusion within the oligomeric structure of the viral envelope and the fact that their formation occurs only after engagement of the viral envelope with CD4, when spatial constraints do not allow binding of relatively large immunoglobulins (Labrijn et al., 2003). Despite viral mechanisms for evading humoral immunity, HIV-1 does elicit NAbs in the natural course of infection. These, however, are considered to be mainly strain-specific, so are only capable of neutralizing autologous virus variants (Moog et al., 1997) and their epitopes are therefore considered irrelevant for vaccine design.
Broadly neutralizing antibodies (BrNAbs) may bypass viral defence mechanisms, as they have the ability to neutralize HIV-1 variants from different subtypes (Binley et al., 2004). Four well-known BrNAbs, b12, 2G12, 2F5 and 4E10, have been isolated from HIV-1-infected individuals. One of the current vaccine strategies is to design an immunogen that mimics the epitopes of these BrNAbs (Burton et al., 2004). However, an effective vaccine would require additional epitope specificities, as a significant proportion (approx. 15 %) of primary subtype A, B, C, D and CRF01-AE is resistant to neutralization by all four BrNAbs mentioned above (Binley et al., 2004; Gray et al., 2006; McKnight & Aasa-Chapman, 2007; Quakkelaar et al., 2007; Richman et al., 2003). The high sequence diversity between HIV-1 variants may underlie the incomplete coverage by BrNAbs. In that light, vaccine-elicited subtype-specific NAbs may be the best alternative to BrNAbs. However, the existence of HIV-1 neutralization serotypes has been questioned (McKnight & Aasa-Chapman, 2007; Moore et al., 2001).
Here, we studied the breadth of serum neutralizing activity in 35 HIV-1 subtype B-infected individuals. We found that sera from seven individuals had highly cross-clade neutralizing activity and that the majority of sera neutralized multiple unrelated subtype B HIV-1 variants, providing evidence for an HIV-1 subtype B neutralization serotype.
RESULTS
Prevalence of strong cross-clade HIV-1-specific neutralizing activity in patient sera
We studied sera from 35 participants in the Amsterdam Cohort Studies for the breadth and titre of HIV-1-specific neutralizing activity. Serum samples were obtained between 24 and 33 months after the estimated day of seroconversion and all participants were therapy-naïve at this point. HIV-1-specific NAb activity was measured in a cell-based infectivity assay using recombinant viruses that carried a luciferase reporter gene and that were pseudotyped with envelope proteins from tier 2–3 HIV-1 subtype A, B, C and D. For comparison, five HIV-1 subtype B reference strains were additionally tested. To monitor neutralizing activity not mediated by antibodies directed against HIV-1 Env-specific antibodies, each plasma sample was also tested against a recombinant virus stock that was pseudotyped with amphotropic murine leukemia virus (aMLV) envelope proteins (gp70SU and p15TM). Typically, neutralization titres, expressed as the reciprocal dilution of plasma that established 50 % inhibition (IC50) of virus infection, were <40 for aMLV controls. No differences in neutralizing activity were observed between sera from long-term non-progressors (LTNPs) and progressors (cross-clade neutralizing activity in three of 20 LTNPs and four of 15 progressors; van Gils et al., 2010). In all sera, neutralizing activity against the reference strains was observed (Tables 1–3).
Table 1.
Breadth and titre of HIV-1-specific neutralizing activity in sera from patients with strong cross-clade neutralizing activity
Patient IDs in italics are LTNPs. Titres <40 are indicated by −. Titres less than three times higher than the negative control are in parentheses. na, Not applicable; nd, not determined.
| Subtype/virus | Virus type | Origin | Patients with strong cross-clade neutralizing activity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 19956 | 19298 | 19554 | 19708 | 19642 | 18969 | 19829 | |||
| Tier 2–3 panel | |||||||||
| A | |||||||||
| MB_pA1 | Primary | Uganda | 72 | 174 | 100 | 284 | 130 | 112 | 94 |
| MB_pA2 | Primary | Uganda | 42 | 129 | − | 210 | 51 | − | 133 |
| MB_pA3 | Primary | Uganda | 88 | 245 | 215 | 520 | 203 | 671 | 309 |
| 94UG103 | AIDS Repository | Uganda | 88 | 254 | 373 | 423 | 258 | 106 | − |
| 92RW020 | AIDS Repository | Rwanda | 201 | 200 | 300 | 569 | 252 | 1118 | 935 |
| B | |||||||||
| APV-16 | Primary | USA | 124 | 307 | 262 | 462 | 188 | 98 | 115 |
| APV-20 | Primary | USA | 427 | 341 | 236 | 273 | 215 | 121 | 59 |
| APV-9 | Primary | USA | 123 | 122 | 148 | 193 | 170 | 67 | 65 |
| 92BR020 | AIDS Repository | Brazil | 320 | 363 | 576 | (63) | 377 | 551 | 431 |
| MB_pB1 | Primary | USA | 146 | 182 | 343 | 136 | 173 | − | 65 |
| MB_pB2 | Primary | USA | 314 | 594 | 990 | 249 | 391 | 285 | 172 |
| C | |||||||||
| MB_pC1 | Primary | Europe | 113 | 207 | 163 | (49) | 110 | − | 73 |
| 93IN905 | AIDS Repository | India | 225 | − | 67 | 114 | 85 | 182 | 48 |
| IAVI_C22 | AIDS Repository | Africa | 42 | 60 | 72 | 72 | (41) | − | − |
| MBC6 | Primary | Africa | 79 | 308 | 136 | 170 | 104 | 181 | 106 |
| MBC3 | Expanded in PBMC | Zimbabwe | 149 | 499 | 372 | 292 | 214 | 892 | 1257 |
| 94IN11246-3 | AIDS Repository | India | 112 | 715 | 241 | 147 | 268 | 1335 | 1707 |
| 93MW960 | AIDS Repository | Malawi | 186 | 315 | 187 | 357 | 207 | 515 | 573 |
| D | |||||||||
| MB_pD1 | Primary | Uganda | 200 | 694 | 189 | 172 | 187 | − | − |
| MB_pD2 | Primary | Uganda | 103 | 365 | 209 | 153 | 100 | 73 | − |
| MB_pD3 | Primary | Uganda | 77 | 95 | 88 | 88 | − | 95 | − |
| 92UG001 | AIDS Repository | Uganda | 187 | 232 | 97 | 165 | 161 | − | − |
| 93UG070 | AIDS Repository | Uganda | 195 | 271 | 204 | 209 | 121 | − | − |
| Reference | |||||||||
| B | |||||||||
| 1196 | Reference strain | USA | 512 | nd | nd | 285 | 491 | 919 | 956 |
| BaL | Reference strain | USA | 1247 | 1754 | nd | 2792 | 898 | 514 | 751 |
| JRCSF | Reference strain | USA | 126 | 348 | 1096 | 708 | 261 | 142 | 67 |
| NL4-3 | Reference strain | USA | 2409 | 1202 | 5239 | 1256 | 2771 | 1153 | 1166 |
| SF162 | Reference strain | USA | 13936 | nd | nd | nd | 5343 | 4784 | 4075 |
| Negative control | |||||||||
| aMLV | Murine leukemia | na | − | − | − | − | − | − | − |
Table 3.
Breadth and titre of HIV-1-specific neutralizing activity in sera from patients with absent cross-reactive neutralizing activity
Patient IDs in italics are LTNPs. Titres <40 are indicated by −. Titres less than three times higher than the negative control are in parentheses. nd, Not determined. For virus type and origin, see Table 1.
| Subtype/virus | Patients with absent cross-reactive neutralizing activity | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19992 | 19943 | 19933 | 18789 | 19974 | 19984 | 19874 | 19659 | 19291 | 19951 | 18880 | 19552 | 19406 | |
| Tier 2–3 panel | |||||||||||||
| A | |||||||||||||
| MB_pA1 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MB_pA2 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MB_pA3 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 94UG103 | − | (42) | − | − | − | − | − | − | − | − | − | − | − |
| 92RW020 | 74 | − | 119 | 42 | − | − | − | − | − | − | − | − | − |
| B | |||||||||||||
| APV-16 | (46) | (40) | 42 | − | (47) | − | − | − | 41 | 57 | − | − | − |
| APV-20 | 60 | (55) | − | 83 | 60 | (54) | 49 | 43 | − | − | 41 | − | − |
| APV-9 | − | − | − | 71 | − | − | − | − | − | − | − | − | − |
| 92BR020 | 123 | 46 | − | − | 91 | 76 | 96 | 54 | 41 | − | − | − | − |
| MB_pB1 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MB_pB2 | − | 132 | − | − | − | − | − | − | − | − | − | − | − |
| C | |||||||||||||
| MB_pC1 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 93IN905 | 68 | − | − | − | − | − | − | − | − | − | − | − | − |
| IAVI_C22 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MBC6 | − | − | 92 | − | − | − | − | − | − | − | − | − | − |
| MBC3 | 60 | − | − | − | − | 55 | − | − | − | − | − | − | − |
| 94IN11246-3 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 93MW960 | − | (49) | − | − | (48) | − | − | − | − | − | − | − | − |
| D | |||||||||||||
| MB_pD1 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MB_pD2 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MB_pD3 | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 92UG001 | − | 106 | − | − | − | − | − | − | − | − | − | − | − |
| 93UG070 | − | 74 | − | − | − | − | − | − | − | − | − | − | − |
| Reference | |||||||||||||
| B | |||||||||||||
| 1196 | 161 | 73 | − | 76 | 150 | 218 | 124 | 98 | 47 | 100 | 44 | 48 | 58 |
| BaL | 309 | 93 | − | 47 | 237 | 216 | 256 | 218 | 71 | 156 | 45 | 54 | − |
| JRCSF | (54) | − | − | − | (47) | 228 | − | − | − | − | − | − | − |
| NL4-3 | 777 | 522 | 44 | 141 | 916 | 958 | 360 | 608 | 177 | 598 | 409 | 187 | 121 |
| SF162 | 2713 | 2212 | 264 | 647 | 3414 | 1530 | 4353 | 1900 | 1067 | 2385 | nd | 497 | 949 |
| Negative control | |||||||||||||
| aMLV | − | − | − | − | − | − | − | − | − | − | − | − | − |
Strong HIV-specific cross-clade neutralizing activity, defined as an IC50≥100 for at least 50 % of the tier 2–3 viruses from at least three different subtypes (so excluding the reference strains), was observed in sera from seven of 35 individuals (20 %) (Table 1). Interestingly, sera from three of these individuals neutralized >80 % of all tier 2–3 viruses in the panel with an IC50≥100 (Table 1; patients 19298, 19642 and 19708).
Prevalence of sera with cross-reactive neutralizing activity against multiple HIV-1 subtype B variants, but less neutralizing activity to viruses from other subtypes
The sera from seven individuals with strong cross-clade neutralizing activity also neutralized five or six of the six tier 2–3 subtype B HIV-1 variants in the panel. Sera from the other 28 of 35 HIV-1 subtype B-infected individuals studied here lacked strong cross-clade neutralizing activity against HIV-1 variants from multiple subtypes, according to the definition described above. Interestingly, while sequence diversity between the envelope genes of the tier 2–3 HIV-1 subtype B variants in the panel, so again excluding the reference strains, varied by up to 12 %, and while phylogenetic analysis did not reveal clustering of the viruses from this panel with autologous viruses of the different patients studied here (data not shown), sera from 26 of these 28 patients (93 %) who lacked strong cross-clade neutralizing activity showed neutralizing activity against at least one of the six unrelated tier 2–3 HIV-1 subtype B variants in the panel (Tables 2 and 3). Strikingly, sera from 15 of these 28 patients (54 %) neutralized three or more of the six unrelated tier 2–3 HIV-1 subtype B variants in the panel (Table 2). Interestingly, four of these patients (19250, 19559, 19663 and 19768) and also two patients with strong cross-clade neutralizing activity (18969 and 19829) showed the same breadth of neutralization against subtype B and subtype C viruses, with even higher neutralizing titres against the subtype C variants than against the subtype B variants.
Table 2.
Breadth and titre of HIV-1-specific neutralizing activity in sera from patients with cross-reactive but mainly subtype B-specific neutralizing activity
Patient IDs in italics are LTNPs. Titres <40 are indicated by −. Titres less than three times higher than the negative control are in parentheses. For virus type and origin, see Table 1.
| Subtype/virus | Patients with cross-reactive but mainly subtype B-specific neutralizing activity | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19250 | 19559 | 19663 | 19768 | 19542 | 18971 | 19999 | 19383 | 18829 | 19335 | 19789 | 19843 | 19417 | 19334 | 19342 | |
| Tier 2–3 panel | |||||||||||||||
| A | |||||||||||||||
| MB_pA1 | 46 | 61 | − | 78 | 47 | 49 | 63 | − | − | − | − | − | − | − | − |
| MB_pA2 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MB_pA3 | 166 | 142 | 126 | 111 | − | 124 | 335 | − | − | − | − | 40 | − | − | − |
| 94UG103 | − | − | − | − | − | 40 | − | − | − | − | − | − | − | − | − |
| 92RW020 | 272 | 143 | 441 | 187 | 58 | 86 | 83 | − | − | − | − | 54 | 44 | − | − |
| B | |||||||||||||||
| APV-16 | 82 | 69 | 116 | 59 | 44 | 47 | 68 | 58 | − | 46 | 61 | 49 | 46 | 40 | 51 |
| APV-20 | 89 | 94 | 105 | − | 96 | 56 | 84 | 53 | 67 | 106 | 54 | 50 | 54 | 88 | 53 |
| APV-9 | 41 | − | 53 | − | 258 | 42 | − | − | 67 | − | − | − | − | − | − |
| 92BR020 | 290 | 220 | 412 | 77 | 62 | 96 | 52 | 77 | 80 | 95 | 79 | 93 | 64 | 81 | 62 |
| MB_pB1 | 49 | 93 | 71 | 78 | − | − | − | 41 | − | − | 41 | − | − | − | − |
| MB_pB2 | 97 | 108 | 106 | 200 | 81 | 82 | − | 80 | 85 | 64 | − | − | 56 | − | − |
| C | |||||||||||||||
| MB_pC1 | 40 | 45 | − | 44 | 71 | − | − | − | − | − | − | − | − | − | − |
| 93IN905 | 149 | 195 | 221 | − | 56 | − | − | − | − | − | − | − | − | − | − |
| IAVI_C22 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MBC6 | 197 | 74 | 59 | 115 | − | − | − | − | − | − | − | − | − | − | − |
| MBC3 | 511 | 290 | 677 | 255 | 141 | (55) | − | − | 80 | − | − | − | − | 65 | 51 |
| 94IN11246-3 | 380 | 340 | 607 | 64 | − | − | − | − | − | − | − | − | − | − | − |
| 93MW960 | 207 | 273 | 267 | 107 | − | 77 | 76 | 47 | − | 48 | 49 | − | − | − | − |
| D | |||||||||||||||
| MB_pD1 | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| MB_pD2 | 146 | 87 | − | − | 54 | − | − | − | − | − | − | − | − | − | − |
| MB_pD3 | − | − | − | − | − | − | 41 | − | − | − | − | − | − | − | − |
| 92UG001 | 153 | 53 | 45 | − | − | − | − | − | − | − | − | − | − | − | − |
| 93UG070 | 189 | 65 | − | 43 | − | − | − | − | − | − | − | − | − | − | − |
| Reference | |||||||||||||||
| B | |||||||||||||||
| 1196 | 546 | 270 | 474 | 135 | 256 | 218 | 284 | 102 | 148 | 175 | 179 | 70 | 164 | 91 | 133 |
| BaL | 820 | 328 | 815 | 358 | 297 | 284 | 104 | 219 | 385 | 243 | 157 | 123 | 478 | 100 | 260 |
| JRCSF | − | 85 | 128 | − | − | 57 | 72 | − | − | − | − | 83 | 74 | − | − |
| NL4-3 | 2654 | 936 | 849 | 338 | 727 | 563 | 371 | 1645 | 1909 | 936 | 668 | 480 | 948 | 676 | 1312 |
| SF162 | 11257 | 2307 | 3624 | 1736 | 4016 | 2781 | 1157 | 3770 | 4019 | 3732 | 2317 | 1497 | 3209 | 1711 | 3066 |
| Negative control | |||||||||||||||
| aMLV | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
The breadth of neutralizing activity against viruses from the other three subtypes was significantly lower, in agreement with the fact that these sera did not have strong cross-clade neutralizing activity. These data show that, apart from the seven sera with strong cross-clade neutralizing activity, the majority of sera had neutralizing activity against multiple and diverse subtype B HIV-1 variants. Indeed, of the total of 35 individuals, 22 (63 %) had neutralizing activity against at least three of the tier 2–3 subtype B viruses in the panel.
Correlation between titre and breadth of HIV-1-specific neutralizing activity in serum
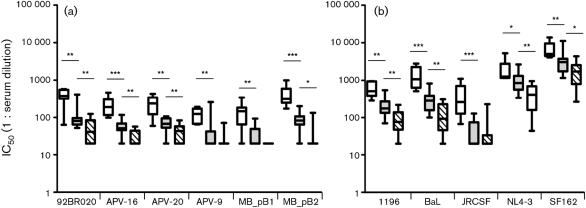
Characteristics of heterologous HIV-1-specific neutralizing serum reactivity are not known in great detail. Here, we observed a strong correlation between the titre of neutralizing activity and the number of different viruses that were neutralized by a serum (Fig. 1). Indeed, for neutralization of each individual virus in the panel of tier 2–3 HIV-1 subtype B viruses, the mean IC50 values were significantly higher for sera that had strong cross-reactive neutralizing activity against viruses from different subtypes (Fig. 1a, empty bars) compared with sera with cross-reactive neutralizing activity against multiple subtype B variants, but not against viruses from other subtypes (Fig. 1a, grey-shaded bars). Additionally, sera from the latter group had, in turn, a significantly higher mean neutralizing titre against four of the six tier 2–3 subtype B HIV-1 variants in the panel (92BR020, APV-16, APV-20 and MB_pB1) compared with the mean neutralizing titres in the 13 patient sera that neutralized up to two of the HIV-1 subtype B viruses in the panel (Fig. 1a, diagonally hatched bars).
Fig. 1.
Correlation between titre and breadth of HIV-1-specific neutralizing humoral immunity in sera of HIV-1-infected individuals. Mean neutralizing titre of sera in defined groups, according to their ability to neutralize the tier 2–3 viruses from the panel, against (a) six unrelated tier 2–3 subtype B HIV-1 variants and (b) five subtype B reference strains. Patient sera were grouped based on neutralizing activity against the tier 2–3 viruses, excluding neutralizing activity against the reference strains: strong cross-clade neutralizing activity (Table 1; ≥50 % of viruses per subtype with IC50≥100 for at least three subtypes, n=7), cross-reactive neutralizing activity against multiple subtype B variants but minimal neutralizing activity against other subtypes (Table 2; ≥50 % of subtype B viruses neutralized, n=15) or absent cross-reactive neutralizing activity, (Table 3; n=13). Serum neutralizing titres required for 50 % inhibition of the tier 2–3 HIV-1 subtype B virus variants in the panel were calculated. Empty bars represent sera with strong cross-clade neutralizing activity (n=7); grey-shaded bars represent sera with cross-reactive neutralizing activity against multiple subtype B variants but minimal reactivity against viruses from other subtypes (n=15); diagonally hatched bars represent sera that lack cross-reactive neutralizing activity (n=13). Neutralizing titres are expressed as the reciprocal of the plasma dilution that inhibited virus infection by 50 %. Significant differences between the three groups are indicated: *P<0.05; **P<0.01; ***P<0.001 (Mann–Whitney U test).
The mean neutralizing titres in the patient sera studied here were higher for some of the reference viruses that were used in this study (1196, BaL, JRCSF, NL4-3 and SF162; Fig. 1b), in agreement with the generally higher neutralization sensitivity of these viruses. Interestingly, also for these reference strains, we observed the same pattern between neutralization breadth and titre. Indeed, the mean neutralizing titre of the seven sera with strong cross-clade neutralizing activity (Fig. 1b, empty bars) was significantly higher for each individual reference virus than the mean neutralizing titre in the 15 sera with subtype B-specific cross-reactive neutralizing activity (Fig. 1b, grey-shaded bars), while the mean neutralizing titres in these sera were again higher than the mean neutralizing titre in the 13 sera that lacked cross-reactivity (Fig. 1b, diagonally hatched bars). For JRCSF, a tier 2 reference strain with a known neutralization-resistant phenotype (Moore et al., 1995), the mean neutralizing titre in the 15 sera with cross-subtype B activity was similar to the mean titre in the 13 sera that lacked cross-reactive neutralizing activity (Fig. 1b).
The neutralizing titres against viruses of subtypes A, C and D also showed a correlation with the neutralization breadth against these viruses, albeit that the differences in titres between groups of sera with strong cross-clade neutralizing activity, cross-subtype B neutralizing activity or almost absent neutralizing activity were less strong (data not shown).
DISCUSSION
All vaccines that provide protection against virus infections elicit at least a potent humoral immune response (Pantaleo & Koup, 2004). In line, HIV-1 vaccine research is aiming for an immunogen in which epitopes for BrNAbs are present (Burton et al., 2004). This is a challenging task, as the HIV-1 envelope has evolved towards a structure in which the relevant epitopes are absent in the native protein, occluded in the oligomeric structure and/or covered by N-linked glycosylation sites. In addition, the HIV-1 envelope gene is highly variable. This variation, which can be up to 35 % between different subtypes (Gaschen et al., 2002; Hemelaar et al., 2006; Taylor et al., 2008), makes it unlikely that a single vaccine will be capable of eliciting a humoral immune response that would cover protection against all possible variants. Indeed, even the best BrNAbs known to date do not neutralize all of the circulating HIV-1 variants (Binley et al., 2004; Gray et al., 2006; McKnight & Aasa-Chapman, 2007; Quakkelaar et al., 2007; Richman et al., 2003). Most HIV-1-infected individuals mount an HIV-1-specific humoral immune response, but these antibodies are considered strain-specific, as neutralizing activity is assumed to be limited to the autologous virus strain. Indeed, the majority of HIV-1-infected individuals do not develop cross-clade neutralizing activity that is capable of neutralizing HIV-1 variants from different subtypes (Li et al., 2007; Piantadosi et al., 2009; Sather et al., 2009). However, cross-reactive neutralization of different HIV-1 variants of the same subtype has received only little attention.
The findings of our present study suggest that subtype-specific differences in HIV-1 neutralization may exist, similar to what is known for influenza virus (Karlsson Hedestam et al., 2008; Webster et al., 1992). Overall, we observed that sera from HIV-1 subtype B-infected individuals had stronger neutralizing activity against multiple unrelated HIV-1 subtype B variants with substantial sequence diversity in their envelopes than against HIV-1 variants from subtypes A, C and D. However, sera from four patients with neutralizing activity against multiple subtype B variants and from two patients with strong cross-clade neutralizing activity had higher neutralizing titres against the subtype C variants in our panel than against the variants from the other subtypes, including subtype B. This may suggest that at least some of the epitopes on the envelope of subtype B variants that elicited cross-clade neutralizing activity may be even better exposed on subtype C variants.
Obviously, it remains to be established whether this observation also holds true for sera from individuals infected with other HIV-1 subtypes. Other studies have not provided evidence for HIV-1 subtype-specific differences in HIV-1-neutralizing activity in serum (Kostrikis et al., 1996; Moore et al., 2001). However, these studies were performed with only a limited number of HIV-1 variants and sometimes with a pool of patient sera in which different neutralizing epitope specificities may have been mixed. Moreover, these studies focused strongly on BrNAbs that, by definition, neutralize HIV-1 variants from different subtypes. Although not emphasized specifically by the authors, some previous reports do include data showing that neutralizing activity in patient sera was stronger against viruses that were from the same subtype as the autologous virus (Binley et al., 2004; Brown et al., 2008; Simek et al., 2009).
The exact nature of the epitopes at which cross-clade neutralizing activity and subtype-specific cross-reactive neutralizing activity is directed remains to be established. It was reported recently that cross-clade neutralizing activity is not directed only against the conserved regions of the envelope, such as the CD4-binding site (Dhillon et al., 2007; Doria-Rose et al., 2009; Guan et al., 2009; Li et al., 2007; Sather et al., 2009; Scheid et al., 2009) or the V3 loop (Li et al., 2009). It is likely that epitopes that are less well-conserved between subtypes, but conserved within a subtype, are capable of eliciting subtype-specific cross-reactive neutralizing activity. Alternatively, the neutralizing activity is mediated by antibodies directed against the V3 loop, similar to the HIV-1 subtype B-specific neutralizing activity of the well-characterized monoclonal antibody 446-52D. This NAb recognizes a GPxR motif that is very well-conserved in the V3 loop of subtype B HIV-1 variants (Conley et al., 1994).
The observation that subtype-specific neutralizing activity in serum may exist can provide a new lead in HIV-1 vaccine development. Indeed, the high sequence diversity between HIV-1 variants of different subtypes may stand in the way of the development of a single vaccine capable of eliciting neutralizing humoral immunity against all circulating HIV-1 variants. Obviously, this approach may be considered once a successful protein vaccine has been developed, which is a major challenge in itself.
Interestingly, we observed relatively strong cross-reactive neutralizing activity against multiple subtype B variants in sera from 63 % of subtype B-infected individuals studied here, suggesting that the epitopes that have elicited these humoral responses are present and accessible on natural HIV-1 variants. Although HIV-1 may escape rapidly from this antibody pressure (Bunnik et al., 2008), escape may be prevented if a vaccine elicits sterilizing immunity that is capable of preventing virus replication completely.
We have also observed that the ability of serum to neutralize different viruses is related directly to the neutralization titre in serum (modelled in Fig. 2). Although this finding does not exclude that highly potent antibody specificities may exist at an average concentration in serum, as was reported recently for two novel cross-clade NAbs, PG9 and PG16 (Walker et al., 2009), it may imply that sera with highly cross-clade neutralizing ability in general harbour multiple epitope specificities or that a high quantity of a single antibody specificity is more potent, even against unrelated HIV-1 variants. This observation indicates that, in general, optimal boosting during vaccination to increase the antibody titre elicited by a future vaccine may also increase the breadth of the neutralizing activity significantly.
Fig. 2.
Correlation between neutralizing breadth and titre in serum. On the x-axis, an increasing neutralization titre is suggested; on the y-axis, an increasing breadth of the response in three categories is depicted. The line represents the association between titre and breadth; increasing depth of shading in the background shows increasing potency of neutralizing activity.
In conclusion, we have found evidence for subtype-specific neutralizing activity and a positive correlation between the titre and breadth of neutralizing activity in patient sera. The design of improved adjuvants that can optimize humoral immune responses, in combination with potentially subtype-specific epitopes, may thus provide new leads on the way to a potent HIV-1 vaccine. Developing and administering multiple HIV vaccines is far less ideal than having a single vaccine that would cover all circulating HIV variants. However, design and delivery of a single vaccine that is capable of eliciting potent and cross-clade neutralizing immunity against HIV-1 have not yet been successful. Although we realize that any vaccine approach will probably have to deal with the complexity of the HIV-1 envelope molecule and the difficulty to mimic it as an immunogen, based on our data we suggest that the approach of subtype-specific vaccines may be worthwhile to consider in current strategies.
METHODS
Patients.
The study group consisted of LTNPs (defined as HIV-1-infected individuals who have ≥10 years of asymptomatic follow-up with stable CD4+ cell counts that were still >400 cells μl−1 in the ninth year of follow-up) and progressors [HIV-1-infected individuals who progressed to AIDS within 7 years after (imputed) seroconversion] who were all participating in the Amsterdam Cohort Studies on HIV and AIDS in homosexual men. All individuals were infected with HIV-1 subtype B and were either seropositive at entry to the cohort studies (seroprevalent cases with an imputed seroconversion date on average 18 months before entry to the cohort; van Griensven et al., 1989; Mascola et al., 2005) or seroconverted during active follow-up in the cohort studies. None of the participants received combination anti-retroviral therapy during the sampling period; samples were obtained on average at 28 months (range, 24–33 months).
The Amsterdam Cohort Studies are conducted in accordance with the ethical principles set out in the Declaration of Helsinki and written consent was obtained prior to data collection from each participant. The study was approved by the Academic Medical Center institutional medical ethics committee.
Viruses.
Sera from all 35 patients were tested for neutralizing activity in a pseudovirus assay developed by Monogram Biosciences. The tier 2–3 virus panel (Table 1) that we used for determining cross-neutralizing activity in serum consisted of HIV-1 pseudoviruses from subtypes A (n=5), B (n=6), C (n=7) and D (n=5) and included recently transmitted isolates and moderately neutralization-sensitive and -resistant primary HIV-1 variants, based on previously determined neutralization sensitivities to subtype B sera and mAbs b12, 2G12 and 4E10 (Binley et al., 2004; Schweighardt et al., 2007; Simek et al., 2009). In addition, five subtype B HIV-1 reference strains were included (1196, BaL, JRCSF, NL4-3 and SF162; AIDS Repository, NIH, Bethesda, MD, USA). Pseudotyped virus particles were produced by cotransfecting HEK293 cells (AIDS Repository, NIH) with an expression vector carrying the patient-derived gp160 gene (eETV) and an HIV-1 genomic vector carrying a luciferase reporter gene (pRTV1.F-lucP.CNDO-ΔU3). Forty-eight hours after transfection, pseudovirus stocks were harvested and small aliquots were tested for infectivity by using U87 target cells (a gift from N. Landau, Department of Microbiology, New York University School of Medicine, NY, USA) expressing CD4, CCR5 and CXCR4. Pseudovirus stocks were then diluted to titres that, as measured by relative light units, fell within a range known to yield reproducible IC50 values.
Neutralization assay.
A recombinant virus assay involving a single round of virus infection was used to measure neutralization (Petropoulos et al., 2000; Richman et al., 2003). Diluted pseudoviruses were incubated for 1 h at 37 °C with serial dilutions of serum, after which the U87 target cells were added. The ability of patient sera to neutralize virus infection was assessed by measuring luciferase activity 72 h after virus inoculation in comparison to a control infection with a virus pseudotyped with the murine leukemia virus envelope (aMLV).
Neutralization titres are expressed as the reciprocal of the plasma dilution that inhibited virus infection by 50 % (IC50). Neutralization titres were considered positive if they were three times greater than the negative aMLV control.
Statistical analyses.
Statistical analyses were performed by using the spss 16 software package. Neutralization titres, expressed as the reciprocal of IC50, and the number of viruses that were neutralized were not distributed normally. Therefore, the non-parametric Kruskal–Wallis test and Mann–Whitney U test were used to compare the neutralization titres between sera that had strong cross-clade neutralizing activity, cross-subtype B-specific neutralizing activity only or no cross-reactive neutralizing activity at all. For the calculation of IC50 values for viruses that were not inhibited by the 1 : 40 serum dilution, we assumed that 50 % inhibition would have occurred at a 1 : 20 serum dilution. A result was considered significant when the P-value was <0.05.
Acknowledgments
The Amsterdam Cohort Studies on HIV infection and AIDS, a collaboration between the Amsterdam Health Service, the Academic Medical Center of the University of Amsterdam, Sanquin Blood Supply Foundation and the University Medical Center Utrecht, are part of the Netherlands HIV Monitoring Foundation and supported financially by the Netherlands National Institute for Public Health and the Environment. This work is supported financially by the Netherlands Organization for Scientific Research (NWO), grant 918.66.628, the European Community's Seventh Framework Programme NGIN (FP7/2007-2013) under grant agreement no. 201433, the European Community's Six Framework Programme Europrise (FP6/2007-2012) under grant no. 037611 and also partially by an NIH Small Business Innovation Research (SBIR) grant (5R44AI062522) awarded to Monogram Biosciences. The funding organizations had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. We are grateful to Jan Albert for his valuable suggestions and to Evelien Bunnik and Andrea Rachinger for their help with the sequence analysis.
References
- Binley, J. M., Wrin, T., Korber, B., Zwick, M. B., Wang, M., Chappey, C., Stiegler, G., Kunert, R., Zolla-Pazner, S. & other authors (2004). Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J Virol 78, 13232–13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, B. K., Wieczorek, L., Sanders-Buell, E., Rosa, B. A., Robb, M. L., Birx, D. L., Michael, N. L., McCutchan, F. E. & Polonis, V. R. (2008). Cross-clade neutralization patterns among HIV-1 strains from the six major clades of the pandemic evaluated and compared in two different models. Virology 375, 529–538. [DOI] [PubMed] [Google Scholar]
- Bunnik, E. M., Pisas, L., van Nuenen, A. C. & Schuitemaker, H. (2008). Autologous neutralizing humoral immunity and evolution of the viral envelope in the course of subtype B human immunodeficiency virus type 1 infection. J Virol 82, 7932–7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton, D. R., Desrosiers, R. C., Doms, R. W., Koff, W. C., Kwong, P. D., Moore, J. P., Nabel, G. J., Sodroski, J., Wilson, I. A. & other authors (2004). HIV vaccine design and the neutralizing antibody problem. Nat Immunol 5, 233–236. [DOI] [PubMed] [Google Scholar]
- Conley, A. J., Gorny, M. K., Kessler, J. A., Boots, L. J., Ossorio-Castro, M., Koenig, S., Lineberger, D. W., Emini, E. A., Williams, C. & other authors (1994). Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447–52D. J Virol 68, 6994–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon, A. K., Donners, H., Pantophlet, R., Johnson, W. E., Decker, J. M., Shaw, G. M., Lee, F. H., Richman, D. D., Doms, R. W. & other authors (2007). Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J Virol 81, 6548–6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria-Rose, N. A., Klein, R. M., Manion, M. M., O'Dell, S., Phogat, A., Chakrabarti, B., Hallahan, C. W., Migueles, S. A., Wrammert, J. & other authors (2009). Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol 83, 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaschen, B., Taylor, J., Yusim, K., Foley, B., Gao, F., Lang, D., Novitsky, V., Haynes, B., Hahn, B. H. & other authors (2002). Diversity considerations in HIV-1 vaccine selection. Science 296, 2354–2360. [DOI] [PubMed] [Google Scholar]
- Gray, E. S., Meyers, T., Gray, G., Montefiori, D. C. & Morris, L. (2006). Insensitivity of paediatric HIV-1 subtype C viruses to broadly neutralising monoclonal antibodies raised against subtype B. PLoS Med 3, e255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, Y., Sajadi, M. M., Kamin-Lewis, R., Fouts, T. R., Dimitrov, A., Zhang, Z., Redfield, R. R., DeVico, A. L., Gallo, R. C. & other authors (2009). Discordant memory B cell and circulating anti-Env antibody responses in HIV-1 infection. Proc Natl Acad Sci U S A 106, 3952–3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemelaar, J., Gouws, E., Ghys, P. D. & Osmanov, S. (2006). Global and regional distribution of HIV-1 genetic subtypes and recombinants in 2004. AIDS 20, W13–W23. [DOI] [PubMed] [Google Scholar]
- Karlsson Hedestam, G. B., Fouchier, R. A., Phogat, S., Burton, D. R., Sodroski, J. & Wyatt, R. T. (2008). The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol 6, 143–155. [DOI] [PubMed] [Google Scholar]
- Kostrikis, L. G., Cao, Y., Ngai, H., Moore, J. P. & Ho, D. D. (1996). Quantitative analysis of serum neutralization of human immunodeficiency virus type 1 from subtypes A, B, C, D, E, F, and I: lack of direct correlation between neutralization serotypes and genetic subtypes and evidence for prevalent serum-dependent infectivity enhancement. J Virol 70, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrijn, A. F., Poignard, P., Raja, A., Zwick, M. B., Delgado, K., Franti, M., Binley, J., Vivona, V., Grundner, C. & other authors (2003). Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J Virol 77, 10557–10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Migueles, S. A., Welcher, B., Svehla, K., Phogat, A., Louder, M. K., Wu, X., Shaw, G. M., Connors, M. & other authors (2007). Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med 13, 1032–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Svehla, K., Louder, M. K., Wycuff, D., Phogat, S., Tang, M., Migueles, S. A., Wu, X., Phogat, A. & other authors (2009). Analysis of neutralization specificities in polyclonal sera derived from human immunodeficiency virus type 1-infected individuals. J Virol 83, 1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim, M. H. & Emerman, M. (2001). HIV-1 sequence variation: drift, shift, and attenuation. Cell 104, 469–472. [DOI] [PubMed] [Google Scholar]
- Mascola, J. R., D'Souza, P., Gilbert, P., Hahn, B. H., Haigwood, N. L., Morris, L., Petropoulos, C. J., Polonis, V. R., Sarzotti, M. & other authors (2005). Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J Virol 79, 10103–10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan, F. E. (2000). Understanding the genetic diversity of HIV-1. AIDS 14 (Suppl. 3), S31–S44. [PubMed] [Google Scholar]
- McKnight, A. & Aasa-Chapman, M. M. (2007). Clade specific neutralising vaccines for HIV: an appropriate target? Curr HIV Res 5, 554–560. [DOI] [PubMed] [Google Scholar]
- Moog, C., Fleury, H. J. A., Pellegrin, I., Kirn, A. & Aubertin, A. M. (1997). Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol 71, 3734–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, J. P., Cao, Y., Qing, L., Sattentau, Q. J., Pyati, J., Koduri, R., Robinson, J., Barbas, C. F., III, Burton, D. R. & other authors (1995). Primary isolates of human immunodeficiency virus type 1 are relatively resistant to neutralization by monoclonal antibodies to gp120, and their neutralization is not predicted by studies with monomeric gp120. J Virol 69, 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, J. P., Parren, P. W. & Burton, D. R. (2001). Genetic subtypes, humoral immunity, and human immunodeficiency virus type 1 vaccine development. J Virol 75, 5721–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo, G. & Koup, R. A. (2004). Correlates of immune protection in HIV-1 infection: what we know, what we don't know, what we should know. Nat Med 10, 806–810. [DOI] [PubMed] [Google Scholar]
- Petropoulos, C. J., Parkin, N. T., Limoli, K. L., Lie, Y. S., Wrin, T., Huang, W., Tian, H., Smith, D., Winslow, G. A. & other authors (2000). A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob Agents Chemother 44, 920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi, A., Panteleeff, D., Blish, C. A., Baeten, J. M., Jaoko, W., McClelland, R. S. & Overbaugh, J. (2009). HIV-1 neutralizing antibody breadth is affected by factors early in infection, but does not influence disease progression. J Virol 83, 10269–10274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quakkelaar, E. D., van Alphen, F. P., Boeser-Nunnink, B. D., van Nuenen, A. C., Pantophlet, R. & Schuitemaker, H. (2007). Susceptibility of recently transmitted subtype B human immunodeficiency virus type 1 variants to broadly neutralizing antibodies. J Virol 81, 8533–8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman, D. D., Wrin, T., Little, S. J. & Petropoulos, C. J. (2003). Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A 100, 4144–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather, D. N., Armann, J., Ching, L. K., Mavrantoni, A., Sellhorn, G., Caldwell, Z., Yu, X., Wood, B., Self, S. & other authors (2009). Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol 83, 757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid, J. F., Mouquet, H., Feldhahn, N., Seaman, M. S., Velinzon, K., Pietzsch, J., Ott, R. G., Anthony, R. M., Zebroski, H. & other authors (2009). Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458, 636–640. [DOI] [PubMed] [Google Scholar]
- Schweighardt, B., Liu, Y., Huang, W., Chappey, C., Lie, Y. S., Petropoulos, C. J. & Wrin, T. (2007). Development of an HIV-1 reference panel of subtype B envelope clones isolated from the plasma of recently infected individuals. J Acquir Immune Defic Syndr 46, 1–11. [DOI] [PubMed] [Google Scholar]
- Shankarappa, R., Margolick, J. B., Gange, S. J., Rodrigo, A. G., Upchurch, D., Farzadegan, H., Gupta, P., Rinaldo, C. R., Learn, G. H. & other authors (1999). Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J Virol 73, 10489–10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simek, M. D., Rida, W., Priddy, F. H., Pung, P., Carrow, E., Laufer, D. S., Lehrman, J. K., Boaz, M., Tarragona-Fiol, T. & other authors (2009). HIV-1 elite neutralizers: individuals with broad and potent neutralizing activity identified using a high throughput neutralization assay together with an analytical selection algorithm. J Virol 83, 7337–7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbing, J. & Moyle, G. (2003). The clades of HIV: their origins and clinical significance. AIDS Rev 5, 205–213. [PubMed] [Google Scholar]
- Taylor, B. S., Sobieszczyk, M. E., McCutchan, F. E. & Hammer, S. M. (2008). The challenge of HIV-1 subtype diversity. N Engl J Med 358, 1590–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gils, M. J., Euler, Z., Schweighardt, B., Wrin, T. & Schuitemaker, H. (2010). Prevalence of cross-reactive HIV-1-neutralizing activity in HIV-1-infected patients with rapid or slow disease progression. AIDS in press [DOI] [PubMed]
- van Griensven, G. J., de Vroome, E. M., Goudsmit, J. & Coutinho, R. A. (1989). Changes in sexual behavior and the fall in incidence of HIV infection among homosexual men. BMJ 298, 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, B. D. & Burton, D. R. (2008). Toward an AIDS vaccine. Science 320, 760–764. [DOI] [PubMed] [Google Scholar]
- Walker, L. M., Phogat, S. K., Chan-Hui, P. Y., Wagner, D., Phung, P., Goss, J. L., Wrin, T., Simek, M. D., Fling, S. & other authors (2009). Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326, 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, R. G., Bean, W. J., Gorman, O. T., Chambers, T. M. & Kawaoka, Y. (1992). Evolution and ecology of influenza A viruses. Microbiol Rev 56, 152–179. [DOI] [PMC free article] [PubMed] [Google Scholar]